Abstract
Introduction
We sought to determine if monitoring heart rate variability (HRV) would enable preclinical detection of secondary complications after subarachnoid hemorrhage (SAH).
Methods
We studied 236 SAH patients admitted within the first 48 hours of bleed onset, discharged after SAH day 5, and had continuous electrocardiogram records available. The diagnosis and date of onset of infections and DCI events were prospectively adjudicated and documented by the clinical team. Continuous ECG was collected at 240 Hz using a high-resolution data acquisition system. The Tompkins Hamilton algorithm was used to identify R-R intervals excluding ectopic and abnormal beats. Time, frequency, and regularity domain calculations of HRV were generated over the first 48 hours of ICU admission and 24 hours prior to the onset of each patient's first complication, or SAH day 6 for control patients. Clinical prediction rules to identify infection and DCI events were developed using bootstrap aggregation and cost sensitive meta-classifiers.
Results
The combined infection and DCI model predicted events 24 hours prior to clinical onset with high sensitivity (87%) and moderate specificity (66%), and was more sensitive than models that predicted either infection or DCI. Models including clinical and HRV variables together substantially improved diagnostic accuracy (AUC 0.83) compared to models with only HRV variables (AUC 0.61).
Conclusions
Changes in HRV after SAH reflect both delayed ischemic and infectious complications. Incorporation of concurrent disease severity measures substantially improves prediction compared to using HRV alone. Further research is needed to refine and prospectively evaluate real-time bedside HRV monitoring after SAH.
Keywords: Subarachnoid hemorrhage, nosocomial infection, delayed cerebral ischemia, cerebral vasospasm, sepsis, heart rate variability
Introduction
Aneurysmal subarachnoid hemorrhage (SAH) is a major public health issue in the United States.1 Prevention, detection, and clinical management of secondary complications generates a large health care burden for SAH patients.2 Heart rate variability (HRV) monitoring has been shown to be an effective method for preclinical detection of sepsis in critically-ill patients.3,4 This work is grounded in the observation that severe infection produces a pro-inflammatory response 5 that results in reductions of parasympathetic nervous system input to the heart. This leads to reductions in heart rate variability 6 that reliably precede the onset of the systemic inflammatory response syndrome (SIRS).7,8
Acute brain injury may also produce a strong pro-inflammatory response.9 SAH patients commonly develop SIRS even in the absence of an infection.10 Systemic inflammation has a crucial and unifying role in the pathogenesis of delayed cerebral ischemia (DCI) from vasospasm after SAH.9-12 DCI has been shown to be the strongest predictor of poor outcome in SAH after the impact of the initial bleeding event and rebleeding.13 The severity of the SIRS response predicts DCI development,10 and monitoring HRV has shown potential for detecting secondary complications after SAH.14 The purpose of this study was to determine whether heart rate variability monitoring of SAH patients can provide preclinical detection of nosocomial infections and DCI a day in advance of symptom onset.
Methods
Study Design
Patients were selected from the Columbia University SAH Outcomes Project, a prospective observational cohort study designed to identify novel risk factors for secondary injury and poor outcome. Subjects for the current analysis were enrolled between April 2006 and June 2011. The study was approved by the Columbia University Medical Center Institutional Review Board; in all cases written informed consent was obtained from the patient or a surrogate. The diagnosis of SAH was established by admission CT or by xanthochromia of cerebrospinal fluid if the initial CT scan was nondiagnostic. Patients with secondary SAH related to trauma, rupture of an AVM, or other causes and age < 18 years are not enrolled in the study.
Patient Selection
Classifiers generally perform poorly when there is an imbalance between the number of positive and negative cases. In the more common instance of many more negative cases than positive, classification results become biased towards labelling almost all instances as negative.15 To counter this we created a dataset with roughly equal numbers of patients with and without complications. Candidates for inclusion were required to have been admitted to the ICU within 48 hours of SAH onset, treated in the ICU until at least SAH bleed day 5, and have continuous high-resolution electrocardiogram (ECG) data available for analysis. Of 447 patients enrolled during the five year screening period, 295 patients met all of these criteria. Among these patients there were 127 good-grade (Hunt-Hess Grade ≤ 2) and 168 poor-grade patients (Hunt-Hess Grade ≥ 3). Roughly half (46%) of poor-grade patients had a documented complication (infection or delayed cerebral ischemia [DCI]) and we included all of these patients in the dataset for analysis. By contrast only a quarter (27%) of good-grade patients had a documented complication. To ensure a relatively balanced dataset for analysis, all 34 good-grade patients with a complication were included and an additional 34 good-grade patients that did not have documented complication were selected at random. The final dataset contained 236 patients with 111 cases and 125 controls.
