Abstract
Relationships between structural and functional variables in asthmatic lungs at local and global (or lobar) levels remain to be discovered. This study aims to investigate local alterations of structural variables [bifurcation angle, circularity, airway wall thickness (WT), and hydraulic diameter (Dh)] in asthmatic subjects, and their correlations with other imaging and pulmonary function test-based global and lobar metrics, including lung shape, air-trapping, regional volume change, and more. Sixty-one healthy subjects, and 67 nonsevere and 67 severe asthmatic subjects were studied. The structural variables were derived from computed tomography images at total lung capacity (TLC). Air-trapping was measured at functional residual capacity, and regional volume change (derived from image registration) was measured between functional residual capacity and TLC. The tracheal diameter and WT predicted by 61 healthy subjects were used to normalize the Dh and WT. New normalization schemes allowed for the dissociation of luminal narrowing and wall thickening effects. In severe asthmatic subjects, the alteration of bifurcation angle was found to be correlated with a global lung shape at TLC, and circularity was significantly decreased in the right main bronchus. While normalized WT increased especially in the upper lobes of severe asthmatic subjects, normalized Dh decreased in the lower lobes. Among local structural variables, normalized Dh was the most representative variable, because it was significantly correlated with alterations of functional variables, including pulmonary function test's data. In conclusion, understanding multiscale phenomena may help to provide guidance in the search for potential imaging-based phenotypes for the development and outcomes assessment of therapeutic intervention.
Keywords: quantitative computed tomography, bifurcation angle, airway circularity, wall thickness, hydraulic diameter
asthma can be characterized by several correlated phenotypes, including airflow obstruction, bronchial hyperresponsiveness, and airway inflammation (8). The hyperresponsiveness and chronic inflammation of airways cause the infiltration of inflammatory cells to smooth muscles and the increase of smooth muscle mass, leading to airway remodeling with thickened wall (and/or narrowed lumen) and subsequent acute or chronic airway obstruction (9, 19, 21). The National Institutes of Health-sponsored multicenter Severe Asthma Research Program (SARP) (15, 20, 28, 29, 34) has acquired quantitative computed tomography (CT) images at total lung capacity (TLC) and functional residual capacity (FRC), enabling the quantitative comparisons of structural and functional phenotypes, e.g., airway wall area (WA), luminal area (LA), and air-trapping percentage (AirT%), among healthy subjects and nonsevere and severe asthmatic subjects.
Imaging studies (2, 22) have demonstrated significant correlations of CT-based WA with epithelial thickness measures at biopsy. The CT-based LA and WA have also been utilized to compare two disease groups, i.e., asthma and chronic obstructive pulmonary disease (23, 24). However, the altered structure of airway dimensions in prior studies of asthma remains controversial. An early study (31) on the apical bronchus of the right upper lobe [right bronchus 1 (RB1)] has reported that WA increases in asthmatic subjects, but LA remains the same compared with that in healthy subjects. A large multicenter study (1) of 123 subjects sponsored by SARP has demonstrated the increased WA percentage [WA%, the ratio of WA to the total cross-sectional area (TA)] in severe asthmatic subjects, relative to healthy subjects and nonsevere asthmatic subjects. However, they also concluded that there is no difference in LA between healthy subjects and asthmatic subjects. In contrast, another study (27) has argued that both LA and WA of asthmatic subjects are smaller than those of healthy subjects. The contradictions between studies may relate to the lack of acceptable normalization schemes.
Regarding functional alterations, air-trapping is known to occur in asthmatic subjects, which can be assessed by CT imaging at either FRC or residual volume (RV). Newman and colleagues (30) demonstrated that air-trapping increases in asthmatic subjects, and the increase of air-trapping is primarily observed in severe asthmatic subjects (7, 11). However, we have recently found that CT density-based air-trapping assessment is sensitive to the imaging protocol. Thus a fraction-based, air-trapping method (11) was introduced to account for variations between data derived from individual centers of a multicenter study. In addition, we employed an image registration technique to observe the characteristics of regional air volume change and lung deformation with two extreme populations, i.e., healthy subjects and severe asthmatic subjects (12). That study demonstrated that reduced air volume changes in the lower lobes of severe asthmatic subjects (air-trapping) are compensated with the increased TLC vs. FRC volume changes in the upper lobes.
The alterations of structural and functional variables in asthmatic subjects have been reported independently by others. Nonetheless, the link between these variables at global, lobar, or more local levels (2, 14) have yet to be established. In this study, we first examine structural alterations of CT-resolved airways with the improved normalization schemes. We then seek to begin the exploration of the links between global and local variables obtained from both CT image and pulmonary function tests (PFT) to identify sensitive CT imaging-based variables. Previous analyses performed within SARP (20, 29) have identified meaningful patient clusters without the use of imaging-based variables. Therefore, the imaging-based variables could help identify meaningful subcategories within asthmatic populations for use in cluster analyses of airway narrowing, altered skeletal structure, increases of wall thickness (WT), and/or air-trapping.
METHODS
Human subject data sets.
A total of 195 subjects, consisting of 61 healthy subjects and 67 nonsevere asthmatic and 67 severe asthmatic subjects, were studied. Twenty-five healthy subjects were from Center 1 (University of Iowa). Fourteen healthy subjects, 26 nonsevere asthmatic subjects, and 30 severe asthmatic subjects were from Center 2 (SARP at University of Pittsburgh); 11 healthy subjects, 16 nonsevere asthmatic subjects, and 22 severe asthmatic subjects were from Center 3 (SARP at Washington University in St. Louis); and 11 healthy subjects, 25 nonsevere asthmatic subjects, and 15 severe asthmatic subjects were from Center 4 (SARP at University of Wisconsin). The aim and potential risks of the study were fully explained to each subject, after which written, informed consent was obtained. These data, except Center 4, were previously used to develop a fraction-based air-trapping measure (11). The imaging protocols for acquiring CT images were approved by the Institutional Review Boards of the respective institutions. Demography and PFTs, and information related to the scanners and protocols of the various institutions, can be found in Tables 1 and 2. Major criteria used to define severe asthma included treatments with oral corticosteroids and high-dose inhaled corticosteroids, along with several minor criteria. At least one major and two minor criteria were required to be classified as severe asthmatic subjects. All asthmatic subjects who did not meet criteria for severe asthmatic subjects were classified as nonsevere asthmatic subjects (40).
Table 1.
Demographic and pulmonary function test information for 61 healthy subjects, 67 nonsevere asthmatic subjects, and 67 severe asthmatic subjects
| Healthy Subjects | Nonsevere Asthmatic Subjects | Severe Asthmatic Subjects | ANOVA (F-test, P Value) | |
|---|---|---|---|---|
| Subjects, n | 61 | 67 | 67 | |
| Age, yr | 35.6 ± 14.2 | 33.1 ± 10.3 | 44.7 ± 13.2 | <0.0001†‡ |
| BMI, kg/m2 | 26.1 ± 5.7 | 28.1 ± 6.7 | 32.6 ± 8.7 | <0.0001†‡ |
| Asthma duration, yr | 20.5 ± 12.2 | 27.3 ± 15.8 | <0.01† | |
| Sex, n (female, %) | 36 (59) | 41 (61) | 42 (63) | 0.913 |
| Race, n (white non-Hispanic/African American/other, %) | 51/3/7 (84/5/11) | 52/9/6 (78/13/9) | 44/17/6 (66/25/9) | <0.05 |
| TLC, %predicted | 103 ± 11 | 101 ± 15 | 105 ± 20 | 0.301 |
| FRC, %predicted | 97 ± 19 | 96 ± 24 | 107 ± 30 | <0.05† |
| RV, %predicted | 109 ± 25 | 115 ± 35 | 145 ± 49 | <0.0001†‡ |
| RV/TLC × 100 | 30 ± 7 | 32 ± 8 | 43 ± 10 | <0.0001†‡ |
| FVC, %predicted | 99 ± 11 | 92 ± 15 | 74 ± 18 | <0.0001*†‡ |
| FEV1, %predicted | 100 ± 12 | 83 ± 18 | 60 ± 21 | <0.0001*†‡ |
| FEV1/FVC × 100 | 83 ± 6 | 74 ± 10 | 64 ± 13 | <0.0001*†‡ |
Values are means ± SD; n, no. of subjects.
BMI, body mass index; TLC, total lung capacity; FRC, functional residual capacity; RV, residual volume; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s.
TLC, FRC, and RV of two healthy and three nonsevere asthmatic and one severe asthmatic subjects were not available, and only FRC of one nonsevere asthmatic subject was not available. ANOVA tests with Tukey's post hoc tests were performed for “populations: healthy subjects vs. nonsevere asthmatic subjects vs. severe asthmatic subjects.”
P < 0.05 for healthy subjects vs. nonsevere asthmatic subjects.
P < 0.05 for nonsevere asthmatic subjects vs. severe asthmatic subjects.
P < 0.05 for healthy subjects vs. severe asthmatic subjects.
Table 2.
