SUMMARY
Existing approaches to tuberculosis (TB) control have been no more than partially successful in areas with high human immunodeficiency virus (HIV) prevalence. In the context of increasingly constrained resources, mathematical modelling can augment understanding and support policy for implementing those strategies that are most likely to bring public health and economic benefits. In this paper, we present an overview of past and recent contributions of TB modelling in this key area, and suggest a way forward through a modelling research agenda that supports a more effective response to the TB-HIV epidemic, based on expert discussions at a meeting convened by the TB Modelling and Analysis Consortium. The research agenda identified high-priority areas for future modelling efforts, including 1) the difficult diagnosis and high mortality of TB-HIV; 2) the high risk of disease progression; 3) TB health systems in high HIV prevalence settings; 4) uncertainty in the natural progression of TB-HIV; and 5) combined interventions for TB-HIV. Efficient and rapid progress towards completion of this modelling agenda will require co-ordination between the modelling community and key stakeholders, including advocates, health policy makers, donors and national or regional finance officials. A continuing dialogue will ensure that new results are effectively communicated and new policy-relevant questions are addressed swiftly.
Keywords: tuberculosis, mathematical modelling, HIV, sub-Saharan Africa, systematic literature review
RESUME
Les approches existantes de la lutte contre la tuberculose (TB) n'ont eu qu'un succès relatif dans les zones à prévalence élevée de virus de l'immunodéficience humaine (VIH). Dans un contexte de ressources de plus en plus limitées, un modèle mathématique peut augmenter la compréhension et soutenir les politiques de mise en œuvre de stratégies plus susceptibles d'offrir des bénéfices en termes d'économie et de santé publique. Dans cet article, nous présentons un aperçu des contributions passées et récentes de la modélisation de la TB dans ce domaine clé et suggérons une façon de répondre plus efficacement à l'épidémie de TB-VIH grâce à un programme de recherche de modélisation en se basant sur des discussions d'experts lors d'une réunion convoquée par le Consortium de modélisation et d'analyse de la TB. Le programme de recherche a identifié des domaines hautement prioritaires pour les futures activités de modélisation, notamment : 1) les difficultés de diagnostic et la mortalité élevée de la TB-VIH ; 2) le risque élevé de progression de la maladie ; 3) le système de prise en charge de la TB dans les zones à haute prévalence du VIH ; 4) l'incertitude de la progression naturelle de la TB-HIV ; et 5) les interventions combinées pour la TB-VIH. Une progression efficace et rapide vers l'achèvement de ce programme de modélisation nécessitera une coordination entre la communauté de modélisation et les partenaires principaux, responsables de plaidoyer, décideurs politiques, donateurs et responsables financiers nationaux ou régionaux. Un dialogue continu s'assurera que les nouveaux résultats sont réellement diffusés et que les nouvelles questions relatives aux politiques sont prises en compte rapidement.
RESUMEN
Los enfoques actuales de lucha contra la tuberculosis (TB) solo han alcanzado una eficacia parcial en las zonas con alta prevalencia de la infección por el virus de la inmunodeficiencia humana (VIH). En el contexto de una restricción progresiva de los recursos, los modelos matemáticos pueden mejorar la comprensión y dar mayor respaldo a las políticas encaminadas a la ejecución de las estrategias con mayor probabilidad de aportar beneficios de salud pública y ventajas económicas. En el presente artículo se examinan las contribuciones pasadas y recientes de la modelización en esta importante aspecto y se propone una forma de avanzar, mediante un programa de investigación en modelización, que respalde una respuesta más eficaz a la epidemia de TB-VIH, a partir de los intercambios de los expertos durante una reunión convocada por el Consorcio de Modelización y Análisis en TB. El programa de investigación encontró esferas de alta prioridad para las futuras iniciativas de modelización, como son: 1) el diagnóstico difícil y la alta mortalidad de la coinfección por el TB-VIH; 2) el alto riesgo de progresión hacia la enfermedad tuberculosa; 3) los sistemas de atención de la TB en los entornos con alta prevalencia del VIH; 4) la incertidumbre sobre la evolución natural de la TB-VIH; y 5) las intervenciones conjuntas en materia de TB-VIH. El progreso eficaz y rápido hacia la culminación de este programa de modelización exigirá una coordinación entre la comunidad de la modelización y los principales interesados directos, entre ellos los promotores, los responsables de elaborar las políticas sanitarias, los donantes y los funcionarios encargados de las cuestiones financieras a escala nacional y regional. Un diálogo sostenido logrará la difusión eficaz de los resultados recientes y el planteamiento oportuno de nuevos aspectos relacionados con las políticas.
WHILE MYCOBACTERIUM TUBERCULOSIS and the human immunodeficiency virus (HIV) are independently responsible for substantial human suffering and death, in areas where these pathogens dually infect the population, their combined effect has been devastating. HIV-related immunosuppression markedly increases the risk for progression to tuberculosis (TB) disease after M. tuberculosis infection,1 and may increase the risk of initial infection; accordingly, in areas of generalised HIV epidemics, there have been steep increases in TB incidence.2 During periods of limited success in HIV prevention and control, standard approaches for TB control have been inadequate in these settings; novel strategies are therefore urgently needed.3
Mathematical models, defined by Garnet et al. as mechanistic representations for how disease burden is established, are useful tools for projecting the potential public health and economic impact of interventions when population-level empirical data, such as from cluster-randomised trials, are unavailable and too expensive, too time consuming or unethical to acquire.4 Models can also provide insight by simplifying complex systems into frameworks that are more easily understood. For example, the relationship between the scale-up of antiretroviral therapy (ART) and the subsequent impact on population-level TB incidence is difficult to predict, but can be understood using a combined model of HIV and TB transmission.5 In a time of limited resources, mathematical modelling, grounded in available data, can be an important guide for the rational use of resources in TB control, development pipelines of new drugs, vaccines or diagnostics, and highlight what empirical data gaps need to be filled.
Recognising the urgency of TB control in high HIV prevalence settings and the potential contributions of modelling, the TB Modelling and Analysis Consortium (TB MAC, Table 1) convened its first meeting between empirical scientists, policy makers and mathematical modellers in September 2012 in Johannesburg, South Africa. The aim of this meeting was to identify a modelling research agenda to advance TB control in high HIV prevalence settings. In the present perspective, we summarise the key historical contributions of TB-HIV modelling following a systematic literature review and identify a future modelling research agenda that would help hasten the reduction of the TB-HIV epidemic.
Table 1.
The TB Modelling and Analysis Consortium

METHODS
A detailed report of the meeting preparations, resources and documents available to the participants and discussion outcomes can be found on the TB MAC website (www.tb-mac.org/WorkAreas/WorkArea/1). In summary, to identify existing TB modelling and cost-effectiveness studies in high HIV prevalence settings (restricted for this review to sub-Saharan Africa or sub-populations with an adult HIV prevalence of over 5%), a systematic literature review was performed in September 2012. We searched PubMed, private libraries, existing reviews and mathematical modelling journals. Further details of the review methods and results are given in the Appendix, including details of the selection process in Figure A.* A formal assessment of model quality was considered to be beyond the scope of this review. Existing research priority agendas were also scanned for potential modelling questions (RMGJH and RGW) to stimulate discussions.6–8
The above documents were used as preparatory material for participants in a 2-day meeting in Johannesburg, South Africa, in September 2012 between key stakeholders. Participants, including empirical scientists, policy makers and mathematical modellers, to discuss modelling research questions in three main areas chosen to cover the breadth of TB care and control: 1) screening and treatment of active TB and latent tuberculous infection (LTBI), 2) TB vaccines and immunology and 3) the economics of TB. Discussions during the meeting focused primarily on the potential opportunities for modelling efforts to hasten the reduction of the TB-HIV epidemic. On behalf of the meeting participants these lists of research questions were consolidated into key themes for TB care and control, which are described below.
