Abstract
Mesenteric panniculitis is a chronic illness that is characterized by fibrosing inflammation of the mesenteries that can lead to intractable abdominal pain. Pain control is a crucial component of the management plan. Most patients will improve with oral corticosteroids treatment, however, some patients will require a trial of other immunosuppressive agents, and a minority of patients will continue to have refractory disease. Endoscopic ultrasound guided celiac plexus block is used frequently to control abdominal pain in patients with pancreatic pathology. To our knowledge there are no case reports describing its use in mesenteric panniculitis patients with refractory abdominal pain.
Keywords: Endoscopic-ultrasound, Abdominal pain, Celiac plexus, Mesenteric panniculitis
Core tip: Mesenteric panniculitis is a rare disorder that can present with refractory and disabling abdominal pain, we describe a novel intervention using endoscopic ultrasonography guided celiac plexus block to control the refractory abdominal pain in a patient with mesenteric panniculitis. This approach is based on the anatomical supply of the epigastric area where the pain is originating.
INTRODUCTION
Mesenteric panniculitis is a rare, benign condition characterized by acute inflammation of the mesenteric adipose tissue that can progress to chronic fibrosis[1]. The disease was first described by Jura[2] in 1924, he used the term retractile mesenteritis to describe this condition. Subsequently, the term mesenteric panniculitis was developed by Aach et al[3] to describe the acute inflammatory phase of the disease. Since then, this has been widely used and adopted term to describe this disease. Mesenteric panniculitis is also known as mesenteric lipodystrophy, primary liposclerosis, isolated lipodystrophy, lipogranuloma, and Weber-Christian disease[4]. The etiology of this disorder remains largely unknown; an association with inflammatory disorders, infection, malignancy (particularly lymphoma)[5], trauma, and abdominal surgery has been described[6]. The prevalence of the disease is estimated to be around 0.6%, and it is more common in Caucasians. The clinical course of mesenteric panniculitis is indolent and favorable[7].
The disease usually progresses slowly and may subside spontaneously[7], around 30%-50% of patients are asymptomatic[5]. However, 20% of patients will have more symptomatic debilitating disease[8]. The most common symptom is chronic abdominal pain, some patients may present with acute abdomen[9]. Abdominal pain can be accompanied by other non-specific symptoms including fever, nausea, vomiting, anorexia and non-intentional weight loss[10]. The diagnosis is usually suggested by high resolution computed tomography (CT) scan[11]. Histological confirmation is rarely required[1]. The majority of patients will respond to systemic corticosteroids[8]. However, some patients will require a more intense immunosuppressive therapy like azathioprine or cyclophosphamide. Only a minority of patients will continue to have refractory disease despite immunosuppressive therapy[12]. Other modalities including progesterone, colchicine, tamoxifen, antibiotics and emetine, or radiotherapy have been used in refractory disease with limited success[13,14]. Surgical resection is reserved for the treatment of complication like intestinal obstruction or ischemia[7]. Refractory abdominal pain can be a major source of morbidity in these patients[15].
CASE REPORT
We report here on a 62-year-old caucasian male who presented with right upper quadrant abdominal pain for several months prior to his first presentation to our institution in 2005. The abdominal pain was not associated with changes in bowel habits, nausea, vomiting, or constitutional symptoms. Initial investigations including complete blood count, liver and kidney function tests, and abdominal ultrasonography were normal. The patient underwent cholecystectomy in 2005 for possible biliary cause of pain, but the pain persisted after surgery. Subsequently, imaging study using CT imaging scan demonstrated thickening and irregularity of the mesentery surrounding the pancreatic head. The radiologic findings were in keeping with the diagnosis of mesenteric panniculitis (Figure 1). Extensive investigations ruled out luminal pathology, pancreatic or adrenal diseases, intermittent porphyria, vascular etiology, and other conditions.
Figure 1.
