Abstract
Adipose triglyceride lipase (ATGL) is the rate-limiting enzyme mediating triacylglycerol hydrolysis in virtually all cells, including adipocytes and skeletal myocytes, and hence, plays a critical role in mobilizing fatty acids. Global ATGL deficiency promotes skeletal myopathy and exercise intolerance in mice and humans, and yet the tissue-specific contributions to these phenotypes remain unknown. The goal of this study was to determine the relative contribution of ATGL-mediated triacylglycerol hydrolysis in adipocytes vs. skeletal myocytes to acute exercise performance. To achieve this goal, we generated murine models with adipocyte- and skeletal myocyte-specific targeted deletion of ATGL. We then subjected untrained mice to acute peak and submaximal exercise interventions and assessed exercise performance and energy substrate metabolism. Impaired ATGL-mediated lipolysis within adipocytes reduced peak and submaximal exercise performance, reduced peripheral energy substrate availability, shifted energy substrate preference toward carbohydrate oxidation, and decreased HSL Ser660 phosphorylation and mitochondrial respiration within skeletal muscle. In contrast, impaired ATGL-mediated lipolysis within skeletal myocytes was not sufficient to reduce peak and submaximal exercise performance or peripheral energy substrate availability and instead tended to enhance metabolic flexibility during peak exercise. Furthermore, the expanded intramyocellular triacylglycerol pool in these mice was reduced following exercise in association with preserved HSL phosphorylation, suggesting that HSL may compensate for impaired ATGL action in skeletal muscle during exercise. These data suggest that adipocyte rather than skeletal myocyte ATGL-mediated lipolysis plays a greater role during acute exercise in part because of compensatory mechanisms that maintain lipolysis in muscle, but not adipose tissue, when ATGL is absent.
Keywords: adipose triglyceride lipase, exercise, lipolysis
physical activity requires a constant supply of energy. The source and type of energy substrates are influenced not only by their availability but also by the intensity and duration of physical activity. In humans, at low exercise intensities (≤30% V̇o2max), carbohydrates account for only 10–30% of total energy production and are derived primarily from circulating glucose (50). At higher exercise intensities (85–100% V̇o2max), carbohydrates become the predominant energy substrate (50) and are derived primarily from skeletal muscle glycogen (26). In contrast, during more prolonged low- to moderate-intensity exercise, fatty acids (FAs) become the primary energy substrate and are derived from intracellular triacylglycerol (TAG) stores within adipocytes (reviewed in Ref. 30) and/or skeletal muscle (60). Although up to 70% of these FAs are derived from adipocyte lipolysis (35, 65), intramyocellular triacylglycerols (IMTGs) can decrease more than 50% in response to moderate intensity exercise (12, 65), suggesting that IMTGs also play an important role in exercising muscle. Since alterations in extra- and/or intramyocellular lipid metabolism occur frequently in both normal physiology (i.e., fasting/feeding, physical activity) and disease (i.e., diabetes, insulin resistance), understanding how FA mobilization from adipose tissue and skeletal muscle influences muscle metabolism and function is of considerable biomedical relevance.
Adipose triglyceride lipase (ATGL) is the rate-limiting enzyme mediating TAG hydrolysis (69) and a major contributor to lipolysis in both adipose tissue (19, 62, 66) and skeletal muscle (54). Indeed, humans (20, 53) and mice (24) with ATGL deficiency exhibit dramatic accumulation of IMTGs in cardiac and skeletal muscle despite markedly reduced adipocyte lipolysis and serum lipids, indicating a critical role for ATGL action in muscle. These effects contribute to early morbidity and mortality in part because of lipotrophic cardiomyopathy. However, although impaired ATGL action within cardiomyocytes clearly contributes to cardiac muscle dysfunction (25, 33), the tissue-specific contribution of ATGL action to skeletal muscle dysfunction remains poorly understood. In mice, global ATGL deletion impairs exercise performance (31, 52). This impairment persists even after reexpressing ATGL in cardiac muscle (52), indicating that skeletal muscle dysfunction cannot be explained entirely by cardiac dysfunction. These data implicate either adipose tissue and/or skeletal muscle ATGL action in exercise performance. Previously, we reported that skeletal muscle-specific targeted deletion or overexpression of ATGL dramatically alters IMTG content but is not sufficient to alter metabolic phenotypes at baseline or in response to nutritional stress (i.e., diet-induced obesity) (54). Whether alterations in ATGL action in skeletal myocytes vs. adipocytes influence acute exercise performance remains unknown.
The primary goal of this study was to determine the relative contribution of adipocyte vs. skeletal myocyte ATGL-mediated TAG hydrolysis to acute exercise performance in mice. To achieve this goal, we generated murine models with both adipocyte- and skeletal myocyte-specific targeted deletion of ATGL. We then subjected these mice to peak and submaximal endurance exercise interventions and assessed their exercise performance and energy substrate metabolism. We found that ATGL deletion from adipocytes, but not skeletal myocytes, alters energy substrate metabolism and impairs acute exercise performance in mice.
MATERIALS AND METHODS
Animals.
B6.129-Pnpla2tm1eek (Atgl-flox) mice were generated as described (54). ATGL-flox mice were crossed to either MyoCre (40) or AdipoqCre (18) mice to generate skeletal myocyte-specific (SMAKO) (54) or adipocyte-specific (AAKO) ATGL knockout mice. For both models, male Atglflox/flox Cre/+ mice were mated to female Atglflox/flox +/+ mice to generate male Atglflox/flox Cre/+ (SMAKO or AAKO) and Atglflox/flox +/+ (control) experimental mice. All mice were congenic (n > 10) on C57BL/6NTac. Animal experiments were approved by the University of Pittsburgh Institutional Animal Care and Use Committee and conducted in conformity with Public Health Service Policy for Care and Use of Laboratory Animals.
Peak exercise protocol.
At 12 wk of age, mice were subjected to a 3-day acclimation protocol with progressively increased intensity and duration of treadmill exposure (RM Exer-3/6 Open Treadmill with Manual Incline; Columbus Instruments). On day 1 of the acclimation protocol, mice were fasted for 4 h, and blood was collected through the tail vein for assessment of baseline circulating substrates. For the peak test, mice were run on an enclosed single-lane treadmill (Modular Enclosed Metabolic Treadmill for Mice; Columbus Instruments) attached to an Oxymax/Comprehensive Laboratory Animal Monitoring System (CLAMS; Columbus Instruments) that allowed for real-time measurements of oxygen consumption (V̇o2) and carbon dioxide production (V̇co2). Mice were fasted for 4 h, with testing being completed between 10 AM and 1 PM. Mice were placed in the treadmill and allowed a 2-min acclimation period prior to the initiation of the peak test. The running protocol was as follows: an initial warmup run was conducted at 6 m/min for 5 min followed by 10 m/min for 2 min. Thereafter, speed was subsequently increased by 1 m/min every minute until fatigue, which was defined as the inability to return to treadmill running after 10 s. Once fatigue was reached, the treadmill was stopped, and respiratory values were collected for 1 min. Mice were then removed from the treadmill, and blood was collected via tail vein for analysis.
Submaximal endurance exercise challenge protocols.
For the initial (nonterminal) submaximal endurance exercise challenge, mice were rested for 7 days following the peak exercise challenge and then subjected to a submaximal endurance exercise challenge at 13 wk of age. Tests were conducted between 10 AM and 3 PM following an ∼3-h fast. The submaximal running velocity was calculated as 55% of the peak running speed obtained in the control mice, an intensity shown to maximize substrate usage, specifically intramyocellular lipids (58). Thus, all mice ran at the same absolute exercise intensity during the submaximal challenge. As with the peak test, mice were run until fatigue, at which point the treadmill was stopped, respiratory measurements were collected for 1 min, and blood was collected via the tail vein. A second (terminal) submaximal endurance exercise challenge was performed in 20-wk-old mice to evaluate for potential differences in tissue substrates, mitochondria, and/or gene/protein expression/phosphorylation. Mice were reacclimated to the treadmill and run for 45 min (representing approximately one-half the total endurance time of the control mice) rather than to fatigue. Following the experiment, blood glucose was determined, and mice were immediately euthanized by CO2 inhalation and blood/tissue collected for analysis (17).
Energy expenditure and metabolic measurements.
V̇o2, V̇co2, and respiratory exchange ratio (RER) were determined using the CLAMS. Respiratory data from the final 30 s of peak and submaximal exercise performance were used in calculations. Respiratory data were normalized to body weight raised to the power of 0.75 (56). Rates of carbohydrate (c) and fat (f) oxidation were calculated using the equations of Frayn: c = (4.55 × V̇co2) − (3.21 × V̇o2) and f = (1.67 × V̇o2) − (1.67 × V̇co2) (protein oxidation ignored due to minimal role during exercise) (21). Results of all respiratory measurements and associated calculations were unchanged after adjusting for fat or lean mass. Whole blood glucose was determined using a One-touch FastTake glucometer (Lifescan). Serum TAGs (Infinity Triglycerides Liquid Stable Reagent; Thermo Scientific) and nonesterified fatty acids [NEFAs; HR Series NEFA-HR(2) Reagents, Wako Diagnostics] were determined by colorimetric assay as described (54).
Tissue and mitochondrial analyses.
Muscle glycogen and TAG were determined in liquid nitrogen-crushed quadriceps samples using a commercially available kit (Glycogen Assay Kit; Cayman Chemical) and Infinity Triglycerides in Liquid Stable Reagent (9). For tissue imaging, muscle samples were cut into 10-μm sections and stained for neutral lipids (54) or glycogen (27). Mitochondrial respiration was assessed in permeabilized muscle fibers in the basal state, as described previously (54). Protein expression in skeletal muscle was determined by Western blot analysis using the following antibodies as described (54): MitoProfile antibody cocktail (MS604; Mitosciences), anti-hormone-sensitive lipase (HSL) (4017s; Cell Signaling Technology), and anti-phosphorylated (p)-HSL (Ser565 and Ser660) (4137S and 4126S; Cell Signaling Technology).
Statistical analysis.
Data are expressed as means ± SE and analyzed using the Statistical Package for the Social Sciences for MAC version 22. Baseline between-group comparisons were determined by one-way analysis of variance (ANOVA). No differences were observed between control littermates for AAKO or SMAKO mice, so data were combined into a single control group for subsequent analyses. Repeated-measures ANOVA was used to evaluate pre- and postexercise test outcome variables and to assess between-group interactions. Univariate analysis addressed the question of genotype and activity (sedentary vs. exercise) for specific outcome variables. When an interaction was identified, a one-way ANOVA on the relative percent change was used to determine group differences. Data were log transformed and reanalyzed if the assumption of homogeneity of variance was invalid. Presentation of the data is nontransformed. Statistical significance was assumed at P ≤ 0.05.
RESULTS
Peak exercise capacity and serum substrate kinetics are impaired by inhibition of adipocyte but not intramyocellular ATGL-mediated lipolysis.
