Abstract
Achilles tendon rupture has been on the rise over recent years due to a variety of reasons. It is a debilitating injury with a protracted and sometimes incomplete recovery. Management strategy is a controversial topic and evidence supporting a definite approach is limited. Opinion is divided between surgical repair and conservative immobilisation in conjunction with functional orthoses. A systematic search of the literature was performed. Pubmed, Medline and EmBase databases were searched for Achilles tendon and a variety of synonymous terms. A recent wealth of reporting suggests that conservative regimens with early weight bearing or mobilisation have equivalent or improved rates of re-rupture to operative regimes. The application of dynamic ultrasound assessment of tendon gap may prove crucial in minimising re-rupture and improving outcomes. Studies employing functional assessments have found equivalent function between operative and conservative treatments. However, no specific tests in peak power, push off strength or athletic performance have been reported and whether an advantage in operative treatment exists remains undetermined.
Keywords: Orthopaedic surgery, Achilles tendon injury, Sports injury, Tendon rupture, Conservative management
Core tip: Achilles tendon rupture is a common injury. Simmonds or Thomas’ test is a reliable diagnostic tool with a sensitivity of between 0.89-0.93. Studies have not shown conclusive superiority of operative repair compared with non-operative and casting techniques. Non-operative management has a more favourable complication profile. There is emerging evidence that the traditional perception that non-operative management is associated with higher re-rupture rates no longer holds true for the new management strategies which assess tendon gap and use a dedicated “Achilles tendon management infrastructure”. It is important that clinicians can recognize the injury and delayed diagnosis can lead to significant morbidity.
INTRODUCTION
The Achilles tendon is the most frequently ruptured tendon in the human body[1]. The incidence of rupture is on the rise and has been so since the 1980s. The yearly incidence of Achilles tendon rupture is rising and reported as 4.7/100000 in 1981 to 6/100000 in 1994 from a Scottish cohort, and 22.1/100000 in 1991 to 32.6/100000 in 2002 from a Danish cohort. The most rapid increase was noted in the male 30 to 39 age group[1,2]. The most hazardous sport appears to be badminton with 83% occurring in males. The mean age of presentation is 35 years with a male:female ratio of 20:1[3,4]. The classical patient is the novice sportsmen in his fourth decade engaging in unaccustomed sport.
The commonest site of rupture is in a region 3 to 6 cm above the os calcis which corresponds to a watershed region of poor vascularisation[5]. Perfusion in this region is further compromised during stretching and contraction[6,7]. With increasing age there is decreased collage-crosslinking and weakening of the tensile strength of the tendon. Maffulli et al[8] and Järvinen et al[9] histologically observed significant collagen degeneration in patients with Achilles tendon rupture. Ruptured Achilles tendon have histologically demonstrated collagen degeneration with a greater content of collagen III and less collagen I[8,9].
Both oral and intratendinous injection of steroids have been implicated in spontaneous tendon rupture[10]. Other risk factors for rupture of the Achilles tendon include steroid therapy, hypercholesterolemia, gout, rheumatoid arthritis, long-term dialysis, and renal transplantation[2,11-15].
PRESENTATION
The patient typically presents with pain, inability to weight bear and a clear popping sensation or sound after an episode of activity during which they sustain a forced dorsiflexion of the ankle. The injury can also be sustained during eccentric contraction. The patient frequently describes the sensation of being kicked, shot or even bitten on the back of the heel.
Acute Achilles tendon rupture can readily be detected on physical examination. Plantarflexion of the foot is understandably weak[16]. The Achilles tendon is best examined with the patient kneeling and the feet hanging over the edge of the chair. In this position soft tissues hang off the Achilles tendon like a tent ridge pole and defects can be readily visualised (Figure 1). There is frequently a visible defect in the Achilles tendon. This is accompanied by swelling due to peritendinous haemotoma.
Figure 1.
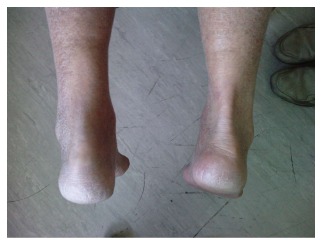
View of the right and left Achilles tendon with the patient prone. The left is ruptured. The right Achilles tendon is well defined and soft tissues hang off it like a tent. The suspension of the soft tissues off the Achilles tendon is not visible on the left side as the tendon is ruptured.
