Abstract
Background
There is limited understanding of cortical neurochemistry and cortical connectivity during ketamine anaesthesia. We conducted a systematic study to investigate the effects of ketamine on cortical acetylcholine (ACh) and electroencephalographic coherence.
Methods
Male Sprague–Dawley rats (n=11) were implanted with electrodes to record electroencephalogram (EEG) from frontal, parietal, and occipital cortices, and with a microdialysis guide cannula for simultaneous measurement of ACh concentrations in prefrontal cortex before, during, and after ketamine anaesthesia. Coherence and power spectral density computed from the EEG, and ACh concentrations, were compared between conscious and unconscious states. Loss of righting reflex was used as a surrogate for unconsciousness.
Results
Ketamine-induced unconsciousness was associated with a global reduction of power (P=0.02) in higher gamma bandwidths (>65 Hz), a global reduction of coherence (P≤0.01) across a broad frequency range (0.5–250 Hz), and a significant increase in ACh concentrations (P=0.01) in the prefrontal cortex. Compared with the unconscious state, recovery of righting reflex was marked by a further increase in ACh concentrations (P=0.0007), global increases in power in theta (4–10 Hz; P=0.03) and low gamma frequencies (25–55 Hz; P=0.0001), and increase in power (P≤0.01) and coherence (P≤0.002) in higher gamma frequencies (65–250 Hz). Acetylcholine concentrations, coherence, and spectral properties returned to baseline levels after a prolonged recovery period.
Conclusions
Ketamine-induced unconsciousness is characterized by suppression of high-frequency gamma activity and a breakdown of cortical coherence, despite increased cholinergic tone in the cortex.
Keywords: acetylcholine, electroencephalography, ketamine, microdialysis, prefrontal cortex, unconsciousness
Editor's key points.
Ketamine, which acts by a unique non-GABAergic mechanism, might have distinct effects on long-range cerebrocortical interactions compared with other general anaesthetics.
The effects of ketamine on electroencephalographic coherence, cortical acetylcholine concentrations, and behaviour were studied in rats.
Ketamine-induced loss of consciousness was associated with global reductions in gamma power and cortical coherence similar to other general anaesthetics.
Ketamine is a unique anaesthetic drug that does not conform to most mechanistic frameworks of anaesthetic-induced unconsciousness.1 Unlike many general anaesthetics, the γ-aminobutyric acid (GABA) receptor is not the primary molecular target of ketamine.2–4 At a systems neuroscience level, ketamine does not appear to activate the sleep-promoting ventrolateral preoptic nucleus,5 as typical GABAergic anaesthetics do.5–7 Instead, ketamine activates the norepinephrine-producing locus coeruleus5 and appears to depend, in part, on noradrenergic transmission.8 At the neurophysiological level, ketamine enhances higher frequency electroencephalographic activity,9,10 which can confound the algorithms in processed electroencephalographic devices intended for monitoring anaesthetic depth.10,11
Recent studies from our laboratory suggest that ketamine shares a network-level property with GABAergic drugs by disrupting information transfer,9 phase relationships,12 or both across the cortex. These findings are consistent with a study of ketamine in a cortical slice model that identified uncoupling of long-range corticocortical interactions.13 However, studies of ketamine and cortical or thalamocortical connectivity in intact animal models are typically conducted with co-administration of xylazine, an α2-adrenergic agonist.14,15 Furthermore, there have been no carefully controlled studies of ketamine-induced unconsciousness in animal models that link disruptions of neurophysiological coupling with neurochemical events. Studies of ketamine and neurochemistry have focused on acetylcholine (ACh) but (i) without concomitant neurophysiological recordings,16,17 (ii) using subanaesthetic doses of ketamine,16,17 or (iii) without formal testing of loss of righting reflex (LORR) as a surrogate of anaesthetic-induced unconsciousness.16 To address this gap in knowledge, the objective of the present study was to identify the relationship of electroencephalographic coherence, cortical ACh, and the state of ketamine-induced unconsciousness.
Methods
Experiments were conducted on adult (3- to 5-month-old) male Sprague–Dawley rats (n=11; Charles River Laboratories, Inc., Kingston, NY, USA) maintained on a 12 h light–12 h dark cycle (lights on at 06.00 h) with ad libitum food and water. The experimental procedures were approved by the University of Michigan Committee on Use and Care of Animals and were in compliance with the Guide for the Care and Use of Laboratory Animals (8th Edition, The National Academies Press, Washington, DC, USA) and the ARRIVE guidelines.
Surgical procedures
Under surgical levels of isoflurane anaesthesia, rats were implanted with screw electrodes to record electroencephalogram (EEG) from frontal (Bregma: anterior 3.0 mm, lateral 2.5 mm), parietal (Bregma: posterior 4.0 mm, lateral 2.5 mm), and occipital areas (Bregma: posterior 8.0 mm, lateral 2.5 mm). A screw electrode was implanted over the nasal commissure to serve as a reference electrode. In addition, a craniotomy was performed over the prefrontal cortex (PFC) (Bregma: anterior 3.0 mm, lateral 0.5 mm, ventral 4.0 mm),18 and a CMA/11 guide cannula (CMA Microdialysis, Harvard Apparatus, Holliston, MA, USA) was implanted 1.0 mm above the target area. Buprenorphine hydrochloride (Buprenex®; Reckitt Benckiser Pharmaceuticals Inc., Richmond, VA, USA) was used for pre- and postsurgical analgesia (0.01 and 0.03 mg kg−1, s.c., respectively), and a presurgical single dose of the antibiotic cefazolin (20 mg kg−1, s.c.; West-Ward Pharmaceutical Corp., Eatontown, NJ, USA) was administered. The EEG electrodes were mated with an electrode pedestal (Plastics One, Roanoke, VA, USA), and the entire assembly along with the guide cannula was affixed to the cranium using dental acrylic.