KEY CONTRIBUTIONS OF TB MODELLING IN HIGH HIV PREVALENCE SETTINGS
The review identified 69 papers. A brief summary of these papers and a selection process flow chart can be found in the Appendix. Despite its public health relevance, modelling activity in TB was limited before 2005, after which six or more papers were published each year. Strikingly, nearly all TB-HIV models started from the perspective of the natural history of TB, adding a simple layer on HIV to the core structure on TB,9 although there are notable exceptions to this trend.10
Natural history of TB in high HIV prevalence setting
In February 1992, Schulzer et al. published the first model to quantify the consequences of the emerging disastrous association between HIV and TB.9 Later confirmed by others, these models predicted the steep rise in TB incidence that was to overwhelm many TB programmes in these, usually low-income, settings.2,11–15
Antiretroviral therapy and isoniazid preventive therapy
In 2003, Williams and Dye used modelling to show how the expansion of access to ART in high HIV prevalence settings would contribute little to controlling TB incidence in the population, unless ART was started early (e.g., at CD4 levels of 500/μl), with very high (85%) effective coverage.10
A large number (n = 17) of the modelling papers incorporated preventive therapy, usually isoniazid preventive therapy (IPT). However, the evaluation showed that assumptions on key parameters such as the level and duration of protection offered by IPT varied widely, complicating the interpretation of these generally positive results. Models assumed between a 34%16 or 100%17 reduction in the risk of TB during IPT, while the assumed duration of protection post-therapy varied between immediate loss of effect18 to lifelong protection.17
Impact of additional interventions for TB-HIV
Mathematical models of TB-HIV have also been used to explore enhancements to DOTS-based programmes, including active case finding19 and expanding access to culture-based diagnosis or drug susceptibility testing.20 These models usually found that such enhancements could provide substantial benefits. In 2010, the World Health Organization endorsed a new TB diagnostic test, Xpert® MTB/RIF (Cepheid, Sunnyvale, CA, USA), generating a need for models that explored costs benefits as well as operational aspects of integrating these novel devices into existing TB care and control infrastructure. While four papers in this review explored the individual benefits and costs,21 none incorporated the population effect of improved diagnosis through a transmission component (note: one paper has since addressed this22).
Implementations of interventions for TB
Models can also inform policy questions on implementation of new interventions and tools. Often such models will include an operational modelling component that explicitly captures key parts of the health system.23 While the importance of the operational modelling of combined TB-HIV interventions was recognised, little work has been done in this area, with only one paper addressing this issue, which evaluated the impact of a novel diagnostic tool.23
A RESEARCH AGENDA FOR MODELLING OF TB CONTROL IN HIGH HIV PREVALENCE SETTINGS
In the following sections, we identify five broad priority areas for TB modelling research for the support for TB care and control in high HIV prevalence settings, discuss the empirical evidence and suggest potential opportunities for future modelling efforts. Specific research questions in each area can be found in Table 2.
Table 2.
Key TB-HIV modelling priority areas and sub-questions

Priority area 1: diagnosis and mortality of TB-HIV
Among people living with HIV (PLHIV), TB is both more difficult to diagnose than in HIV-negative individuals and a major cause of death if untreated.2 To reduce mortality, early diagnosis and the resulting access to lifesaving treatment for TB and often HIV, is key. It is therefore likely that intensified case-finding strategies (which aim to diagnose individuals at earlier stages of disease)24 and improved diagnosis20 might have a disproportionate morbidity and mortality benefit among HIV-positive individuals with active TB disease. However, given the likely much shorter duration of overall TB disease and higher probability of smear-negative (i.e., presumably less infectious) disease,2 the impact of such strategies on M. tuberculosis transmission and future TB disease incidence may be less pronounced.
Although the population-level benefits of intensified TB case finding and improved TB diagnosis among PLHIV have not been conclusively demonstrated,24 models can use the best available data to help identify the approaches to diagnosis and case-finding that are likely to be most cost-effective if scaled up at the population level. For example, models that incorporate routes of care-seeking and diagnosis among individuals with HIV and TB can augment these findings by relating them to existing systems of care. Progress in this area is therefore clearly dependent on increasing the empirical evidence base related to the organisation of health systems for HIV and TB in resource-constrained settings (Priority area 3) as well as the progression (Priority area 2) and pathogenesis (Priority area 4) of TB-HIV.
Priority area 2: high risk of progression to active TB-HIV
HIV dramatically increases both the rate of TB infection progressing to disease (as measured by recurrent TB episodes with novel molecular fingerprints),25 and the rate of progression from LTBI (as measured by a positive tuberculin skin test) to active TB disease.26 Thus, the potential role of preventing progression (e.g., by using IPT or other preventive therapy regimens, ART or post-exposure vaccines) may be especially pronounced among this population. While IPT has been demonstrated to reduce the risk of active TB during treatment,27 there is ongoing uncertainty about the duration of protection among PLHIV after therapy completion.28 Recent modelling studies have suggested that the failure of isoniazid to sterilise is at least part of the explanation,29 although this may improve following immune recovery with ART co-therapy.30
The population-level effects of TB preventive interventions remain unclear. While models of the impact of preventive therapy among PLHIV should reflect this uncertainty, their guidance is needed to inform decisions regarding the scale-up of preventive therapy.
Advances in diagnostic tools to identify those individuals at highest likelihood for progression, coupled with better understanding of latency and partial immunity in TB-HIV (Priority area 4), can help to better inform the structure and parameterisation of these models. Earlier economic work on the potential cost-effectiveness and cost savings from preventive therapies will need to be revisited to incorporate updated understanding about the duration of effectiveness, individual level effects and costs of maintaining adherence to treatment.
Priority area 3: TB health systems in high HIV prevalence settings
TB requires intensive, often directly observed, therapy with a short course of inexpensive drugs, whereas HIV requires lifelong, mostly unsupervised treatment with expensive agents and regular therapeutic monitoring. However, in HIV-endemic regions, the patients taking these drugs are often the same, and synergistic efforts at linkage to TB and HIV care can improve systems of diagnosis and treatment at relatively low cost.31
Health systems in these settings must therefore adapt to this reality. Operational and economic models have great potential to inform decisions about how to structure health systems, in particular to inform the optimal level of service integration. Such models can identify ways to resolve both allocative inefficiencies (e.g., by combining resources in optimal ways to provide services for those who are co-infected) and technical inefficiencies (e.g., by combining services in optimal ways to improve outcomes). They can also assist in understanding the delays and costs that service users face, and how to reduce them.
Combined health system and economic models could also help identify the corresponding health systems investments needed to support the efficient operation of both TB and HIV services. To date, such health system models have been underutilised;23 however, as resources for TB and HIV care become increasingly constrained, and new technologies continue to be scaled up, health systems models will become increasingly important in helping to maximise value for money.