Clinical Management
Management algorithms for SAH patients at Columbia University Medical Center have been described previously 13 and conforms to guidelines set forth by the American Heart Association.16 All patients were followed with daily or every-other-day with transcranial Doppler (TCD) sonography and received oral nimodipine and intravenous hydration with 0.9% saline with supplemental fluids as needed to maintain equal fluid balance and a normal central venous pressure (5-10 mm Hg). Hypertensive hypervolemic therapy (HHT) was initiated for symptomatic vasospasm or when severe angiographic vasospasm was diagnosed in poor grade patients by increasing systolic blood pressure (SBP) from 180 to 220 mmHg as required to reverse the neurological deficit.17 CT was performed serially when clinically needed. All patients with clinical deterioration underwent CT or MRI scanning to identify causes of deterioration other than vasospasm whenever clinically feasible. When clinical evidence of DCI persisted for more than 2 hours despite HHT, cerebral angiography was used to identify vasospasm and balloon angioplasty or intra-arterial administration of verapamil was performed whenever feasible.
Complication Definitions
We recorded demographics and past medical history, baseline clinical status, imaging results, and treatment and complications during hospitalization as described previously.13 The presence and timing of infectious complications and DCI were adjudicated and documented prospectively in weekly meetings by the clinical team. Infectious complications were classified as pneumonia (new infiltrate on CXR with fever or purulent sputum), urinary tract infection (urine white cell count >5/hpf and positive urine culture), and bloodstream infection (positive blood cultures with local IV erythema or SIRS).
Delayed cerebral ischemia (DCI) was defined as (1) clinical deterioration (i.e. a new focal deficit, decrease in level of consciousness, or both), and/or (2) a new infarct on CT that was not visible on the admission or immediate postoperative scan, when the cause was thought by the research team to be vasospasm. Other potential causes of clinical deterioration or CT lucencies, such as hydrocephalus, rebleeding, cerebral edema, retraction injury, ventriculitis, metabolic derangements, and seizures were rigorously excluded. DCI and cerebral infarcts due to spasm were diagnosed by the treating study neurointensivist, and confirmed in a weekly review of each subject's clinical course by the study team. In each patient with DCI, symptomatic territories were identified, and clinical deficits, angiographic, and CT findings were recorded. Evidence of arterial spasm by transcranial Doppler (TCD) ultrasonography (mean flow velocity >120 cm/s) or angiography was used to support the diagnosis but was not mandatory.
Feature Construction
Continuous ECG data was collected at 240 Hz using a high-resolution data acquisition system (BedmasterEx, Excel Medical, Jupiter, FL). The Tompkins Hamilton algorithm was implemented on a streaming analytic platform (InfoSphere Streams version 3.1, IBM, Armonk, NY) to identify valid R-R intervals while excluding ectopic and abnormal beats.18 Time, frequency, and regularity domain calculations of heart rate variability were generated (Table 1). HRV calculations during the 48-hours post bleed onset were averaged to determine a baseline for each HRV measurement. HRV calculations during the day preceding complication onset or SAH bleed day 6 for control patients were also averaged. A difference score between baseline and event was generated resulting in three HRV features for each metric including baseline, event, and the difference between baseline and event measurements. The highest measured TCD velocity and white blood cell count prior to the onset of a first complication, or by SAH day 6 in the case of control patients, was recorded. In 4 patients the first observed complication was adjudicated as clinically-silent cerebral infarction due to cerebral vasospasm. Due to the inability to determine the absolute timing of infarction in these patients, we took a very conservative approach and defined the event time as 4 days prior to the emergence of new infarct on CT (mean SAH day = 11).
