Abstract
BACKGROUND:
Exposure to household air pollution (HAP) causes 4 million deaths annually, and strategies to reduce HAP exposure are urgently required.
OBJECTIVE:
To evaluate the acceptability and feasibility of conducting a trial of a cookstove intervention in rural Malawi.
DESIGN:
Non-smoking women were randomised to continuing to use an open fire (control) or to using a wood-burning clay cookstove (intervention). Symptom burden, oxygen saturation and exhaled carbon monoxide (eCO) were assessed at baseline and 7-day follow-up. A subset of women underwent HAP exposure monitoring.
RESULTS:
Of 51 women recruited, 50 (98%) completed the main study. The methodology was acceptable to participants. Headache, back pain and cough were the most commonly reported symptoms at baseline and follow-up. Median eCO was within normal limits, but with a difference of 0.5 parts per million (ppm) in median change of eCO from baseline to follow-up seen between the two groups (P ∙ 0.035). The peak ambient CO concentration detected was 150 ppm.
CONCLUSION:
This study suggests that a large cookstove intervention trial in Malawi would be feasible with careful community sensitisation. Monitoring exposure to HAP is challenging, and further studies evaluating potential biomarkers of exposure, including eCO, should be undertaken.
Keywords: pollution, smoke, respiratory symptoms, carbon monoxide, biomarker
RÉSUMÉ
CONTEXTE :
L’exposition à la pollution domestique est à l’origine de 4 millions de décès chaque année ; il faut donc élaborer d’urgence des stratégies de lutte contre cette pollution.
OBJECTIF :
Evaluer l’acceptabilité et la faisabilité d’une intervention liée aux fours de cuisine dans une zone rurale du Malawi.
SCHÉMA :
Des femmes non fumeuses ont été réparties de façon aléatoire en deux groupes, le premier groupe (témoins) continuant à utiliser un feu ouvert, le deuxième groupe (intervention) recourant à un four amélioré en argile brûlant du bois. Les symptômes présentés par les femmes, leur saturation en oxygène et le taux de monoxyde de carbone expiré (COe) ont été mesurés au début et après 7 jours. Un sous-groupe de femmes a bénéficié d’un suivi de son exposition à la pollution domestique.
RÉSULTATS :
Sur 51 femmes recrutées, 50 (98%) ont terminé l’étude principale. La méthode a été bien acceptée par les participants. Les céphalées, les lombalgies et la toux étaient les symptômes les plus fréquents au départ et pendant le suivi. Le COe médian restait dans des limites normales, mais on notait une différence de 0.5 ppm de modification médiane entre les deux groupes (P = 0.035). La concentration maximale de CO ambiant était de 150 ppm.
CONCLUSION :
Cette étude suggère qu’une vaste intervention en termes de mise à disposition de fours améliorés serait faisable au Malawi à condition de bien sensibiliser les communautés. Le suivi de l’exposition à la pollution domestique constitue un défi et il serait judicieux d’entreprendre d’autres évaluations de marqueurs biologiques potentiels de cette exposition, notamment le COe.
RESUMEN
MARCO DE REFERENCIA:
La exposición a la contaminación del aire interior causa 4 millones de muertes cada año. Se precisan con urgencia estrategias que reduzcan esta exposición.
OBJETIVO:
Evaluar la aceptabilidad y la factibilidad de la realización de un estudio de intervención sobre las estufas de cocción en una zona rural de Malawi.
MÉTODOS:
Se asignaron en forma aleatoria mujeres no fumadoras a un grupo que continuó la cocción en un fogón al aire libre (grupo testigo) o a otro grupo que cocinó en un fogón de barro con combustión de madera (grupo experimental). Se evaluaron los síntomas, la saturación de oxígeno y el monóxido de carbono espirado (COe) al comienzo del estudio y al séptimo día de seguimiento. En un subgrupo de mujeres se supervisó la exposición a la contaminación del aire interior.
RESULTADOS:
Participaron en el estudio 51 mujeres; 50 de ellas completaron la parte principal de la investigación (98%). El método fue aceptable para las participantes. Los síntomas referidos con mayor frecuencia fueron cefalea, dorsalgia y tos al comienzo del estudio y durante el seguimiento. La mediana de la concentración de COe estuvo dentro de los límites normales, pero se observó una diferencia entre ambos grupos de 0,5 partes por millón (ppm) en la mediana del cambio entre el comienzo del estudio y el seguimiento (P = 0,035). La concentración máxima de CO detectada en el ambiente fue 150 ppm.