Priority area 4: uncertainty in the natural history of TB-HIV
All epidemiological models of infectious diseases are limited by the current state of knowledge of the natural history of the pathogen. In the case of TB-HIV, we must consider not only two individual natural histories, but also the interaction of these two (potentially) chronic infectious diseases. The need for a more thorough understanding of the natural history of TB-HIV is clear.
The natural history of untreated HIV is well-known from cohort studies of HIV-infected individuals before the availability of ART, and some insight exists into the natural history of TB from the pre-chemotherapy era (1950s). However, the availability of TB chemotherapy throughout the HIV era has meant that, for ethical and methodological reasons, studies to acquire this information in HIV-positive TB patients are not possible – including the infectious duration of untreated TB32 (and its relationship to CD4 count and/or ART), TB mortality risk,33 and the risk of re-infection and progression to disease relative to non-HIV-infected individuals (Priority area 2).
To accurately project the impact of interventions for TB in HIV-endemic regions, it is essential to better understand these elements of natural history. For example, the impact of early diagnosis cannot be accurately estimated without knowing the duration of infectiousness likely to be averted. Empirical studies that provide further insight into these areas are urgently needed; in the interim, models using existing data may be able to better define and communicate the bounds of our uncertainty.
Priority area 5: combined interventions to control TB-HIV
If we are ultimately to achieve aggressive targets for TB control in HIV-endemic regions, it is unlikely that we can rely on a single intervention.34 Strategies for the control of complex epidemics such as HIV and TB will include multiple interventions for the foreseeable future. As such, we must deploy a combination of interventions that are intelligently targeted at different steps of the M. tuberculosis transmission cycle.
Given the growing number of potential interventions for the control of TB-HIV, including alternative screening and diagnostic approaches (Priority area 1), preventive strategies (Priority area 2) and alternative models for integrated care (Priority area 3), there is an opportunity for models to help identify which interventions will likely perform best when combined. For example, a combination of preventive treatment (targeting LTBI) and intensified case finding (i.e., targeting active disease) may be more effective than one of intensified case finding plus better passive diagnosis (i.e., both targeting active disease).35 Economic considerations are also critical, as certain interventions may be more efficient to combine than others,36 as both provider and patient costs are reduced through economies of scope.
To be able to provide such insight, new models with flexible structures capable of simulating the impact and cost-effectiveness of relevant combinations of interventions are urgently required. Such models could provide a platform for comparing alternative combinations of existing and novel interventions that go beyond current policy in terms of both impact and resource requirements, thus helping to chart the fastest, most cost-effective course possible for the elimination of TB in settings of high HIV prevalence.
CONCLUSION
In this paper, we have identified five critical areas in which TB-HIV models can help advance TB control in high HIV prevalence settings. These include questions ranging from improved understanding of the natural history of TB-HIV to comparative impact and cost-effectiveness achievable from implementation and combination of TB-HIV control strategies. However, efficient and rapid progress towards the completion of this modelling agenda will require coordination between the modelling community and key stakeholders, including advocates, health policy makers, donors and national or regional finance officials. They will be faced with decisions that will increasingly reflect not simple, idealised comparisons of individual interventions, but rather a complex assessment of the optimal combination of available options in real-world settings. As such, effective models will incorporate operational components and inform the prioritisation, sequencing and expected consequences of combination approaches that involve both scaling up new techniques and improving existing systems.
Acknowledgments
The authors thank O Ross-Hurst for her invaluable support during the systematic literature review process and meeting organisation.
TB MAC TB-HIV Meeting participants:
G Garnett (Bill and Melinda Gates Foundation, Seattle, WA, USA); K Fielding, E L Corbett, K Kranzer, P Dodd, A Grant, L Rodrigues, E Vynnycky (London School of Hygiene & Tropical Medicine, London, UK); A Suthar, P Glaziou, C Fitzpatrick (World Health Organization, Geneva, Switzerland; B Williams, W Hanekom, (South African Tuberculosis Vaccine Initiative, Institute of Infectious Diseases, University of Cape Town, Cape Town, South Africa); S Verver (KNCV Tuberculosis Foundation, The Hague, The Netherlands); N A Menzies, T Bärnighausen (Harvard University, Boston, MA, USA); G Churchyard, P Hippner, K Velen, (Aurum Institute, Johannesburg, South Africa); T Hallett (Imperial College, London, UK); A Nanni (AERAS, Rockville, MD, USA); D Maher (Wellcome Trust, London, UK); G Gomez (Amsterdam Institute for Global Health and Development, Amsterdam, The Netherlands); N Foster (Health Economics Unit, University of Cape Town, Cape Town, South Africa); C Sizemore (National Institute of Allergy and Infectious Diseases, Bethesda, MD, USA); T Bärnighausen (Africa Centre for Health and Population Studies, Mtubatuba, South Africa); C Pretorius (Futures Institute, Palo Alto, CA, USA); E Vynnycky (Public Health England, London, UK).
This work was supported by the Bill and Melinda Gates Foundation through the TB Modelling and Analysis Consortium (TB MAC) grant (OPP1084276). The funder was involved in the decision to focus on TB in high HIV prevalence settings, but had no role in the design of the systematic review, scientific content of the meeting or writing the first draft of this manuscript.
Conflict of interest: none declared.
APPENDIX
Full search query for systematic literature review
We carried out a systematic literature review to identify existing tuberculosis (TB) modelling and cost-effectiveness studies in high human immunodeficiency virus (HIV) prevalence settings, with the aim of highlighting gaps in existing work against current research priorities and give an overview of the modelling methods used to date.
We searched the medical literature using a PubMed query which identified papers from an earlier narrative review in 2008.1 The following search query was used in September 2012: (tuberculosis OR TB) AND ((mathem* AND (model OR models)) OR (mathem* modell*) OR (mathem* modeling) OR (modeling OR modelling) OR ‘Population dynamics’[MeSH Terms] OR ‘Population dynamics’ OR ‘System dynamics’ OR ‘Computer simulation’ OR ‘Computer simulation’[MeSH Terms]).
We searched mathematical modelling journals for any papers on TB (search for ‘tuberculosis’ OR ‘TB’) and scanned references from existing reviews for relevant papers. We also searched the personal libraries of TB MAC steering committee members (RGW, CD, AV, TC and DD) who kindly made their personal libraries available. To identify those relevant for high HIV prevalence settings, we included those papers that included any of the terms ‘HIV’, ‘AIDS’, ‘human imm* or ‘Africa’ in their title, keywords or abstract.
Papers were eligible for full-text review if they were written in English and described a mathematical model of TB. For the purposes of this review, in defining ‘mathematical model’, we followed Garnett et al.2 and included decision analytic, cohort, transmission, operational or within-host models, but excluded purely statistical models and studies using models to estimate only resource requirements. Papers were also excluded if they did not model or use data from populations with high HIV prevalence (restricted to sub-Saharan Africa or sub-populations with an adult HIV prevalence of over 5%). Details of the selection process are given in the Figure.3
Figure A.