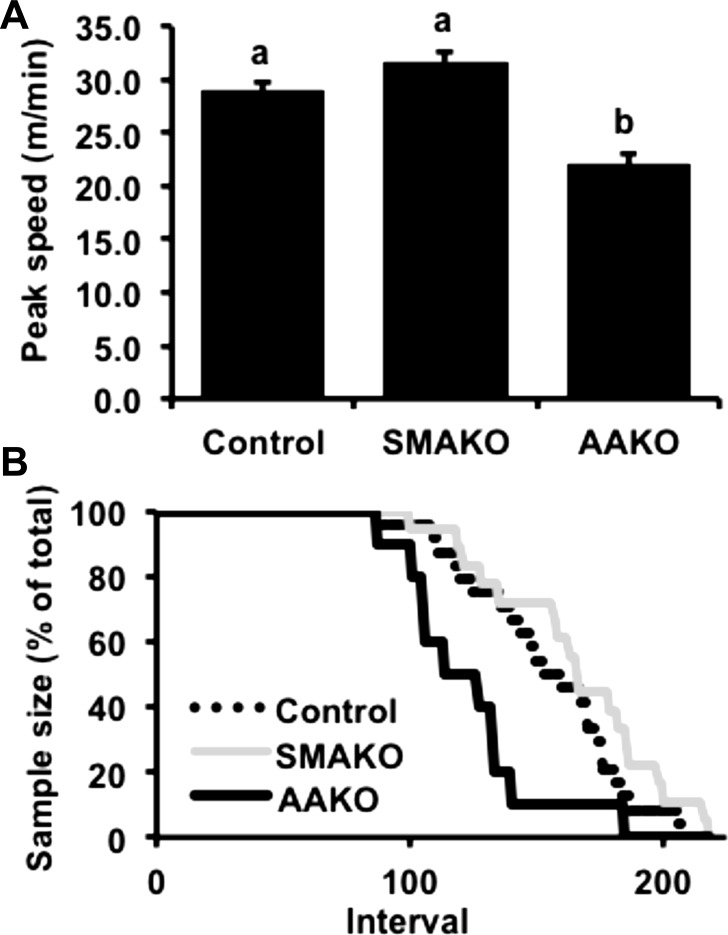
In humans (10) and rodents (6), peak exercise performance is highly dependent on circulating energy substrates. Previous studies have reported that adipocyte- (2, 66) but not skeletal myocyte-specific (54) deletion of ATGL in mice reduces circulating glucose and FA substrates in the basal (nonexercised) state. To determine the relative impact of adipocyte vs. skeletal myocyte ATGL action on energy substrates in response to peak exercise, we first assessed these parameters in age- and weight-matched (26.27 ± 0.22 g) control, SMAKO, and AAKO mice subjected to a peak exercise challenge. Peak running speeds were similar in control (28.98 ± 0.89 m/min) and SMAKO (31.68 ± 0.97 m/min) mice but lower in AAKO mice (22.04 ± 1.05 m/min, P < 0.01) (Fig. 1A). Likewise, dropout rates during a peak exercise challenge were similar for control and SMAKO mice but greater for AAKO mice (Fig. 1B). Baseline circulating energy substrates, including serum glucose, TAGs, and NEFAs, were comparable between control and SMAKO mice but lower in AAKO mice (Table 1). In response to a peak exercise challenge, control and SMAKO mice demonstrated similar changes in both circulating glucose and TAGs, i.e., increased serum glucose and reduced serum TAGs. In contrast, AAKO mice demonstrated the opposite response, i.e., decreased serum glucose and increased serum TAGs. Serum NEFAs tended to respond differently between the genotypes (P = 0.06 for group interaction) with decreased NEFAs in SMAKO (post hoc 2-tailed pairwise comparison, P = 0.05) but not in control or AAKO mice. Thus, inhibition of ATGL action in adipocytes, but not skeletal myocytes, alters circulating energy substrate availability and impairs peak exercise performance in mice.
Fig. 1.
Peak exercise performance. A: peak exercise speed. B: dropout rates during peak exercise. Interval, 1–10 s. For each group/variable (i.e. all data), male mice were used [12 wk, fasted 4 h; n = 26 control, n = 14 skeletal myocyte-specific adipose triglyceride lipase (ATGL) knockout (SMAKO) mice, and n = 15 adipocyte-specific ATGL knockout (AAKO) mice]. a,bP ≤ 0.05 for group difference; noncorresponding letters are different.
Table 1.
Substrate kinetics before and after a peak exercise challenge in untrained mice
| Control |
SMAKO |
AAKO |
||||
|---|---|---|---|---|---|---|
| Basal | Peak | Basal | Peak | Basal | Peak | |
| Body weight, g | 26.17 ± 0.30 | 26.21 ± 0.58 | 27.42 ± 0.22 | |||
| Glucose, mg/dl | 173.27 ± 5.66a | 246.26 ± 9.37*c | 181.29 ± 5.93a | 225.21 ± 19.63*c | 147.67 ± 11.62b | 111.14 ± 14.61*d |
| TAG, μg/μl | 0.72 ± 0.05a | 0.50 ± 0.03*d | 0.86 ± 0.09a | 0.54 ± 0.07*d | 0.22 ± 0.02b | 0.28 ± 0.05*c |
| NEFA, mEq/l | 0.45 ± 0.04a | 0.54 ± 0.06 | 0.56 ± 0.08a | 0.35 ± 0.05 | 0.22 ± 0.03b | 0.27 ± 0.06 |
Data are means ± SE; 12 wk, fasted 4 h; n = 26 control, 14 skeletal muscle adipose triglyceride lipase (ATGL) knockout (SMAKO) mice, and 15 adipocyte-specific ATGL knockout (AAKO) mice.
TAG, triacylglycerol; NEFA, nonesterified fatty acids.
P ≤ 0.05 for baseline group effect; noncorresponding letters are different.
P ≤ 0.05 for exercise effect.
P ≤ 0.05 for group effect (interaction); noncorresponding letters are different.
Maximal aerobic capacity and metabolic flexibility in response to peak exercise are impaired by inhibition of adipocyte but not intramyocellular ATGL-mediated lipolysis.
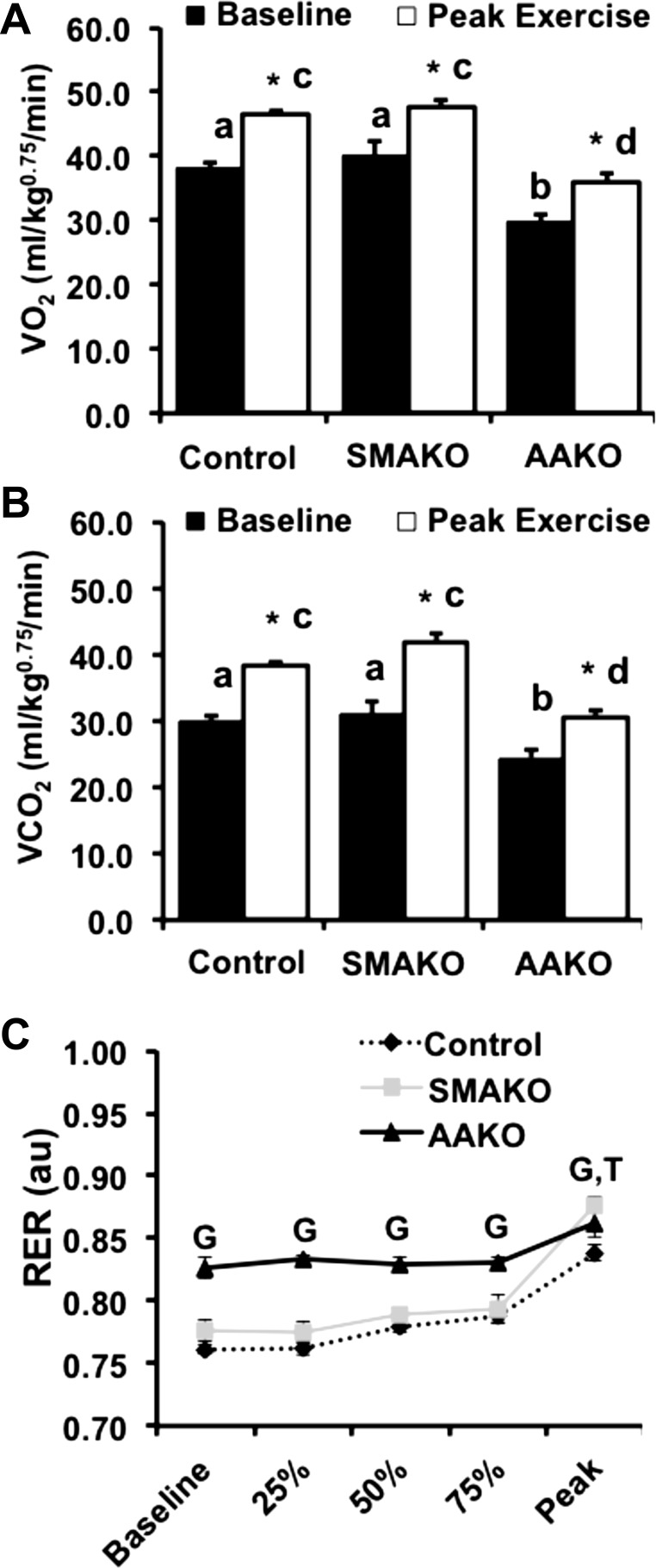
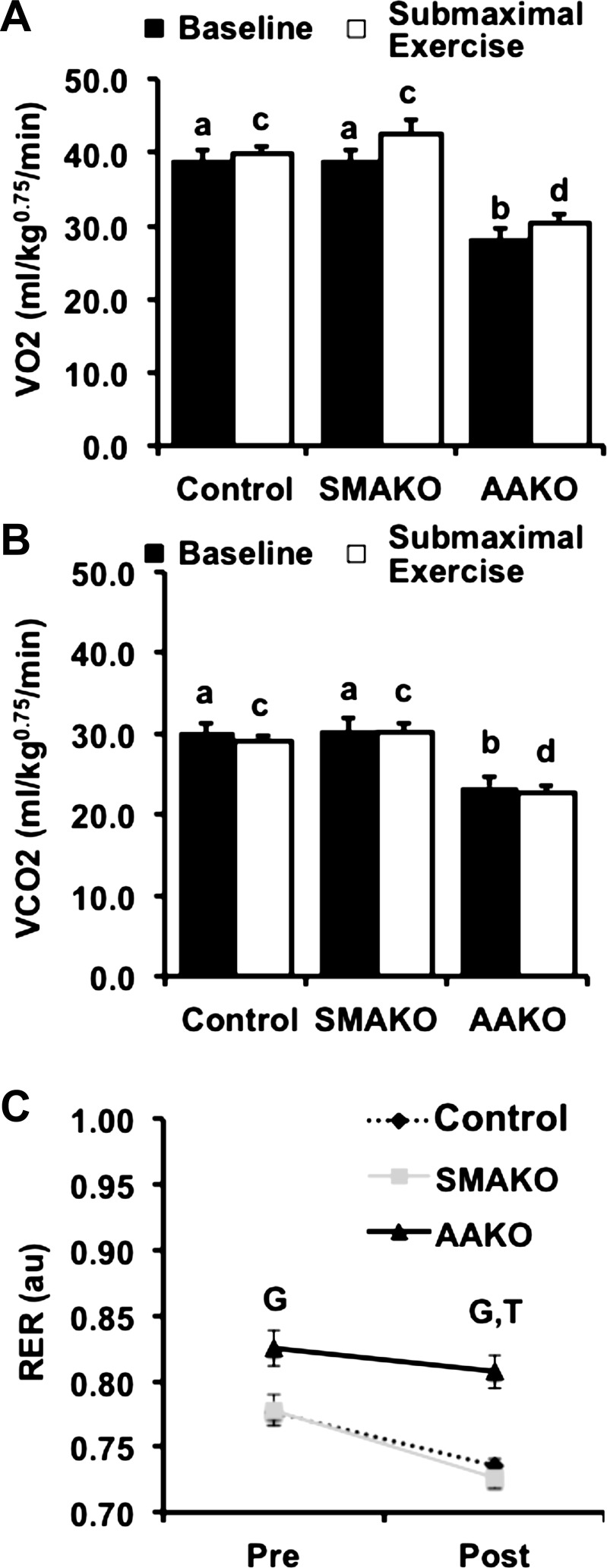
Peak exercise represents maximal aerobic capacity and is regulated by neuronal, cardiopulmonary, and metabolic factors (63). Among these, substrate availability and oxidation play key roles in exercise performance. To determine the relative effects of adipocyte vs. skeletal myocyte ATGL action on maximal aerobic capacity and relative substrate oxidation, we measured whole body gas exchange in response to a peak exercise challenge. At baseline, O2 consumption (V̇o2; Fig. 2A) and CO2 production (V̇co2; Fig. 2B) were similar between control and SMAKO mice but lower in AAKO mice. In response to a peak exercise challenge, O2 consumption and CO2 production increased for all genotypes but remained lower in AAKO mice compared with the other groups. The baseline RER (RER = V̇co2/V̇o2) in AAKO mice was higher than in control and SMAKO mice, consistent with a baseline preference for carbohydrate over FA oxidation (Fig. 2C). In response to peak exercise, the RER increased for all genotypes (P < 0.01); however, the magnitude of this increase in RER relative to baseline was smaller for AAKO [0.03 ± 0.01 arbitrary units (AU), P < 0.01] compared with control (0.07 ± 0.01 AU) or SMAKO (0.10 ± 0.01 AU) mice. In contrast, the RER at peak exercise was higher in SMAKO (0.88 ± 0.01, P < 0.05) compared with control (0.84 ± 0.01) mice, as was the magnitude of the increase in RER relative to baseline (P < 0.05). Thus, inhibition of ATGL action in adipocytes impairs maximal aerobic capacity and metabolic flexibility (i.e., the ability to switch between carbohydrate and FA oxidation) at peak exercise, whereas inhibition of ATGL action in skeletal myocytes does not alter maximal aerobic capacity and may enhance metabolic flexibility at peak exercise.
