Abstract
Thiazide-sensitive sodium chloride cotransporter (NCC) plays an important role in maintaining blood pressure. Aldosterone is known to modulate NCC abundance. Previous studies reported that dietary salts modulated NCC abundance through either WNK4 [with no lysine (k) kinase 4]-SPAK (Ste20-related proline alanine-rich kinase) or WNK4-extracellular signal-regulated kinase-1 and -2 (ERK1/2) signaling pathways. To exclude the influence of SPAK signaling pathway on the role of the aldosterone-mediated ERK1/2 pathway in NCC regulation, we investigated the effects of dietary salt changes and aldosterone on NCC abundance in SPAK knockout (KO) mice. We found that in SPAK KO mice low-salt diet significantly increased total NCC abundance while reducing ERK1/2 phosphorylation, whereas high-salt diet decreased total NCC while increasing ERK1/2 phosphorylation. Importantly, exogenous aldosterone administration increased total NCC abundance in SPAK KO mice while increasing DUSP6 expression, an ERK1/2-specific phosphatase, and led to decreasing ERK1/2 phosphorylation without changing the ratio of phospho-T53-NCC/total NCC. In mouse distal convoluted tubule (mDCT) cells, aldosterone increased DUSP6 expression while reducing ERK1/2 phosphorylation. DUSP6 Knockdown increased ERK1/2 phosphorylation while reducing total NCC expression. Inhibition of DUSP6 by (E)-2-benzylidene-3-(cyclohexylamino)-2,3-dihydro-1H-inden-1-one increased ERK1/2 phosphorylation and reversed the aldosterone-mediated increments of NCC partly by increasing NCC ubiquitination. Therefore, these data suggest that aldosterone modulates NCC abundance via altering NCC ubiquitination through a DUSP6-dependent ERK1/2 signal pathway in SPAK KO mice and part of the effects of dietary salt changes may be mediated by aldosterone in the DCTs.
Keywords: aldosterone, dietary salt, sodium chloride cotransporter, dual-specificity protein phosphatase 6, ERK1/2, SPAK KO mice
the thiazide-sensitive sodium chloride cotransporter (NCC), located in the apical membrane of the distal convoluted tubule (DCT) of the kidney, is responsible for 5–7% of sodium reabsorption (6). NCC plays a key role in the regulation of blood pressure and electrolytes homeostasis (6, 12). Loss-of-function mutations in NCC have been reported to cause Gitelman's syndrome (GS), which is characterized by salt-wasting and low blood pressure (15). NCC is known to be modulated by two different types of regulation, type 1–altering its membrane expression, and type 2–altering the transporter kinetics (17).
Aldosterone has been shown to increase the thiazide-sensitive NCC protein in rats (20). Previous studies reported that the regulation of NCC by aldosterone is mediated through a WNK4 [with no lysine (k) kinase 4]-SPAK (Ste20-related proline alanine-rich kinase)-dependent pathway in response to the dietary salt changes (8, 9, 29, 32, 34). In addition to the WNK-mediated SPAK/oxidative stress responsive kinase 1 (OSR1) signaling pathway, the mitogen-activated protein kinases (MAPKs) were also found to be involved in NCC regulation. Phorbol ester and parathyroid hormone were also found to regulate NCC activity and protein expression via Ras-GRP1 and MAPK extracellular signal-regulated kinase-1 and -2 (ERK1/2) signaling pathways (21, 22). Furthermore, we showed that the ERK1/2 signaling pathway is involved in NCC regulation both in vivo and in vitro (24, 39). Knockdown of ERK1/2 expression also increased the total and membrane NCC protein levels in mouse distal convoluted tubule (mDCT) cells (39). We found that high-salt (HS) diet significantly decreased NCC abundance while enhancing ERK1/2 phosphorylation, whereas low-salt (LS) diet increased NCC abundance while reducing ERK1/2 phosphorylation (24). Finally, we observed that aldosterone infusion in HS-fed rats increased NCC expression while decreasing ERK1/2 phosphorylation (24). These data suggest that dietary salt changes modulate NCC expression through an aldosterone-mediated ERK1/2 signaling pathway (24).
ERK1/2 kinases are activated by MAP/ERK kinase 1 (MEK 1) and MEK 2 (referred to as MEK 1/2). Inactivation of ERK1/2 is mediated by a dual-specificity protein phosphatase (DUSP) (1). DUSPs, also known as MAPK phosphatases (MKPs), are a heterogeneous group of protein phosphatases that dephosphorylate both phospho-tyrosine and phospho-serine/phospho-threonine residues (11). DUSPs have been implicated as major modulators of signaling pathways affecting various physiological processes (11). A recent study showed that hypotonic stress stimulates β-epithelial sodium channel (ENaC) and γ-ENaC mRNA expression through suppression of ERK by inducing MKP-1 (28). DUSP6, also known as MAP kinase phosphatase 3 (MKP-3), is specifically responsible for ERK1/2 dephosphorylation (1, 26). Since the ERK1/2 signaling pathway has been implicated in NCC regulation (23, 24, 39), whether DUSP6 is involved in NCC regulation remains to be determined.
To assess the influence of SPAK/OSR1 signaling pathway on the role of aldosterone-ERK1/2 signaling pathway in the regulation of NCC, we investigated the effects of dietary salt changes on NCC protein expression in SPAK knockout (KO) mice. Here, we report that LS diet stimulates aldosterone secretion and increases total NCC abundance while suppressing ERK1/2 phosphorylation, whereas HS diet reduces aldosterone secretion and decreases total NCC abundance, while increasing ERK1/2 phosphorylation. Importantly, exogenous aldosterone administration increases total NCC abundance in SPAK KO mice while increasing DUSP6 expression led to decreasing ERK1/2 phosphorylation without changing the ratio of phospho-T53-NCC/total NCC. Aldosterone also increases the expression of DUSP6 while reducing ERK1/2 phosphorylation and knocking down DUSP6 expression increases ERK1/2 phosphorylation while reducing total NCC expression in mDCT cells. Inhibition of DUSP6 by (E)-2-benzylidene-3-(cyclohexylamino)-2,3-dihydro-1H-inden-1-one (BCI), a specific DUSP6 inhibitor, increases ERK1/2 phosphorylation and reverses the aldosterone-mediated increments of NCC partly by increasing NCC ubiquitination. Altogether, these data suggest that in SPAK KO mice aldosterone modulates NCC protein abundance by altering NCC ubiquitination through a DUSP6-dependent ERK1/2 signal pathway that does not require intact SPAK signaling pathway and part of the effects of dietary salt changes may be mediated by aldosterone in the DCTs.
MATERIALS AND METHODS
Experimental animals and dietary manipulation.