Scanners and the scanning protocols used for healthy and nonsevere asthmatic and severe asthmatic subjects in different institutions
| Imaging Center | Center 1 | Center 2 | Center 3 | Center 4 |
|---|---|---|---|---|
| Scanner model | Siemens Sensation 64 slice | GE VCT 64 slice | Siemens Sensation 16 Slice | GE |
| Scan type | Spiral | Helical | Spiral | Helical |
| Rotation time, s | 0.5 | 0.5 | 0.5 | 0.5 |
| Detector configuration channel no. × mm | 64 × 0.6 mm | 64 × 0.625 mm | 16 × 0.75 mm | 16 × 1.25 mm |
| Pitch | 1.0 | 0.984 | 1.5 | 1.675 |
| Peak kilovoltage, kVp | 120 | 120 | 120 | 120 |
| Siemens = effective mAs* | Effective | mA | Effective | mA 50 |
| GE = mAs* | mAs 100 | S-145; M-180; L-270 | mAs 33 | |
| Dose modulation | Care dose OFF | Auto mA OFF | Care dose OFF | Smart mA off |
| Reconstruction algorithm | B35 | Standard or detail | B30 | Standard or detail |
| Lung algorithm | None | None | None | None |
| Additional image filters | No selection | No selection | No selection | No selection |
| Thickness, mm | 0.75 | 0.625 | 1.0 | 1.25 |
| Interval, mm | 0.5 | 0.5–0.625 | 0.5–1.0 | 0.625 |
| Iterative reconstruction (noise reduction algorithm) | No selection | No selection | No selection | No selection |
| Scan Time (30-cm length), s | <10 | <10 | <15 | <20 |
GE, General Electric; mAs, millampere second.
mA was varied for center 2 protocol based on BMI size [small (S): BMI <20; medium (M): 20 ≤ BMI ≤30, large (L): BMI >30].
Structural variables.
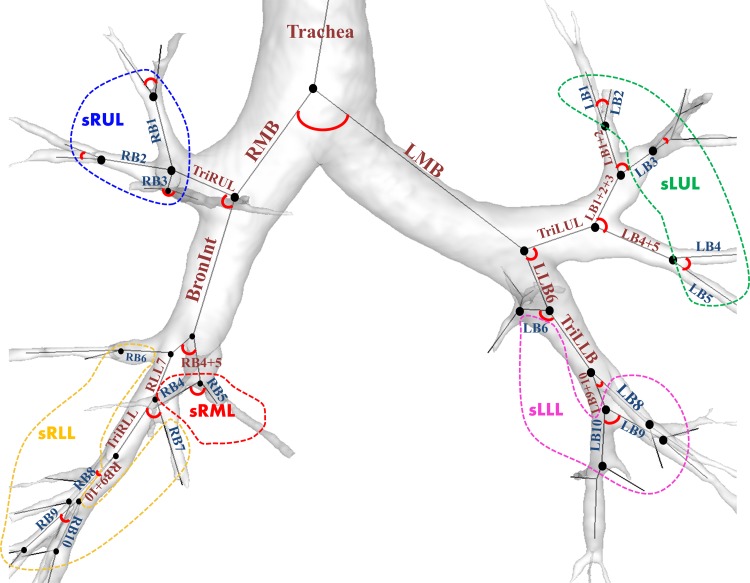
We extracted average airway LA, TA, perimeter of LA (Pe), and the one-dimensional (1D) skeleton of the tracheobronchial tree using the VIDA pulmonary analysis software (Pulmonary Work Station and Apollo; VIDA Diagnostics, Coralville, IA) with the CT images at TLC. The LA, TA, Pe, and 1D skeleton were estimated from three-dimensional airway masks (Fig. 1) via a robust airway segmentation algorithm (38). All of the airway values were averaged from the middle region (30–70%) of an airway segment by excluding the first 30% of the proximal region and the last 30% of the distal region.
Fig. 1.
Labels of 35 airway segments and 21 subgroup regions of interest (ROIs). Each bifurcation angle of the airway segments represents the angle between two daughter branches. See text for definition of acronyms.
First, the 1D airway skeleton was used to calculate bifurcation angle θ as follows.
| (1) |
where d1 and d2, (dot), and | | denote directional vectors of daughter branches, inner product of two vectors, and magnitude of the vector, respectively. In this study, the angle of a named segment (Fig. 1) represents θ between daughter branches of the corresponding segment.
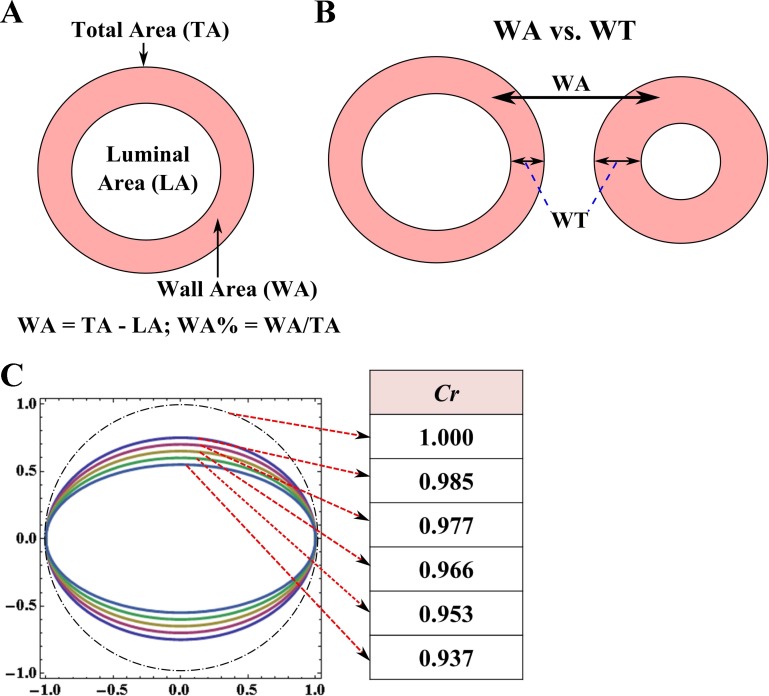
Next, WA was calculated by the subtraction of LA from TA, and the percentage of airway WA was defined as follows (Fig. 2A).
| (2) |
Fig. 2.
A: schematic of luminal area (LA), total area (TA), airway wall area (WA), and WA percentage (WA%). B: the difference between WA and WT. C: a schematic of circularity Cr from 0.937 to 1.0.
Average luminal diameter (Dave) and outer diameter (Douter) were calculated by Dave = and Douter = , respectively. WT was calculated by the subtraction of Dave from Douter. WT may exhibit different characteristics from WA because of airway constriction (Fig. 2B).
In addition, circularity Cr (39) was employed to assess the degree of noncircularity:
| (3) |
Figure 2C shows that Cr equals 1 if luminal shape is circular, whereas it decreases with increasing noncircularity. An important parameter related to pulmonary airflow resistance, i.e., hydraulic diameter (Dh) was defined as follows:
| (4) |
where Dh is associated with either “LA and Pe” or “Cr and Pe”, determining the airflow resistance of noncircular airways.
To sum up, θ, Cr, WT, and Dh could be utilized to evaluate altered skeletal structure, heterogeneity of airway shapes, wall thickening, and luminal narrowing, respectively.
Functional variables.
To control inter- and intracenter variability, instead of the existing method that is based on a fixed threshold of CT density (I less than −850) (7), we used a fraction-based method (11) employing air fraction (βair,threshold = 0.9) to calculate adjusted thresholds (Ithreshold) as follows:
| (5) |
where HUair,trachea is Hounsfield units of air in the trachea and was extracted from the trachea, and HUtissue is Hounsfield units of tissue and was set to 55. A voxel was regarded as an air-trapped voxel if I is below Ithreshold, then AirT% is defined as the ratio of the number of air-trapped voxels to the number of voxels in the whole lung (or respective lobes) at FRC. Furthermore, via an image registration technique (12), the Jacobian (J) was calculated to estimate local volume expansion from FRC to TLC scans at the voxel level. To reduce the size of these very large data sets, we calculated the median of J in each lobe for assessment of their correlations with the structural variables. In addition, our laboratory's previous study (11) has derived two sensitive imaging-based global variables. One method utilizes the ratio of air-volume change in the upper lobes to that in the middle and lower lobes, denoted by U/(M + L)|v. This metric represents the lobar distribution of air volume change between TLC and FRC. A second method utilizes the ratio of apical-to-basal distance to ventral-to-dorsal distance at TLC, called a lung shape metric. The former is considered as a global functional variable, and the latter a global structural variable. In asthmatic populations, the U/(M + L)|v increases, whereas the lung shape decreases. The two global variables were employed to explain local alterations of θ and WT*.
Regions of interests.
Based on anatomical labeling, 35 segmental regions of interest (ROIs) were chosen (Fig. 1). The aforementioned structural variables can be classified into two types: orientation independence vs. orientation dependence. The orientation-independent variables included LA, TA, WA, WT, Cr, and Dh. Analysis of the orientation-independent variables allows grouping of ROIs in proximity to facilitate data analysis. We grouped relatively small segments into five subgroups to study the correlations of average “local” structural variables with “global” PFT-based and “lobar” image-based functional variables. Here, among 35 segmental ROIs, 19 relatively small segments were grouped into five subgroups of right-upper-lobe (sRUL), right-middle-lobe (sRML), right-lower-lobe (sRLL), left-upper-lobe (sLUL), and left-lower-lobe (sLLL). They are sRUL = (RB1, RB2, RB3), sRML = (RB4, RB5), sRLL = (RB6, RB7, RB8, RB9, RB10), sLUL = [left bronchus 1 (LB1), LB2, LB3, LB4, LB5], and sLLL = (LB6, LB8, LB9, LB10). Those grouped branches belong to the respective lobes, as illustrated in Fig. 1. Thus, for the analysis of orientation-independent variables, we analyzed 21 ROIs: 16 large-segmental ROIs, and 5 grouped ROIs. On the other hand, θ is an orientation-dependent variable, depending on the alignment of child branches, e.g., with the apical-to-basal axis, the ventral-to-dorsal axis, or the left-to-right axis. For the analysis of orientation-dependent variables, we analyze only 20 branches up to 3 (upper lobes) or 5 (lower lobes) generations (Fig. 1).
Normalization of dimensional variables.