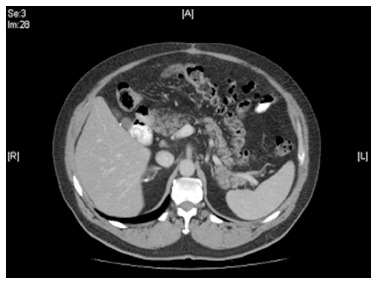
Computed tomography of the abdomen. Computed tomography scan of the abdomen showing mesenteric irregularity and thickness.
Given the radiologic findings and the patient symptoms, the patient was started on prednisone 40 mg once daily for two months. This was associated with a significant improvement in the severity of abdominal pain. However, prednisone therapy was complicated by severe systemic side effects, including worsening of pre-existing depressive disorder, hypertension and cataracts. For this reason, the patient was subsequently weaned off corticosteroids. He remained symptom free for 6 mo after discontinuation of steroid therapy, and then had recurrence of abdominal pain. Because of the chronicity and severity of the symptoms, the patient underwent diagnostic laparoscopy primarily to rule out malignant process. The operative findings showed thickening of the mesentery with no discrete visible masses. Samples from the thickened mesentery were obtained. The pathology results confirmed the diagnosis of mesenteric panniculitis (Figure 2). The patient was started on a steroid-sparing agent (azathioprine) for 6-mo with no response. Further attempts using 3 to 6 mo courses of tamoxifen and subsequently thalidomide failed to improve his symptoms. Different non-opiate analgesic agents were unsuccessful in controlling his symptoms, including acetaminophen and non-steroidal anti-inflammatory drugs. Eventually the patient was started on opioids (oxycodone and morphine) and a serotonin-norepinephrine reuptake inhibitor for pain control. A follow-up CT imaging of the abdomen showed similar findings.
Figure 2.
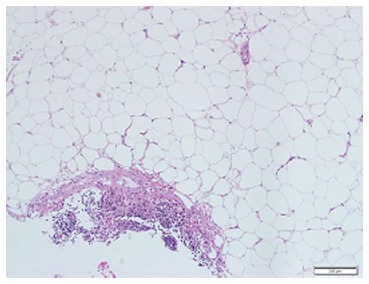
Mesenteric biopsy. Mesenteric biopsy showing fibrotic band of dense collagen infiltrated by mixed inflammatory cells (lymphocytes, plasma cells and neutrophils). There is fat necrosis, no vasculitis or malignancy seen. There is no cellular atypia or lipoblast identified in the biopsy.
After discussion with the patient, the patient was referred for endoscopic ultrasonography (EUS) guided celiac plexus block in an attempt to control relief the intractable abdominal pain and minimize the use of narcotics.
After obtaining consent from the patient, the linear echoendoscope was advanced through the oral cavity into the stomach; the celiac trunk was identified using the ultrasound images (Figure 3). A 19-gauge needle (Echotip; Wilson-Cook) was used to inject 40 mg of Triamcinilone and 10 mL of 0.25% bupivicaine on both sides of the celiac trunk. This protocol is similar to that described by Gress et al[16]. Intravenous crystalloids were administered during the procedure to prevent hypotension caused by the procedure. The patient tolerated the procedure well and was discharged home within few hours.
Figure 3.
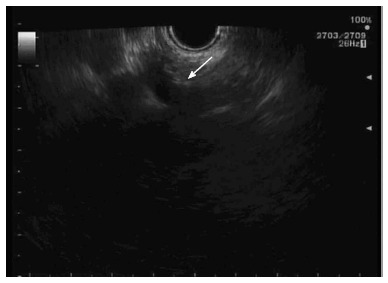
Ultrasonographic image of celiac plexus. This image is showing the celiac artery and celiac plexus (arrow).
Within the first week after the procedure, the patient noticed a dramatic improvement in his symptoms. Within 2 mo, he was weaned off narcotics with complete resolution of his symptoms. However, symptoms recurred 6 mo after the procedure. Given the initial response to this therapy, the procedure was repeated using identical protocol. Few days after the procedure, the patient developed a back injury that led to a surreptitious diagnosis of a 1 cm schwannoma at T12-L1 spinal levels. Surgical resection of the spinal cord tumor was done soon after celiac block, which confounded the assessment of pain. Three months after the second EUS-guided celiac plexus block, the patient was pain free and off all analgesics.
