Abstract
OBJECTIVE:
To review the current literature concerning the effects of physical exercise on several metabolic variables related to childhood obesity.
DATA SOURCE:
A search was performed in Pubmed/MEDLINE and Web of Science databases. The keywords used were as follows: Obesity, Children Obesity, Childhood Obesity, Exercise and Physical Activity. The online search was based on studies published in English, from April 2010 to December 2013.
DATA SYNTHESIS:
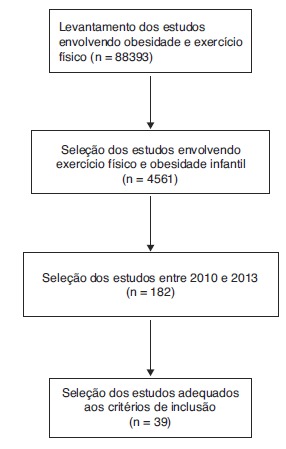
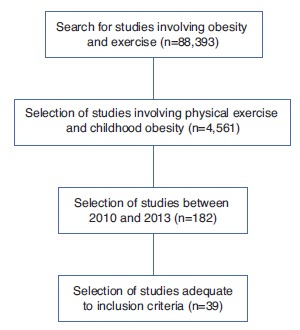
Search queries returned 88,393 studies based on the aforementioned keywords; 4,561 studies were selected by crossing chosen keywords. After applying inclusion criteria, four studies were selected from 182 eligible titles. Most studies found that aerobic and resistance training improves body composition, lipid profile and metabolic and inflammatory status of obese children and adolescents; however, the magnitude of these effects is associated with the type, intensity and duration of practice.
CONCLUSIONS:
Regardless of the type, physical exercise promotes positive adaptations to childhood obesity, mainly acting to restore cellular and cardiovascular homeostasis, to improve body composition, and to activate metabolism; therefore, physical exercise acts as a co-factor in fighting obesity.
Keywords: Exercise, Pediatric obesity, Child nutrition, Metabolism
Introduction
Obesity is a metabolic disorder characterized by a chronic inflammatory condition and excessive accumulation of body fat, which represents a health risk and contributes to the development of other diseases, such as type 2 diabetes, hypercholesterolemia, arterial hypertension, cardiovascular disease, obstructive sleep apnea syndrome, musculoskeletal impairments and several types of cancers.1 , 2
The etiology of obesity seems to be associated with several factors, such as genetic polymorphisms,3 , 4 dysfunction of the hypothalamic hormone signaling related to satiety, appetite and hunger,5 , 6 increased release of proinflammatory adipokines by white adipose tissue, and positive energy balance, in which the high total calorie intake, mainly high intake of energy-dense foods rich in saturated fats,7 sugar and salt exceeds daily calorie requirement.8
The development of obesity in the early stages of life is associated with the maintenance of the physiopathological state during adulthood. Childhood obesity can be defined as a condition of excessive accumulation of body fat in adipose tissue during childhood, with negative implications for health.9 The worldwide prevalence of childhood obesity is rapidly increasing in recent decades, being characterized as a global epidemic.9 In recent decades, children have become less active, probably encouraged by technological advances and socioeconomic factors.10 Obesity in childhood is the most important known risk factor for cardiovascular disease in adulthood, and these factors, when present in childhood, increase later in life, so it is necessary to fight them since the early stages of life, especially in relation to the life habits observed during this period.11
The benefits that physical exercises have on individuals' health have been well established, by improving cardiorespiratory fitness, body composition, and psychosocial well-being, among others. Physical exercise has been used as an important tool in the prevention and treatment of obesity12 by developing physical qualities that positively alter body composition, metabolic activity and by attenuating the comorbidities associated with excess weight.4 , 13 - 15
An inverse association has been demonstrated between physical activity level and development of obesity, mainly in the early stages of life,9 , 11 , 16 , 17 which justifies adherence to these practices, especially by children. While physical activity recommendations are well established for the adult population to fight obesity and its effects, the magnitude of the volume, intensity and frequency of physical activity is still controversial in the pediatric population.12
Considering that most clinical recommendations for treatment of obesity are based on the combination of several interventions, such as changing eating habits, medication use, regular physical activity and others, it is necessary to identify, assess or quantify the magnitude of the contribution of the possible types of treatment. Therefore, given the multifactorial nature of obesity, it is necessary to explain, in fact, the degree of contribution of physical exercise in the reduction and treatment of childhood obesity and its associated comorbidities.