CONCLUSIÓN:
Los resultados del presente estudio indican que una intervención sobre las estufas de cocción en gran escala en Malawi sería factible, tras una cuidadosa sensibilización de la comunidad. La medición de la exposición a la contaminación del aire interior es difícil y es necesario realizar nuevas investigaciones que evalúen los posibles biomarcadores de la exposición, entre ellos el CO en el aire espirado.
EXPOSURE TO AIR POLLUTION resulting from burning solid fuels (wood, charcoal, animal dung, crop residues, coal) for essential household activities, such as cooking and heating, is thought to be responsible for 4 million deaths per year.1 Household air pollution (HAP) is associated with childhood pneumonia (the biggest killer in under-fives worldwide), poor neonatal outcomes and chronic obstructive pulmonary disease.1–8 Due to its relative affordability compared to cleaner fuels such as electricity and gas, solid fuel use is widespread; in Malawi, 95% of people rely on solid fuels as their domestic energy source.9 Cooking on open fires in poorly ventilated homes can lead to pollutant levels much greater than is considered safe by the World Health Organization (WHO).10
Interventions to reduce HAP exposure—which need to be efficient, cost-effective and acceptable to the target populations—are varied, and address factors including type of stove, ventilation, fuel type and behaviour modification.11 None of these factors can be evaluated in isolation, making intervention studies in this field complex. When combined with the logistical difficulties of conducting research in resource-poor settings and potential cultural barriers, studies of such interventions can be challenging.
Cookstoves are a promising intervention strategy for reducing HAP exposure. Three randomised controlled trials (RCTs) of cookstove interventions have been conducted to date and have shown improvements in symptom burden, respiratory function and childhood pneumonia, but their findings were limited by poor adherence to the intervention and exposure misclassification.12–14 Furthermore, their findings are not directly transferable to sub-Saharan Africa, given the wide variation in cooking practices and fuels across the globe.
The Global Alliance for Clean Cookstoves (GACC; www.cleancookstoves.org) has been established to address the public health burden of HAP. GACC recognises the need for further studies to assess the health impact, effectiveness and acceptability of interventions in different settings, to inform policy decisions and justify large-scale intervention programmes. Given the previously discussed challenges of conducting these studies, it is vital that emphasis is placed on ensuring that the study design is appropriate for the setting. The primary aim of this study was therefore to assess the feasibility and acceptability of conducting an RCT of a cookstove intervention in rural Malawi to inform future larger studies in this or similar settings.
The Chitetezo stove is a simple clay cookstove (Figure 1) for burning solid fuels that was chosen as the intervention in this study because it reduces fuel consumption by approximately 40% compared to a traditional ‘three-stone’ fire,15 it is produced by local women’s groups using local materials and it is low cost (approximately US$2).
Figure 1.

The Chitetezo stove is a simple clay cookstove for burning solid fuels that aims to reduce exposure to household air pollution by burning fuel more efficiently than an open fire, thereby reducing fuel consumption and producing fewer waste combustion products.
METHODS
Participants
Women living in the Ntcheu District of Malawi who cooked on traditional open wood fires, but wished to purchase a Chitetezo stove, were invited to take part in this pilot, parallel RCT. For logistical reasons, community engagement meetings were held in different villages on different days to inform women about the study. Informed written consent was obtained in private and was completed in Chichewa, the local language. Individuals were excluded if they were current smokers or lived with a smoker.
Randomisation
Women were individually randomised to one of two parallel groups in a 1:1 ratio with block randomisation (block size 10), generated using a random number table. Randomisation was concealed using pre-prepared, sequentially numbered, opaque sealed envelopes.
Intervention
Women in the intervention group purchased a Chitetezo stove on the day of recruitment or the following day. They were asked to stop using their traditional open wood fire and commence cooking on their Chitetezo stove with immediate effect, following instructions from the study workers regarding the use and maintenance of their Chitetezo stove. Women in the control group were asked to continue cooking on a traditional open wood fire and were able to purchase their Chitetezo stove 7 days later, at the end of the study.