Fig. 2.
Whole body gas exchange and respiratory exchange ratio (RER) during peak exercise. A and B: whole body oxygen consumption (V̇o2; A) and carbon dioxide production (V̇co2; B) normalized to body weight (kg) raised to the 0.75 power at baseline (black bars) and at peak exercise (open bars) by indirect calorimetry. C: RER (V̇co2/V̇o2) at baseline and 25, 50, 75, and 100% of peak exercise run duration. For each group/variable (i.e. all data), male mice were used (12 wk, fasted 4 h; n = 26 control, 14 SMAKO, and 15 AAKO). a,bP ≤ 0.05 for baseline group difference; noncorresponding letters are different. c,dP < 0.05 for peak exercise group difference; noncorresponding letters are different. *P < 0.05, effect of exercise; GP < 0.05 for effect of genotype, AAKO > control and SMAKO; TP < 0.05 for effect of time (i.e., baseline compared with peak), all groups.
Substrate kinetics during peak exercise are impaired by inhibition of adipocyte but not intramyocellular ATGL-mediated lipolysis.
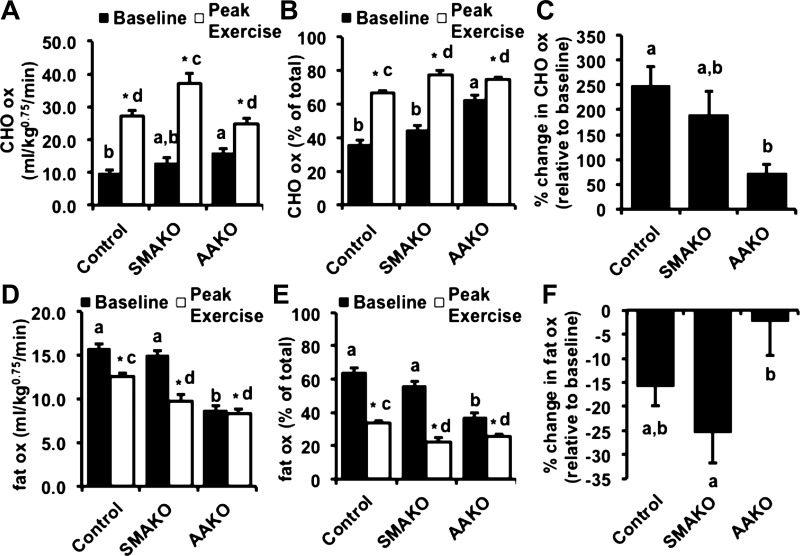
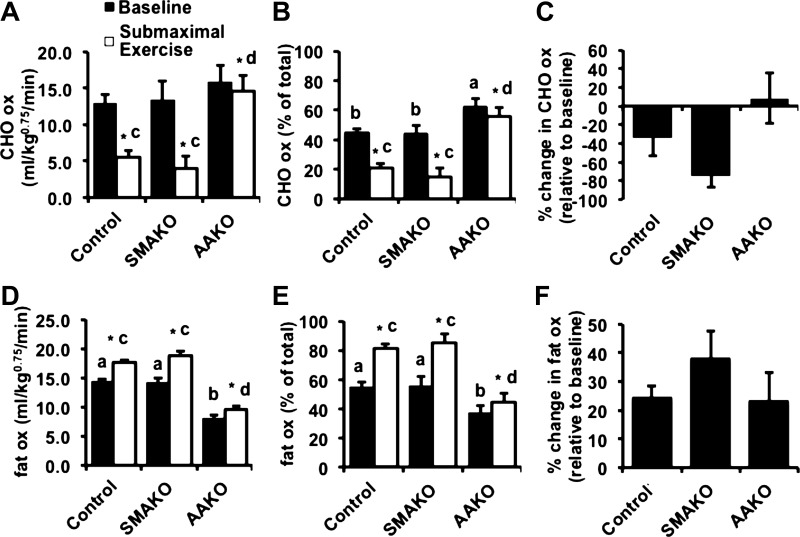
To more specifically characterize energy substrates during peak exercise, we next determined the relative rates of carbohydrate and fat oxidation by respirometry at baseline and in response to a peak exercise challenge (21). For carbohydrate oxidation (Fig. 3, A–C), absolute rates of carbohydrate oxidation (Fig. 3A) as well as percent carbohydrate relative to total oxidation (Fig. 3B) were similar between control and SMAKO mice but higher in AAKO mice at baseline. In response to a peak exercise challenge, carbohydrate oxidation (both absolute and %total) increased in all genotype groups (Fig. 3, A and B), but the magnitude of this increase was much smaller in AAKO (9.03 ± 1.26 mg·kg0.75·min−1) compared with control (15.35 ± 1.33 mg·kg0.75·min−1) and SMAKO (19.69 ± 2.24, mg·kg0.75·min−1) mice (Fig. 3A). Likewise, the percent change in carbohydrate oxidation relative to baseline increased for all genotype groups, but the magnitude of this response was attenuated in AAKO mice (Fig. 3C). For fat oxidation (Fig. 3, D–F), both absolute rates of fat oxidation (Fig. 3D) as well as percent fat to total oxidation (Fig. 3E) were similar between control and SMAKO mice but lower in AAKO mice at baseline. In response to a peak exercise challenge, fat oxidation (both absolute and %total) decreased for all genotype groups (Fig. 3, D and E), but the magnitude of this decrease was much lower in AAKO (−0.45 ± 0.61 mg·kg0.75·min−1, absolute) compared with control (−2.87 ± 0.65 mg·kg0.75·min−1, absolute) and SMAKO (−4.08 ± 1.11 mg·kg0.75·min−1, absolute) mice (Fig. 3D). Likewise, the percent change in fat oxidation relative to baseline decreased for all genotype groups, with the greatest reduction in SMAKO mice and the smallest reduction in AAKO mice (Fig. 3F). Thus, inhibition of ATGL action in adipocytes, but not skeletal myocytes, reduces the ability to adjust energy substrate oxidation in response to peak exercise.
Fig. 3.
Substrate kinetics in response to peak exercise. A and D: carbohydrate (CHO; A) and fat oxidation (D) normalized to body weight (kg) raised to the 0.75 power at baseline (black bars) and at peak exercise (open bars) by indirect calorimetry. B and E: %change in CHO (B) and fat oxidation (E) relative to baseline. C and F: CHO (C) and fat oxidation (F) as %total oxidation. For each group/variable (i.e. all data), male mice were used (12 wk, fasted 4 h; n = 26 control, 14 SMAKO, and 15 AAKO). a,bP ≤ 0.05 for group difference; noncorresponding letters are different. c,dP < 0.05 for peak exercise group difference; noncorresponding letters are different. *P < 0.05, effect of exercise.
Submaximal endurance exercise capacity is impaired by inhibition of adipocyte but not intramyocellular ATGL-mediated lipolysis.
