Abstract
The field of redox proteomics focuses to a large extent on analyzing cysteine oxidation in proteins under different experimental conditions and states of diseases. The identification and localization of oxidized cysteines within the cellular milieu is critical for understanding the redox regulation of proteins under physiological and pathophysiological conditions, and it will in turn provide important information that are potentially useful for the development of novel strategies in the treatment and prevention of diseases associated with oxidative stress. Antioxidant enzymes that catalyze oxidation/reduction processes are able to serve as redox biomarkers in various human diseases, and they are key regulators controlling the redox state of functional proteins. Redox regulators with antioxidant properties related to active mediators, cellular organelles, and the surrounding environments are all connected within a network and are involved in diseases related to redox imbalance including cancer, ischemia/reperfusion injury, neurodegenerative diseases, as well as normal aging. In this review, we will briefly look at the selected aspects of oxidative thiol modification in antioxidant enzymes and thiol oxidation in proteins affected by redox control of antioxidant enzymes and their relation to disease. [BMB Reports 2015; 48(4): 200-208]
Keywords: Antioxidant enzyme, Cysteine oxidation, Disease, ROS, Redox proteomics
REDOX BIOLOGY AND REDOX PROTEOMICS
Since the first published article appeared in 1945, it has become clear that reactive oxygen species (ROS) exert a broad array of biological effects, ranging from physiological regulatory functions to damaging alterations, contributing to the pathogenesis of various diseases (1). ROS are highly reactive molecules produced mainly by the mitochondrial electron transport chain, but they are also formed as byproducts of several cellular enzymes including NAD(P)H oxidase and nitric oxide synthase (1, 2). Because of the reactive nature of ROS, living organisms must endeavor to maintain redox homeostasis under tight regulation through an intricate system of antioxidants, which include the thioredoxin (TRX) and glutathione (GSH) systems as well as low-molecular weight antioxidants that reside within the cell membrane (3, 4). The actions of redox regulators with antioxidant properties related to active mediators, cellular organelles, and the surrounding environment are all connected in the context of ROS-related diseases resulting from chronic redox imbalance, which include cancer, diabetes mellitus, atherosclerosis, inflammatory diseases, neurodegenerative diseases, rheumatoid arthritis, ischemia/reperfusion injury, as well as normal aging (2, 5-10). Several antioxidant enzymes have been investigated in vitro and/or in animal models to assess their potential therapeutic effects in the conditions linked to oxidative stress related to redox imbalance (11, 12). The results highlight the importance of understanding the relation between redox homeostasis and the status of ROS-related disease.
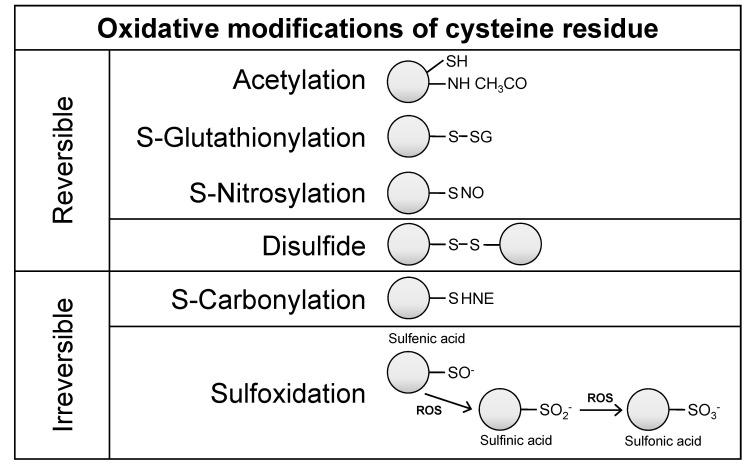
A key feature of ROS-related disease is the induction of oxidative post-translational modifications (PTMs), which include carbonylation, nitrosylation, acetylation, and glutathionylation, which may directly alter a protein’s activity (13). Cysteine (Cys), for example, is a highly redox-sensitive target for oxidation by ROS. Cysteine is one of the least abundant amino acid residues, and it is commonly localized in functionally active regions of proteins, where it may contribute to the maintenance of protein structural stability, the regulation of protein/enzymatic activity, and the binding of cofactors and other proteins (14). In that way, the redox state of functional cysteines may influence such cellular processes as proliferation, differentiation, migration, metastasis/angiogenesis, and inflammation (15-17). Oxidation of cysteine thiol groups leads to diverse modifications (e.g., disulfide, sulfenic acid, sulfinic acid, sulfonic acid, nitrosylation and glutathionylation), which can be reversible or irreversible, making it a challenge to monitor cysteine redox status (Fig. 1). Proteomic approaches are often used as an effort to further the understanding of the cysteine redox state in ROS-related diseases. Recently, a redox proteomics, which is a variation of functional proteomics, was employed to couple mass spectrometry with oxidation-sensitive cysteines under conditions of oxidative stress (18, 19).
Fig. 1. Oxidative post-translational modification of cysteine residues in proteins. Cysteine is commonly located on the surface and at the active site of proteins, either alone (monothiols) or in close proximity to another cysteine residue (vicinal dithiols). Vicinal dithiols tend to form disulfides upon oxidation, whereas monothiols undergo reversible oxidation to sulfenic acid. Under strongly oxidizing conditions, sulfenic acid is further oxidized to sulfinic and sulfonic acids. Other modifications that also occur include acetylation, glutathionylation, nitrosylation, and carbonylation of protein cysteines. These changes can result in alterations in protein-protein interactions, enzyme activity, DNA and/or RNA binding, and membrane interactions. HNE, 4hydroxynonenal; ROS, reactive oxygen species.
The primary focus of redox proteomics is to quantify the different redox levels between two or more physiological redox states. In that effort, various chemical labeling tags have been integrated into the process of redox proteomics. Iodoacetamide (IAM) and N-ethylmaleimide (NEM) are typical tags that both react only with free Cys-thiol (SH) groups but have different mass shifts. IAM reacts with thiols in alkaline solutions (pH 8-8.5) in a nucleophilic substitution, while NEM is usually thiol-specific at pH 7 (20). Biotin-tagged IAM (BIAM) and isotope coded affinity tag (ICAT) have also been used to detect or enrich free Cys-thiol groups (21), and most free sulfhydryl groups are easily labeled by generating acrylamide adducts (propionamide, PAM-Cys) in acrylamide gel electrophoresis. In addition to the free Cys-thiol group, sulfenic acid (Cys-SOH), which is an oxidatively modified intermediate of the Cys-thiol group, can be specifically labeled with dimedone (22). Despite the trials of using various labeling chemicals for redox proteomics, only a limited number of proteins have been described as being sensitive to oxidation/reduction processes.