The defect in the Achilles tendon is typically palpable with a sensitivity of 0.71 and specificity of 0.89. Maffulli compared the sensitivity and specificity of the principal clinical tests designed to determine Achilles tendon rupture[17]. Specific tests include Simmonds or Thompsons’ test with sensitivity of 0.98 and specificity of 0.93. Lesser known are the O’Brien and Copeland tests both with a sensitivities of 0.8. Early reports suggest that up to 20% of Achilles tendon injuries can be missed by clinical assessment alone[18].
RADIOLOGY
In patients with equivocal clinical signs diagnostic imaging is required.
Ultrasound is readily available, cheap, non-invasive but user dependant. Ultrasound has a diagnostic reported sensitivity, specificity and accuracy of 100%, 89.9% and 94.4% respectively[19].
It can discriminate between partial and complete tears except those located at the proximal pole or musculotendinous junction of the tendon where sensitivity and specificity drop to 0.5 and 0.81 respectively[20]. An additional advantage in ultrasound in the dynamic observation of tendon gaps which have been shown to correlate strongly with those observed during operative repair[21]. Some authors argue that if the gap between the tendon ends is greater than 5 mm as assessed by ultrasound in full equinus than surgical intervention is indicated[22].
Magnetic resonance imaging remains the gold standard for the diagnosis of the Achilles tendon rupture with a sensitivity of 100% and a specificity of 0.03[23].
TREATMENT
There is a dichotomy of therapeutic options: operative and conservative. Both are accepted forms of management for acute rupture and the optimal regimen remains contentious. The article discusses cases of acute tendoachilles rupture. In cases of delayed diagnosis the likely success of conservative management may be limited by a lack of apposition of the tendon ends due to scarring and retraction. Therefore, surgical repair is advocated[24]. Cases of chronic rupture of the tendoachilles by their very nature will not respond to conservative treatment and therefore will require repair utilising graft[25].
Conservative
The aim of non-operative means of treatment is to restore and maintain contact between the two ends of the ruptured Achilles tendon to facilitate healing. Conservative treatment regimens vary greatly but commonly involve immobilisation with rigid casting or functional bracing. The foot is initially placed in full equinus (30° namely full plantarflexion). The foot is then brought into neutral sequentially over a period of 8-12 wk. Once ankle position permits it, weight bearing is allowed. There is currently no clinical consensus on whether the cast should extend above the knee or if a below knee cast is sufficient. The above knee plaster is applied with the knee in slight flexion which serves to defunction gastrocnemius, having an origin over the posterior aspect of the femora condyles. However, one study shows the position of the knee does not influence gap between the torn ends of the Achilles tendon[26].
Little evidence exists to recommend one regimen over another. The current evidence is summarised in Table 1. These studies demonstrate that patients can be allowed to weight-bear early in an off-the-shelf orthosis/CAM walker/Sheffield splint with no detriment in any long term outcomes[27,28]. This has obvious practical advantages compared to the traditional treatment of prolonged non weight-bearing in a below knee equinus cast. This is particularly true for frail or elderly patients where non-operative treatment tends to be preferred. Petersen et al also suggest that this may also actually decrease the risk of re-rupture although this was not found to be significant (P = 0.066). Saleh et al[29] also suggested that their splint allowed patients to regain mobility significantly more quickly and that patients preferred the splint to the cast. These findings are in keeping with the literature on operatively managed acute Achilles tendon ruptures which suggests that early weight bearing and mobilisation improve outcomes.
Table 1.
