Abstract
Systemic lupus erythematosus is an autoimmune disease characterized by the development of auto antibodies against a variety of self-antigens and deposition of immune complexes that lead to inflammation, fibrosis, and end-organ damage. Up to 60% of lupus patients develop nephritis and renal dysfunction leading to kidney failure. N-acetyl-seryl-aspartyl-lysyl-proline, i.e., Ac-SDKP, is a natural tetrapeptide that in hypertension prevents inflammation and fibrosis in heart, kidney, and vasculature. In experimental autoimmune myocarditis, Ac-SDKP prevents cardiac dysfunction by decreasing innate and adaptive immunity. It has also been reported that Ac-SDKP ameliorates lupus nephritis in mice. We hypothesize that Ac-SDKP prevents lupus nephritis in mice by decreasing complement C5-9, proinflammatory cytokines, and immune cell infiltration. Lupus mice treated with Ac-SDKP for 20 wk had significantly lower renal levels of macrophage and T cell infiltration and proinflammatory chemokine/cytokines. In addition, our data demonstrate for the first time that in lupus mouse Ac-SDKP prevented the increase in complement C5-9, RANTES, MCP-5, and ICAM-1 kidney expression and it prevented the decline of glomerular filtration rate. Ac-SDKP-treated lupus mice had a significant improvement in renal function and lower levels of glomerular damage. Ac-SDKP had no effect on the production of autoantibodies. The protective Ac-SDKP effect is most likely achieved by targeting the expression of proinflammatory chemokines/cytokines, ICAM-1, and immune cell infiltration in the kidney, either directly or via C5-9 proinflammatory arm of complement system.
Keywords: Ac-SDKP, lupus nephritis, complement system proteins, inflammation, GFR, renal damage
systemic lupus erythematosus (SLE) is an autoimmune chronic disease that can affect almost any organ of the body. SLE affects an estimated 1.5 million Americans, with 90% of them being women (9). Currently, SLE is a noncurable disease and the treatment is based on controlling the symptoms by immunosuppressive and anti-inflammatory therapies (20, 24). In lupus patients with nephritis and hypertension, angiotensin-converting enzyme inhibitors (ACEi) or angiotensin receptor blockers are added to the therapy (7).
SLE is characterized by the development of auto antibodies against a variety of nuclear and cytoplasmic self-antigens and by deposition of immune complexes in the various tissues. These changes lead to complement activation and increase in the production of cytokines, chemokines, and other proinflammatory factors that promote inflammation, fibrosis, and end-organ damage. Renal and cardiovascular morbidity are the leading causes of mortality in SLE (20). The most common form of kidney disease in SLE patients is glomerulonephritis with histological changes spanning from mild mesangial proliferation to severe inflammation, glomerular and tubulointerstitial fibrosis, vascular lesions, and end-stage renal disease (8, 57). Several different mouse models of SLE have been established for studying the underlying mechanisms of this autoimmune disorder, such as BXSB, NZBWF1, and MRL/lpr mice (48). BXSB and NZBWF1 models are most useful for studying SLE-related coronary artery disease and hypertension, respectively. Since our study focused on the kidney damage associated with SLE, here we used MRL/MpJ-Faslpr/2J (Lupus) mice that develop an aggressive form of lupus nephritis with no hypertension (47). We used age-matched MRL-MpJ mice as controls (Ctrl).
N-acetyl-seryl-aspartyl-lysyl-proline, i.e., Ac-SDKP is a natural tetrapeptide that is released from its precursor thymosin β4, by prolyl oligopeptidase (12). Ac-SDKP is found in human plasma and circulating mononuclear cells (43) and is inactivated by ACE (3). Ac-SDKP was previously shown to have anti-inflammatory and anti-fibrotic properties (2, 59) and a decrease in endogenous Ac-SDKP levels promoted heart and kidney fibrosis (11). These Ac-SDKP anti-inflammatory and anti-fibrotic effects were shown to be in part the result of a decrease in macrophage infiltration and transforming growth factor (TGF)-β expression (59). Ac-SDKP role in inflammation and fibrosis was mainly explored in hypertension and heart failure animal models (29, 38). However, the role of Ac-SDKP in autoimmune processes is less clear. Indirect evidence on potential beneficial effect of Ac-SDKP in autoimmune diseases came from the recent clinical study on lupus nephritis showing that ACE inhibitors delayed the renal involvement and decreased risk of disease activity in SLE patients (15). On the other hand, autoimmune animal models were used only by two studies that both reported structural and functional improvement in response to Ac-SDKP. The first study used rat model of EAM where Ac-SDKP decreased the expression of proinflammatory cytokines, chemokines and cell adhesion molecules, and cardiac infiltration by macrophages, dendritic and T cells (35). The second study used lupus-prone mouse model where Ac-SDKP attenuated the progression of renal damage, decreased proinflammatory cell infiltration and fibrosis, and these actions were suggested to be achieved by downregulation of TNF-α and TGF-β pathways (54). While these data indicate that Ac-SDKP targets immune cells migration and tissue infiltration, it is still not clear what is the underlying mechanism involved in attenuating this cellular response. Therefore, in the present study, we tested the hypothesis that in MRL/lpr mice lupus model Ac-SDKP prevents inflammation and renal end-organ damage by decreasing complement C5/C5a and C5b-9 activation, proinflammatory cytokines, chemokines, and cell adhesion protein expression, and as a consequence causes a decrease in macrophage and T cell infiltration in the kidney. The Ac-SDKP dose chosen in this study was previously shown to correlate to the increase in plasma Ac-SDKP levels caused by ACEi administration (25, 46).
MATERIALS AND METHODS
Animals.
Female MRL/MpJ-Faslpr/2J (lupus) and age-matched control MRL-MpJ mice (Ctrl), 10 wk of age (Jackson Laboratory), were used in the studies. Before all surgical procedures, mice underwent analgesia and anesthesia with butorphanol (2 mg/kg sc) and pentobarbital sodium (50 mg/kg ip), respectively. This study was approved by the Henry Ford Hospital Institutional Animal Care and Use Committee and was in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Experimental protocols.
Mice of 12 wk of age were randomly divided into four groups: 1) control mice receiving vehicle (Veh-Ctrl; n = 6), 2) control mice receiving Ac-SDKP (Ac-SDKP-Ctrl; n = 6), 3) lupus mice receiving vehicle (Veh-Lupus; n = 10), and 4) lupus mice receiving Ac-SDKP (Ac-SDKP-Lupus; n = 10). The vehicle (0.01N acetic acid saline) and Ac-SDKP (800 μg·kg−1·day−1) were infused subcutaneously for 20 wk via osmotic mini-pumps implanted in the back of mice. Systolic blood pressure (SBP) was monitored using computerized tail-cuff system (BP 2000, Visitech) (27). At the end of the treatment, glomerular filtration rate (GFR) was measured, urine and blood samples were collected, animals were euthanized, and kidney tissues were collected for further histological and protein analysis.
Urinary Ac-SDKP and albuminuria.
Mice were allowed to adapt to metabolic cages for 24 h after which they underwent 24 h of fasting and urine collection. ACE inhibitor lisinopril (10−5 M) was applied to the collecting funnels and tubes to prevent Ac-SDKP degradation. Urine Ac-SDKP was measured using EIA KIT (SPI Biolaboratories), as previously described (30). Albuminuria was determined by ELISA kit (Cayman Chemicals).
GFR.
GFR was measured as previously described (32). Data were expressed as microliters per minute per 100 mg of kidney weight (kidney wt).
Glomerular matrix analysis.
