Abstract
We tested the hypothesis that attention, memory, and executive function are impaired to a greater extent in passively heat-stressed older adults than in passively heat-stressed younger adults. In a randomized, crossover design, 15 older (age: 69 ± 5 yr) and 14 younger (age: 30 ± 4 yr) healthy subjects underwent passive heat stress and time control trials. Cognitive tests (outcomes: accuracy and reaction time) from the CANTAB battery evaluated attention [rapid visual processing (RVP), choice reaction time (CRT)], memory [spatial span (SSP), pattern recognition memory (PRM)], and executive function [one touch stockings of Cambridge (OTS)]. Testing was undertaken on two occasions during each trial, at baseline and after internal temperature had increased by 1.0 ± 0.2°C or after a time control period. For tests that measured attention, reaction time during RVP and CRT was slower (P ≤ 0.01) in the older group. During heat stress, RVP reaction time improved (P < 0.01) in both groups. Heat stress had no effect (P ≥ 0.09) on RVP or CRT accuracy in either group. For tests that measured memory, accuracy on SSP and PRM was lower (P < 0.01) in the older group, but there was no effect of heat stress (P ≥ 0.14). For tests that measured executive function, overall, accuracy on OTS was lower, and reaction time was slower in the older group (P ≤ 0.05). Reaction time generally improved during heat stress, but there was no effect of heat stress on accuracy in either group. These data indicate that moderate increases in body temperature during passive heat stress do not differentially compromise cognitive function in younger and older adults.
Keywords: cognitive function, aging, hyperthermia, thermal comfort
adults over the age of ∼65 yr are at an increased risk of illness, injury, hospitalization, and death during heat waves (23, 29, 33–36, 63, 64). Age-related impairments in physiological responses to heat stress undoubtedly contribute to this increased risk (32). Heat-stressed older adults have attenuated increases in skin blood flow (30, 31) and sweating (26, 27), as well as impaired cardiovascular adjustments (42, 43). However, both physiological and psychological states influence health and safety (52, 53). Thus, a variety of factors may mediate the deleterious outcomes observed in the older population during heat waves.
Healthy aging is associated with a general cognitive decline (49). Aspects of memory (4, 10, 12), attention (1, 12, 40), executive functioning (12, 58, 60), and processing speed (59) are typically [but not always (37, 48)] impaired with advancing age. This chronological cognitive decline may contribute to the risk of deleterious outcomes during heat waves in older adults by, for instance, leading to poor decision-making. Interestingly, perhaps because of the deleterious impact of heat stress on cerebral blood flow (46, 61) and/or disruptions in cerebral functional connectivity (66), many cognitive processes are impaired in heat-stressed younger adults [e.g., aspects of attention (19, 22, 65), memory (6, 19, 28, 41, 54), and executive function (16, 17, 67)], although this is not always observed (3, 50, 61). If heat stress-induced impairments in cognitive function are amplified with age, this might suggest that the contribution of cognitive factors to the risk of morbidity and mortality during heat waves would be exacerbated in older adults. However, the combined effect of heat stress and age on cognitive function remains unknown. Therefore, the purpose of this study was to test the hypothesis that indices of attention, memory, and executive function during passive heat stress will be reduced to a greater extent in healthy older, compared with younger, adults. The testing of this hypothesis will help define the role of psychological factors potentially contributing to the increased risk of morbidity and mortality during heat waves in the older population.
METHODS
Subjects
Fifteen older and fourteen younger, healthy subjects participated in this study. Each subject was fully informed of the experimental procedures and possible risks before giving informed written consent. The protocol and consent were approved by the Institutional Review Boards at the University of Texas Southwestern Medical Center at Dallas and Texas Health Presbyterian Hospital of Dallas. The subject characteristics are presented in Table 1. All subjects were nonsmokers, free of any cardiac, metabolic, neurological, or psychological diseases, and had normal, or corrected to normal, vision. Subjects taking drugs were excluded, with the exception of multivitamins and, in the older subjects only, prescription drugs for hypertension and hypercholesteremia. Subjects were mostly right-handed, were of normal, or above normal, cognitive abilities for their age (57), and most identified themselves as being physically active.
Table 1.
Subject characteristics
| Younger | Older | |
|---|---|---|
| Number of subjects | 14 | 15 |
| Age, yr | 30 ± 4 | 69 ± 5* |
| Sex (male/female) | 6/8 | 5/10 |
| Height, m | 1.7 ± 0.2 | 1.7 ± 0.1 |
| Weight, kg | 74.5 ± 19.7 | 74.0 ± 14.6 |
| Body mass index, kg/m2 | 25.3 ± 5.4 | 26.3 ± 4.1 |
| Body surface area, m2 | 1.9 ± 0.3 | 1.8 ± 0.2 |
| Handedness (right/left) | 13/1 | 14/1 |
| Montreal Cognitive Assessment scorea | 29 ± 1 | 28 ± 1* |
| Corrected vision (yes/no) | 4/10 | 10/5 |
| Physical activity (high/moderate/low)b | 7/6/1 | 2/11/2 |
Values are expressed as means ± SD.
Significantly different from younger (P ≤ 0.016).
All subjects were within the normal range for their age group [younger: ≥26; older: ≥23; (52, 53)].
Stratified according to Craig et al. (9).
Subjects visited the laboratory on three occasions. Visit 1 was a familiarization trial that involved a health assessment, inclusive of vital signs (e.g., blood pressure and 12 lead ECG) and a complete health history, assessment of handedness (47), subjective levels of physical activity (9), and baseline cognitive ability (44). During this visit, subjects also completed the cognitive battery one time through, and they were given the opportunity to ask questions and repeat tests that they did not fully understand. This familiarized the subjects with the tests that they completed in the subsequent experimental trials. Visits 2 and 3 comprised the experimental trials, which are described in detail below. For these trials, subjects arrived at the laboratory euhydrated (confirmed via urine specific gravity: 1.014 ± 0.008) and having refrained from strenuous exercise, alcohol, and caffeine for a period of 24 h. For premenopausal females, the experimental trials were completed within 10 days following menstruation.
Instrumentation and Measurements
Approximately 90 min prior to experimental testing, each subject swallowed a telemetry pill (HQ, Palmetto, FL), which has been shown to provide a reliable measure of central body temperature (51). Two subjects had contraindications for taking the telemetry pill. In these subjects, rectal temperature was measured at a depth ∼10 cm past the anal sphincter using a general purpose thermocouple (Mon-a-therm, Mallinckrodt Medical, St. Louis, MO). Mean skin temperature was measured as the weighted average of six thermocouples attached to the skin. Body temperature was controlled via a water-perfused tube-lined suit (Med-Eng, Ottawa, ON, Canada) that covered the entire body except the head, hands, and feet. The internal-to-skin temperature gradient was calculated as the difference of internal and mean skin temperatures. Heart rate was continually recorded from an electrocardiogram (HP Patient Monitor, Agilent, Santa Clara, CA) interfaced with a cardiotachometer (CWE, Ardmore, PA). Blood pressure was measured intermittently via auscultation of the brachial artery by electrosphygmomanometry (Tango+, SunTech, Raleigh, NC), and mean arterial pressure was calculated as diastolic pressure plus 1/3 pulse pressure. Pretrial and posttrial nude body weight was also measured, providing an index of percent changes in body fluid loss.