PROTEOMIC APPROACHES FOR CYSTEINE OXIDATION OF ANTIOXIDANT ENZYMES IN ROS-RELATED DISEASES
The enzymatic antioxidant defense systems that neutralize such ROS as hydroxyl radicals (·OH), superoxide anions (O2·-), and hydrogen peroxide (H2O2) representatively include superoxide dismutase (SOD), glutathione peroxidase (GPX), catalase (CAT), and thioredoxin-peroxiredoxin (TRX-Prdx) (23, 24). These antioxidant enzymes can serve as redox biomarkers in various human diseases, because they are the first to indicate the redox state through oxidation/reduction processes. Proteomic approaches toward identification of oxidation-sensitive cysteines in antioxidant enzymes are summarized in Table 1.
Table 1. The identification of cysteine (thiol group) oxidation of antioxidant enzymes in disease-related condition.
| Disease-related conditon | Species | Tissue | Oxidative stress | Proteomic strategy | Oxidatively modified antioxidant enzyme | Ref. |
|---|---|---|---|---|---|---|
|
| ||||||
| Cancer | Mouse | B16F10 melanoma cell line | Treatment of H2O2 | 2DE, LC-MS/MS | Prdx6 (Cys47, Cys91) | 26 |
| Human | HepG2 hepatoblastoma cell line | Treatment of H2O2 | 2DE, MS/MS | Prdx1 (Cys52), Prdx2 (Cys51), Prdx6 (Cys46) | 27 | |
| Human | CCD-966SK skin fibroblast line | Treatment of UVB | 2DE, MS/MS | Prdx1, Prdx4 | 28 | |
| Human | A431 epidermoid carcinoma cells | Photodynamic treatment | LC-MS/MS | Prdx1, Prdx6, Catalase, TrxR1, GR, GSTo1, GSS | 29 | |
| Kidney hypertension | Rat | Kidney medulla | Spontaneously hyper tensive rat model | 2DE, LC-MS/MS | SOD1, Prdx2, Prdx3, Prdx5, Prdx6, GSS | 31 |
| Ischemia/Reperfusion | Rat | Liver tissue | Segmental ischemia/reperfusion | 2DE, LC-MS/MS | Prdx1 (Cys52, Cys173) | 35 |
| Mouse | Heart tissue | Ischemia/reperfusion, or ischemic preconditioning | 2DE, LC-MS/MS | Prdx6 (Cys47) | 36 | |
| Alzheimer’s disease | Human | Brain tissue (hippocampus and cortex) | AD patient | 2DE, MS/MS | SOD2, Prdx6 | 39 |
| Human | Brain tissue (inferior parietal lobule) | Early Alzheimer's disease (EAD) | 2DE, MS/MS | SOD2 | 42 | |
| Aging | Rat | Brain tissue (cortex) | 12- and 28-month-age | 2DE, MS/MS | Prdx2 | 43 |
UVB: ultraviolet B, GR: glutathione reductase, GSTo1: glutathione transferase omega-1, GSS, glutathione synthetase.
Cancer
Chronic disturbances of the redox balance within cells can lead to cancer through induction of cellular adaptation, DNA mutations, and genetic instability (25), and many oxidative cysteine modifications have been identified in tumor cells through the use of comprehensive redox proteomics approaches. When Prdx6 from a mouse melanoma cell line with oxidative stress was separated on 2D-PAGE, hyperoxidation (e.g. sulfinic acid, sulfonic acid) was observed at Cys47 and Cys91 (26). Comprehensive analysis of these modifications revealed that in the cells subjected to oxidative stress, Prdx6 exists as a heterogeneous mixture of molecules containing a multitude of oxidative modifications. For example, when the HepG2 human hepatoblastoma cells were treated with H2O2, the oxidation kinetics of the peroxiredoxin isoforms were all extremely rapid, but the affected cysteine was isoform-specific. Oxidation of Cys52 (hPrdx1), Cys51 (hPrdx2), and Cys46 (hPrdx6) were interpreted as unique modifications of peroxiredoxin polypeptides responsible for measured acidic shifts in HepG2 cells following oxidative insult (27). Especially, the oxidized hPrdx1 and hPrdx2 were recovered into reduced form by recovery experiments. The results of that study suggest that hPrdx1 has special functions as an early antioxidant-buffering molecule in hepatocytes and that its oxidation could potentially serve as a sensitive marker for oxidative stress during ischemia/reperfusion (I/R) after liver transplantation.
Cysteine-labeled 2D-DIGE (2D-differential gel electrophoresis) has been used to measure oxidation-sensitive proteins and to study the effect on thiol activity of UVB-induced oxidative stress during the development of skin cancer (28). In that study, the thiol activities of 37 proteins containing redox regulator (e.g., hPrdx1 and hPrdx4) were altered by UVB-induced oxidative stress. Similarly, cysteine sulfenylation was identified in epithelial carcinoma cells using a proteomic approach involving oxidative site-specific biotinylation, during photodynamically induced oxidative stress (29). While a significant fraction of cabonylated proteins were mostly classified as abundant structural proteins and chaperones, redox homeostasis regulators including the antioxidant enzymes hPrdx1, hPrdx6, hCatalase, hTrxR1, hGR, hGSTo1, and hGSS enabled the detection of even scarce sulfenylation. To maintain redox homeostasis, GSH reductase maintains high levels of the major cellular antioxidant GSH in the cytosol, and glutathione synthetase catalyzes the second step of GSH synthesis. Oxidation of these proteins may impair their activity by reducing the cell’s ability to resist oxidative stress and facilitating the progression of apoptosis triggered by photodynamic treatment.