Table 1. Heart Rate Variability Measurements.
| Measurement | Unit | Definition |
|---|---|---|
| Very Low Frequency (VLF) | ms2 | Power in VLF (≤0.04 Hz) range |
| Normalized Low Frequency (nLF) | nu | LF (0.04-0.15 Hz) Power in normalized units (LF/(total power – VLF) × 100 |
| Normalized High Frequency (nHF) | nu | HF (0.15-0.4 Hz) Power in normalized units (HF/(total power – VLF) × 100 |
| LF/HF Ratio | Ratio LF [ms2]/HF[ms2] | |
| RMSSD | ms | The square root of the mean of the sum of the squares of differences between adjacent RR intervals |
| Standard Deviation RRIDX | ms | Mean of the standard deviations of all RR intervals for all 5-minute segments of the recording period |
| Sample Entropy | Measure of time series complexity that is small for low variability and large for high variability sequences | |
| 1 / f | Slope of the linear interpolation of the spectrum in a log-log scale (≈≤0.04 Hz) |
Predictive Models
All measurements were normalized to a mean of 0 and standard deviation of 1, and strongly skewed features were either log transformed to normal or categorized. Bootstrap aggregation (i.e., boosting) and cost sensitive meta-classifiers were implemented to build and evaluate standard random forest, decision tree, back propagation neural network, support vector machine, and logistic regression models. Higher costs (1.5) were assigned to missed detection compared to false alarms (1.0). Tenfold cross-validation was used to evaluate each model whereby the sample was partitioned into ten approximately equally sized disjoint subsamples. Nine subsamples were used as training data and validated against the tenth subsample with the process repeated until each of the ten subsamples was used for validation exactly once. The ten results from each of the repetitions were then averaged to produce a single estimation for the model. The computation was performed using Weka (Weka, version 3.7, the University of Waikato, New Zealand).
Statistical analysis
Univariate data analyses of patient characteristics were performed with R statistical software (R, version 2.12.2, R Project). P ≤ 0.05 was considered significant. The discrimination ability of each model was determined by measuring the accuracy, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), likelihood ratios for positive and negative predictions, and the area under the receiver operating characteristic (ROC) curves (AUC). A cut-off value corresponding to the minimal false negative and false positive results was selected to report the sensitivity and specificity of each prediction model. The Delong-Delong method was used to compare model performance on AUC whereas bootstrapping was used to compare partial AUC (pAUC).19
Results
A total of 111 (47%) of 236 SAH patients in this dataset experienced an infection, DCI, or both. Of these 111 patients, the first complication documented was an infection in 77 patients (median day 6, IQR 4 to 9) and DCI in 34 (median day 6, IQR 5 to 8). Of the patients that experienced both complications types, 8 infection patients went on to have DCI whereas 10 DCI patients went on to have an infection later in their course. Compared to controls, patients that experienced infections or DCI were more likely to have hydrocephalus treated with CSF diversion, have their aneurysm secured by a clipping procedure, experience a fever, require an external ventricular drain, and have an abnormal white blood cell count; DCI patients were more likely to have a maximum TCD flow velocity greater than 120 cm/sec. DCI patients were more likely to be on vasopressor support (Table 2).
Table 2. Univariate Associations 24 Hours Prior to Complication Onset.
| Controls (N=125) |
Infection (N=77) |
DCI (N=34) |
Statistic a | P | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age ≥ 53b | 74 (59%) | 51 (66%) | 17 (50%) | 2.66 | 0.26 |