Electroencephalographic data acquisition
The electrode over the nasal commissure was used as a reference for monopolar EEG recordings from the frontal, parietal, and occipital cortices. The choice of monopolar EEG recordings was based on previously published animal studies19–21 and on a study from our laboratory demonstrating that monopolar reference is best suited to detect genuine EEG phase synchronization.22 Electroencephalographic signals were amplified (×5000) and filtered (0.1–300 Hz) on a Grass Model 15 LT system (15A54 Quad Amplifier, Warwick, RI, USA). Data were digitized at 1 kHz using an MP150 system and AcqKnowledge data acquisition software (version 4.1; Biopac Systems, Inc., Goleta, CA, USA).
Coherence and power spectral analysis
Data were first down-sampled to 500 Hz to reduce computation time, and an IIR notch filter was applied to remove 60 Hz line noise. We calculated coherence across cortical electrodes at individual frequencies from 0.5 to 250 Hz (in 0.5 Hz intervals) as magnitude squared coherence using the ‘mscohere.m’ function in the MATLAB Signal Processing Toolbox (MathWorks Inc., Natick, MA, USA).21 To control for spurious coherence, the surrogate data method was used, wherein phases were randomized but the spectral content of the signals was maintained.23 These estimates of spurious coherence were then statistically compared with the empirical data. Furthermore, frequencies at which obvious artifact was present on the individual spectrogram or coherogram were excluded from quantitative analysis and statistical comparison.
Absolute power spectral density (PSD) between 0.5 and 250 Hz was calculated based on the short-time Fourier transform using the ‘spectrogram.m’ function in the MATLAB Signal Processing Toolbox.21 Relative power was calculated for each epoch by dividing the mean absolute power of each frequency band by the total power across the entire frequency range. Coherence and PSD were calculated for the following frequency bands: delta (δ: 0.5–4 Hz), theta (θ: 4–10 Hz), alpha (α: 10–15 Hz), beta (β: 15–25 Hz), low gamma (γ1: 25–55 Hz), medium gamma (γ2: 65–125 Hz), high gamma (γ3: 125–175 Hz), and ultrahigh gamma (γ4: 185–250 Hz). The data are reported as changes in global coherence, which was obtained by averaging the coherence values for individual channel pairs. Likewise, PSD values for individual channels were averaged and reported as changes in global PSD.
Quantification of acetylcholine release in prefrontal cortex
A CMA/11 microdialysis probe (1 mm cuprophane membrane, 0.24 mm diameter, 6 kDa) was perfused continuously with Ringer's solution (147 mM NaCl, 2.4 mM CaCl2, 4.0 mM KCl, and 10 µM neostigmine; pH 5.8–6.2) at 2.0 µl min−1 using a CMA/400 syringe pump. Microdialysis samples were collected every 12.5 min, which yielded 25 µl of dialysate, out of which 22 µl was used for ACh quantification. Acetylcholine concentrations were quantified using high-performance liquid chromatography coupled with electrochemical detection (BASi Inc., West Lafayette, IN, USA and Showa Denko America, Inc., New York, NY, USA). Chromatograms were digitized and quantified using LC Real Time Analysis Program (Showa Denko America, Inc.) and a seven-point ACh–choline standard curve (from 0.05 to 1.0 pmol).
Experimental design
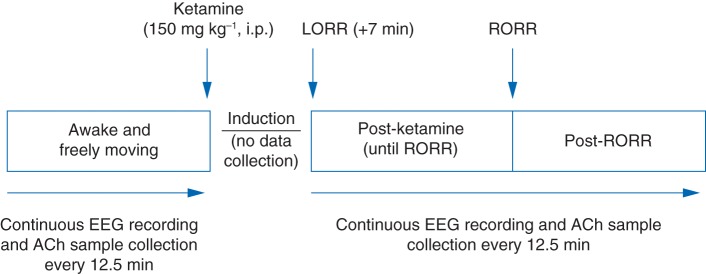
The experimental design is illustrated in Fig. 1. All experiments were conducted between 10.00 and 18.00 h. Rats were permitted 7–10 days of postsurgical recovery, during which they were conditioned to the EEG recording and microdialysis set-up. On the day of the experiment, rats were connected to the EEG recording system, and a microdialysis probe was lowered into the PFC. After allowing 45 min to ensure stable ACh concentrations, simultaneous monopolar EEG recording and ACh sample collection were started. Rats were kept awake by gentle tapping on the recording chamber to hold the behavioural state constant. Data collection was stopped after collection of the fourth ‘Wake’ ACh sample, and animals were given a single intraperitoneal dose (150 mg kg−1) of ketamine (Ketamine Hydrochloride; Hospira. Inc., Lake Forest, IL, USA). The dose for ketamine was based on previous literature,24 as well as dose–response experiments conducted in our laboratory, and titrated to achieve LORR, which occurred within 3–5 min of ketamine injection. Immediately after LORR, a rectal probe (Model 7001H; Physitemp Instruments, Clifton, NJ, USA) was inserted to monitor body temperature, and a foot sensor (MouseOx; Starr Life Science Corp., Oakmont, PA, USA) was positioned to monitor heart rate and oxygen saturation. To obtain a pure ACh sample corresponding to the unconscious state, data collection was resumed after LORR and after an additional 7 min elapsed in order to purge the dead space volume in the ACh collection tubing. Three of 11 rats were excluded from the data analysis because they continued to show whisker or slight body movements, or both, after LORR. In the remaining eight rats, EEG recording and ACh sample collection were continued until three ACh samples were obtained after return of righting reflex (RORR), a surrogate for recovery from anaesthesia in rodents. Additionally, in three of these eights rats, data collection was extended until ACh concentrations returned to those observed during the waking state. The location of the dialysis probe was confirmed histologically (see Supplementary methods).
Fig 1.
Schematic diagram illustrating the temporal course of experimental intervention and data collection. ACh, acetylcholine; i.p., intraperitoneal; LORR, loss of righting reflex; RORR, return of righting reflex.