Kidney hypertension
Redox proteomics studies have also been applied to kidney-related pathology. Renal redox regulation, which plays a central role in the balancing of electrolyte and physiological buffer systems, varies greatly across the kidney from the well-perfused cortex to the near-anoxic medulla, reflecting differences in blood flow and oxygenation (30). In addition, the total free thiol content is significantly lower in the renal medulla of spontaneously hypertensive rats than in their normotensive controls. In that study, the observed enhancement of protein sulfenation may be due to the reduction of thioredoxin reductase activity (31). Using gel-based redox proteomics with biotin-maleimide, sulfenylated proteins involved in metabolism, antioxidant defense, and the regulation of nitric oxide synthase were identified. These proteins include rSOD1, rPrdx2, rPrdx3, rPrdx5, rPrdx6, and rGS. Moreover, Cys146 of SOD1 is reportedly hyperoxidized to sulfonic acid in patients with Alzheimer’s or Parkinson’s disease (32). In addition, increases in plasma protein thiol oxidation and carbonylation have been observed in patients with chronic kidney disease (33). Given that redox imbalance leads to kidney dysfunction stemming from high levels of oxidative modification, redox proteomics examining the relation between kidney disease and oxidative PTM could contribute to the development of new methods for early diagnosis.
Ischemia/Reperfusion
The pathology most affected by oxidative stress is ischemia/reperfusion (I/R) injury associated with organ transplantation. Oxidative stress is the major initiator of a signaling cascade that leads to inflammation, cell death, and organ failure during hepatic I/R injury, especially during the early stage of the process (34). In a rat model of segmental hepatic ischemia, 2D-DIGE was used to identify cytosolic proteins altered early during I/R (35). Of the 18 identified proteins, there was a three-fold increase in Prdx1 which was over-oxidized at Cys173 (resolving cysteine). Interestingly, in contrast to other studies, typical over-oxidation of Prdx1 at Cys52 (peroxidatic cysteine) was not detected. In another recent study, protein oxidation and S-nitrosylation were simultaneously measured during various perfusion protocols involving myocardial ischemic preconditioning (IPC) (36). The two types of thiol oxidation products caused by I/R injury, thiol oxidation, and S-nitrosylation have different redox activities, which makes their simultaneous measurement difficult to perform. In the abovementioned study (36), different techniques were used in parallel to selectively block free thiols and reduce modified thiols, and to enrich thiol peptides through resin-assisted capture. S-nitrosylation has also been used to protect cysteine residues against potential oxidative damage from ROS (37). S-nitrosylation is a transient modification that can potentially shield cysteine residues from irreversible oxidation during reperfusion, when it is elicited during ischemic preconditioning (IPC). Consequently, cysteine oxidation of Prdx6 on Cys47 was increased during I/R but was decreased during IPC (36). This suggests that S-nitrosylation could potentially exert cardioprotective effects by inhibiting cysteine oxidation. The nitroso-redox balance, which is essential for the maintenance of normal myocardial function, can be vitally affected by the widespread oxidation that occurs with I/R injury (38).
Alzheimer’s disease
Several studies have shown that oxidative damage due to accumulation of oxidatively modified proteins is a characteristic feature of many neurodegenerative diseases (39). For instance, oxidative dysfunction of proteins involved in ATP production, excitotoxicity, detoxification, protein degradation, neuritic abnormalities, and mitochondrial abnormalities are likely involved in the neurodegeneration at different stages of dementia. Indeed, several lines of evidence support the notion that oxidative/nitrosative stress plays a major role in the pathogenesis of Alzheimer’s disease (AD). Oxidative stress in the AD brain is indexed by increased total levels of protein carbonyls, protein nitration, and protein-bound HNE (4-hydroxynonenal) (18). Proteomics studies identified regionally specific HNE-modification of antioxidant proteins in AD, including SOD2 in the hippocampus and Prdx6 in the cortex (40). In addition, elevated levels of nitrated Prdx6 have been detected in the inferior parietal lobule (IPL) of the subjects with mild cognitive impairment (MCI), which is an early form of AD, suggesting the potential involvement of this enzyme in the pathogenesis and progression of AD (41). In addition, SOD2 is excessively nitrated in the brains and neurons of human double mutant APP/PS-1 knock-in mice, a model of AD (42). Also, SOD2 is found to be excessively bound with HNE in the IPL of individuals with early AD, which is the intermediary stage between MCI and late-stage AD (43). It is thus evident from redox proteomic analyses that protein oxidation is strongly correlated with the clinical features, pathology, and biochemistry of AD, which confirms the utility of biomarker screening using redox proteomics. Aging is considered as one of the most significant risk factors for age-related neurodegenerative diseases, such as AD, and it leads to a general decline of CNS functionality, which is particularly vulnerable to oxidative injury.
Aging
Prdx2 was found to have significantly higher levels of carbonylated oxidation in the cortex of senescent (28 months old) rats than less aged (12 months old) rats, though this was not seen in the hippocampus, striatum, or cerebellum (44). On the other hand, GSH levels were significantly lower and GSSG was significantly higher in all regions of the brain in senescent rats, compared to the control rats with lower age. This highlights the fact that thiol oxidation is specifically regulated in concert by the environment and the oxidative state, which are dependent on the health and age of the individual. Advances in redox proteomics have provided a means to gain new insight into the relationships between redox biology and age-related diseases.
IDENTIFICATION OF CYSTEINE OXIDATION UNDER CONTROL OF AN ANTIOXIDANT SYSTEM
Reversible oxidation of cysteine moieties has been shown to act as a redox switch to regulate protein interactions, enzyme activity, protein conformation, transporter activity, DNA binding, and protein trafficking and degradation (45). Thiol oxidation occurs as a result of ROS generation due to electron leakage from intrinsic mitochondrial metabolism or extrinsic environmental sources. Because uncontrolled oxidative stress may interfere with signaling via thiol oxidation of key redox-sensitive proteins, maintenance of thiol redox homeostasis is critical, and it is achieved through the actions of GSH-dependent and TRX oxidoreductase systems. Table 2 summarizes the proteomic approaches that have been used to identify oxidative modifications of protein thiol groups under the control of antioxidant enzymes.