| Women | 82 (66%) | 51 (66%) | 26 (76%) | 1.41 | 0.49 |
| Non-white Ethnicity | 71 (57%) | 52 (68%) | 20 (59%) | 2.17 | 0.34 |
| Clinical Factors | |||||
| Loss of consciousness at ictus | 69 (55%) | 36 (47%) | 15 (45%) | 2.22 | 0.33 |
| Hunt-Hess Grade ≥ 4 | 60 (48%) | 32 (42%) | 13 (38%) | 1.56 | 0.46 |
| Glasgow Coma Scale ≤ 8 | 58 (47%) | 29 (38%) | 9 (26%) | 5.25 | 0.07 |
| APACHE II score - Physiological | 8 (6,13) | 7 (5,11) | 7 (5,9) | 3.77 | 0.02 |
| Fever > 38° C | 40 (32%) | 52 (68%) | 21 (62%) | 26.14 | <0.001 |
| External Ventricular Drain | 56 (45%) | 48 (62%) | 20 (59%) | 6.51 | 0.04 |
| Aneurysm Characteristics | |||||
| Size ≥ 10 mm | 16 (13%) | 9 (12%) | 4 (12%) | 0.08 | 0.96 |
| Clipping Procedure | 37 (30%) | 45 (58%) | 27 (79%) | 29.91 | <0.001 |
| Admission Echocardiogram | |||||
| Ejection Fraction ≤ 40% | 15 (12%) | 16 (21%) | 7 (21%) | 3.21 | 0.20 |
| Any Wall Motion Abnormalities | 17 (14%) | 10 (13%) | 5 (15%) | 0.06 | 0.97 |
| Admission CT | |||||
| Admission Cerebral Edema | 67 (54%) | 48 (62%) | 21 (62%) | 1.59 | 0.45 |
| Hijdra SAH Sum Score ≥15 | 79 (63%) | 44 (57%) | 25 (76%) | 3.84 | 0.15 |
| IVH present | 81 (65%) | 59 (79%) | 21 (64%) | 4.08 | 0.13 |
| ICH present | 22 (18%) | 14 (19%) | 10 (30%) | 2.57 | 0.28 |
| Bicaudate Index ≥16 | 48 (38%) | 44 (62%) | 16 (50%) | 6.35 | 0.04 |
| TCD Flow Velocity | |||||
| Maximum Velocity > 120 cm/sec | 11 (9%) | 13 (17%) | 18 (53%) | 35.38 | <0.001 |
| White Blood Cell Count | |||||
| <4000/mm3 or >12000/mm3 | 13 (10%) | 39 (51%) | 18 (53%) | 46.83 | <0.001 |
| Medications | |||||
| Beta Blockers | 2 (2%) | 5 (6%) | 0 (0%) | 5.18 | 0.08 |
| Vasopressor support | 15 (12%) | 17 (22%) | 11 (32%) | 8.57 | 0.01 |
| Propofol | 12 (10%) | 16 (21%) | 4 (12%) | 5.19 | 0.08 |
| Analgesia and Sedation | 12 (10%) | 11 (14%) | 5 (15%) | 1.31 | 0.52 |
Values are N (%), median (25%, 75%);
Chi-square test for dichotomized variables, general linear model for continuous variables;
Dichotomized at median age
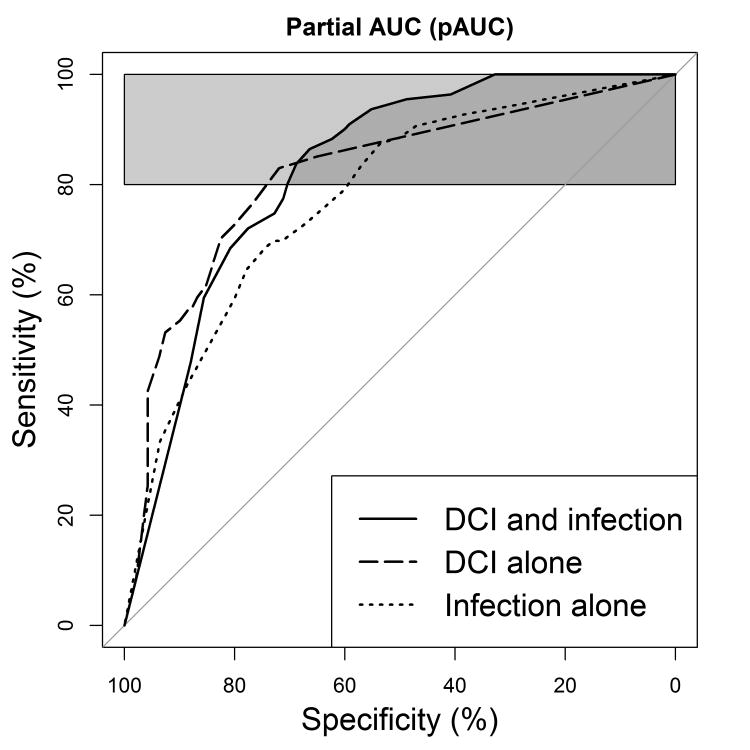
We sought to minimize the number of attributes (i.e., variables) that would require manual data entry (e.g., location of blood or edema presence on CT, Apache-II score) at the bedside if deployed. A supervised gain ratio attribute evaluator was utilized to identify the best attributes for model building. This process is akin to performing univariate analysis to identify candidate variables for a multifactorial model. The worth of an attribute is reported as a value whereby higher values represent greater relatedness to the outcome class, which in this case is complication status. VLF power, standard deviation RRIDX, and total power HRV measurements provided the most worth to classify complication status over white blood cell count, transcranial Doppler measurements, ventilation changes, aneurysm clipping procedure, and fever (Table 3). The models utilizing random forest classification were the most accurate and are reported. Performance of the models comparing a combined complication category (i.e., both infection and DCI), DCI alone, or infection alone are compared using the ‘best’ threshold (Table 4). The overall area under the curve for the three models did not differ significantly but the combined complication model was the most sensitive of the three (Figure 1). To determine if HRV variables would be sufficient to identify patients we removed clinical variables from the combined complication model. Statistical comparison of the ROC curves revealed that the HRV only model performed significantly worse (AUC: 0.83 versus 0.61, Z=5.74, P<0.001). Model performance statistics suggest that HRV is sensitive but not specific for secondary complications without the addition of clinical variables (Table 5).