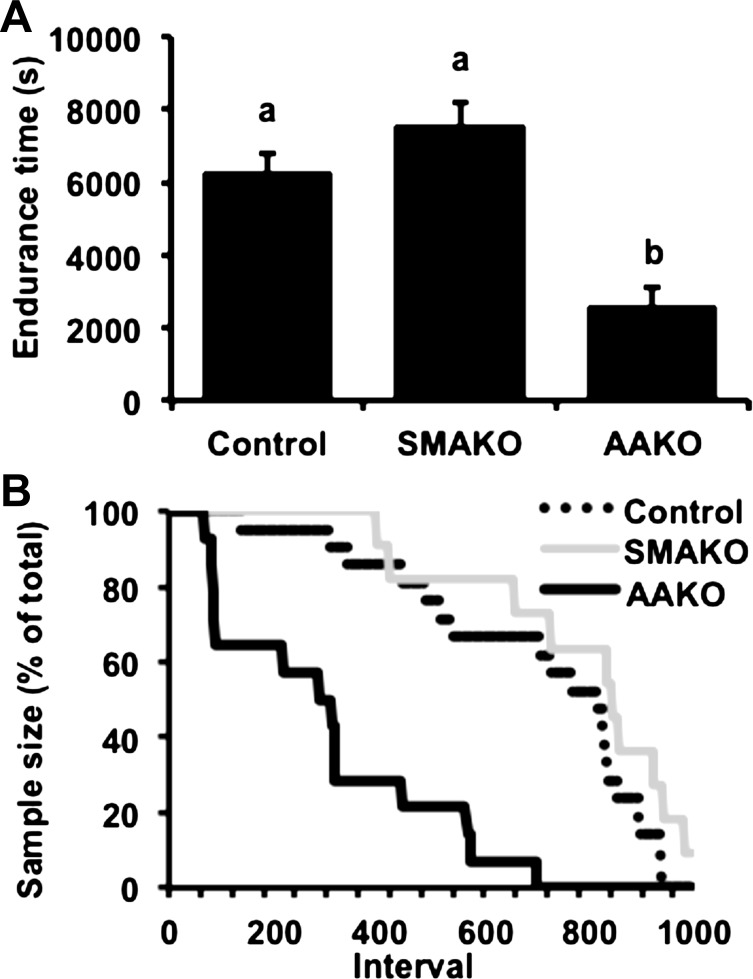
In contrast to peak exercise, energy production during submaximal endurance exercise generally relies more heavily on fat oxidation (50), with substrates derived from both adipocyte (65) and skeletal muscle TAG stores (58). To assess the relative impact of adipocyte vs. skeletal myocyte ATGL action on energy substrates in response to submaximal endurance exercise, we next assessed these parameters in age- and weight-matched (27.04 ± 0.19 g) control, SMAKO, and AAKO mice subjected to a submaximal endurance exercise challenge (i.e., mice run ∼55% of control group peak run speed until exhaustion). Time to fatigue was similar in control (6,289.61 ± 483.48 s) and SMAKO (7,528.56 ± 660.10 s) mice but lower in AAKO mice (2,558.79 ± 562.59 s, P < 0.01) (Fig. 4A). Likewise, dropout rates during the submaximal endurance exercise challenge were similar for control and SMAKO mice but greater for AAKO mice (Fig. 4B). Baseline circulating energy substrates, including serum glucose, TAGs, and NEFAs, were comparable between control and SMAKO mice but lower in AAKO mice (Table 2). In response to the submaximal endurance exercise challenge, an interaction effect was observed for circulating energy substrates. Specifically, serum TAGs decreased in control and SMAKO mice but not AAKO mice. Serum NEFAs increased in all genotypes, but the magnitude of this increase was lowest in AAKO mice (Table 2). Conversely, serum glucose decreased in all genotypes, and the magnitude of this decrease was greatest in AAKO mice. These data suggest that reduced circulating substrate availability in response to impaired ATGL action in adipocytes, but not myocytes, limits submaximal endurance exercise performance.
Fig. 4.
Submaximal endurance exercise performance. A: endurance time until exhaustion. B: dropout rates during submaximal endurance exercise; 1 interval = 10 s. For each group/variable (i.e. all data), male mice were used (13 wk, fasted 3 h; n = 23 control, 11 SMAKO, and 14 AAKO). a,bP ≤ 0.05 for group difference; noncorresponding letters are different.
Table 2.
Substrate kinetics before and after an exhaustive submaximal endurance challenge in untrained mice
| Control |
SMAKO |
AAKO |
||||
|---|---|---|---|---|---|---|
| Basal | Postexercise | Basal | Postexercise | Basal | Postexercise | |
| Body weight, g | 26.87 ± 0.27 | 27.27 ± 0.54 | 27.25 ± 0.23 | |||
| Glucose, mg/dl | 165.58 ± 6.82a | 161.73 ± 13.32c | 169.96 ± 11.3a | 139.21 ± 18.04*c | 146.11 ± 14.31b | 85.64 ± 10.06*d |
| TAG, μg/μl | 0.76 ± 0.07a | 0.46 ± 0.07* | 0.78 ± 0.06a | 0.42 ± 0.03* | 0.21 ± 0.02b | 0.22 ± 0.02 |
| NEFA, mEq/l | 0.46 ± 0.05a | 1.24 ± 0.08*c | 0.53 ± 0.09a | 1.38 ± 0.19*c | 0.23 ± 00.04b | 0.35 ± 0.04*d |
Data are means ± SE; 13 wk, fasted 3 h; n = 23 control, 14 SMAKO, and 12 AAKO.
P < 0.05 for baseline group effect; noncorresponding letters are different.
Difference in postexercise values; noncorresponding letters are different.
P < 0.05 for exercise effect.
Sustained aerobic capacity and metabolic flexibility in response to submaximal endurance exercise are impaired by inhibition of adipocyte but not intramyocellular ATGL-mediated lipolysis.
To determine the effects of adipocyte vs. skeletal myocyte ATGL action on sustained aerobic capacity and substrate oxidation, we measured whole body gas exchange during submaximal endurance exercise to exhaustion. At baseline, O2 consumption (Fig. 5A) and CO2 production (Fig. 5B) were similar between control and SMAKO mice but lower in AAKO mice. In response to a submaximal endurance exercise challenge, neither O2 consumption nor CO2 production changed significantly in any of the genotype groups. In contrast, RER decreased in all groups, consistent with a switch to FAs as the preferred energy substrate for oxidation. At baseline, RER was higher in AAKO mice compared with control and SMAKO mice and remained higher at fatigue (Fig. 5C), suggesting a sustained preference for carbohydrate over FA oxidation. In contrast, RER was comparable in control and SMAKO mice both at baseline and in response to submaximal endurance exercise. Thus, in contrast to inhibition of ATGL action in adipocytes, inhibition of ATGL action in skeletal myocytes is not sufficient to impair sustained aerobic capacity or the ability to enhance FA oxidation in response to submaximal endurance exercise.
Fig. 5.
Whole body gas exchange and RER during submaximal endurance exercise. A and B: whole body oxygen consumption (A) and CO2 production (B) normalized to body weight (kg) raised to the 0.75 power at baseline (black bars) and at peak exercise (open bars) by indirect calorimetry. C: RER (V̇co2/V̇o2) at baseline and following the endurance exercise challenge. For each group/variable (i.e. all data), male mice were used (13 wk, fasted 3 h; n = 23 control, 11 SMAKO, and 14 AAKO). a,bP ≤ 0.05 for baseline group difference; noncorresponding letters are different. c,dP < 0.05 for peak exercise group difference; noncorresponding letters are different. GP < 0.05 for genotype, AAKO > control and SMAKO; TP < 0.05 for peak compared with baseline.
Substrate kinetics with submaximal endurance exercise is impaired by inhibition of adipocyte but not intramyocellular ATGL-mediated lipolysis.
To more specifically characterize energy substrate use during submaximal endurance exercise, we next determined the rates of carbohydrate and FA oxidation by respirometry at baseline and in response to a submaximal endurance exercise challenge. For carbohydrate oxidation (Fig. 6, A–C), the rates of carbohydrate oxidation were similar between control and SMAKO mice baseline (Fig. 6, A and B) but higher in AAKO mice when expressed as percent carbohydrate to total oxidation (Fig. 6B). In response to a submaximal endurance exercise challenge, carbohydrate oxidation (both absolute and %total) decreased in all genotype groups (Fig. 6, A and B), but the magnitude of this decrease was much smaller in AAKO (−2.85 ± 2.74 mg·kg0.75·min−1) compared with control (−7.98 ± 1.64 mg·kg0.75·min−1) and SMAKO (−12.04 ± 2.62 mg·kg0.75·min−1) mice (Fig. 6A). Furthermore, the percent change in carbohydrate oxidation relative to baseline tended (P = 0.06 for interaction effect) to be greater for control and SMAKO mice compared with AAKO mice (Fig. 6C). For FA oxidation (Fig. 6, D–F), both absolute rates of FA oxidation (Fig. 6D) as well as percent FA to total oxidation (Fig. 6E) were similar between control and SMAKO mice but lower in AAKO mice at baseline. In response to a submaximal endurance exercise challenge, FA oxidation (both absolute and %total) increased for all genotype groups (Fig. 6, D and E). Although the percent changes in FA oxidation relative to baseline were similar between groups (Fig. 6F), the absolute magnitude of this decrease was lower in AAKO (1.51 ± 0.77 mg·kg0.75·min−1) compared with control (3.23 ± 0.52 mg·kg0.75·min−1) and SMAKO (4.90 ± 0.84 mg·kg0.75·min−1) mice (Fig. 6A). Thus, inhibition of ATGL-mediated lipolysis in adipocytes, but not skeletal myocytes, reduces the ability to adjust energy substrate oxidation in response to submaximal endurance exercise.
Fig. 6.
Substrate kinetics in response to submaximal endurance exercise. A and D: CHO (A) and fat oxidation (D) normalized to body weight (kg) raised to the 0.75 power at baseline (black bars) and at peak exercise (open bars) by indirect calorimetry. B and E: %change in CHO (B) and fat oxidation (E) relative to baseline. C and F: CHO (C) and fat oxidation (F) as %total oxidation. For each group/variable (i.e. all data), male mice were used (13 wk, fasted 3 h; n = 26 control, 14 SMAKO, and 15 AAKO). a,bP ≤ 0.05 for group difference; noncorresponding letters are different. c,dP < 0.05 for peak exercise group difference; noncorresponding letters are different. *P < 0.05, effect of exercise.
ATGL-mediated lipolysis in adipocytes vs. skeletal myocytes differentially influences skeletal muscle substrates, lipolytic enzyme, and mitochondria in response to submaximal endurance exercise.
To better understand intramyocellular factors contributing to differences in acute exercise performance in mice lacking ATGL in either adipocyte or skeletal myocytes, we next evaluated skeletal muscle substrates, mitochondria, and lipolytic enzymes in age- and weight-matched control, AAKO, and SMAKO mice. For muscle substrate and protein analysis (Fig. 7, A–C), mice were exercised just as they were for the nonterminal submaximal exercise challenge, except they were euthanized after 45 min of running so that all mice could be examined after the same run duration. Sedentary (nonexercised) age-, weight-, and genotype-matched mice were used for comparison. Consistent with results of the nonterminal submaximal exercise study, the terminal submaximal exercise study (Table 3) revealed that circulating energy substrates (glucose, TAGs, and NEFAs) were comparable between control and SMAKO mice but lower in both sedentary and exercised AAKO mice. Likewise, both sedentary and exercise AAKO mice demonstrated a greater preference for carbohydrate relative to FA oxidation than control or SMAKO mice.
Fig. 7.