Summary of evidence for non-operative management of acute tendo-achilles rupture
| Ref. | Patient group | Study type (level of evidence) | Outcomes | Key results | Study weaknesses |
| Costa et al[27] | 48 adult patients with acute achilles tendon rupture who chose to have non-operative treatment. Randomised to either six weeks in an off-the-shelf, carbon-fibre orthosis with three 1.5 cm heel raises that were encouraged to mobilise fully weight-bearing and move the ankle within the orthosis (trial group) or to six weeks in a below knee gravity equinus cast that were non weight-bearing (control group). This was followed by serial removal of heel raises or casting in increasing dorsiflexion over 6 further weeks. Immobilisation was discontinued at 12 wk. Reviews at 3, 6 and 12 mo | PRCT | Numbers returning to sport | No significant difference found (P = 1.0); 56% trial group vs 52% control group | Of the original 48 patients only 40 were available for review at one year. All patients who presented out of hours were initially placed in below-knee equinus plaster backslab |
| Time to return to normal activities | No significant differences found. Sport- (P = 0.631) 18 wk trial group vs 21 wk control group. Walking- (P = 0.765) 16 wk trial group vs 22 wk control group. Stair climbing- (P = 0.484) 16 wk trial group vs 22 wk control group. Work- (P = 0.370) 13 wk trial group vs 17 wk control group | ||||
| EuroQol health status questionnaire- EQoL Domain | No significant differences found. 3 mo- (P = 0.372) 80 trial group vs 85 control group. 6 mo (P = 0.598) 89 trial group vs 88 control group. 12 mo- (P = 0.122) 85 trial group vs 91 control group | ||||
| EuroQol health status questionnaire- E5D Domain | No significant differences found. 3 mo- (P = 0.450) 0.73 trial group vs 0.69 control group. 6 mo- (P = 0.810) 0.80 trial group vs 0.80 control group. 12 mo- (P = 0.888) 0.85 trial group vs 0.85 control group | ||||
| Deficit in calf diameter in mm | No significant difference found (P = 0.634). 1.37 trial group vs 1.11 control group | ||||
| Loss of movement in degrees | No significant differences found. Dorsiflexion (P = 0.879) -0.7 trial group vs 0.27 control group. Plantarflexion (P = 0.248) 4.13 trial group vs 7.27 control group | ||||
| Deficit in total concentric and eccentric work | No significant differences found | ||||
| Complications | 1 re-rupture in trial group vs 1 re-rupture, 1 failure of tendon healing and 1 PE in control group | ||||
| Petersen et al[28] | 50 adult patients with acute achilles tendon ruptures. Randomised to either a CAM walker and were encouraged to weight bear (trial group) or to a below knee full equinus cast and were non-weight bearing (control group). Both groups were immobilised for 8 wk. Reviews at 4 and 12 mo | PRCT | Re-rupture rate | No significant difference found (P = 0.066) but suggestive of a trend towards increased re-rupture in the control group. The risk of a type II error was 44% and it was thought likely that should the numbers of patients recruited have been larger this may have become a significant difference. 0% trial group, vs 17% control group | Number lost to follow-up: 8. Length of time between injury and treatment not stated although delayed presentations were excluded |
| Patient satisfaction | No significant difference | ||||
| Saleh et al[29] | 40 adult patients with acute achilles tendon ruptures. Randomised to either a below knee full equinus cast for 2 wk followed by, a mid equinus cast for 1 wk and then controlled early mobilisation in a Sheffield splint with full weight-bearing (trial group) or to a full-leg cast, with the ankle in full equinus, for four weeks, followed by two weeks in a below-knee cast with the ankle in mid-equinus, and then two more weeks with the ankle in the neutral position with weight-bearing allowed during the final two weeks only (control group). Review at 3, 6 and 12 mo. The Sheffield splint is an ankle-foot orthosis which holds the ankle at 15 degrees of plantar flexion, but allows some movement at the metatarsophalangeal joints. The orthosis is used in conjunction with an insole within an extra-depth shoe. It is removed to allow controlled movement during physiotherapy | PRCT | Strength of plantar flexion | No significant difference found at 3, 6 or 12 mo | Randomisation method not stated |
| Range of plantar flexion (degrees) | No significant difference found at 3, 6 or 12 mo | ||||
| Range of dorsiflexion (degrees) | Significantly more in trial group at 3, 6 and 12 mo (P < 0.001) | ||||
| Time to walking indoors | Significantly quicker in trial group (P < 0.001). 6 wk trial group vs 11 wk control group | ||||
| Time to walking outdoors | Significantly quicker in trial group (P < 0.001). 9 wk trial group vs 15 wk control group | ||||
| Complications | 1 re-rupture in each group | ||||
| Patient preference | All patients in the trial group preferred the time spent in the Sheffield splint to the time spent in the cast |
Newer splints for immobilisation have been developed with encouraging initial results. The Vacoped© is a cast in which the patient’s ankle is supported by an air cushion which is then inflated. The cushion is encased in a robust shell. The design of the cast allows the degree of equinus to be dialled from 30° (full) to 15° (mid) and 0° (neutral). In addition there is latch which allows users the facility to perform a restricted range (-10°-10°) of plantar and dorsiflexion. The Vacoped regimen recommends 2 wk in full equinus followed by a further 2 wk in partial equinus. Then the ankle is held in neutral for 1 wk and then restricted (10°) dorsi- plantar flexion for the final week. The Vacoped allows the patient to touch weight bear for two weeks, and partial weight bear from for one week after that. Full weight-bearing is commenced at 3 wk[30]. In addition the periodic insufflations and deflation of air facilitates venous drainage theoretically reducing the risk of deep vein thrombosis. Furthermore, the support is buoyant and supple avoiding the risk of pressure areas.