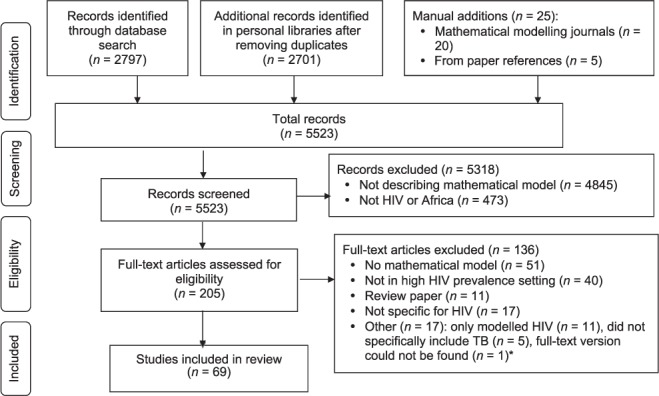
Systematic review flow chart for selection of papers. HIV = human immunodeficiency virus; TB = tuberculosis.3 Note: File with references can be downloaded at http://www.tb-mac.org/resources.
Paper selection was done by RMGJH; data extraction was done by RMGJH with support from RGW.
Table A.
Summary of studies identified in the systematic review
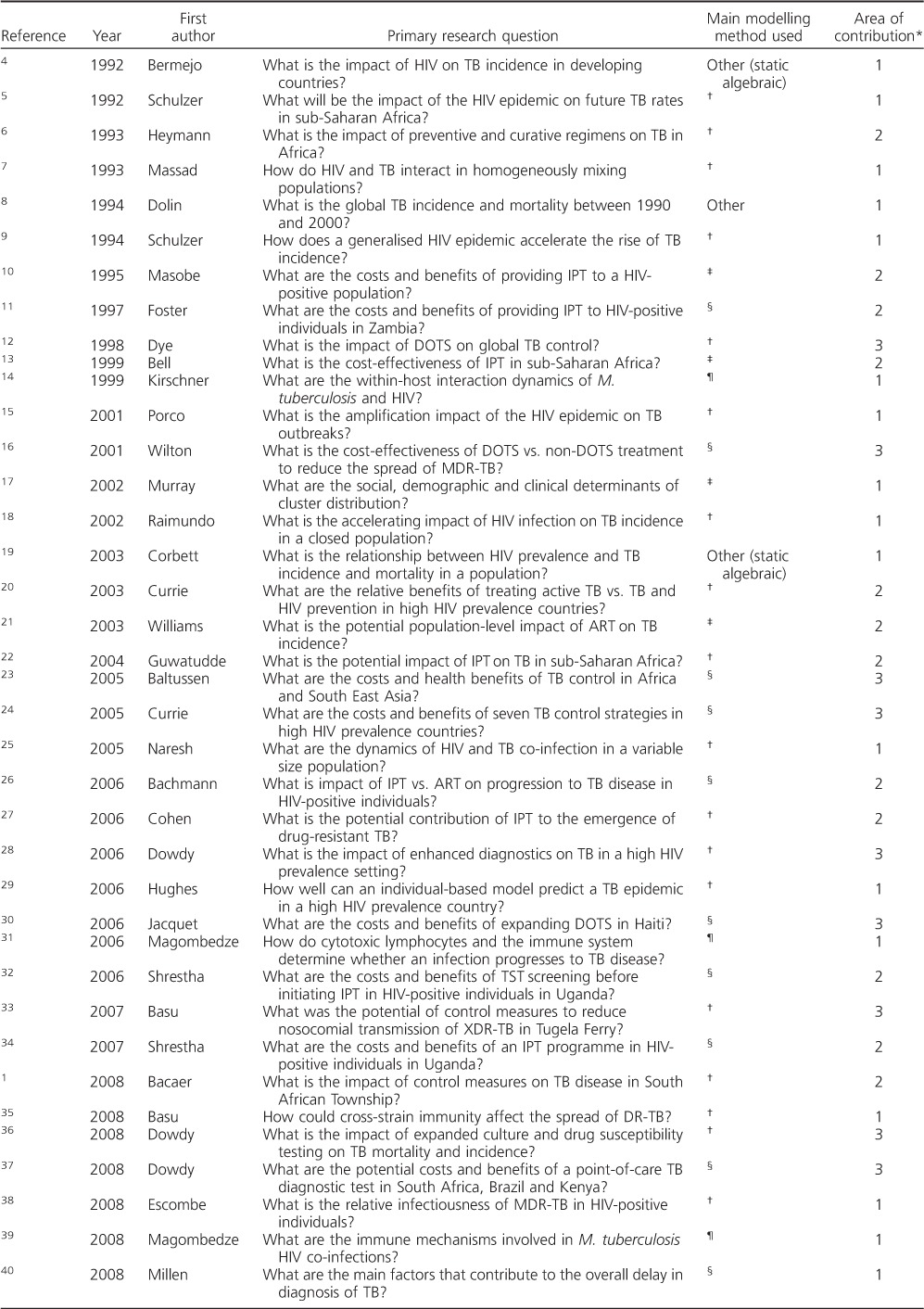
Table.
(continued)
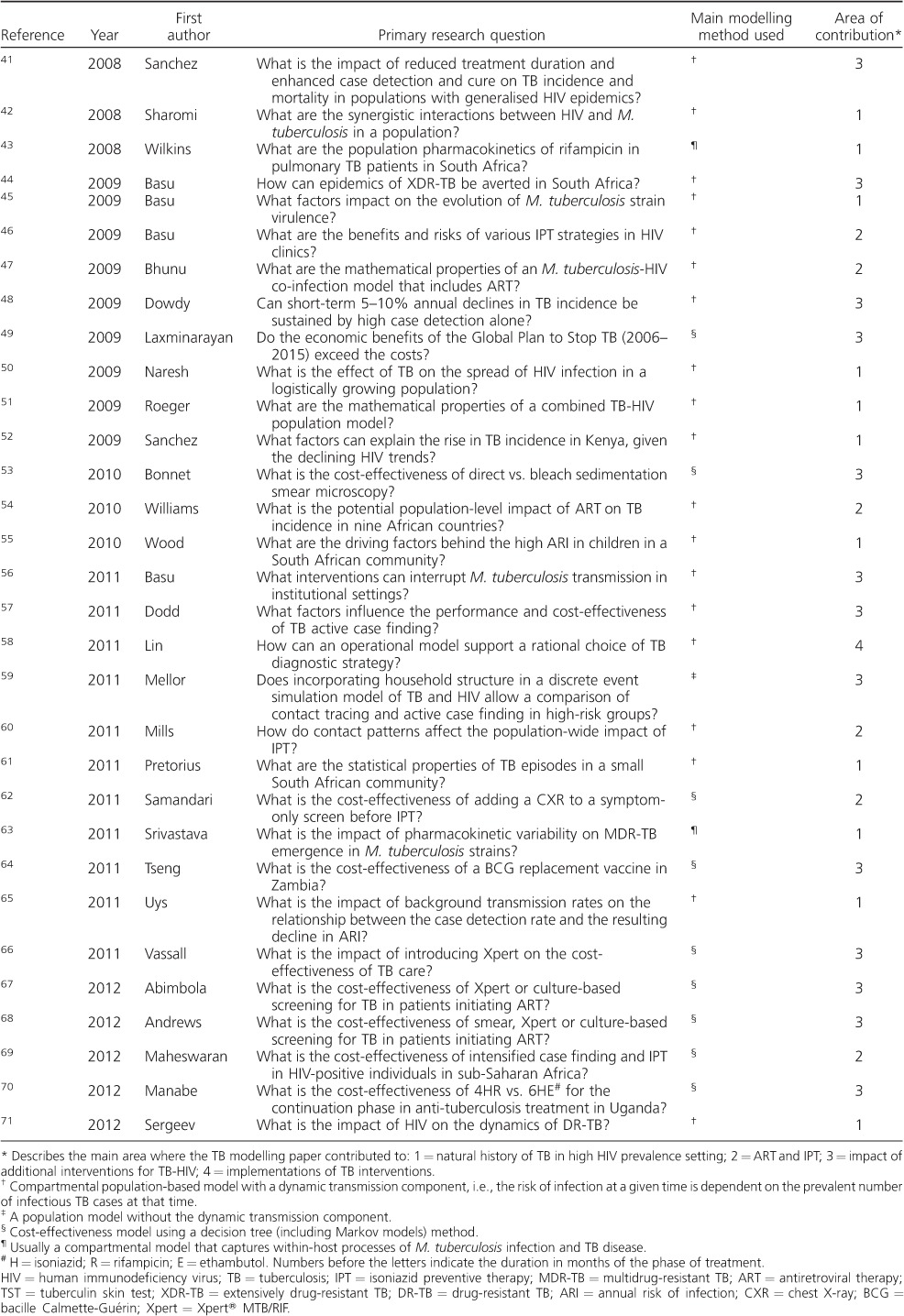
Footnotes
[A version in French of this article is available from the Editorial Office in Paris and from the Union website www.theunion.org]
* The Appendix is available in the online version of this article, at http://www.ingentaconnect.com/content/iuatld/ijtld/2014/00000018/00000005/art00004
References
- 1.Getahun H, Gunneberg C, Granich R, Nunn P. HIV infection-associated tuberculosis: the epidemiology and the response. Clin Infect Dis. 2010;50(Suppl 3):S201–S207. doi: 10.1086/651492. [DOI] [PubMed] [Google Scholar]
- 2.Corbett E L, Watt C J, Walker N et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009–1021. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 3.Lienhardt C, Glaziou P, Uplekar M, Lönnroth K, Getahun H, Raviglione M. Global tuberculosis control: lessons learnt and future prospects. Nat Rev Microbiol. 2012;10:407–416. doi: 10.1038/nrmicro2797. [DOI] [PubMed] [Google Scholar]
- 4.Garnett G P, Cousens S, Hallett T B, Steketee R, Walker N. Mathematical models in the evaluation of health programmes. Lancet. 2011;378:515–525. doi: 10.1016/S0140-6736(10)61505-X. [DOI] [PubMed] [Google Scholar]
- 5.Williams B G, Granich R, De Cock K M, Glaziou P, Sharma A, Dye C. Antiretroviral therapy for tuberculosis control in nine African countries. Proc Natl Acad Sci USA. 2010;107:19485–19489. doi: 10.1073/pnas.1005660107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization Stop TB Partnership. Priority research questions for tuberculosis/human immunodeficiency virus (TB/HIV) in HIV-prevalent and resource-limited settings. Geneva, Switzerland: WHO; 2010. WHO/HTM/TB/2010.8. WHO/HTM/HIV/2010.10. [Google Scholar]
- 7.World Health Organization Stop TB Partnership. Priorities in operational research to improve tuberculosis care and control. Geneva, Switzerland: WHO; 2011. [Google Scholar]
- 8.World Health Organization Stop TB Partnership. An international roadmap for tuberculosis research: towards a world free of tuberculosis. Geneva, Switzerland: WHO; 2011. [Google Scholar]
- 9.Schulzer M, Fitzgerald J M, Enarson D A, Grzybowski S. An estimate of the future size of the tuberculosis problem in sub-Saharan Africa resulting from HIV infection. Tubercle Lung Dis. 1992;73:52–58. doi: 10.1016/0962-8479(92)90080-4. [DOI] [PubMed] [Google Scholar]
- 10.Williams B G, Dye C. Antiretroviral drugs for tuberculosis control in the era of HIV/AIDS. Science. 2003;301:1535–1537. doi: 10.1126/science.1086845. [DOI] [PubMed] [Google Scholar]
- 11.Bermejo A, Veeken H, Berra A. Tuberculosis incidence in developing countries with high prevalence of HIV infection. AIDS. 1992;6:1203–1206. doi: 10.1097/00002030-199210000-00022. [DOI] [PubMed] [Google Scholar]
- 12.Dolin P J, Raviglione M C, Kochi A. Global tuberculosis incidence and mortality during 1990–2000. Bull World Health Organ. 1994;72:213–220. [PMC free article] [PubMed] [Google Scholar]
- 13.Schulzer M, Radhamani M P, Grzybowski S, Mak E, Fitzgerald J M. A mathematical model for the prediction of the impact of HIV infection on tuberculosis. Int J Epidemiol. 1994;23:400–407. doi: 10.1093/ije/23.2.400. [DOI] [PubMed] [Google Scholar]
- 14.Naresh R, Tripathi A. Modelling and analysis of HIV-TB co-infection in a variable size population. Math Model Anal. 2005;10:275–286. [Google Scholar]
- 15.Hughes G R, Currie C S M, Corbett E L. Monterey, CA, USA: Winter Simulation Conference; 2006. Modeling tuberculosis in areas of high HIV prevalence; pp. 459–465. Proceedings of the 38th Conference on Winter Simulation, 3–6 December 2006. pp. [Google Scholar]
- 16.Bachmann M O. Effectiveness and cost effectiveness of early and late prevention of HIV/AIDS progression with antiretrovirals or antibiotics in Southern African adults. AIDS Care. 2006;18:109–120. doi: 10.1080/09540120500159334. [DOI] [PubMed] [Google Scholar]
- 17.Mills H L, Cohen T, Colijn C. Modelling the performance of isoniazid preventive therapy for reducing tuberculosis in HIV-endemic settings: the effects of network structure. J R Soc Interface. 2011;8:1510–1520. doi: 10.1098/rsif.2011.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Currie C S, Williams B G, Cheng R C, Dye C. Tuberculosis epidemics driven by HIV: is prevention better than cure? AIDS. 2003;17:2501–2508. doi: 10.1097/01.aids.0000096903.73209.ac. [DOI] [PubMed] [Google Scholar]
- 19.Dodd P J, White R G, Corbett E L. Periodic active case finding for TB: when to look? PLOS ONE. 2011;6:e29130. doi: 10.1371/journal.pone.0029130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dowdy D W, Chaisson R E, Moulton L H, Dorman S E. The potential impact of enhanced diagnostic techniques for tuberculosis driven by HIV: a mathematical model. AIDS. 2006;20:751–762. doi: 10.1097/01.aids.0000216376.07185.cc. [DOI] [PubMed] [Google Scholar]