Table 3. Supervised Gain Ratio Feature Evaluation for Complication Status.
| Gain Ratio | Feature |
|---|---|
| 0.2049 | Log transformed VLF power (60 min window) at baseline |
| 0.2012 | Log transformed VLF power (30 min window) at baseline |
| 0.1934 | Increase in propofol from Baseline |
| 0.1902 | Log transformed Total Power (5 min window) at baseline |
| 0.1864 | Log transformed VLF (30 min window) 24 hours prior to event |
| 0.1826 | Standard deviation RRIDX 24 hours prior to event |
| 0.1826 | Standard deviation RRIDX Baseline |
| 0.1786 | Maximum white blood count 24 hours prior to event |
| 0.1582 | Maximum TCD Lindengaard Ratio: Mean Velocity |
| 0.1582 | Maximum TCD Lindengaard Ratio: Peak Velocity |
| 0.1440 | Propofol dosage at baseline |
| 0.1212 | Change in ventilation status |
| 0.0877 | Clipping aneurysm repair procedure |
| 0.0845 | Maximum TCD Mean Velocity: any vessel |
| 0.0838 | Temperature > 38.3 |
The gain ratio is the worth of an attribute whereby higher values represent greater relatedness to the presence of a complication.
Table 4. Random Forest Classification Performance: Combined Complication Model versus Specific Models for Infection or DCI.
| Performance Metric | Combined Complication | Infection | DCI | |||
|---|---|---|---|---|---|---|
| AUC | 0.83 (0.77-0.88) | 0.78 (0.72-0.84) | 0.82 (0.75-0.89) | |||
| Accuracy (%) | 75.8 | 72.0 | 74.2 | |||
| Sensitivity (%) | 86.5 (71.3-94.2) | 68.8 (55.2-78.6) | 83.0 (69.2-92.6) | |||
| Specificity (%) | 66.4 (54.1-75.6) | 74.3 (59.8-83.8) | 72.0 (42.6-83.1) | |||
| LR + | 2.6 (2.2-3.0) | 2.7 (2.3-3.2) | 3.0 (2.3-3.8) | |||
| LR - | 0.2 (0.1-0.4) | 0.4 (0.3-0.6) | 0.2 (0.1-0.5) | |||
| PPV | 69.6 (61.9-77.2) | 64.7 (55.4-74.0) | 42.4 (32.3-52.5) | |||
| NPV | 84.7 (77.6-91.8) | 77.6 (70.6-84.7) | 94.4 (90.7-98.2) | |||
|
| ||||||
| Classified as --> | No | Yes | No | Yes | No | Yes |
|
|
||||||
| Actual No | 83 | 42 | 104 | 36 | 136 | 53 |
|
|
||||||
| Actual Yes | 15 | 96 | 30 | 66 | 8 | 39 |
AUC, area under receiver operating curve; LR + an LR -, positive and negative likelihood ratios; PPV, positive predictive value; NPV, negative predictive value
Figure 1.
Receiver operating characteristic curves of the predictive models. Depicts 80%-100% partial AUC for combined DCI and infection model (pAUC = 76.3%) versus infection alone (66.9%) and DCI alone (68.0%). The combined model was significantly more sensitive than the infection alone model (P=0.017) and DCI alone (P=0.004) models. Infection alone was not significantly more sensitive than the DCI alone model (P=0.734).
Table 5. Random Forest Classification Performance: Clinical and HRV Variables versus HRV Variables Alone.