Thus, the aim of this study was to review the current literature regarding the effects of exercise on several metabolic variables of childhood obesity.
Method
A literature review was performed, focusing on studies that reported the effects of exercise on several metabolic variables involved in childhood obesity. The databases used for this review were PubMed/MEDLINE and Web of Science. The descriptors applied were: Obesity, Children Obesity, Childhood Obesity, Exercise and Physical Activity. The electronic search was based on studies published from April 2010 to December 2013. Therefore, we aimed to focus on the most current findings in the literature on the subject. Inclusion criteria were randomized controlled studies involving the pediatric population around 12 years old, published in English, which associated the effects of physical exercise on metabolic variables associated with childhood obesity. Initially, after a wide selection, the articles were systematically read, analyzed and listed, aiming to confront the variables of interest in the study with the literature findings. Figure 1 shows the design of the study selection.
Figure 1. Article selection for the study.
Results and discussion
The main identified metabolic effects of physical exercise on childhood obesity are described in Table 1.
Table 1. Main metabolic effects of exercise on childhood obesity.
| Reference | Origin | (n) F– M | Age(yrs.) | Nutritional status | Assessed Parameters | Type of Exercise | Results |
|---|---|---|---|---|---|---|---|
| Militão et al, 201337 | Brazilians | 34 (17-17) | 9-11 | Overweight and obese | Energy expenditure and health habits | Recreational activities | ↓%F ↓SBP ↓TC ↓TG ↓LDL ↑VO2max |
| Laguna et al, 201329 | Spaniards | 437 (227-210) | 8-11 | Obese and normal weight | HRV and Cardiometabolic risk | Cycle ergometer | Inverse association between HRV and BMI |
| Schranz et al, 201338 | Australians | 56 (0-56) | 13-17 | Overweight and obese | Resistance Training and Body Composition | Resistance exercises | ↑MM; ↓%F and ↓BMI |
| Lai et al, 20133 | Chinese | 88 (48-40) | 10-16 | Obese | Genetic Polymorphism and Exercise | Aerobics | ↓resting HR; ↓%F ↓GI ↓Dyslipidemia |
| Lee et al, 201235 | North-Americans | 45 (0-45) | 12-16 | Obese | Metabolic effects of aerobic and resistance exercises | Aerobics and Resistance | ↑MM; ↓%F; ↓BMI ↑VO2max ; ↓BW |
| Davis et al, 201236 | North-Americans | 222 (128-94) | 9 -10 | Overweight and obese | Dose of exercise and risk of T2DM | Aerobics | ↓GI; ↓IR; ↓%F; ↓BMI |
| Araujo et al, 201232 | Brazilian | 30 (21-9) | 8-12 | Obese | Endurance and Resistance training | Aerobics and Resistance | ↑VO2max; ↓GI; ↓Insulinemia; ↓BMI |
| Park et al, 201228 | Koreans | 29 (15-14) | 11-12 | Overweight and obese | Physical Activity and Endothelial Dysfunction | Aerobics and Resistance | ↑VO2max; ↓AC ↓BMI; ↑NO; ↑Vasodilation |
| Makni et al, 201220 | Tunisians | 131 (63-68) | 12-14 | Obese | Field Testing and lipolytic rate | Walking | Correlation VO2max and %F |
| Legantis et al, 201230 | Greeks | 48 (23-25) | 10-11 | Obese and normal weight | Cardiorespiratory Fitness and Hemodynamic Response | Isometric hand grip | ↑SNA; ↑CO; ↑SBP |
| Woo et al, 201226 | Koreans | 39 (19-20) | 10-12 | Obese and normal weight | Detraining, Adipokines and Lipid Profile | Aerobics | Negative effect of ↓LPA on lipid profile |
| Plonka et al, 201125 | Polish | 59 (59-0) | 9-15 | Normal weight | Physical Activity Level and Leptin | Daily Energy Expenditure | Negative correlation between LPA, leptin and fat accumulation |
| Zorba et al, 201118 | Turkish | 40 (0-40) | 11-12 | Obese | Effects of Exercise on Cardiometabolic Risk | Aerobics and Recreational activities | ↓%F; ↓TC; ↓TG; ↓LDL; ↓Insulin; ↑HDL |
| Rosa et al, 201123 | North-Americans | 66 (32-34) | 11-14 | Obese and normal weight | Physical Exercise and Inflammatory Cytokines | Aerobics with Interval | ↑acute Inflammation in obese individuals |
| Velez et al, 201039 | Hispanics | 28 (13-15) | 15-16 | Overweight and obese | Resistance Training and Body Composition | Resistance | ↑MM ; ↓%F; ↓BMI |