Outcomes
Data were collected at baseline (before randomisation) and at 7-day follow-up. The primary outcome of this study was to assess the feasibility of conducting a cookstove intervention study in this setting, including exploring any logistical challenges faced. The acceptability to the study population of the methodology used was also considered. Data regarding health and HAP exposure were collected as secondary outcomes, although given the pilot nature of this study, we did not aim to assess the performance or impact of the Chitetezo stove. Questionnaires regarding demographics, household details and symptom burden, pulse oximetry for oxygen saturation (SpO2) and exhaled carbon monoxide (eCO) levels using a handheld monitor were all used.
Exposure to pollution was monitored for a minority of women who volunteered to take part, and limited by the number of monitors available. A Sidepak monitor (TSI Inc, Shoreview, MN, USA), used to measure air particulate matter (PM < 2.5 μm in size), was placed in a woman’s home for 24 h at baseline and 7-day follow-up. Personal CO monitors (Lascar USB Dataloggers; Lascar Electronics, Salisbury, UK), which clip to the clothes, were worn continuously for 24 h at baseline and 7-day follow-up.
Data were collected in the villages using a paper-based case report form and then collated in an Excel (Microsoft, Redmond, WA, USA) spreadsheet by a study worker.
Sample size
A sample size of 50 (including both groups) was chosen based on the requirement to assess the feasibility of study methodology, rather than to detect any clinical difference between the two groups.
Compensation
The women were compensated for the inconvenience with a gift to the value of approximately US$2. This gift varied between villages, depending on what was agreed before the recruitment process, and included either pigeon pea seeds for cultivation or a Chite-tezo stove.
Statistical methods and analysis
IBM SPSS Statistics 19 was used to analyse data (IBM Corporation, Somers, NY, USA). Histograms were reviewed to assess data distribution, χ2 tests or Fisher’s exact tests were used for comparison of categorical data between the two groups, and Mann Whitney U-tests for continuous data. P < 0.05 was considered statistically significant. Missing data were excluded from the analysis on a case-by-case basis for health and exposure outcomes. Feasibility and acceptability aspects were reviewed through dialogue with all members of the study group during and after completion of the study.
Ethical approval
This study was a collaboration between the Liverpool School for Tropical Medicine (LSTM), Concern Universal (CU; www.concernuniversal.org) and Clioma Ltd (Malawi). Staff from CU provided the study group with access to the communities, and the approval of village elders was sought.
The study received ethical approval from the LSTM Research Ethics Committee (11.74) and the College of Medicine Research Ethics Committee, University of Malawi, Zomba, Malawi (P.07/11/1103). It was registered with the Pan-African Clinical Trials Registry (PACTR201110000324321).
RESULTS
Recruitment and randomisation
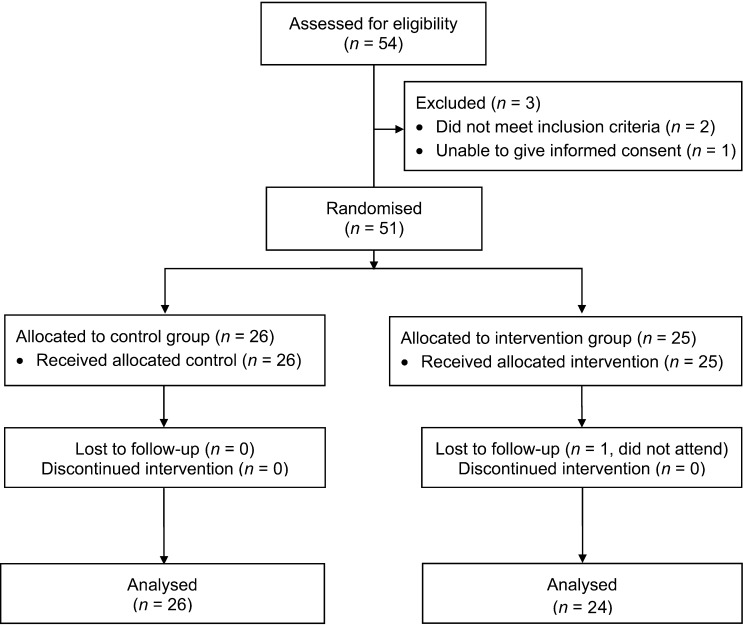
The study took place in November–December 2011. All women approached were keen to participate. Two women were not eligible for participation due to the exclusion criteria (Figure 2). Following assessment by two study workers, one elderly woman was deemed unable to give informed consent due to a lack of understanding. A total of 51 women were recruited from five villages, and recruitment stopped once the desired sample size had been reached: 26 women (51%) were randomised to the control group and 25 (49%) to the intervention group (Figure 2).