| Performance Metric | Clinical + HRV | HRV alone | ||
|---|---|---|---|---|
| AUC | 0.83 (0.77-0.88) | 0.61 (0.54-0.68) | ||
| Accuracy (%) | 75.8 | 58.9 | ||
| Sensitivity (%) | 86.5 (71.3-94.2) | 95.5 (75.7-99.1) | ||
| Specificity (%) | 66.4 (54.1-75.6) | 26.4 (15.7-33.6) | ||
| LR + | 2.6 (2.2-3.0) | 1.3 (1.1-1.6) | ||
| LR - | 0.2 (0.1-0.4) | 0.2 (0.1-0.4) | ||
| PPV | 69.6 (61.9-77.2) | 53.5 (46.6-60.5) | ||
| NPV | 84.7 (77.6-91.8) | 86.8 (76.1-97.6) | ||
|
| ||||
| Classified as --> | No | Yes | No | Yes |
|
|
||||
| Actual No | 83 | 42 | 46 | 79 |
|
|
||||
| Actual Yes | 15 | 96 | 26 | 85 |
AUC, area under receiver operating curve; LR + an LR -, positive and negative likelihood ratios; PPV, positive predictive value; NPV, negative predictive value
Discussion
There is currently great interest in the development of ICU-based early warning systems that can more effectively process standard physiological monitoring data to predict life-threatening complications. This study demonstrates that HRV monitoring in SAH patients may be beneficial for preclinical detection of nosocomial infections and DCI at least up to the day prior of symptom onset. Classification models performed best when nosocomial infections and DCI were combined into a single complication category, rather than trying to predict either complication alone. Gain ratio attribute evaluation methods identified several HRV variables that were predictive of these complications, but these will require further testing in other patient populations before they can be generally applied. HRV measures were highly sensitive for secondary complications but lacked sufficient specificity without incorporating standard clinical measures of illness severity, such as sedative use, TCD findings, white blood cell count, and fever. HRV monitoring for preclinical detection of complications after SAH will not be able to differentiate ischemic from infectious complications, and will require automated incorporation of additional clinical inputs for maximal effectiveness.
This work is grounded on the observations that pro-inflammatory responses 5 result in reductions in parasympathetic nervous system activity. The heart rate variability measurements that were most important to the predictive model in this study were VLF power and its time domain surrogate measurement, standard deviation RRIDX. Although mechanisms for VLF are poorly understood 20 it has been connected to parasympathetic modulation of heart rate.20-22 Sympathetic blockage has little effect while parasympathetic blockage totally abolishes VLF power.21 It has been suggested that VLF power may reflect parasympathetic modulation of heart rate without being confounded by nonrespiratory sinus arrhythmia.23 Changes in RMSSD, which reflects high frequency parasympathetic outflow to the heart,24 has previously shown promise to predict complications in SAH.14 It remains unclear what measures of parasympathetic activity best discriminate patients with complications and this should be studied further.
Utilizing heart rate variability monitoring for preclinical detection of complications in SAH patients poses unique challenges. SAH patients often develop a pro-inflammatory response from the bleeding event itself 9 and commonly develop SIRS even in the absence of an infection.10 In our study HRV abnormalities were present for many SAH patients that did not develop secondary complications. While we found that HRV was highly sensitive for the development of secondary complications, it was not a specific marker and inclusion of clinical variables was essential to improving overall model performance. Further, DCI from cerebral vasospasm, and not sepsis, poses the greatest risk to morbidity and mortality after SAH.25 Analyses of non-specific inflammatory markers after SAH have found higher levels in patients with ischemic complications from vasospasm, even after correcting for the presence of infectious complications.26-28 Although sepsis is rare after SAH, pneumonia and urinary tract infections are common, and frequently patients that experience DCI have infections during their intensive care.25
Our data suggest that DCI cases are difficult to discriminate pre-clinically from infectious cases using HRV even when including TCD sonography, white blood cell count, and other predictive factors. The AUC for models predicting either DCI or infection alone was artificially boosted by the relative increase of true negatives compared to positive cases. Testing the sensitivity of the models showed the combined complication model significantly outperformed the single complication DCI or infection models. The DCI model missed nearly two times as many cases as it accurately identified either in the form of false alarms or false negative regardless of the threshold used. The infection model was better, identifying about as many cases as it missed. The combined model clearly performed best identifying almost twice as many positive cases as it missed. For every 10 positive cases 1 to 2 cases were not identified and 2 positive cases were identified for every false alarm. The clinical value of the combined prediction model would be to alert clinicians to examine a patient for possible early stages of a complication rather than quantifying risk for any one type of problem.
A validation study using a new cohort of patients is needed to confirm the findings of this study in which several model enhancements may be possible. Clinical protocols for DCI and infections are fundamentally different and the practical clinical utility of this work would be enhanced by the capability to discriminate between such cases. A larger pool of patients will allow multiclass or tiered model approaches to be explored that may prove better at discriminating each patient group. Additional patients would also enable the use of methods more amenable to real-time implementation to be employed.
We limited the number of factors in the model that would require manual entry at the bedside. As electronic patient data becomes more available in real-time from the electronic health record and other digital sources it may also be possible to deploy models that integrate a complex combination of high resolution physiology, patient characteristics, intervention information, and laboratory test values. In this case a model could incorporate a multitude of risk factors for nosocomial infections and DCI without increasing the burden on nursing staff to manually enter this information into a separate computer or device.