DISCUSSION
To our knowledge, there are no published reports of applying this unique intervention to control refractory abdominal pain in a patient with mesenteric panniculitis. Mesenteric panniculitis is a rare disorder that is characterized by chronic inflammation leading to fibrosis of the mesentery. Patients’ presentation varies from asymptomatic incidental radiologic findings to severe abdominal pain, vomiting, changes in bowel habits, and constitutional symptoms[17,18]. Associated malignancy is not uncommon, with one report showing that 70% of included patients had radiological findings consistent with malignant disorders[19].
Due to the low incidence of this condition, the prognosis of the disease is not well defined. One report with more than 5 years of follow-up showed that mortality rate approaches 45%, majority of fatalities (50%) were related to co-existing malignancy[20]. Several case reports showed that immunosuppressive medications are effective in controlling disease activity[4,21,22]. However, there are no published reports on the utility of celiac plexus block for controlling refractory symptoms.
Celiac plexus is composed of sympathetic efferent fibres, which are derived from the greater, lesser, and least splanchnic (T5-T12) nerves[23]. The visceral afferent fibres supplying the distal esophagus down to the transverse colon pass through the celiac plexus, before ending in the spinal cord. Therefore, pain originating from pancreatic disease may respond to celiac plexus block. In fact, the most studied application of EUS-guided celiac block is in pancreas-related pain[1,24-26]. The application of this procedure in other disorders is very limited. There are few case reports on the use EUS-guided block in the management of pelvic cancer pain[27], acute intermittent porphyria[28], and pain caused by diabetic gastroparesis[29]. No published literature on the utility of this intervention in patients with mesenteric panniculitis related pain.
Theoretically, pain originating from upper abdominal organs could be alleviated by this procedure. However, this was not tested in clinical trials or observational studies. More research in this area is required in order to ascertain or dispute our observation.
COMMENTS
Case characteristics
Recurrent right upper quadrant abdominal pain.
Clinical diagnosis
Mesenteric panniculitis.
Differential diagnosis
Upper endoscopy ruled out an intraluminal pathology, computed tomography (CT) scan finding was not suggestive of neuroendocrine or pancreatic malignancy, diagnostic laparoscopy done to rule out intra-abdominal malignant process.
Laboratory diagnosis
Extensive investigations including complete blood count, Lipase, Liver enzymes, kidney function, porphyria screening, radiological imaging with CT scan, the diagnosis was confirmed with histology.
Imaging diagnosis
Imaging study using CT scan demonstrated thickening and irregularity of the mesentery surrounding in keeping with the diagnosis of mesenteric panniculitis.
Pathological diagnosis
Mesenteric biopsy showing fibrotic band of dense collagen infiltrated by mixed inflammatory cells (lymphocytes, plasma cells and neutrophils) in keeping with the diagnosis of mesenteric panniculitis.
Treatment
The patient was treated with multiple pharmacological agents including prednisone, azathioprine, tamoxifen and thalidomide that failed control his symptoms. Subsequently, responded to endoscopic ultrasonography (EUS)-guided celiac plexus block.
Related reports
Nicholson et al reported that mesenteric panniculitis in merseyside: a case series and a review of the literature in 2010.
Experiences and lessons
Mesenteric panniculitis is a rare disorder that can present with refractory and disabling abdominal pain, the authors describe a novel intervention using EUS guided celiac plexus block to relieve refractory abdominal pain in a patient with mesenteric panniculitis.
Peer-review
Novel intervention for a rare disease is based on the anatomical supply of the epigastric area where the pain is originating.
Footnotes
Informed consent: Patient consented to publish the case, consent attached.