Figure 2.
Consort flow diagram showing recruitment to the study, randomisation and loss to follow-up.
Retention
The majority of the women (n = 50, 98%) completed the main study—one woman from the intervention group did not attend the follow-up session.
Feasibility
No objections were raised to the randomisation process or to a delay in receiving a Chitetezo stove. Although all of the recruited women wished to purchase a Chitetezo stove, many were unable to do so on the day of the visit, and many therefore opted to have a Chitetezo stove as their compensation gift to allow them to participate. No adverse events occurred during the study.
All of the recruited women completed the questionnaire, with no objections raised to any of the questions asked. No problems with measuring SpO2 or eCO were encountered.
One of the four women who volunteered to wear the CO monitor declined to do so at follow-up due to superstitious beliefs regarding the monitor removing oxygen from the air. One static PM monitor was placed in the home of a participant for 24 h at baseline and follow-up (data not shown). As it was not possible to leave the monitor in the separate building where this woman cooked due to security concerns, it was instead placed inside her main living area (where there was no fire).
Baseline data
As shown in Table 1, limited resources were available to the participants. Sources of HAP exposure are reported in Table 2: 98% used wood as their primary fuel source, and most cooked inside a building separate from their main house in both dry and wet seasons. The majority of the women did not use any form of heating. Simple battery powered light-emitting diode lights were used by 90% of the women. All the women denied any other forms of smoke exposure.
Table 1.
Baseline characteristics of the study participants
| Control group (n = 26) |
Intervention group (n = 25) |
||||
|---|---|---|---|---|---|
| Parameter | n (%) | Missing data n (%) | n (%) | Missing data n (%) | Total (N = 51) n (%) |
| Age, years, median [IQR] | 36.5 [36.0] | 2 (7.7) | 33.0 [18.0] | 0 | 38.1 [15.5] |
| Rooms in household, mean ± SD | 2.5 ± 0.9 | 0 | 2.6 ± 1.0 | 0 | 2.5 ± 0.9 |
| Adults in household, mean ± SD | 1.8 ± 0.4 | 2 (7.7) | 2.2 ± 0.6 | 1 (4.0) | 2.0 ± 0.5 |
| Children in household, median [IQR] | 1.5 [2.0] | 2 (7.7) | 2.0 [2.0] | 1 (4.0) | 1.9 [1.6] |
| Roof type | 0 | 0 | |||
| Corrugated iron | 21 (80.8) | 22 (88.0) | 43 (84.3) | ||
| Grass | 5 (19.2) | 3 (12.0) | 8 (15.7) | ||
| Window type | 2 (7.7) | 2 (8.0) | |||
| No window | 6 (25.0) | 2 (8.7) | 8 (17.0) | ||
| Space only | 10 (41.7) | 12 (52.2) | 22 (46.8) | ||
| Glass | 8 (33.3) | 9 (39.9) | 17 (36.2) | ||
| Water supply type | 0 | 0 | |||
| Communal pipe | 5 (19.2) | 7 (28.0) | 12 (23.5) | ||
| Well/bore hole | 8 (30.8) | 10 (40.0) | 18 (35.3) | ||
| River/other | 1 (3.8) | 0 | 1 (2.0) | ||
| Communal pipe and well or bore hole | 12 (46.2) | 8 (32.0) | 20 (39.2) | ||
| Owned by a member of the household | 0 | 0 | |||
| Car | 0 | 0 | 0 | ||
| Motorcycle | 0 | 0 | 0 | ||
| Bicycle | 10 (38.5) | 11 (44.0) | 21 (41.2) | ||
| Radio | 7 (26.9) | 11 (44.0) | 18 (35.3) | ||
| Refrigerator | 0 | 0 | 0 | ||
| Television | 1 (3.8) | 0 | 1 (2.0) | ||
| Telephone | 5 (19.2) | 7 (28.0) | 12 (23.5) | ||
| Computer | 0 | 0 | 0 | ||
IQR = interquartile range; SD = standard deviation.
Table 2.
