Abstract
Prevalence and severity of postmyocardial infarction heart failure continually escalate in type 2 diabetes via incompletely understood mechanisms. The discovery of the cardiac secretomes, collectively known as “cardiokines”, has significantly enhanced appreciation of the local microenvironment's influence on disease development. Recent studies demonstrated that C1q-TNF-related protein-9 (CTRP9), a newly discovered adiponectin (APN) paralog, is highly expressed in the heart. However, its relationship with APN (concerning diabetic cardiovascular injury in particular) remains unknown. Plasma CTRP9 levels are elevated in APN knockout and reduced in diabetic mice. In contrast to APN, which circulates as full-length multimers, CTRP9 circulates in the plasma primarily in the globular domain isoform (gCTRP9). Recombinant full-length CTRP9 (fCTRP9) was cleaved when incubated with cardiac tissue extracts, generating gCTRP9, a process inhibited by protease inhibitor cocktail. gCTRP9 rapidly activates cardiac survival kinases, including AMPK, Akt, and endothelial NOS. However, fCTRP9-mediated kinase activation is much less potent and significantly delayed. Kinase activation by fCTRP9, but not gCTRP9, is inhibited by protease inhibitor cocktail. These results demonstrate for the first time that the novel cardiokine CTRP9 undergoes proteolytic cleavage to generate gCTRP9, the dominant circulatory and actively cardioprotective isoform. Enhancing cardiac CTRP9 production and/or its proteolytic posttranslational modification are of therapeutic potential, attenuating diabetic cardiac injury.
Keywords: adiponectin, cardiokine, CTRP9, proteolysis, diabetes
mortality from ischemic heart disease exceeds 60% in type 2 diabetic patients (1). Affecting 23.6 million people in the United States, diabetes continues to increase in prevalence, a trend unlikely to change in the short term. Improved reperfusion technologies and strategies have decreased mortality in nondiabetic patients following acute myocardial infarction (AMI), but both severity and prevalence of post-AMI heart failure (HF) in type 2 diabetic patients have increased (12). Complex unknown mechanisms are responsible for exacerbated diabetic heart injury after ischemic insult, leading to deleterious cardiac remodeling and poor outcome in diabetic patients. As diabetes and its collateral consequences continually escalate, so does the urgency of identifying new therapeutic strategies ameliorating diabetic ischemic injuries.
Adiponectin (APN) is an abundant protein primarily produced by the body's adipocytes. Since its discovery in 1995, it has been intensely investigated by researchers of various body system fields. Among APN's major metabolic functions, its vasculoprotective and anti-ischemic properties are of great therapeutic interest in the setting of diabetic ischemic injury. However, several limitations detract from the employability of APN as a therapeutic ideal. First, although we have demonstrated that APN is indeed locally produced in the heart (31a), such quantities are dwarfed by the circulatory amounts produced by the body's adipose reserves. Second, APN-deficient animals manifest an unimpressive phenotype not exhibiting derangement until metabolically challenged, suggesting the existence of compensatory mechanisms at play in the absence of APN. Third, the most biologically active isoform of APN is its globular COOH-terminal domain (gAPN), postulated to exist in vivo (8). However, in practical terms, circulatory gAPN levels are virtually undetectable.
The C1q tumor necrosis factor (TNF)-related proteins (CTRPs) are a newly discovered family of adiponectin paralogs (5, 37). Studies have described various metabolic properties of select CTRP members 1 through 15. Of all CTRPs identified thus far, CTRP9 shares the greatest amino acid overlap with APN (36). Similarly to APN, total plasma CTRP9 levels are significantly reduced in diabetic animals (27). However, a very recent study demonstrated that, in dissimilar fashion to APN-deficient mice (which lack phenotypic changes under physiological conditions), CTRP9-deficient mice were obese and insulin resistant and developed hepatic steatosis with reduced skeletal muscle AMPK activation and mitochondrial content (41). Such results provided genetic evidence for a physiological role of CTRP9, suggesting that CTRP9 may play a significant role in cardiovascular homeostasis.
The cellular secretome influences the local microenvironment, controlling disease development (18). The secretomes produced by the heart, collectively known as “cardiokines”, are increasingly recognized as essential regulators of cardiac physiology and pathology (25). Emerging evidence indicates that CTRP9, unlike APN, is not a typical adipokine and may function as a cardiokine. The initial CTRP tissue distribution study (employing semiquantitative PCR) demonstrated that the heart is the third most abundantly CTRP9-expressing organ (36). We (27) recently demonstrated that CTRP9 is the most abundantly expressed adipokine in the heart, exceeding local APN expression more than 100-fold, with local cardiac CTRP9 levels exceeding plasma CTRP9 levels more than twofold. A very recent study utilizing fully quantitative PCR reported that CTRP9 mRNA is predominantly expressed in the heart, at levels 2.5-fold greater than adipocyte expression levels (2). Moreover, several recent studies, including those from our laboratory, demonstrated that CTRP9 is a potent cardioprotective molecule capable of attenuating acute ischemia/reperfusion injury, post-MI pathological ventricular remodeling, and ischemic heart failure in nondiabetic and diabetic animals (14, 27, 28). Collectively, considerable evidence exists supporting CTRP9 as a novel protective cardiokine attenuating diabetic cardiovascular injury.
The aims of the current study were to determine 1) whether CTRP9 production is altered in diabetes and the APN knockout (KO) condition, 2) whether CTRP9 is a novel cardiokine, and 3) whether CTRP9 is cleaved, producing a globular domain format, as recently reported for CTRP12.
MATERIALS AND METHODS
Materials and animals.
Antibody specific against globular CTRP9 was kindly provided by Dr. Wong (Dept. of Physiology, Johns Hopkins University School of Medicine, Baltimore, MD). Antibodies against p-AMPK (Thr172)/AMPK, p-Akt (Ser473)/Akt, p-eNOS (Ser1177)/eNOS, and horseradish peroxidase-conjugated secondary antibody were purchased from Cell Signaling Technology (Danvers, MA). Monoclonal antibody for ANTI-FLAG M2 was purchased from Sigma (St. Louis, MO). Monoclonal Anti-c-Myc-tag antibody was purchased from Millipore (Billerica, MA). All primers employed in gene cloning and vector construction were obtained from Integrated DNA Technologies (IDT, Coralville, IA). All animal experiments in this study were performed in accordance with the National Institutes of Health Guidelines on the Use of Laboratory Animals and were approved by the Thomas Jefferson University Committee on Animal Care. The high-fat diet-induced type 2 diabetic mouse model utilized was established as previously reported (41). Adult (6 wk old) male C57BL/6J mice were randomized and fed a high-fat diet (HFD; 60% kcal% fat, Research Diets, D12492i) or normal diet (ND, 10% kcal% fat, D12450Bi) for 8–16 wk.