- 21.Vassall A, van Kampen S, Sohn H et al. Rapid diagnosis of tuberculosis with the Xpert MTB/RIF assay in high burden countries: a cost-effectiveness analysis. PLOS MED. 2011;8:e1001120. doi: 10.1371/journal.pmed.1001120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menzies N A, Cohen T, Lin H H, Murray M, Salomon J A. Population health impact and cost-effectiveness of tuberculosis diagnosis with Xpert MTB/RIF: a dynamic simulation and economic evaluation. PLOS MED. 2012;9:e1001347. doi: 10.1371/journal.pmed.1001347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin H H, Langley I, Mwenda R et al. A modelling framework to support the selection and implementation of new tuberculosis diagnostic tools. Int J Tuberc Lung Dis. 2011;15:996–1004. doi: 10.5588/ijtld.11.0062. [DOI] [PubMed] [Google Scholar]
- 24.Kranzer K, Afnan-Holmes H, Tomlin K et al. The benefits to communities and individuals of screening for active tuberculosis disease: a systematic review. Int J Tuberc Lung Dis. 2013;17:432–446. doi: 10.5588/ijtld.12.0743. [DOI] [PubMed] [Google Scholar]
- 25.Sonnenberg P, Murray J, Glynn J R, Shearer S, Kambashi B, Godfrey-Faussett P. HIV-1 and recurrence, relapse, and reinfection of tuberculosis after cure: a cohort study in South African mineworkers. Lancet. 2001;358:1687–1693. doi: 10.1016/S0140-6736(01)06712-5. [DOI] [PubMed] [Google Scholar]
- 26.Selwyn P A, Hartel D, Lewis V A et al. A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. N Engl J Med. 1989;320:545–550. doi: 10.1056/NEJM198903023200901. [DOI] [PubMed] [Google Scholar]
- 27.Akolo C, Adetifa I, Shepperd S, Volmink J. Treatment of latent tuberculosis infection in HIV-infected persons. Cochrane Database Syst Rev. 2010:CD000171. doi: 10.1002/14651858.CD000171.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samandari T, Agizew T B, Nyirenda S et al. 6-month versus 36-month isoniazid preventive treatment for tuberculosis in adults with HIV infection in Botswana: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377:1588–1598. doi: 10.1016/S0140-6736(11)60204-3. [DOI] [PubMed] [Google Scholar]
- 29.Houben R M G J, Sumner T, Grant A D, White R G. Ability of preventive therapy to cure latent Myobacterium tuberculosis infection in HIV-infected individuals in high-burden settings. PNAS. doi: 10.1073/pnas.1317660111. [In press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rangaka M X, Boulle A, Wilkinson R J Washington DC, USA: 2012. Randomized controlled trial of isoniazid preventive therapy in HIV-infected persons on antiretroviral therapy. XIX International AIDS Conference, 22–27 July 2012. [THLBB03 - Oral Abstract] [Google Scholar]
- 31.Terris-Prestholt F, Kumaranayake L, Ginwalla R et al. Integrating tuberculosis and HIV services for people living with HIV: costs of the Zambian ProTEST Initiative. Cost Eff Resour Alloc. 2008;6:2. doi: 10.1186/1478-7547-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wood R, Middelkoop K, Myer L et al. Undiagnosed tuberculosis in a community with high HIV prevalence: implications for tuberculosis control. Am J Respir Crit Care Med. 2007;175:87–93. doi: 10.1164/rccm.200606-759OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Straetemans M, Glaziou P, Bierrenbach A L, Sismanidis C, van der Werf M J. Assessing tuberculosis case fatality ratio: a meta-analysis. PLOS ONE. 2011;6:e20755. doi: 10.1371/journal.pone.0020755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dye C, Glaziou P, Floyd K, Raviglione M. Prospects for tuberculosis elimination. Annual Rev Public Health. 2013;34:271–286. doi: 10.1146/annurev-publhealth-031912-114431. [DOI] [PubMed] [Google Scholar]
- 35.Kasaie P 1, Andrews J R, Kelton W D, Dowdy D W. Timing of tuberculosis transmission and the impact of household contact tracing: an agent-based simulation model. Am J Respir Crit Care Med. 2014 Feb 21 doi: 10.1164/rccm.201310-1846OC. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 36.Sweeney S, Obure C D, Maier C B, Greener R, Dehne K, Vassall A. Costs and efficiency of integrating HIV/AIDS services with other health services: a systematic review of evidence and experience. Sex Transm Infect. 2012;88:85–99. doi: 10.1136/sextrans-2011-050199. [DOI] [PubMed] [Google Scholar]
References
- 1.Bacaer N, Ouifki R, Pretorius C, Wood R, Williams B. Modeling the joint epidemics of TB and HIV in a South African township. J Math Biol. 2008;57:557–593. doi: 10.1007/s00285-008-0177-z. [DOI] [PubMed] [Google Scholar]
- 2.Garnett G P, Cousens S, Hallett T B, Steketee R, Walker N. Mathematical models in the evaluation of health programmes. Lancet. 2011;378:515–525. doi: 10.1016/S0140-6736(10)61505-X. [DOI] [PubMed] [Google Scholar]
- 3.Clarelli F, Natalini R. A pressure model of immune response to Mycobacterium tuberculosis infection in several space dimensions. Math Biosci Eng. 2010;7:277–300. doi: 10.3934/mbe.2010.7.277. [DOI] [PubMed] [Google Scholar]
- 4.Bermejo A, Veeken H, Berra A. Tuberculosis incidence in developing countries with high prevalence of HIV infection. AIDS. 1992;6:1203–1206. doi: 10.1097/00002030-199210000-00022. [DOI] [PubMed] [Google Scholar]