Participants smoke exposure at baseline
| Missing data |
||||
|---|---|---|---|---|
| Parameter | Control group (n = 26) n (%) | Intervention group (n = 25) n (%) | Control group n (%) | Intervention group n (%) |
| Primary cooking method | 1 (3.8) | 1 (4.0) | ||
| Wood | 24 (96.0) | 24 (100.0) | ||
| Mbaula* | 1 (4.0) | 0 | ||
| Secondary cooking method | 1 (3.8) | 1 (4.0) | ||
| None used | 21 (84.0) | 24 (100.0) | ||
| Wood fire | 1 (4.0) | 0 | ||
| Mbaula* | 2 (8.0) | 0 | ||
| Crop residue fire | 1 (4.0) | 0 | ||
| Dry season primary cooking location | 1 (3.8) | 0 | ||
| Inside main living area | 0 | 1 (4.0) | ||
| Elsewhere inside main house | 2 (8.0) | 2 (8.0) | ||
| Separate building | 17 (68.0) | 21 (84.0) | ||
| Outside | 6 (24.0) | 1 (4.0) | ||
| Wet season primary cooking location | 1 (3.8) | 0 | ||
| Inside main living area | 0 | 1 (4.0) | ||
| Elsewhere inside main house | 2 (8.0) | 2 (8.0) | ||
| Separate building | 17 (68.0) | 21 (84.0) | ||
| Outside | 6 (24.0) | 1 (4.0) | ||
| Primary heating method | 0 | 0 | ||
| No heating used | 21 (80.8) | 21 (84.0) | ||
| Wood fire | 3 (11.5) | 4 (16.0) | ||
| Mbaula* | 2 (7.7) | 0 | ||
| Primary lighting method | 0 | 3 (12.0) | ||
| No lighting used | 2 (7.7) | 0 | ||
| Paraffin/kerosene lamp | 2 (7.7) | 0 | ||
| Battery powered torch | 21 (80.8) | 22 (100.0) | ||
| Hurricane lamp | 1 (3.8) | 0 | ||
Traditional stove.
Symptom burden and oxygen saturation
Headache, back pain and cough were the most commonly reported symptoms at baseline (Table 3). Of those reporting a headache at baseline, the median number of days the headache had been experienced in the 7 days preceding baseline was 3 (interquartile range [IQR] 2–4), with those reporting back pain having experienced it for a median of 5 days (IQR 2–7). Symptom burden at follow-up was very similar (Table 3). There was no significant difference in change in symptoms over the 7-day period between the two groups (data not shown). Median SpO2 when breathing room air were respectively 98% and 99% in the control group and intervention group at baseline, and 99% in both groups at follow-up.
Table 3.
Symptoms reported at baseline and follow-up
| Missing data |
Number of individuals reporting symptom |
||||
|---|---|---|---|---|---|
| Symptom | Control group (n = 26) n (%) | Intervention group (n = 25) n (%) | Control group n (%) | Intervention group n (%) | P value |
| Baseline | |||||
| Cough | 0 | 0 | 9 (34.6) | 7 (28.0) | 0.611* |
| Mucus | 0 | 0 | 2 (7.7) | 0 | 0.490† |
| Shortness of breath | 0 | 0 | 2 (7.7) | 3 (12.0) | 0.668† |
| Wheezing or whistling in chest | 1 (3.8) | 0 | 1 (4.0) | 1 (4.0) | 1.000† |
| Sneezing or runny nose | 1 (3.8) | 1 (4.0) | 7 (28.0) | 2 (8.3) | 0.138† |
| Headache | 0 | 1 (4.0) | 12 (46.2) | 13 (54.2) | 0.571* |
| Burning or watery eyes | 0 | 0 | 6 (23.1) | 3 (12.0) | 0.465† |
| Back pain | 0 | 1 (4.0) | 12 (46.2) | 5 (20.8) | 0.059* |
| Burns | 2 (7.7) | 2 (8.0) | 2 (8.3) | 1 (4.3) | 1.000† |
| Family member with burns | 0 | 0 | 2 (7.7) | 1 (4.0) | 1.000† |
| Follow-up | |||||
| Cough | 0 | 1 (4.0) | 5 (19.2) | 5 (20.8) | 1.000† |
| Mucus | 0 | 1 (4.0) | 2 (7.7) | 1 (4.2) | 1.000† |
| Shortness of breath | 0 | 1 (4.0) | 1 (3.8) | 0 | 1.000† |
| Wheezing or whistling in chest | 1 (3.8) | 1 (4.0) | 1 (4.0) | 0 | 1.000† |
| Sneezing or runny nose | 0 | 1 (4.0) | 3 (11.5) | 5 (20.8) | 0.456† |
| Headache | 0 | 1 (4.0) | 8 (30.8) | 6 (25.0) | 0.650* |
| Burning or watery eyes | 0 | 1 (4.0) | 6 (23.1) | 1 (4.2) | 0.100† |
| Back pain | 1 (3.8) | 1 (4.0) | 6 (24.0) | 2 (8.3) | 0.247† |
| Burns | 0 | 1 (4.0) | 5 (19.2) | 3 (12.5) | 0.704† |
| Family member with burns | 0 | 1 (4.0) | 0 | 0 | — |
The statistical difference between the two groups for ‘Number of individuals reporting symptom’ was tested using the Χ2 test.