CTRP9 gene cloning, protein purification, and 3T3-L1 cell transfection.
Total RNA was extracted from mouse adipose tissue by RNeasy Lipid Tissue Mini Kit (Qiagen, Valencia, CA), and 5 μg of total RNA was used for reverse transcription with a SuperScript III First-Strand Synthesis System Kit (Life Technologies, Grand Island, NY). The synthesized cDNA was used to amplify gCTRP9 (AA 194-333) or full-length CTRP9 (fCTRP9) gene by CloneAmp HiFi PCR Premix (Clontech Laboratories, Mountain View, CA). The DNA sequences of gCTRP9 were inserted into prokaryotic expression vector pET45b (Novagen, Billerica, MA) using an In-Fusion HD Plus EcoDry Cloning System (Clontech Laboratories, Mountain View, CA). DE3 bacteria carrying gCTRP9 plasmids were cultured in LB broth, and gCTRP9 protein was purified by Ni-NTA column resin under native conditions, as previously reported (41). Endotoxin was removed (<1 EU/μg protein) by ActiClean Etox exdotoxin cut-off resin (Sterogene, Carlsbad, CA). Proteins were concentrated and desalted by Amicon Ultra-15 filter (Millipore, Billerica, MA) in PBS buffer. The fCTRP9 gene was inserted into COOH-terminal-FLAG Tag eukaryotic expression vector pCMV Tag 4A (Stratagene, La Jolla, CA) or dual-Tag eukaryotic expression vector p3xFLAG-Myc-CMV-24 vector (Sigma) by In-Fusion HD Plus EcoDry Cloning System (Clontech Laboratories). The endotoxin-free plasmid was prepared by PureYield Plasmid Miniprep System (Promega, Madison, WI) for HEK 293T cell transfection by calcium phosphate method as reported before (4). CTRP9 in culture medium (containing fCTRP9 and its cleaved fragments) and cell lysates (containing fCTRP9 only) was purified by Anti-Flag M2 Affinity Gel and eluted via 100–150 μg/ml FLAG peptides, concentrated, and desalted as described above.
3T3-L1 preadipocytes were cultured in high-glucose DMEM complete culture medium supplemented with 10% fetal bovine serum and antibiotics. At around 70–80% confluence, cells were transfected by Xfect Transfection Reagent (Clontech Laboratories) per the manufacturer's instructions. After 48 h culture and 3× PBS washes, the transfected medium and cell lysates were analyzed by Western blot.
Adult cardiac cell isolation and culture.
Hearts were removed under 2% isoflurane anesthesia and perfused at 37°C for 30 s in Langendorff perfusion system with a calcium-free bicarbonate-based buffer. Enzymatic digestion was initiated by adding collagenase type B/D (Roche) to the perfusion solution. Ca2+ (50 μM) was added to the enzyme solution 5 min after digestion. The heart was continually perfused for another 10 min. The left ventricle was then removed, cut into several sections, and further digested in a shaker for 10 min at 37°C in the same enzyme solution. The supernatant containing the dispersed cardiac cells (cardiomyocytes and cardiac fibroblast cells) was filtered into a sterilized tube, and centrifuged at 800 g for 1 min. The cell pellet was then resuspended in bicarbonate-based buffer containing 125 μM Ca2+ and planted in laminin precoated culture dishes. CTRP9 protein expression levels in cultured cardiac cell medium were detected by mouse CTRP9 ELISA kit (Aviscera Bioscience) per the manufacturer's instructions.
Quantitative real-time PCR.
Epididymal fat pad and heart were removed under isoflurane anesthesia. Total tissue RNA was extracted via RNeasy Mini Kit (Qiagen). First-strand cDNA was synthesized from total RNA by Superscript III First Strand cDNA Synthesis Kit (Life Technologies, Grand Island, NY). Real-time PCR was performed on the ABI 7900 using SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA) per the manufacturer's protocol. Forward/reverse primer sets for CTRPs, APN, and 18S were established per previously published protocols (36).
Western blot analysis.
Proteins in serum (purified using a Pierce Albumin and IgG Removal Kit; Pierce, Rockford, IL), cell lysates, or conditioned medium were separated on sodium dodecyl sulfate-polyacrylamide electrophoresis gels and transferred to PVDF membrane (Millipore, Billerica, MA) by semidry transfer system (Bio-Rad, Hercules, CA). After 5% nonfat milk blocking, the membrane was incubated with primary antibodies at 4°C overnight followed by horseradish peroxidase-conjugated secondary antibody. The membrane was developed with a SuperSignal West Femto Chemiluminescent Substrate detection kit (Pierce) and visualized with Kodak Image Station 4000R Pro (Rochester, NY).
Statistical analysis.
All values in the text and figures are presented as means ± SE of n independent experiments. All data (except Western blot density) were subjected to ANOVA followed by Tukey correction for post hoc t-test. Western blot densities were analyzed by Kruskal-Wallis test followed by Dunn's post hoc test. Probabilities of 0.05 or less were considered statistically significant.
RESULTS
Identification of CTRP9 as an important molecule in diabetes.
Because CTRPs are novel APN paralogs (and APN is a potent cardiovascular protective molecule), we were intrigued that CTRPs might play significant roles in diabetic cardiovascular complications. To determine which of the fifteen CTRP members identified to date might act as overlapping regulators compensating the beneficial actions of APN in APN-KO animals, two experiments were performed.