The Institutional Animal Care and Use Committees from Emory University and the Atlanta Veterans Administration Medical Center approved all animal-related procedures. The generation of SPAK KO (SPAK −/−) mice has been described in detail previously (14). SPAK KO mice were assigned to different dietary groups for 2 wk: 1) control diet group fed with normal-sodium diet (NS; 0.49% NaCl, TD96208); 2) low-sodium group given pelleted sodium-deficient diet (LS; 0.01–0.02% NaCl, TD90228); 3) high-sodium group fed a sodium diet (HS; 4% NaCl, TD92034). All diets were purchased from Harlan Laboratories. Mice were individually housed in metabolic cages for 24 h before and after dietary salt manipulations as well as aldosterone osmotic minipump (250 μg·kg−1·day−1). Urine and blood samples were collected for analysis before and after the experiments. Tail-cuff blood pressures were measured using a tail-cuff technique with the noninvasive BP-2000 Blood Pressure Analysis System (Visitech Systems) before and after the experiments as previously described (35).
Cell culture, transfection, and aldosterone treatments.
mDCT cells expressing endogenous NCC were obtained from Dr. Robert Hoover (Formerly at University of Chicago and now at Emory University) and were maintained on 60-mm cell culture plates and grown to 90% confluence in DMEM/F12 (1:1; Invitrogen) as previously described (24, 39). RNAiMAX was used for transfection of DUSP6 siRNA and SPAK siRNA into mDCT cells as previously described (39). The DUSP6 siRNA and SPAK siRNA were purchased from Santa Cruz (Dallas, TX).
Cell surface biotinylation, immunoprecipitation, and Western blot analysis.
The cell surface biotinylation was performed as previously described (5). Immunoprecipitation (IP) was performed for ubiquitination experiments as previously described (40). Briefly, the mDCT cells were treated with aldosterone with or without BCI before cell lysates were made. The cell lysates were incubated with anti-ubiquitin antibody (Thermo Scientific Pierce, Rockford, IL) for 2 h, then protein G/A sepharose beads were added, and the lysates were further mixed and incubated overnight at 4°C. After being washed with lysis buffer three times, beads were then eluted with Laemmli buffer (Bio-Rad). The eluted proteins were separated by SDS-PAGE, transferred onto PVDF membrane, and probed with anti-NCC.
Western blot analysis was performed as previously described (24, 39). BCI was purchased from EMD Chemicals (Gibbstown, NJ). NCC antibody was provided by Dr. Robert Hoover at Emory University School of Medicine. Anti-p53-NCC antibody was a gift of Dr. David Ellison (Oregon Health & Science University). All antibodies were purchased from the following companies: p-ERK1/2 and t-ERK1/2 (Cell Signaling Technology, Boston, MA), anti-actin (Sigma, St. Louis, MO), t-SPAK and p-SPAK S373 (University of Dundee, Scotland, UK), and anti-DUSP6 (Abcam, Cambridge, MA).
Statistical analysis.
The data are presented as means ± SE. Statistical significance was determined using either a Student's t-test when two groups were compared or by a one-way ANOVA, followed by Bonferroni's post hoc tests when multiple groups were compared. We assigned significance at P < 0.05.
RESULTS
Phenotypic characteristics of SPAK KO mice and effects of dietary salt changes on serum electrolytes, aldosterone level, and urinary volume and electrolytes.
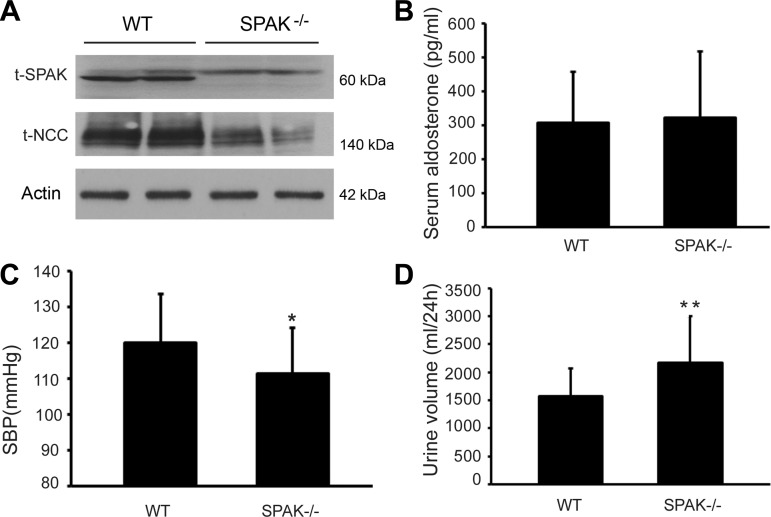
Wild-type (WT) and SPAK KO mice were identified by Western blot analysis. As shown in Fig. 1A, SPAK protein was not detected in SPAK KO mice. The total NCC abundance decreased in SPAK KO mice compared with WT mice. However, serum aldosterone level was not significantly different between the two groups (308.5 ± 150.7 vs. 323.8 ± 193.5 pg/ml), as shown in Fig. 1B. Moreover, SPAK KO mice exhibited hypotension and polyuria compared with WT mice, which is consistent with the clinical phenotype of GS (Fig. 1, C and D). The systolic arterial blood pressure decreased by 7.17% in SPAK KO mice compared with WT mice (111.3 ± 12.8 vs. 119.9 ± 13.7, *P < 0.05) and 24-h urine volume increased by 37.5% in the SPAK KO group compared with the WT group (2,169 ± 834 vs. 1,578 ± 497 ml/24 h, **P < 0.01).
Fig. 1.
Difference between wild-type (WT) and SPAK knockout (KO) mice. WT and SPAK KO mice were fed with normal-salt diets for a week and kidneys were then harvested for Western blot analysis. A: protein expressions of SPAK and total thiazide-sensitive sodium chloride cotransporter (NCC) in the SPAK KO (SPAK−/−) mice were determined by Western blot analysis. B: serum aldosterone level was not different between the WT and SPAK KO groups. C: systolic blood pressure (SBP) was lower in SPAK KO group than that in WT group (*P < 0.05). D: 24-h urine volume in SPAK KO mice was increased compared with WT control (**P < 0.01); n = 12.
After 14 days with dietary salt challenges in SPAK KO mice, the systolic blood pressure (SBP), urinary volume, urinary electrolytes, and serum aldosterone level were determined before and after the special diets. As shown in Table 1, LS-fed mice exhibited a very low sodium excretion at the end of the experiments, whereas HS-fed mice showed a significant increase in urinary sodium excretion. Aldosterone levels were significantly elevated in LS-fed mice, while decreased in HS-fed mice.
Table 1.