The statistical difference between the two groups for ‘Number of individuals reporting symptom’ was tested using Fisher’s exact test.
Exposure measurements
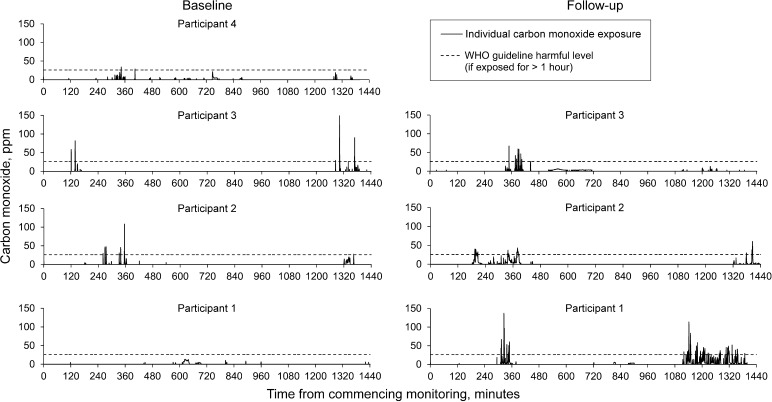
Median eCO was respectively 2 (IQR 2) and 3 (IQR 2) for the control and intervention group at baseline. At follow-up, median eCO was respectively 3 (IQR 2) and 2 (IQR 1) for these groups. The median change in eCO from baseline to follow-up was significantly different between the two groups (median change in eCO 0.0 parts per million [ppm] [IQR 3] and −0.5 ppm [IQR 3] for the control group and intervention respectively, P = 0.035). Four women wore personal CO monitors for 24 h at baseline, and peaks of up to 150 ppm were detected (Figure 3). The results for the three women at follow-up are shown in Figure 3.
Figure 3.
Personal exposures to CO at baseline and at follow-up in four participants. These graphs show the detected level of ambient CO over two separate 24-h periods, measured by personal CO monitors (Lascar USB Dataloggers), which clipped to the clothes of four participants at baseline (left) and three participants at 7-day follow-up (right). A peak reading of 150 ppm was detected. The black dotted line indicates the level the WHO considers unsafe if exposed for >1 hour (26 ppm). WHO = World Health Organization; ppm = parts per million; CO = carbon monoxide.
DISCUSSION
This study has demonstrated that it is feasible to conduct an RCT of a cookstove intervention in rural Malawi. With the high level of interest we observed in participating villages, including village elders, we are encouraged that a larger trial will be possible. Recruitment and retention rates over this short study period were excellent. The main findings of this feasibility study were that the methodologies used, including randomisation, the short delay in receiving the cookstove, the personal health questionnaires and SpO2/exhaled CO measurement, were acceptable to the participants. Difficulties relating to exposure measurements and superstitious beliefs were encountered with only a minority of women. Larger trials will require careful community sensitisation to address superstitious beliefs and achieve excellent retention rates.
The Chitetezo stove was chosen for this study due to its low cost and easy availability. However, despite this, many of the women were unable to afford a Chitetezo stove and so received their stove as a compensation gift. Future studies aiming to detect changes in clinical outcomes should consider the ability of the stoves to significantly reduce HAP exposure. A study of childhood pneumonia following a cookstove intervention showed that greater health benefit is achieved with larger reductions in HAP exposure.13 Stoves that are proven to achieve larger reductions in exposure are available, but are considerably more expensive than the Chitetezo. Future studies or intervention programmes may need to consider subsidising cookstoves, but this may have implications for study validity.
As only a brief snapshot of pollution exposure was captured by the present study, conclusions regarding differences between baseline and follow-up cannot be inferred. However, CO peak levels of 150 ppm detected in these households suggest worrying levels of HAP exposure; the WHO Air Quality Guidelines recommend that individuals should not be exposed to CO concentrations of >26 ppm for >1 h (Figure 3) or to concentrations exceeding 87 ppm for >15 min.16 Monitoring exposure to air pollution was logistically challenging, requiring additional visits to retrieve the equipment. In a resource-poor setting, this significant additional demand on transport and fuel resources should be considered when planning larger studies.