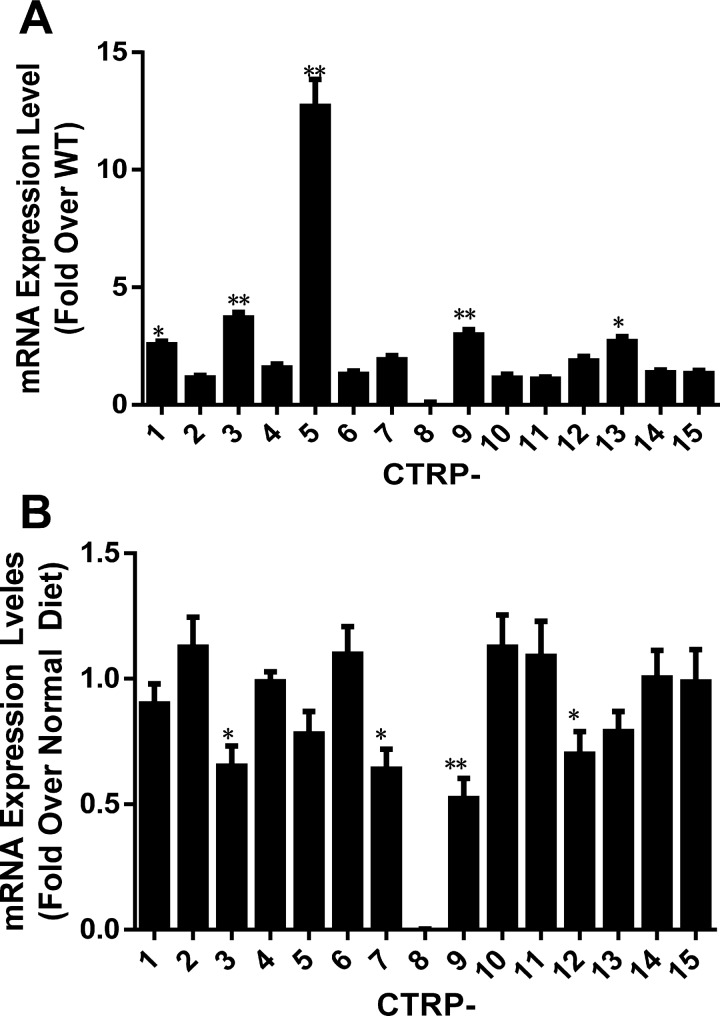
First, CTRP transcript levels in the adipose tissues of wild-type (WT) and APN-KO mice fed normal diet (ND) were screened. As summarized in Fig. 1A, among 15 CTRPs, five CTRPs were significantly upregulated (>2-fold) in APN-KO mice compared with WT mice. Second, CTRP transcript levels were determined in the adipose tissues of C57BL/6J mice fed ND or HFD for 8 wk, a time point previously demonstrated by our laboratory at which HFD-induced type 2 diabetes develops in C57BL/6J mice. As summarized in Fig. 1B, four CTRPs were significantly (>25% reduction) reduced in HFD-induced diabetic animals. Taken together, the data from APN-KO and HFD-induced diabetic animals suggest that two CTRPs (CTRP3 and CTRP9) are promising CTRP candidates involved in diabetic cardiovascular protection.
Fig. 1.
Gene expression changes of 15 C1q/TNF-related proteins (CTRPs) in adiponectin knockout (APN-KO) mice and type 2 diabetic mice. A: CTRP mRNA expression levels in adipose tissues of APN-KO mice normalized against WT mice. B: adipose CTRP mRNA expression levels in high-fat diet (HFD, 8 wk) induced type 2 diabetic mice normalized against mice fed normal diet (ND); n = 4–6 mice/group. *P < 0.05, **P < 0.01 vs. control.
CTRP9 is a novel cardiokine.
Cardiac cells (including myocytes, fibroblasts, vascular cells, and progenitor cells) secrete various regulatory proteins, collectively known as cardiokines (6, 25), which regulate cardiac function/structure. As cardiac APN expression levels are several orders of magnitude below those of adipocytes (<1:100,000), APN is an unlikely physiologically relevant cardiokine (31a). To determine whether any CTRP might function as a cardiokine, several experiments were performed.
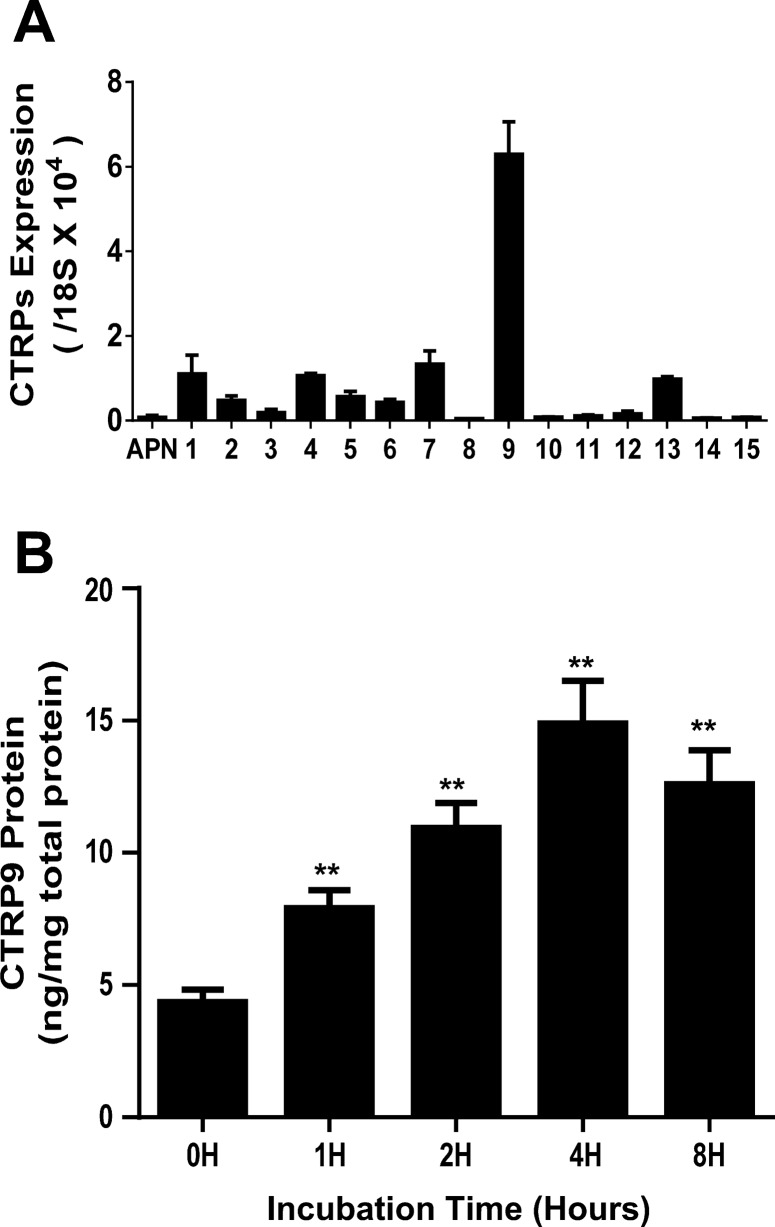
First, mRNA expression levels of 15 CTRPs were determined concomitantly with APN in C57BL/6J mouse hearts. Several CTRPs (including CTRP1, CTRP4, CTRP7, CTRP9, and CTRP13) were expressed in the heart at levels significantly greater than that of APN. Most notably, the mRNA level of CTRP9 exceeded APN's more than 100-fold in cardiac tissue (Fig. 2A). Second, adult cardiac cells were isolated and cultured in vitro. Conditioned medium was collected 1–8 h after culture, and CTRP9 protein levels were determined by ELISA. A significant increase of CTRP9 in conditioned medium was detected in as early as 1 h in culture, steadily accumulating thereafter (Fig. 2B). These results provided evidence that CTRP9 is a novel cardiokine with significantly inhibited expression when mice are fed HFD for 8 wk or longer, a time point when HFD-induced type 2 diabetes develops (41).