Effects of dietary salt changes on BP, UV, and serum aldosterone level
| Low Salt |
High Salt |
|||
|---|---|---|---|---|
| Before | After | Before | After | |
| Weight change, g | 24.1 ± 4.1 | 23.6 ± 4.5* | 22.3 ± 3.4 | 22.6 ± 3.4* |
| SBP, mmHg | 113.9 ± 17.0 | 114.0 ± 19.4 | 104.9 ± 13.5 | 115.4 ± 14.8 |
| UV, ml/24 h | 2,078.2 ± 998.1 | 1,533.4 ± 544.9* | 1,687.5 ± 780.5 | 5,163.6 ± 2,059.7†‡ |
| Serum aldosterone, pg/ml | 464.9 ± 161.8 | 1,349.1 ± 816.2† | 320.9 ± 169.3 | 154.3 ± 97.6*‡ |
| Urinary sodium, μmol | 193.4 ± 40.8 | 7.0 ± 6.4† | 202.7 ± 83.3 | 1,305.9 ± 411.8†‡ |
Values are means ± SE. The SPAK knockout (KO) mice were fed with either low-salt diet or high-salt diet for 2 wk. Systolic blood pressure (SBP) was measured using tail-cuff technique. Metabolic cage studies were performed before dietary salt change was started and after the completion of 2 wk of dietary challenges. Urine volume (UV) and blood sample were collected and sent out for aldosterone level determination. n = 8;
P < 0.05 and
P < 0.01, compared with the mice before feeding with special diet.
P < 0.01, compared with mice fed with low-salt diets.
Effects of dietary salt on total NCC and ERK1/2 phosphorylation in SPAK KO mice.
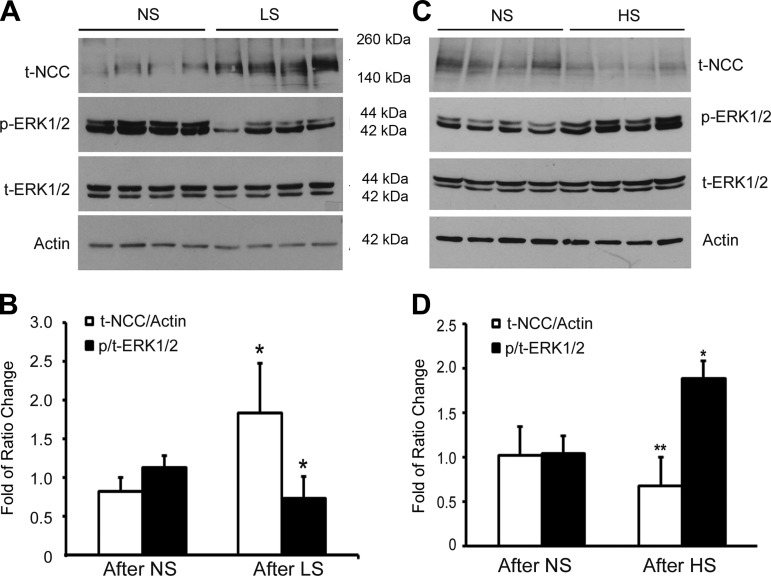
After 14 days with special diets, Western blot analysis from kidney tissues showed that NCC protein abundance increased by 226% while the phospho-ERK1/2 decreased to 65% in the LS-fed SPAK KO mice group compared with NS-fed SPAK KO group (n = 8, P < 0.05) as shown in Fig. 2, A and B, while total NCC protein expression significantly decreased to 60.6% (n = 8, **P < 0.01) and phospho-ERK1/2 remarkably increased by 181% (*P < 0.05) in HS-fed SPAK KO mice compared with NS-fed SPAK KO mice (Fig. 2, C and D). These data suggest that dietary salt changes modulate NCC protein abundance and ERK1/2 phosphorylation. As shown in Table 1, aldosterone levels significantly increased in LS-fed mice and decreased significantly in HS-fed SPAK KO mice. All of these data suggest that the dietary salt changes modulate NCC expression by altering aldosterone-mediated ERK1/2 phosphorylation.
Fig. 2.
Effects of dietary salts on total NCC and extracellular signal-regulated kinase-1 and -2 (ERK1/2) phosphorylation in SPAK KO mice. SPAK KO mice were fed with low salt (LS), normal salt (NS), and high salt (HS) for total 14 days before the kidney tissues were harvested. A: representative immunoblot of NCC and phospho-ERK1/2 (p-ERK1/2) and total ERK1/2 (t-ERK1/2) in SPAK KO mice fed with NS or LS diet. B: bar graphs representation of average band density from A. *P < 0.05; n = 8. C: representative immunoblot of NCC and phospho-ERK1/2 and total ERK1/2 in SPAK KO mice fed with NS or HS diet. D: bar graphs representation of average band density from C. *P < 0.05 and **P < 0.01; n = 8.
Effect of aldosterone on total NCC and ERK1/2 phosphorylation in SPAK KO mice.
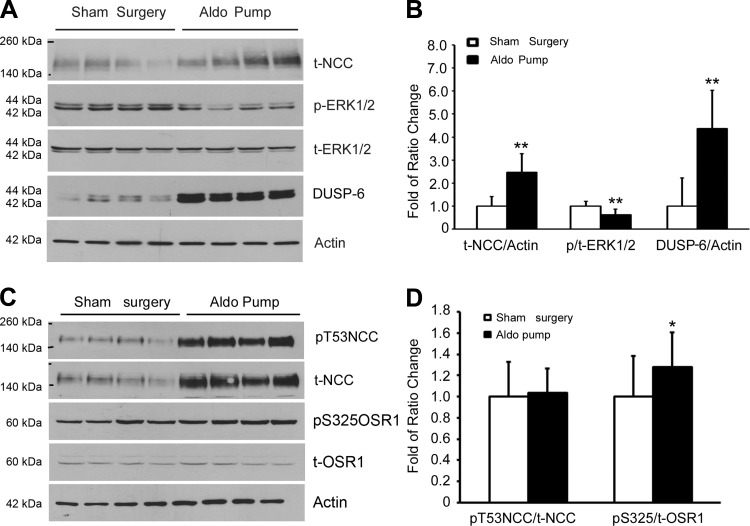
Since our initial results suggested that aldosterone-mediated ERK1/2 signaling pathway was involved in NCC regulation in SPAK KO mice, we sought to further confirm the effects of aldosterone on NCC modulation by exogenous administration of aldosterone (250 μg·kg−1·day−1) via osmotic minipumps for 14 days in SPAK KO mice. At the end of the experiments, serum aldosterone levels were significantly increased compared with the levels in sham-operated animals (2,310.0 ± 367.9 vs. 357.3 ± 72.1 pg/ml, n = 8, **P < 0.001). Total NCC expression was increased by 258%, while phospho-ERK1/2 decreased to 62% in the aldosterone-infused group compared with the sham-operated group (Fig. 3, A and B). Importantly, we found that aldosterone significantly increased the protein expression of DUSP-6, a specific ERK1/2 phosphatase, which is responsible for the reduction of ERK1/2 phosphorylation. Aldosterone also increased OSR1 (a functionally closely related to SPAK kinase) phosphorylation (Fig. 3, C and D). However, aldosterone did not change the ratio of phospho-T53-NCC/total NCC although it significantly increased total NCC abundance (Fig. 3, C and D), suggesting that SPAK-mediated NCC phosphorylation was not altered. These findings further support the notion that in the absence of SPAK signaling, aldosterone still upregulates NCC expression through DUSP6-mediated ERK1/2 signaling pathway. Aldosterone is able to affect ERK1/2 phosphorylation, regardless of SPAK/OSR1 phosphorylation levels, indicating that the SPAK signaling pathway is not required for an aldosterone-mediated effect on ERK1/2 phosphorylation.