Cognitive testing.
Subjects performed six different cognitive tests from the CANTAB Eclipse battery (Cambridge Cognition, Cambridge, UK). This computerized battery, which has multiple alternate forms and established norms, has been used in multiple research settings (11, 56). The chosen CANTAB tests assess aspects of attention, memory, executive function, and subjective perceptions and required ∼30–40 min to complete. Importantly, these tests have been used repeatedly in studies investigating cognitive changes in heat-stressed younger adults (16, 17, 19, 54). Subjects completed the battery of tests on five occasions: one time during visit 1 and two times during each of the two experimental sessions. During the experimental sessions, the order of the tests within a battery was randomized, and four different versions of each test were used. The order of these different versions was quasi-randomized, such that each subject completed all four versions. The test batteries were completed in a dimly lit, quiet, and temperature-controlled (average: 24 ± 1°C) laboratory. Tests were performed on a rapid-response (5 ms), 43.2-cm capacitance touchscreen monitor (One World Touch, Austin, TX) that was kept at a fixed distance from each subject's eyes across both test sessions (52 ± 6 cm). For tests of reaction time, a rapid-response (1 ms) press pad was used (Cambridge Cognition, Cambridge, UK). The tests comprising the cognitive testing battery are described as follows:
Rapid visual processing test (RVP) is a measure of sustained visual attention that required ∼7 min. Numbers from 2 to 9 were presented at a rate of 100 digits/min in the center of the screen in a pseudo-random order. Subjects were instructed to detect target sequences of digits (2–4–6, 3–5–7, and 4–6–8) and to register responses using the press pad. The test was delivered in two parts: a 2-min practice test stage that was not scored, and a 3-min test stage. The number of responses that occurred within 1,800 ms of the final digit presented for each of the target sequences was calculated. Outcome measures were the number of missed sequences (accuracy), mean latency (reaction time), and the number of false alarms (impulsivity).
Choice reaction time test (CRT) is a measure of attention and motor speed that requires ∼7 min. A right or left pointing arrow was displayed on the screen. Subjects were instructed to press the left button on the press pad if the arrow pointed left and the right button if arrow pointed right. Subjects were instructed to respond as quickly as possible. The direction of the arrows and the delay between the arrows were presented in a random order. The test was delivered in two parts: a 24-trial practice stage and two assessment stages that comprised 50 trials each. Outcome measures were mean latency (reaction time) and the percentage of correct responses (accuracy).
Pattern recognition memory test (PRM) assesses visual memory in ∼5 min. Subjects were presented with a series of 12 visual patterns one at a time every 3 s in the center of the screen. These patterns were designed so that they could not easily be given verbal labels. Following display of 12 patterns, subjects were required to choose between a pattern they had already seen and a novel pattern, the patterns were presented in reverse order. The subjects completed two 12-pattern sequences. The outcome measure was the percentage of correct responses (accuracy).
Spatial span test (SSP) is a measure of working memory that required ∼5 min. Subjects were presented with a screen in which white squares were shown. Some of these squares briefly changed color in a variable sequence. Subjects were instructed to touch the boxes that changed color in the same order in which they were displayed. The number of boxes increased from two at the start of the test to nine at the end. There were three possible attempts at each level. However, as soon as the subject successfully completed a sequence at each level, they progressed to the next level. If all three sequences were unsuccessfully completed, the test was terminated. Outcome measures were the longest sequence of successfully recalled boxes (accuracy) and the total number of errors (accuracy).
One touch stockings of Cambridge test (OTS) requires executive function, spatial planning, and working memory. The duration was 10–15 min. Subjects were presented with two displays containing three colored balls. The displays were presented such that they could be perceived as stacks of colored balls held in stockings suspended from a beam. Along the bottom of the screen there was a row of numbered boxes. Subjects were initially shown how to move the balls in the lower display to copy the pattern in the upper display. The experimenter completed one demonstration problem, where the solution required one move. Then the subject completed three further practice problems, one each of two, three, and four moves. For the test itself, subjects were shown further problems, and had to mentally calculate the minimum number of moves required to solve the problems, and then to touch the corresponding box at the bottom of the screen to indicate their response. Outcome measures were the number of problems solved on the first choice and mean choices to the correct choice (accuracy), as well as mean latency to first choice and mean latency to correct choice (response time). Data are presented for overall performance, as well as across two levels of complexity: simple: those requiring two moves, and complex: those requiring six moves, similar to that done previously (16, 17). For this latter analysis, within a testing session, each measure was obtained by averaging the score obtained over four trials.
Visual analog scales (VAS) assessed subjective indices of mood, calmness, and alertness. Duration was ∼2 min. Sixteen questions were answered using computerized VAS. These 16 questions were grouped according to Bond and Lader (2) (100-point scales) for measures of mood (0 = good, 100 = bad), calmness (0 = calm, 100 = excited), and alertness (0 = drowsy, 100 = alert).
Immediately prior to commencing and upon terminating each cognitive battery, thermal discomfort (4 point scale: 1 = comfortable, 4 = very uncomfortable) (14), thermal sensation (7 point scale: 1 = cold, 7 = hot) (14), and affect (11 point scale: −5 = feeling bad, +5 = feeling good) (24) were assessed on standardized scales. The duration was ∼30 s. To reflect average levels of these perceptions during the cognitive battery, these data are presented as a mean of perception levels before and after each cognitive testing battery.
Experimental Protocol (Visits 2 and 3)
Following instrumentation, subjects rested quietly in a semirecumbent position, while 34°C water perfused the suit. Following at least 30 min of quiet rest, baseline cognitive testing was completed, after which the subjects underwent either whole body passive heat stress or a time control period, both of which were ∼40–60 min in duration (average duration − heat stress: 51 ± 5 min, time control: 52 ± 11 min, no differences between groups: P ≥ 0.518). Whole body passive heat stress was induced by perfusing 48°C water through the suit, sufficient to increase internal temperature ∼1.0°C above baseline, while 34°C water was perfused through the suit during the time control trial. Immediately following the heating/time control period, the subjects completed another cognitive assessment. During the heat stress trial, the temperature of the water perfusing the suit was not adjusted, thereby ensuring uncompensable heat stress and allowing internal temperature to continue rising throughout cognitive testing. This was by design, as the achievement of heat balance, independent of the magnitude of the increase in body temperature, can restore cognitive functioning (22). Whole body cooling was commenced immediately following completion of heat-stressed cognitive testing. The time control trial was utilized to ensure there was no effect of time, independent of heat stress, which might confound the interpretation of the findings during the heat stress trial. Subjects were not allowed to drink fluids at any time during either trial. Both experimental trials were conducted in a randomized manner. There were at least 48 h between the two trials, and both trials were conducted at the same time of day.