%F, Percentage of fat; SBP, systolic blood pressure; TC, Total Cholesterol; TG, Triglycerides; LDL, Low-density lipoprotein; VO2max, maximal oxygen uptake; HDL, high-density lipoprotein; HRV, heart rate variability; MM, muscle mass; BMI, Body Mass Index; HR, heart rate; GI, Glucose Intolerance; BW, body weight; T2DM, type 2 Diabetes Mellitus; IR, Insulin Resistance; AC, abdominal circumference; NO, Nitric Oxide; SNA, Sympathetic Nervous Activity; CO, cardiac output; LPA, level of physical activity.
Physical exercise, metabolic rate and lipid profile
The results of this review demonstrate the effect of systematic and targeted physical exercise on metabolic variables associated with childhood obesity.9 , 10 , 16 - 18 The evidence associates the practice of exercises to body composition improvement, promoting physiological potentials that involve positive changes regarding the promotion of health and physical fitness.
The main physiological and metabolic effects resulting from both acute and chronic exercise, in general, are: increase in skeletal muscle mass, strength and proprioception gain, decrease in fat stores, increase in caloric expenditure, increased metabolic rate at rest, increased tolerance to glucose use as energy substrate, improved insulin sensitivity, decreased inflammatory state, among others.12 , 17 , 18
The increase in energy expenditure secondary to physical exercise occurs by stimulating the metabolic reactions and the enhancement of energy substrate use by active muscles. This occurs both acutely and by physiological adaptations that stimulate metabolism throughout the day.14 Leisure activities of moderate intensity and practiced for fun for 12 weeks were effective in attenuating dyslipidemia and hemodynamic factors associated with the worsening of the health status of obese children, with a mean body mass index (BMI) of 40 kg/m².18
A study carried out by Escalante et al19 reported that physical exercise can reduce low-density lipoproteins (LDL) by 35% and triglycerides by 40%, and increase high-density lipoproteins (HDL) in up to 25%. Therefore, exercise is considered by many authors as the main tool to attenuate the damage associated with childhood obesity.9 , 12 , 16 Makni et al20 evaluated the correlation between the 6-minute walk test and the use of fat as an energy substrate (FatMax) in 131 obese children (12.4±0.4 years). The study showed that the distance traveled during the test correlated significantly with the maximum heart rate achieved at the end of the walk, with this correlation being positive for boys (r=0.88) and girls (r=0.81). Thus, the researchers demonstrated that the field test is capable of quantifying the lipolytic rate of the obese child, i.e., how much the child is able to metabolize fat as an energy substrate, which makes the walk test a good clinical tool to estimate caloric expenditure. In the absence of an ergospirometer, this simple field test could be used to estimate VO2max and stratify aerobic physical training loads in obese young individuals.20
It is noteworthy, therefore, the beneficial role of exercise in regulating the lipid profile of obese children and as an attenuator of risk factors associated with metabolic syndrome, a pathological condition that involves, in addition to the dyslipidemic and obesogenic characteristics, the presence of hypertension, insulin resistance and altered fasting glucose.11 , 16 , 17 , 18 , 21 , 22
Physical exercise and inflammatory status
It is known that one of the characteristics of obesity is triggering a systemic inflammatory process caused by a regulatory hormonal dysfunction arising mainly from increased release of proinflammatory cytokines in the bloodstream, even during physical exercise.23 However, studies demonstrate that regular physical exercise is associated with the reduction of the systemic inflammatory state observed in childhood obesity.24
Lai et al3 evaluated, in 88 Chinese children, the association of the polymorphism of the recently discovered adipokine vistatin on metabolic variables. The researchers investigated the association of vistatin and the effect of an aerobic training program (20-40% of heart rate reserve), performed four times a week for four weeks. There was a marked decrease in triacylglycerol levels and insulin sensitivity in children that had the polymorphism of this pro-inflammatory adipokine. Rosa et al24 reported a 92% reduction in the levels of the proinflammatory adipokine interleukin-6 and oxidative metabolites of myeloperoxidase in 47 obese children undergoing intermittent training with cycling sprints at 80% VO2max.