Operative
There are a variety of approaches to the surgical management of this injury. Contention exists over the surgical approach (open or percutaneous), suture repair method and suture type.
In addition to isolated direct tendon repair, various means of augmentation of the tendon have been described. Gastrocnemius augmentation involves raising a flap 2 cm wide by 8 cm long which is reflected across the repair and sutured. The Plantaris tendon can also be used (Figure 2). It is either weaved around the tendon or may be expanded into a membrane which is sutured around the repair. The evidence supporting augmentation is weak. Pajala et al[31] performed a large prospective study of tendoachilles repair and found no benefits between augmented and simple end-to-end repair.
Figure 2.
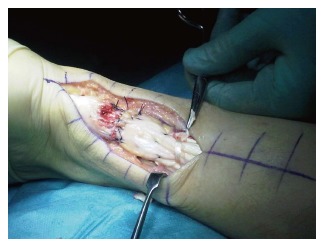
Achilles tendon repair with plantaris tendon reinforcement.
Percutaneous repair has been described involving minimally invasive stab incisions on the medial and lateral aspect of Achilles tendon and a suture passer. Reduced infection rates have been shown compared to open repair[32]. Increased rates of Sural nerve injury have been demonstrated with this technique[33].
Patient factors have been demonstrated to influence post-operative wound breakdown and infection rates. These included diabetes mellitus, steroid therapy, smoking and rheumatoid disease[34].
Post-operative regime: Postoperatively the patient can progressively increase the extent of weightbearing. Typically, at 6 wk the patient commences active and assisted movement of the ankle. Isokinetic strengthening is commenced 2 to 4 wk. The patient can usually expect full strength and endurance 4 mo after surgery. Although this represents the standard post-operative regimen, the optimum post-operative rehabilitation remains to be determined. Suchak et al[35] explored the effect of early weight bearing at 2 wk vs weight bearing at 6 wk in their randomised controlled trial. They observed that early weight-bearing had statistically significant improvement in quality of life indices (such as social functioning), vitality scores and physical functioning[35]. However, by 6 mo postoperatively there was no difference in the groups. Other sources of clinical controversy include post-operative early mobilisation versus rigid immobilisation for 6 wk. Kangas et al[36] explored this in a randomised controlled trial. They reported that early mobilisation was associated with improved isokinetic calf strength at 60 wk. The re-rupture rate was higher in the immobilisation cohort. However, the difference did not reach statistical significance[36]. Mortenson’s group observed that patients who were allowed to perform early restricted motion had a shorter rehabilitation time when compared to a below knee cast for 8 wk[37].
Conservative or operative: which is better?
No published studies conclusively demonstrate the definitive superiority of one modality over another. Meta-analyses of studies have shown that the re-rupture rates are higher in cases of non-operative management: 13% for conservative management compared with 4% for surgically repaired Achilles tendons[31,38]. Meta-analyses report a re-rupture rate of 2% for percutaneous reparative techniques.
Tendon elongation and weaker plantar flexion are also associated with non-operative management. However, recent studies suggest that these benefits of operative repair over conservative management in plaster are only short-lived. Keating and collaborators in the recent prospective randomised trial found that operative repair was associated initially with increased range of ankle movement and plantarflexion power when compared with cast management[39]. However by 26 wk there was no difference between the two groups.
Plaster or simple immobilisation alone avoids the inherent risks of surgery. These include wound infection (4%), fistula formation, skin necrosis, suture granuloma and damage to the sural nerve[31,37]. The skin necrosis can result in significant morbidity and require extensive plastic soft tissue procedures to ensure coverage of the tendon. Operative repair is associated with more rapid rehabilitation and return to work.