Fig. 3.
Effect of aldosterone (Aldo) infusion on total NCC, phospho-NCC, phosphorylations of ERK1/2 and OSR1 in SPAK KO mice. SPAK KO mice were fed with NS diets and aldosterone osmotic minipumps (250 μg·kg−1·day−1) were implanted for total 14 days before the kidney tissues were harvested. A: representative immunoblot for total NCC, phospho-ERK1/2, total ERK1/2, and dual-specificity protein phosphatase 6 (DUSP6) in sham surgery or aldosterone minipump group. B: bar graph representation of average band density from A. **P < 0.01; n = 7. C: representative immunoblot for total NCC (t-NCC), phospho-NCC (pT53-NCC), total OSR1 and phospho-OSR1 (pS325OSR1). D: bar graph representation of average band density from C. *P < 0.05; n = 7.
Effect of aldosterone on total NCC expression and DUSP6-dependent ERK1/2 phosphorylation in mDCT cells.
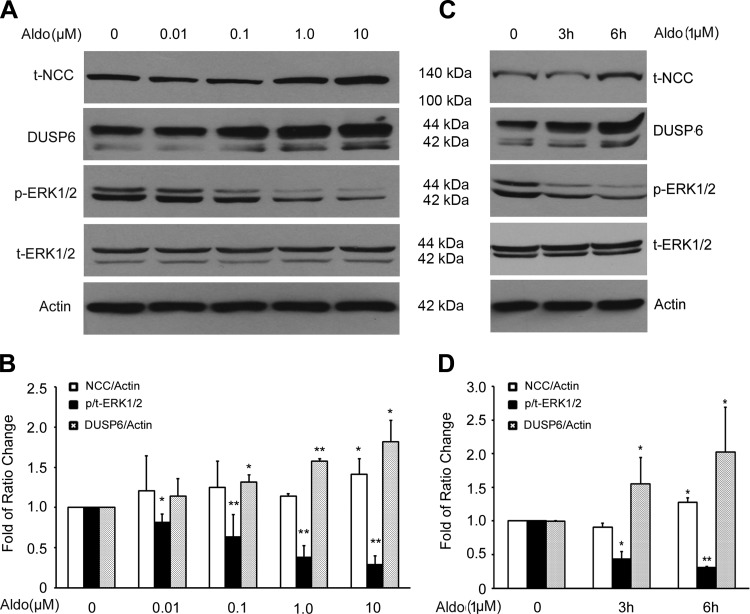
To further confirm the effect of aldosterone on NCC via DUSP6-dependent ERK1/2 phosphorylation in vitro, we tested the effects of aldosterone on NCC, DUSP6, and phospho-ERK1/2 in mDCT cells. As shown in Fig. 4, A and B, aldosterone significantly increased NCC protein while reducing ERK1/2 phosphorylation in a dose-dependent manner. Aldosterone also increased DUSP6 expression in a dose-dependent manner. In addition, aldosterone increased NCC and DUSP6 expressions while reducing ERK1/2 phosphorylation in a time-dependent manner (Fig. 4, C and D). These data suggest that aldosterone increases NCC protein expression through increasing DUSP6 expression, leading to enhanced dephosphorylation of ERK1/2, rather than activating ERK1/2 phosphorylation.
Fig. 4.
Effect of aldosterone on total NCC expression, DUSP6, and ERK1/2 phosphorylation in mouse distal convoluted tubule (mDCT) cells. The mDCT cells were grown to 100% confluence and the cells were treated with aldosterones as indicated. A: representative immunoblot for expressions of total NCC, DUSP6, phospho-ERK1/2, and total ERK1/2 in mDCT cells treated for 3 h with increasing doses of aldosterone. B: bar graph representing average band density from 4 independent experiments. C: representative immunoblot for expressions of total NCC, DUSP6, phospho-ERK1/2, and total ERK1/2 in mDCT cells treated with aldosterone (1.0 μM) for 0, 1 h, 3 h, and 6 h. D: bar graph representing average band density from 4 independent experiments. *P < 0.05, **P < 0.01 compared with control group (Aldosterone at 0); n = 4.
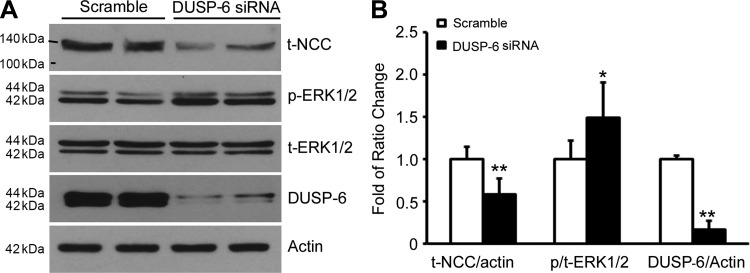
Effects of knocking down DUSP6 expression on ERK1/2 phosphorylation and NCC protein expression.
ERK1/2 activity is tightly regulated by its specific phosphatase, DUSP6. To demonstrate whether DUSP6 is responsible for ERK1/2-mediated downregulation of NCC, we performed a DUSP6 knockdown experiment. As shown in Fig. 5, A and B, when the mDCT cells were transfected with either DUSP6 siRNA or scramble siRNA, DUSP6 expression was significantly reduced as expected in DUSP6 siRNA group compared with scramble siRNA group. The ERK1/2 phosphorylation was significantly increased while NCC protein expression level was decreased. Combined with above results in Figs. 4 and 5, these data further confirm that DUSP6 mediates the aldosterone's regulation of NCC through an ERK1/2 signaling pathway.