Paraffin-embedded tissues sections (4 μm) were stained with periodic acid Schiff (PAS). Thirty five glomeruli within randomly chosen fields of renal cortex were imaged at ×400 magnification. The dark pink color was considered a positive staining representing the extracellular matrix. Glomerular matrix was analyzed by computerized image analysis system (Microsuite Biological imaging software, Olympus America, Center Valley, PA) and positive staining was expressed as the percentage of glomerular area. All of the images shown in this study were captured and analyzed using the same imaging system, unless otherwise specified.
Collagen deposition.
Picrosirius red staining was used to quantify renal interstitial and perivascular collagen deposition (38, 53). Randomly chosen fields within corticomedullar junction were imaged at ×200 magnification. Interstitial collagen fraction was calculated as the ratio of the collagen-positive area to the imaging area.
Collagen content.
A piece of apical renal cortex was used for hydroxyproline assay as described previously (39). Data were expressed as micrograms of collagen per milligram of dry weight (14).
Glomerular nephrin and complement C5b-9 expression.
Frozen sections (6 μm) were stained with goat anti-nephrin antibody (1:50; R&D Systems) and rabbit anti-C5b-9 antibody (1:500; Abcam) and positive signals were visualized using Alexa 488-conjugated species' appropriate secondary antibody. Areas of positive staining within the glomeruli were measured in each section and expressed as percentage of glomerular area (29).
Plasma anti-dsDNA antibodies.
Plasma anti-dsDNA antibody levels were measured by ELISA kit according to the manufacturer's protocol (Alpha Diagnostic International, San Antonio, TX).
Proinflammatory protein array.
Kidney cortex tissue samples were analyzed for protein expression levels of 29 inflammatory mediators using “Proteome Profiler Mouse Chemokine Array Kit,” according to the manufacturer's protocol (R&D Systems). This array included complement C5/5a, monocyte chemotactic protein 5 (MCP-5), regulated on activation normal T cells expressed and secreted (RANTES), and macrophage colony stimulating factor (M-CSF). Data were expressed as arbitrary units (AU) representing the optical density (OD) values of protein of interest divided by the positive control OD values.
Intercellular adhesion molecule-1 expression.
Kidney protein extracts (120 μg/sample) were analyzed by Western blot. Antibodies used were primary anti-intercellular adhesion molecule (ICAM)-1 antibody (1:4,000; R&D Systems), primary anti-GAPDH antibody (1:50,000; Cell Signaling Technology), and the appropriate peroxidase-conjugated secondary antibody (1:20,000; Santa Cruz Biotechnology). Positive signals were visualized using ECL-plus detection system (Amersham Biosciences). Data were expressed as the ratio of ICAM-1 to the GAPDH.
Macrophage and T cell infiltration.
Cryosections (6 μm) were used for the immunohistochemistry to detect macrophages (rat anti-mouse CD68, 1:200, AbD Serotec) and CD3+ T cells (hamster anti-mouse CD3, 1:200, AbD Serotec). Detection system was ABC kit (Vectastain Elite ABC peroxidase kit, Vector Lab) and 3-amino-9-ethylcarbazole substrate. Data were expressed as number of cells per millimeter squared.
Facial lesions score.
Facial lesions assessment was performed independently by three different unbiased investigators. Facial rash was scored according to the following scale: 1 for normal, 2 for mild, 3 for moderate, and 4 for severe.
Data analysis.
All data are expressed as means ± SE. ANOVA and nonparametric Wilcoxon tests were used to compare mean values of the various parameters between different groups. Hochberg's method for multiple comparisons was used to adjust the alpha level of significance (22).
RESULTS
Serum anti-dsDNA antibody concentration.
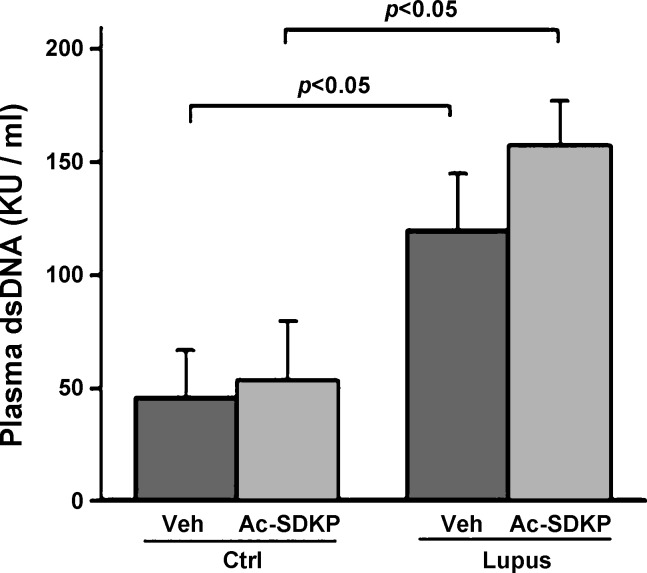
Serum concentrations of anti-dsDNA antibodies were significantly elevated in vehicle-treated lupus mice (Veh-Lupus), compared with vehicle-treated control nonlupus mice (Veh-Ctrl) animals. Ac-SDKP treatment did not decrease the serum concentrations of anti-dsDNA antibodies in neither, Ac-SDKP-treated control, nonlupus mice (Ac-SDKP-Ctrl), nor Ac-SDKP-treated lupus mice (Ac-SDKP-Lupus; Fig. 1).
Fig. 1.
Serum anti-dsDNA antibody concentration. Serum concentration of anti-dsDNA antibodies was significantly elevated in the lupus mice receiving vehicle (Veh-Lupus), compared with control mice receiving vehicle (Veh-Ctrl). Ac-SDKP treatment did not decrease the serum concentrations of anti-dsDNA antibodies in neither, control mice receiving Ac-SDKP (Ac-SDKP-Ctrl) nor lupus mice receiving Ac-SDKP (Ac-SDKP-Lupus).
Complement C5/5a and C5b-9, MCP-5, RANTES, and M-CSF expression in the kidney.
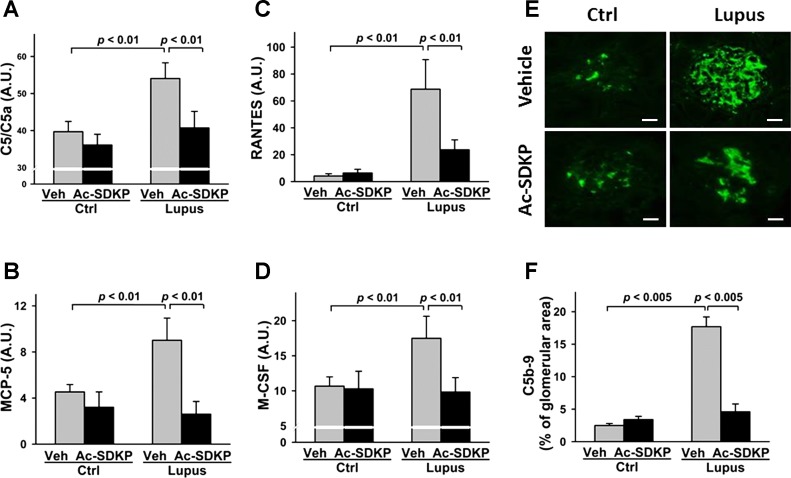
Protein array analysis of C5/C5a, MCP-5, RANTES, and M-CSF, and immunohistochemistry analysis of C5b-9 revealed that the expression levels of these factors were significantly higher in the Veh-Lupus mice, compared with Veh-Ctrl mice (P < 0.001 and P < 0.005, respectively). These levels were significantly lower in Ac-SDKP-Lupus mice, compared with Veh-Lupus mice (P < 0.01). No effect of Ac-SDKP treatment on the expression levels of these factors was detected in Ac-SDKP-Ctrl mice (Fig. 2, A–F).