Statistical analyses
The Center for Statistical Consultation and Research at the University of Michigan was consulted for data analyses. A priori power analysis (nQuery Advisor+nTerim; Statistical Solutions Ltd, Boston, MA, USA) was conducted to design the study with >80% power at an α value of 0.05. Although EEG data and ACh sample collection were continuous linear processes, the data set was divided into 12.5 min epochs corresponding to the time required for collection of each dialysis sample. Five epochs reflecting different behavioural states were selected for statistical comparisons, as follows: (i) ‘Wake’, the last epoch of the awake period; (ii) ‘Ketamine’, the first epoch after both LORR and exclusion of microdialysis dead space volume; (iii) ‘Pre-RORR’, one epoch before RORR; (iv) ‘Post-RORR’, the first epoch after RORR; and (v) ‘Recovery’, the last epoch from rats with the prolonged/extended recovery. Acetylcholine concentrations, and coherence and PSD across each frequency band for Wake, Ketamine, Pre-RORR, and Post-RORR were compared using repeated measures analysis of variance (anova) with Tukey's multiple comparisons test. Acetylcholine and EEG parameters for the Recovery epoch were compared with the Wake epoch using a one-tailed Wilcoxon test. Surrogate EEG data were compared with the raw unshuffled EEG data using Student's paired t-test. Changes in blood oxygen saturation, heart rate, and rectal temperature between Ketamine and Pre-RORR epochs were compared using a one-tailed Wilcoxon test. Statistical comparisons were performed with Graph Pad Prism 6.05 (Graph Pad Software, Inc., La Jolla, CA, USA).
Results
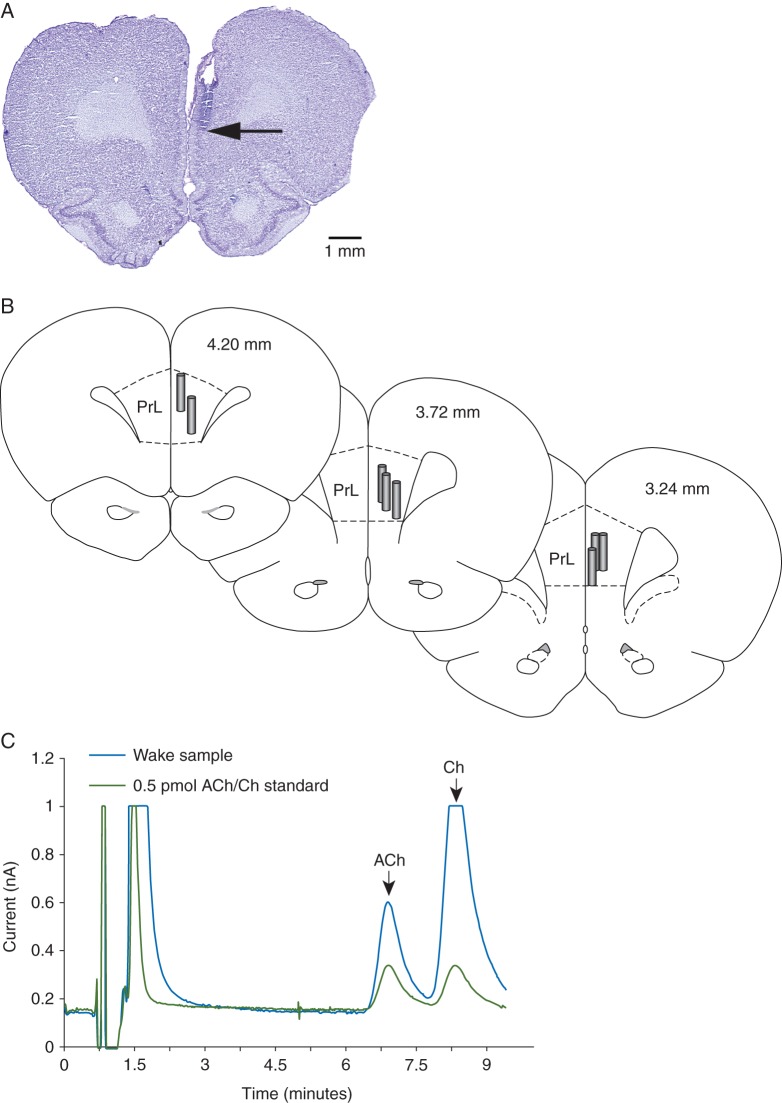
The site of microdialysis was confirmed to be within the PFC for all rats (Fig. 2a and b). Representative ACh peaks from a Wake sample and a known standard are shown in Fig. 2c.
Fig 2.
(a) Cresyl violet-stained representative coronal brain section through prefrontal cortex showing the dialysis probe track and the site of microdialysis. Arrow indicates the ventral tip of the dialysis membrane (1 mm long). (b) Coronal brain section drawings from the rat brain atlas of Paxinos and Watson18 to illustrate the location of microdialysis probes (vertical cylinders) within the prefrontal cortex. The numbers within coronal sections show the anterior–posterior location of each coronal section relative to bregma. (c) Representative acetylcholine (ACh) chromatogram showing the signal-to-noise ratio and the retention time for ACh and choline (Ch) peaks. PrL, prelimbic area in the prefrontal cortex.
Effect of ketamine on behaviour
Eight of 11 rats were completely immobile and maintained LORR without any body movements during the first 12.5 min epoch after ketamine administration; the three rats showing movements were excluded from analysis. Behavioural observations were accompanied by EEG and ACh data collection (Ketamine epoch). After the first epoch after ketamine administration, rats exhibited varying degrees of purposeful movements in limbs, head, and torso until RORR. The Post-RORR period was characterized by continuous circling movements. These stereotyped movements ceased, and rats returned to normal, calm behaviour after a prolonged recovery period (14–22 epochs, or 175–275 min Post-RORR), which was also marked by return of ACh concentrations to values recorded during the Wake period. Data on temperature, heart rate, and oxygen saturation could be collected only between loss and recovery of consciousness and were collected from all except one rat. There was no significant change in blood oxygen saturation during the Ketamine (anaesthetized) epoch compared with Pre-RORR (P=0.14); however, we observed a significant increase in rectal temperature (P=0.007) and heart rate (P=0.01) at the point of RORR compared with the Ketamine epoch.