Conclusions
Heart rate variability monitoring may be a viable method to help identify SAH patients in the preclinical stage of infectious complications and delayed cerebral ischemia. The pro-inflammatory response to SAH that is known to contribute to the development of DCI is difficult to discriminate from the pro-inflammatory response to nosocomial infection using heart rate variability measurements. A validation study is needed to confirm these findings and to explore methods that would enhance the clinical utility of such a model by enabling the discrimination of DCI from infection patients.
Acknowledgments
Sources of Funding: The project described was supported by Grant Number KL2 RR024157 (JMS) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research, and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at NCRR Website. Information on Re-engineering the Clinical Research Enterprise can be obtained from NIH Roadmap website. Additional support was provided by the Charles A. Dana Foundation (SAM, JMS) and an IBM Faculty Research Award (JMS).
Contributor Information
J. Michael Schmidt, Department of Neurology, Columbia University Medical Center, New York, New York.
Daby Sow, IBM Research, T. J. Watson Research Center, Yorktown, New York.
Michael Crimmins, Department of Neurology, Columbia University Medical Center, New York, New York.
David Albers, Department of Biomedical Informatics, Columbia University Medical Center, New York, New York.
Sachin Agarwal, Department of Neurology, Columbia University Medical Center, New York, New York; Department of Neurosurgery, Columbia University Medical Center, New York, New York.
Jan Claassen, Department of Neurology, Columbia University Medical Center, New York, New York; Department of Neurosurgery, Columbia University Medical Center, New York, New York.
E. Sander Connolly, Department of Neurosurgery, Columbia University Medical Center, New York, New York.
Mitchell S. V. Elkind, Department of Neurology, Columbia University Medical Center, New York, New York; Department of Epidemiology, Mailman School of Public Health, Columbia University, New York NY.
George Hripcsak, Department of Biomedical Informatics, Columbia University Medical Center, New York, New York.
Stephan A. Mayer, Department of Neurology, Columbia University Medical Center, New York, New York; Department of Neurosurgery, Columbia University Medical Center, New York, New York.
References
- 1.Qureshi AI, Suri MF, Nasar A, et al. Trends in hospitalization and mortality for subarachnoid hemorrhage and unruptured aneurysms in the United States. Neurosurgery. 2005;57:1–8. doi: 10.1227/01.neu.0000163081.55025.cd. discussion 1-8. [DOI] [PubMed] [Google Scholar]
- 2.Diringer MN. Management of aneurysmal subarachnoid hemorrhage. Critical Care Medicine. 2009;37:432. doi: 10.1097/CCM.0b013e318195865a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moorman JR, Carlo WA, Kattwinkel J, et al. Mortality reduction by heart rate characteristic monitoring in very low birth weight neonates: a randomized trial. The Journal of pediatrics. 2011 doi: 10.1016/j.jpeds.2011.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmad S, Ramsay T, Huebsch L, et al. Continuous multi-parameter heart rate variability analysis heralds onset of sepsis in adults. PLoS One. 2009;4:e6642. doi: 10.1371/journal.pone.0006642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brun-Buisson C. The epidemiology of the systemic inflammatory response. Intensive Care Medicine. 2000;26:S064–S74. doi: 10.1007/s001340051121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117:289–96. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–6. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 8.Lenz A, Franklin GA, Cheadle WG. Systemic inflammation after trauma. Injury. 2007;38:1336–45. doi: 10.1016/j.injury.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Provencio JJ, Vora N. Subarachnoid hemorrhage and inflammation: bench to bedside and back. Semin Neurol. 2005;25:435–44. doi: 10.1055/s-2005-923537. [DOI] [PubMed] [Google Scholar]