Fig. 2.
Effect of Ac-SDKP on C5/5a and C5b-9 complement, monocyte chemotactic protein (MCP)-5, regulated on activation normal T cells expressed and secreted (RANTES), and macrophage colony stimulating factor (M-CSF) expression. Renal expression of C5/C5a (A), MCP-5 (B), RANTES (C), and M-CSF (D) was measured with mouse protein array kit. Data were expressed as means ± SE; n = 6. P < 0.01, Veh-Ctrl vs. Veh-Lupus mice; Ac-SDKP-Lupus vs. Veh-Lupus mice. E: glomerular C5b-9 expression detected by immunofluorescent staining. Green indicates positive C5b-9 staining. Tissue section images captured using ×40 objective. Scale bar = 25 μm. F: analysis of C5b-9 expression in lupus mice. Data were calculated as a percentage of the glomerular area and expressed as means ± SE; n = 6. P < 0.005, Veh-Ctrl vs. Veh-Lupus mice; P < 0.005, Ac-SDKP-Lupus vs. Veh-Lupus mice.
ICAM-1 expression in the kidney.
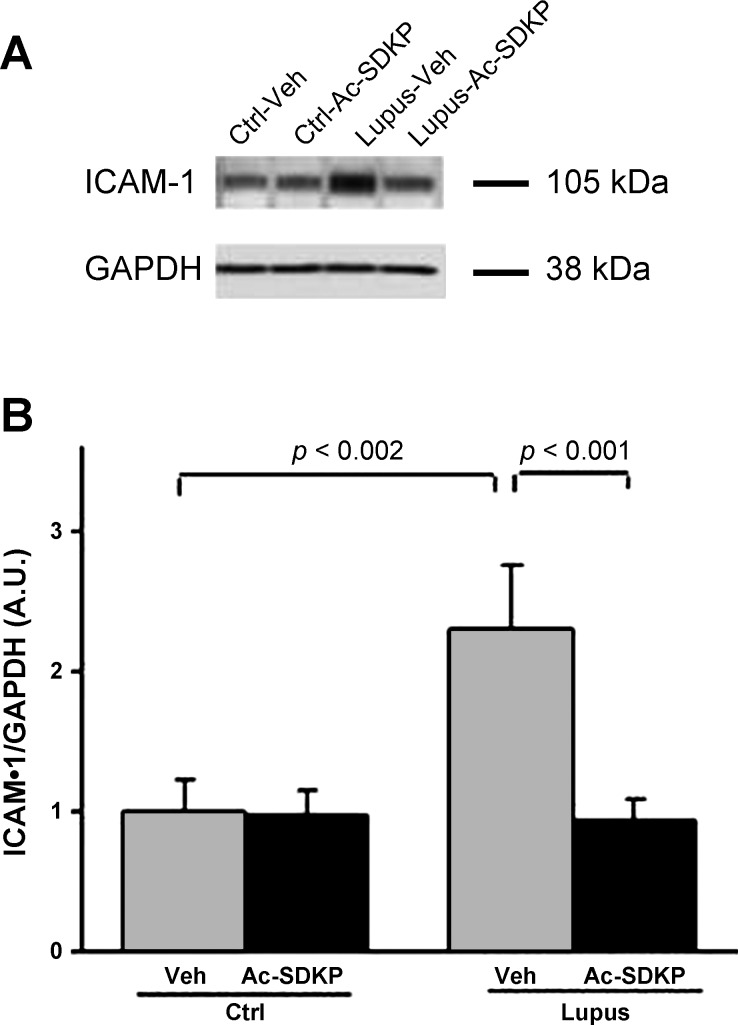
Veh-Lupus mice expressed significantly higher amount of ICAM-1, compared with Veh-Ctrl mice (P < 0.002; Fig. 2, A and B). Ac-SDKP-Lupus mice had significantly lower amount of ICAM-1, compared with the Veh-Lupus mice (P < 0.001), and these ICAM-1 amounts were comparable with the basal ICAM-1 expression observed in Veh-Ctrl mice. These basal ICAM-1 levels in Ac-SDKP-Ctrl mice were not affected by Ac-SDKP treatment (Fig. 3, A and B).
Fig. 3.
Effect of Ac-SDKP on intercellular adhesion molecule (ICAM)-1 expression. A: renal tissue Western blot analysis of ICAM-1 and GAPDH. ICAM-1 band density was quantified after normalization with GAPDH band density. B: ICAM-1 expression. Data were expressed as means ± SE; n = 6. P < 0.002, Veh-Ctrl vs. Veh-Lupus mice; P < 0.001, Ac-SDKP-Lupus vs. Veh-Lupus mice.
Macrophage and T cell infiltration in the kidney.
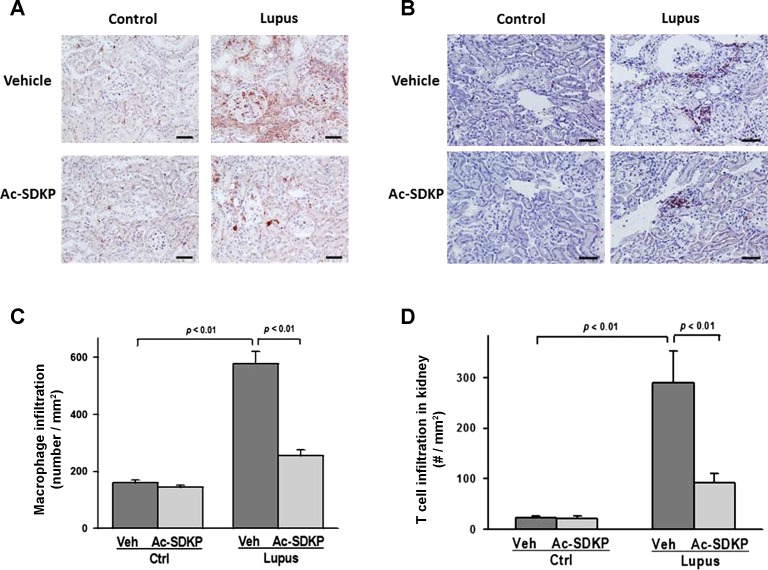
Quantitative immunohistochemistry analysis showed significantly higher numbers of infiltrating macrophages and T cells in Veh-Lupus mice, compared with Veh-Ctrl mice (P < 0.01). In Ac-SDKP-Lupus mice, numbers of infiltrating cells were significantly lower, compared with Veh-Lupus mice (P < 0.01). In the Ac-SDKP-Ctrl mice, macrophage or T cell infiltration was not altered by treatment with Ac-SDKP (Fig. 4, A–D). Representative images showed macrophage (Fig. 4A) and T cell kidney infiltration (Fig. 4B).
Fig. 4.
Effect of Ac-SDKP on macrophage and T cell infiltration. Representative images of renal macrophage (A) and T cell infiltration (B). Macrophages and T cells were identified using anti-CD68 and anti-CD3 antibody, respectively. Positive signals were detected as reddish staining in the cytoplasm. Shown are images captured using ×40 microscope objective. Scale bar = 100 μm. C, D: quantitative analysis of captured images showed that Ac-SDKP significantly reduced macrophage and T cell infiltration in lupus mice. Data were calculated as a number of cells per mm2 and expressed as means ± SE; n = 6–10. P < 0.01, Veh-Ctrl vs. Veh-Lupus mice; P < 0.001, Ac-SDKP-Lupus vs. Veh-Lupus mice.
GFR and albuminuria.