Fig. 5.
Effect of knocking down DUSP expression on total NCC and ERK1/2 phosphorylation in mDCT cells. The mDCT cells were transfected with siRNA DUSP6 (10 nM). Forty-eight hours after transfection cells were lyzed for Western blot analysis. A: representative immunoblot for expressions of total NCC, phospho-ERK1/2, total ERK1/2, and DUSP6 in mDCT cells in both siRNA DUSP6 and scramble control groups. B: bar graph representing average band density from 4 independent experiments. *P < 0.05, **P < 0.01 compared with control group; n = 6.
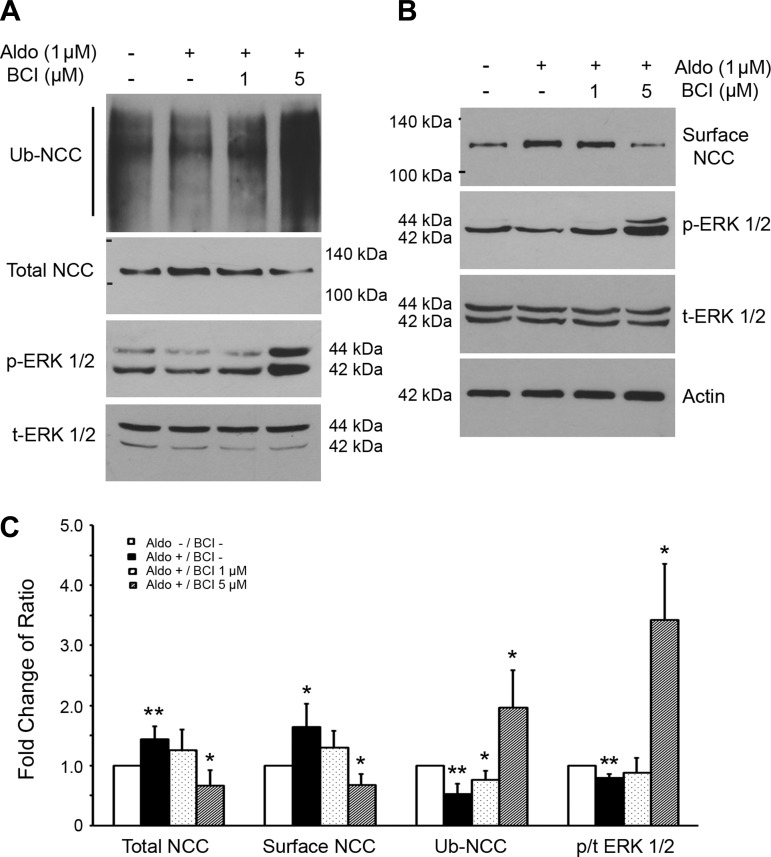
DUSP6 phosphatase-specific inhibitor reversed aldosterone-mediated stimulation of NCC expression.
To confirm the effect of knockdown of DUSP6 expression using siRNA technique on NCC expression, we used a DUSP6 phosphatase-specific inhibitor, BCI (27), and assessed its effect on surface expression of NCC and ERK1/2 phosphorylation in mDCT cells. As shown in Fig. 6, aldosterone treatment alone significantly increased both total NCC and NCC surface expression, while reducing ERK1/2 phosphorylation (Fig. 6C). BCI at 1 μM did not affect total NCC and NCC surface expression as well as ERK1/2 phosphorylation. However, when a higher dose of BCI (5 μM) was used, ERK1/2 phosphorylation was significantly increased while total NCC and NCC surface expression were significantly decreased. These data indicate that aldosterone upregulates NCC surface expression through dephosphorylation of ERK1/2.
Fig. 6.
Effects of (E)-2-benzylidene-3-(cyclohexylamino)-2,3-dihydro-1H-inden-1-one (BCI), a specific DUSP6 inhibitor, on aldosterone-mediated NCC surface expression and NCC ubiquitination through through ERK1/2 dephosphorylation in mDCT cells. The mDCT cells were pretreated with different concentrations of BCI and then treated with 1 μM aldosterone for 6 h. Cells were then lyzed for Western blot analysis. A: representative blots for the ubiquitinated NCC (Ub-NCC), total NCC, total ERK1/2, and phospho-ERK1/2 were detected by respective antibodies using coimmunoprecipitation and Western blot analysis. B: representative blots for the surface NCC, total ERK1/2, and phospho-ERK1/2 were detected by respective antibodies using cell surface biotinylation and Western blot analysis. C: bar graph representing average band density from 4 independent experiments from A and B. *P < 0.05, **P < 0.01 compared with the control group without aldosterone (Aldo) and BCI treatments.
Aldosterone stimulates NCC protein expression by reducing NCC ubiquitination via altering ERK1/2 phosphorylation.
To determine how aldosterone upregulates NCC, we analyzed NCC ubiquitination in mDCT cells. As shown in Fig. 6A, when mDCT cells were treated with 1 μM aldosterone, total NCC expression was significantly increased while NCC ubiquitination was significantly reduced (Fig. 6C). At the same time, ERK1/2 phosphorylation was decreased. When cells were treated with 1 and 5 μM BCI, NCC ubiquitination was significantly increased in a dose-dependent manner (Fig. 6C). When cells were treated with 5 μM BCI, total NCC and surface NCC expression were significantly reduced while ERK1/2 phosphorylation was significantly enhanced; this was accompanied by a large increase in ubiquitinated NCC (Fig. 6C). These data demonstrated that aldosterone stimulates NCC protein expression in part by reducing NCC ubiquitination and consequently NCC degradation. These changes occur via an ERK1/2-dependent signaling pathway.
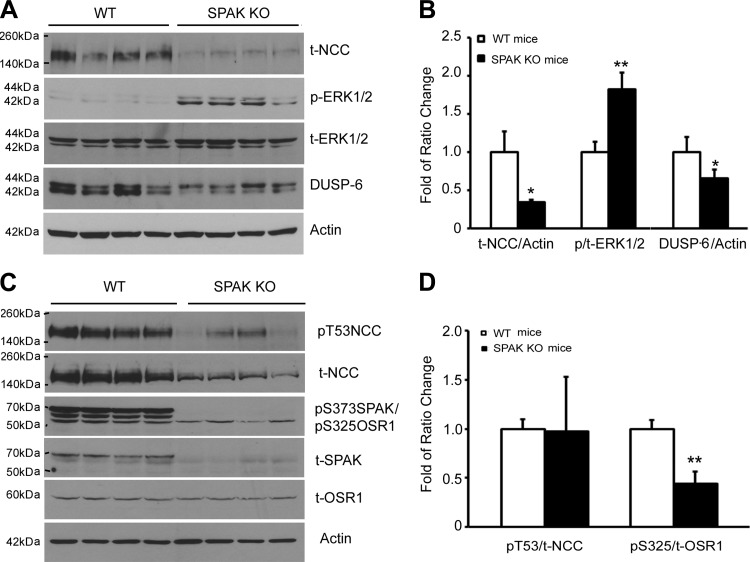
Effects of SPAK signaling on ERK1/2 phosphorylation and on DUSP6 and NCC protein expression.