Effect of ketamine on electroencephalographic/cortical coherence
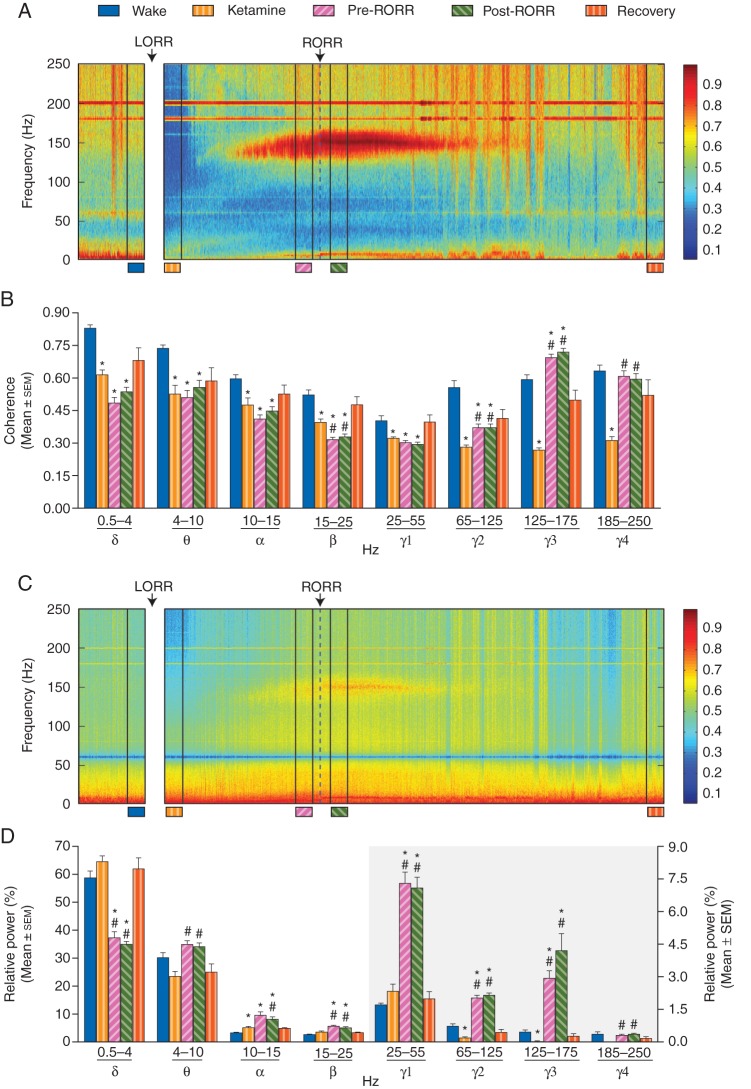
A significant and global depression of coherence was observed across all frequency bands during ketamine-induced unconsciousness (Fig. 3a and b, Table 1). Repeated measures anova between Wake, Ketamine, Pre-RORR, and Post-RORR revealed a significant effect of ketamine on coherence across all frequency bands [delta (P=0.0004), theta (P=0.008), alpha (P=0.05), beta (P=0.0006), low gamma (P=0.02), medium gamma (P<0.0001), high gamma (P<0.0001), and ultrahigh gamma (P=0.0003)]. Return of consciousness (Post-RORR), compared with the Ketamine epoch, was marked by a significant increase in coherence in the medium gamma (P=0.002), high gamma (P<0.0001), and ultrahigh gamma (P=0.0007) bands, while there was a significant decrease in coherence in the beta frequency band (P=0.009). The coherence was not significantly different and remained at Ketamine concentrations in delta (P=0.13), theta (P=0.93), alpha (P=0.81) and low gamma (P=0.13) bands. A remarkable feature of the emergence process from unconsciousness (i.e. during the Pre-RORR epoch) was a dramatic increase in higher gamma frequencies [medium (P=0.003), high (P<0.0001), and ultrahigh (P=0.0005)]. Comparison between Wake and Post-RORR showed that at the return of consciousness, except for the ultrahigh gamma (P=0.35), the coherence in delta (P<0.0001), theta (P=0.0006), alpha (P=0.0002), beta (P=0.0001), low gamma (P=0.003), and medium gamma (P=0.0004) remained significantly lower and did not return to the Wake levels; the coherence increased in high gamma band (P=0.002). As would be predicted, coherence across all frequency bands returned to basal Wake levels after a prolonged recovery period (Recovery epoch). Coherence for empirical data was significantly higher than for the surrogate data (Supplementary Fig. S1), which controls for the possibility that spurious coherence resulting from spectral changes accounted for the findings. The changes in global coherence (Fig. 3b) were generally consistent with coherence changes between individual channel pairs (Supplementary Fig. S2).
Fig 3.
Effect of ketamine on coherence and power spectral density (PSD). (a) Representative coherogram showing a significant decrease in coherence during ketamine-induced unconsciousness (Ketamine) and the appearance of high-frequency gamma coherence during emergence and after the return of righting reflex (RORR). (b) Statistical comparisons (repeated measures anova) of changes in coherence between Wake (blue), Ketamine-induced unconsciousness (gold), Pre-RORR (Pink), and Post-RORR (green; n=8 animals). The fifth bar (orange) represents the Recovery epoch from the rats with extended recovery and was compared (one-tailed Wilcoxon text) with the Wake state from the same rats. (c) Representative spectrogram showing a significant decrease in power in higher gamma frequencies during ketamine-induced unconsciousness (Ketamine) and the appearance of high-frequency gamma power during emergence and after RORR. (d) Statistical comparisons (repeated measures anova) of changes in PSD between Wake (blue), Ketamine-induced unconsciousness (gold), Pre-RORR (Pink), and Post-RORR (green; n=8 animals). The fifth bar (orange) represents the Recovery epoch from the rats with extended recovery and was compared (one-tailed Wilcoxon text) with the Wake state from the same rats. The right axis applies only to the grey shaded area. *P<0.05 significant compared with Wake; #P<0.05 significant compared with Ketamine. Most of the comparisons are highly significant, but in order to avoid crowding the figure with the significance symbols, all the significance values are reported as P<0.05. The accurate P-values are reported in the text in the Results section.