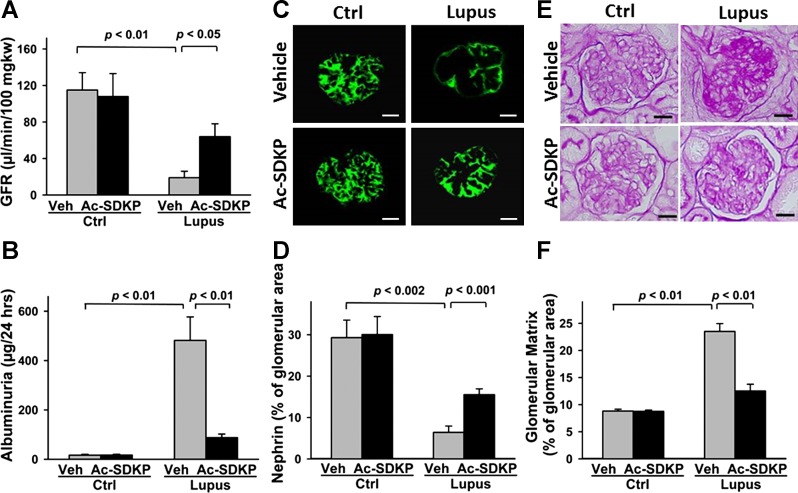
At 32 wk of age, Veh-Lupus mice had significantly lower GFR, compared with Veh-Ctrl mice (P < 0.01). In Ac-SDKP-Lupus mice, GFR was significantly higher, compared with Veh-Lupus mice (P < 0.01). Ac-SDKP had no effect on GFR in Ac-SDKP-Ctrl mice (Fig. 5A). Veh-Lupus mice also developed significantly higher albuminuria, compared with Veh-Ctrl (P < 0.01) and treated Ac-SDKP-Lupus (P < 0.01) animals. In the AcSDKP-Ctrl mice, Ac-SDKP had no effect on albumin in the urine (Fig. 5B).
Fig. 5.
Effect of Ac-SDKP on glomerular filtration rate (GFR) and albuminuria, renal nephrin expression, and glomerular matrix deposition. GFR (A) and 24-h urinary albumin excretion (B) at the end of 20-wk treatment. Data were expressed as means ± SE; n = 6–10. P < 0.01, Veh-Ctrl vs. Veh-Lupus mice and Ac-SDKP-Lupus vs. Veh-Lupus mice. C: glomerular nephrin expression images captured using ×40 microscope objective. Scale bar = 25 μm. D: quantitative data analysis of nephrin expression. Data are expressed as a percentage of the glomerular area and expressed as means ± SE; n = 6–10. P < 0.002, Veh-Ctrl vs. Veh-Lupus mice; P < 0.001, Ac-SDKP-Lupus vs. Veh-Lupus mice. E: periodic acid Schiff staining of kidney at the end of 20-wk treatment. Images captured using ×40 microscope objective. Scale bar = 25 μm. F: quantitative analysis. Data expressed as means ± SE; n = 6–10. P < 0.01, Veh-Ctrl vs. Veh-Lupus mice and Ac-SDKP-Lupus vs. Veh-Lupus mice.
Glomerular nephrin expression.
In Veh-Ctrl or Ac-SDKP-Ctrl mice, positive strong staining for nephrin expression was detected. In the Veh-Lupus mice, nephrin staining was decreased, compared with Veh-Ctrl mice, and it was partially restored in response to Ac-SDKP in Ac-SDKP-Lupus (Fig. 5C). Quantitative analysis demonstrated that Veh-Lupus mice had significantly lower levels of glomerular nephrin expression, compared with Veh-Ctrl (P = 0.002). In Ac-SDKP-Lupus mice, nephrin levels were significantly higher, compared with the Veh-Lupus mice (P = 0.001). In Ac-SDKP-Ctrl, Ac-SDKP had no effect on nephrin expression (Fig. 5D).
Glomerular matrix analysis.
Veh-Lupus mice developed glomerular matrix depositions, detected by PAS staining as dark purple regions within the glomeruli. The percentage of glomerular area positive for PAS was significantly higher in Veh-Lupus compared with Veh-Ctrl and Ac-SDKP-Lupus animals (P < 0.01). In Ac-SDKP-Ctrl mice, Ac-SDKP had no effect on the percentage of PAS staining in the glomeruli (Fig. 5, E–F).
Renal interstitial collagen.
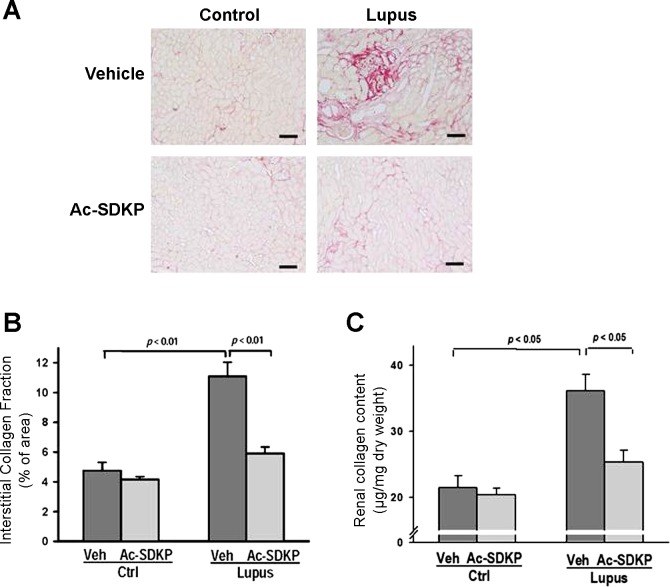
Deposits of interstitial collagen were analyzed by picrosirius red staining. Veh-Lupus mice had significantly higher percentage of the collagen-positive areas, compared with Veh-Ctrl (P < 0.01). In Ac-SDKP-Lupus mice, the percentage of these positive areas was significantly lower, compared with Veh-Ctrl (P < 0.01). Ac-SDKP had no effect on renal interstitial collagen in Ac-SDKP-Ctrl mice (Fig. 6, A and B).
Fig. 6.
Effect of Ac-SDKP on renal collagen. A: representative images of interstitial collagen depositions detected by picrosirius red staining of kidney tissues collected at the end of 20-wk treatment with either vehicle or Ac-SDKP. Shown are digitized sections captured using ×20 microscope objective. Scale bar = 200 μm. B: quantitative analysis of images showed a significantly lower amount of interstitial collagen depositions in Ac-SDKP-Lupus mice, compared with Veh-Lupus mice. Data were calculated as a percentage of the fibrotic area and expressed as means ± SE; n = 6–10. P < 0.01, Veh-Ctrl vs. Veh-Lupus and Ac-SDKP-Lupus vs. Veh-Lupus mice. C: quantitative analysis of total renal collagen content determined by hydroxyproline assay. Significantly lower renal collagen content was observed in Ac-SDKP-Lupus mice compared with Veh-Lupus mice. Data were calculated as a microgram of collagen per milligram of dry tissue weight and expressed as means ± SE; n = 6–10. P < 0.05, Veh-Ctrl vs. Veh-Lupus mice and Ac-SDKP-Lupus vs. Veh-Lupus mice.
Renal collagen content.
In Veh-Lupus mice, collagen content, measured by the hydroxyproline assay, was significantly higher, compared with Veh-Ctrl mice (P < 0.05). In Ac-SDKP-Lupus mice, collagen content was significantly lower compared with the Veh-Lupus mice (P < 0.05). Ac-SDKP had no effect on collagen content in Ac-SDKP-Ctrl mice (Fig. 6C).