Conflict-of-interest: None of the authors have any conflict of interest to disclose.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: September 20, 2014
First decision: September 28, 2014
Article in press: March 5, 201
P- Reviewer: Franceschi F, Giannopoulos GA, Li XL, Richardson WS S- Editor: Tian YL L- Editor: A E- Editor: Wu HL
References
- 1.Issa I, Baydoun H. Mesenteric panniculitis: various presentations and treatment regimens. World J Gastroenterol. 2009;15:3827–3830. doi: 10.3748/wjg.15.3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jura V. Sulla mesenterite retrattile sclerosante. Policlinico (sezprat) 1924;31:575–581. [Google Scholar]
- 3.Aach RD, Kahn LI, Frech RS. Obstruction of the small intestine due to retractile mesenteritis. Gastroenterology. 1968;54:594–598. [PubMed] [Google Scholar]
- 4.Mitchinson MJ. Systemic idiopathic fibrosis and systemic Weber-Christian disease. J Clin Pathol. 1965;18:645–649. doi: 10.1136/jcp.18.5.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piessen G, Mariette C, Triboulet JP. Mesenteric panniculitis. Ann Chir. 2006;131:85–90. doi: 10.1016/j.anchir.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 6.van der Hulst RW, Rauws EA, Tytgat GN. Mesenteritis secondary to the use of a pneumatic jackhammer. Eur J Gastroenterol Hepatol. 1995;7:573–575. [PubMed] [Google Scholar]
- 7.Ferrari TC, Couto CM, Vilaça TS, Xavier MA, Faria LC. An unusual presentation of mesenteric panniculitis. Clinics (Sao Paulo) 2008;63:843–844. doi: 10.1590/S1807-59322008000600023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akram S, Pardi DS, Schaffner JA, Smyrk TC. Sclerosing mesenteritis: clinical features, treatment, and outcome in ninety-two patients. Clin Gastroenterol Hepatol. 2007;5:589–596; quiz 523-524. doi: 10.1016/j.cgh.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 9.Satheesh SP, HO S, Grendell JH. Retractile Mesenteritis: A Case Report and Review of the Literature. Practical Gastroenterol. 2003:40–44. [Google Scholar]
- 10.Shah AN, You CH. Mesenteric lipodystrophy presenting as an acute abdomen. South Med J. 1982;75:1025–1026. doi: 10.1097/00007611-198208000-00031. [DOI] [PubMed] [Google Scholar]
- 11.Horton KM, Lawler LP, Fishman EK. CT findings in sclerosing mesenteritis (panniculitis): spectrum of disease. Radiographics. 2003;23:1561–1567. doi: 10.1148/rg.1103035010. [DOI] [PubMed] [Google Scholar]
- 12.Tytgat GN, Roozendaal K, Winter W, Esseveld MR. Successful treatment of a patient with retractile mesenteritis with prednisone and azathioprine. Gastroenterology. 1980;79:352–356. [PubMed] [Google Scholar]
- 13.Mazure R, Fernandez Marty P, Niveloni S, Pedreira S, Vazquez H, Smecuol E, Kogan Z, Boerr L, Mauriño E, Bai JC. Successful treatment of retractile mesenteritis with oral progesterone. Gastroenterology. 1998;114:1313–1317. doi: 10.1016/s0016-5085(98)70438-x. [DOI] [PubMed] [Google Scholar]
- 14.Miyake H, Sano T, Kamiya J, Nagino M, Uesaka K, Yuasa N, Oda K, Nimura Y. Successful steroid therapy for postoperative mesenteric panniculitis. Surgery. 2003;133:118–119. doi: 10.1067/msy.2003.54. [DOI] [PubMed] [Google Scholar]