Table 1.
Coherence during Wake, ketamine-induced unconsciousness (Ketamine) and recovery states (n=8 animals). Data are shown as mean coherence (sem) and [95% confidence intervals] *significant; NS, not significant
| Frequency band (Hz) | Repeated measures anova |
Wilcoxon test |
||||||
|---|---|---|---|---|---|---|---|---|
| Wake | Ketamine | Pre-RORR | Post-RORR | F statistic, P-value | Wake | Recovery | P-value | |
| δ (0.5–4) | 0.83 (0.017) [0.79–0.87] | 0.61 (0.023) [0.56–0.67] | 0.48 (0.026) [0.42–0.55] | 0.54 (0.022) [0.48–0.59] | F(3,7)=67.4 *P<0.0001 | 0.85 (0.024) [0.74–0.95] | 0.68 (0.058) [0.43–0.93] | NSP=0.13 |
| θ (4–10) | 0.74 (0.016) [0.70–0.77] | 0.53 (0.040) [0.43–0.62] | 0.51 (0.032) [0.43–0.59] | 0.56 (0.032) [0.48–0.63] | F(3,7)=15.8 *P=0.0017 | 0.74 (0.033) [0.60–0.88] | 0.59 (0.062) [0.32–0.85] | NSP=0.13 |
| α (10–15) | 0.60 (0.019) [0.55–0.64] | 0.48 (0.036) [0.40–0.55] | 0.41 (0.019) [0.36–0.46] | 0.45 (0.020) [0.40–0.50] | F(3,7)=19.5 *P=0.0007 | 0.60 (0.033) [0.45–0.74] | 0.53 (0.041) [0.35–0.70] | NSP=0.25 |
| β (15–25) | 0.52 (0.023) [0.47–0.58] | 0.40 (0.015) [0.36–0.43] | 0.32 (0.011) [0.29–0.34] | 0.33 (0.013) [0.30–0.36] | F(3,7)=77.0 *P<0.0001 | 0.54 (0.053) [0.31–0.77] | 0.47 (0.040) [0.30–0.65] | NSP=0.13 |
| γ1 (25–55) | 0.40 (0.023) [0.35–0.46] | 0.32 (0.006) [0.31–0.34] | 0.30 (0.009) [0.28–0.33] | 0.30 (0.009) [0.27–0.32] | F(3,7)=23.6 *P=0.0002 | 0.41 (0.037) [0.25–0.57] | 0.40 (0.033) [0.25–0.54] | NSP=0.13 |
| γ2 (65–125) | 0.56 (0.033) [0.48–0.63] | 0.28 (0.010) [0.26–0.30] | 0.37 (0.017) [0.33–0.41] | 0.37 (0.016) [0.33–0.41] | F(3,7)=68.9 *P<0.0001 | 0.54 (0.049) [0.33–0.75] | 0.41 (0.041) [0.24–0.59] | NSP=0.13 |
| γ3 (125–175) | 0.59 (0.022) [0.54–0.64] | 0.27 (0.009) [0.25–0.29] | 0.69 (0.016) [0.66–0.73] | 0.72 (0.016) [0.68–0.76] | F(3,7)=203 *P<0.0001 | 0.63 (0.020) [0.54–0.71] | 0.50 (0.046) [0.30–0.70] | NSP=0.13 |
| γ4 (185–250) | 0.63 (0.027) [0.57–0.70] | 0.31 (0.017) [0.27–0.35] | 0.61 (0.026) [0.55–0.67] | 0.59 (0.026) [0.53–0.66] | F(3,7)=50.5 *P<0.0001 | 0.62 (0.031) [0.49–0.76] | 0.52 (0.071) [0.22–0.83] | NSP=0.25 |
Effect of ketamine on power spectral density
Repeated measures anova between Wake, Ketamine, Pre-RORR, and Post-RORR showed a significant effect of ketamine on global PSD (Fig. 3c and d, Table 2). Post hoc Tukey's multiple comparisons test showed a significant decrease in higher gamma activity [medium (P=0.02) and high (P=0.02)] during ketamine-induced unconsciousness compared with the waking state; there was no effect on other frequency bands [delta (P=0.37), theta (P=0.22), beta (P=0.19), low gamma (P=0.32), and ultrahigh gamma (P=0.06)], with the exception of a small increase in alpha (P=0.05). Compared with the Ketamine epoch, Post-RORR was characterized by a significant decrease in delta (P<0.0001) activity, whereas there was an increase in theta (P=0.03), alpha (P=0.04), beta (P=0.002), and gamma activity [low (P<0.0001), medium (P<0.0001), high (P=0.005), and ultrahigh (P=0.001)]. Emergence (Pre-RORR) was also characterized by a decrease in delta (P=0.0002) and an increase in theta (P=0.009), beta (P=0.006), and gamma activity [low (P=0.0002), medium (P<0.0001), high (P=0.0003), and ultrahigh (P=0.003)]. Post-RORR was marked by return of theta and ultrahigh gamma activity to the Wake levels, while power increased in alpha (P=0.001), beta (P=0.0003), and lower gamma frequencies [(low (P<0.0001), medium (P=0.0003), and high (P=0.01)]; there was a significant decrease in delta activity (P=0.0005). Power across all frequency bands returned to basal Wake levels after a prolonged recovery period (Recovery epoch). The changes in global PSD (Fig. 3d) were generally consistent with changes in PSD at individual channels across the cortex (Supplementary Fig. S3).
Table 2.