Plonka et al25 evaluated the association between serum leptin and physical activity level in 59 obese schoolchildren. Girls that spent at least two hours daily in physical activity were considered active. It was concluded that among the active students, serum leptin was three times lower than among the sedentary ones, suggesting an improvement in sensitivity to leptin action in active girls. Corroborating the findings, Woo et al26 reported a significant reduction in serum leptin and increased adiponectin in obese Korean children between 10 and 12 years submitted to moderate-intensity aerobic training for 12 weeks. Moreover, even after cessation of training, serum concentrations of these adipokines remained unchanged for three subsequent months, during which the children did not engage in physical exercise.
Physical exercise has shown to be able to decrease the systemic inflammatory state, one of the physiopathological factors of obesity. The decrease in this clinical picture leads to improved function of several systems. In parallel, cellular signaling repair at the molecular level is able to act positively on cell communication and all cascades of biochemical reactions associated with metabolic systems and utilization of glucose, amino acids and fatty acids as an energy source.
Physical exercise and cardiovascular and neural risk factors
The metabolic and hormonal dysfunction triggered by childhood obesity is associated with cardiovascular risk factors by inducing systemic changes that, later in life, can cause cardiovascular injury, whose outcomes can culminate in death. Therefore, it is necessary to encourage preventive or remedial measures to attenuate such risk factors.27
It has been demonstrated that regular physical exercise can promote, as early as in childhood, positive cardiovascular adaptations. Park et al28 evaluated the effect of an aerobic and resistance training program on endothelial function in 29 obese children for 12 weeks. Aerobic training consisted of 30 minutes of brisk walking (approximately 60% of heart rate reserve). Resistance exercise consisted in a circuit with three exercises for the upper limbs and four for the lower limbs, with 8-12 repetitions and intensity of 60% of maximum repetitions (MR). Researchers showed a two-fold higher increase in three types of endothelial progenitor cells, that is, physical training was able to stimulate an increase in endothelial vasodilator capacity, which increases blood flow to the body and decreases the strength of ventricular ejection, decreasing cardiac overload.
The heart rate recovery time after physical exercise can be used as an important tool to measure autonomic control of the heart. Thus, the magnitude of the decrease in the number of heartbeats after performing an activity, within a short time, seems to reflect an individual's level of cardiovascular fitness. However, obese individuals have an imbalance, as early as in childhood, of this involuntary control over the heart, i.e., they require more time to decrease heart rate after physical exertion. Laguna et al29 performed maximal exercise test on a cycle ergometer in 437 obese Spanish children, with a mean age of 9 years, and found a positive association between the time of heart rate recovery after exercise and cardiometabolic risk factors in this population, i.e., the longer it took for the heart rate to be restored to resting rate, the lower the efficiency of cardiac work.
Corroborating these findings, Legantis et al30 evaluated the effect of cardiorespiratory fitness and obesity on the hemodynamic response of 24 obese children, physically active and inactive, submitted to isometric handgrip exercise at 30% MR for three minutes. Inactive obese children had higher systolic blood pressure at rest and during isometric muscle contraction, when compared to active obese children. Additionally, higher levels of muscle sympathetic nerve activity, cardiac output and oxygen consumption were observed in the inactive children. Physical inactivity promotes a decrease in the individual's mechanical efficiency in the presence of a certain exertion, that is, obesity reduces the metabolic capacity to generate work and support the energy demands of physical activity. Thus, the lower the individual's aerobic efficiency in the presence of a stimulus, the less capable the individual is to endure the intensity of a task over time. These findings demonstrate that adequate physical conditioning is a good predictor of cardiovascular health, regardless of obesity, i.e., cardiorespiratory fitness may play a protective role in the heart of the obese individuals, even during childhood.