Fig. 2.
CTRP expression in WT mouse heart tissue and cardiac cells. A: CTRP mRNA expression levels compared with APN in WT mice heart; n = 4–6 mice/group. B: CTRP9 protein concentration in adult cardiac cell culture medium at different time points (h); n = 8–10 mice/group. **P < 0.01 vs. control.
CTRP9 undergoes proteolytic cleavage, producing gCTRP9.
Proteolysis is one of the most important posttranslational modifications of the proteome. Whereas it is debated whether APN undergoes proteolytic cleavage with biological relevance in vivo, recent studies provide clear evidence that CTRPs (particularly CTRP12) circulate primarily as globular domain isoforms. Additionally, full-length CTRP12 (fCTRP12) and the globular domain of CTRP12 (gCTRP12) preferentially activate different downstream signaling pathways (34). Our next discovery relating to the proteolytic processing of CTRP9 originated from our initial CTRP9 experiments.
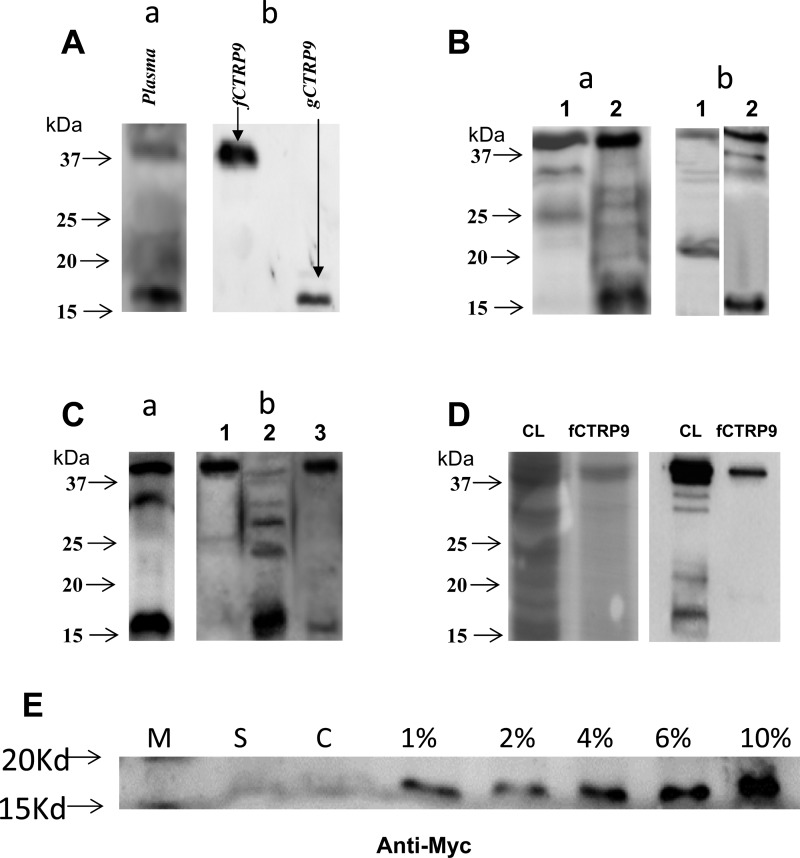
During our initial CTRP9 experiments, two bands were observed when Western blot was run on plasma utilizing antibody against CTRP9. The antibody we employed to detect CTRP9 specifically recognized an epitope against COOH-terminal sequences of CTRP9. The upper band exhibited molecular mass slightly exceeding 37 kDa, corresponding to intact CTRP9 with glycosylation, as previously reported (36). We did not initially pay special attention to the lower band, believing it to be a nonspecific reaction. However, as we repeated Western blots in independent experiments, we consistently found tht the lower band was a CTRP9 fragment consisting of the COOH-terminal sequences. In purified plasma samples, the lower band is much stronger than the upper band (Fig. 3Aa). Possessing a molecular mass of ∼16 kDa, the lower band corresponded to the calculated molecular mass of the COOH-terminal gCTRP9. We have obtained the following evidence supporting that this cleaved fragment is indeed gCTRP9.
Fig. 3.
A: identification of 2 CTRP9 isoforms in plasma. gCTRP9, globular domain isoform; fCTRP9, recombinant full-length isoform. a: mouse plasma CTRP9 determined by anti-gCTRP9. b: fCTRP9 and gCTRP9 expressed in E. coli. Typical blots from ≥5 independent experiments. B: overexpressed CTRP9 in mammalian cell produce 2 protein isoforms in medium. a: COOH-terminal FLAG-tagged fCTRP9 was expressed in 3T3-L1 cells, CTRP9 in cell lysates (lane 1) or conditioned medium (lane 2) was detected by antibody against FLAG. b: Flag-Myc duo-tagged fCTRP9 was expressed in HEK 293T cells, and CTRP9 in medium was detected by antibody against either Flag (lane 1) or Myc (lane 2). Typical blots from ≥5 independent experiments. C: purified fCTRP9 protein is proteolytic modified by heart tissue lyses. a: cardiac endogenous CTRP9 was detected with antibody against gCTRP9. b: HEK 293T cells expressed fCTRP9-FLAG-Tag (purified from cell lysate) was incubated with homogenized tissue buffer (lane 1) or cardiac tissue extracts in the absence (lane 2) or presence (lane 3) of protease inhibitor cocktail. CTRP9 is detected with antibody against FLAG. Typical blots from ≥5 independent experiments. D: Coomassie blue and Western blot analysis of purified fCTRP9 protein. Left: Coomassie brilliant blue staining (on M2 affinity gel) of fCTRP9-transfacted HEK 293T cell lysates (CL) or purified fCTRP9 from transfected cells (fCTRP9). Right: Western blot analysis (anti-CTRP9) of fCTRP9-transfacted HEK 293T cell lysates (CL) or purified fCTRP9 from the transfected cells (fCTRP9). E: Western blot determining presence of gCTRP9 fragment (anti-Myc) in cultured medium with varying FBS %concentration. M, marker; S, nontransfected HEK 293 cells, cultured in 10% FBS-MEM; C, pCMV24-3XFLAG-fCTRP9-Myc transfected HEK 293T cells, cultured in 0% FBS-MEM medium; 1%–10%: pCMV24-3XFLAG-fCTRP9-Myc transfected HEK 293T cells, cultured in indicated %FBS-MEM medium for 48 h.
First, Eschericchia coli-expressed gCTRP9 (Fig. 3Ab, lane 2) was detected at the same molecular location with the CTRP9 fragment detected in mouse plasma (Fig. 3Aa). However, gCTRP9 was not detected when fCTRP9 was expressed in E. coli, indicating fCTRP9 is not cleaved in E. coli.