Power spectral density during Wake, ketamine-induced unconsciousness (Ketamine) and the recovery states (n=8 animals). Data are shown as mean relative power (sem) and [95% confidence intervals] *significant; NS, not significant
| Frequency band (Hz) | Repeated measures anova |
Wilcoxon test |
||||||
|---|---|---|---|---|---|---|---|---|
| Wake | Ketamine | Pre-RORR | Post-RORR | F statistic, P-value | Wake | Recovery | P-value | |
| δ (0.5–4) | 59 (2.4) [53–64] | 65 (2.0) [60–69] | 37 (2.3) [32–42] | 35 (1.1) [32–37] | F(3,7)=52.1 *P<0.0001 | 65 (1.0) [60–69] | 62 (3.9) [45–79] | NSP=0.38 |
| θ (4–10) | 30. (1.8) [26–34] | 23 (1.9) [19–28] | 35 (1.4) [32–38] | 34 (1.4) [31–37] | F(3,7)=8.60 *P=0.0022 | 25 (0.78) [22–28] | 25 (3.2) [11–38] | NSP=0.5 |
| α (10–15) | 3.2 (0.18) [2.8–3.6] | 5.1 (0.47) [3.9–6.2] | 9.5 (1.3) [6.5–12] | 8.1 (0.83) [6.2–10.] | F(3,7)=17.3 *P=0.0011 | 2.9 (0.34) [1.4–4.4] | 4.9 (0.29) [3.7–6.1] | NSP=0.13 |
| β (15–25) | 2.6 (0.11) [2.4–2.9] | 3.5 (0.37) [2.6–4.4] | 5.6 (0.40) [4.6–6.5] | 5.1 (0.31) [4.3–5.8] | F(3,7)=32.9 *P<0.0001 | 2.6 (0.22) [1.6–3.5] | 3.31 (0.21) [2.4–4.2] | NSP=0.13 |
| γ1 (25–55) | 1.7 (0.077) [1.5–1.9] | 2.3 (0.33) [1.5–3.1] | 7.3 (0.52) [6.1–8.5] | 7.1 (0.50) [5.9–8.3] | F(3,7)=92.3 *P<0.0001 | 1.6 (0.15) [0.99–2.3] | 2.0 (0.34) [0.53–3.4] | NSP=0.38 |
| γ2 (65–125) | 0.72 (0.11) [0.47–0.98] | 0.18 (0.050) [0.063–0.30] | 2.0 (0.14) [1.7–2.3] | 2.1 (0.10) [1.9–2.3] | F(3,7)=99.0 *P<0.0001 | 0.53 (0.11) [0.058–0.99] | 0.44 (0.13) [−0.12 to 1.0] | NSP=0.38 |
| γ3 (125–175) | 0.45 (0.092) [0.23–0.67] | 0.040 (0.012) [0.012–0.068] | 2.9 (0.34) [2.1–3.7] | 4.2 (0.78) [2.3–6.1] | F(3,7)=25.3 *P=0.0008 | 0.34 (0.055) [0.10–0.58] | 0.27 (0.11) [−0.20 to 0.74] | NSP=0.38 |
| γ4 (185–250) | 0.36 (0.11) [0.10–0.62] | 0.007 (0.002) [0.003–0.011] | 0.30 (0.050) [0.19–0.42] | 0.35 (0.052) [0.23–0.47] | F(3,7)=6.47 *P=0.027 | 0.23 (0.047) [0.030–0.43] | 0.16 (0.085) [−0.21 to 0.52] | NSP=0.38 |
Effect of ketamine on cortical acetylcholine
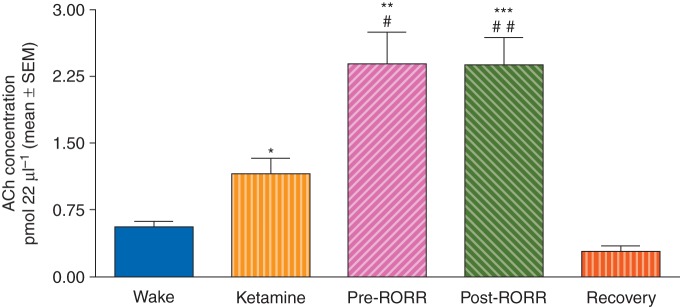
Acetylcholine concentrations increased significantly during ketamine-induced unconsciousness compared with the waking state (P=0.01) and showed more than a four-fold increase during Pre- (P=0.002) and Post-RORR epochs (P=0.0007). Acetylcholine concentrations returned to baseline values by the end of the extended recovery period (Fig. 4, Table 3).
Fig. 4.
Increase in ACh concentrations in the prefrontal cortex during ketamine-induced unconsciousness (Ketamine) and Pre- and Post-RORR (n=8 animals). Acetylcholine concentrations returned to the basal Wake concentrations after the prolonged recovery period. *P<0.05, **P<0.01, ***P<0.001 compared with Wake; #P<0.05, ##P<0.01 compared with Ketamine.
Table 3.
Absolute acetylcholine concentrations during Wake, ketamine-induced unconsciousness (Ketamine) and the recovery states. Data are shown as mean picomoles (sem) and [95% confidence intervals] *significant; NS, not significant
| Repeated measures anova |
Wilcoxon test |
||||||
|---|---|---|---|---|---|---|---|
| Wake | Ketamine | Pre-RORR | Post-RORR | F statistic, P-value | Wake | Recovery | P-value |
| 0.56 (0.060) [0.42–0.70] | 1.2 (0.18) [0.74–1.6] | 2.4 (0.35) [1.6–3.2] | 2.4 (0.31) [1.7–3.1] | F(3,7)=31.7 *P=0.0002 | 0.55 (0.14) [−0.042 to 1.1] | 0.29 (0.057) [0.041–0.53] | NSP=0.13 |
Discussion
We identified a depression of EEG coherence and high-frequency gamma power during ketamine-induced unconsciousness in rats. Given that similar decreases in high-frequency gamma activity25,26 and phase synchronization27 have been reported for primarily GABAergic anaesthetics, this study supports the hypothesis that there are common EEG features of unconsciousness induced by both GABAergic and non-GABAergic anaesthetics.9,12,28 In contrast to high gamma frequencies, there was no decrease in global or regional low gamma (25–55 Hz) power; the increase in low gamma power in certain areas of the cortex (Supplementary Fig. S3) parallels our earlier investigation of ketamine-induced unconsciousness in surgical patients.9
The emergence and recovery processes were characterized by increased high gamma activity and coherence. Similar increases in high gamma power and coherence have been reported after subanaesthetic doses of ketamine in rats29–31 and humans.32 In addition, subanaesthetic ketamine in rats induces hyperlocomotion,29–31 which has been attributed to increases in power and coherence in higher gamma frequencies in the basal ganglia motor circuit.31 However, our observation of increased cortical gamma activity and coherence in both the absence and the presence of hyperlocomotion (i.e. before and after RORR) suggests that these may be independent effects of ketamine.