Percutaneous operative techniques have been found to have a complication profile superior to that of both conservative and open operative techniques. Meta-analysis report a re-rupture rate of 2%[31,37]. Studies involving functional bracing suggest that the disparity between surgical and conservative management may not be as marked as originally suspected. More recent studies show that re-rupture rates in patients treated operatively vs functional bracing are comparative[19,40-42]. In a recent study the result of percutaneous operative management were compared with those achieved by functional bracing using the Vacoped. Investigators found that the incidence of re-rupture to be 3.9% for percutaneous repair and 3.4% for functional bracing with the Vacoped[43]. The difference did not reach statistical significance. The Vacoped allows early weight-bearing. The protective effect of early weight-bearing appears to be reproduced when patients are allowed early ankle movements. A recent prospective randomised controlled trial observed no difference in rupture rates between operative and non-operatively managed Achilles tendon rupture when both groups are permitted early movement in a functional brace[29].
Assessment of the gap between the ends of the tendon as determined by magnetic resonance imaging or ultrasound may influence re-rupture rates in patients managed conservatively. Kotnis et al[22] elected to manage conservatively only those patients whose gap in full equinus was less than 5 mm. All others were managed operatively. They observed no statistically significant difference in re-rupture rates between the groups[22]. Wallace et al[44] based in Belfast, Ireland and Sheffield, United Kingdom studied 875 non-operatively treated Achilles tendon ruptures. The decision to manage patients non-operatively was based on the presence of opposition of the tendon ends on dorsiflexion. The observed re-rupture rate was 2.9%. A recent series studied by the Swansea and Maudsley group used a protocol of dynamic ultrasound and a tendon gap of less than 1 cm in full equinus. Furthermore, a dedicated clinic and service was established to treat and rehabilitate the patients. They found only a single case of re-rupture (< 1%) in 151 conservatively treated patients since 2008. This was comparable to the single case in the operative group of 63 patients[45]. In two of these series the re-rupture rate is superior to any anything achieved in a published operative series.
In Wallace’s published series of excellent non-operative results patients were managed in a dedicated “Tendo Achilles” clinic. Patients were placed in an equinus non-weight bearing cast for the first four weeks. For the next four weeks they were place placed in a pneumatic walker with heel-raises, which were sequentially removed over a period of 4 wk. The combined time in the equinus cast and boot walker was 8 wk. After this patients engaged in a specialist physiotherapy programme involving gait training, strength and mobility training. Final assessment and discharge was at 14 wk from injury or 6 wk from the time of removing the walking boot. It is uncertain how much the dedicated Achilles tendon clinic contributed to the favourable outcome. Patients were attended to by a specialist physiotherapist and were only discharged when the latter deemed that ankle strength was satisfactory.
Some authors would argue the merits of operative intervention in high performing athletes. The rationale for this argument is the potential loss of power or push off strength with conservative management which is lessened by operative repair. The most recent studies quoted using specific treatment and rehabilitation regimes do not identify a functional benefit to operative repair. It is possible that the cohort may not reflect the athlete group. Furthermore, the measurement tools and assessments may not be sensitive enough to detect a deficit at the high functioning sporting level. For this reason the authors would exercise caution in treating this cohort as it is difficult to draw definitive conclusions.
CONCLUSION
Tendoachilles rupture causes significant burden. Recovery is slow and potentially incomplete. Simmonds is a sensitive and reliable test. Avoiding missed diagnosis is imperative in good outcome. Both MRI and Ultrasound have potential diagnostic value. The argument of conservative vs operative treatment will no doubt continue; evidence is beginning to shift towards underpinning the benefits of non-operative treatment. Tendon gap assessment may be an important tool in deciding treatment modality. Intensive and specific post-operative regimes are being employed with seemingly positive results. The relative impacts of these factors in not known but certainly it has been demonstrated that favourable re-rupture rates are achievable in the non-operative group.