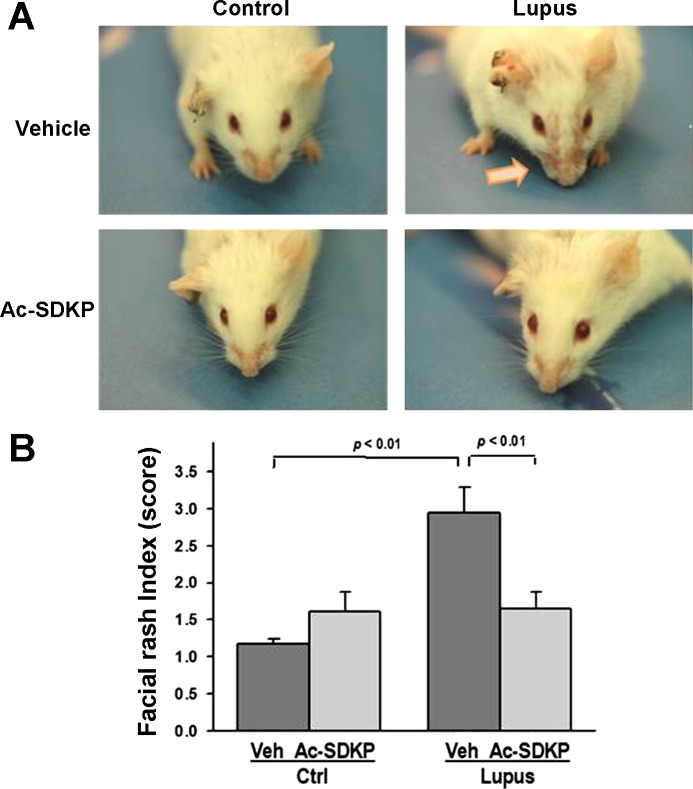
Facial lesions.
Veh-Lupus mice developed a symmetrical facial skin rash at age of 25 wk. The lesions persisted until the end of the study period (32 wk of age). Ac-SDKP-Lupus mice had little or no facial lesions during the entire treatment period (Fig. 7A). Quantitative assessment demonstrated significantly higher lesion index score in Veh-Lupus mice, compared with Veh-Ctrl mice (P < 0.01). In Ac-SDKP-Lupus mice, this score was significantly lower compared with Veh-Lupus mice (P < 0.01). Ac-SDKP had no effect on the skin rash in Ac-SDKP-Ctrl mice (Fig. 7B).
Fig. 7.
Effect of Ac-SDKP on facial lesion formation. A: representative images of mice at the age of 25 wk, with lupus mice exhibiting noticeable facial lesions. Ac-SDKP-Lupus mice exhibited little or no facial lesions. B: quantitative data expressed as facial lesion index determined using 1–4 scoring scale. Data were expressed as means ± SE; n = 6–10. P < 0.01, Veh-Ctrl vs. Veh-Lupus mice; Ac-SDKP-Lupus vs. Veh-Lupus mice.
Urine Ac-SDKP concentration.
Concentration of Ac-SDKP in urine collected for 24 h was significantly higher in Ac-SDKP-Ctrl and Ac-SDKP-Lupus mice, compared with their respective vehicle-treated controls. Urine Ac-SDKP concentration in Veh-Lupus mice was higher than in Veh-Ctrl mice, most likely due to renal damage; however, the observed difference between these two groups of animals did not reach statistical significance (Table 1).
Table 1.
Urinary Ac-SDKP, SBP, and body and kidney wt
| Groups | Veh-Ctrl | Ac-SDKP-Ctrl | Veh-Lupus | Ac-SDKP-Lupus |
|---|---|---|---|---|
| Urinary Ac-SDKP, ng/24 h | 54.7 ± 5.7 * | 185.2 ± 19.9 | 90.6 ± 17.1 | 248.7 ± 22.4 † |
| SBP, mmHg | 113 ± 3 | 108 ± 3 | 112 ± 2 | 113 ± 1 |
| Body wt, g | 41.6 ± 1.9 | 41.2 ± 2.2 | 38.7 ± 0.9 | 37.3 ± 0.7 |
| KW/BW, ng/10 g | 109.2 ± 1.5 | 111.9 ± 3.7 | 142.0 ± 9.3‡ | 122.6 ± 3.5 |
Data are means ± SE; n= 6–10.
SBP, systolic blood pressure; KW/BW, kidney weight/body weight (wt).
P < 0.001 Veh-Ctrl vs. Ac-SDKP-Ctrl.
P < 0.001 Ac-SDKP-Lupus vs. Veh-Lupus.
P < 0.01 Veh-Ctrl vs. Veh-Lupus.
SBP, body weight, and organ weight.
Twenty weeks after treatment with Ac-SDKP no difference was observed in SBP among the groups. Kidney weight-to-body weight ratio increased significantly in Veh-Lupus mice, compared with the Veh-Ctrl mice. In Ac-SDKP-Lupus mice, kidney weight-to-body weight ratio was decreased compared with Veh-Lupus mice; however, the difference did not reach significance. Left ventricle-to-body weight ratio was not different among the groups (Table 1).
DISCUSSION
In this study, we examined the possible mechanisms by which Ac-SDKP decreases nephritis in mouse model of SLE (20). We found, as previously reported by Tan et al. (54), that in lupus mice Ac-SDKP treatment decreases renal nephritis, inflammation and fibrosis. These beneficial effects occurred without changes in blood pressure and anti-dsDNA antibody concentrations. We hypothesized that Ac-SDKP beneficial effects in lupus are due to a significant decrease in: 1) C5/C5a, 2) C5b-9, 3) RANTES, 4) M-CSF, 5) MCP-5, and 6) ICAM-1, and these decreases could be responsible for the decrease in inflammation (macrophage and T cell kidney infiltration). Since Ac-SDKP neither altered the levels of auto anti-ds DNA antibodies nor, as previously reported, did it have any effect on C3 complement and IgGs deposition (54), these data indicate that in lupus mouse model Ac-SDKP may affect molecules downstream from immune complex formation and complement C3 activation. Therefore, to further dissect the mechanisms of Ac-SDKP action, we analyzed complement C5a and C5b-9, as well as their downstream effectors. Previous studies implicated that overexpression of complement C5a plays a role in the pathogenesis of autoimmune diseases, such as SLE (23). In addition, blocking of C5a receptor significantly decreased the severity of renal damage in mouse lupus models (4, 58), while deletion or blocking C3 or C3a receptor had no effect on murine lupus nephritis development (50, 51). In our study, Veh-Lupus mice expressed significantly higher levels of C5a, compared with Veh-Ctrl mice. However, the levels of C5a were significantly lower in Ac-SDKP-Lupus animals. C5a is a strong anaphylatoxin for immune cells that increases their chemotaxis and cytokine release (28). Since our data showed that in Ac-SDKP-Lupus mice renal infiltration with macrophages and T cells was of less magnitude than in Veh-Lupus mice, it is possible that this effect was in part due to C5a downregulation. In addition to generating C5a, complement activation involves the formation of C5b-9, i.e., membrane attack complex (MAC). Although it is still unclear which of the complement activation products are most important in lupus nephritis, C5b-9 was clearly demonstrated in glomeruli and peritubular basement membranes of kidneys from SLE nephritis patients (6). Our data showed that Veh-Lupus mice expressed significantly higher levels of C5b-9 deposits that were significantly reduced in response to Ac-SDKP treatment. As MAC, C5b-9 inserts into the phospholipid bilayers and causes cell lysis (5). However, MAC was also shown to trigger cellular reactions that can produce renal injury (45), mainly by mediating proinflammatory response of resident glomerular cells (49) and macrophages (21) and these are the actions that were most likely downregulated in response to Ac-SDKP treatment.