Data and Statistical Analysis
Heart rate and thermal data were sampled continuously at 50 Hz via a data acquisition system (Biopac MP150, Santa Barbara, CA). Subject characteristics between groups were compared using independent sample t-tests. All other data were analyzed using mixed-model ANOVA with one between- (age) and two within- (trial, time) subject factors. These data were assessed for approximation to a normal distribution and sphericity, and no corrections were necessary. When the ANOVA revealed a significant F test, post hoc pair-wise comparisons were made incorporating a Bonferroni adjustment. Data were analyzed using SPSS Statistics (version 22; IBM, Armonk, NY) with a priori statistical significance set at P ≤ 0.05. All data are reported as means ± SD.
RESULTS
Thermal Changes
Physiological data immediately prior to cognitive testing at baseline (i.e., before heat stress or time control period), during heat stress, and following the time control period are presented in Table 2. Baseline internal and mean skin temperatures, heart rate, and mean arterial pressure were not different between groups and trials, the exception being that in the younger group baseline internal temperature was slightly, but significantly, lower (by −0.1 ± 0.3°C, P = 0.031) in the heat stress trial than in the time control trial. As expected, internal (by +1.0 ± 0.2°C, P < 0.001) and mean skin (by +4.0 ± 0.7°C, P < 0.001) temperatures, as well as heart rate (by +33 ± 12 bpm, P < 0.001) increased equally (P ≥ 0.504) between groups with heat stress, while mean arterial pressure was maintained in both groups (P ≥ 0.121). Mean skin temperature increased slightly (by +0.4 ± 0.6°C, P ≤ 0.021) during the time control period and was slightly lower (P = 0.006) in the older group at that time point. During the heat stress trial, body weight decreased (P < 0.001) in both groups, but the magnitude was greater (P = 0.003) in the younger group (Younger: −1.2 ± 1.0%, Older: −0.6 ± 0.8%). Body weight was unchanged in both groups during the time control trial (Younger: 0.0 ± 0.2%, Older: +0.1 ± 0.5%).
Table 2.
Physiological data immediately prior to commencing each cognitive test battery
| Heat Stress Trial |
Time Control Trial |
|||
|---|---|---|---|---|
| Younger | Older | Younger | Older | |
| Baseline | ||||
| Internal temperature, °C | 36.9 ± 0.2∝ | 37.0 ± 0.4 | 37.0 ± 0.2 | 37.0 ± 0.3 |
| Mean skin temperature, °C | 34.2 ± 0.4 | 33.9 ± 0.5 | 34.1 ± 0.2 | 33.8 ± 0.4 |
| Internal-to-skin temperature gradient, °C | 2.6 ± 0.4 | 3.1 ± 0.5* | 2.9 ± 0.9 | 3.2 ± 0.6 |
| Heart rate, bpm | 67 ± 10 | 68 ± 9 | 67 ± 11 | 69 ± 14 |
| Mean arterial pressure, mmHg | 86 ± 12 | 93 ± 10 | 87 ± 14 | 94 ± 9 |
| Heat/Control | ||||
| Internal temperature, °C | 38.0 ± 0.2†∝ | 37.9 ± 0.3†∝ | 37.1 ± 0.2 | 37.0 ± 0.3 |
| Mean skin temperature, °C | 38.3 ± 0.6†∝ | 38.4 ± 0.6†∝ | 34.6 ± 0.3† | 34.2 ± 0.4†* |
| Internal-to-skin temperature gradient, °C | −0.3 ± 0.6†∝ | −0.4 ± 0.7†∝ | 2.5 ± 0.4 | 2.8 ± 0.5† |
| Heart rate, bpm | 100 ± 12†∝ | 100 ± 16†∝ | 66 ± 13 | 67 ± 9 |
| Mean arterial pressure, mmHg | 80 ± 12 | 91 ± 11 | 86 ± 13 | 98 ± 10 |
Values are expressed as means ± SD.
Significantly different from younger within trial at same time point (P ≤ 0.022).
Significantly different from baseline within trial and age group (P ≤ 0.021).
Significantly different from time control trial at same time point (P ≤ 0.033).
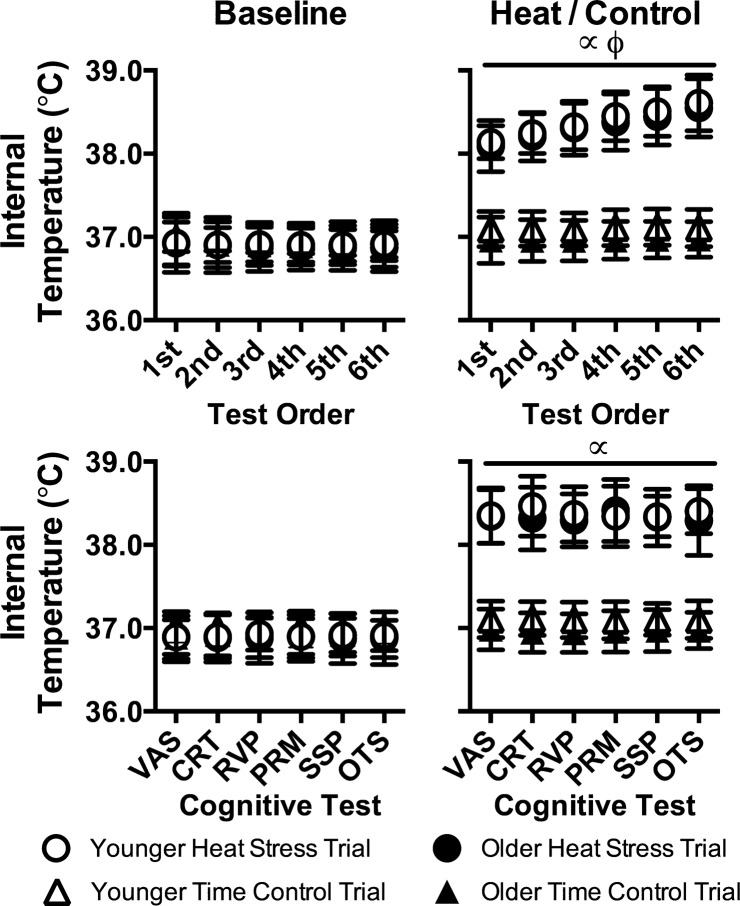
Internal temperature throughout cognitive testing is presented in Fig. 1. With the exception of heat stress cognitive testing, during which internal temperature was higher (P < 0.001), internal temperature was stable throughout cognitive testing (P ≥ 0.215) and did not differ between groups (P ≥ 0.437) or trials (P ≥ 0.236). By design, however, internal temperature continued to rise over time (P < 0.001) during heat stress cognitive testing, the magnitude of which was not different between groups (Younger: +0.6 ± 0.3°C, Older: +0.6 ± 0.2°C, P = 0.732). Conversely, because of randomization, internal temperature during each of the six cognitive tests was not different (P ≥ 0.808) during heat stress. At baseline, mean skin temperatures were not different (P = 0.945), but increased (P < 0.001) slightly over time during cognitive testing (by +0.5 ± 1.2°C), which was not different between groups (P = 0.116). During heat stress, mean skin temperature was higher (P < 0.001) during cognitive testing compared with following the time control period, and during cognitive testing, mean skin temperatures remained stable during both trials (average changes for Control: 0.0 ± 0.5°C, Heat stress: +0.2 ± 0.3°C) with no differences between groups (P ≥ 0.116). Mean skin temperature did not differ (P ≥ 0.256) during each test within a given cognitive battery. At baseline, heart rate was stable throughout cognitive testing (P = 0.695) and did not differ between groups (P = 0.444) or trials (P = 0.240). Likely because of further increases in internal temperature, during heat stress, heart rate rose (by 8 ± 8 bpm) throughout cognitive testing (P < 0.001), and it was higher (P < 0.001) compared with following the time control period, but it did not differ between groups (P = 0.785).