- 15.Nicholson JA, Smith D, Diab M, Scott MH. Mesenteric panniculitis in Merseyside: a case series and a review of the literature. Ann R Coll Surg Engl. 2010;92:W31–W34. doi: 10.1308/147870810X12699662981393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gress F, Schmitt C, Sherman S, Ciaccia D, Ikenberry S, Lehman G. Endoscopic ultrasound-guided celiac plexus block for managing abdominal pain associated with chronic pancreatitis: a prospective single center experience. Am J Gastroenterol. 2001;96:409–416. doi: 10.1111/j.1572-0241.2001.03551.x. [DOI] [PubMed] [Google Scholar]
- 17.Durst AL, Freund H, Rosenmann E, Birnbaum D. Mesenteric panniculitis: review of the leterature and presentation of cases. Surgery. 1977;81:203–211. [PubMed] [Google Scholar]
- 18.Emory TS, Monihan JM, Carr NJ, Sobin LH. Sclerosing mesenteritis, mesenteric panniculitis and mesenteric lipodystrophy: a single entity? Am J Surg Pathol. 1997;21:392–398. doi: 10.1097/00000478-199704000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Daskalogiannaki M, Voloudaki A, Prassopoulos P, Magkanas E, Stefanaki K, Apostolaki E, Gourtsoyiannis N. CT evaluation of mesenteric panniculitis: prevalence and associated diseases. AJR Am J Roentgenol. 2000;174:427–431. doi: 10.2214/ajr.174.2.1740427. [DOI] [PubMed] [Google Scholar]
- 20.Kipfer RE, Moertel CG, Dahlin DC. Mesenteric lipodystrophy. Ann Intern Med. 1974;80:582–588. doi: 10.7326/0003-4819-80-5-582. [DOI] [PubMed] [Google Scholar]
- 21.Bala A, Coderre SP, Johnson DR, Nayak V. Treatment of sclerosing mesenteritis with corticosteroids and azathioprine. Can J Gastroenterol. 2001;15:533–535. doi: 10.1155/2001/462823. [DOI] [PubMed] [Google Scholar]
- 22.Kikiros CS, Edis AJ. Mesenteric panniculitis resulting in bowel obstruction: response to steroids. Aust N Z J Surg. 1989;59:287–290. doi: 10.1111/j.1445-2197.1989.tb01565.x. [DOI] [PubMed] [Google Scholar]
- 23.Loukas M, Klaassen Z, Merbs W, Tubbs RS, Gielecki J, Zurada A. A review of the thoracic splanchnic nerves and celiac ganglia. Clin Anat. 2010;23:512–522. doi: 10.1002/ca.20964. [DOI] [PubMed] [Google Scholar]
- 24.Yan BM, Myers RP. Neurolytic celiac plexus block for pain control in unresectable pancreatic cancer. Am J Gastroenterol. 2007;102:430–438. doi: 10.1111/j.1572-0241.2006.00967.x. [DOI] [PubMed] [Google Scholar]
- 25.Puli SR, Reddy JB, Bechtold ML, Antillon MR, Brugge WR. EUS-guided celiac plexus neurolysis for pain due to chronic pancreatitis or pancreatic cancer pain: a meta-analysis and systematic review. Dig Dis Sci. 2009;54:2330–2337. doi: 10.1007/s10620-008-0651-x. [DOI] [PubMed] [Google Scholar]
- 26.Kaufman M, Singh G, Das S, Concha-Parra R, Erber J, Micames C, Gress F. Efficacy of endoscopic ultrasound-guided celiac plexus block and celiac plexus neurolysis for managing abdominal pain associated with chronic pancreatitis and pancreatic cancer. J Clin Gastroenterol. 2010;44:127–134. doi: 10.1097/MCG.0b013e3181bb854d. [DOI] [PubMed] [Google Scholar]
- 27.Mishra S, Bhatnagar S, Gupta D, Thulkar S. Anterior ultrasound-guided superior hypogastric plexus neurolysis in pelvic cancer pain. Anaesth Intensive Care. 2008;36:732–735. doi: 10.1177/0310057X0803600518. [DOI] [PubMed] [Google Scholar]
- 28.Ferrari AP, Ardengh JC. Endosonography-guided celiac plexus neurolysis in the treatment of pain secondary to acute intermittent porphyria. Endoscopy. 2002;34:341–342. doi: 10.1055/s-2002-23636. [DOI] [PubMed] [Google Scholar]
- 29.Wu DJ, Dib C, Hoelzer B, McMahon M, Mueller P. Coeliac plexus block in the management of chronic abdominal pain due to severe diabetic gastroparesis. BMJ Case Rep. 2009;2009 doi: 10.1136/bcr.06.2009.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