Table 2. Proteomic approaches for identification of oxidative modified proteins under control of antioxidant system.
| Antioxidant system | Tissue | Treatment | Labeling tag for thiol | Proteomic strategy | Ref. |
|---|---|---|---|---|---|
|
| |||||
| TRX system | Plant leave (Arabidopsis thaliana) | - | [12C]IAM, PEO-iodoacetylbiotin, or 4-vinylpyridine | 2DE, MS/MS | 47 |
| Plant germinated Barley seed embryo | Treatment of recombinant TRX | ICATLight /ICATHigh | LC-MS/MS | 48 | |
| Human HT29 intestinal epithelial cells | Treatement of auranofin | ICATLight /ICATHigh | LC-MS/MS | 49 | |
| Human lung adenocarcinoma H1299 cells | - | Interaction with Trx1 C35S mutant | LC-MS/MS | 51 | |
| Mouse heart | Cardiac hypertrophy using cardiac specific overexpression of TRX1 | ICATLight /ICATHigh | LC-MS/MS | 67 | |
| GSH system | Human HT29 intestinal epithelial cells | Treatment of L-buthionine sulfoximine | ICATLight /ICATHigh | LC-MS/MS | 49 |
| Prdx1 | Human hepatoblastoma HepG2 cells | Stable expreesion of Prdx1 shRNA | 5-iodoacetamidofluorescein | 2DE, MS/MS | 58 |
| Prdx1&Prdx3 | Mouse hepatoma Hepa 1-6 cell | Simulteneous treatment of siRNA of Prdx1, Prdx3, and GCLC | 5-iodoacetamidofluorescein | 2DE, MS/MS | 60 |
| Prdx2 | Mouse red blood cells | Knockout mice | Iodoacetamide (IAM) | LC-MS/MS | 66 |
Thioredoxin system
The TRX system consists of TRX, TRX reductase (TrxR), TRX peroxidase/Prdx, and NADPH. It is involved in redox regulation through catalysis of thiol-disulfide interchanges that lead to modification of enzyme activity or provide thiol-dependent reductases with redox power (46). The TRX family contains two catalytically conserved active cysteine residues that can reduce the disulfide bonds of target proteins in the thiol proteome. These proteins include, for example, apoptosis signaling kinase-1 (ASK-1), Trx1-interacting protein (Txnip), various transcription factors, and actin (47). In addition to its catalytic activity, TRXs bind to target proteins, further controlling their cellular activity and biological function.
To identify proteins that are able to interact with TRXs, several studies have been carried out in plants, as photosynthetic organisms harbor more than 20 genes encoding TRXs. In (Arabidopsis thaliana), 73 TRX-linked targets were identified using three approaches: radioactive labeling reduction, affinity chromatography on an avidin column, and affinity chromatography on a mutated thioredoxin column (48). The identified proteins were analyzed for known functions based on genome annotation, and some isoforms exhibited new functions with different compartmental localization. For example, some identified proteins containing TRX-reducible cysteines had already been described in other organisms (ATP sulfurylase, catalase, formate dehydrogenase, malate dehydrogenase, two putative aldolases, alanine aminotransferase, GST, OEE2, and Prdxs), but other proteins were considered as new targets (tyrosine aminotransferase, xyloglucan endotransglycosylase XET, and plastocyanin). In the seeds of barley cultivar, a thiol-specific differential ICAT labeling approach was used with recombinant TRX to quantify TRX-catalyzed disulfide reduction. Disulfide targets were identified in 104 of the 199 identified ICAT-labeled peptides (49). TRX-reduced disulfides were found in several previously identified target proteins, including Prdxs and cyclophilin, as well as in a wide range of new targets, including several ribosomal proteins. Of the identified proteins, the functions of the most extensively reduced TRX targets were mainly classified to redox control (e.g., dehydroascorbate reductase and 1-Cys Prdx), metabolism (e.g., adenosylhomocysteinase, enolase, and alcohol dehydrogenase1), translation (e.g., seryl-tRNA synthetase 1 and ribosomal protein S12), protein folding (e.g. cyclophilin) and seed-related proteins (e.g., B1 hordein and Bowman-Birk type trypsin inhibitor). This study also found that the catalytic Cys19 in dehydroascorbate reductase was extensively reduced (62%), which suggests that TRX has an important role in the ascorbate-glutathione cycle.
In human intestinal epithelial cells, redox proteomics showed that selectively inhibiting the TRX system using auranofin resulted in a 1.3-fold or greater increase in the oxidation of 96 peptidyl cysteines over control (50). Interestingly, auranofin and aurothioglucose are gold-containing compounds that inhibit the catalytic activity of TrxR and are used to treat rheumatoid arthritis (51). The functional pathways affected by TRX inhibition were mainly categorized as glycolysis/gluconeogenesis, cytoskeleton remodeling, protein synthesis, and folding processes. For instance, redox proteomics-identified proteins involved in glycolysis/gluconeogenesis pathways include aldolase A, enolase 3, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), lactate dehydrogenase A, malate dehydrogenase, phosphoglycerate mutase1, and pyruvate kinase isozyme 2. Among these, oxidation of Cys93 in malate dehydrogenase was increased by more than 3.5-fold upon inhibition of the TRX system. Similarly, in adenocarcinoma cells of human lung, a substrate trap (mass action trapping) proteomics approach was used to identify more than 100 proteins as Trx1 redox targets within the DTT eluate of Flag-TRX1 C35S-expressing cells (52). These proteins included two known Trx1-interacting proteins, Prdx1 and GAPDH, which validated this approach. The identified proteins were associated with antioxidant (9.4%), cell signaling (7.5%), and stress response/chaperone functions (19.8%). Specifically, two isoforms of heat shock protein 90 (HSP90AA1 and HSP90AB1) were identified as TRX1 substrate targets, and the redox-mediated interaction with TRX1 was simultaneously confirmed using a HSP 90 antibody. TRX may also play a specific role in angiogenesis, as HSP90 is a critical chaperone that promotes vascular development through stabilization of HIF-1α and regulation of nitric oxide synthase activity (53, 54).