Multiple linear regressions of tracheal diameter (Dtrachea) and tracheal WT (WTtrachea) were performed with explanatory variables of age, sex, and height for 61 healthy subjects. The explanatory variables were selected based on previous studies of airway sizes and lung volumes (5, 6, 13, 16, 35). Next, the predicted values of Dtrachea and WTtrachea from healthy subjects (Dtrachea,pred and WTtrachea,pred) were utilized as references to normalize the corresponding airway variables of healthy and asthmatic populations. Thus LA, TA, WA (area), and Dh (length) were normalized by Dtrachea,pred2 and Dtrachea,pred, respectively, whereas WT was normalized by WTtrachea,pred. It is noted that WA could be normalized by Dtrachea,pred2, because WA was significantly correlated with Dave2 (r = 0.86) in trachea, whereas WT required an independent normalization scheme because WT was less significantly correlated with Dave in trachea (r = 0.51). These normalized quantities were denoted with an asterisk as LA*, TA*, WA*, WT*, and Dh*.
Statistical analysis.
Kruskal-Wallis tests, along with Nemenyi's post hoc tests with χ2 approximation (32) were performed to examine whether the regional airway features differ between healthy subjects, nonsevere asthmatic subjects, and severe asthmatic subjects. We have taken 0.05 as the significance level in all tests, and this controls the false discovery rate (FDR) of multiple comparisons at 12.5% (e.g., 4.7% for P = 0.01), estimated by the reported Kruskal-Wallis P values and using the method of Benjamini and Hochberg (4). Furthermore, Pearson and Spearman linear correlation tests were employed, and the significance level of the correlation tests was taken as 0.01, which controls the FDR of multiple comparisons at 2.9%. The software R (37) was used for statistical analysis (http://www.R-project.org).
RESULTS
Normalization.
With the multiple linear regression of 61 healthy subjects, the Dtrachea,pred was predicted as follows:
| (6) |
where [sex, age, height] take the value/unit of [0 (male) or 1 (female), year, meter]. An optimal combination of the multiple regression was determined by acquiring the largest adjusted R2 (=0.59) and the smallest Akaike's information criterion (AIC = 203). This model that includes age and height only within interaction terms better predicted Dtrachea,pred of healthy subjects than a model with additional main effects of age and height (adjusted R2 = 0.58 and AIC = 206). Among the explanatory variables, sex showed the most significant correlation with the Dtrachea (r = −0.69), but sex is neglected in body surface area (BSA)-based normalization. We compared linear correlations of tracheal Dave with BSA1/2 and Dtrachea,pred in healthy subjects. Dtrachea,pred is more correlated with Dtrachea than BSA1/2 (r = 0.79 vs. r = 0.48, P < 0.001 for two correlations). The correlations between Dtrachea,pred and Dave in left main bronchus (LMB) and right main bronchus (RMB) were also improved (r = 0.68 and 0.71), compared with BSA1/2 (r = 0.45 and 0.43) (P < 0.05 between correlations). While height was an important factor in the above equation, adding weight did not reach a level of significance.
With the same healthy subjects, WTtrachea,pred was predicted as follows:
| (7) |
The adjust R2 and AIC were 0.37 and 53, respectively, for this regression model, which was a better prediction than the case with the main effects of height and age (adjusted R2 = 0.36 and AIC = 56). WTtrachea also shows the most significant correlation with sex (r = −0.60) as in Dtrachea, which is neglected in BSA-based normalization.
Bifurcation angle (θ).
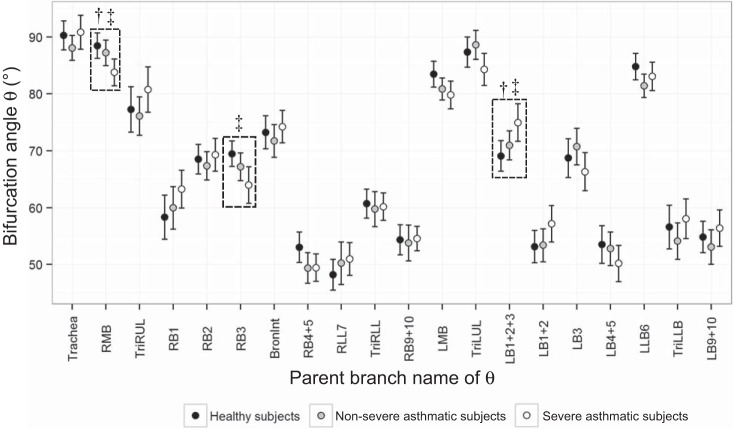
Figure 3 shows that θ of RMB and RB3 in severe asthmatic subjects were smaller than those in healthy subjects, and θ of LB1+2+3 in severe asthmatic subjects was larger than that in healthy subjects. Next, θ of severe asthmatic subjects was smaller in RMB and larger in LB1+2+3 than those of nonsevere asthmatic subjects. The FDR rates for respective P values in RMB, RB3, and LB1+2+3 were 4.2, 1.7, and 8.6%, respectively. In addition, the differences of θ in RB1 (P = 0.085), LMB (P = 0.058), and LB1+2 (P = 0.069) were close to the borderline of significance. The alterations of θ were largely associated with the lung shape (the ratio of apical-basal distance to ventral-dorsal distance). For instance, the decreased θ in RMB, RB3, and LMB were correlated with a decrease of lung shape, as r = 0.23, 0.33, and 0.23 (P < 0.01), respectively. In contrast, the increased θ of LB1+2+3 was also correlated with the decrease of lung shape, as r = −0.38 (P < 0.0001), respectively. In addition, we found that the increased θ of apical bronchi, e.g., RB1 (r = 0.30) and LB1+2 (r = 0.24), were correlated with the increased air volume change in upper lobes, i.e., increased U/(M + L)|v (P < 0.01).
Fig. 3.
Bifurcation angles (θ) between daughter branches in 20 segmental regions of healthy subjects and nonsevere and severe asthmatic subjects. See text for definition of acronyms. †P < 0.05 for nonsevere asthmatic subjects vs. severe asthmatic subjects. ‡P < 0.05 for healthy subjects vs. severe asthmatic subjects.
Cr.
The decrease of Cr (i.e., increase of noncircularity) in severe asthmatic subjects was mostly seen in ROIs of RMB, TriRLL (RLL trifurcation), and sRML (Table 3), compared with that in healthy subjects. The statistical difference of Cr was the most significant in RMB between healthy subjects and severe asthmatic subjects, and the mean Cr of nonsevere asthmatic subjects fell between two extreme groups. The decreased Cr has significant correlations with PFT's variables, e.g., forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) (r = 0.27) and RV/TLC (r = −0.25) (P < 0.001). On the other hand, Cr of TriLUL (LUL trifurcation) in severe asthmatic subjects tended to be larger than that of healthy subjects (P < 0.001), and it was found to be different from the general characteristics of decreased Cr in severe asthmatic subjects. It is presumably due to its short length. In fact, if the segmental length between proximal node and distal node is short, Cr may decrease due to branching patterns. Our analysis also demonstrated that the segmental lengths of severe asthmatic subjects (13.3 ± 3.0 mm) and nonsevere asthmatic subjects (13.3 ± 3.2 mm) were greater than those of healthy subjects (12.0 ± 3.3 mm) in TriLUL (P < 0.05), and Cr had a positive correlation with the segmental length (r = 0.34, P < 0.0001).
Table 3.
Kruskal-Wallis tests with Nemenyi's post hoc tests of circularity among healthy subjects, nonsevere asthmatic subjects, and severe asthmatic subjects in each ROI
| 21 ROIs | Healthy Subjects | Nonsevere Asthmatic Subjects | Severe Asthmatic Subjects | Kruskal-Wallis (P Value) |
|---|---|---|---|---|
| Trachea | 0.986 ± 0.005 | 0.984 ± 0.008 | 0.982 ± 0.009 | 0.116 |
| RMB | 0.970 ± 0.013 | 0.966 ± 0.016 | 0.959 ± 0.017 | <0.001†‡ |
| TriRUL | 0.941 ± 0.024 | 0.930 ± 0.053 | 0.939 ± 0.036 | 0.878 |
| BronInt | 0.955 ± 0.017 | 0.959 ± 0.018 | 0.957 ± 0.019 | 0.394 |
| RB4 + 5 | 0.975 ± 0.014 | 0.975 ± 0.015 | 0.971 ± 0.019 | 0.326 |
| RLL7 | 0.969 ± 0.036 | 0.973 ± 0.032 | 0.964 ± 0.042 | 0.255 |
| TriRLL | 0.970 ± 0.030 | 0.969 ± 0.033 | 0.965 ± 0.024 | <0.05‡ |
| RB9 + 10 | 0.967 ± 0.037 | 0.970 ± 0.030 | 0.968 ± 0.023 | 0.110 |
| sRUL | 0.967 ± 0.015 | 0.963 ± 0.019 | 0.962 ± 0.016 | 0.071 |
| sRML | 0.966 ± 0.011 | 0.956 ± 0.024 | 0.955 ± 0.029 | <0.05‡ |
| sRLL | 0.961 ± 0.013 | 0.959 ± 0.014 | 0.956 ± 0.015 | 0.111 |
| LMB | 0.975 ± 0.011 | 0.972 ± 0.010 | 0.972 ± 0.010 | 0.068 |
| TriLUL | 0.922 ± 0.068 | 0.951 ± 0.043 | 0.953 ± 0.049 | <0.0001*‡ |
| LB1 + 2 +3 | 0.955 ± 0.051 | 0.945 ± 0.065 | 0.969 ± 0.023 | 0.549 |
| LB1 + 2 | 0.961 ± 0.031 | 0.966 ± 0.027 | 0.947 ± 0.060 | 0.167 |
| LB4 + 5 | 0.956 ± 0.052 | 0.968 ± 0.029 | 0.963 ± 0.037 | 0.513 |
| LLB6 | 0.950 ± 0.037 | 0.946 ± 0.044 | 0.942 ± 0.055 | 0.949 |
| TriLLB | 0.981 ± 0.008 | 0.978 ± 0.015 | 0.975 ± 0.018 | 0.327 |
| LB9 + 10 | 0.949 ± 0.044 | 0.955 ± 0.034 | 0.955 ± 0.028 | 0.786 |
| sLUL | 0.957 ± 0.015 | 0.954 ± 0.014 | 0.952 ± 0.017 | 0.160 |
| sLLL | 0.963 ± 0.015 | 0.961 ± 0.013 | 0.956 ± 0.019 | 0.051 |
Values are means ± SD.