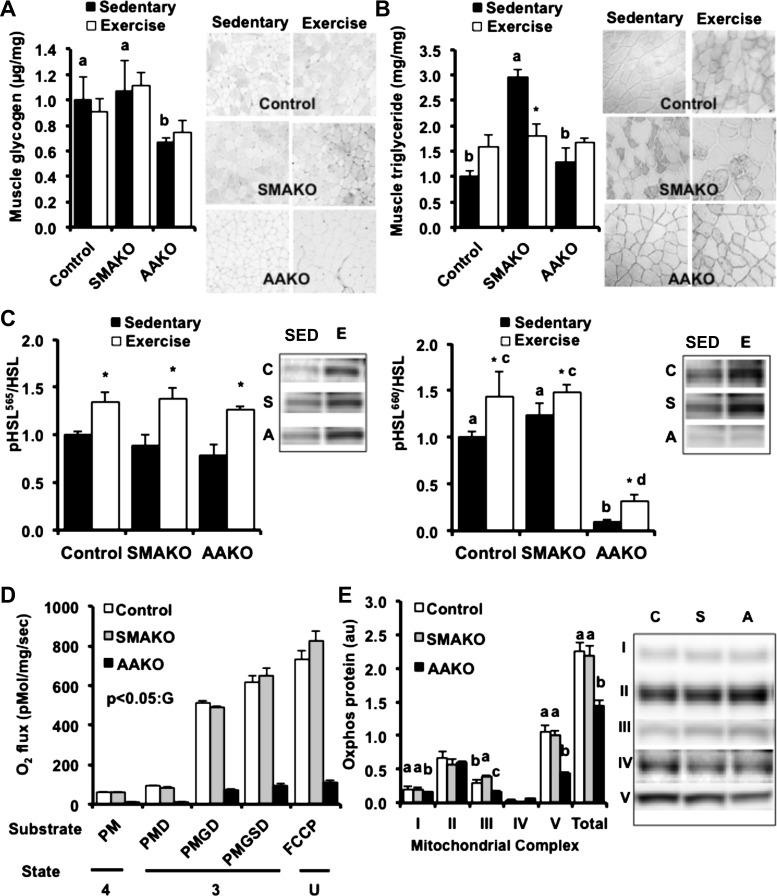
Skeletal muscle substrates, mitochondrial performance and content, and lipase phosphorylation with exercise. A and B: skeletal muscle glycogen (A) and triacylglycerol content (B) under sedentary (black bars) and submaximal endurance exercise (open bars) conditions {20 wk, fasted 3 h, biochemical analysis [red quadriceps], histology [gastrocnemius-plantaris-soleus complex (GPS)]; n = 4–6/group} normalized to control sedentary representative images (right) of histological analyses (GPS; n = 3/group). Interaction effect for triacylglycerol (P = 0.04). *P < 0.05 for exercise effect in SMAKO only. C: protein expression of phosphorylated hormone-sensitive lipase (p-HSL; Ser565, Ser660) normalized to total HSL and representative immunoblots (right) under sedentary (SED) and submaximal endurance exercise (E) conditions for control (C), SMAKO (S), and AAKO (A) (20 wk, fasted 3 h, tibialis anterior; n = 4–6/group). a,bP ≤ 0.05 for group difference; noncorresponding letters are different. c,dP < 0.05 for submaximal endurance exercise group difference, noncorresponding letters are different. *P < 0.05, effect of exercise. D: mitochondrial respiration in permeabilized muscle fibers (20 wk, fasted 12 h, soleus; n = 6/group). Oxygen consumption was measured following the sequential addition of the following substrates: palmitoylcarnitine, malate, ADP, glutamate, succinate, and FCCP. The corresponding respiratory states are noted: ADP-driven respiration (state 3), respiration in the absence of ADP (state 4), and uncoupled respiration (state U). GP < 0.05, effect of genotype, AAKO < control and SMAKO for each state. PM, palmitoylcarnitine + malate; PMD, palmitoylcarnitine + malate + ADP; PMGD, palmitoylcarnitine + malate + glutamate + ADP; PMGSD, palmitoylcarnitine + malate + ADP + glutamate + succinate. E: oxidative phosphorylation protein content of complexes I–V [NDUFB8 (complex I), SDHB (complex II), UQCRC2 (complex III), MTCO1 (complex IV), and ATP5A (complex V)] (20 wk, fasted 3 h, tibialis anterior; n = 6/group). Data are normalized to protein expression of β-actin. a,b,cP < 0.05, effect of genotype; noncorresponding letters are different. Representative immunoblots (right) for control (C), SMAKO (S), and AAKO (A).
Table 3.
Substrate kinetics following a preexhaustive submaximal endurance exercise challenge in untrained mice
| Control |
SMAKO |
AAKO |
||||
|---|---|---|---|---|---|---|
| Sedentary | Exercise | Sedentary | Exercise | Sedentary | Exercise | |
| Body weight, g | 31.04 ± 00.78 | 32.39 ± 00.82 | 32.50 ± 0.54 | 32.06 ± 1.42 | 29.30 ± 0.84 | 28.95 ± 0.80 |
| Glucose, mg/dl | 239.86 ± 8.25a | 291.71 ± 19.47* | 233.33 ± 3.82a | 253.57 ± 13.71 | 174.00 ± 33.81b | 143.33 ± 30.31 |
| TAG, μg/μl | 0.87 ± 0.11a | 0.83 ± 0.08 | 0.91 ± 0.13a | 0.80 ± 0.06 | 0.19 ± 0.02b | 0.17 ± 0.02 |
| NEFA, mEq/l | 0.54 ± 0.09 | 0.80 ± 0.07* | 0.68 ± 0.09 | 0.82 ± 0.06* | 0.30 ± 0.06 | 0.25 ± 0.03 |
| CHO oxidation, mg·kg0.75·min−1 | 9.89 ± 1.05 | 12.63 ± 0.86 | 9.87 ± 0.71 | 11.28 ± 2.57 | 8.29 ± 1.10 | 15.22 ± 1.77* |
| Fat oxidation, mg·kg0.75·min−1 | 13.81 ± 0.44 | 14.10 ± 0.42 | 12.35 ± 1.54 | 15.28 ± 0.37* | 7.21 ± 0.65 | 7.93 ± 0.74 |
Data are means ± SE; 20 wk, fasted 3 h; n = 23 control, 14 SMAKO, and 12 AAKO.
CHO, carbohydrate.
P ≤ 0.05 for baseline group effect; noncorresponding letters are different.
P ≤ 0.05 for exercise effect.
To better understand the role of intramyocellular energy substrates in the above metabolic and functional phenotypes during exercise, we next measured glycogen and TAG content within several different skeletal muscle types using both biochemical and histological methods (Fig. 7, A and B). Skeletal muscle glycogen content was comparable in control and SMAKO mice but lower in both sedentary and exercised AAKO mice (Fig. 7A). Skeletal muscle glycogen did not decrease significantly with submaximal exercise in any of the groups. As we have shown previously (54), IMTG content was markedly higher in sedentary SMAKO mice compared with control and AAKO mice (Fig. 7B). In response to exercise, IMTG content decreased in SMAKO mice but not in control or AAKO mice. To explore the possibility that HSL might be contributing to IMTG hydrolysis in SMAKO mice, we next assessed skeletal muscle HSL phosphorylation at Ser565 and Ser660 [sites phosphorylated by AMP-activated protein kinase and PKA, respectively, during acute exercise, resulting in increased HSL lipolytic activity (64)] (Fig. 7C). Indeed, phosphorylation of HSL at both Ser565 and Ser660 was increased in skeletal muscle of exercised compared with sedentary mice for all genotype groups, suggesting that HSL might be sufficient to maintain IMTG hydrolysis during exercise despite the loss of ATGL in SMAKO mice. Notably, both basal and exercise-stimulated phosphorylation of HSL at Ser660 was markedly reduced in AAKO mice, a finding consistent with decreased PKA-stimulated lipolysis in skeletal muscle (37). Finally, given the differences in muscle substrate profiles and lower rates of gas exchange in AAKO mice, we next assessed mitochondrial function in skeletal muscle of sedentary control, SMAKO, and AAKO mice (Fig. 7, D and E). As we demonstrated previously (54), mitochondrial respiration in isolated skeletal muscle fibers of control and SMAKO mice was similar under basal, substrate-stimulated, and uncoupled conditions (Fig. 7D). In contrast, mitochondrial respiration was dramatically lower in AAKO mice during all of the above conditions. Consistent with these results, expression of mitochondrial oxidative phosphorylation proteins was similar between control and SMAKO mice (Fig. 7E), with the exception of slightly higher complex III in SMAKO mice. In contrast, AAKO mice had lower expression of complex I, ATP synthase, and total OXPHOS proteins (Fig. 7D). Normalization of the respirometry data to expression of OXPHOS proteins suggested that AAKO mice had intrinsic mitochondrial dysfunction that was not solely due to decreased mitochondrial content. Thus, ATGL-mediated lipolysis in adipocytes vs. skeletal myocytes differentially influences intramyocellar factors that regulate substrate metabolism during submaximal endurance exercise.
DISCUSSION
The overall goal of this study was to determine the relative contribution of adipocyte vs. skeletal myocyte ATGL-mediated TAG hydrolysis to acute exercise performance in untrained mice. To achieve this goal, we evaluated exercise performance and energy substrate metabolism in response to peak and submaximal exercise in mice lacking ATGL in either adipocytes or skeletal myocytes compared with control mice. This study revealed several novel findings. First, impaired adipocyte lipolysis due to loss of ATGL action in adipocytes has a greater impact on both peak and submaximal exercise performance in untrained mice than loss of ATGL action in skeletal myocytes. This decrease in exercise performance in AAKO mice is likely to be mediated, at least in part, by 1) reduced systemic energy substrate availability (FAs and glucose), 2) reduced metabolic flexibility (decreased ability to switch between FA and glucose substrates, depending on energy availability and/or energy requirements), 3) reduced phosphorylation of HSL at Ser660 in skeletal muscle, and/or 4) reduced mitochondrial respiration in skeletal muscle. Notably, the latter is profound and may independently be sufficient to reduce exercise performance. Second, impaired intramyocellular lipolysis due to loss of ATGL action in skeletal myocytes is not sufficient to reduce peak or submaximal exercise performance in untrained mice. This lack of an effect on acute exercise performance in SMAKO mice may be due to 1) sustained/adequate peripheral energy substrate delivery, 2) maintenance (or even enhancement) of metabolic flexibility, and/or 3) preserved HSL phosphorylation and HSL-mediated TAG hydrolysis of an expanded IMTG pool. Together, these data suggest that ATGL action in adipocytes plays a greater role than in skeletal myocytes in generating energy for peak and submaximal exercise in untrained mice.