Fig. 1.
Internal temperature during cognitive testing in younger (n = 14) and older (n = 15) subjects during heat stress and time control trials at baseline (left) and during heat stress (i.e., heat) and following a time control period (i.e., control; right). Top: temporal changes in internal temperature across the six cognitive tests independent of the test being undertaken. Bottom: internal temperature during each cognitive test. Data are presented as means ± SD. VAS, visual analog scales; CRT, choice reaction time test; RVP, rapid visual processing test; PRM, pattern recognition memory test, SSP, spatial span test; OTS, one touch stockings of Cambridge test. ∝Significantly different from time control trial (P < 0.001). ϕSignificant increase over time (P < 0.001).
Cognitive Function and Perceptual Indices
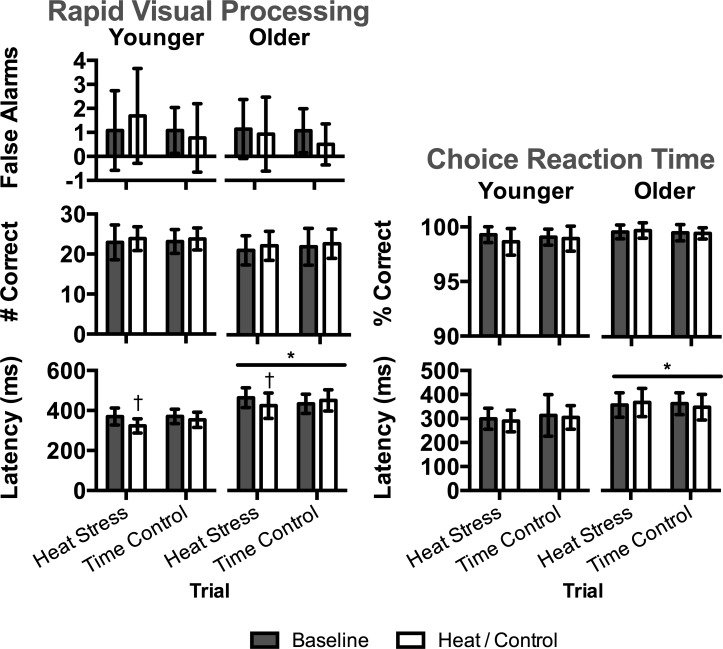
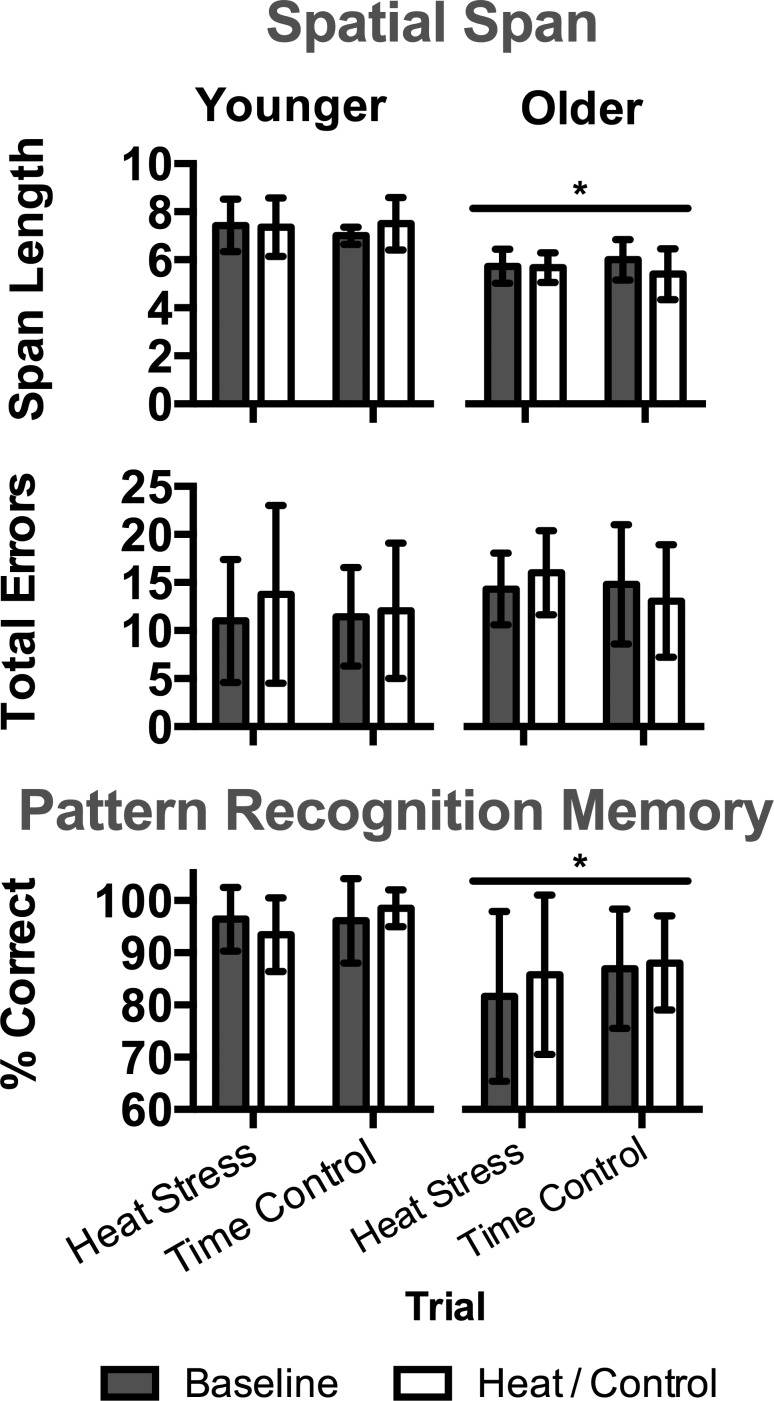
Performance on the computerized tests evaluating aspects of attention (RVP, CRT) is presented in Fig. 2. As expected, reaction time during RVP and CRT was slower (P ≤ 0.003) in the older group. During heat stress, reaction time improved from baseline (P ≤ 0.001) in both groups during RVP, and heat stress had no effect (P ≥ 0.094) on RVP or CRT accuracy in either group. Performance on tests evaluating aspects of memory (PRM, SSP) is presented in Fig. 3. Overall accuracy on SSP and PRM was lower (P < 0.001) in the older group compared with the younger group, but there was no effect of heat stress (P ≥ 0.142). Performance on OTS, evaluating aspects of executive function, is presented in Table 3. Overall, accuracy was lower, and reaction time was slower in the older group compared with the younger group (P ≤ 0.050). Reaction time generally improved during heat stress, but there was no effect of heat stress on aspects of accuracy (P ≥ 0.218). OTS performance on simple (those requiring two moves) and complex (those requiring six moves) tasks were identical to that observed for the overall performance (those requiring 1–6 moves, data not shown).