In addition, we showed that the levels of proinflammatory RANTES, MCP-5, and MCSF chemokines/cytokines were also significantly reduced in Ac-SDKP-Lupus mice. These chemokines/cytokines are produced mainly by macrophages and are increased in the kidneys of lupus mice before the occurrence of proteinuria and renal damage (33, 40). The observed decrease in RANTES, MCP-5, and MCSF levels in response to Ac-SDKP is probably related to the combination of the decrease in the numbers of infiltrating macrophages and the decrease in macrophage cytokine expression. In addition, both effects may be due to Ac-SDKP affecting macrophages directly or via the decrease in C5a/C5b-9, or due to the combination of both. Our previous work showed that Ac-SDKP inhibited macrophage differentiation, activation, migration, and release of TNF-α (52).
MCP-5 and M-CSF have been previously associated with renal damage in lupus (13, 16). In mouse lupus model, MCP-5 kidney expression was increased (55) and 8-wk treatment with Ac-SDKP inhibited this expression (54). At the same time, depletion of M-CSF improved renal function and decreased systemic illness in lupus mice (17).
In addition to precipitating the reduction in macrophage influx, decrease in RANTES may also explain the significantly lower numbers of kidney-infiltrating T cells in Ac-SDKP-Lupus mice. RANTES is a strong stimulator of T cell chemotaxis, proliferation and cytokine production, and its depletion in lupus mice markedly decreased numbers of CD4−/CD8− T cells (56). Here, we document for the first time the decrease in RANTES levels and consequently numbers of infiltrating T cells in the kidneys of lupus mice in response to Ac-SDKP. We previously showed that in rat model of autoimmune myocarditis Ac-SDKP prevented tissue infiltration of T helper cells (35). The overall decline in chemokine/cytokine levels in response to Ac-SDKP treatment most likely further decreases T cells and macrophage influx as well as their proliferation, survival, and differentiation.
Besides induction by chemokines, recruitment of immune cells to the inflammatory sites strongly depends on the interaction and binding to endothelial cell adhesion molecules. We analyzed the expression of ICAM-1, an endothelial cell adhesion molecule important in autoimmune processes, especially for migration of autoreactive T cells (44). Here, we demonstrated that in Ac-SDKP-Lupus mice ICAM-1 expression was significantly lower than in Veh-Lupus mice. Again, this ICAM-1 downregulation may be in part due to the decrease in C5a levels, since in endothelial cells ICAM-1 was shown to be upregulated in response to C5a via NF-κB pathway (1). Our previous study in rat model of autoimmune myocarditis also showed significantly lower levels of ICAM-1 in Ac-SDKP-treated animals (34).
Inflammation, glomerular, and interstitial fibrosis cause a decrease in renal function in lupus. In MRL lupus mouse, loss of renal function develops between 12 and 24 wk of age (20). Here, we show that 32-wk-old Veh-Lupus mice developed significant albuminuria and a decrease in GFR that was improved after treatment with Ac-SDKP for 20 wk. Our results are in agreement with the recent report showing that, in this model of lupus, treatment with Ac-SDKP ameliorates progression of lupus nephritis (54). In lupus, glomerulosclerosis is the main reason for GFR decline and our data showed significant increase in extracellular matrix within the glomeruli of Veh-Lupus mice. These deposits were significantly lower in Ac-SDKP-lupus mice. Recent studies implicated that glomerular sclerotic changes in lupus are accompanied with a decrease in the expression of podocyte slit diaphragm proteins, such as nephrin (41). Indeed, we detected significant decrease in nephrin expression in Veh-Lupus mice that was partially prevented with Ac-SDKP treatment. Thus, significant increase in nephrin expression together with ∼50% decrease in glomerular matrix deposits and renal collagen expression are the most probable reason for the observed improvement in GFR and decrease in proteinuria in Ac-SDKP-Lupus mice, where GFR values were three times higher (65 ml/min) than in Veh-Lupus (19 ml/ml). It is important to note that if in patients treated for renal failure the GFR improved by 200%, the therapy outcome would be considered as extremely successful. Tissue injury in kidneys of the lupus patients is not restricted to glomeruli only, but it affects interstitial tissue as well (37). Here, we demonstrated that interstitial collagen fraction increased significantly in the kidneys of Veh-Lupus mice and that Ac-SDKP treatment significantly prevented the occurrence of these collagen deposits. These results were consistent with what we previously reported on the anti-fibrotic effect of Ac-SDKP in various disease models including autoimmune myocarditis (35). However, the exact mechanisms of Ac-SDKP-mediated decrease in fibrosis are still not clear. As in other diseases characterized by fibrosis, TGF-β has been associated with renal fibrosis (31) and one of the mechanisms of Ac-SDKP anti-fibrotic effects may be a decrease in collagen formation via suppression of TGF-β pathway. Since proinflammatory cytokines other than TGF-β can stimulate fibrosis, especially through promoting fibroblast/epithelial/endothelial mesenchymal transition (10), downregulation of C5-9-induced cytokine pathways may be an additional mechanism contributing to the observed decrease in renal fibrosis in response to Ac-SDKP.
In addition to renal and cardiovascular diseases, skin lesions occur in 90% of patients with SLE (42). The most intensively studied model for cutaneous lupus is the MRL/lpr mouse that usually develops spontaneous skin lesions by 5 mo of age (18, 26). Here, we demonstrated that 20-wk treatment with Ac-SDKP significantly decreased severity of facial skin lesions in these mice. Skin lesions in lupus mice were characterized by T cell-rich infiltrates (19) and shown to strongly depend on local ICAM-1 expression (36). Therefore, it is possible that the observed Ac-SDKP effect on skin lesions in part relies on the above postulated mechanisms involving Ac-SDKP modulation of renal T cell migration and ICAM-1 expression.
In summary, Ac-SDKP prevented renal damage and dysfunction in the lupus mouse model. These protective Ac-SDKP effects were in part manifested as a prevention of inflammation and fibrosis. Ac-SDKP inhibited renal T cell and macrophage infiltration and proinflammatory chemokine expression, and collagen production. In addition, our data demonstrate for the first time that in lupus mouse models Ac-SDKP prevented an increase in C5a, C5b-9, and ICAM-1 expression. C5a was shown to be important in lupus development and is possibly playing an essential role in perpetuating inflammatory cell infiltration and activation, endothelial cell activation including the expression of ICAM-1, and proinflamatory cytokine/chemokine and collagen production, and downregulation of these actions may be central to the mechanisms involved in the observed AcSDKP anti-inflammatory effects that likely involve alteration of inflammasome (proinflammatory cytoplasmic complex) function and/or signaling cascades such as NF-κB pathway. Ac-SDKP treatment may be a novel and useful therapeutic strategy for the treatment of progressive autoimmune diseases.
GRANTS
This work was supported by National Institutes of Health Grant HL028982.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.-D.L., M.A.D., and O.A.C. conception and design of research; T.-D.L., P.N., and M.A.D. performed experiments; T.-D.L., P.N., B.J., M.A.D., M.E.W., and E.L.P. analyzed data; T.-D.L., P.N., B.J., M.E.W., N.-E.R., X.-P.Y., and O.A.C. interpreted results of experiments; T.-D.L. and B.J. prepared figures; T.-D.L. and B.J. drafted manuscript; T.-D.L., P.N., B.J., M.E.W., E.L.P., X.-P.Y., and O.A.C. edited and revised manuscript; T.-D.L., P.N., B.J., M.A.D., M.E.W., E.L.P., N.-E.R., X.-P.Y., and O.A.C. approved final version of manuscript.