Although IMTG metabolism has been studied intensively, the precise physiological relevance of IMTGs under different physiological and/or pathophysiological conditions remains poorly understood (11, 15). In the context of physical activity, adipocyte lipolysis contributes to as much as 75% of FA substrates for moderate-intensity exercise in humans (44). Other studies have suggested that IMTG lipolysis also contributes substantially to fat oxidation for energy during exercise (35, 49, 55). In humans (36) and rodents (31, 52), global inhibition of ATGL-mediated lipolysis impairs exercise performance, and yet the tissue-specific contributions of ATGL action to exercise performance are poorly understood. Using our previously established model of skeletal myocyte-specific ATGL deletion (54) and our newly developed model of adipocyte-specific ATGL deletion, we address this important question. Of particular relevance, inhibition of ATGL-mediated lipolysis in adipocyte but not skeletal myocytes results in reduced peripheral energy substrate availability, specifically circulating NEFAs and TAGs as well as glucose (52). This depletion of circulating glucose in AAKO mice underscores the glucose-sparing effect of adipocyte FA mobilization during exercise. Indeed, impaired adipocyte ATGL action decreased O2 consumption and CO2 production and enhanced rates of whole body carbohydrate oxidation at rest. Our data parallel those of Huijsman et al. (31), who demonstrated higher RERs during the light cycle in global ATGL as well as HSL knockout mice compared with control mice, reflecting an inability to switch from carbohydrate to fat oxidation under fasting conditions. These data indicate that a lack of circulating lipids due to impaired adipocyte lipolysis results in a shift toward carbohydrate oxidation (48). Thus, adipocyte-specific ATGL action is critical for whole body energy substrate availability and oxidation, whereas inhibition of skeletal muscle ATGL action is not sufficient to alter systemic substrate metabolism (54).
Peak exercise performance is the product of cardiovascular dynamics and tissue energy metabolism. Although impaired skeletal myocyte-specific ATGL action is not sufficient to influence energy homeostasis or insulin action at baseline or in response to nutritional stress (i.e., high-fat diet feeding) in mice (54), we hypothesized that it would be required for FA mobilization in the context of a functional stress such as acute exercise. Our data indicate that mice with impaired ATGL action in skeletal myocytes exhibit normal responses to increasing exercise intensity. There are several possible explanations for this observation. First, the inhibition of ATGL-mediated lipolysis in skeletal myocytes results in a compensatory metabolic shift toward greater carbohydrate metabolism as well as total whole body substrate oxidation (i.e., fat + carbohydrate oxidation) during peak exercise. These data suggest a compensatory upregulation of muscle glycolysis (46), presumably through a greater reliance on type II (glycolytic) fibers, which are also more abundant in mice than humans (1). Second, peak exercise does not require a significant contribution of fat metabolism for energy production. As exercise intensity increases, rates of both glycolysis and gluconeogenesis are adjusted to maintain adequate glucose availability (57). Thus, despite the loss of ATGL-mediated intramyocellular lipolysis, peak exercise could remain unaffected. In contrast, reduction of adipocyte ATGL action resulted in a metabolic shift toward whole body carbohydrate oxidation and a reduction in overall substrate availability. Therefore, similarly to global ATGL (31, 52) as well as HSL (31) knockout mice, the increased energy demand of exercise likely resulted in a preferential utilization and depletion of glycogen stores in mice lacking adipocyte lipolysis, coupled with the lack of substrate for gluconeogenesis, thereby contributing to an overall decrease in exercise performance. These observations can be further explained by dysregulation of counterregulatory mechanisms (19) associated with hypoglycemia. Studies in humans have demonstrated that pharmacological inhibition of lipolysis following hypoglycemia results in suppression of endogenous glucose production (42), which is rescued by administration of exogenous lipid. Thus, inhibition of adipocyte but not intramyocellular ATGL action impairs substrate availability necessary for peak exercise performance.
In contrast to the anaerobic energy demand of maximal-intensity exercise, submaximal endurance exercise energy requirements in humans are generally met through the oxidation of FAs (13) derived from adipocyte (28) and/or IMTG stores (22, 58, 60). In mice and humans, IMTG content is highest in type I slow-oxidative fibers (54, 59, 60), which are physically associated with mitochondria (34) and correlated with ATGL content (32, 54). We hypothesized that submaximal endurance exercise performance would be impaired in SMAKO mice due to reduced intramyocellular FA mobilization. Likewise, we hypothesized that submaximal endurance exercise performance would be impaired in AAKO mice due to reduced circulating lipid substrate. Unexpectedly, however, submaximal endurance exercise capacity was not affected by the inhibition of skeletal myocyte-specific ATGL action. To the contrary, SMAKO mice demonstrated a robust switch to greater rates of fat oxidation in the face of moderate-intensity exercise. Although the exact mechanism for this result is unclear, one possible explanation is that the lipolytic action of HSL, which is nonfiber type specific (39) and activated by skeletal muscle contraction (38), is sufficient to compensate for loss of ATGL action in skeletal muscle but not adipocytes during exercise. This is supported by the observation that TAG hydrolase activity is present in ATGL-deficient skeletal muscle (5, 54). Furthermore, the higher IMTG content in SMAKO mice would provide more substrate for HSL-mediated lipolysis, as suggested by the dramatic reduction in IMTG content in exercised SMAKO mice. Indeed, both ATGL and HSL are activated by similar pathways during muscle contraction (43). This would explain our observation of appropriate whole body fat oxidation and a tendency for greater relative increases in fat oxidation with skeletal myocyte-specific ATGL deletion. In contrast, and in agreement with our peak exercise data, inhibition of adipocyte ATGL action attenuates exercise performance due to a greater reliance on carbohydrate oxidation and metabolic inflexibility. Our results are consistent with the body of literature related to substrate competition first proposed by Randle et al. (48) demonstrating that a pharmacological decrease in FA availability in both rodents (14, 67) and humans (8, 29, 41, 68) leads to an overall switch toward carbohydrate oxidation and compensatory reduction in FA oxidation. Together, these data point to metabolic inflexibility within the context of submaximal endurance exercise with the inhibition of adipocyte ATGL-mediated lipolysis.
The acute peak and submaximal endurance exercise interventions indicate that impaired ATGL action in adipocytes rather than myocytes has a greater impact on acute exercise performance. To better understand the potential mechanisms associated with this observation, we examined skeletal muscle substrates, mitochondrial performance and content, and markers of lipolysis. Inhibition of available FA substrate through the targeted deletion of ATGL in adipocyte results in decreased basal skeletal muscle glycogen content (31) but does not appear to affect IMTG content. Thus, adipocyte but not skeletal muscle ATGL action is required to maintain circulating and intramyocellular carbohydrates at rest and during physical activity. The lack of an exercise-induced reduction in IMTGs in AAKO mice is somewhat surprising. However, because baseline IMTG content is low in AAKO and control mice (in contrast to SMAKO mice), a further reduction in IMTG content may be below the limit of detection. Alternatively, there may be differences in substrate delivery and/or utilization in AAKO and control mice. Nevertheless, our data from control and AAKO mice are in agreement with previous reports of unchanged IMTG content under acute submaximal exercise conditions in untrained mice (31, 52). These data are in contrast to acute exercise studies in trained humans (60) and under conditions of pharmacological inhibition of adipose tissue lipolysis (61) as well as maximal tetanic stimulation in mice (5). Together, these data emphasize the importance of species (mice and men), training status, and exercise and/or contraction stimulus in the regulation of IMTG.
We demonstrate that impaired adipocyte ATGL action dramatically reduces both mitochondrial performance and content. Similarly, global and cardiomyocyte-specific ATGL deficiency markedly reduces peroxisome proliferator-activated receptor target gene expression and mitochondrial function in cardiomyocytes, resulting in severe cardiac dysfunction (25). This dramatic phenotype is probably intensified by the constant high energy demand of cardiac muscle. In contrast, skeletal muscle fluctuates between low and high energy demand during rest and exercise, respectively. Thus, the relative importance of skeletal myocyte vs. adipocyte ATGL action may only manifest certain physiological situations in which sustained high energy demand is required (i.e., chronic exercise training). Also, in contrast to cardiac muscle, global (47) but not skeletal muscle-specific (54) ATGL deficiency downregulates genes associated with electron transport and ATP synthesis in skeletal muscle. Interestingly, mitochondrial capacity, as measured by 31P-MRS in the mixed fiber-type calf complex, does not seem to be impaired with global ATGL deficiency under conditions of maximal tetanic electrical stimulation (45). Since exercise training markedly promotes mitochondrial biogenesis in rodents (23, 51), additional studies are required to define the role of adipocyte and skeletal myocyte ATGL action in skeletal muscle metabolism, mitochondrial function, and exercise performance in response to chronic exercise training. Our study suggests that, in the setting of acute exercise, adipocyte- but not skeletal myocyte-specific ATGL-mediated lipolysis is essential for the effect on mitochondrial function in skeletal muscle.
It is important to reiterate that although skeletal muscle-specific ATGL deletion was not sufficient to impair acute peak and submaximal endurance exercise performance in this study, these data do not exclude an important role for ATGL action in skeletal muscle lipolysis. To the contrary, a recent study by Alsted et al. (5) demonstrated that ATGL and HSL together account for greater than 98% of contraction-stimulated lipolysis in skeletal muscle. Furthermore, the absence of HSL is not sufficient to impair contraction-stimulated skeletal muscle lipolysis (5) or exercise performance (31). These data suggest that ATGL not only plays an important role in skeletal muscle lipolysis but is sufficient to compensate for loss of HSL. Likewise, in our study, absence of ATGL action in skeletal muscle is not sufficient to impair acute exercise performance, suggesting that other factors, such as HSL action or systemic delivery of energy substrates, are sufficient to compensate for loss of ATGL in skeletal muscle in the context of acute peak and submaximal exercise. In addition, our data do not exclude an important role for ATGL action in skeletal muscle lipolysis in humans. IMTGs accumulate in both mice (54) and humans (20, 53) with ATGL deficiency. Substantial evidence supports the importance of ATGL action in human skeletal muscle lipid metabolism (4, 7). Thus, inherent differences between mice and men, such as the percentage of oxidative fibers, may exist. Given the severe myopathy in ATGL-deficient humans, understanding the relative contribution of ATGL action in adipocytes and muscle metabolism function is important. Additional studies are required to more precisely delineate the relative contribution of ATGL and HSL-mediated IMTG hydrolysis to muscle function in both mice and humans.