Glutathione system
The GSH system is dependent on NADPH and consists of GSH, GSSG reductase, and glutaredoxins. Redox interactions between GSH and cysteine are catalyzed by glutaredoxins, which are independently controlled in mammalian cells. The GSH system defends against acute but not chronic oxidative stress (55); its function can differ from that of TRX within the redox organizational structure, which controls redox cysteine, and it highlights the need to examine the GSH-dependent redox proteome. Redox ICAT analysis identified 50 proteins oxidized by treatment with buthionine sulfoximine, which selectively depletes GSH (50). Of those, 41 proteins were also oxidized in the presence of auranofin and were involved in insulin regulation of translation, lipid metabolism, and cell adhesion, but not glycolysis or cytoskeletal remodeling. For example, disruption of the TRX and GSH systems leads to oxidation of Cys511 in plakoglobin, which is highly homologous to β-catenin and is found in adherence junctions (56). Although redox regulation of junction proteins has not been confirmed, this finding highlights the need for detailed studies to assess the redox-dependence of cell-cell adhesion pathways as possible redox modules regulated by the TRX and GSH systems.
Peroxiredoxins
The mammalian Prdx family is composed of six proteins that contain essential catalytic cysteine residues and uses thioredoxin as the electron donor to reduce peroxides. Prdx1 appears to play opposing roles in tumorigenesis, functioning as both a tumor promoter and tumor suppressor. For instance, the lifespans of mice lacking Prdx1 are shortened by the development of severe hemolytic anemia and several malignant cancers (57). In contrast, Prdx1 expression is abnormally upregulated in several cancers, including liver cancer, and this elevated expression leads to a decline of overall survival, poor clinical outcome, and resistance of cancer cells to radiotherapy and chemotherapy (58). Down-regulation of Prdx1 by using Prdx1 shRNA is stably expressed in HepG2s cells, which shows that silencing Prdx1 significantly increased the thiol oxidation of 9 proteins that were functionally categorized as pathways related to metabolism, cell proliferation, and transcription: ENOA, TPIS, DHE3, AL1A1, ECH1, SMC1A, GSLG1, ZN682, and PRD15 (59). Cys112 of DHE3 (glutamate dehydrogenase 1, mitochondrial) is prone to reversible oxidation in Prdx1-silenced cells, suggesting that Cys112 may play a role in the regulation of DHE3 activity. Similarly, Cys112 or Cys146 of DHE3 is also oxidized under oxidative stress induced by simultaneous silencing of the antioxidant defenses (Prdx1, Prdx3, and the catalytic subunit of the glutamate-cysteine ligase; GCLC) that regulate the rate-limiting step in GSH biosynthesis (60). Glutamine is typically converted into α-ketoglutarate in two steps; glutaminase catalyzes the conversion of glutamine to glutamate, which is in turn converted to α-ketoglutarate by glutamate dehydrogenase. Glutamine metabolism, with the Warburg effect, is a major component of the general metabolic phenotype of proliferating tumor cells. (61). Accordingly, we suggest that Prdx1 acts as a cancer promoter in hepatocellular carcinoma cells.
Levels of Prdx2, which is highly homologous with Prdx1, are also increased in several human cancers. Overexpression of Prdx2 causes leukemia, and it makes the gastric cancer cells to become resistant to chemotherapeutic agents; and downregulation of Prdx2 makes head-and-neck cancer cells to become susceptible to radiation and gastric carcinoma cells to become susceptible to cisplatin (62, 63). These findings suggest that Prdx2 also provokes resistance to therapy and contributes to a strong pro-survival phenotype in cancer cells.
Prdx2 has also been well studied in red blood cells (RBCs), where it is the third most abundant protein (64, 65). Mice lacking Prdx2 develop severe hemolytic anemia and exhibit symptoms that include reductions in both hemoglobin content and hematocrit (implying hemolysis), increased reticulocyte counts (indicating erythropoietic compensation to maintain hematologic homeostasis), and splenomegaly (reflecting the need to destroy large numbers of abnormal erythrocytes) (66). In addition, their RBCs contained aggregations of oxidatively denatured hemoglobin known as Heinz bodies. Using the thiolmodifying reagent BIAM, a number of RBC proteins were shown to be highly oxidized in Prdx2-deficient mice, as compared to wild-type mice. The RBC proteome was recently used to identify oxidation-sensitive proteins in the cytosolic and membrane fractions of RBCs, from Prdx2 knockout mice. Fifty-four proteins containing 61 oxidation-sensitive cysteines were analyzed in healthy RBCs from wild-type mice and in both healthy and abnormal RBCs from Prdx2 knockout mice (67). These proteins are concerned with cellular functions related with RBC lifespan maintenance, and they include cytoskeletal proteins, stress-induced proteins, metabolic enzymes, signal transducers, and transporters. For example, the reduced forms of Cys177 or Cys192 in ACTG1 (actin, gamma1) and Cys247 in GAPDH were diminished in abnormal RBCs from Prdx2 knockout mice. GAPDH Cys247 is also reported to be greatly reduced in cardiac tissue from transgenic mice overexpressing TRX1 (68). These results show that the cysteine oxidation state is an important regulatory factor affecting protein functionality, and it can eventually lead to redox imbalances in cancer and other diseases.
CONCLUSION
Because the redox state of cysteine residues can affect protein-protein interactions and cellular signaling, it must be tightly regulated by antioxidant enzymes. As discussed in this article, redox proteomic approaches have shown that cysteine residues in several antioxidant enzymes are specifically oxidized in ROS-related diseases including cancer, I/R, neurodegenerative diseases, as well as normal aging. Thus the thiol oxidation state of antioxidant enzymes could potentially serve as a redox biomarker for the pathogenesis of human diseases. Moreover, identification of target proteins, whose cysteine oxidation state is regulated by antioxidant enzymes, could provide critical clues to the pathology of diseases in which ROS are out of balance. But despite the continuous trials and advances in redox proteomics, there have only been few studies until now, in which antioxidant enzyme systems were used to shed light on the oxidation/reduction processes of target proteins. In particular, redox proteomic approaches toward examining thiol oxidation in antioxidant enzymes represent potentially effective means of identifying novel oxidation/reduction-sensitive targets and therapeutic strategies for ROS-related diseases.
Acknowledgments
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (2011-0030121), and by a Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (2014R1A2A2A01005448). HY Yang was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2014R1A1A2004303).