The practice of physical exercises promotes important neural adaptations in the cardiovascular system, positively stimulating neural pathways connected to the heart muscle and endothelial smooth muscle. This has a positive effect on hemodynamic factors, such as blood pressure, heart rate and peripheral vascular resistance, which increases the strength and capacity of cardiac ejection, distribution of blood flow and thus maximizes the availability and utilization of nutrients by the skeletal muscles.
Effect of the types of physical exercises
Increased aerobic capacity is inversely associated with fat accumulation and cardiovascular risk factors. According to a meta-analysis by Saavedra et al, 31 improved aerobic fitness triggers a series of physiological stimuli that enhance oxygen uptake and utilization of fatty acids as an energy source, which reduces body fat deposits, thus decreasing obesity rates.
A study by Araujo et al32 demonstrated an increase in VO2max in 39 obese children submitted to a 12 week-training protocol, using resistance training at 80% of maximum heart rate intercalated with sprints. There was a significant increase in absolute VO2 (26% vs. 19%) and relative peak VO2 (13.1% vs. 14.6%), as well as in insulin sensitivity. However, this proposed training showed no significant reduction in body fat, or serum glucose, cholesterol, triacylglycerols and lipoproteins. However, only measurements of subcutaneous fat were performed, as visceral fat was not assessed. It is known that visceral fat is more metabolically active and associated with cardiometabolic comorbidities. In this sense, these results should be considered with caution, as most studies show positive responses related to metabolic parameters related to obesity and physical exercise, both aerobic and resistance.31 , 33
A study carried out by Ando et al34 showed an increased use of fat as energy substrate 24 hours after the practice of continuous or intermittent aerobic exercise. However, the magnitude of fat utilization during the 24 hours after the exercise was higher in patients submitted to continuous exercise, suggesting that the intensity, in spite of the importance of volume loads, may be a factor that modulates the level of energy expenditure. Thus, it is suggested that fractionated activities throughout the day, with higher intensity and lower volume, may result in greater impact on daily energy expenditure.
Lee et al35 assessed, for a period of three months, the effects of aerobic and resistance exercise on the accumulation of abdominal, hepatic and intramyocellular fat and insulin sensitivity in 32 obese pre-adolescent boys. Both types of exercises promoted decrease in visceral and intramyocellular fat; however, only the counter-resistance exercise caused a significant increase in insulin sensitivity. The researchers attributed this increased sensitivity to the fact that resistance exercises enhance the level of localized muscle contraction and provide a further stimulus to glucose transporter proteins into the cell.
Regarding the training volume Davis et al36 evaluated the effect of different volumes of aerobic training in 222 overweight and obese children during 13 weeks, with five training sessions a week. The intensity of the aerobic training was 65% of VO2max, the volumes were 20 and 40 minutes per session and the analyzed parameters were insulin resistance and visceral fat accumulation. Both 20- and 40-minute training sessions resulted in improved insulin sensitivity and reduced visceral fat. However, the 40-minute group showed an improvement of 83% in insulin sensitivity and the same occurred for visceral fat reduction, whose decrease was 72% higher compared to the control group.
Recreational activities are also effective to promote the attenuation of risk factors of childhood obesity. Militão et al37 followed 34 obese schoolchildren between 9 and 11 years during the interval between classes. The study demonstrated that a 10-week program of recreational exercise combined with a program promoting healthy lifestyle habits was able to increase the values of VO2max, reduce LDL, triglycerides and total cholesterol as well as blood pressure levels.