ROI, region of interest; RMB, right main bronchus; LMB, left main bronchus; RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe; LUL, left upper lobe;LLL, left lower lobe; RB, right bronchus; LB, left bronchus; Tri, trifurcation; s, segment; BronInt, bronchus intermedius.
P < 0.05 for healthy subjects vs. nonsevere asthmatic subjects.
P < 0.05 for nonsevere asthmatic subjects vs. severe asthmatic subjects.
P < 0.05 for healthy subjects vs. severe asthmatic subjects.
LA*, TA*, WA*.
LA*, TA* and WA* were significantly different at some regions (Tables 4 and 5). For instance, in severe asthmatic subjects, LA* tended to be larger in trachea and LMB, whereas they were smaller in the RB4+5, TriRLL, RB9+10, sRML, sRLL, LB4+5, TriLLB, sLUL, and sLLL, compared with that in healthy subjects. Those of nonsevere asthmatic subjects fell between healthy subjects and severe asthmatic subjects. Most regions of elevated or reduced LA* were consistent with the regions of TA*, e.g., trachea, LMB, TriRLL, RB9+10, sRML, sRLL, TriLLB, LB9+10, and sLLL (Table 4). The reduced LA* and TA* were mainly observed in middle and lower lobes. WA* of severe asthmatic subjects were larger only in trachea and LMB than those of healthy subjects (Table 5).
Table 4.
Kruskal-Wallis tests with Nemenyi's post hoc tests of luminal area and total area normalized by Dtrachea pred2 among healthy subjects and nonsevere and severe asthmatic subjects in each ROI
| LA* |
TA* |
|||||||
|---|---|---|---|---|---|---|---|---|
| 21 ROI | Healthy subjects | Nonsevere asthmatic subjects | Severe asthmatic subjects | Kruskal-Wallis (P value) | Healthy subjects | Nonsevere asthmatic subjects | Severe asthmatic subjects | Kruskal-Wallis (P value) |
| Trachea | 0.789 ± 0.11 | 0.824 ± 0.15 | 0.857 ± 0.16 | 0.052 | 1.340 ± 0.16 | 1.387 ± 0.21 | 1.447 ± 0.25 | <0.05‡ |
| RMB | 0.575 ± 0.08 | 0.576 ± 0.10 | 0.579 ± 0.12 | 0.992 | 1.006 ± 0.16 | 1.012 ± 0.20 | 1.009 ± 0.20 | 0.974 |
| TriRUL | 0.264 ± 0.06 | 0.279 ± 0.14 | 0.264 ± 0.14 | 0.376 | 0.501 ± 0.11 | 0.522 ± 0.22 | 0.508 ± 0.21 | 0.625 |
| BronInt | 0.334 ± 0.05 | 0.341 ± 0.06 | 0.360 ± 0.07 | 0.111 | 0.600 ± 0.08 | 0.604 ± 0.09 | 0.643 ± 0.11 | 0.096 |
| RB4 + 5 | 0.128 ± 0.03 | 0.119 ± 0.04 | 0.110 ± 0.04 | <0.05‡ | 0.285 ± 0.06 | 0.272 ± 0.06 | 0.262 ± 0.07 | 0.089 |
| RLL7 | 0.209 ± 0.05 | 0.190 ± 0.06 | 0.185 ± 0.06 | <0.05 | 0.412 ± 0.08 | 0.384 ± 0.10 | 0.381 ± 0.10 | <0.05 |
| TriRLL | 0.162 ± 0.04 | 0.146 ± 0.04 | 0.137 ± 0.04 | <0.005‡ | 0.337 ± 0.07 | 0.313 ± 0.07 | 0.304 ± 0.07 | <0.05‡ |
| RB9 + 10 | 0.132 ± 0.05 | 0.113 ± 0.05 | 0.103 ± 0.04 | <0.001‡ | 0.284 ± 0.07 | 0.255 ± 0.08 | 0.246 ± 0.06 | <0.01‡ |
| sRUL | 0.090 ± 0.02 | 0.080 ± 0.02 | 0.083 ± 0.03 | <0.05* | 0.215 ± 0.04 | 0.199 ± 0.04 | 0.208 ± 0.05 | <0.05* |
| sRML | 0.067 ± 0.02 | 0.061 ± 0.02 | 0.057 ± 0.02 | <0.001*‡ | 0.175 ± 0.04 | 0.161 ± 0.05 | 0.156 ± 0.04 | <0.01*‡ |
| sRLL | 0.084 ± 0.03 | 0.073 ± 0.03 | 0.070 ± 0.03 | <0.01*‡ | 0.202 ± 0.05 | 0.182 ± 0.05 | 0.180 ± 0.05 | <0.01*‡ |
| LMB | 0.364 ± 0.07 | 0.362 ± 0.07 | 0.392 ± 0.08 | <0.05 | 0.648 ± 0.11 | 0.658 ± 0.12 | 0.706 ± 0.13 | <0.05‡ |
| TriLUL | 0.319 ± 0.15 | 0.269 ± 0.09 | 0.279 ± 0.11 | 0.057 | 0.608 ± 0.25 | 0.523 ± 0.15 | 0.540 ± 0.20 | 0.078 |
| LB1 + 2 +3 | 0.181 ± 0.08 | 0.185 ± 0.09 | 0.163 ± 0.06 | 0.382 | 0.369 ± 0.12 | 0.376 ± 0.13 | 0.350 ± 0.10 | 0.574 |
| LB1 + 2 | 0.092 ± 0.03 | 0.089 ± 0.04 | 0.102 ± 0.07 | 0.707 | 0.224 ± 0.05 | 0.217 ± 0.06 | 0.245 ± 0.12 | 0.607 |
| LB4 + 5 | 0.155 ± 0.07 | 0.127 ± 0.05 | 0.132 ± 0.07 | <0.01‡ | 0.325 ± 0.11 | 0.282 ± 0.09 | 0.294 ± 0.12 | <0.05 |
| LLB6 | 0.272 ± 0.06 | 0.271 ± 0.09 | 0.280 ± 0.11 | 0.633 | 0.522 ± 0.10 | 0.518 ± 0.15 | 0.532 ± 0.18 | 0.682 |
| TriLLB | 0.195 ± 0.05 | 0.174 ± 0.05 | 0.168 ± 0.05 | <0.01‡ | 0.393 ± 0.08 | 0.358 ± 0.08 | 0.356 ± 0.09 | <0.05‡ |
| LB9 + 10 | 0.185 ± 0.08 | 0.147 ± 0.05 | 0.144 ± 0.07 | <0.05* | 0.371 ± 0.12 | 0.314 ± 0.08 | 0.312 ± 0.11 | <0.01* |
| sLUL | 0.062 ± 0.01 | 0.056 ± 0.02 | 0.057 ± 0.02 | <0.01*‡ | 0.160 ± 0.03 | 0.148 ± 0.03 | 0.153 ± 0.04 | <0.05* |
| sLLL | 0.101 ± 0.03 | 0.089 ± 0.03 | 0.085 ± 0.03 | <0.005*‡ | 0.235 ± 0.04 | 0.215 ± 0.05 | 0.212 ± 0.05 | <0.01‡ |
Values are means ± SD. Dtrachea pred2, predicted values of tracheal diameter;
LA*, normalized luminal area; TA*, normalized total cross-sectional area.
P < 0.05 for healthy subjects vs. nonsevere asthmatic subjects.
P < 0.05 for nonsevere asthmatic subjects vs. severe asthmatic subjects.
P < 0.05 for healthy subjects vs. severe asthmatic subjects.
Table 5.