Second, a previous study suggested that gAPN is produced extracellularly by leukocyte-derived elastase (31). However, a recent study reported that gCTRP12 is produced intracellularly (34). To determine whether CTRP9 is cleaved extra- or intracellularly, COOH-terminal FLAG-tagged fCTRP9 was expressed in 3T3-L1 adipocytes. CTRP9 was assessed in conditioned medium or cells (after extensive washing) via Western blot using anti-FLAG antibody. Only intact CTRP9 was detected in cell lysis samples (Fig. 3Ba, lane 1). In contrast, intact and fragmented CTRP9 (similar to that observed in plasma) were detected in conditioned medium (Fig. 3Ba, lane 2). This result indicates that CTRP9 is cleaved during and/or after its secretion from 3T3-L1 cells into culture medium. Notably, mammalian (Fig. 3Ba, lane 2), but not bacterial (Fig. 3Ab, lane 1), cells have the ability to generate fragmented CTRP9.
Third, to obtain more direct evidence that CTRP9 is cleaved in mammalian cells, dual-tag-fCTRP9 plasmid (pCMV24-3XFLAG-fCTRP9-Myc) was generated and transfected into HEK 293T cells. CTRP9 in conditioned medium was detected by anti-FLAG (NH2-terminal) or anti-Myc (COOH-terminal) antibody. Two bands were clearly detected with antibody against either NH2-terminal FLAG or COOH-terminal Myc (Fig. 3Bb). Intact CTRP9 localizes at the same position regardless of the antibody employed. However, fragmented CTRP9 is detected at different positions dependent on the antibody employed. A cleaved form (size 20–25 kDa) was detected with antibody against NH2-terminal tagged FLAG (Fig. 3Bb, lane 1), whereas a cleaved form (size 15–20 kDa) was detected with antibody against COOH-terminal tagged Myc (Fig. 3Bb, lane 2). These results provide clear evidence that full-length CTRP9 is cleaved, generating smaller COOH-terminal fragments and larger NH2-terminal fragments in mammalian cells.
Fourth, since CTRP9 is highly expressed in the heart, we determined whether locally produced CTRP9 is cleaved in the heart. Both intact and fragmented CTRP9 were detected in cardiac tissue after lysis, via antibody specific against the COOH-terminal region of CTRP9 (Fig. 3Ca). In addition, the COOH-terminal FLAG-tagged fCTRP9 protein purified from HEK 293T cells was incubated with cardiac tissue extracts for 1–4 h in the presence or absence of a protease inhibitor cocktail. CTRP9 was detected by antibody against FLAG. As illustrated in Fig. 3Cb, fragmented CTRP9 was not detected in samples incubated with homogenized buffer solution (lane 1) but was clearly detected in samples incubated with cardiac tissue extract for 2 h (lane 2). Protease inhibitor administration blocked this process (lane 3). The purity of the recombinant fCTRP9 protein was determined by a coomasie blue gel and Western blot analysis (Fig. 3D).
CTRP9 was first reported by Wong et al. (36), but the production of gCTRP9 was not described in that study. Since the FBS concentration utilized during the transfection was different in these two experiments (0 vs. 10% in the current study), we thought the presence of FBS might be required for gCTRP9 cleavage. A new experiment was conducted to test this hypothesis. pCMV24-3XFLAG-fCTRP9-Myc plasmid was transfected into HEK 293T cells via calcium phosphate method in 0% FBS medium. After transfection, FBS was added in concentrations of 1, 2, 4, 6, and 10%. Western blot for the gCTRP9 fragment commenced after 48 h of culture. In FBS dose-dependent fashion, the presence of the gCTRP9 fragment was significantly increased when detected by antibody against Myc (which does not recognize plasma CTRP9; Fig. 3E).
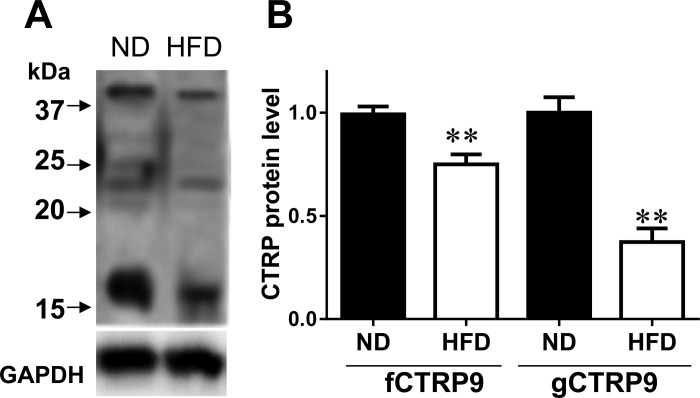
Having demonstrated that gCTRP9 is the primary isoform both produced in the heart and circulating in the plasma, we determined whether gCTRP9 levels are reduced in the diabetic heart. As illustrated in Fig. 4, both fCTRP9 and gCTRP9 were significantly reduced in the diabetic heart, with gCTRP9 reduction being more significant. This result suggests that diabetes inhibits cardiac CTRP9 gene expression as well as its proteolytic cleavage.
Fig. 4.
Levels of fCTRP9 and gCTRP9 in mouse heart are significantly reduced after 8 wk of HFD feeding. A: fCTRP9 and gCTRP9 proteins were detected by antibody against COOH terminus of CTRP9. B: blot densities of fCTRP9 and gCTRP9 were analyzed. Data normalized against mean of ND-fed animals; n = 6–7 animals/group. **P < 0.01 vs. ND.
gCTRP9 is the biologically active isoform.