As for resistance training, studies with obese children are limited due to the difficulty in quantifying training loads. However, studies that reported the effects of resistance training on metabolic variables in obese children reported positive results regarding the potential damage the disease brings to the individual.13 These factors are associated with the gain of fat-free mass and decrease in fat tissue, as well as the reduction of hemodynamic pressure levels and risk factors associated with the development of cardiovascular diseases.33
Schranz et al38 evaluated the effects of a 6-month resistance training program in 56 obese adolescents aged 13-17 years. In addition to the metabolic benefits brought by the practice, such as increase in muscle mass and decrease in body fat percentage, it was observed that this type of intervention also promotes benefits related to the self-esteem of obese individuals, a factor that is indirectly associated with the psychosocial aspect related to obesity.38 , 39
Similarly, it was demonstrated that resistance training promotes many metabolic benefits, such as improved insulin sensitivity, increased glucose utilization as energy substrate and improved lipid profile, factors closely associated with childhood obesity impairment.13
Resistance exercises performed in the traditional way, such as in fitness centers, are usually not well accepted by the pediatric population. Thus, it is important that recreational activities such as games and/or sports that involve the body's own resistance or the peers' are encouraged. Sports practices that include gymnastics or combat sports in general, with special emphasis on judo, are interesting ways of working the force component in this population, mainly by stimulating the practice of pleasurable activities that require anaerobic and neuromuscular power.
Exercises with predominant aerobic characteristics should also be performed. Unlike adults, who can bear periods of continuous exercise on a cycle ergometer or running, children do not tolerate well this type of prescription. Because of this, it is interesting to encourage the practice of recreational activities and sports that involve a large amount of body mass such as soccer, futsal, basketball, handball and water polo. Swimming and activities with roller skates usually represent well-tolerated activities, which are also interesting to increase energy expenditure and improve aerobic capacity.
Thus, alternating different types of physical activity throughout the week, totaling four to six days, would have a fundamental role in daily energy expenditure, constituting a strategy to fight, prevent or mitigate the deleterious effects of childhood obesity.
Conclusion
The practice of physical exercises has shown to promote positive adaptations on childhood obesity and act as adjuvant for its prevention and treatment. The magnitude of benefits may vary with the exercise. The main effects arising from the exercises are mainly related to the restoration of the lipid profile, autonomic and hemodynamic restoration, improved body composition, use of energy substrates and metabolic activation.
Funding Statement
Fundação de Amparo à Pesquisa de Minas de Gerais - FAPEMIG, Brazil; Edital de Primeiros Projetos Process CBB-APQ 04173-10.
Footnotes
Funding Fundação de Amparo à Pesquisa de Minas de Gerais - FAPEMIG, Brazil; Edital de Primeiros Projetos Process CBB-APQ 04173-10.
References
- 1.Pereira-Lancha LO, Campos-Ferraz PL, Lancha AH., Jr Obesity: considerations about etiology, metabolism, and the use of experimental models. Diabetes Metab Syndr Obes. 2012;5:75–87. doi: 10.2147/DMSO.S25026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Preis SR, Massaro JM, Robins SJ, Hoffmann U, Vasan RS, Irlbeck T. Abdominal subcutaneous and visceral adipose tissue and insulin resistance in the Framingham heart study. Obesity (Silver Spring) 2010;18:2191–2198. doi: 10.1038/oby.2010.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai A, Chen W, Helm K. Effects of visfatin gene polymorphism RS4730153 on exercise-induced weight loss of obese children and adolescents of Han Chinese. Int J Biol Sci. 2013;9:16–21. doi: 10.7150/ijbs.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC. A PGC1-a-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;11:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arruda GP, Milanski M, Velloso LA. Hypothalamic inflammation and thermogenesis: the brown adipose tissue connection. J Bioenerg Biomembr. 2011;43:53–58. doi: 10.1007/s10863-011-9325-z. [DOI] [PubMed] [Google Scholar]