In summary, the coordinated effects of adipocyte and skeletal muscle lipolysis provide critical substrates for energy homeostasis during rest and physical activity. Dysregulation of lipolysis contributes to insulin resistance and other disease states, whereas modulation of lipolysis can improve health outcomes (3, 16). Understanding the effects of altered lipolysis within the context of physical activity may provide novel insights into these processes that can be therapeutically targeted to improve metabolic disease. Our study provides evidence that inhibition of ATGL-mediated lipolysis in adipocytes, but not skeletal muscle, results in significant impairments in exercise performance. This dramatic phenotype is characterized by robust depletion of circulating and skeletal muscle substrates as well as impaired mitochondrial performance and content. Additional studies are warranted to further characterize the tissue-specific contribution of ATGL action to skeletal muscle function in normal physiology (i.e., in response to exercise training) and disease (myopathy in neutral lipid storage disease). Given the fundamental role of TAG hydrolysis in metabolism, these studies are likely to provide important insights into the critical role of extra- and intramyocellular FA mobilization in overall muscle health and function.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant R01-DK-090166, a Howard Hughes Medical Institute Physician-Scientist Early Career Award, and a University of Pittsburgh Department of Medicine Junior Scholar Award (to E. E. Kershaw); NIDDK Grant T32-DK-007052 (to M. T. Sitnick); and an Erwin Schrödinger Fellowship-J3221-B19 funded by the Austrian Science Fund (to G. Schoiswohl).
DISCLOSURES
The authors report no conflicts of interest, financial or otherwise.
AUTHOR CONTRIBUTIONS
E.E.K., J.J.D., and M.T.S. conception and design of research; E.E.K., J.J.D., M.T.S., G.S., R.C.W., M.K.B., and L.C. performed experiments; E.E.K., J.J.D., M.T.S., G.S., and T.P. analyzed data; E.E.K., J.J.D., M.T.S., G.S., and T.P. interpreted results of experiments; E.E.K. and J.J.D. prepared figures; E.E.K. and J.J.D. drafted manuscript; E.E.K., J.J.D., M.T.S., G.S., R.C.W., M.K.B., L.C., and T.P. edited and revised manuscript; E.E.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the University of Pittsburgh Department of Medicine Metabolic Research Center.
Present address of M. T. Sitnick: Dept. of Biology and Molecular Biology, Montclair State University, Montclair, NJ 07043.
Present address of G. Schoiswohl: Inst. of Molecular Biosciences, University of Graz, Graz 8010, Austria.
Present address of M. K. Basantani: Inst. of Bioscience and Technology, Shri Ramswaroop Memorial University, Uttar Pradesh 225003, India.
REFERENCES
- 1.Agbulut O, Noirez P, Beaumont F, Butler-Browne G. Myosin heavy chain isoforms in postnatal muscle development of mice. Biol Cell 95: 399–406, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Ahmadian M, Abbott MJ, Tang T, Hudak CS, Kim Y, Bruss M, Hellerstein MK, Lee HY, Samuel VT, Shulman GI, Wang Y, Duncan RE, Kang C, Sul HS. Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype. Cell Metab 13: 739–748, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmadian M, Duncan RE, Varady KA, Frasson D, Hellerstein MK, Birkenfeld AL, Samuel VT, Shulman GI, Wang Y, Kang C, Sul HS. Adipose overexpression of desnutrin promotes fatty acid use and attenuates diet-induced obesity. Diabetes 58: 855–866, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alsted TJ, Nybo L, Schweiger M, Fledelius C, Jacobsen P, Zimmermann R, Zechner R, Kiens B. Adipose triglyceride lipase in human skeletal muscle is upregulated by exercise training. Am J Physiol Endocrinol Metab 296: E445–E453, 2009. [DOI] [PubMed] [Google Scholar]
- 5.Alsted TJ, Ploug T, Prats C, Serup AK, Høeg L, Schjerling P, Holm C, Zimmermann R, Fledelius C, Galbo H, Kiens B. Contraction-induced lipolysis is not impaired by inhibition of hormone-sensitive lipase in skeletal muscle. J Physiol 591: 5141–5155, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arogyasami J, Sellers TL, Wilson GI, Jones JP, Duan C, Winder WW. Insulin-induced hypoglycemia in fed and fasted exercising rats. J Appl Physiol 72: 1991–1998, 1992. [DOI] [PubMed] [Google Scholar]
- 7.Badin PM, Louche K, Mairal A, Liebisch G, Schmitz G, Rustan AC, Smith SR, Langin D, Moro C. Altered skeletal muscle lipase expression and activity contribute to insulin resistance in humans. Diabetes 60: 1734–1742, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bajaj M, Suraamornkul S, Romanelli A, Cline GW, Mandarino LJ, Shulman GI, DeFronzo RA. Effect of a sustained reduction in plasma free fatty acid concentration on intramuscular long-chain fatty Acyl-CoAs and insulin action in type 2 diabetic patients. Diabetes 54: 3148–3153, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Basantani MK, Sitnick MT, Cai L, Brenner DS, Gardner NP, Li JZ, Schoiswohl G, Yang K, Kumari M, Gross RW, Zechner R, Kershaw EE. Pnpla3/Adiponutrin deficiency in mice does not contribute to fatty liver disease or metabolic syndrome. J Lipid Res 52: 318–329, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bender PR, Martin BJ. Ventilatory and treadmill endurance during acute semistarvation. J Appl Physiol 60: 1823–1827, 1986. [DOI] [PubMed] [Google Scholar]
- 11.Bergman BC, Butterfield GE, Wolfel EE, Casazza GA, Lopaschuk GD, Brooks GA. Evaluation of exercise and training on muscle lipid metabolism. Am J Physiol Endocrinol Metab 276: E106–E117, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Brechtel K, Niess AM, Machann J, Rett K, Schick F, Claussen CD, Dickhuth HH, Haering HU, Jacob S. Utilisation of intramyocellular lipids (IMCLs) during exercise as assessed by proton magnetic resonance spectroscopy (1H-MRS). Horm Metab Res 33: 63–66, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Brooks GA, Mercier J. Balance of carbohydrate and lipid utilization during exercise: the “crossover” concept. J Appl Physiol 76: 2253–2261, 1994. [DOI] [PubMed] [Google Scholar]
- 14.Bryson JM, Cooney GJ, Wensley VR, Phuyal JL, Caterson ID. The effects of the inhibition of fatty acid oxidation on pyruvate dehydrogenase complex activity in tissues of lean and obese mice. Int J Obes Relat Metab Disord 20: 738–744, 1996. [PubMed] [Google Scholar]
- 15.Coen PM, Goodpaster BH. Role of intramyocelluar lipids in human health. Trends Endocrinol Metab 23: 391–398, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das SK, Eder S, Schauer S, Diwoky C, Temmel H, Guertl B, Gorkiewicz G, Tamilarasan KP, Kumari P, Trauner M, Zimmermann R, Vesely P, Haemmerle G, Zechner R, Hoefler G. Adipose triglyceride lipase contributes to cancer-associated cachexia. Science 333: 233–238, 2011. [DOI] [PubMed] [Google Scholar]
- 17.Dubé JJ, Amati F, Stefanovic-Racic M, Toledo FG, Sauers SE, Goodpaster BH. Exercise-induced alterations in intramyocellular lipids and insulin resistance: the athlete's paradox revisited. Am J Physiol Endocrinol Metab 294: E882–E888, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eguchi J, Wang X, Yu S, Kershaw EE, Chiu PC, Dushay J, Estall JL, Klein U, Maratos-Flier E, Rosen ED. Transcriptional control of adipose lipid handling by IRF4. Cell Metab 13: 249–259, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fanelli C, Calderone S, Epifano L, De Vincenzo A, Modarelli F, Pampanelli S, Perriello G, De Feo P, Brunetti P, Gerich JE, et al. Demonstration of a critical role for free fatty acids in mediating counterregulatory stimulation of gluconeogenesis and suppression of glucose utilization in humans. J Clin Invest 92: 1617–1622, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischer J, Lefevre C, Morava E, Mussini JM, Laforet P, Negre-Salvayre A, Lathrop M, Salvayre R. The gene encoding adipose triglyceride lipase (PNPLA2) is mutated in neutral lipid storage disease with myopathy. Nat Genet 39: 28–30, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol 55: 628–634, 1983. [DOI] [PubMed] [Google Scholar]
- 22.Frayn KN. Fat as a fuel: emerging understanding of the adipose tissue-skeletal muscle axis. Acta Physiol (Oxf) 199: 509–518, 2010. [DOI] [PubMed] [Google Scholar]
- 23.Gollnick PD, King DW. Effect of exercise and training on mitochondria of rat skeletal muscle. Am J Physiol 216: 1502–1509, 1969. [DOI] [PubMed] [Google Scholar]
- 24.Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, Heldmaier G, Maier R, Theussl C, Eder S, Kratky D, Wagner EF, Klingenspor M, Hoefler G, Zechner R. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science 312: 734–737, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Haemmerle G, Moustafa T, Woelkart G, Buttner S, Schmidt A, van de Weijer T, Hesselink M, Jaeger D, Kienesberger PC, Zierler K, Schreiber R, Eichmann T, Kolb D, Kotzbeck P, Schweiger M, Kumari M, Eder S, Schoiswohl G, Wongsiriroj N, Pollak NM, Radner FP, Preiss-Landl K, Kolbe T, Rulicke T, Pieske B, Trauner M, Lass A, Zimmermann R, Hoefler G, Cinti S, Kershaw EE, Schrauwen P, Madeo F, Mayer B, Zechner R. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-alpha and PGC-1. Nat Med 17: 1076–1085, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hargreaves M, Richter EA. Regulation of skeletal muscle glycogenolysis during exercise. Can J Sport Sci 13: 197–203, 1988. [PubMed] [Google Scholar]
- 27.He J, Kelley DE. Muscle glycogen content in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab 287: E1002–E1007, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Helge JW, Biba TO, Galbo H, Gaster M, Donsmark M. Muscle triacylglycerol and hormone-sensitive lipase activity in untrained and trained human muscles. Eur J Appl Physiol 97: 566–572, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Hinderling VB, Schrauwen P, Langhans W, Westerterp-Plantenga MS. The effect of etomoxir on 24-h substrate oxidation and satiety in humans. Am J Clin Nutr 76: 141–147, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Horowitz JF. Fatty acid mobilization from adipose tissue during exercise. Trends Endocrinol Metab 14: 386–392, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Huijsman E, van de Par C, Economou C, van der Poel C, Lynch GS, Schoiswohl G, Haemmerle G, Zechner R, Watt MJ. Adipose triacylglycerol lipase deletion alters whole body energy metabolism and impairs exercise performance in mice. Am J Physiol Endocrinol Metab 297: E505–E513, 2009. [DOI] [PubMed] [Google Scholar]