To investigate whether SPAK/OSR1 kinases affect DUSP6-mediated ERK1/2 signaling pathway, we analyzed the protein levels of DUSP6, ERK1/2 phosphorylation, and NCC in both WT and SPAK KO mice. As shown in Fig. 7, A and B, DUSP6 protein abundance was significantly reduced while ERK1/2 phosphorylation was increased with reduction of total NCC protein abundance in SPAK KO mice compared with those in WT mice. However, as shown in Fig. 7, C and D, the SPAK/OSR1 phosphorylation of NCC at T53 residue was decreased in proportion to the reduction of total NCC level and the ratio of pT53-NCC/total NCC was not altered in SPAK KO mice compared with that in WT mice, suggesting that OSR1 kinase activity is not compensatorily upregulated in SPAK KO mice. In addition, OSR1 kinase phosphorylation itself (at residue S325) was rather decreased. These data suggest that SPAK kinase activity may have basal stimulation for DUSP6 expression, and lead to inhibition of ERK1/2 phosphorylation and upregulation of NCC.
Fig. 7.
Expressions of total NCC, phospho-NCC, DUSP6, total ERK1/2, phospho-ERK1/2, total SPAK/OSR1, and phospho-OSR1 in both WT and SPAK KO mice. A: representative immunoblot for total NCC, p-ERK1/2, t-ERK1/2, and DUSP6 in both WT and SPAK KO mice. B: bar graph representing average band density from 4 independent experiments from A. C: representative immunoblot for phospho-NCC, total NCC, p-SPAK(S373)/p-OSR1 (S325), total SPAK, and total OSR1 in both WT and SPAK KO mice. D: bar graph representing average band density from 4 independent experiments from C. *P < 0.05, **P < 0.01 compared with WT mice.
DISCUSSION
In this study, we demonstrated that aldosterone modulates NCC abundance via altering NCC ubiquitination through a DUSP6-dependent ERK1/2 signal pathway in SPAK KO mice and part of the effects of dietary salt changes may be mediated by aldosterone in the DCTs. Although the SPAK/OSR1 signaling pathway plays a critical role in regulating NCC activity (19), the MAPK signaling pathway also plays an important role in the regulation of SLC12 cation chloride cotransporter family members such as NCC and NKCC (7, 21, 36, 37). Our results are the first to demonstrate that aldosterone modulates NCC through a DUSP6/ERK1/2 signaling pathway that does not require intact SPAK/OSR1 signaling pathway.
Dietary salt changes have been shown to affect NCC protein abundance (33). Previous studies showed that LS diet and aldosterone enhance NCC abundance and its phosphorylation by activating the SPAK/OSR1 signaling pathway (9). We also reported that LS diets enhanced NCC protein abundance by the aldosterone-mediated inhibition of WNK4-ERK1/2 signaling pathway (24). These two signaling pathways distinctly affect NCC function: the SPAK signaling pathway positively regulates NCC function by increasing the activity of transporters, whereas the ERK1/2 negatively modulates NCC protein abundance. The next question that needed to be answered is whether these two signaling pathways work independently or in a complementary way in regulating the cotransporter. In the present study, we demonstrated that aldosterone-mediated ERK1/2 signaling pathway plays an important role in NCC regulation in response to dietary salt changes even in the absence of SPAK kinase. Although in SPAK KO mice DCT segment of nephron was atrophied, we found that LS diet was still able to stimulate aldosterone secretion and increase total NCC abundance while suppressing ERK1/2 phosphorylation, whereas HS diet reduces aldosterone secretion and decreases total NCC abundance while increasing ERK1/2 phosphorylation. These findings are consistent with previous results obtained in WT Sprague-Dawley rats subjected to similar dietary salt challenges (24, 38) and in WT C57/B6 mice (9). The present study provides evidence that aldosterone-mediated ERK1/2 signaling pathway plays an important role in NCC regulation in addition to the aldosterone-mediated SPAK signaling pathway. Importantly, we also found that aldosterone stimulates DUSP6 expression that leads to reduction of ERK1/2 phosphorylation, ultimately upregulated NCC expression, even in the absence of SPAK kinase. Since absence of SPAK kinase without compensatory upregulation of OSR1 activity in SPAK KO mice resulted in reduction of DUSP6 expression, enhancement of ERK1/2 phosphorylation, and reduction of NCC abundance, these combined results suggest that diet salt changes modulate NCC abundance partly by altering aldosterone-mediated DUSP6-dependent ERK1/2 signaling pathway in which the intact SPAK signaling pathway is not required. While SPAK signaling pathway was found to affect ERK1/2 signaling pathway, the exact relationship of these two signaling pathways in the regulation of NCC needs to be further explored. Since dietary salt changes could also affect other NCC modulators, such as angiotensin II, the roles of angiotensin II and other NCC modulators in DUSP6/ERK1/2-mediated regulation of NCC need to be further investigated.
The phosphorylation state of ERK1/2 is balanced by both activator of ERK1/2 kinase and activator of ERK1/2 phosphatase. DUSP is a dual-specificity protein phosphatase (11). DUSP6 is specifically responsible for ERK1/2 dephosphorylation (1, 26). Our present study showed that aldosterone enhanced NCC protein expression along with increasing DUSP6 protein expression in a dose- and time-dependent manner while simultaneously decreasing ERK1/2 phosphorylation. Knockdown of DUSP6 expression resulted in an increase in ERK1/2 phosphorylation and downregulation of NCC protein expression. The aldosterone-mediated increase of NCC and decrease of ERK1/2 phosphorylation were reversed by BCI, a DUSP6-specific inhibitor, demonstrating that aldosterone modulates NCC protein expression through altering the DUSP6-mediated ERK1/2 signaling pathway. Since we used a pharmacological dose of aldosterone for the in vitro experiments, the effects of aldosterone on DUSP6, ERK1/2 phosphorylation, and NCC protein expression might be overestimated.