- 6.Thaler JP, Choi SJ, Schwartz MW, Wisse BE. Hypothalamic inflammation and energy homeostasis: resolving the paradox. Frontiers in Neuroendocrinology. 2010;31:79–84. doi: 10.1016/j.yfrne.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Borg ML, Omran SF, Weir J, Meikle PJ, Watt MJ. Consumption of a high-fat diet, but not regular endurance exercise training, regulates hypothalamic lipid accumulation in mice. J Physiol. 2012;1(590):4377–4389. doi: 10.1113/jphysiol.2012.233288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drewnowski A, Mennela JA, Johnson SL, Bellisle F. Sweetness and food preference. J Nutr. 2012;142:1142S–11142S. doi: 10.3945/jn.111.149575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guinhouya BC. Physical activity in the prevention of childhood obesity. Paediatr Perinat Epidemiol. 2012;26:438–447. doi: 10.1111/j.1365-3016.2012.01269.x. [DOI] [PubMed] [Google Scholar]
- 10.Landry BW, Driscoll SW. Physical activity in children and adolescents. PM R. 2012;4:826–832. doi: 10.1016/j.pmrj.2012.09.585. [DOI] [PubMed] [Google Scholar]
- 11.Brambilla P, Pozzobon G, Pietrobelli A. Physical activity as the main therapeutic tool for metabolic syndrome in childhood. Int J Obes (Lond) 2011;35:16–28. doi: 10.1038/ijo.2010.255. [DOI] [PubMed] [Google Scholar]
- 12.Kelley GA, Kelley KS. Effects of exercise in the treatment of overweight and obese children and adolescents: a systematic review of meta-analyses. J Obes. 2013:783103–783103. doi: 10.1155/2013/783103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alberga AS, Sigal RJ, Kenny GP. A review of resistance exercise training in obese adolescents. Phys Sportsmed. 2011;39:50–63. doi: 10.3810/psm.2011.05.1895. [DOI] [PubMed] [Google Scholar]
- 14.Church T. Exercise in obesity, metabolic syndrome, and diabetes. Prog Cardiovasc Dis. 2011;53:412–418. doi: 10.1016/j.pcad.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 15.Alberga AS, Farnesi BC, Lafleche A, Legault L, Komorowski J. The effects of resistance exercise training on body composition and strength in obese prepubertal children. Phys Sportsmed. 2013;41:103–109. doi: 10.3810/psm.2013.09.2028. [DOI] [PubMed] [Google Scholar]
- 16.Guinhouya BC, Hubert H. Insight into physical activity in combating the infantile metabolic syndrome. Environ Health Prev Med. 2011;16:144–147. doi: 10.1007/s12199-010-0185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim Y, Park H. Does regular exercise without weight loss reduce insulin resistance in children and adolescents. Int J Endocrinol. 2013;2013:402592–402592. doi: 10.1155/2013/402592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zorba E, Cengiz T, Karacabey K. Exercise training improves body composition, blood lipid profile and serum insulin levels in obese children. J Sports Med Phys Fitness. 2011;51:664–669. [PubMed] [Google Scholar]
- 19.Escalante Y, Saavedra JM, García-Hermoso A, Domínguez AM. Improvement of the lipid profile with exercise in obese children: a systematic review. Prev Med. 2012;54:293–301. doi: 10.1016/j.ypmed.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Makni E, Moalla W, Trabelsi Y, Lac G, Brun JF, Tabka Z. Six-minute walking test predicts maximal fat oxidation in obese children. Int J Obes (Lond) 2012;36:908–913. doi: 10.1038/ijo.2011.257. [DOI] [PubMed] [Google Scholar]
- 21.Parrett AL, Valentine RJ, Arngrímsson SA, Castelli DM, Evans EM. Adiposity and aerobic fitness are associated with metabolic disease risk in children. Appl Physiol Nutr Metab. 2011;36:72–79. doi: 10.1139/H10-083. [DOI] [PubMed] [Google Scholar]
- 22.Lee S, Kim Y. Effects of exercise alone on insulin sensitivity and glucose tolerance in obese youth. Diabetes Metab J. 2013;4:225–232. doi: 10.4093/dmj.2013.37.4.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosa JS, Heydari S, Oliver SR, Flores RL, Pontello AM, Ibardolaza M. Inflammatory cytokine profiles during exercise in obese, diabetic, and healthy children. J Clin Res Pediatr Endocrinol. 2011;3:115–121. doi: 10.4274/jcrpe.v3i3.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosa JS, Oliver SR, Flores RL, Ngo J, Milne GL, Zaldivar FP. Altered inflammatory, oxidative, and metabolic responses to exercise in pediatric obesity and type 1 diabetes. Pediatr Diabetes. 2011;12:464–472. doi: 10.1111/j.1399-5448.2010.00724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plonka M, Toton-Morys A, Adamski P, Suder A, Bielanski W, Dobrzanska MJ. Association of the physical activity with leptin blood serum level, body mass indices and obesity in schoolgirls. J Phys and Pharm. 2011;62:647–656. [Google Scholar]