Kruskal-Wallis tests with Nemenyi's post hoc tests of wall area normalized by Dtrachea pred2 among healthy subjects and nonsevere and severe asthmatic subjects in each ROI
| 21 ROIs | Healthy Subjects | Nonsevere Asthmatic Subjects | Severe Asthmatic Subjects | Kruskal-Wallis Tests (P value) |
|---|---|---|---|---|
| Trachea | 0.551 ± 0.06 | 0.563 ± 0.08 | 0.590 ± 0.10 | <0.05‡ |
| RMB | 0.431 ± 0.08 | 0.435 ± 0.10 | 0.430 ± 0.09 | 0.947 |
| TriRUL | 0.237 ± 0.05 | 0.243 ± 0.08 | 0.244 ± 0.08 | 0.933 |
| BronInt | 0.265 ± 0.04 | 0.264 ± 0.04 | 0.283 ± 0.05 | 0.062 |
| RB4 + 5 | 0.157 ± 0.03 | 0.152 ± 0.03 | 0.152 ± 0.03 | 0.486 |
| RLL7 | 0.203 ± 0.04 | 0.194 ± 0.04 | 0.197 ± 0.04 | 0.202 |
| TriRLL | 0.175 ± 0.03 | 0.167 ± 0.03 | 0.167 ± 0.03 | 0.295 |
| RB9 + 10 | 0.153 ± 0.03 | 0.143 ± 0.03 | 0.143 ± 0.03 | 0.197 |
| sRUL | 0.126 ± 0.02 | 0.119 ± 0.02 | 0.125 ± 0.03 | 0.122 |
| sRML | 0.108 ± 0.02 | 0.100 ± 0.02 | 0.100 ± 0.02 | 0.061 |
| sRLL | 0.118 ± 0.02 | 0.108 ± 0.02 | 0.110 ± 0.02 | <0.05* |
| LMB | 0.285 ± 0.05 | 0.295 ± 0.06 | 0.313 ± 0.06 | <0.05‡ |
| TriLUL | 0.289 ± 0.11 | 0.254 ± 0.07 | 0.261 ± 0.09 | 0.212 |
| LB1 + 2 +3 | 0.188 ± 0.05 | 0.191 ± 0.04 | 0.186 ± 0.04 | 0.762 |
| LB1 + 2 | 0.132 ± 0.03 | 0.128 ± 0.03 | 0.143 ± 0.06 | 0.329 |
| LB4 + 5 | 0.171 ± 0.04 | 0.154 ± 0.03 | 0.162 ± 0.05 | 0.105 |
| LLB6 | 0.251 ± 0.06 | 0.247 ± 0.06 | 0.252 ± 0.07 | 0.822 |
| TriLLB | 0.198 ± 0.04 | 0.184 ± 0.03 | 0.188 ± 0.04 | 0.055 |
| LB9 + 10 | 0.186 ± 0.04 | 0.167 ± 0.03 | 0.168 ± 0.04 | <0.05* |
| sLUL | 0.098 ± 0.01 | 0.092 ± 0.02 | 0.097 ± 0.02 | 0.051 |
| sLLL | 0.135 ± 0.02 | 0.126 ± 0.03 | 0.127 ± 0.03 | 0.069 |
Values are means ± SD.
P < 0.05 for healthy subjects vs. nonsevere asthmatic subjects.
P < 0.05 for nonsevere asthmatic subjects vs. severe asthmatic subjects.
P < 0.05 for healthy subjects vs. severe asthmatic subjects.
WT*.
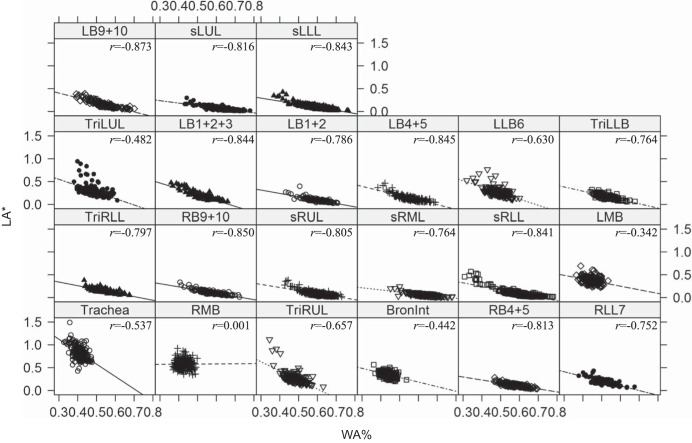
WA% has been used to assess the degree of airway WT (2). However, the increased WA% was significantly correlated with the reduced LA* (Fig. 4). Thus it is difficult to conclude that WA% only reflects airway wall thickening in an absolute sense, because the increase of WA% may merely reflect the decrease of LA* without changing WA. In addition, WT* appears equivalent to WA*, but WT* could vary with fixed WA* due to airway constriction/enlargement (Fig. 2B). Therefore, we focused on WT* to assess wall thickening (Table 6), rather than WA* and WA%. Specifically, WT* of RB4+5, sRUL, LMB, LB1+2+3, and LB1+2 in severe asthmatic subjects were greater than those in healthy subjects. WT* of BronInt (bronchus intermedius), RB4+5, sRUL, sRLL, LB1+2, TriLLB, and sLUL in severe asthmatic subjects were also greater than those in nonsevere asthmatic subjects. Overall, the mean WT* in severe asthmatic subjects increased relative to healthy subjects and nonsevere asthmatic subjects, and the most significant increases (P < 0.01) were observed in upper lobar bronchi. Whereas WT* was little correlated with PFTs and AirT%, the elevated WT* was significantly correlated with the U/(M + L)|v in sRUL (r = 0.27) and LB1+2 (r = 0.26) (P < 0.001).
Fig. 4.
Linear regressions between normalized LA (LA*) and WA% in the 21 respective subgroup ROIs with all subjects, i.e., healthy subjects and nonsevere and severe asthmatic subjects. These data support the notion that the use of WA% is a poor metric when seeking to reflect airway wall remodeling because of the strong interdependence with LA*.
Table 6.
Kruskal-Wallis tests with Nemenyi's post hoc tests of normalized wall thickness among healthy subjects and nonsevere asthmatic subjects and severe asthmatic subjects in each ROI
| 21 ROIs | Healthy Subjects | Nonsevere Asthmatic Subjects | Severe Asthmatic Subjects | Kruskal-Wallis Tests (P Value) |
|---|---|---|---|---|
| Trachea | 1.000 ± 0.07 | 1.000 ± 0.08 | 1.031 ± 0.11 | 0.081 |
| RMB | 0.903 ± 0.12 | 0.905 ± 0.13 | 0.904 ± 0.11 | 0.990 |
| TriRUL | 0.717 ± 0.09 | 0.719 ± 0.09 | 0.743 ± 0.09 | <0.05 |
| BronInt | 0.726 ± 0.07 | 0.718 ± 0.05 | 0.750 ± 0.07 | <0.05† |
| RB4 + 5 | 0.655 ± 0.06 | 0.652 ± 0.04 | 0.675 ± 0.06 | <0.05†‡ |
| RLL7 | 0.685 ± 0.07 | 0.683 ± 0.06 | 0.702 ± 0.06 | 0.061 |
| TriRLL | 0.662 ± 0.06 | 0.658 ± 0.05 | 0.679 ± 0.06 | <0.05 |
| RB9 + 10 | 0.636 ± 0.07 | 0.633 ± 0.05 | 0.656 ± 0.06 | <0.05 |
| sRUL | 0.612 ± 0.05 | 0.608 ± 0.04 | 0.630 ± 0.05 | <0.005†‡ |
| sRML | 0.589 ± 0.06 | 0.568 ± 0.05 | 0.587 ± 0.05 | 0.112 |
| sRLL | 0.597 ± 0.05 | 0.580 ± 0.04 | 0.599 ± 0.05 | <0.05† |
| LMB | 0.748 ± 0.09 | 0.773 ± 0.09 | 0.794 ± 0.09 | <0.005‡ |
| TriLUL | 0.793 ± 0.14 | 0.756 ± 0.09 | 0.765 ± 0.11 | 0.606 |
| LB1 + 2 +3 | 0.676 ± 0.07 | 0.685 ± 0.05 | 0.698 ± 0.06 | <0.05‡ |
| LB1 + 2 | 0.631 ± 0.06 | 0.620 ± 0.05 | 0.662 ± 0.08 | <0.005†‡ |
| LB4 + 5 | 0.662 ± 0.07 | 0.648 ± 0.05 | 0.676 ± 0.07 | 0.053 |
| LLB6 | 0.746 ± 0.10 | 0.738 ± 0.09 | 0.747 ± 0.10 | 0.768 |
| TriLLB | 0.687 ± 0.07 | 0.671 ± 0.04 | 0.696 ± 0.07 | <0.05† |
| LB9 + 10 | 0.672 ± 0.06 | 0.658 ± 0.04 | 0.676 ± 0.06 | 0.147 |
| sLUL | 0.559 ± 0.04 | 0.547 ± 0.04 | 0.571 ± 0.04 | <0.005† |
| sLLL | 0.626 ± 0.05 | 0.614 ± 0.05 | 0.630 ± 0.05 | 0.175 |
Values are means ± SD.
P < 0.05 for healthy subjects vs. nonsevere asthmatic subjects.
P < 0.05 for nonsevere asthmatic subjects vs. severe asthmatic subjects.
P < 0.05 for healthy subjects vs. severe asthmatic subjects.
Dh*.
While LA has been mostly analyzed in existing studies to assess airway narrowing, Dh* is the most relevant variable in terms of airflow resistance (41), especially in noncircular airways. Table 7 shows the slightly increased Dh* of severe asthmatic subjects in the trachea and LMB, relative to healthy subjects and nonsevere asthmatic subjects, respectively. On the other hand, Dh* of severe asthmatic subjects were significantly decreased in most ROIs, such as RB4+5, TriRLL, RB9+10, sRUL, sRML, sRLL, LB4+5, TriLLB, LB9+10, sLUL, and sLLL, compared with those of healthy subjects. We observed that most of the statistical differences between healthy subjects and severe asthmatic subjects were found in lower lobar bronchi. In addition, Dh* of nonsevere asthmatic subjects decreased in sRUL, sRML, sRLL, TriLLB, LB9+10, sLUL, and sLLL, relative to healthy subjects, but the statistical difference between healthy subjects and nonsevere asthmatic subjects was less significant than between healthy subjects and severe asthmatic subjects. Figure 5 shows the generational distributions of Dh* for three populations. In asthmatic subjects, Dh* in the 0th generation (trachea) tends to be larger, whereas it remained similar in respective 1st and 2nd generations, including RMB, LMB, TriRUL, TriLUL, BronInt, and LLB6, compared with that in healthy subjects. Dh* then significantly decreased beginning from the 3rd generation of the airways corresponding to the subsegmental airways residing in the lung lobes.
Table 7.