Although cortical ACh decreases during administration of GABAergic anaesthetics,16,33,34 the present findings show a significant increase in ACh concentrations during ketamine-induced unconsciousness. However, this increase was considerably lower than that reported by Kikuchi and colleagues16 (∼105% in the present study vs ∼281% in the study by Kikuchi and colleagues).16 We demonstrate that a dramatic increase (∼325%) in cortical ACh is associated with emergence and recovery, which could result from increased arousal because of decreased plasma ketamine concentrations.
Higher ACh concentrations are typically correlated with an activated cortex, as in the waking state35 or rapid eye movement sleep,35 which is also characterized by decreased gamma coherence.19 We suggest that ketamine, at least at the dose used in this study, leads to dissociation of cholinergic tone and cortical activation. This might be mediated by suppression of NMDA-type glutamate receptors on both pyramidal neurones and inhibitory interneurones, as opposed to the preferential inhibition of NMDA receptors on interneurones that has been hypothesized for subanaesthetic doses of ketamine36 based on a study of the NMDA antagonist MK801.37 Of note, ketamine-induced activation of subcortical wake-promoting nuclei5 and increased cortical ACh concentrations suggest active arousal systems in the setting of an unconscious state (as indicated by LORR), depressed high-frequency EEG, and disrupted cortical coherence. Therefore, deactivation of ascending arousal signals does not appear to be required for anaesthetic-induced unconsciousness, which has been consistently correlated in humans with fragmentation of cortical networks across diverse anaesthetics.9,28,38,39
In conclusion, we demonstrate that ketamine-induced unconsciousness in rats is correlated with suppression of high-frequency EEG activity and disruption of cortical coherence despite increased cholinergic tone in the prefrontal cortex. The EEG correlates of ketamine-induced unconsciousness might be more similar to those induced by GABAergic anaesthetics than previously thought, even in the setting of activated arousal systems.
Authors' contributions
D.P. and G.A.M. designed the study, interpreted the findings and wrote the manuscript. D.P. and V.S.H. collected and analysed the data. B.H.S. conducted data analysis.
Supplementary material
Supplementary material is available at British Journal of Anaesthesia online.
Declaration of interest
None declared.
Funding
National Institutes of Health (Bethesda, MD, USA; R01 GM098578 to G.A.M.); Department of Anesthesiology, University of Michigan Medical School.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the contribution of Stella Wisidagamage and Shivam Thakur to data collection.
References
- 1.Mashour GA. Top-down mechanisms of anesthetic-induced unconsciousness. Front Syst Neurosci 2014; 8: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anis NA, Berry SC, Burton NR, Lodge D. The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. Br J Pharmacol 1983; 79: 565–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamamura T, Harada K, Okamura A, Kemmotsu O. Is the site of action of ketamine anesthesia the N-methyl-D-aspartate receptor? Anesthesiology 1990; 72: 704–10 [DOI] [PubMed] [Google Scholar]
- 4.Antkowiak B. Different actions of general anesthetics on the firing patterns of neocortical neurons mediated by the GABAA receptor. Anesthesiology 1999; 91: 500–11 [DOI] [PubMed] [Google Scholar]
- 5.Lu J, Nelson LE, Franks N, Maze M, Chamberlin NL, Saper CB. Role of endogenous sleep-wake and analgesic systems in anesthesia. J Comp Neurol 2008; 508: 648–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson LE, Guo TZ, Lu J, Saper CB, Franks NP, Maze M. The sedative component of anesthesia is mediated by GABAA receptors in an endogenous sleep pathway. Nat Neurosci 2002; 5: 979–84 [DOI] [PubMed] [Google Scholar]
- 7.Moore JT, Chen J, Han B, et al. Direct activation of sleep-promoting VLPO neurons by volatile anesthetics contributes to anesthetic hypnosis. Curr Biol 2012; 22: 2008–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kushikata T, Yoshida H, Kudo M, Kudo T, Kudo T, Hirota K. Role of coerulean noradrenergic neurones in general anaesthesia in rats. Br J Anaesth 2011; 107: 924–9 [DOI] [PubMed] [Google Scholar]
- 9.Lee U, Ku S, Noh G, Baek S, Choi B, Mashour GA. Disruption of frontal-parietal communication by ketamine, propofol, and sevoflurane. Anesthesiology 2013; 118: 1264–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maksimow A, Särkelä M, Långsjö JW, et al. Increase in high frequency EEG activity explains the poor performance of EEG spectral entropy monitor during S-ketamine anesthesia. Clin Neurophysiol 2006; 117: 1660–8 [DOI] [PubMed] [Google Scholar]
- 11.Hirota K, Kubota T, Ishihara H, Matsuki A. The effects of nitrous oxide and ketamine on the bispectral index and 95% spectral edge frequency during propofol-fentanyl anaesthesia. Eur J Anaesthesiol 1999; 16: 779–83 [DOI] [PubMed] [Google Scholar]
- 12.Blain-Moraes S, Lee U, Ku S, Noh G, Mashour GA. Electroencephalographic effects of ketamine on power, cross-frequency coupling, and connectivity in the alpha bandwidth. Front Syst Neurosci 2014; 8: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voss LJ, Baas CH, Hansson L, Steyn-Ross DA, Steyn-Ross M, Sleigh JW. Investigation into the effect of the general anaesthetics etomidate and ketamine on long-range coupling of population activity in the mouse neocortical slice. Eur J Pharmacol 2012; 689: 111–7 [DOI] [PubMed] [Google Scholar]