References
- 1.Alfadda AA, Sallam RM. Reactive oxygen species in health and disease. J Biomed Biotechnol. (2012);2012:936486. doi: 10.1155/2012/936486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. (2002);82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 3.Tsantes AE, Bonovas S, Travlou A, Sitaras NM. Redox imbalance, macrocytosis, and RBC homeostasis. Antioxid Redox Signal. (2006);8:1205–1216. doi: 10.1089/ars.2006.8.1205. [DOI] [PubMed] [Google Scholar]
- 4.Nickel C, Rahlfs S, Deponte M, Koncarevic S, Becker K. Thioredoxin networks in the malarial parasite Plasmodium falciparum. Antioxid Redox Signal. (2006);8:1227–1239. doi: 10.1089/ars.2006.8.1227. [DOI] [PubMed] [Google Scholar]
- 5.Wolff SP. Diabetes mellitus and free radicals. Free radicals, transition metals and oxidative stress in the aetiology of diabetes mellitus and complications. Br Med Bull. (1993);49:642–652. doi: 10.1093/oxfordjournals.bmb.a072637. [DOI] [PubMed] [Google Scholar]
- 6.Dopheide JF, Doppler C, Scheer M, et al. Critical limb ischaemia is characterised by an increased production of whole blood reactive oxygen species and expression of TREM-1 on neutrophils. Atherosclerosis. (2013);229:396–403. doi: 10.1016/j.atherosclerosis.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 7.Leitemperguer MR, Tatsch E, Kober H, De Carvalho JA, Moresco RN, Da Silva JE. Assessment of ischemia-modified albumin levels in patients with rheumatoid arthritis. Clin Lab. (2014);60:1065–1070. doi: 10.7754/clin.lab.2013.130143. [DOI] [PubMed] [Google Scholar]
- 8.Pierola J, Alemany A, Yanez A, et al. NADPH oxidase p22phox polymorphisms and oxidative stress in patients with obstructive sleep apnoea. Respir Med. (2011);105:1748–1754. doi: 10.1016/j.rmed.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Hsieh HL, Yang CM. Role of redox signaling in neuroinflammation and neurodegenerative diseases. Biomed Res Int. (2013);2013:484613. doi: 10.1155/2013/484613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park JG, Oh GT. The role of peroxidases in the pathogenesis of atherosclerosis. BMB Rep. (2011);44:497–505. doi: 10.5483/BMBRep.2011.44.8.497. [DOI] [PubMed] [Google Scholar]
- 11.Milani P, Ambrosi G, Gammoh O, Blandini F, Cereda C. SOD1 and DJ-1 converge at Nrf2 pathway: a clue for antioxidant therapeutic potential in neurodegeneration. Oxid Med Cell Longev. (2013);2013:836760. doi: 10.1155/2013/836760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saeidnia S, Abdollahi M. Toxicological and pharmacological concerns on oxidative stress and related diseases. Toxicol Appl Pharmacol. (2013);273:442–455. doi: 10.1016/j.taap.2013.09.031. [DOI] [PubMed] [Google Scholar]
- 13.Rinalducci S, Murgiano L, Zolla L. Redox proteomics: basic principles and future perspectives for the detection of protein oxidation in plants. J Exp Bot. (2008);59:3781–3801. doi: 10.1093/jxb/ern252. [DOI] [PubMed] [Google Scholar]
- 14.Marino SM, Gladyshev VN. Analysis and functional prediction of reactive cysteine residues. J Biol Chem. (2012);287:4419–4425. doi: 10.1074/jbc.R111.275578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giannoni E, Buricchi F, Raugei G, Ramponi G, Chiarugi P. Intracellular reactive oxygen species activate Src tyrosine kinase during cell adhesion and anchorage-dependent cell growth. Mol Cell Biol. (2005);25:6391–6403. doi: 10.1128/MCB.25.15.6391-6403.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim YM, Kim KE, Koh GY, Ho YS, Lee KJ. Hydrogen peroxide produced by angiopoietin-1 mediates angiogenesis. Cancer Res. (2006);66:6167–6174. doi: 10.1158/0008-5472.CAN-05-3640. [DOI] [PubMed] [Google Scholar]
- 17.Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. (2006);312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 18.Butterfield DA, Gu L, Di Domenico F, Robinson RA. Mass spectrometry and redox proteomics: applications in disease. Mass Spectrom Rev. (2014);33:277–301. doi: 10.1002/mas.21374. [DOI] [PubMed] [Google Scholar]
- 19.Kim HJ, Ha S, Lee HY, Lee KJ. ROSics: Chemistry and proteomics of cysteine modifications in redox biology. Mass Spectrom Rev. (2014) doi: 10.1002/mas.21430. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansen RE, Winther JR. An introduction to methods for analyzing thiols and disulfides: Reactions, reagents, and practical considerations. Anal Biochem. (2009);394:147–158. doi: 10.1016/j.ab.2009.07.051. [DOI] [PubMed] [Google Scholar]
- 21.Kim JR, Yoon HW, Kwon KS, Lee SR, Rhee SG. Identification of proteins containing cysteine residues that are sensitive to oxidation by hydrogen peroxide at neutral pH. Anal Biochem. (2000);283:214–221. doi: 10.1006/abio.2000.4623. [DOI] [PubMed] [Google Scholar]
- 22.Charles RL, Schroder E, May G, et al. Protein sulfenation as a redox sensor: proteomics studies using a novel biotinylated dimedone analogue. Mol Cell Proteomics. (2007);6:1473–1484. doi: 10.1074/mcp.M700065-MCP200. [DOI] [PubMed] [Google Scholar]
- 23.Mates JM, Perez-Gomez C, Nunez de Castro I. Antioxidant enzymes and human diseases. Clin Biochem. (1999);32:595–603. doi: 10.1016/S0009-9120(99)00075-2. [DOI] [PubMed] [Google Scholar]
- 24.Kinnula VL, Paakko P, Soini Y. Antioxidant enzymes and redox regulating thiol proteins in malignancies of human lung. FEBS Lett. (2004);569:1–6. doi: 10.1016/j.febslet.2004.05.045. [DOI] [PubMed] [Google Scholar]
- 25.Scibior-Bentkowska D, Czeczot H. [Cancer cells and oxidative stress]. Postepy Hig Med Dosw (Online) (2009);63:58–72. [PubMed] [Google Scholar]