NCC belongs to the superfamily of the cation chloride cotransporter (CCC) which also includes sodium-potassium-chloride cotransporter (NKCC) and potassium-chloride transporter (KCC) (12). The activity of CCCs is dependent on their phosphorylation state and therefore regulated by both kinases and phosphatases. Phosphatases were found to directly regulate NCC activity. Inhibition of protein phosphatases (PP) 1 and 2B reversed WNK3-mediated activation of KCC (10). Inhibition of PP2B was also found to prevent WNK4 dead-kinase-mediated activation of KCC2 and KCC3 (13). A previous study reported that PP4 inhibited NCC activity without affecting NCC surface trafficking (16). PP1 inhibitor-1 was found to increase NCC activity, whereas knockdown of PP1 inhibitor-1 reduced NCC phosphorylation and its activity (30). In addition to WNK-mediated SPAK and ERK1/2 signaling, parvalbumin (3), serum and glucocorticoid-inducible kinase 1 (Sgk 1), ubiquitin ligase Nedd 4-2 (2, 31), Kelch-like 3 (4, 25), and cullin 3 (4, 25) were also found to modulate NCC activity. We demonstrated that either reduction or inhibition of DUSP6 enhanced ERK1/2 phosphorylation. The enhanced ERK1/2 phosphorylation ultimately increased NCC ubiquitination partly leading to reduction of NCC expression by increasing degradation of NCC which is consistent with previous findings (23). Our present findings on DUSP6 clarify another layer of NCC regulation.
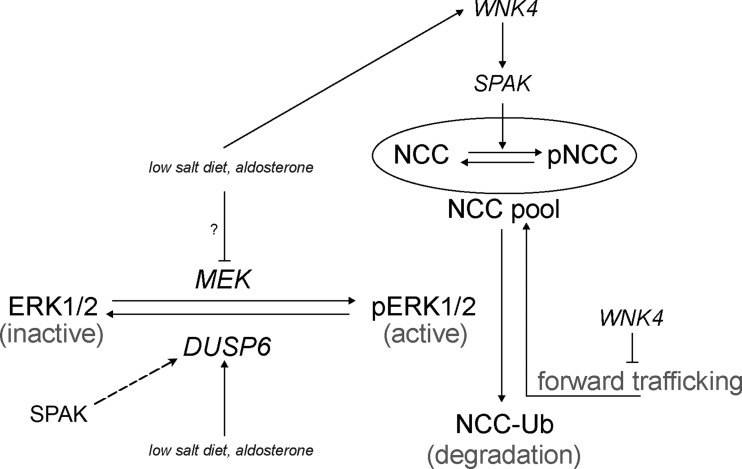
Although the SPAK signaling pathway is not required for aldosterone-mediated DUSP6-dependent ERK1/2 signaling pathway in NCC regulation, our results showed that absence of SPAK kinase itself reduced DUSP6 expression associated with increasing ERK1/2 phosphorylation and leading to reduction of NCC abundance as shown in Fig. 7. These data suggest that ERK1/2 signaling pathway plays a complementary role to SPAK signaling in NCC regulation. Aldosterone can modulate NCC through DUSP6-ERK1/2 signaling pathway and SPAK signaling itself could also affect ERK1/2 signaling pathway via altering DUSP6 that opens a gate for further investigating the role of SPAK signaling in ERK1/2-mediated regulation of NCC. Thus, we propose a model showing how LS diet and aldosterone modulate NCC through both ERK1/2 and SPAK signaling pathways (Fig. 8). Aldosterone might modulate NCC phosphorylation acutely through SPAK signaling pathway, whereas it might affect NCC abundance through ERK1/2 signaling pathway in a rather chronic fashion. In our model, we suggest that LS diet and aldosterone could affect WNK4 and subsequently modulate NCC phosphorylation and its activity through SPAK signaling pathway (9, 31). WNK4 also affects NCC forward trafficking (5, 18, 40). On the other hand, our present study suggests that SPAK signaling has basal regulation on DUSP6 expression, and LS diet and aldosterone modulate NCC protein abundance through DUSP6-dependent ERK1/2 signaling pathway.
Fig. 8.
Working model on how aldosterone modulates NCC through both SPAK signaling pathway and DUSP6-dependent ERK1/2 signaling pathway. NCC within oval circle indicates NCC accumulation in plasma membrane which consists of NCC and phosphorylated NCC (pNCC). Majority of NCC pool are present in the cytoplasm. Long arrow lines indicate either activation of the kinases or direction of chemical reaction. Long broken arrow line indicates upregulation with unknown mechanism. A t-shaped line indicates inhibition effect. NCC-Ub indicates ubiquitinated NCC. pERK1/2 indicates phospho-ERK1/2. MEK is a MAP/ERK kinase, the upstream kinase of ERK1/2. DUSP6 is a dual-specificity protein phosphatase 6 (the specific ERK1/2 phosphatase).
In summary, in the present study we demonstrated that in SPAK KO mice aldosterone modulates NCC abundance via altering NCC ubiquitination through a DUSP6-dependent ERK1/2 signal pathway that does not require intact SPAK signaling pathway and part of the effects of dietary salt changes may be mediated by aldosterone in the DCTs. We also showed that aldosterone modulates NCC protein expression by reducing ERK1/2-mediated NCC ubiquitination by altering the ERK1/2-specific phosphatase DUSP6. These data suggest that ERK1/2 phosphatase DUSP6 plays an important role in regulation of NCC ubiquitination leading to NCC degradation.
GRANTS
This work is supported by Norman S. Coplon Satellite grant (to H. Cai), the Department of Veteran Affairs MERIT Award 5I01BX000994 (to H. Cai), National Institutes of Health (NIH) Grant K08 DK068226S-1 (to H. Cai), NIH Grants GM074771 and DK093501 (to E. Delpire), Zhejiang Provincial Natural Science Foundation of China #Y2090529 (to D. Gu), and Wenzhou City Science and Technology Cooperation Program H20090022 (to J. Zhuang).
Part of the data was presented at the annual ASN meetings in 2011 and 2012.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: X.F., X.H.W., E.D., D.G., and H.C. conception and design of research; X.F., Y.Z., N.S., Y.W., Z.Z., P.W., M.J.L., and Y.L. performed experiments; X.F., Y.Z., N.S., Y.W., Z.Z., P.W., M.J.L., Y.L., J.Z., D.G., and H.C. analyzed data; X.F., X.H.W., J.Z., E.D., D.G., and H.C. interpreted results of experiments; X.F., Y.Z., N.S., Y.W., Z.Z., E.D., and H.C. prepared figures; X.F. drafted manuscript; X.F., Y.Z., Y.W., P.W., X.H.W., J.Z., E.D., D.G., and H.C. edited and revised manuscript; X.F., Y.Z., N.S., Y.W., Z.Z., P.W., M.J.L., Y.L., X.H.W., J.Z., E.D., D.G., and H.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Janet Klein for suggestions and critical reading of this manuscript. We also thank Dr. Robert Hoover for providing the NCC antibody and mDCT cell lines.