- 5.Schulzer M, Fitzgerald J M, Enarson D A, Grzybowski S. An estimate of the future size of the tuberculosis problem in sub-Saharan Africa resulting from HIV infection. Tubercle Lung Dis. 1992;73:52–58. doi: 10.1016/0962-8479(92)90080-4. [DOI] [PubMed] [Google Scholar]
- 6.Heymann S J. Modelling the efficacy of prophylactic and curative therapies for preventing the spread of tuberculosis in Africa. Trans R Soc Trop Med Hyg. 1993;87:406–411. doi: 10.1016/0035-9203(93)90014-h. [DOI] [PubMed] [Google Scholar]
- 7.Massad E. Modeling the interaction between aids and tuberculosis. Math Comput Model. 1993;17:7–21. [Google Scholar]
- 8.Dolin J, Raviglione M C, Kochi A. Global tuberculosis incidence and mortality during 1990–2000. Bull World Health Organ. 1994;72:213–220. [PMC free article] [PubMed] [Google Scholar]
- 9.Schulzer M, Radhamani M P, Grzybowski S, Mak E, Fitzgerald J M. A mathematical model for the prediction of the impact of HIV infection on tuberculosis. Int J Epidemiol. 1994;23:400–407. doi: 10.1093/ije/23.2.400. [DOI] [PubMed] [Google Scholar]
- 10.Masobe P, Lee T, Price M. Isoniazid prophylactic therapy for tuberculosis in HIV-seropositive patients—a least-cost analysis. S Afr Med J. 1995;85:75–81. [PubMed] [Google Scholar]
- 11.Foster S, Godfrey-Faussett P, Porter J. Modelling the economic benefits of tuberculosis preventive therapy for people with HIV: the example of Zambia. AIDS. 1997;11:919–925. doi: 10.1097/00002030-199707000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Dye C, Garnett G P, Sleeman K, Williams B G. Prospects for worldwide tuberculosis control under the WHO DOTS strategy. Directly observed short-course therapy. Lancet. 1998;352:1886–1891. doi: 10.1016/s0140-6736(98)03199-7. [DOI] [PubMed] [Google Scholar]
- 13.Bell J C, Rose D N, Sacks H S. Tuberculosis preventive therapy for HIV-infected people in sub-Saharan Africa is cost-effective. AIDS. 1999;13:1549–1556. doi: 10.1097/00002030-199908200-00016. [DOI] [PubMed] [Google Scholar]
- 14.Kirschner D. Dynamics of co-infection with M. tuberculosis and HIV-1. Theor Popul Biol. 1999;55:94–109. doi: 10.1006/tpbi.1998.1382. [DOI] [PubMed] [Google Scholar]
- 15.Porco T C, Small M, Blower S M. Amplification dynamics: predicting the effect of HIV on tuberculosis outbreaks. J Acquir Immune Defic Syndr. 2001;28:437–444. doi: 10.1097/00042560-200112150-00005. [DOI] [PubMed] [Google Scholar]
- 16.Wilton P, Smith R D, Coast J, Millar M, Karcher A. Directly observed treatment for multidrug-resistant tuberculosis: an economic evaluation in the United States of America and South Africa. Int J Tuberc Lung Dis. 2001;5:1137–1142. [PubMed] [Google Scholar]
- 17.Murray M. Determinants of cluster distribution in the molecular epidemiology of tuberculosis. Proc Natl Acad Sci USA. 2002;99:1538–1543. doi: 10.1073/pnas.022618299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raimundo S M, Yang H M, Bassanezi R C, Ferreira M A C. The attracting basins and the assessment of the transmission coefficients for HIV and M. tuberculosis infections among women inmates. J Biol Syst. 2002;10:61–83. [Google Scholar]
- 19.Corbett E L, Watt C J, Walker N et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009–1021. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 20.Currie C S, Williams B G, Cheng R C, Dye C. Tuberculosis epidemics driven by HIV: is prevention better than cure? AIDS. 2003;17:2501–2508. doi: 10.1097/01.aids.0000096903.73209.ac. [DOI] [PubMed] [Google Scholar]
- 21.Williams B G, Dye C. Antiretroviral drugs for tuberculosis control in the era of HIV/AIDS. Science. 2003;301:1535–1537. doi: 10.1126/science.1086845. [DOI] [PubMed] [Google Scholar]
- 22.Guwatudde D, Debanne S M, Diaz M, King C, Whalen C C. A re-examination of the potential impact of preventive therapy on the public health problem of tuberculosis in contemporary sub-Saharan Africa. Prev Med. 2004;39:1036–1046. doi: 10.1016/j.ypmed.2004.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baltussen R, Floyd K, Dye C. Cost-effectiveness analysis of strategies for tuberculosis control in developing countries. BMJ. 2005;331:1364. doi: 10.1136/bmj.38645.660093.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Currie C S, Floyd K, Williams B G, Dye C. Cost, affordability and cost-effectiveness of strategies to control tuberculosis in countries with high HIV prevalence. BMC Public Health. 2005;5:130. doi: 10.1186/1471-2458-5-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naresh R, Tripathi A. Modelling and analysis of HIV-TB co-infection in a variable size population. Math Model Anal. 2005;10:275–286. [Google Scholar]
- 26.Bachmann M O. Effectiveness and cost effectiveness of early and late prevention of HIV/AIDS progression with antiretrovirals or antibiotics in Southern African adults. AIDS Care. 2006;18:109–120. doi: 10.1080/09540120500159334. [DOI] [PubMed] [Google Scholar]
- 27.Cohen T, Lipsitch M, Walensky R P, Murray M. Beneficial and perverse effects of isoniazid preventive therapy for latent tuberculosis infection in HIV-tuberculosis coinfected populations. Proc Natl Acad Sci USA. 2006;103:7042–7047. doi: 10.1073/pnas.0600349103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dowdy D W, Chaisson R E, Moulton L H, Dorman S E. The potential impact of enhanced diagnostic techniques for tuberculosis driven by HIV: a mathematical model. AIDS. 2006;20:751–762. doi: 10.1097/01.aids.0000216376.07185.cc. [DOI] [PubMed] [Google Scholar]