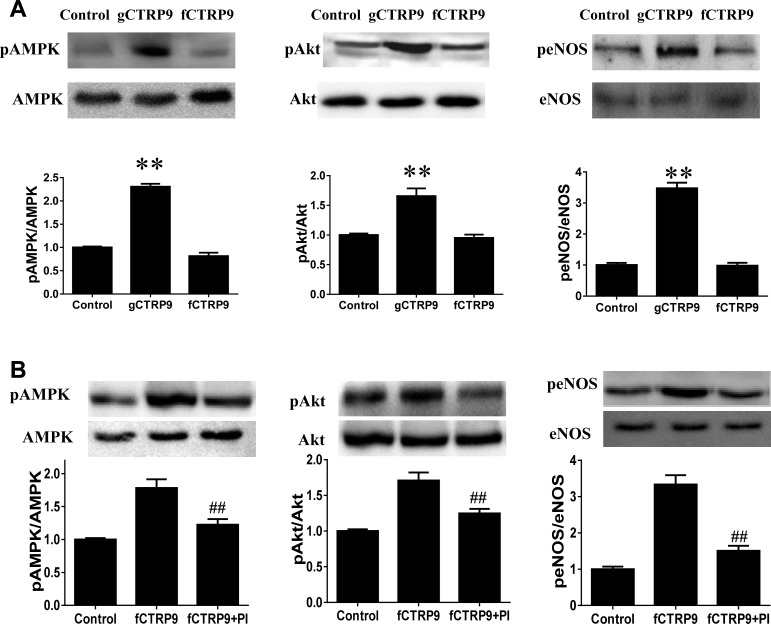
To gain more insight into the biological significance of gCTRP9 production, isolated adult cardiomyocytes were treated with gCTRP9 or fCTRP9 (from HEK 239 cell lysis) for 30 min. As summarized in Fig. 5A, treatment of isolated adult cardiomyocytes with gCTRP9 (500 ng/ml) significantly activated key survival kinases, including Akt, AMPK, and eNOS. However, treatment of cardiomyocytes with fCTRP9 at concentrations up to 3 μg/ml for 30 min yielded no significant effect on survival kinases. However, when the incubation time of fCTRP9 was extended to 2 h, significant AMPK, Akt, and eNOS phosphorylation was observed (Fig. 5B). Most importantly, kinase activation activity of fCTRP9 was inhibited when a protease inhibitor cocktail was added (Fig. 5B, rightmost bar). Our preliminary data demonstrated that protease cocktail inhibitors in isolation do not have any direct effect on AMPK/Akt/eNOS phosphorylation (data not shown). These results indicate that proteolytic cleavage of fCTRP9 is a necessary posttranslational modification that results in generation of the biologically active isoform, gCTRP9.
Fig. 5.
gCTRP9 is the active form of CTRP9 protein. A: treatment with gCTRP9 (0.5 μg/ml) but not fCTRP9 (3 μg/ml) for 30 min activates AMPK, Akt, and eNOS in adult cardiomyocytes. B: treatment with fCTRP9 (3 μg/ml) for 2 h activates AMPK, Akt, and eNOS. Cotreatment with protease inhibitor (PI) cocktail blocked kinase activation by fCTRP9; n = 10–12 dishes from ≥3 mice/group. **P < 0.01 vs. control; ##P < 0.01 vs. fCTRP9.
DISCUSSION
Our current study demonstrates several novel findings. First, CTRP9 mRNA is abundantly expressed in the heart, supporting the reclassification of CTRP9 as a cardiokine. Second, CTRP9 primarily circulates in the plasma as the gCTRP9 isoform. This is different from APN, which circulates in the plasma as multimers containing full-length APN. Third, cardiac tissue lysates possess a strong ability to cleave fCTRP9 into gCTRP9. Fourth, gCTRP9, but not fCTRP9, is capable of activating key cardiac survival kinases.
Diabetes is costly, both financially and in terms of health complications. Cardiovascular complications, particularly ischemic heart disease, are the primary cause of death in diabetic patients, who have substantially increased risk for heart failure (HF) development after similar initial ischemic insult, and endure worse prognosis and greater mortality (2- to 6-fold) compared with nondiabetic HF patients (3, 13, 22). Post-MI mortality in diabetic patients is not decreased by tightened glycemic control; recent large-scale clinical trials failed to demonstrate the cardiovascular mortality benefit of strict glycemic control in diabetic patients (7, 16). More than ever, efficacious therapies attenuating diabetic cardiovascular complications are needed.
Adiponectin, the adipokine regarded as “adipocyte-derived insulin”, is the most extensively investigated cardioprotective adipokine. Reduced APN levels correlate with increased AMI risk (11, 15, 17), as well as worse cardiac functional recovery after MI with reperfusion (23, 24). Exogenous APN supplementation significantly protects the heart against ischemic injury (24, 29). However, the cardioprotective effects of APN are significantly attenuated in diabetic animals (41). Moreover, complete APN abrogation results in only mild phenotypic change unless pathologically challenged (e.g., by high-fat diet or ischemia), suggesting existent overlapping regulators. Efforts to identify such regulators have led to the discovery of a family of APN paralogs, designated the C1q/TNF-related proteins (CTRP1 to CTRP15) (20, 37).
All CTRPs are secreted proteins sharing the same modular organization: a signal peptide, a short amino-terminal region, a variable number of Gly-X-Y collagen repeats, and a carboxyl-terminal globular C1q domain. Although the CTRP family member roster has rapidly grown since their initial discovery nine years ago, the biological functions of CTRPs have been realized only in recent years. Thus far, most published studies focus upon the beneficial metabolic-regulatory functions of CTRPs (19, 21, 35). Among these studies, CTRP12 is an insulin-sensitizing, anti-inflammatory adipokine, downregulated by obesity (8). In the current study, adipose CTRP expression levels in WT and APN-KO mice were screened. Five of 15 total CTRPs screened are significantly upregulated in APN-KO mice, and four CTRPs are significantly reduced in diet-induced diabetic animals. From these data, CTRP3 and CTRP9 were promising CTRP candidates involved in diabetic cardiovascular protection.
Further studies honed our focus upon CTRP9. We demonstrate that CTRP9 mRNA exceeds APN by more than 100-fold in cardiac tissue. CTRP9 can be detected in medium from isolated cardiac cells as early as 1 h after culture. The abundant production of CTRP9 in the heart distinguished it from APN and lent support to the notion it is a cardiokine, part of the secretome milieu produced by the heart, increasingly recognized as essential regulators of cardiac physiology and pathology (25). Cardiokines maintain normal cardiac function and control pathologic remodeling in response to injury via modulation of myocyte death, fibroblast activation, inflammation, and vascular growth/regression (18, 26). Cellular secretomes change significantly in response to pathologic stresses (32). Indeed, we demonstrated that cardiac CTRP9 mRNA levels were significantly reduced 8 and 16 wk in animals fed a high-fat diet, a time period when diet-induced type 2 diabetes develops (41). Despite increased CTRP9 expression in the APN-KO mouse, the APN-deficient mouse still exhibits exacerbated myocardial ischemia/reperfusion injury compared with WT, suggesting that increased CTRP9 expression alone is insufficient to compensate for intact adiponectin signaling.
Every protein undergoes proteolysis during its synthesis and maturation and again upon inactivation and degradation. Extracellular proteolysis can either activate or inactivate bioactive molecules regulating physiological and pathological processes (10). For example, matrix metalloproteinase cleaves a number of chemokines/cytokines, including CXCL5/CXCL8, TNFα, IL-1β, and TGFβ, converting inactivate forms to active forms via proteolysis (30). Since APN's initial discovery, its proteolytic cleavage and pertinent biological relevance have been debated. A proteolytic APN cleavage product containing the globular COOH-terminal domain (gAPN) was originally postulated to exist in vivo (9). gAPN exhibits much stronger (>20-fold) biological activity than full-length APN (fAPN) and exerts significantly faster biological function upon exogenous administration (39, 40). Despite the extensive utilization of gAPN as a tool in APN research, its biological relevance remains questioned because circulatory gAPN levels are extremely low. Leukocyte elastase, secreted from activated monocytes and/or neutrophils, may cleave APN and generate gAPN (31), raising the intriguing possibility that high-molecular-weight APN might be the storage form of APN. gAPN might be generated locally, functioning as the terminal active isoform. However, direct evidence supporting this notion is not currently available, limiting the feasibility of gAPN as a therapeutic agent.