Footnotes
P- Reviewer: Chen YK, Mohseni-Bandpei MA, Yamakado K S- Editor: Qi Y L- Editor: A E- Editor: Lu YJ
Conflict-of-interest: The authors declare that are no conflicts of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: September 4, 2014
First decision: January 20, 2015
Article in press: April 18, 2015
References
- 1.Maffulli N, Waterston SW, Squair J, Reaper J, Douglas AS. Changing incidence of Achilles tendon rupture in Scotland: a 15-year study. Clin J Sport Med. 1999;9:157–160. doi: 10.1097/00042752-199907000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Sode J, Obel N, Hallas J, Lassen A. Use of fluroquinolone and risk of Achilles tendon rupture: a population-based cohort study. Eur J Clin Pharmacol. 2007;63:499–503. doi: 10.1007/s00228-007-0265-9. [DOI] [PubMed] [Google Scholar]
- 3.Möller A, Astron M, Westlin N. Increasing incidence of Achilles tendon rupture. Acta Orthop Scand. 1996;67:479–481. doi: 10.3109/17453679608996672. [DOI] [PubMed] [Google Scholar]
- 4.Leppilahti J, Puranen J, Orava S. Incidence of Achilles tendon rupture. Acta Orthop Scand. 1996;67:277–279. doi: 10.3109/17453679608994688. [DOI] [PubMed] [Google Scholar]
- 5.Beddy P, Dunne R, de Blacam C. Achilles wiiitis. AJR Am J Roentgenol. 2009;192:W79. doi: 10.2214/AJR.08.1654. [DOI] [PubMed] [Google Scholar]
- 6.Carr AJ, Norris SH. The blood supply of the calcaneal tendon. J Bone Joint Surg Br. 1989;71:100–101. doi: 10.1302/0301-620X.71B1.2914976. [DOI] [PubMed] [Google Scholar]
- 7.Komi PV, Fukashiro S, Järvinen M. Biomechanical loading of Achilles tendon during normal locomotion. Clin Sports Med. 1992;11:521–531. [PubMed] [Google Scholar]
- 8.Maffulli N, Ewen SW, Waterston SW, Reaper J, Barrass V. Tenocytes from ruptured and tendinopathic achilles tendons produce greater quantities of type III collagen than tenocytes from normal achilles tendons. An in vitro model of human tendon healing. Am J Sports Med. 2000;28:499–505. doi: 10.1177/03635465000280040901. [DOI] [PubMed] [Google Scholar]
- 9.Järvinen M, Józsa L, Kannus P, Järvinen TL, Kvist M, Leadbetter W. Histopathological findings in chronic tendon disorders. Scand J Med Sci Sports. 1997;7:86–95. doi: 10.1111/j.1600-0838.1997.tb00124.x. [DOI] [PubMed] [Google Scholar]
- 10.Newnham DM, Douglas JG, Legge JS, Friend JA. Achilles tendon rupture: an underrated complication of corticosteroid treatment. Thorax. 1991;46:853–854. doi: 10.1136/thx.46.11.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee WT, Collins JF. Ciprofloxacin associated bilateral achilles tendon rupture. Aust N Z J Med. 1992;22:500. [PubMed] [Google Scholar]
- 12.Poon CC, Sundaram NA. Spontaneous bilateral Achilles tendon rupture associated with ciprofloxacin. Med J Aust. 1997;166:665. doi: 10.5694/j.1326-5377.1997.tb123308.x. [DOI] [PubMed] [Google Scholar]
- 13.McGarvey WC, Singh D, Trevino SG. Partial Achilles tendon ruptures associated with fluoroquinolone antibiotics: a case report and literature review. Foot Ankle Int. 1996;17:496–498. doi: 10.1177/107110079601700811. [DOI] [PubMed] [Google Scholar]
- 14.Donck JB, Segaert MF, Vanrenterghem YF. Fluoroquinolones and Achilles tendinopathy in renal transplant recipients. Transplantation. 1994;58:736–737. [PubMed] [Google Scholar]
- 15.West MB, Gow P. Ciprofloxacin, bilateral Achilles tendonitis and unilateral tendon rupture--a case report. N Z Med J. 1998;111:18–19. [PubMed] [Google Scholar]