Previous studies have used intensive methods of monitoring PM and CO at individual and household levels.10,13 However, these techniques are expensive and challenging, and interpretation of results is not standardised. To effectively evaluate the impact of HAP reduction strategies, a standardised and convenient approach to assessing HAP exposure is required. Development of a biomarker that is representative of HAP exposure over the preceding weeks or months could remove the need for complex air sampling, and aid in the timely delivery of effective interventions to the market. Several potential biomarkers have been explored, but none are yet suitable for routine use.17–19 In this study, measurement of eCO was trialled but the majority of readings were within the normal range. However, a significant reduction of 0.5 ppm was seen at follow-up in the intervention group, and although this is unlikely to be clinically significant, small changes in eCO levels may be a sensitive and responsive marker of HAP exposure. Further testing of a larger sample size is required to establish this. Furthermore, in this study, the eCO measurements were taken outdoors, away from the fires/stoves; measuring eCO in the vicinity of smoke exposure may improve the sensitivity of eCO as a biomarker. Alternatively, alveolar macrophage carbon load (AMCL) obtained by induced sputum, which may represent a longer exposure period than eCO, may prove a useful biomarker. Increased AMCL has been detected in individuals who report exposure to HAP compared to those who do not,20 although further development of this methodology for this application is required.
A weakness of this study is that participants’ experiences of using the stove were not formally assessed and actual use was not quantified. Individuals may initially be reluctant to use a new stove exclusively in the place of traditional methods. In a future study, assessing use and the impact this has on exposure, fuel consumption and expenditure should be a key consideration.
This pilot study was not large enough to detect clinical changes; a study with more participants and longer follow-up period would be required to detect any differences in symptom burden between these groups. As the SpO2 levels measured were within normal limits at both time points, this is unlikely to be a useful variable for detecting changes after intervention. Although not used in this pilot study, lung function testing would enable an objective assessment of airway damage in future studies, although depending on the length of follow-up in the study, changes in these parameters following intervention may be difficult to detect. Spirometry testing in the community has been shown to be successful in Malawi in the recently conducted Blantyre Health Study (publication in progress), and will be extensively used in a planned RCT of a cookstove intervention in Malawi (Cooking and Pneumonia Study, www.capstudy.org).
CONCLUSIONS
Further studies exploring strategies for HAP reduction and their impact on health are urgently needed. Although the complex nature of these interventions means that such studies are challenging, we are encouraged that a larger scale RCT of a cookstove intervention will be feasible and acceptable in rural Malawi. Adequate community sensitisation and careful consideration of appropriate outcome measures will be required. Intensive monitoring of HAP exposure levels or development of a biomarker of exposure are warranted, but significant investment will be required to achieve this.
Conflict of interest: none declared.
Acknowledgments
The authors thank all of the participants and village elders for their kind cooperation, and Concern Universal (CU), Blantyre, Malawi, for providing access to local communities, staff time and logistical support. The authors also thank all staff at CU, Malawi and Clioma Ltd, Malawi, who supported this study and to the Liverpool School of Tropical Medicine for providing a Research Development Fund grant which enabled this work. HRJ is a Wellcome Trust Funded Clinical PhD Fellow.