REFERENCES
- 1.Albrecht EA, Chinnaiyan AM, Varambally S, Kumar-Sinha C, Barrette TR, Sarma JV, Ward PA. C5a-induced gene expression in human umbilical vein endothelial cells. Am J Pathol 164: 849–859, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azizi M, Ezan E, Reny JL, Wdzieczak-Bakala J, Gerineau V, Ménard J. Renal and metabolic clearance of N-acetyl-seryl-aspartyl-lysyl-proline (AcSDKP) during angiotensin-converting enzyme inhibition in humans. Hypertension 33: 879–886, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Azizi M, Rousseau A, Ezan E, Guyene TT, Michelet S, Grognet JM, Lenfant M, Corvol P, Menard J. Acute angiotensin-converting enzyme inhibition increases the plasma level of the natural stem cell regulator N-acetyl-seryl-aspartyl-lysyl-proline. J Clin Invest 97: 839–844, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bao L, Osawe I, Puri T, Lambris JD, Haas M, Quigg RJ. C5a promotes development of experimental lupus nephritis which can be blocked with a specific receptor antagonist. Eur J Immunol 35: 2496–2506, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Biesecker G. Membrane attack complex of complement as a pathologic mediator. Lab Invest 49: 237–249, 1983. [PubMed] [Google Scholar]
- 6.Biesecker G, Katz S, Koffler D. Renal localization of the membrane attack complex in systemic lupus erythematosus nephritis. J Exp Med 154: 1779–1794, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bomback AS, Appel GB. Updates on the treatment of lupus nephritis. J Am Soc Nephrol 21: 2028–2035, 2010. [DOI] [PubMed] [Google Scholar]
- 8.Borchers AT, Leibushor N, Naguwa SM, Cheema GS, Shoenfeld Y, Gershwin ME. Lupus nephritis: a critical review. Autoimmun Rev 12: 174–194, 2012. [DOI] [PubMed] [Google Scholar]
- 9.Borchers AT, Naguwa SM, Shoenfeld Y, Gershwin ME. The geoepidemiology of systemic lupus erythematosus. Autoimmun Rev 9: A277–A287, 2010. [DOI] [PubMed] [Google Scholar]
- 10.Borthwick LA, Wynn TA, Fisher AJ. Cytokine mediated tissue fibrosis. Biochim Biophys Acta 1832: 1049–1060, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavasin MA, Liao TD, Yang XP, Yang JJ, Carretero OA. Decreased endogenous levels of Ac-SDKP promote organ fibrosis. Hypertension 50: 130–136, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Cavasin MA, Rhaleb NE, Yang XP, Carretero OA. Prolyl oligopeptidase is involved in release of the antifibrotic peptide Ac-SDKP. Hypertension 43: 1140–1145, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan RW, Lai FM, Li EK, Tam LS, Chow KM, Lai KB, Li PK, Szeto CC. Intrarenal cytokine gene expression in lupus nephritis. Ann Rheum Dis 66: 886–892, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiariello M, Ambrosio G, Cappelli-Bigazzi M, Perrone-Filardi P, Brigante F, Sifola C. A biochemical method for the quantitation of myocardial scarring after experimental coronary artery occlusion. J Mol Cell Cardiol 18: 283–290, 1986. [DOI] [PubMed] [Google Scholar]
- 15.Duran-Barragan S, McGwin G Jr, Vila LM, Reveille JD, Alarcon GS, LUMINA (LIX): a multiethnic US cohort. Angiotensin-converting enzyme inhibitors delay the occurrence of renal involvement and are associated with a decreased risk of disease activity in patients with systemic lupus erythematosus–results from LUMINA (LIX): a multiethnic US cohort. Rheumatology (Oxford) 47: 1093–1096, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eriksson C, Eneslatt K, Ivanoff J, Rantapaa-Dahlqvist S, Sundqvist KG. Abnormal expression of chemokine receptors on T-cells from patients with systemic lupus erythematosus. Lupus 12: 766–774, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Fleetwood AJ, Dinh H, Cook AD, Hertzog PJ, Hamilton JA. GM-CSF- and M-CSF-dependent macrophage phenotypes display differential dependence on type I interferon signaling. J Leukoc Biol 86: 411–421, 2009. [DOI] [PubMed] [Google Scholar]
- 18.Furukawa F, Tanaka H, Sekita K, Nakamura T, Horiguchi Y, Hamashima Y. Dermatopathological studies on skin lesions of MRL mice. Arch Dermatol Res 276: 186–194, 1984. [DOI] [PubMed] [Google Scholar]
- 19.Ghoreishi M, Dutz JP. Murine models of cutaneous involvement in lupus erythematosus. Autoimmun Rev 8: 484–487, 2009. [DOI] [PubMed] [Google Scholar]
- 20.Grande JP. Experimental models of lupus nephritis. Contrib Nephrol 169: 183–197, 2011. [DOI] [PubMed] [Google Scholar]
- 21.Hansch GM, Seitz M, Martinotti G, Betz M, Rauterberg EW, Gemsa D. Macrophages release arachidonic acid, prostaglandin E2, and thromboxane in response to late complement components. J Immunol 133: 2145–2150, 1984. [PubMed] [Google Scholar]
- 22.Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat Med 9: 811–818, 1990. [DOI] [PubMed] [Google Scholar]
- 23.Hopkins P, Belmont HM, Buyon J, Philips M, Weissmann G, Abramson SB. Increased levels of plasma anaphylatoxins in systemic lupus erythematosus predict flares of the disease and may elicit vascular injury in lupus cerebritis. Arthritis Rheum 31: 632–641, 1988. [DOI] [PubMed] [Google Scholar]
- 24.Javierre BM, Richardson B. A new epigenetic challenge: systemic lupus erythematosus. In: Epigenetic Contributions in Autoimmune Disease, edited by Ballestar E. New York: Springer, 2011, p. 117–136. [DOI] [PubMed] [Google Scholar]
- 25.Junot C, Nicolet L, Ezan E, Gonzales MF, Menard J, Azizi M. Effects of angiotensin-converting enzyme inhibition on plasma, urine, and tissue concentrations of hemoregulatory peptide Acetyl-Ser-Asp-Lys-Pro in rats. J Cardiovasc Pharmacol 291: 982–987, 1999. [PubMed] [Google Scholar]
- 26.Kanauchi H, Furukawa F, Imamura S. Characterization of cutaneous infiltrates in MRL/lpr mice monitored from onset to the full development of lupus erythematosus-like skin lesions. J Invest Dermatol 96: 478–483, 1991. [DOI] [PubMed] [Google Scholar]
- 27.Krege JH, Hodgin JB, Hagaman JR, Smithies O. A noninvasive computerized tail-cuff system for measuring blood pressure in mice. Hypertension 25: 1111–1115, 1995. [DOI] [PubMed] [Google Scholar]
- 28.Lee H, Whitfeld PL, Mackay CR. Receptors for complement C5a. The importance of C5aR and the enigmatic role of C5L2. Immunol Cell Biol 86: 153–160, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Liao TD, Yang XP, D'Ambrosio M, Zhang Y, Rhaleb NE, Carretero OA. N-acetyl-seryl-aspartyl-lysyl-proline attenuates renal injury and dysfunction in hypertensive rats with reduced renal mass: Council for High Blood Pressure Research. Hypertension 55: 459–467, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liao TD, Yang XP, Liu YH, Shesely EG, Cavasin MA, Kuziel WA, Pagano PJ, Carretero OA. Role of inflammation in the development of renal damage and dysfunction in angiotensin II-induced hypertension. Hypertension 52: 256–263, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int 69: 213–217, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Lorenz JN, Gruenstein E. A simple, nonradioactive method for evaluating single-nephron filtration rate using FITC-inulin. Am J Physiol Renal Physiol 276: F172–F177, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Moore KJ, Wada T, Barbee SD, Kelley VR. Gene transfer of RANTES elicits autoimmune renal injury in MRL-Fas(1pr) mice. Kidney Int 53: 1631–1641, 1998. [DOI] [PubMed] [Google Scholar]