Kruskal-Wallis tests with Nemenyi's post hoc tests of normalized hydraulic diameter among healthy subjects and nonsevere and severe asthmatic subjects in each ROI
| 21 ROIs | Healthy Subjects | Nonsevere Asthmatic Subjects | Severe Asthmatic Subjects | Kruskal-Wallis Tests (P Value) |
|---|---|---|---|---|
| Trachea | 0.986 ± 0.07 | 1.004 ± 0.10 | 1.022 ± 0.10 | 0.117 |
| RMB | 0.828 ± 0.06 | 0.825 ± 0.08 | 0.820 ± 0.09 | 0.780 |
| TriRUL | 0.542 ± 0.06 | 0.538 ± 0.09 | 0.529 ± 0.11 | 0.503 |
| BronInt | 0.622 ± 0.05 | 0.629 ± 0.06 | 0.645 ± 0.06 | 0.106 |
| RB4 + 5 | 0.390 ± 0.05 | 0.376 ± 0.06 | 0.358 ± 0.07 | <0.05‡ |
| RLL7 | 0.495 ± 0.05 | 0.472 ± 0.06 | 0.462 ± 0.08 | <0.05 |
| TriRLL | 0.436 ± 0.05 | 0.413 ± 0.06 | 0.397 ± 0.06 | <0.01‡ |
| RB9 + 10 | 0.389 ± 0.06 | 0.358 ± 0.07 | 0.345 ± 0.07 | <0.001‡ |
| sRUL | 0.321 ± 0.03 | 0.300 ± 0.04 | 0.303 ± 0.05 | <0.01*‡ |
| sRML | 0.280 ± 0.04 | 0.261 ± 0.05 | 0.251 ± 0.05 | <0.001*‡ |
| sRLL | 0.303 ± 0.04 | 0.281 ± 0.05 | 0.274 ± 0.05 | <0.005*‡ |
| LMB | 0.661 ± 0.06 | 0.658 ± 0.06 | 0.684 ± 0.07 | <0.05 |
| TriLUL | 0.570 ± 0.09 | 0.549 ± 0.06 | 0.557 ± 0.08 | 0.422 |
| LB1 + 2 +3 | 0.447 ± 0.06 | 0.444 ± 0.08 | 0.434 ± 0.07 | 0.554 |
| LB1 + 2 | 0.325 ± 0.05 | 0.319 ± 0.06 | 0.325 ± 0.08 | 0.897 |
| LB4 + 5 | 0.412 ± 0.07 | 0.381 ± 0.06 | 0.380 ± 0.09 | <0.01‡ |
| LLB6 | 0.555 ± 0.06 | 0.548 ± 0.07 | 0.550 ± 0.09 | 0.574 |
| TriLLB | 0.485 ± 0.06 | 0.456 ± 0.06 | 0.446 ± 0.08 | <0.005*‡ |
| LB9 + 10 | 0.447 ± 0.08 | 0.406 ± 0.06 | 0.398 ± 0.09 | <0.01*‡ |
| sLUL | 0.262 ± 0.03 | 0.246 ± 0.03 | 0.248 ± 0.04 | <0.01*‡ |
| sLLL | 0.338 ± 0.04 | 0.316 ± 0.05 | 0.307 ± 0.05 | <0.001*‡ |
Values are means ±SD.
P < 0.05 for healthy subjects vs. nonsevere asthmatic subjects.
P < 0.05 for nonsevere asthmatic subjects vs. severe asthmatic subjects.
P < 0.05 for healthy subjects vs. severe asthmatic subjects.
Fig. 5.
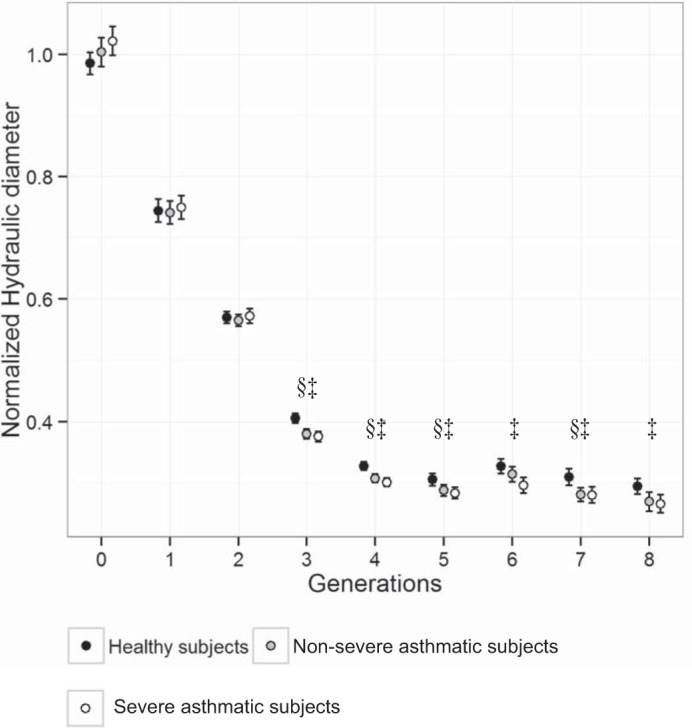
Normalized hydraulic diameter (Dh*) according to generation numbers with only the selected 35 segments. Values are presented as means ± 95% confidence interval. §P < 0.05 for healthy subjects vs. nonsevere asthmatic subjects. †P < 0.05 for nonsevere asthmatic subjects vs. severe asthmatic subjects. ‡P < 0.05 for healthy subjects vs. severe asthmatic subjects.
Correlation of structural variables with functional variables.
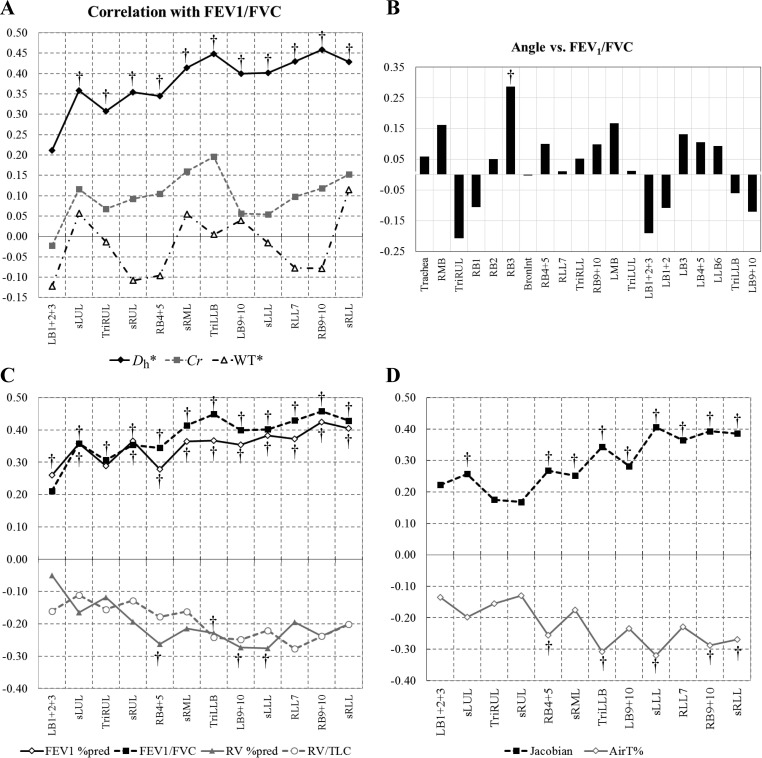
We performed correlation tests of the structural variables at the local segmental level with functional variables of PFTs at global level and AirT% and J at lobar level. Figure 6, A and B, shows that Dh* is the most significantly correlated with FEV1/FVC, compared with the other structural variables. We also confirmed that, unlike Dh*, the θ, Cr, and WT* were less correlated with the other PFTs and image-based AirT% and J. In addition, Fig. 6, C and D, shows that Dh* exhibits the significant correlations with FEV1% predicted, RV% predicted, and RV/TLC, lobar AirT%, and J. Among PFT variables, Dh* exhibited the most significant correlation with FEV1/FVC, especially in TriLLB and RB9+10 (Fig. 6C). Next, Dh* was inversely correlated with AirT%, especially in RB4+5, TriLLB, sLLL, RB9+10, and sRLL (P < 0.001, Fig. 6D). In addition, reduced Dh* was significantly correlated with reduced J, especially in lower lobes. These results confirmed that constricted airways visible on CT are associated with elevated air-trapping and reduced volume change.
Fig. 6.
Spearman's rank correlations for Dh*, Cr, and normalized WT (WT*) with forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) (A); θ with FEV1/FVC (B); Dh* with pulmonary function test measurements (FEV1 %predicted, FEV1/FVC, RV %predicted, and RV/TLC; C); and Dh* with air-trapping percentage (AirT%) and the median of Jacobian J in each lobe (D). †P < 0.001 (false discovery rate = 0.5%). Note the stronger correlations of Dh* within the middle and lower lobes.
DISCUSSION
We investigated structural alterations of bronchial airways in asthmatic subjects and their correlations with functional quantities, to understand the relationship of global (or lobar) vs. local variables. Linking the multiscale phenomena leads to an alternative approach to the better identification of imaging-based phenotypes, which is a critical step to classifying asthmatic subjects into subgroups. In this study, we focused on alterations of four structural variables of θ, WT, Cr, and Dh in asthmatic subjects to evaluate altered skeletal structure, wall thickening, heterogeneous airway shape, and luminal constriction, respectively. The imaging-based structural metrics could potentially be utilized as objective measures for alterations of local bronchi, leading to an efficient targeted therapy, such as in the application of bronchial thermoplasty. Furthermore, we believe that the tools explored in this study will help in the search for novel phenotypes, aiding in the exploration and application of new therapies.