Fig. 2.
Performance on tests evaluating aspects of attention in younger (n = 14) and older (n = 15) subjects during heat stress and time control trials at baseline and during heat stress (i.e., heat) and following a time control period (i.e., control). Left: rapid visual processing test (RVP). Right: choice reaction time test (CRT). Data are presented as means ± SD. *Significantly different from younger within trial(s) at same time point(s) (P ≤ 0.003). †Significantly different from baseline within trial and age group (P ≤ 0.001).
Fig. 3.
Performance on tests evaluating aspects of memory in younger (n = 14) and older (n = 15) subjects during heat stress and time control trials at baseline and during heat stress (i.e., heat) and following a time control period (i.e., control). Top: spatial span test (SSP). Bottom: pattern recognition memory test (PRM). Data are presented as means ± SD. *Significantly different from younger within trial(s) at same time point(s) (P < 0.001).
Table 3.
One touch stockings of Cambridge performance
| Heat Stress Trial |
Time Control Trial |
|||
|---|---|---|---|---|
| Younger | Older | Younger | Older | |
| Overall (1–6 moves) |
||||
| Baseline | ||||
| Problems solved on 1st choice | 21.1 ± 2.2 | 18.5 ± 3.3* | 21.2 ± 2.6 | 19.3 ± 2.7 |
| Choices to correct answer | 1.1 ± 0.1 | 1.3 ± 0.2* | 1.2 ± 0.2 | 1.3 ± 0.3 |
| Latency to 1st choice, s | 14.4 ± 4.6 | 24.2 ± 10.1* | 15.3 ± 6.3 | 25.1 ± 8.5* |
| Latency to correct, s | 17.2 ± 6.6 | 31.3 ± 12.5* | 19.5 ± 14.8 | 33.9 ± 13.6* |
| Heat/control | ||||
| Problems solved on 1st choice | 20.9 ± 2.5 | 17.9 ± 3.2* | 21.3 ± 2.1 | 19.4 ± 2.5* |
| Choices to correct answer | 1.2 ± 0.2 | 1.4 ± 0.3*∝ | 1.1 ± 0.1 | 1.3 ± 0.2* |
| Latency to 1st choice, s | 10.4 ± 3.2† | 18.0 ± 7.6*∝ | 13.4 ± 5.0† | 21.6 ± 9.2*† |
| Latency to correct, s | 12.1 ± 3.6† | 24.0 ± 11.3*† | 16.5 ± 8.7 | 27.3 ± 13.0*† |
Values are expressed as means ± SD.
Significantly different from younger within trial at the same time point (P ≤ 0.050).
Significantly different from baseline within trial and age group (P ≤ 0.049).
Significantly different from time control trial at same time point (P ≤ 0.049).
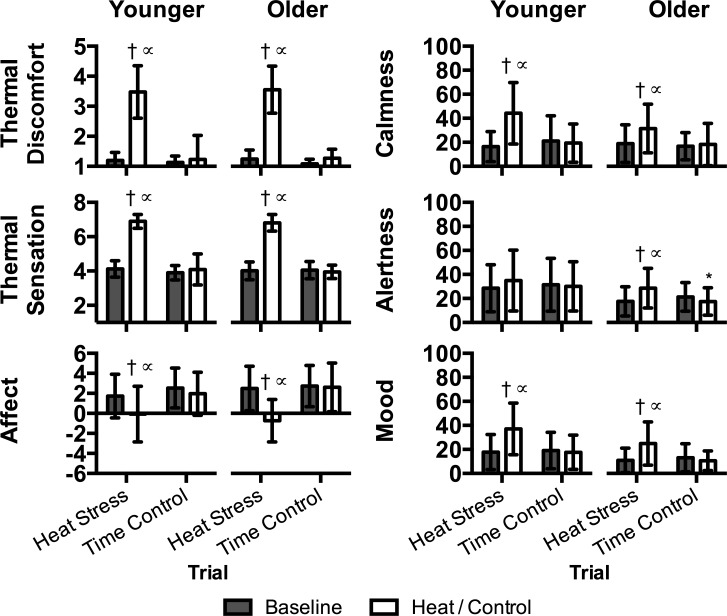
Perceptual indices are presented in Fig. 4. Heat stress increased (P ≤ 0.022) thermal discomfort, sensations of warmth, reduced affect, worsened mood, and increased excitement, and there were no differences between groups (P ≥ 0.148). Heat stress was associated with increased reporting of alertness, but only in the older group (P = 0.028).
Fig. 4.
Perceptual indices in younger (n = 14) and older (n = 15) subjects during heat stress and time control trials at baseline and during heat stress (i.e., heat) and following a time control period (i.e., control). Data are presented as means ± SD. *Significantly different from younger within trial at same time point (P = 0.049). †Significantly different from baseline within trial and age group (P ≤ 0.028). ∝Signifcantly different from time control trial at same time point (P ≤ 0.027).
DISCUSSION
This study tested the hypothesis that passive heat stress, sufficient to increase internal temperature 1.0–1.6°C (Table 2, Fig. 1) and profoundly narrow the internal-to-skin temperature gradient (Table 2), impairs cognitive function to a greater extent in older adults. In contrast to this hypothesis, accuracy on computerized tests evaluating aspects of attention (Fig. 2), memory (Fig. 3), and executive function (Table 3) was found to be unaffected by passive heat stress in both younger and older subjects. As expected, age-related differences in processing speed (Fig. 2, Table 3), memory (Fig. 3), and executive function (Table 3) were apparent, independent of increases in body temperature. These data suggest that, independent of age, moderate passive heat stress does not impair many aspects of cognitive function, as measured by the tests used herein.
Elevations in Body Temperature and Cognitive Function
Various aspects of attention (19, 22, 65), memory (6, 19, 28, 41, 54), and executive function (16, 17, 67) are reduced in heat-stressed younger adults. Therefore, our findings that passive heat stress did not affect the measured indices of cognitive function are unexpected (Figs. 2 and 3, Table 3). This finding is even more surprising given that performance on these exact tests was reduced in heat-stressed younger adults utilizing a similar sample size (range: 8–18 subjects), e.g., OTS (16, 17), RVP (19), PRM (19, 54), and SSP (19, 54). Thus, we are confident that our findings are not due to a lack of sensitivity, as has been speculated as a confounding factor in other similar studies (e.g., 3, 61).