References
- 1.Lim S S, Vos T, Flaxman A D, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2013;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levesqu B, Allaire S, Gauvin D, et al. Wood–burning appliances and indoor air quality. Sci Total Environ. 2001;281:47–62. doi: 10.1016/s0048-9697(01)00834-8. [DOI] [PubMed] [Google Scholar]
- 3.Shen M, Chapman R S, Vermeulen R, et al. Coal use, stove improvement, and adult pneumonia mortality in Xuanwei, China: a retrospective cohort study. Environ Health Perspect. 2009;117:261–266. doi: 10.1289/ehp.11521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ezzati M, Kammen D. Indoor air pollution from biomass combustion and acute respiratory infections in Kenya: an exposure-response study. Lancet. 2001;358:619–624. doi: 10.1016/s0140-6736(01)05777-4. [DOI] [PubMed] [Google Scholar]
- 5.Dherani M, Pope D, Mascarenhas M, Smith K R, Weber M, Bruce N. Indoor air pollution from unprocessed solid fuel use and pneumonia risk in children aged under five years: a systematic review and meta-analysis. Bull World Health Organ. 2008;86:390–398C. doi: 10.2471/BLT.07.044529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torres-Duque C, Maldonado D, Perez-Padilla R, Ezzati M, Viegi G. Biomass fuels and respiratory diseases: a review of the evidence. Proc Am Thorac Soc. 2008;5:577–590. doi: 10.1513/pats.200707-100RP. [DOI] [PubMed] [Google Scholar]
- 7.Diette G B, Accinelli R A, Balmes J R, et al. Obstructive lung disease and exposure to burning biomass fuel in the indoor environment. Global Heart. 2012;7:265–270. doi: 10.1016/j.gheart.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sram R J, Binkova B, Dejmek J, Bobak M. Ambient air pollution and pregnancy outcomes: a review of the literature. Environ Health Perspect. 2005;113:375–382. doi: 10.1289/ehp.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization . Geneva: Switzerland; World health statistics. WHO, 2012. http://www.who.int/healthinfo/EN_WHS2012_Full.pdf Accessed November 2013. [Google Scholar]
- 10.Fullerton D G, Semple S, Kalambo F, et al. Biomass fuel use and indoor air pollution in homes in Malawi. Occup Environ Med. 2009;66:777–783. doi: 10.1136/oem.2008.045013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fullerton D G, Bruce N, Gordon S B. Indoor air pollution from biomass fuel smoke is a major health concern in the developing world. Trans R Soc Trop Med Hyg. 2008;102:843–851. doi: 10.1016/j.trstmh.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romieu I, Riojas-Rodriguez H, Marron-Mares A T, Schilmann A, Perez-Padilla R, Masera O. Improved biomass stove intervention in rural Mexico: impact on the respiratory health of women. Am J Respir Crit Care Med. 2009;180:649–656. doi: 10.1164/rccm.200810-1556OC. [DOI] [PubMed] [Google Scholar]
- 13.Smith K R, McCracken J P, Weber M W, et al. Effect of reduction in household air pollution on childhood pneumonia in Guatemala (RESPIRE): a randomised controlled trial. Lancet. 2011;378:1717–1726. doi: 10.1016/S0140-6736(11)60921-5. [DOI] [PubMed] [Google Scholar]
- 14.Hanna R, Duflo E, Greenstone M. NBER Working Paper No 18033. Cambridge, MA, USA: National Bureau of Economic Research, 2012; Up in smoke: the influence of household behaviour on the long-run impact of improved cooking stoves. [Google Scholar]
- 15.Malinski B. Pretoria: South Africa; Impact of Chitetezo Mbaula—improved household firewood stove in rural Malawi. Gesellschaft fur Technische Zusammenarbeit and Programme for Basic Energy and Conservation, 2008. http://www.probec.org/fileuploads/fl09222008074344_Impact_Assessment_of_Chitetezo_Mbaula_Improved_Household_Firewood_Stove_in_Rural_Malawi.pdf Accessed November 2013. [Google Scholar]
- 16.Raub J. 2nd ed. Geneva, Switzerland: World Health Organization, 1999; Environmental Health Criteria 213: carbon monoxide. [Google Scholar]
- 17.Behera D, Dash S, Yadav S P. Carboxyhaemoglobin in women exposed to different cooking fuels. Thorax. 1991;46:344–346. doi: 10.1136/thx.46.5.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lam N, Nicas M, Ruiz-Mercado I, Thompson L M, Romero C, Smith K R. Non-invasive measurement of carbon monoxide burden in Guatemalan children and adults following wood-fired temazcal (sauna-bath) use. J Environ Monit. 2011;13:2172–2181. doi: 10.1039/c1em10172b. [DOI] [PubMed] [Google Scholar]
- 19.Dills R L, Zhu X, Kalman D A. Measurement of urinary methoxyphenols and their use for biological monitoring of wood smoke exposure. Environ Res. 2001;85:145–158. doi: 10.1006/enrs.2000.4107. [DOI] [PubMed] [Google Scholar]
- 20.Kulkarni N S, Prudon B, Panditi S L, Abebe Y, Grigg J. Carbon loading of alveolar macrophages in adults and children exposed to biomass smoke particles. Sci Total Environ. 2005;345:23–30. doi: 10.1016/j.scitotenv.2004.10.016. [DOI] [PubMed] [Google Scholar]