Among structural variables, WT and Dh are associated with dimensional units; thus they require normalization schemes. In many existing studies (18, 27, 36), BSA or body mass index (BMI) (determined by height and weight) has been used for normalization to control intersubject variability. However, the relationship between BSA (or BMI) and airway size is yet to be confirmed. Earlier studies have reported that Dtrachea is correlated with sex and height (5, 13, 16), and that TLC predicted value is determined by sex and height (35). In addition, Brooks et al. (6) have shown that tracheal area is mainly dependent on sex, rather than on body size. Our multiple linear regression models with sex, age, and height have demonstrated significantly improved correlations, compared with BSA, when evaluating in the trachea, LMB, and RMB in healthy subjects. Furthermore, we have found that sex is the most important variable in determining airway diameter and WT, which is not considered in a calculation of BSA (or BMI).
There have been controversies concerning the increase or decrease of LA and WA in asthmatic subjects. Some studies (3, 27) have reported a decrease of LA in asthmatic subjects, whereas some (2, 31) have reported no difference between healthy subjects and asthmatic subjects. The controversies of LA and WA among existing studies may be attributable to the lack of an acceptable normalization scheme. In addition, most of the studies (2, 18, 27) employed WA% for analysis, because WA and WA normalized by BSA of asthmatic subjects are similar to or even decreased relative to healthy subjects. However, the measure of WA% depends on the net effect of alterations in both LA and WA. In other words, WA% could increase even with reduced LA alone, regardless of the increase of WA (Fig. 6). Thus caution must be taken in using WA% when evaluating airway wall thickening. It has recently been demonstrated that the use of WA% can lead to erroneous conclusions in the assessment of chronic obstructive pulmonary disease-related airway alterations (33). In addition, constant WA may yield different WT due to airway constriction (Fig. 2B). Therefore, it is reasonable to use WT rather than WA or WA% in the characterization of airway wall thickening (inflammation) dissociated from the airway narrowing (hyperresponsiveness).
Our laboratory's previous study (11) found that the global variable of lung shape, i.e., the ratio of apical-basal distance to ventral-dorsal distance, at TLC is a sensitive variable in differentiating asthmatic subjects from healthy subjects. Specifically, asthmatic subjects tend to have a smaller lung shape than healthy subjects. In this study, we investigated how the alteration of lung shape at a global level may affect the airway skeletal structure at TLC, and subsequently θ at a local level. The results have demonstrated that θ of several specific branches in severe asthmatic subjects are greater or smaller than those in healthy subjects. Intuitively, their spatial correlations with lung shape should be associated with reduced deformation on the apical-basal axis and with an elevated deformation on ventral-dorsal axis. Indeed, the co-planes of the daughter branches of the above branching angles are mostly aligned with one of these axes.
The bronchial noncircular shape may be caused by heterogeneous distributions of tissue or smooth muscle mass in circumference. The analysis showed that Cr significantly decreases in RMB, TriRLL, and sRML of severe asthmatic subjects, compared with that in healthy subjects (Table 3), particularly in RMB. WT (measured by WT*) increases in airway segments of the sRUL, LMB, LB1+2, and sLUL in severe asthmatic subjects. It is noted that the elevated WT is mainly observed in upper lobes of severe asthmatic subjects, wheras WT of nonsevere asthmatic subjects remain similar to those of healthy subjects. In fact, the alterations of θ, Cr, and WT* likely have little association with airway resistance, because their correlations with FEV1/FVC were weak (Fig. 6, A and B). This analysis supports that PFT-based functional alterations are less associated with airway wall thickening, consistent with the study of Kosciuch et al. (25). In their study, basement membrane thickness of bronchial airways was not correlated with PFT's variables. However, we also found that alterations of θ and WT* mainly observed in upper lobes are significantly correlated with lung hyperinflation of upper lobes measured by U/(M + L)|v. Thus we speculate that the altered θ and Cr, along with increased air volume change in the upper lobes, might increase regional particle deposition. This in turn could potentially cause local inflammation of the airway wall and subsequently lead to elevated WT at specific bronchi. Hypothetically, the altered θ and Cr reflecting airway remodeling observed in large airways could affect wall shear stress, particle deposition, and water loss, which require further investigation via computational fluid dynamics (10, 26, 42).
Although LA* and Dh* show similar characteristics, use of Dh* is more appropriate than LA* in quantitative assessment of airway narrowing, because it is directly associated with pressure drop of noncircular airways (41). We tested correlations of structural variables with different PFT measurements, expiratory image AirT% (air trapping), and J (volume change) between two CT scans at inspiratory and expiratory volumes using image registration. Among PFT variables, FEV1/FVC was most strongly correlated with Dh* in the lower lobar regions (Fig. 6C), indicating that the reduced Dh* in CT resolved airways was associated with the functional defect of initial forced expiration. Furthermore, RV% predicted values, AirT%, and volume change (J) were significantly correlated with Dh*. Thus we have confirmed that the constricted airways detected at TLC image are associated with the trapped air at FRC and the reduced volume changes between FRC and TLC in especially lower lobes.
Recently, Gupta et al. (17) performed imaging-phenotype-based cluster analysis. They grouped asthmatic subjects into three clusters: 1) severe air-trapping with wall thickening and luminal dilation; 2) moderate air-trapping; and 3) severe air-trapping and luminal constriction. Their severe asthmatic subjects were mostly grouped into clusters 1 and 3, and nonsevere asthmatic subjects were largely grouped into cluster 2. However, they only focused on luminal volume and wall volume of one specific branch (RB1) and air-trapping for the whole lung. In contrast, the present study examined local structural variables of all of the CT-resolved branches and functional variables at lobar level. Our findings demonstrate that airway narrowing is observed in lower lobar bronchi, and wall thickening is observed in upper lobar bronchi, indicating preferential regional occurrences of variable alterations. Therefore, it is desirable to perform more comprehensive cluster analyses including global (or lobar) variables of AirT% at FRC, Jacobian J and U/(M + L)|v between TLC and FRC, and lung shape at TLC as well as local structural variables of θ, Cr, WT*, and Dh*, which should classify subasthmatic populations more effectively. The imaging-derived clusters can be potentially associated with clinical phenotypes, such as allergy, onset of asthma, and FEV1 (20).
In conclusion, we examined local alterations of imaging-based structural variables in asthmatic subjects and their correlations with global and lobar variables. We found that θ in asthmatic subjects were locally different from those in healthy subjects, and the local alterations were associated with the lung shape at TLC and U/(M + L)|v. In addition, Cr of severe asthmatic subjects decreases, especially in RMB. More importantly, by using the improved normalization schemes instead of WA%, it was enabled to dissociate the effects of the airway constriction and wall thickening. Thus we demonstrated that Dh* is smaller in the lower lobes, and WT* is greater in the upper lobes, of severe asthmatic subjects. We also found that the local variable of Dh* observed at TLC is the variable most closely correlated with PFT-based variables, especially FEV1/FVC, and lobar variables of air-trapping at FRC (AirT%) and volume expansion from FRC to TLC (J). These findings suggest that these image-based variables may serve as sensitive metrics for assessing local airway alterations and for refining the cluster analysis and subgroupings of the asthmatic population.
GRANTS
This study was supported in part by National Institutes of Health Grants U01 HL-114494, R01 HL-094315, HL-064368, HL-112986, HL-69174, HL-069116, and S10 RR-022421.
DISCLOSURES
E. A. Hoffman is a shareholder in VIDA Diagnostics, which is commercializing lung image analysis software derived by the University of Iowa lung imaging group. He is also a member of the Siemens CT advisory board.
AUTHOR CONTRIBUTIONS
Author contributions: S.C. and C.-L.L. conception and design of research; S.C., E.A.H., S.E.W., M.C., and S.B.F. performed experiments; S.C., K.C., and C.-L.L. analyzed data; S.C., E.A.H., S.B.F., M.L.S., and C.-L.L. interpreted results of experiments; S.C. prepared figures; S.C. drafted manuscript; S.C., E.A.H., S.E.W., M.C., S.B.F., N.N.J., M.L.S., K.C., and C.-L.L. edited and revised manuscript; S.C., E.A.H., S.E.W., M.C., S.B.F., N.N.J., M.L.S., K.C., and C.-L.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Jiwoong Choi and Jered Sieren for assisting with data acquisition and analysis.