It is noteworthy that in the aforementioned studies, subjects were exposed to extremely hot environments (range: 44–50°C, 30–50% RH) for 20–120 min, in which internal temperature either did not change (16, 17) or increased to a similar extent as that in the current study (∼1.5°C) (16, 19, 54). As a result, a rationale for our divergent findings is likely the water-perfused suit method with which we induced moderate heat stress. This method was chosen given that it allows tight control of the magnitude of increases in body temperature during heat stress between groups, which allowed us to independently evaluate the effect of increases in body temperature on aspects of perception and cognitive function. This passive heat stress method resulted in increases in thermal discomfort, sensations of warmth, reductions in mood and affect (Fig. 4), and general enhancements in processing speed (reaction time) (Fig. 2, Table 3). However, it did not affect any measure of cognitive function accuracy (Figs. 2 and 3, Table 3). The rationale underlying the current observations remains unknown, especially in light of recent findings, indicating that negative affect and thermal discomfort associated with heat stress impairs aspects of executive function (specifically, OTS) (16, 19, 54). It should be noted that these decrements in aspects of executive function were observed during heat stress that induced dynamic increases in skin temperature (17), whereas mean skin temperature remained stable during the cognitive tests in the current study. As such, it may be that the potential deleterious cognitive impact of negative affect and discomfort is constrained to instances in which only skin temperature is increasing (17), as opposed to when skin temperature is stable and only internal temperature is rising (Fig. 1).
Impact of Age
Healthy aging is generally associated with reductions in aspects of memory (e.g., 17), attention (4, 10, 12), executive functioning (1, 12, 40), and processing speed (12, 58, 60). The current findings generally support this, as accuracy on the measured indices of memory (Fig. 3) and executive functioning (Table 3) were lower, and processing speed was slower (Fig. 2, Table 3) in the older group. Such findings suggest the current tests, in combination with the moderate sample size, were sensitive to cognitive differences typically associated with aging. Importantly, however, a novel aspect of this study is that age-related differences were not exacerbated with moderate increases in body temperature in the older group (Figs. 2 and 3, Table 3). Thus, the measured aspects of cognitive function were unaffected by passive heat stress in both younger and older subjects.
Aging heightens the cerebral neuronal demands of a cognitive task, and when the demand is greater than the neuronal resources available, task accuracy and reaction time are diminished (59). Interestingly, the deleterious effects of heat stress on prior assessments of cognitive function appear to be dictated by a similar mechanism, such that heat stress increases the neuronal demand of a cognitive task (39, 55) and disrupts cerebral functional connectivity (18, 25, 38). Given that passive heat stress did not affect accuracy on any of the measured aspects of cognitive function in either group, but that age-related differences persisted (Figs. 2 and 3, Table 3), it can be speculated that the neuronal demand of the cognitive tasks in the current paradigm was unaffected by the passive heat stress method utilized in this study.
The ability to perceive warm and cool temperatures is generally reduced with age (66). Furthermore, a given reduction in internal temperature elicits less thermal discomfort in older, compared with younger, adults (21). The current findings indicate that moderate passive heat stress is perceived as similarly warm and uncomfortable in both younger and older subjects (Fig. 4). Such findings were particularly surprising given that discomfort dictates the decision to initiate adaptive behavioral responses during thermal stress (e.g., change the temperature in a room, etc.) (13) and that the decision to initiate such behavior is incumbent upon greater changes in body temperature in older adults (62). Thus, it may be that older adults are less sensitive to reductions in body temperature, but sensitivity to increases in body temperature is well maintained. However, direct evidence for such an arrangement is required.
Other Considerations
Because of sweat production, dehydration typically accompanies heat stress and subsequent increases in body temperature (8, 45, 68). Notably, mild dehydration (i.e., ≤2% body weight loss) is usually associated with impairments in various aspects of cognitive function, including aspects of attention (5), memory (7, 15, 20), and executive function (6, 7, 20). Although this study was not designed to evaluate hydration status as a modulator of cognitive function, it is notable that body weight decreased on average less than 2% in the younger and older subjects. Given that the measured aspects of cognitive function were unchanged during heat stress, which induced mild dehydration (Figs. 2 and 3, Table 3), such findings suggest that mild dehydration has little impact on attention, memory, and executive function during passive heat stress as measured herein. Interestingly, given that the magnitude of dehydration was less in the older subjects (−1.2% vs. −0.6), it remains unknown whether dehydration during passive heat stress equally impacts cognitive function in both younger and older adults.
Perspectives and Significance
Older adults are at an increased risk of morbidity and mortality during heat waves (15, 20). Impaired physiological responses to heat stress likely contribute to this increased risk (23, 29, 33–36, 63, 64). However, given that both physiological and psychological responses dictate health and safety (32), psychological factors may also modulate the increased risk of deleterious outcomes during heat waves in the older population. The current study indicates that changes in aspects of cognitive function are not exacerbated with advancing age during heat stress. That said, during passive heat stress, classic age-related differences in aspects of cognitive function persisted. Such findings do not discount a potential cognitive contribution to the increased risk of morbidity and mortality during heat waves in the older population, but rather suggest that the cognitive contribution is not exacerbated by moderate increases in body temperature. It should also be noted that while the computerized tests used in the present study have been shown to be sensitive to abnormal cognitive function and did show the expected age-related differences, it remains to be seen whether other neuropsychological measures might prove sensitive to the hypothesized effects of heat stress. Clearly, further studies are required to understand the multifaceted nature of this risk. Such information is important, as understanding such risk will allow for the development of interventions and countermeasures aimed at protecting the older population during heat waves.
Conclusions
The present study indicates that moderate increases in body temperature and a narrowing of the internal-to-skin temperature gradient during passive heat stress do not compromise the assessed aspects of attention, memory, or executive function in younger or older adults. That said, the expected age-related differences in cognitive performance were apparent independent of changes in body temperature. The present findings also indicate that both older and younger adults generally perceive increases in body temperature similarly.
GRANTS
Awards from the National Institutes of Health (Grants F32AG04328 and HL61388) supported this study.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: Z.J.S., C.M.C., and C.G.C. conception and design of research; Z.J.S., D.G., A.A., and E.R. performed experiments; Z.J.S. and A.A. analyzed data; Z.J.S., D.G., C.M.C., and C.G.C. interpreted results of experiments; Z.J.S. prepared figures; Z.J.S. drafted manuscript; Z.J.S., D.G., A.A., E.R., C.M.C., and C.G.C. edited and revised manuscript; Z.J.S., D.G., A.A., E.R., C.M.C., and C.G.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the subjects for participating in our study. We would also like to thank registered nurses Jena Kern and Naomi Kennedy for their technical assistance.