- 29.Hughes G R, Currie C S M, Corbett E L. Modeling tuberculosis in areas of high HIV prevalence. :459–465. In: Proceedings of the 38th conference on Winter Simulation 2006, Winter Simulation Conference, 3–6 December 2006, Monterey, California. pp. [Google Scholar]
- 30.Jacquet V, Morose W, Schwartzman K et al. Impact of DOTS expansion on tuberculosis related outcomes and costs in Haiti. BMC Public Health. 2006;6:209. doi: 10.1186/1471-2458-6-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Magombedze G, Garira W, Mwenje E. Modelling the human immune response mechanisms to Mycobacterium tuberculosis infection in the lungs. Math Biosci Eng. 2006;3:661–682. doi: 10.3934/mbe.2006.3.661. [DOI] [PubMed] [Google Scholar]
- 32.Shrestha R K, Mugisha B, Bunnell R et al. Cost-effectiveness of including tuberculin skin testing in an IPT program for HIV-infected persons in Uganda. Int J Tuberc Lung Dis. 2006;10:656–662. [PubMed] [Google Scholar]
- 33.Basu S, Andrews J R, Poolman E M et al. Prevention of nosocomial transmission of extensively drug-resistant tuberculosis in rural South African district hospitals: an epidemiological modelling study. Lancet. 2007;370:1500–1507. doi: 10.1016/S0140-6736(07)61636-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shrestha R K, Mugisha B, Bunnell R et al. Cost-utility of tuberculosis prevention among HIV-infected adults in Kampala, Uganda. Int J Tuberc Lung Dis. 2007;11:747–754. [PubMed] [Google Scholar]
- 35.Basu S, Orenstein E, Galvani A. The theoretical influence of immunity between strain groups on the progression of drug-resistant tuberculosis epidemics. J Infect Dis. 2008;198:1502–1513. doi: 10.1086/592508. [DOI] [PubMed] [Google Scholar]
- 36.Dowdy D W, Chaisson R E, Maartens G, Corbett E L, Dorman S E. Impact of enhanced tuberculosis diagnosis in South Africa: a mathematical model of expanded culture and drug susceptibility testing. Proc Natl Acad Sci USA. 2008;105:11293–11298. doi: 10.1073/pnas.0800965105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dowdy D W, O'Brien M A, Bishai D. Cost-effectiveness of novel diagnostic tools for the diagnosis of tuberculosis. Int J Tuberc Lung Dis. 2008;12:1021–1029. [PubMed] [Google Scholar]
- 38.Escombe A R, Moore D A, Gilman R H et al. The infectiousness of tuberculosis patients coinfected with HIV. PLOS MED. 2008;5:e188. doi: 10.1371/journal.pmed.0050188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Magombedze G, Garira W, Mwenje E. In-vivo mathematical study of co-infection dynamics of HIV-1 and Mycobacterium tuberculosis. J Biol Syst. 2008;16:357–394. [Google Scholar]
- 40.Millen S J, Uys P W, Hargrove J, van Helden P D, Williams B G. The effect of diagnostic delays on the drop-out rate and the total delay to diagnosis of tuberculosis. PLOS ONE. 2008;3:e1933. doi: 10.1371/journal.pone.0001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanchez M S, Lloyd-Smith J O, Porco T C et al. Impact of HIV on novel therapies for tuberculosis control. AIDS. 2008;22:963–972. doi: 10.1097/QAD.0b013e3282f7cb4b. [DOI] [PubMed] [Google Scholar]
- 42.Sharomi O, Podder C N, Gumel A B, Song B. Mathematical analysis of the transmission dynamics of HIV/TB coinfection in the presence of treatment. Math Biosci Eng. 2008;5:145–174. doi: 10.3934/mbe.2008.5.145. [DOI] [PubMed] [Google Scholar]
- 43.Wilkins J J, Savic R M, Karlsson M O et al. Population pharmacokinetics of rifampin in pulmonary tuberculosis patients, including a semimechanistic model to describe variable absorption. Antimicrob Agents Chemother. 2008;52:2138–2148. doi: 10.1128/AAC.00461-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Basu S, Friedland G H, Medlock J et al. Averting epidemics of extensively drug-resistant tuberculosis. Proc Natl Acad Sci USA. 2009;106:7672–7677. doi: 10.1073/pnas.0812472106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Basu S, Galvani A. The evolution of tuberculosis virulence. Bull Math Biol. 2009;71:1073–1088. doi: 10.1007/s11538-009-9394-x. [DOI] [PubMed] [Google Scholar]
- 46.Basu S, Maru D, Poolman E, Galvani A. Primary and secondary tuberculosis preventive treatment in HIV clinics: simulating alternative strategies. Int J Tuberc Lung Dis. 2009;13:652–658. [PubMed] [Google Scholar]
- 47.Bhunu C, Garira W, Mukandavire Z. Modeling HIV/AIDS and tuberculosis coinfection. Bull Math Biol. 2009;71:1745–1780. doi: 10.1007/s11538-009-9423-9. [DOI] [PubMed] [Google Scholar]
- 48.Dowdy DW, Chaisson RE. The persistence of tuberculosis in the age of DOTS: reassessing the effect of case detection. Bull World Health Organ. 2009;87:296–304. doi: 10.2471/BLT.08.054510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laxminarayan R, Klein E Y, Darley S, Adeyi O. Global investments in TB control: economic benefits. Health Aff (Millwood) 2009;28:w730–w742. doi: 10.1377/hlthaff.28.4.w730. [DOI] [PubMed] [Google Scholar]
- 50.Naresh R, Sharma D, Tripathi A. Modelling the effect of tuberculosis on the spread of HIV infection in a population with density-dependent birth and death rate. Math Comput Model. 2009;50:1154–1166. [Google Scholar]