- 14.Kim SP, Hwang E, Kang JH, Kim S, Choi JH. Changes in the thalamocortical connectivity during anesthesia-induced transitions in consciousness. Neuroreport 2012; 23: 294–8 [DOI] [PubMed] [Google Scholar]
- 15.Hwang E, Kim S, Han K, Choi JH. Characterization of phase transition in the thalamocortical system during anesthesia-induced loss of consciousness. PLoS ONE 2012; 7: e50580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kikuchi T, Wang Y, Shinbori H, Sato K, Okumura F. Effects of ketamine and pentobarbitone on acetylcholine release from the rat frontal cortex in vivo. Br J Anaesth 1997; 79: 128–30 [DOI] [PubMed] [Google Scholar]
- 17.Nelson CL, Burk JA, Bruno JP, Sarter M. Effects of acute and repeated systemic administration of ketamine on prefrontal acetylcholine release and sustained attention performance in rats. Psychopharmacology (Berl) 2002; 161: 168–79 [DOI] [PubMed] [Google Scholar]
- 18.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6th Edn. London: Academic Press, 2007 [Google Scholar]
- 19.Castro S, Falconi A, Chase MH, Torterolo P. Coherent neocortical 40-Hz oscillations are not present during REM sleep. Eur J Neurosci 2013; 37: 1330–9 [DOI] [PubMed] [Google Scholar]
- 20.Castro S, Cavelli M, Vollono P, Chase MH, Falconi A, Torterolo P. Inter-hemispheric coherence of neocortical gamma oscillations during sleep and wakefulness. Neurosci Lett 2014; 578: 197–202 [DOI] [PubMed] [Google Scholar]
- 21.Borjigin J, Lee U, Liu T, et al. Surge of neurophysiological coherence and connectivity in the dying brain. Proc Natl Acad Sci USA 2013; 110: 14432–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee U, Lee H, Müller M, Noh GJ, Mashour GA. Genuine and spurious phase synchronization strengths during consciousness and general anesthesia. PLoS ONE 2012; 7: e46313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schreiber T, Schmitz A. Surrogate time series. Physica D: Nonlinear Phenomena 2000; 142: 346–82 [Google Scholar]
- 24.Kelland MD, Soltis RP, Boldry RC, Walters JR. Behavioral and electrophysiological comparison of ketamine with dizocilpine in the rat. Physiol Behav 1993; 54: 547–54 [DOI] [PubMed] [Google Scholar]
- 25.Hudetz AG, Vizuete JA, Pillay S. Differential effects of isoflurane on high-frequency and low-frequency γ oscillations in the cerebral cortex and hippocampus in freely moving rats. Anesthesiology 2011; 114: 588–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reed SJ, Plourde G, Tobin S, Chapman CA. Partial antagonism of propofol anaesthesia by physostigmine in rats is associated with potentiation of fast (80–200 Hz) oscillations in the thalamus. Br J Anaesth 2013; 110: 646–53 [DOI] [PubMed] [Google Scholar]
- 27.Nicolaou N, Georgiou J. Global field synchrony during general anaesthesia. Br J Anaesth 2014; 112: 529–39 [DOI] [PubMed] [Google Scholar]
- 28.Casali AG, Gosseries O, Rosanova M, et al. A theoretically based index of consciousness independent of sensory processing and behavior. Sci Transl Med 2013; 5: 198ra105. [DOI] [PubMed] [Google Scholar]
- 29.Pinault D. N-methyl d-aspartate receptor antagonists ketamine and MK-801 induce wake-related aberrant γ oscillations in the rat neocortex. Biol Psychiatry 2008; 63: 730–5 [DOI] [PubMed] [Google Scholar]
- 30.Hakami T, Jones NC, Tolmacheva EA, et al. NMDA receptor hypofunction leads to generalized and persistent aberrant γ oscillations independent of hyperlocomotion and the state of consciousness. PLoS ONE 2009; 4: e6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicolás MJ, López-Azcárate J, Valencia M, et al. Ketamine-induced oscillations in the motor circuit of the rat basal ganglia. PLoS One 2011; 6: e21814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong LE, Summerfelt A, Buchanan RW, et al. Gamma and delta neural oscillations and association with clinical symptoms under subanesthetic ketamine. Neuropsychopharmacology 2010; 35: 632–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kikuchi T, Wang Y, Sato K, Okumura F. In vivo effects of propofol on acetylcholine release from the frontal cortex, hippocampus and striatum studied by intracerebral microdialysis in freely moving rats. Br J Anaesth 1998; 80: 644–8 [DOI] [PubMed] [Google Scholar]
- 34.Shichino T, Murakawa M, Adachi T, Arai T, Miyazaki Y, Mori K. Effects of inhalation anaesthetics on the release of acetylcholine in the rat cerebral cortex in vivo. Br J Anaesth 1998; 80: 365–70 [DOI] [PubMed] [Google Scholar]
- 35.Marrosu F, Portas C, Mascia MS, et al. Microdialysis measurement of cortical and hippocampal acetylcholine release during sleep-wake cycle in freely moving cats. Brain Res 1995; 671: 329–32 [DOI] [PubMed] [Google Scholar]
- 36.Brown EN, Purdon PL, Van Dort CJ. General anesthesia and altered states of arousal: a systems neuroscience analysis. Annu Rev Neurosci 2011; 34: 601–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci 2007; 27: 11496–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewis LD, Weiner VS, Mukamel EA, et al. Rapid fragmentation of neuronal networks at the onset of propofol-induced unconsciousness. Proc Natl Acad Sci USA 2012; 109: E3377–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blain-Moraes S, Tarnal V, Vanini G, et al. Neurophysiological correlates of sevoflurane-induced unconsciousness. Anesthesiology 2015; 122: 307–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.