- 16.Saxena A, Bareither D. Magnetic resonance and cadaveric findings of the “watershed band” of the achilles tendon. J Foot Ankle Surg. 2001;40:132–136. doi: 10.1016/s1067-2516(01)80078-8. [DOI] [PubMed] [Google Scholar]
- 17.Maffulli N. The clinical diagnosis of subcutaneous tear of the Achilles tendon. A prospective study in 174 patients. Am J Sports Med. 1998;26:266–270. doi: 10.1177/03635465980260021801. [DOI] [PubMed] [Google Scholar]
- 18.Saltzman CL, Tearse DS. Achilles tendon injuries. J Am Acad Orthop Surg. 1998;6:316–325. doi: 10.5435/00124635-199809000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Rockett MS, Waitches G, Sudakoff G, Brage M. Use of ultrasonography versus magnetic resonance imaging for tendon abnormalities around the ankle. Foot Ankle Int. 1998;19:604–612. doi: 10.1177/107110079801900907. [DOI] [PubMed] [Google Scholar]
- 20.Kayser R, Mahlfeld K, Heyde CE. Partial rupture of the proximal Achilles tendon: a differential diagnostic problem in ultrasound imaging. Br J Sports Med. 2005;39:838–842; discussion 838-842. doi: 10.1136/bjsm.2005.018416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Margetić P, Miklić D, Rakić-Ersek V, Doko Z, Lubina ZI, Brkljacić B. Comparison of ultrasonographic and intraoperative findings in Achilles tendon rupture. Coll Antropol. 2007;31:279–284. [PubMed] [Google Scholar]
- 22.Kotnis R, David S, Handley R, Willett K, Ostlere S. Dynamic ultrasound as a selection tool for reducing achilles tendon reruptures. Am J Sports Med. 2006;34:1395–1400. doi: 10.1177/0363546506288678. [DOI] [PubMed] [Google Scholar]
- 23.Garras DN, Raikin SM, Bhat SB, Taweel N, Karanjia H. MRI is unnecessary for diagnosing acute Achilles tendon ruptures: clinical diagnostic criteria. Clin Orthop Relat Res. 2012;470:2268–2273. doi: 10.1007/s11999-012-2355-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Den Hartog BD. Surgical strategies: delayed diagnosis or neglected achilles’ tendon ruptures. Foot Ankle Int. 2008;29:456–463. doi: 10.3113/FAI.2008.0456. [DOI] [PubMed] [Google Scholar]
- 25.Maffulli N, Ajis A, Longo UG, Denaro V. Chronic rupture of tendo Achillis. Foot Ankle Clin. 2007;12:583–596, vi. doi: 10.1016/j.fcl.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Trickett RW, Hodgson P, Lyons K, Thomas R. Effect of knee position on gap size following acute Achilles rupture. Foot Ankle Int. 2011;32:1–4. doi: 10.3113/FAI.2011.0001. [DOI] [PubMed] [Google Scholar]
- 27.Costa ML, MacMillan K, Halliday D, Chester R, Shepstone L, Robinson AH, Donell ST. Randomised controlled trials of immediate weight-bearing mobilisation for rupture of the tendo Achillis. J Bone Joint Surg Br. 2006;88:69–77. doi: 10.1302/0301-620X.88B1.16549. [DOI] [PubMed] [Google Scholar]
- 28.Petersen OF, Nielsen MB, Jensen KH, Solgaard S. [Randomized comparison of CAM walker and light-weight plaster cast in the treatment of first-time Achilles tendon rupture] Ugeskr Laeger. 2002;164:3852–3855. [PubMed] [Google Scholar]
- 29.Saleh M, Marshall PD, Senior R, MacFarlane A. The Sheffield splint for controlled early mobilisation after rupture of the calcaneal tendon. A prospective, randomised comparison with plaster treatment. J Bone Joint Surg Br. 1992;74:206–209. doi: 10.1302/0301-620X.74B2.1544953. [DOI] [PubMed] [Google Scholar]
- 30. Available from: http: //vacoped.com/vacoped/en/principles.php.