- 32.Jocken JW, Smit E, Goossens GH, Essers YP, van Baak MA, Mensink M, Saris WH, Blaak EE. Adipose triglyceride lipase (ATGL) expression in human skeletal muscle is type I (oxidative) fiber specific. Histochem Cell Biol 129: 535–538, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kienesberger PC, Pulinilkunnil T, Nagendran J, Young ME, Bogner-Strauss JG, Hackl H, Khadour R, Heydari E, Haemmerle G, Zechner R, Kershaw EE, Dyck JR. Early structural and metabolic cardiac remodelling in response to inducible adipose triglyceride lipase ablation. Cardiovasc Res 99: 442–451, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koves TR, Sparks LM, Kovalik JP, Mosedale M, Arumugam R, DeBalsi KL, Everingham K, Thorne L, Phielix E, Meex RC, Kien CL, Hesselink MK, Schrauwen P, Muoio DM. PPARgamma coactivator-1alpha contributes to exercise-induced regulation of intramuscular lipid droplet programming in mice and humans. J Lipid Res 54: 522–534, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krssak M, Petersen KF, Bergeron R, Price T, Laurent D, Rothman DL, Roden M, Shulman GI. Intramuscular glycogen and intramyocellular lipid utilization during prolonged exercise and recovery in man: a 13C and 1H nuclear magnetic resonance spectroscopy study. J Clin Endocrinol Metab 85: 748–754, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Laforêt P,Ørngreen M, Preisler N, Andersen G, Vissing J. Blocked muscle fat oxidation during exercise in neutral lipid storage disease. Arch Neurol 69: 530–533, 2012. [DOI] [PubMed] [Google Scholar]
- 37.Lampidonis AD, Rogdakis E, Voutsinas GE, Stravopodis DJ. The resurgence of Hormone-Sensitive Lipase (HSL) in mammalian lipolysis. Gene 477: 1–11, 2011. [DOI] [PubMed] [Google Scholar]
- 38.Langfort J, Ploug T, Ihlemann J, Holm C, Galbo H. Stimulation of hormone-sensitive lipase activity by contractions in rat skeletal muscle. Biochem J 351: 207–214, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Langfort J, Ploug T, Ihlemann J, Saldo M, Holm C, Galbo H. Expression of hormone-sensitive lipase and its regulation by adrenaline in skeletal muscle. Biochem J 340: 459–465, 1999. [PMC free article] [PubMed] [Google Scholar]
- 40.Li S, Czubryt MP, McAnally J, Bassel-Duby R, Richardson JA, Wiebel FF, Nordheim A, Olson EN. Requirement for serum response factor for skeletal muscle growth and maturation revealed by tissue-specific gene deletion in mice. Proc Natl Acad Sci USA 102: 1082–1087, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liang H, Tantiwong P, Sriwijitkamol A, Shanmugasundaram K, Mohan S, Espinoza S, Defronzo RA, Dube JJ, Musi N. Effect of a sustained reduction in plasma free fatty acid concentration on insulin signalling and inflammation in skeletal muscle from human subjects. J Physiol 591: 2897–2909, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lucidi P, Rossetti P, Porcellati F, Pampanelli S, Candeloro P, Andreoli AM, Perriello G, Bolli GB, Fanelli CG. Mechanisms of insulin resistance after insulin-induced hypoglycemia in humans: the role of lipolysis. Diabetes 59: 1349–1357, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mason RR, Meex RC, Lee-Young R, Canny BJ, Watt MJ. Phosphorylation of adipose triglyceride lipase Ser404 is not related to 5′-AMPK activation during moderate-intensity exercise in humans. Am J Physiol Endocrinol Metab 303: E534–E541, 2012. [DOI] [PubMed] [Google Scholar]
- 44.Mittendorfer B, Horowitz JF, Klein S. Effect of gender on lipid kinetics during endurance exercise of moderate intensity in untrained subjects. Am J Physiol Endocrinol Metab 283: E58–E65, 2002. [DOI] [PubMed] [Google Scholar]
- 45.Nunes PM, van de Weijer T, Veltien A, Arnts H, Hesselink MK, Glatz JF, Schrauwen P, Tack CJ, Heerschap A. Increased intramyocellular lipids but unaltered in vivo mitochondrial oxidative phosphorylation in skeletal muscle of adipose triglyceride lipase-deficient mice. Am J Physiol Endocrinol Metab 303: E71–E81, 2012. [DOI] [PubMed] [Google Scholar]
- 46.Picard M, Hepple RT, Burelle Y. Mitochondrial functional specialization in glycolytic and oxidative muscle fibers: tailoring the organelle for optimal function. Am J Physiol Cell Physiol 302: C629–C641, 2012. [DOI] [PubMed] [Google Scholar]
- 47.Pinent M, Hackl H, Burkard TR, Prokesch A, Papak C, Scheideler M, Hammerle G, Zechner R, Trajanoski Z, Strauss JG. Differential transcriptional modulation of biological processes in adipocyte triglyceride lipase and hormone-sensitive lipase-deficient mice. Genomics 92: 26–32, 2008. [DOI] [PubMed] [Google Scholar]
- 48.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1: 785–789, 1963. [DOI] [PubMed] [Google Scholar]
- 49.Roepstorff C, Steffensen CH, Madsen M, Stallknecht B, Kanstrup IL, Richter EA, Kiens B. Gender differences in substrate utilization during submaximal exercise in endurance-trained subjects. Am J Physiol Endocrinol Metab 282: E435–E447, 2002. [DOI] [PubMed] [Google Scholar]
- 50.Romijn JA, Coyle EF, Sidossis LS, Gastaldelli A, Horowitz JF, Endert E, Wolfe RR. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Physiol Endocrinol Metab 265: E380–E391, 1993. [DOI] [PubMed] [Google Scholar]
- 51.Rowe GC, El-Khoury R, Patten IS, Rustin P, Arany Z. PGC-1alpha is dispensable for exercise-induced mitochondrial biogenesis in skeletal muscle. PLoS One 7: e41817, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schoiswohl G, Schweiger M, Schreiber R, Gorkiewicz G, Preiss-Landl K, Taschler U, Zierler KA, Radner FP, Eichmann TO, Kienesberger PC, Eder S, Lass A, Haemmerle G, Alsted TJ, Kiens B, Hoefler G, Zechner R, Zimmermann R. Adipose triglyceride lipase plays a key role in the supply of the working muscle with fatty acids. J Lipid Res 51: 490–499, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schweiger M, Lass A, Zimmermann R, Eichmann TO, Zechner R. Neutral lipid storage disease: genetic disorders caused by mutations in adipose triglyceride lipase/PNPLA2 or CGI-58/ABHD5. Am J Physiol Endocrinol Metab 297: E289–E296, 2009. [DOI] [PubMed] [Google Scholar]
- 54.Sitnick MT, Basantani MK, Cai L, Schoiswohl G, Yazbeck CF, Distefano G, Ritov V, DeLany JP, Schreiber R, Stolz DB, Gardner NP, Kienesberger PC, Pulinilkunnil T, Zechner R, Goodpaster BH, Coen P, Kershaw EE. Skeletal muscle triacylglycerol hydrolysis does not influence metabolic complications of obesity. Diabetes 62: 3350–3361, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stellingwerff T, Boon H, Jonkers RA, Senden JM, Spriet LL, Koopman R, van Loon LJ. Significant intramyocellular lipid use during prolonged cycling in endurance-trained males as assessed by three different methodologies. Am J Physiol Endocrinol Metab 292: E1715–E1723, 2007. [DOI] [PubMed] [Google Scholar]
- 56.Taylor CR, Maloiy GM, Weibel ER, Langman VA, Kamau JM, Seeherman HJ, Heglund NC. Design of the mammalian respiratory system. III Scaling maximum aerobic capacity to body mass: wild and domestic mammals. Respir Physiol 44: 25–37, 1981. [DOI] [PubMed] [Google Scholar]
- 57.Trimmer JK, Schwarz JM, Casazza GA, Horning MA, Rodriguez N, Brooks GA. Measurement of gluconeogenesis in exercising men by mass isotopomer distribution analysis. J Appl Physiol 93: 233–241, 2002. [DOI] [PubMed] [Google Scholar]
- 58.van Loon LJ. Intramyocellular triacylglycerol as a substrate source during exercise. Proc Nutr Soc 63: 301–307, 2004. [DOI] [PubMed] [Google Scholar]
- 59.van Loon LJ, Koopman R, Manders R, van der Weegen W, van Kranenburg GP, Keizer HA. Intramyocellular lipid content in type 2 diabetes patients compared with overweight sedentary men and highly trained endurance athletes. Am J Physiol Endocrinol Metab 287: E558–E565, 2004. [DOI] [PubMed] [Google Scholar]
- 60.van Loon LJ, Koopman R, Stegen JH, Wagenmakers AJ, Keizer HA, Saris WH. Intramyocellular lipids form an important substrate source during moderate intensity exercise in endurance-trained males in a fasted state. J Physiol 553: 611–625, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Loon LJ, Manders RJ, Koopman R, Kaastra B, Stegen JH, Gijsen AP, Saris WH, Keizer HA. Inhibition of adipose tissue lipolysis increases intramuscular lipid use in type 2 diabetic patients. Diabetologia 48: 2097–2107, 2005. [DOI] [PubMed] [Google Scholar]
- 62.Villena JA, Roy S, Sarkadi-Nagy E, Kim KH, Sul HS. Desnutrin, an adipocyte gene encoding a novel patatin domain-containing protein, is induced by fasting and glucocorticoids: ectopic expression of desnutrin increases triglyceride hydrolysis. J Biol Chem 279: 47066–47075, 2004. [DOI] [PubMed] [Google Scholar]
- 63.Wagner PD. New ideas on limitations to VO2max. Exerc Sport Sci Rev 28: 10–14, 2000. [PubMed] [Google Scholar]
- 64.Watt MJ, Holmes AG, Pinnamaneni SK, Garnham AP, Steinberg GR, Kemp BE, Febbraio MA. Regulation of HSL serine phosphorylation in skeletal muscle and adipose tissue. Am J Physiol Endocrinol Metab 290: E500–E508, 2006. [DOI] [PubMed] [Google Scholar]
- 65.Wolfe RR, Klein S, Carraro F, Weber JM. Role of triglyceride-fatty acid cycle in controlling fat metabolism in humans during and after exercise. Am J Physiol Endocrinol Metab 258: E382–E389, 1990. [DOI] [PubMed] [Google Scholar]
- 66.Wu JW, Wang SP, Casavant S, Moreau A, Yang GS, Mitchell GA. Fasting energy homeostasis in mice with adipose deficiency of desnutrin/adipose triglyceride lipase. Endocrinology 153: 2198–2207, 2012. [DOI] [PubMed] [Google Scholar]
- 67.Wu M, Singh SB, Wang J, Chung CC, Salituro G, Karanam BV, Lee SH, Powles M, Ellsworth KP, Lassman ME, Miller C, Myers RW, Tota MR, Zhang BB, Li C. Antidiabetic and antisteatotic effects of the selective fatty acid synthase (FAS) inhibitor platensimycin in mouse models of diabetes. Proc Natl Acad Sci USA 108: 5378–5383, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zderic TW, Schenk S, Davidson CJ, Byerley LO, Coyle EF. Manipulation of dietary carbohydrate and muscle glycogen affects glucose uptake during exercise when fat oxidation is impaired by β-adrenergic blockade. Am J Physiol Endocrinol Metab 287: E1195–E1201, 2004. [DOI] [PubMed] [Google Scholar]
- 69.Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger G, Eisenhaber F, Hermetter A, Zechner R. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 306: 1383–1386, 2004. [DOI] [PubMed] [Google Scholar]