REFERENCES
- 1.Aysola R, de Lange EE, Castro M, Altes TA. Demonstration of the heterogeneous distribution of asthma in the lungs using CT and hyperpolarized helium-3 MRI. J Magn Reson Imaging 32: 1379–1387, 2010. [DOI] [PubMed] [Google Scholar]
- 2.Aysola RS, Hoffman EA, Gierada D, Wenzel S, Cook-Granroth J, Tarsi J, Zheng J, Schechtman KB, Ramkumar TP, Cochran R, Xueping E, Christie C, Newell J, Fain S, Altes TA, Castro M. Airway remodeling measured by multidetector CT is increased in severe asthma and correlates with pathology. Chest 134: 1183–1191, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beigelman-Aubry C, Capderou A, Grenier PA, Straus C, Becquemin MH, Similowski T, Zelter M. Mild intermittent asthma: ct assessment of bronchial cross-sectional area and lung attenuation at controlled lung volume. Radiology 223: 181–187, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 57: 289–300, 1995. [Google Scholar]
- 5.Breatnach E, Abbott GC, Fraser RG. Dimensions of the normal human trachea. AJR Am J Roentgenol 142: 903–906, 1984. [DOI] [PubMed] [Google Scholar]
- 6.Brooks LJ, Byard PJ, Helms RC, Fouke JM, Strohl KP. Relationship between lung-volume and tracheal area as assessed by acoustic reflection. J Appl Physiol 64: 1050–1054, 1988. [DOI] [PubMed] [Google Scholar]
- 7.Busacker A, Newell JD Jr, Keefe T, Hoffman EA, Granroth JC, Castro M, Fain S, Wenzel S. A multivariate analysis of risk factors for the air-trapping asthmatic phenotype as measured by quantitative CT analysis. Chest 135: 48–56, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Busse WW, Lemanske RF Jr. Asthma. N Engl J Med 344: 350–362, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Carroll N, Cooke C, James A. The distribution of eosinophils and lymphocytes in the large and small airways of asthmatics. Eur Respir J 10: 292–300, 1997. [DOI] [PubMed] [Google Scholar]
- 10.Choi J, Tawhai MH, Hoffman EA, Lin CL. On intra- and intersubject variabilities of airflow in the human lungs. Phys Fluids (1994) 21: 101901, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi S, Hoffman EA, Wenzel SE, Castro M, Lin CL. Improved CT-based estimate of pulmonary gas trapping accounting for scanner and lung volume variations in a multicenter study. J Appl Physiol 117: 593–603, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi S, Hoffman EA, Wenzel SE, Tawhai MH, Yin Y, Castro M, Lin CL. Registration-based assessment of regional lung function via volumetric ct images of normal subjects vs. severe asthmatics. J Appl Physiol 115: 730–742, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Jong PA, Long FR, Wong JC, Merkus PJ, Tiddens HA, Hogg JC, Coxson HO. Computed tomographic estimation of lung dimensions throughout the growth period. Eur Respir J 27: 261–267, 2006. [DOI] [PubMed] [Google Scholar]
- 14.de Jong PA, Muller NL, Pare PD, Coxson HO. Computed tomographic imaging of the airways: relationship to structure and function. Eur Respir J 26: 140–152, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Fain SB, Peterson ET, Sorkness RL, Wenzel S, Castro M, Busse WW. Severe asthma research program-phenotyping and quantification of severe asthma. Imaging Decisions MRI 13: 24–27, 2009. [Google Scholar]
- 16.Griscom NT, Wohl ME. Dimensions of the growing trachea related to age and gender. AJR Am J Roentgenol 146: 233–237, 1986. [DOI] [PubMed] [Google Scholar]
- 17.Gupta S, Hartley R, Khan UT, Singapuri A, Hargadon B, Monteiro W, Pavord ID, Sousa AR, Marshall RP, Subramanian D, Parr D, Entwisle JJ, Siddiqui S, Raj V, Brightling CE. Quantitative computed tomography-derived clusters: redefining airway remodeling in asthmatic patients. J Allergy Clin Immunol 133: 729–738, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta S, Siddiqui S, Haldar P, Entwisle JJ, Mawby D, Wardlaw AJ, Bradding P, Pavord ID, Green RH, Brightling CE. Quantitative analysis of high-resolution computed tomography scans in severe asthma subphenotypes. Thorax 65: 775–781, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.James AL, Paré PD, Hogg JC. The mechanics of airway narrowing in asthma. Am Rev Respir Dis 139: 242–246, 1989. [DOI] [PubMed] [Google Scholar]
- 20.Jarjour NN, Erzurum SC, Bleecker ER, Calhoun WJ, Castro M, Comhair SA, Chung KF, Curran-Everett D, Dweik RA, Fain SB, Fitzpatrick AM, Gaston BM, Israel E, Hastie A, Hoffman EA, Holguin F, Levy BD, Meyers DA, Moore WC, Peters SP, Sorkness RL, Teague WG, Wenzel SE, Busse WW, and for the NHLBI Severe Asthma Research Program (SARP). Severe asthma: lessons learned from the National Heart, Lung, and Blood Institute Severe Asthma Research Program. Am J Respir Crit Care Med 185: 356–362, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeffery PK. Remodeling in asthma and chronic obstructive lung disease. Am J Respir Crit Care Med 164: S28–S33, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Kasahara K, Shiba K, Ozawa T, Okuda K, Adachi M. Correlation between the bronchial subepithelial layer and whole airway wall thickness in patients with asthma. Thorax 57: 242–246, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kosciuch J, Krenke R, Gorska K, Zukowska M, Maskey-Warzechowska M, Chazan R. Airway dimensions in asthma and COPD in high resolution computed tomography: can we see the difference? Respir Care 58: 1335–1342, 2013. [DOI] [PubMed] [Google Scholar]
- 24.Kosciuch J, Krenke R, Gorska K, Zukowska M, Maskey-Warzechowska M, Chazan R. Relationship between airway wall thickness assessed by high-resolution computed tomography and lung function in patients with asthma and chronic obstructive pulmonary disease. J Physiol Pharmacol 60: 71–76, 2009. [PubMed] [Google Scholar]
- 25.Kościuch J, Przybyłowski T, Górska K, Krenke R, Baran W, Kujawa M, Hildebrand K, Chazan R. Relationship between airway basement membrane thickness and lung function tests in patients with asthma. Pneumonol Alergol Pol 77: 256–263, 2009. [PubMed] [Google Scholar]
- 26.Miyawaki S, Tawhai MH, Hoffman EA, Lin CL. Effect of carrier gas properties on aerosol distribution in a CT-based human airway numerical model. Ann Biomed Eng 40: 1495–1507, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montaudon M, Lederlin M, Reich S, Begueret H, Tunonde-Lara JM, Marthan R, Berger P, and Laurent F. Bronchial measurements in patients with asthma: comparison of quantitative thin-section CT findings with those in healthy subjects and correlation with pathologic findings. Radiology 253: 844–853, 2009. [DOI] [PubMed] [Google Scholar]
- 28.Moore WC, Bleecker ER, Curran-Everett D, Erzurum SC, Ameredes BT, Bacharier L, Calhoun WJ, Castro M, Chung KF, Clark MP, Dweik RA, Fitzpatrick AM, Gaston B, Hew M, Hussain I, Jarjour NN, Israel E, Levy BD, Murphy JR, Peters SP, Teague WG, Meyers DA, Busse WW, Wenzel SE, and for the National Heart Lung Blood Institute's Severe Asthma Research Program. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute's Severe Asthma Research Program. J Allergy Clin Immunol 119: 405–413, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, D'Agostino R Jr, Castro M, Curran-Everett D, Fitzpatrick AM, Gaston B, Jarjour NN, Sorkness R, Calhoun WJ, Chung KF, Comhair SA, Dweik RA, Israel E, Peters SP, Busse WW, Erzurum SC, Bleecker ER, and for the National Heart Lung Blood Institute's Severe Asthma Research Program. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med 181: 315–323, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newman KB, Lynch DA, Newman LS, Ellegood D, Newell JD. Quantitative computed tomography detects air trapping due to asthma. Chest 106: 105–109, 1994. [DOI] [PubMed] [Google Scholar]
- 31.Niimi A, Matsumoto H, Amitani R, Nakano Y, Mishima M, Minakuchi M, Nishimura K, Itoh H, Izumi T. Airway wall thickness in asthma assessed by computed tomography: relation to clinical indices. Am J Respir Crit Care Med 162: 1518–1523, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Pohlert T. The Pairwise Multiple Comparison of Mean Ranks Package (PMCMR). Vienna, Austria: R Package, 2014. [Google Scholar]
- 33.Smith BM, Hoffman EA, Rabinowitz D, Bleecker E, Christenson S, Couper D, Donohue KM, Han MK, Hansel NN, Kanner RE, Kleerup E, Rennard S, Barr RG. Comparison of spatially matched airways reveals thinner airway walls in COPD. The Multi-Ethnic Study of Atherosclerosis (MESA) COPD Study and the Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS). Thorax 69: 987–996, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sorkness RL, Bleecker ER, Busse WW, Calhoun WJ, Castro M, Chung KF, Curran-Everett D, Erzurum SC, Gaston BM, Israel E, Jarjour NN, Moore WC, Peters SP, Teague WG, Wenzel SE, and for the National Heart Lung and Blood Institute's Severe Asthma Research Program. Lung function in adults with stable but severe asthma: air trapping and incomplete reversal of obstruction with bronchodilation. J Appl Physiol 104: 394–403, 2008. [DOI] [PubMed] [Google Scholar]
- 35.Stocks J, Quanjer PH. Reference values for residual volume, functional residual capacity and total lung capacity. Eur Respir J 8: 492–506, 1995. [DOI] [PubMed] [Google Scholar]
- 36.Svenningsen S, Kirby M, Starr D, Coxson HO, Paterson NA, McCormack DG, Parraga G. What are ventilation defects in asthma? Thorax 69: 63–71, 2014. [DOI] [PubMed] [Google Scholar]
- 37.The R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2013. [Google Scholar]
- 38.Tschirren J, Hoffman EA, McLennan G, Sonka M. Intrathoracic airway trees: segmentation and airway morphology analysis from low-dose CT scans. IEEE Trans Med Imaging 24: 1529–1539, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wadell H. Sphericity and roundness of rock particles. J Geol 41: 310–331, 1933. [Google Scholar]
- 40.Wenzel SE, Busse WW; National Heart Lung, and Blood Institute's Severe Asthma Research Program. Severe asthma: lessons from the severe asthma research program. J Allergy Clin Immunol 119: 14–21, 2007. [DOI] [PubMed] [Google Scholar]
- 41.White FM. Fluid Mechanics. New York: McGraw-Hill, 2011. [Google Scholar]
- 42.Wu D, Tawhai MH, Hoffman EA, Lin CL. A numerical study of heat and water vapor transfer in mdct-based human airway models. Ann Biomed Eng 42: 2117–2131, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]