- 31.Pajala A, Kangas J, Siira P, Ohtonen P, Leppilahti J. Augmented compared with nonaugmented surgical repair of a fresh total Achilles tendon rupture. A prospective randomized study. J Bone Joint Surg Am. 2009;91:1092–1100. doi: 10.2106/JBJS.G.01089. [DOI] [PubMed] [Google Scholar]
- 32.Khan RJ, Carey Smith RL. Surgical interventions for treating acute Achilles tendon ruptures. Cochrane Database Syst Rev. 2010;8:CD003674. doi: 10.1002/14651858.CD003674.pub4. [DOI] [PubMed] [Google Scholar]
- 33.Aibinder WR, Patel A, Arnouk J, El-Gendi H, Korshunov Y, Mitgang J, Uribe J. The rate of sural nerve violation using the Achillon device: a cadaveric study. Foot Ankle Int. 2013;34:870–875. doi: 10.1177/1071100712473097. [DOI] [PubMed] [Google Scholar]
- 34.Saxena A, Maffulli N, Nguyen A, Li A. Wound complications from surgeries pertaining to the Achilles tendon: an analysis of 219 surgeries. J Am Podiatr Med Assoc. 2008;98:95–101. [PubMed] [Google Scholar]
- 35.Suchak AA, Bostick GP, Beaupré LA, Durand DC, Jomha NM. The influence of early weight-bearing compared with non-weight-bearing after surgical repair of the Achilles tendon. J Bone Joint Surg Am. 2008;90:1876–1883. doi: 10.2106/JBJS.G.01242. [DOI] [PubMed] [Google Scholar]
- 36.Kangas J, Pajala A, Siira P, Hämäläinen M, Leppilahti J. Early functional treatment versus early immobilization in tension of the musculotendinous unit after Achilles rupture repair: a prospective, randomized, clinical study. J Trauma. 2003;54:1171–1180; discussion 1180-1181. doi: 10.1097/01.TA.0000047945.20863.A2. [DOI] [PubMed] [Google Scholar]
- 37.Mortensen HM, Skov O, Jensen PE. Early motion of the ankle after operative treatment of a rupture of the Achilles tendon. A prospective, randomized clinical and radiographic study. J Bone Joint Surg Am. 1999;81:983–990. doi: 10.2106/00004623-199907000-00011. [DOI] [PubMed] [Google Scholar]
- 38.Khan RJ, Fick D, Keogh A, Crawford J, Brammar T, Parker M. Treatment of acute achilles tendon ruptures. A meta-analysis of randomized, controlled trials. J Bone Joint Surg Am. 2005;87:2202–2210. doi: 10.2106/JBJS.D.03049. [DOI] [PubMed] [Google Scholar]
- 39.Keating JF, Will EM. Operative versus non-operative treatment of acute rupture of tendo Achillis: a prospective randomised evaluation of functional outcome. J Bone Joint Surg Br. 2011;93:1071–1078. doi: 10.1302/0301-620X.93B8.25998. [DOI] [PubMed] [Google Scholar]
- 40.Roberts CP, Palmer S, Vince A, Deliss LJ. Dynamised cast management of Achilles tendon ruptures. Injury. 2001;32:423–426. doi: 10.1016/s0020-1383(00)00195-9. [DOI] [PubMed] [Google Scholar]
- 41.McComis GP, Nawoczenski DA, DeHaven KE. Functional bracing for rupture of the Achilles tendon. Clinical results and analysis of ground-reaction forces and temporal data. J Bone Joint Surg Am. 1997;79:1799–1808. doi: 10.2106/00004623-199712000-00005. [DOI] [PubMed] [Google Scholar]
- 42.Nilsson-Helander K, Silbernagel KG, Thomeé R, Faxén E, Olsson N, Eriksson BI, Karlsson J. Acute achilles tendon rupture: a randomized, controlled study comparing surgical and nonsurgical treatments using validated outcome measures. Am J Sports Med. 2010;38:2186–2193. doi: 10.1177/0363546510376052. [DOI] [PubMed] [Google Scholar]
- 43.Marx RC, Mizel MS. What’s new in foot and ankle surgery. J Bone Joint Surg Am. 2010;92:512–523. doi: 10.2106/JBJS.I.01502. [DOI] [PubMed] [Google Scholar]
- 44.Wallace RG, Heyes GJ, Michael AL. The non-operative functional management of patients with a rupture of the tendo Achillis leads to low rates of re-rupture. J Bone Joint Surg Br. 2011;93:1362–1366. doi: 10.1302/0301-620X.93B10.26187. [DOI] [PubMed] [Google Scholar]
- 45.Hutchison AM, Beard DJ, Evans RM, Topliss CJ, Williams PR. Reduction in re-rupture rate using a new standardised Achilles tendon rupture pathway and protocol in a dedicated clinic - Personal Communication. Swansea: Morriston Hospital, Abertawe Bro Morgannwg University Health Board; 2012. [Google Scholar]