- 34.Muller-Esterl W, Fritz H, Machleidt W, Ritonja A, Brzin J, Kotnik M, Turk V, Kellermann J, Lottspeich F. Human plasma kininogens are identical with alpha-cysteine proteinase inhibitors. Evidence from immunological, enzymological and sequence data. FEBS Lett 182: 310–314, 1985. [DOI] [PubMed] [Google Scholar]
- 35.Nakagawa P, Liu Y, Liao TD, Chen X, Gonzalez GE, Bobbitt KR, Smolarek D, Peterson EL, Kedl R, Yang XP, Rhaleb NE, Carretero OA. Treatment with N-acetyl-seryl-aspartyl-lysyl-proline prevents experimental autoimmune myocarditis in rats. Am J Physiol Heart Circ Physiol 303: H1114–H1127, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Norman MU, James WG, Hickey MJ. Differential roles of ICAM-1 and VCAM-1 in leukocyte-endothelial cell interactions in skin and brain of MRL/faslpr mice. J Leukoc Biol 84: 68–76, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nowling TK, Gilkeson GS. Mechanisms of tissue injury in lupus nephritis. Arthritis Res Ther 13: 250, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peng H, Carretero OA, Liao TD, Peterson EL, Rhaleb NE. Role of N-acetyl-seryl-aspartyl-lysyl-proline in the antifibrotic and anti-inflammatory effects of the angiotensin-converting enzyme inhibitor captopril in hypertension. Hypertension 49: 695–703, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peng H, Carretero OA, Raij L, Yang F, Kapke A, Rhaleb NE. Antifibrotic effects of N-acetyl-seryl-aspartyl-Lysyl-proline on the heart and kidney in aldosterone-salt hypertensive rats. Hypertension 37: 794–800, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perez De LG, Maier H, Nieto E, Vielhauer V, Luckow B, Mampaso F, Schlondorff D. Chemokine expression precedes inflammatory cell infiltration and chemokine receptor and cytokine expression during the initiation of murine lupus nephritis. J Am Soc Nephrol 12: 1369–1382, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Perysinaki GS, Moysiadis DK, Bertsias G, Giannopoulou I, Kyriacou K, Nakopoulou L, Boumpas DT, Daphnis E. Podocyte main slit diaphragm proteins, nephrin and podocin, are affected at early stages of lupus nephritis and correlate with disease histology. Lupus 20: 781–791, 2011. [DOI] [PubMed] [Google Scholar]
- 42.Petri M. Dermatologic lupus: Hopkins Lupus Cohort. Semin Cutan Med Surg 17: 219–227, 1998. [DOI] [PubMed] [Google Scholar]
- 43.Pradelles P, Frobert Y, Creminon C, Liozon E, Masse A, Frindel E. Negative regulator of pluripotent hematopoietic stem cell proliferation in human white blood cells and plasma as analysed by enzyme immunoassay. Biochem Biophys Res Commun 170: 986–993, 1990. [DOI] [PubMed] [Google Scholar]
- 44.Pummerer CL, Grassl G, Sailer M, Bachmaier KW, Penninger JM, Neu N. Cardiac myosin-induced myocarditis: target recognition by autoreactive T cells requires prior activation of cardiac interstitial cells. Lab Invest 74: 845–852, 1996. [PubMed] [Google Scholar]
- 45.Raij L, Dalmasso AP, Staley NA, Fish AJ. Renal injury in DOCA-salt hypertensive C5-sufficient and C5-deficient mice. Kidney Int 36: 582–592, 1989. [DOI] [PubMed] [Google Scholar]
- 46.Rasoul S, Carretero OA, Peng H, Cavasin MA, Zhuo J, Sanchez-Mendoza A, Brigstock DR, Rhaleb NE. Antifibrotic effect of Ac-SDKP and angiotensin-converting enzyme inhibition in hypertension. J Hypertens 22: 593–603, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rudofsky UH, Dilwith RL, Roths JB, Lawrence DA, Kelley VE, Magro AM. Differences in the occurrence of hypertension among (NZB X NZW)F1, MRL-lpr, and BXSB mice with lupus nephritis. Am J Pathol 116: 107–114, 1984. [PMC free article] [PubMed] [Google Scholar]
- 48.Ryan MJ. The pathophysiology of hypertension in systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol 296: R1258–R1267, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schonermark M, Deppisch R, Riedasch G, Rother K, Hansch GM. Induction of mediator release from human glomerular mesangial cells by the terminal complement components C5b-9. Int Arch Allergy Appl Immunol 96: 331–337, 1991. [DOI] [PubMed] [Google Scholar]
- 50.Sekine H, Reilly CM, Molano ID, Garnier G, Circolo A, Ruiz P, Holers VM, Boackle SA, Gilkeson GS. Complement component C3 is not required for full expression of immune complex glomerulonephritis in MRL/lpr mice. J Immunol 166: 6444–6451, 2001. [DOI] [PubMed] [Google Scholar]
- 51.Sekine H, Ruiz P, Gilkeson GS, Tomlinson S. The dual role of complement in the progression of renal disease in NZB/W F(1) mice and alternative pathway inhibition. Mol Immunol 49: 317–323, 2011. [DOI] [PubMed] [Google Scholar]
- 52.Sharma U, Rhaleb NE, Pokharel S, Harding P, Rasoul S, Peng H, Carretero OA. Novel anti-inflammatory mechanisms of N-Acetyl-Ser-Asp-Lys-Pro in hypertension-induced target organ damage. Am J Physiol Heart Circ Physiol 294: H1226–H1232, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sweat F, Puchtler H, Rosenthal SI. Sirius Red F3BA as a stain for connective tissue. Arch Pathol 78: 69–72, 1964. [PubMed] [Google Scholar]
- 54.Tan H, Zhao J, Wang S, Zhang L, Wang H, Huang B, Liang Y, Yu X, Yang N. Ac-SDKP ameliorates the progression of lupus nephritis in MRL/lpr mice. Int Immunopharmacol 14: 401–409, 2012. [DOI] [PubMed] [Google Scholar]
- 55.Tesch GH, Maifert S, Schwarting A, Rollins BJ, Kelley VR. Monocyte chemoattractant protein 1-dependent leukocytic infiltrates are responsible for autoimmune disease in MRL-Fas(lpr) mice. J Exp Med 190: 1813–1824, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsukahara T, Makino Y, Fujii T, Ogawa M, Saisho H, Hamano Y, Ueda S, Akikusa B, Danoff TM. Role of RANTES in the development of autoimmune tissue injuries in MRL-Fas lpr mice. Clin Immunol 103: 89–97, 2002. [DOI] [PubMed] [Google Scholar]
- 57.Weening JJ, D'Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, Balow JE, Bruijn JA, Cook T, Ferrario F, Fogo AB, Ginzler EM, Hebert L, Hill G, Hill P, Jennette JC, Kong NC, Lesavre P, Lockshin M, Looi LM, Makino H, Moura LA, Nagata M. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol 15: 241–250, 2004. [DOI] [PubMed] [Google Scholar]
- 58.Wenderfer SE, Ke B, Hollmann TJ, Wetsel RA, Lan HY, Braun MC. C5a receptor deficiency attenuates T cell function and renal disease in MRLlpr mice. J Am Soc Nephrol 16: 3572–3582, 2005. [DOI] [PubMed] [Google Scholar]
- 59.Yang F, Yang XP, Liu YH, Xu J, Cingolani O, Rhaleb NE, Carretero OA. Ac-SDKP reverses inflammation and fibrosis in rats with heart failure after myocardial infarction. Hypertension 43: 229–236, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]