- 26.Jeong J, Kim Y, Kyung Seong J, Lee KJ. Comprehensive identification of novel post-translational modifications in cellular peroxiredoxin 6. Proteomics. (2012);12:1452–1462. doi: 10.1002/pmic.201100558. [DOI] [PubMed] [Google Scholar]
- 27.Cesaratto L, Vascotto C, D'Ambrosio C, et al. Overoxidation of peroxiredoxins as an immediate and sensitive marker of oxidative stress in HepG2 cells and its application to the redox effects induced by ischemia/reperfusion in human liver. Free Radic Res. (2005);39:255–268. doi: 10.1080/10715760400029603. [DOI] [PubMed] [Google Scholar]
- 28.Wu CL, Chou HC, Cheng CS, et al. Proteomic analysis of UVB-induced protein expression- and redox-dependent changes in skin fibroblasts using lysine- and cysteinelabeling two-dimensional difference gel electrophoresis. J Proteomics. (2012);75:1991–2014. doi: 10.1016/j.jprot.2011.12.038. [DOI] [PubMed] [Google Scholar]
- 29.Tsaytler PA, C O'Flaherty M, Sakharov DV, Krijgsveld J, Egmond MR. Immediate protein targets of photodynamic treatment in carcinoma cells. J Proteome Res. (2008);7:3868–3878. doi: 10.1021/pr800189q. [DOI] [PubMed] [Google Scholar]
- 30.Thongboonkerd V, Malasit P. Renal and urinary proteomics: current applications and challenges. Proteomics. (2005);5:1033–1042. doi: 10.1002/pmic.200401012. [DOI] [PubMed] [Google Scholar]
- 31.Tyther R, Ahmeda A, Johns E, McDonagh B, Sheehan D. Proteomic profiling of perturbed protein sulfenation in renal medulla of the spontaneously hypertensive rat. J Proteome Res. (2010);9:2678–2687. doi: 10.1021/pr1001719. [DOI] [PubMed] [Google Scholar]
- 32.Choi J, Rees HD, Weintraub ST, Levey AI, Chin LS, Li L. Oxidative modifications and aggregation of Cu, Zn-superoxide dismutase associated with Alzheimer and Parkinson diseases. J Biol Chem. (2005);280:11648–11655. doi: 10.1074/jbc.M414327200. [DOI] [PubMed] [Google Scholar]
- 33.Matsuyama Y, Terawaki H, Terada T, Era S. Albumin thiol oxidation and serum protein carbonyl formation are progressively enhanced with advancing stages of chronic kidney disease. Clin Exp Nephrol. (2009);13:308–315. doi: 10.1007/s10157-009-0161-y. [DOI] [PubMed] [Google Scholar]
- 34.Elias-Miró M, Jiménez-Castro MB, Rodés J, Peralta C. Current knowledge on oxidative stress in hepatic ischemia/reperfusion. Free Radic Res. (2013);47:555–568. doi: 10.3109/10715762.2013.811721. [DOI] [PubMed] [Google Scholar]
- 35.Wilson CH, Zeile S, Chataway T, Nieuwenhuijs VB, Padbury RT, Barritt GJ. Increased expression of peroxiredoxin 1 and identification of a novel lipid-metabolizing enzyme in the early phase of liver ischemia reperfusion injury. Proteomics. (2011);11:4385–4396. doi: 10.1002/pmic.201100053. [DOI] [PubMed] [Google Scholar]
- 36.Kohr MJ, Sun J, Aponte A, et al. Simultaneous measurement of protein oxidation and S-nitrosylation during preconditioning and ischemia/reperfusion injury with resin-assisted capture. Circ Res. (2011);108:418–426. doi: 10.1161/CIRCRESAHA.110.232173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun J, Murphy E. Protein S-nitrosylation and cardioprotection. Circ Res. (2010);106:285–296. doi: 10.1161/CIRCRESAHA.109.209452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li T, Li J, Liu J, et al. Polymerized placenta hemoglobin attenuates ischemia/reperfusion injury and restores the nitroso-redox balance in isolated rat heart. Free Radic Biol Med. (2009);46:397–405. doi: 10.1016/j.freeradbiomed.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 39.Butterfield DA, Perluigi M, Reed T, et al. Redox proteomics in selected neurodegenerative disorders: from its infancy to future applications. Antioxid Redox Signal. (2012);17:1610–1655. doi: 10.1089/ars.2011.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perluigi M, Sultana R, Cenini G, et al. Redox proteomics identification of 4-hydroxynonenal-modified brain proteins in Alzheimer's disease: Role of lipid peroxidation in Alzheimer's disease pathogenesis. Proteomics Clin Appl. (2009);3:682–693. doi: 10.1002/prca.200800161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sultana R, Reed T, Perluigi M, Coccia R, Pierce WM, Butterfield DA. Proteomic identification of nitrated brain proteins in amnestic mild cognitive impairment: a regional study. J Cell Mol Med. (2007);11:839–851. doi: 10.1111/j.1582-4934.2007.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anantharaman M, Tangpong J, Keller JN, et al. Betaamyloid mediated nitration of manganese superoxide dismutase: implication for oxidative stress in a APPNLH/NLH X PS-1P264L/P264L double knock-in mouse model of Alzheimer's disease. Am J Pathol. (2006);168:1608–1618. doi: 10.2353/ajpath.2006.051223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reed TT, Pierce WM, Markesbery WR, Butterfield DA. Proteomic identification of HNE-bound proteins in early Alzheimer disease: Insights into the role of lipid peroxidation in the progression of AD. Brain Res. (2009);1274:66–76. doi: 10.1016/j.brainres.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 44.Perluigi M, Di Domenico F, Giorgi A, et al. Redox proteomics in aging rat brain: involvement of mitochondrial reduced glutathione status and mitochondrial protein oxidation in the aging process. J Neurosci Res. (2010);88:3498–3507. doi: 10.1002/jnr.22500. [DOI] [PubMed] [Google Scholar]