- 10.Dhar R, Diringer MN. The burden of the systemic inflammatory response predicts vasospasm and outcome after subarachnoid hemorrhage. Neurocrit Care. 2008;8:404–12. doi: 10.1007/s12028-008-9054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshimoto Y, Tanaka Y, Hoya K. Acute systemic inflammatory response syndrome in subarachnoid hemorrhage. Stroke. 2001;32:1989–93. doi: 10.1161/hs0901.095646. [DOI] [PubMed] [Google Scholar]
- 12.Dumont AS, Dumont RJ, Chow MM, et al. Cerebral vasospasm after subarachnoid hemorrhage: putative role of inflammation. Neurosurgery. 2003;53:123–33. doi: 10.1227/01.neu.0000068863.37133.9e. discussion 33-5. [DOI] [PubMed] [Google Scholar]
- 13.Wartenberg KE, Schmidt JM, Temes RE, et al. Medical complications after subarachnoid hemorrhage: Frequency and impact on outcome. Stroke. 2005;36:521. [Google Scholar]
- 14.Park S, Kaffashi F, Loparo KA, Jacono FJ. The use of heart rate variability for the early detection of treatable complications after aneurysmal subarachnoid hemorrhage. Journal of Clinical Monitoring and Computing. 2013:1–9. doi: 10.1007/s10877-013-9467-0. [DOI] [PubMed] [Google Scholar]
- 15.Akbani R, Kwek S, Japkowicz N. Machine Learning: ECML 2004. Springer; 2004. Applying support vector machines to imbalanced datasets; pp. 39–50. [Google Scholar]
- 16.Bederson JB, Connolly ES, Jr, Batjer HH, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009;40:994–1025. doi: 10.1161/STROKEAHA.108.191395. [DOI] [PubMed] [Google Scholar]
- 17.Komotar RJ, Schmidt JM, Starke RM, et al. Resuscitation and critical care of poor-grade subarachnoid hemorrhage. Neurosurgery. 2009;64:397–411. doi: 10.1227/01.NEU.0000338946.42939.C7. [DOI] [PubMed] [Google Scholar]
- 18.Hamilton PS, Tompkins WJ. Quantitative investigation of QRS detection rules using the MIT/BIH arrhythmia database. Biomedical Engineering, IEEE Transactions on. 1986:1157–65. doi: 10.1109/tbme.1986.325695. [DOI] [PubMed] [Google Scholar]
- 19.Robin X, Turck N, Hainard A, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleiger RE, Stein PK, Bigger JT. Heart rate variability: measurement and clinical utility. Annals of Noninvasive Electrocardiology. 2005;10:88–101. doi: 10.1111/j.1542-474X.2005.10101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor JA, Carr DL, Myers CW, Eckberg DL. Mechanisms underlying very-low-frequency RR-interval oscillations in humans. Circulation. 1998;98:547–55. doi: 10.1161/01.cir.98.6.547. [DOI] [PubMed] [Google Scholar]
- 22.Campbell B, Sturani A, Reid J. Evidence of parasympathetic activity of the angiotensin converting enzyme inhibitor, captopril, in normotensive man. Clin Sci. 1985;68:49–56. doi: 10.1042/cs0680049. [DOI] [PubMed] [Google Scholar]
- 23.Stein PK, Schmieg RE, Jr, El-Fouly A, Domitrovich PP, Buchman TG. Association between heart rate variability recorded on postoperative day 1 and length of stay in abdominal aortic surgery patients. Critical Care Medicine. 2001;29:1738–43. doi: 10.1097/00003246-200109000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Electrophysiology TFotESoCtNASoP. Heart Rate Variability : Standards of Measurement, Physiological Interpretation, and Clinical Use. Circulation. 1996;93:1043–65. [PubMed] [Google Scholar]
- 25.Wartenberg KE, Schmidt JM, Claassen J, et al. Impact of medical complications on outcome after subarachnoid hemorrhage. Crit Care Med. 2006;34:617–23. doi: 10.1097/01.ccm.0000201903.46435.35. quiz 24. [DOI] [PubMed] [Google Scholar]
- 26.Rothoerl RD, Axmann C, Pina AL, Woertgen C, Brawanski A. Possible role of the C-reactive protein and white blood cell count in the pathogenesis of cerebral vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg Anesthesiol. 2006;18:68–72. doi: 10.1097/01.ana.0000181693.30750.af. [DOI] [PubMed] [Google Scholar]
- 27.Kubo Y, Ogasawara K, Kakino S, et al. Serum inflammatory adhesion molecules and high-sensitivity C-reactive protein correlates with delayed ischemic neurologic deficits after subarachnoid hemorrhage. Surg Neurol. 2008;69:592–6. doi: 10.1016/j.surneu.2008.02.014. discussion 6. [DOI] [PubMed] [Google Scholar]
- 28.Badjatia N, Carpenter A, Fernandez L, et al. Relationship between C-reactive protein, systemic oxygen consumption, and delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Stroke. 2011;42:2436–42. doi: 10.1161/STROKEAHA.111.614685. [DOI] [PubMed] [Google Scholar]