- 26.Woo J, Shin KO, Yoo JH, Park S, Kang S. The effects of detraining on blood adipokines and antioxidant enzyme in Korean overweight children. Eur J Pediatr. 2012;171:235–243. doi: 10.1007/s00431-011-1518-2. [DOI] [PubMed] [Google Scholar]
- 27.Zhang H, Zhang C. Adipose "talks" to distant organs to regulate insulin sensitivity and vascular function. Obesity (Silver Spring) 2010;18:2071–2076. doi: 10.1038/oby.2010.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park J, Miyashita M, Kwon Y, Park H, Kim E, Park J. A 12-week after-school physical activity programme improves endothelial cell function in overweight and obese children: a randomized controlled study. BMC Pediatrics. 2012;12:111–111. doi: 10.1186/1471-2431-12-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laguna M, Aznar S, Lara MT, Lucía A, Ruiz JR. Heart rate recovery is associated with obesity traits and related cardiometabolic risk factors in children and adolescents. Nutr Metab Cardiovasc Dis. 2013;10:995–1001. doi: 10.1016/j.numecd.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Legantis CD, Nassis GP, Dipla K, Vrabas IS, Sidossis LS, Geladas ND. Role of cardiorespiratory fitness and obesity on hemodynamic responses in children. J Sports Med Phys Fitness. 2012;52:311–318. [PubMed] [Google Scholar]
- 31.Saavedra JM, Escalante Y, Garcia-Hermoso A. Improvement of aerobic fitness in obese children: a meta-analysis. Int J Pediatr Obes. 2011;6:169–177. doi: 10.3109/17477166.2011.579975. [DOI] [PubMed] [Google Scholar]
- 32.Araujo AC, Roschel H, Picanço AR, Prado DM, Villares SM, Pinto AL. Similar health benefits of endurance and high-intensity interval training in obese children. PlosOne. 2012;7: doi: 10.1371/journal.pone.0042747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dietz P, Hoffmann S, Lachtermann E, Simon P. Influence of exclusive resistance training on body composition and cardiovascular risk factors in overweight or obese children: a systematic review. Obes Facts. 2012;5:546–560. doi: 10.1159/000341560. [DOI] [PubMed] [Google Scholar]
- 34.Ando T, Usui C, Ohkawara K, Miyake R, Miyashita M, Park J. Effects of intermittent physical activity on fat utilization over a whole day. Med Sci Sports Exerc. 2013;45:1410–1418. doi: 10.1249/MSS.0b013e3182885e4b. [DOI] [PubMed] [Google Scholar]
- 35.Lee S, Bacha F, Hannon T, Kuk JL, Boesch C, Arslanian S. Effects of aerobic versus resistance exercise without caloric restriction on abdominal fat, intrahepatic lipid, and insulin sensitivity in obese adolescent boys: a randomized, controlled trial. Diabetes. 2012;61:2787–2795. doi: 10.2337/db12-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis CL, Pollock NK, Waller JL, Allison JD, Dennis BA, Bassali R. Exercise dose and diabetes risk in overweight and obese children: a randomized controlled trial. JAMA. 2012;308:1103–1112. doi: 10.1001/2012.jama.10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Militão AG, de Oliveira Karnikowski MG, da Silva FR, Garcez Militão ES, Dos Santos Pereira RM, Grubert Campbell CS. Effects of a recreational physical activity and healthy habits orientation program, using an illustrated diary, on the cardiovascular risk profile of overweight and obese schoolchildren: a pilot study in a public school in Brasilia, Federal District, Brazil. Diabetes Metab Syndr Obes. 2013;6:445–451. doi: 10.2147/DMSO.S52166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schranz N, Tomkinson G, Parletta N, Petkov J, Olds T. Can resistance training change the strength, body composition and self-concept of overweight and obese adolescent males? A randomised controlled trial. Br J Sports Med. 2013 doi: 10.1136/bjsports-2013-092209. [DOI] [PubMed] [Google Scholar]
- 39.Velez A, Golem DL, Arent SM. The impact of a 12-week resistance training program on strength, body composition, and self-concept of Hispanic adolescents. J Strength Cond Res. 2010;24:1065–1073. doi: 10.1519/JSC.0b013e3181cc230a. [DOI] [PubMed] [Google Scholar]