REFERENCES
- 1.Anderson ND, Craik FI, Naveh-Benjamin M. The attentional demands of encoding and retrieval in younger and older adults. 1. Evidence from divided attention costs. Psychol Aging 13: 405–423, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Bond A, Lader M. The use of analogue scales in rating subjective feelings. Br J Med Psychol 47: 211–218, 1974. [Google Scholar]
- 3.Caldwell JN, Patterson MJ, Taylor NA. Exertional thermal strain, protective clothing and auxiliary cooling in dry heat: evidence for physiological but not cognitive impairment. Eur J Appl Physiol 112: 3597–3606, 2012. [DOI] [PubMed] [Google Scholar]
- 4.Cherry KE, Park DC. Individual difference and contextual variables influence spatial memory in younger and older adults. Psychol Aging 8: 517, 1993. [DOI] [PubMed] [Google Scholar]
- 5.Cheuvront SN, Kenefick RW. Dehydration: physiology, assessment, and performance effects. Compr Physiol 4: 457–485, 2014. [DOI] [PubMed] [Google Scholar]
- 6.Cian C, Barraud PA, Melin B, Raphel C. Effects of fluid ingestion on cognitive function after heat stress or exercise-induced dehydration. Int J Psychophysiol 42: 243–251, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Cian C, Koulmann N, Barraud PA, Raphel C, Jimenez C, Melin B. Influence of variations in body hydration on cognitive function: Effect of hyperhydration, heat stress, and exercise-induced dehydration. J Psychophysiol 14: 29–36, 2000. [Google Scholar]
- 8.Collins KJ, Extonsmith AN, Dore C. Urban hypothermia—preferred temperature and thermal perception in old age. Br Med J 282: 175–177, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 35: 1381–1395, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Craik FIM, Mcdowd JM. Age-differences in recall and recognition. J Exp Psychol Learn 13: 474–479, 1987. [Google Scholar]
- 11.De Luca CR, Wood SJ, Anderson V, Buchanan JA, Proffitt TM, Mahony K, Pantelis C. Normative data from the CANTAB. I. Development of executive function over the lifespan. J Clin Exp Neuropsychol 25: 242–254, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Drag LL, Bieliauskas LA. Contemporary review 2009: cognitive aging. J Geriatr Psychiatry Neurol 23: 75–93, 2010. [DOI] [PubMed] [Google Scholar]
- 13.Frank SM, Raja SN, Bulcao C, Goldstein DS. Age-related thermoregulatory differences during core cooling in humans. Am J Physiol Regul Integr Comp Physiol 279: R349–R354, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Gagge AP, Stolwijk JA, Hardy JD. Comfort and thermal sensations and associated physiological responses at various ambient temperatures. Environ Res 1: 1–20, 1967. [DOI] [PubMed] [Google Scholar]
- 15.Ganio MS, Armstrong LE, Casa DJ, McDermott BP, Lee EC, Yamamoto LM, Marzano S, Lopez RM, Jimenez L, Le Bellego L, Chevillotte E, Lieberman HR. Mild dehydration impairs cognitive performance and mood of men. Br J Nutr 106: 1535–1543, 2011. [DOI] [PubMed] [Google Scholar]
- 16.Gaoua N, Grantham J, El Massioui F, Girard O, Racinais S. Cognitive decrements do not follow neuromuscular alterations during passive heat exposure. Int J Hyperthermia 27: 10–19, 2011. [DOI] [PubMed] [Google Scholar]
- 17.Gaoua N, Grantham J, Racinais S, El Massioui F. Sensory displeasure reduces complex cognitive performance in the heat. J Environ Psychol 32: 158–163, 2012. [Google Scholar]
- 18.Gaoua N, Herrera CP, Racinais S, El Massioui F. Heat induces an overload during complex cognitive performance. Med Sci Sport Exer 44: S230, 2012. [Google Scholar]
- 19.Gaoua N, Racinais S, Grantham J, El Massioui F. Alterations in cognitive performance during passive hyperthermia are task dependent. Int J Hyperthermia 27: 1–9, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gopinathan PM, Pichan G, Sharma VM. Role of dehydration in heat stress-induced variations in mental performance. Arch Environ Health 43: 15–17, 1988. [DOI] [PubMed] [Google Scholar]
- 21.Guergova S, Dufour A. Thermal sensitivity in the elderly: a review. Ageing Res Rev 10: 80–92, 2011. [DOI] [PubMed] [Google Scholar]
- 22.Hancock PA. Sustained attention under thermal stress. Psychol Bull 99: 263–281, 1986. [PubMed] [Google Scholar]
- 23.Hansen AL, Bi P, Nitschke M, Ryan P, Pisaniello D, Tucker G. The effect of heatwaves on mental health in a temperate Australian city. Epidemiology 19: S85, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardy CJ, Rejeski WJ. Not what, but how one feels: The measurement of affect during exercise. J Sport Exerc Psychol 11: 304–317, 1989. [Google Scholar]
- 25.Hocking C, Silberstein RB, Lau WM, Stough C, Roberts W. Evaluation of cognitive performance in the heat by functional brain imaging and psychometric testing. Comp Biochem Physiol A 128: 719–734, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Inoue Y, Havenith G, Kenney WL, Loomis JL, Buskirk ER. Exercise-and methylcholine-induced sweating responses in older and younger men: effect of heat acclimation and aerobic fitness. Int J Biometeorol 42: 210–216, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Inoue Y, Shibasaki M, Ueda H, Ishizashi H. Mechanisms underlying the age-related decrement in the human sweating response. Eur J Appl Physiol Occup Physiol 79: 121–126, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Jiang Q, Yang X, Liu K, Li B, Li L, Li M, Qian S, Zhao L, Zhou Z, Sun G. Hyperthermia impaired human visual short-term memory: An fMRI study. Int J Hyperthermia 29: 219–224, 2013. [DOI] [PubMed] [Google Scholar]
- 29.Johnson H, Kovats RS, McGregor G, Stedman J, Gibbs M, Walton H, Cook L, Black E. The impact of the 2003 heat wave on mortality and hospital admissions in England. Health Stat Q 6–11, 2005. [PubMed] [Google Scholar]
- 30.Kenney WL. Control of heat-induced cutaneous vasodilatation in relation to age. Eur J Appl Physiol 57: 120–125, 1988. [DOI] [PubMed] [Google Scholar]
- 31.Kenney WL. Decreased cutaneous vasodilation in aged skin: mechanisms, consequences and interventions. J Therm Biol 26: 263–271, 2001. [Google Scholar]
- 32.Kenney WL, Craighead DH, Alexander LM. Heat waves, aging and human cardiovascular health. Med Sci Sport Exer 46: 1891–1899, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kenny GP, Yardley J, Brown C, Sigal RJ, Jay O. Heat stress in older individuals and patients with common chronic diseases. CMAJ 182: 1053–1060, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim Y, Kim H, Shin SD, Hong YC. Different influence of outdoor temperature on traumatic and nontraumatic injuries. J Trauma Acute Care Surg 73: 944–949, 2012. [DOI] [PubMed] [Google Scholar]