- 45.Go YM, Jones DP. The redox proteome. J Biol Chem. (2013);288:26512–26520. doi: 10.1074/jbc.R113.464131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thom SR, Bhopale VM, Milovanova TN, Yang M, Bogush M. Thioredoxin reductase linked to cytoskeleton by focal adhesion kinase reverses actin S-nitrosylation and restores neutrophil beta(2) integrin function. J Biol Chem. (2012);287:30346–30357. doi: 10.1074/jbc.M112.355875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saitoh M, Nishitoh H, Fujii M, et al. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. (1998);17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marchand C, Le Maréchal P, Meyer Y, Decottignies P. Comparative proteomic approaches for the isolation of proteins interacting with thioredoxin. Proteomics. (2006);6:6528–6537. doi: 10.1002/pmic.200600443. [DOI] [PubMed] [Google Scholar]
- 49.Hägglund P, Bunkenborg J, Maeda K, Svensson B. Identification of thioredoxin disulfide targets using a quantitative proteomics approach based on isotope-coded affinity tags. J Proteome Res. (2008);7:5270–5276. doi: 10.1021/pr800633y. [DOI] [PubMed] [Google Scholar]
- 50.Go YM, Roede JR, Walker DI, et al. Selective targeting of the cysteine proteome by thioredoxin and glutathione redox systems. Mol Cell Proteomics. (2013);12:3285–3296. doi: 10.1074/mcp.M113.030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Finkelstein AE, Walz DT, Batista V, Mizraji M, Roisman F, Misher A. Auranofin. New oral gold compound for treatment of rheumatoid arthritis. Ann Rheum Dis. (1976);35:251–257. doi: 10.1136/ard.35.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Floen MJ, Forred BJ, Bloom EJ, Vitiello PF. Thioredoxin-1 redox signaling regulates cell survival in response to hyperoxia. Free Radic Biol Med. (2014);75:167–177. doi: 10.1016/j.freeradbiomed.2014.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Isaacs JS, Jung YJ, Mimnaugh EG, Martinez A, Cuttitta F, Neckers LM. Hsp90 regulates a von Hippel Lindau-independent hypoxia-inducible factor-1 alpha-degradative pathway. J Biol Chem. (2002);277:29936–29944. doi: 10.1074/jbc.M204733200. [DOI] [PubMed] [Google Scholar]
- 54.Aschner JL, Foster SL, Kaplowitz M, Zhang Y, Zeng H, Fike CD. Heat shock protein 90 modulates endothelial nitric oxide synthase activity and vascular reactivity in the newborn piglet pulmonary circulation. Am J Physiol Lung Cell Mol Physiol. (2007);292:L1515–1525. doi: 10.1152/ajplung.00252.2006. [DOI] [PubMed] [Google Scholar]
- 55.Spector D, Labarre J, Toledano MB. A genetic investigation of the essential role of glutathione: mutations in the proline biosynthesis pathway are the only suppressors of glutathione auxotrophy in yeast. J Biol Chem. (2001);276:7011–7016. doi: 10.1074/jbc.M009814200. [DOI] [PubMed] [Google Scholar]
- 56.Fukunaga Y, Liu H, Shimizu M, Komiya S, Kawasuji M, Nagafuchi A. Defining the roles of beta-catenin and plakoglobin in cell-cell adhesion: isolation of beta-catenin/plakoglobin-deficient F9 cells. Cell Struct Funct. (2005);30:25–34. doi: 10.1247/csf.30.25. [DOI] [PubMed] [Google Scholar]
- 57.Neumann CA, Krause DS, Carman CV, et al. Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defence and tumour suppression. Nature. (2003);424:561–565. doi: 10.1038/nature01819. [DOI] [PubMed] [Google Scholar]
- 58.Zhang B, Wang Y, Su Y. Peroxiredoxins, a novel target in cancer radiotherapy. Cancer Lett. (2009);286:154–160. doi: 10.1016/j.canlet.2009.04.043. [DOI] [PubMed] [Google Scholar]
- 59.Aguilar-Melero P, Prieto-Álamo MJ, Jurado J, Holmgren A, Pueyo C. Proteomics in HepG2 hepatocarcinoma cells with stably silenced expression of PRDX1. J Proteomics. (2013);79:161–171. doi: 10.1016/j.jprot.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 60.Fuentes-Almagro CA, Prieto-Alamo MJ, Pueyo C, Jurado J. Identification of proteins containing redox-sensitive thiols after PRDX1, PRDX3 and GCLC silencing and/or glucose oxidase treatment in Hepa 1-6 cells. J Proteomics. (2012);77:262–279. doi: 10.1016/j.jprot.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 61.DeBerardinis RJ, Cheng T. Q's next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene. (2010);29:313–324. doi: 10.1038/onc.2009.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park SH, Chung YM, Lee YS, et al. Antisense of human peroxiredoxin II enhances radiation-induced cell death. Clin Cancer Res. (2000);6:4915–4920. [PubMed] [Google Scholar]
- 63.Chung YM, Yoo YD, Park JK, Kim YT, Kim HJ. Increased expression of peroxiredoxin II confers resistance to cisplatin. Anticancer Res. (2001);21:1129–1133. [PubMed] [Google Scholar]
- 64.Rocha S, Vitorino RM, Lemos-Amado FM, et al. Presence of cytosolic peroxiredoxin 2 in the erythrocyte membrane of patients with hereditary spherocytosis. Blood Cells Mol Dis. (2008);41:5–9. doi: 10.1016/j.bcmd.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 65.Matte A, Low PS, Turrini F, et al. Peroxiredoxin-2 expression is increased in beta-thalassemic mouse red cells but is displaced from the membrane as a marker of oxidative stress. Free Radic Biol Med. (2010);49:457–466. doi: 10.1016/j.freeradbiomed.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee TH, Kim SU, Yu SL, et al. Peroxiredoxin II is essential for sustaining life span of erythrocytes in mice. Blood. (2003);101:5033–5038. doi: 10.1182/blood-2002-08-2548. [DOI] [PubMed] [Google Scholar]
- 67.Yang HY, Kwon J, Choi HI, et al. In-depth analysis of cysteine oxidation by the RBC proteome: advantage of peroxiredoxin II knockout mice. Proteomics. (2012);12:101–112. doi: 10.1002/pmic.201100275. [DOI] [PubMed] [Google Scholar]
- 68.Fu C, Wu C, Liu T, et al. Elucidation of thioredoxin target protein networks in mouse. Mol Cell Proteomics. (2009);8:1674–1687. doi: 10.1074/mcp.M800580-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]