- 51.Roeger L I, Feng Z, Castillo-Chavez C. Modeling TB and HIV co-infections. Math Biosci Eng. 2009;6:815–837. doi: 10.3934/mbe.2009.6.815. [DOI] [PubMed] [Google Scholar]
- 52.Sanchez M S, Lloyd-Smith J O, Williams B G et al. Incongruent HIV and tuberculosis co-dynamics in Kenya: interacting epidemics monitor each other. Epidemics. 2009;1:14–20. doi: 10.1016/j.epidem.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bonnet M, Tajahmady A, Hepple P et al. Added value of bleach sedimentation microscopy for diagnosis of tuberculosis: a cost-effectiveness study. Int J Tuberc Lung Dis. 2010;14:571–577. [PubMed] [Google Scholar]
- 54.Williams B G, Granich R, De Cock K M, Glaziou P, Sharma A, Dye C. Antiretroviral therapy for tuberculosis control in nine African countries. Proc Natl Acad Sci USA. 2010;107:19485–19489. doi: 10.1073/pnas.1005660107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wood R, Johnstone-Robertson S, Uys P et al. Tuberculosis transmission to young children in a South African community: modeling household and community infection risks. Clin Infect Dis. 2010;51:401–408. doi: 10.1086/655129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Basu S, Stuckler D, McKee M. Addressing institutional amplifiers in the dynamics and control of tuberculosis epidemics. Am J Trop Med Hyg. 2011;84:30–37. doi: 10.4269/ajtmh.2011.10-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dodd J, White R G, Corbett E L. Periodic active case finding for TB: when to look? PLOS ONE. 2011;6:e29130. doi: 10.1371/journal.pone.0029130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin H H, Langley I, Mwenda R et al. A modelling framework to support the selection and implementation of new tuberculosis diagnostic tools. Int J Tuberc Lung Dis. 2011;15:996–1004. doi: 10.5588/ijtld.11.0062. [DOI] [PubMed] [Google Scholar]
- 59.Mellor G R, Currie C S M, Corbett E L. Incorporating household structure into a discrete-event simulation model of tuberculosis and HIV. ACM Trans Model Comput Simul. 2011;21:1–17. [Google Scholar]
- 60.Mills H L, Cohen T, Colijn C. Modelling the performance of isoniazid preventive therapy for reducing tuberculosis in HIV endemic settings: the effects of network structure. J R Soc Interface. 2011;8:1510–1520. doi: 10.1098/rsif.2011.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pretorius C, Dodd P, Wood R. An investigation into the statistical properties of TB episodes in a South African community with high HIV prevalence. J Theor Biol. 2011;270:154–163. doi: 10.1016/j.jtbi.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 62.Samandari T, Bishai D, Luteijn M et al. Costs and consequences of additional chest X-ray in a tuberculosis prevention program in Botswana. Am J Respir Crit Care Med. 2011;183:1103–1111. doi: 10.1164/rccm.201004-0620OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Srivastava S, Pasipanodya J G, Meek C, Leff R, Gumbo T. Multidrug-resistant tuberculosis not due to non-compliance but to between-patient pharmacokinetic variability. J Infect Dis. 2011;204:1951–1959. doi: 10.1093/infdis/jir658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tseng C L, Oxlade O, Menzies D, Aspler A, Schwartzman K. Cost-effectiveness of novel vaccines for tuberculosis control: a decision analysis study. BMC Public Health. 2011;11:55. doi: 10.1186/1471-2458-11-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Uys P, Marais B J, Johnstone-Robertson S, Hargrove J, Wood R. Transmission elasticity in communities hyperendemic for tuberculosis. Clin Infect Dis. 2011;52:1399–1404. doi: 10.1093/cid/cir229. [DOI] [PubMed] [Google Scholar]
- 66.Vassall A, van Kampen S, Sohn H et al. Rapid diagnosis of tuberculosis with the Xpert MTB/RIF assay in high-burden countries: a cost-effectiveness analysis. PLOS MED. 2011;8:e1001120. doi: 10.1371/journal.pmed.1001120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abimbola T O, Marston B J, Date A A, Blandford J M, Sangrujee N, Wiktor S Z. Cost-effectiveness of tuberculosis diagnostic strategies to reduce early mortality among persons with advanced HIV infection initiating antiretroviral therapy. J Acquir Immune Defic Syndr. 2012;60:e1–e7. doi: 10.1097/QAI.0b013e318246538f. [DOI] [PubMed] [Google Scholar]
- 68.Andrews J R et al. The cost-effectiveness of routine tuberculosis screening with Xpert® MTB/RIF prior to initiation of antiretroviral therapy: a model-based analysis. AIDS. 2012;26:987–995. doi: 10.1097/QAD.0b013e3283522d47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maheswaran H, Barton P. Intensive case finding and isoniazid preventative therapy in HIV-infected individuals in Africa: economic model and value of information analysis. PLOS ONE. 2012;7:e30457. doi: 10.1371/journal.pone.0030457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Manabe Y C, Hermans S M, Lamorde M, Castelnuovo B, Mullins C D, Kuznik A. Rifampicin for continuation phase tuberculosis treatment in Uganda: a cost-effectiveness analysis. PLOS ONE. 2012;7:e39187. doi: 10.1371/journal.pone.0039187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sergeev R, Colijn C, Murray M, Cohen T. Modeling the dynamic relationship between HIV and the risk of drug-resistant tuberculosis. Sci Transl Med. 2012;4:135ra67. doi: 10.1126/scitranslmed.3003815. [DOI] [PMC free article] [PubMed] [Google Scholar]