- 35.Knowlton K, Rotkin-Ellman M, King G, Margolis HG, Smith D, Solomon G, Trent R, English P. The 2006 California heat wave: impacts on hospitalizations and emergency department visits. Environ Health Perspect 117: 61, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kovats RS, Hajat S. Heat stress and public health: a critical review. Annu Rev Public Health 29: 41–55, 2008. [DOI] [PubMed] [Google Scholar]
- 37.Light LL, Albertson SA. Direct and indirect tests of memory for category exemplars in young and older adults. Psychol Aging 4: 487–492, 1989. [DOI] [PubMed] [Google Scholar]
- 38.Liu K, Sun G, Li B, Jiang Q, Yang X, Li M, Li L, Qian S, Zhao L, Zhou Z. The impact of passive hyperthermia on human attention networks: An fMRI study. Behav Brain Res 243: 220–230, 2013. [DOI] [PubMed] [Google Scholar]
- 39.Mattay VS, Fera F, Tessitore A, Hariri AR, Berman KF, Das S, Meyer-Lindenberg A, Goldberg TE, Callicott JH, Weinberger DR. Neurophysiological correlates of age-related changes in working memory capacity. Neurosci Lett 392: 32–37, 2006. [DOI] [PubMed] [Google Scholar]
- 40.McDowd JM, Craik FI. Effects of aging and task difficulty on divided attention performance. J Exp Psychol Hum Percept Perform 14: 267–280, 1988. [DOI] [PubMed] [Google Scholar]
- 41.McMorris T, Swain J, Smith M, Corbett J, Delves S, Sale C, Harris RC, Potter J. Heat stress, plasma concentrations of adrenaline, noradrenaline, 5-hydroxytryptamine and cortisol, mood state and cognitive performance. Int J Psychophysiol 61: 204–215, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Minson CT, Wladkowski SL, Cardell AF, Pawelczyk JA, Kenney WL. Age alters the cardiovascular response to direct passive heating. J Appl Physiol 84: 1323–1332, 1998. [DOI] [PubMed] [Google Scholar]
- 43.Minson CT, Wladkowski SL, Pawelczyk JA, Kenney WL. Age, splanchnic vasoconstriction, and heat stress during tilting. Am J Physiol Regul Integr Comp Physiol 276: R203–R212, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53: 695–699, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Natsume K, Ogawa T, Sugenoya J, Ohnishi N, Imai K. Preferred ambient temperature for old and young men in summer and winter. Int J Biometeorol 36: 1–4, 1992. [DOI] [PubMed] [Google Scholar]
- 46.Ogoh S, Sato K, Okazaki K, Miyamoto T, Hirasawa A, Morimoto K, Shibasaki M. Blood flow distribution during heat stress: cerebral and systemic blood flow. J Cereb Blood Flow Metab 33: 1915–1920, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113, 1971. [DOI] [PubMed] [Google Scholar]
- 48.Park DC, Puglisi JT, Smith AD. Memory for pictures: does an age-related decline exist? Psychol Aging 1: 11–17, 1986. [DOI] [PubMed] [Google Scholar]
- 49.Park DC, Schwarz editors N. Aging and Cognition: A Primer. Philadelphia, PA: Psychology Press, 2000. [Google Scholar]
- 50.Parker SM, Erin JR, Pryor RR, Khorana P, Suyama J, Guyette FX, Reis SE, Hostler D. The effect of prolonged light intensity exercise in the heat on executive function. Wilderness Environ Med 24: 203–210, 2013. [DOI] [PubMed] [Google Scholar]
- 51.Pearson J, Ganio MS, Seifert T, Overgaard M, Secher NH, Crandall CG. Pulmonary artery and intestinal temperatures during heat stress and cooling. Med Sci Sports Exerc 44: 857–862, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Porter BE, Bliss JP, Sleet DA. Human factors in injury control. Am J Lifestyle Med 4: 90–97, 2010. [Google Scholar]
- 53.Prince M, Patel V, Saxena S, Maj M, Maselko J, Phillips MR, Rahman A. No health without mental health. Lancet 370: 859–877, 2007. [DOI] [PubMed] [Google Scholar]
- 54.Racinais S, Gaoua N, Grantham J. Hyperthermia impairs short-term memory and peripheral motor drive transmission. J Physiol 586: 4751–4762, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reuter-Lorenz PA, Cappell KA. Neurocognitive aging and the compensation hypothesis. Curr Dir Psychol Sci 17: 177–182, 2008. [Google Scholar]
- 56.Robbins TW, James M, Owen AM, Sahakian BJ, McInnes L, Rabbitt P. Cambridge Neuropsychological Test Automated Battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia 5: 266–281, 1994. [DOI] [PubMed] [Google Scholar]
- 57.Rossetti HC, Lacritz LH, Cullum CM, Weiner MF. Normative data for the Montreal Cognitive Assessment (MoCA) in a population-based sample. Neurology 77: 1272–1275, 2012. [DOI] [PubMed] [Google Scholar]
- 58.Salthouse TA. Mediation of adult age differences in cognition by reductions in working memory and speed of processing. Psychol Sci 2: 179–183, 1991. [Google Scholar]
- 59.Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev 103: 403–428, 1996. [DOI] [PubMed] [Google Scholar]
- 60.Salthouse TA, Babcock RL. Decomposing adult age differences in working memory. Dev Psychol 27: 763–776, 1991. [Google Scholar]
- 61.Schlader ZJ, Lucas RA, Pearson J, Crandall CG. Hyperthermia does not alter the increase in cerebral perfusion during cognitive activation. Exp Physiol 98: 1597–1607, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schlader ZJ, Simmons SE, Stannard SR, Mundel T. The independent roles of temperature and thermal perception in the control of human thermoregulatory behavior. Physiol Behav 103: 217–224, 2011. [DOI] [PubMed] [Google Scholar]
- 63.Semenza JC, McCullough JE, Flanders WD, McGeehin MA, Lumpkin JR. Excess hospital admissions during the July 1995 heat wave in Chicago [see comments]. Am J Prev Med 16: 269–277, 1999. [DOI] [PubMed] [Google Scholar]
- 64.Semenza JC, Rubin CH, Falter KH, Selanikio JD, Flanders WD, Howe HL, Wilhelm JL. Heat-related deaths during the July 1995 heat wave in Chicago. N Engl J Med 335: 84–90, 1996. [DOI] [PubMed] [Google Scholar]
- 65.Simmons SE, Saxby BK, McGlone FP, Jones DA. The effect of passive heating and head cooling on perception, cardiovascular function and cognitive performance in the heat. Eur J Appl Physiol 104: 271–280, 2008. [DOI] [PubMed] [Google Scholar]
- 66.Sun G, Qian S, Jiang Q, Liu K, Li B, Li M, Zhao L, Zhou Z, von Deneen KM, Liu Y. Hyperthermia-induced disruption of functional connectivity in the human brain network. PLos One 8: e61157, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sun G, Yang X, Jiang Q, Liu K, Li B, Li L, Zhao L, Li M. Hyperthermia impairs the executive function using the Attention Network Test. Int J Hyperthermia 28: 621–626, 2012. [DOI] [PubMed] [Google Scholar]
- 68.Taylor NAS, Allsopp NK, Parkes DG. Preferred room-temperature of young vs aged males—the influence of thermal sensation, thermal comfort, and affect. J Gerontol A Biol Sci Med Sci 50: M216–M221, 1995. [DOI] [PubMed] [Google Scholar]