In contrast to APN, several recent studies have provided clear evidence that CTRPs, particularly CTRP12, circulate in plasma primarily as globular domain isoforms. Endopeptidase cleavage of full-length CTRP12 (fCTRP12) by PCSK3/furinat in amino acid position Lys91 generates the gCTRP12 isoform, a process stimulated by insulin. fCTRP12 forms trimers and larger complexes, but gCTRP12 exists predominantly in dimeric form. More importantly, fCTRP12 and gCTRP12 preferentially activate different downstream signaling pathways. Whereas fCTRP12 activates Akt, gCTRP12 activates MAPK signaling (34). In the current study, we demonstrated that fCTRP9 is cleaved during and/or after its secretion extracellularly. Most importantly, we have demonstrated that cleavage of fCTRP9 and production of gCTRP9 is a necessary step to generate a biologically active form of CTRP9. gCTRP9, but not fCTRP9, significantly activated key survival kinases, including Akt, AMPK, and eNOS. This is yet another feature distinguishing CTRP9 from APN, as both fAPN and gAPN activate similar intracellular signaling systems (although gAPN does so more potently). These results strongly suggest that proteolytic cleavage of CTRP9 is a physiologically relevant and biologically important posttranslational modification.
Several limitations exist in this study. As no single study can address all meritorious questions, further work is necessary. The specific cell types in the heart responsible for CTRP9 production remain unknown at this time. Furthermore, the precise mechanisms of posttranslational CTRP9 modification (and by what specific proteases) are complex and warrant further investigation. Additionally, divergent or similar signaling pathways stimulated by gCTRP9/fCTRP9 were not the primary focus of the current investigation. As mentioned earlier, gCTRP12 and fCTRP12 do not share similar signaling pathways (34). As such, studies determining commonalities and differences between gCTRP9/fCTRP9 signaling pathways are also ongoing in our laboratory.
In conclusion, we have demonstrated that CTRP9 is an important molecule in diabetes, and its levels are decreased in a high-fat diet-induced diabetic mouse model. Our data support CTRP9 as a novel cardiokine, produced locally in the heart in concentrations exceeding systemic circulation. We have demonstrated that CTRP9 undergoes proteolytic cleavage to generate gCTRP9, its dominant circulatory and biologically active isoform. Additional studies dissecting the molecular mechanisms responsible for extracellular CTRP9 cleavage in the heart are currently ongoing.
GRANTS
This research project was supported by NIH Grants HL-123404 and HL-096686 and American Diabetes Association Grants 7-11-BS-93 (X. L. Ma) and 1-11-JF56 (Y. Wang).
AUTHOR CONTRIBUTIONS
W.B.L, Y.W. and X.L.M conception and design of research; Y.Y., H.S., Y.S., W.Y. and Y.D. performed experiments; Y.Y. and W.B.L analyzed data; Y.Y., Y.W., and W.B.L interpreted results of experiments; Y.Y. and W.B.L. drafted manuscript; T.A.C, B.L., and X.L.M. edited and revised manuscript; X.L.M. approved final version of manuscript.
REFERENCES
- 1.American Diabetes Association, National Heart, Lung, and Blood Institute, Juvenile Diabetes Foundation International, National Institute of Diabetes and Digestive and Kidney Diseases, American Heart Association. Diabetes mellitus: a major risk factor for cardiovascular disease: a joint editorial statement by the American Diabetes Association; the National Heart, Lung, and Blood Institute; the Juvenile Diabetes Foundation International; the National Institute of Diabetes and Digestive and Kidney Diseases; and the American Heart Association. Circulation 100: 1132–1133, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Breitbart AH, Brandes F, Müller O, Froese N, Korf-Klingebiel M, Schrameck U, Wollert KC, Bauersachs J, Heineke J. CTRP9—a novel cardiac derived secreted factor with anti-hypertrophic properties (Abstract). Circ Res 113: A182, 2013. [Google Scholar]
- 3.Chandler MP, Morgan EE, McElfresh TA, Kung TA, Rennison JH, Hoit BD, Young ME. Heart failure progression is accelerated following myocardial infarction in type 2 diabetic rats. Am J Physiol Heart Circ Physiol 293: H1609–H1616, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y. Calcium phosphate transfection of eukaryotic cells. Bio-protocol 1: e86, 2011. [Google Scholar]
- 5.Davis KE, Scherer PE. Adiponectin: no longer the lone soul in the fight against insulin resistance? Biochem J 416: e7–e9, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Doroudgar S, Glembotski CC. The cardiokine story unfolds: ischemic stress-induced protein secretion in the heart. Trends Mol Med 17: 207–214, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 360: 129–139, 2009. [DOI] [PubMed] [Google Scholar]
- 8.Enomoto T, Ohashi K, Shibata R, Kambara T, Uemura Y, Yuasa D, Kataoka Y, Miyabe M, Matsuo K, Joki Y, Hayakawa S, Hiramatsu-Ito M, Ito M, Murohara T, Ouchi N. Transcriptional regulation of an insulin-sensitizing adipokine adipolin/CTRP12 in adipocytes by Kruppel-like factor 15. PloS One 8: e83183, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, Bihain BE, Lodish HF. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci USA 98: 2005–2010, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gioia M, Foster LJ, Overall CM. Cell-based identification of natural substrates and cleavage sites for extracellular proteases by SILAC proteomics. Methods Mol Biol (Clifton, NJ) 539: 131–153, 2009. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein BJ, Scalia R. Adipokines and vascular disease in diabetes. Curr Diabetes Rep 7: 25–33, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Greenberg BH, Abraham WT, Albert NM, Chiswell K, Clare R, Stough WG, Gheorghiade M, O'Connor CM, Sun JL, Yancy CW, Young JB, Fonarow GC. Influence of diabetes on characteristics and outcomes in patients hospitalized with heart failure: a report from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF). Am Heart J 154: 277 e271–e277, e278, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Ishihara M, Inoue I, Kawagoe T, Shimatani Y, Kurisu S, Nishioka K, Kouno Y, Umemura T, Nakamura S, Sato H. Diabetes mellitus prevents ischemic preconditioning in patients with a first acute anterior wall myocardial infarction. J Am Coll Cardiol 38: 1007–1011, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Kambara T, Ohashi K, Shibata R, Ogura Y, Maruyama S, Enomoto T, Uemura Y, Shimizu Y, Yuasa D, Matsuo K, Miyabe M, Kataoka Y, Murohara T, Ouchi N. CTRP9 protein protects against myocardial injury following ischemia-reperfusion through AMP-activated protein kinase (AMPK)-dependent mechanism. J Biol Chem 287: 18965–18973, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumada M, Kihara S, Sumitsuji S, Kawamoto T, Matsumoto S, Ouchi N, Arita Y, Okamoto Y, Shimomura I, Hiraoka H, Nakamura T, Funahashi T, Matsuzawa Y. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol 23: 85–89, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Mazzone T. Intensive glucose lowering and cardiovascular disease prevention in diabetes: reconciling the recent clinical trial data. Circulation 122: 2201–2211, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA 291: 1730–1737, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Ranganath Sudhir H, Levy O, Inamdar Maneesha S, Karp Jeffrey M. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell 10: 244–258, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schaffler A, Buechler C. CTRP family: linking immunity to metabolism. Trends Endocrinol Metab 23: 194–204, 2012. [DOI] [PubMed] [Google Scholar]
- 20.Seldin MM, Peterson JM, Byerly MS, Wei Z, Wong GW. Myonectin (CTRP15), a novel myokine that links skeletal muscle to systemic lipid homeostasis. J Biol Chem 287: 11968–11980, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seldin MM, Tan SY, Wong GW. Metabolic function of the CTRP family of hormones. Rev Endocr Metab Dis 15: 111–123, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah AM, Uno H, Kober L, Velazquez EJ, Maggioni AP, MacDonald MR, Petrie MC, McMurray JJ, Califf RM, Pfeffer MA, Solomon SD. The inter-relationship of diabetes and left ventricular systolic function on outcome after high-risk myocardial infarction. Eur J Heart Fail 12: 1229–1237, 2010. [DOI] [PubMed] [Google Scholar]
- 23.Shibata R, Numaguchi Y, Matsushita K, Sone T, Kubota R, Ohashi T, Ishii M, Kihara S, Walsh K, Ouchi N, Murohara T. Usefulness of adiponectin to predict myocardial salvage following successful reperfusion in patients with acute myocardial infarction. Am J Cardiol 101: 1712–1715, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med 11: 1096–1103, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimano M, Ouchi N, Walsh K. Cardiokines: recent progress in elucidating the cardiac secretome. Circulation 126: e327–e332, 2012. [DOI] [PubMed] [Google Scholar]
- 26.Stastna M, Van Eyk JE. Investigating the secretome: lessons about the cells that comprise the heart. Circ Cardiovasc Genet 5: o8–o18, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Su H, Yuan Y, Wang XM, Lau WB, Wang Y, Wang X, Gao E, Koch WJ, Ma XL. Inhibition of CTRP9, a novel and cardiac-abundantly expressed cell survival molecule, by TNFalpha-initiated oxidative signaling contributes to exacerbated cardiac injury in diabetic mice. Basic Res Cardiol 108: 315, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun Y, Yi W, Yuan Y, Lau WB, Yi D, Wang X, Wang Y, Su H, Wang X, Gao E, Koch WJ, Ma XL. C1q/tumor necrosis factor-related protein-9, a novel adipocyte-derived cytokine, attenuates adverse remodeling in the ischemic mouse heart via protein kinase A activation. Circulation 128: S113–120, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tao L, Gao E, Jiao X, Yuan Y, Li S, Christopher TA, Lopez BL, Koch W, Chan L, Goldstein BJ, Ma XL. Adiponectin cardioprotection after myocardial ischemia/reperfusion involves the reduction of oxidative/nitrative stress. Circulation 115: 1408–1416, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Van Den Steen PE, Wuyts A, Husson SJ, Proost P, Van Damme J, Opdenakker G. Gelatinase B/MMP-9 and neutrophil collagenase/MMP-8 process the chemokines human GCP-2/CXCL6, ENA-78/CXCL5 and mouse GCP-2/LIX and modulate their physiological activities. Eur J Biochem 270: 3739–3749, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Waki H, Yamauchi T, Kamon J, Kita S, Ito Y, Hada Y, Uchida S, Tsuchida A, Takekawa S, Kadowaki T. Generation of globular fragment of adiponectin by leukocyte elastase secreted by monocytic cell line THP-1. Endocrinology 146: 790–796, 2005. [DOI] [PubMed] [Google Scholar]
- 31a.Wang Y, Lau WB, Gao E, Tao L, Yuan Y, Li R, Wang X, Koch WJ, Ma XL. Cardiomyocyte-derived adiponectin is biologically active in protecting against myocardial ischemia-reperfusion injury. Am J Physiol Endocrinol Metab 298: E663–E670, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weber KT, Sun Y, Bhattacharya SK, Ahokas RA, Gerling IC. Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat Rev Cardiol 10: 15–26, 2013. [DOI] [PubMed] [Google Scholar]
- 33.Wei Z, Lei X, Petersen PS, Aja S, Wong GW. Targeted deletion of C1q/TNF-related protein 9 increases food intake, decreases insulin sensitivity, and promotes hepatic steatosis in mice. Am J Physiol Endocrinol Metab 306: E28–E35, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei Z, Lei X, Seldin MM, Wong GW. Endopeptidase cleavage generates a functionally distinct isoform of C1q/tumor necrosis factor-related protein-12 (CTRP12) with an altered oligomeric state and signaling specificity. J Biol Chem 287: 35804–35814, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei Z, Peterson JM, Lei X, Cebotaru L, Wolfgang MJ, Baldeviano GC, Wong GW. C1q/TNF-related protein-12 (CTRP12), a novel adipokine that improves insulin sensitivity and glycemic control in mouse models of obesity and diabetes. J Biol Chem 287: 10301–10315, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong GW, Krawczyk SA, Kitidis-Mitrokostas C, Ge G, Spooner E, Hug C, Gimeno R, Lodish HF. Identification and characterization of CTRP9, a novel secreted glycoprotein, from adipose tissue that reduces serum glucose in mice and forms heterotrimers with adiponectin. FASEB J 23: 241–258, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong GW, Wang J, Hug C, Tsao TS, Lodish HF. A family of Acrp30/adiponectin structural and functional paralogs. Proc Natl Acad Sci USA 101: 10302–10307, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 8: 1288–1295, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 7: 941–946, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Yi W, Sun Y, Gao E, Wei X, Lau WB, Zheng Q, Wang Y, Yuan Y, Wang X, Tao L, Li R, Koch W, Ma XL. Reduced cardioprotective action of adiponectin in high-fat diet-induced type II diabetic mice and its underlying mechanisms. Antiox Redox Signal 15: 1779–1788, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]