REFERENCES
- 1.Arkell RS, Dickinson RJ, Squires M, Hayat S, Keyse SM, Cook SJ. DUSP6/MKP-3 inactivates ERK1/2 but fails to bind and inactivate ERK5. Cell Signal 20: 836–843, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Arroyo JP, Lagnaz D, Ronzaud C, Vazquez N, Ko BS, Moddes L, Ruffieux-Daidie D, Hausel P, Koesters R, Yang B, Stokes JB, Hoover RS, Gamba G, Staub O. Nedd4-2 modulates renal Na+-Cl− cotransporter via the aldosterone-SGK1-Nedd4-2 pathway. J Am Soc Nephrol 22: 1707–1719, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belge H, Gailly P, Schwaller B, Loffing J, Debaix H, Riveira-Munoz E, Beauwens R, Devogelaer JP, Hoenderop JG, Bindels RJ, Devuyst O. Renal expression of parvalbumin is critical for NaCl handling and response to diuretics. Proc Natl Acad Sci USA 104: 14849–14854, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyden LM, Choi M, Choate KA, Nelson-Williams CJ, Farhi A, Toka HR, Tikhonova IR, Bjornson R, Mane SM, Colussi G, Lebel M, Gordon RD, Semmekrot BA, Poujol A, Valimaki MJ, De Ferrari ME, Sanjad SA, Gutkin M, Karet FE, Tucci JR, Stockigt JR, Keppler-Noreuil KM, Porter CC, Anand SK, Whiteford ML, Davis ID, Dewar SB, Bettinelli A, Fadrowski JJ, Belsha CW, Hunley TE, Nelson RD, Trachtman H, Cole TR, Pinsk M, Bockenhauer D, Shenoy M, Vaidyanathan P, Foreman JW, Rasoulpour M, Thameem F, Al-Shahrouri HZ, Radhakrishnan J, Gharavi AG, Goilav B, Lifton RP. Mutations in kelch-like 3 and cullin 3 cause hypertension and electrolyte abnormalities. Nature 482: 98–102, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai H, Cebotaru V, Wang YH, Zhang XM, Cebotaru L, Guggino SE, Guggino WB. WNK4 kinase regulates surface expression of the human sodium chloride cotransporter in mammalian cells. Kidney Int 69: 2162–2170, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Campean V, Kricke J, Ellison D, Luft FC, Bachmann S. Localization of thiazide-sensitive Na+-Cl− cotransport and associated gene products in mouse DCT. Am J Physiol Renal Physiol 281: F1028–F1035, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Capo-Aponte JE, Wang Z, Bildin VN, Pokorny KS, Reinach PS. Fate of hypertonicity-stressed corneal epithelial cells depends on differential MAPK activation and p38MAPK/Na-K-2Cl cotransporter1 interaction. Exp Eye Res 84: 361–372, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castaneda-Bueno M, Cervantes-Perez LG, Vazquez N, Uribe N, Kantesaria S, Morla L, Bobadilla NA, Doucet A, Alessi DR, Gamba G. Activation of the renal Na+:Cl− cotransporter by angiotensin II is a WNK4-dependent process. Proc Natl Acad Sci USA 109: 7929–7934, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiga M, Rai T, Yang SS, Ohta A, Takizawa T, Sasaki S, Uchida S. Dietary salt regulates the phosphorylation of OSR1/SPAK kinases and the sodium chloride cotransporter through aldosterone. Kidney Int 74: 1403–1409, 2008. [DOI] [PubMed] [Google Scholar]
- 10.de Los HP, Kahle KT, Rinehart J, Bobadilla NA, Vazquez N, San CP, Mount DB, Lifton RP, Hebert SC, Gamba G. WNK3 bypasses the tonicity requirement for K-Cl cotransporter activation via a phosphatase-dependent pathway. Proc Natl Acad Sci USA 103: 1976–1981, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dickinson RJ, Keyse SM. Diverse physiological functions for dual-specificity MAP kinase phosphatases. J Cell Sci 119: 4607–4615, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Gamba G. Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiol Rev 85: 423–493, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Garzon-Muvdi T, Pacheco-Alvarez D, Gagnon KB, Vazquez N, Ponce-Coria J, Moreno E, Delpire E, Gamba G. WNK4 kinase is a negative regulator of K+-Cl− cotransporters. Am J Physiol Renal Physiol 292: F1197–F1207, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Geng Y, Hoke A, Delpire E. The Ste20 kinases Ste20-related proline-alanine-rich kinase and oxidative-stress response 1 regulate NKCC1 function in sensory neurons. J Biol Chem 284: 14020–14028, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gitelman HJ, Graham JB, Welt LG. A new familial disorder characterized by hypokalemia and hypomagnesemia. Trans Assoc Am Physicians 79: 221–235, 1966. [PubMed] [Google Scholar]
- 16.Glover M, Mercier ZA, Figg N, O'shaughnessy KM. The activity of the thiazide-sensitive Na+-Cl− cotransporter is regulated by protein phosphatase PP4. Can J Physiol Pharmacol 88: 986–995, 2010. [DOI] [PubMed] [Google Scholar]
- 17.Glover M, O'Shaughnessy KM. SPAK and WNK kinases: a new target for blood pressure treatment? Curr Opin Nephrol Hypertens 20: 16–22, 2011. [DOI] [PubMed] [Google Scholar]
- 18.Golbang AP, Cope G, Hamad A, Murthy M, Liu CH, Cuthbert AW, O'Shaughnessy KM. Regulation of the expression of the Na/Cl cotransporter by WNK4 and WNK1: evidence that accelerated dynamin-dependent endocytosis is not involved. Am J Physiol Renal Physiol 291: F1369–F1376, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Grimm PR, Taneja TK, Liu J, Coleman R, Chen YY, Delpire E, Wade JB, Welling PA. SPAK isoforms and OSR1 regulate sodium-chloride co-transporters in a nephron-specific manner. J Biol Chem 287: 37673–37690, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim GH, Masilamani S, Turner R, Mitchell C, Wade JB, Knepper MA. The thiazide-sensitive Na-Cl cotransporter is an aldosterone-induced protein. Proc Natl Acad Sci USA 95: 14552–14557, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ko B, Cooke LL, Hoover RS. Parathyroid hormone (PTH) regulates the sodium chloride cotransporter via Ras guanyl releasing protein 1 (Ras-GRP1) and extracellular signal-regulated kinase (ERK)1/2 mitogen-activated protein kinase (MAPK) pathway. Transl Res 158: 282–289, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ko B, Joshi LM, Cooke LL, Vazquez N, Musch MW, Hebert SC, Gamba G, Hoover RS. Phorbol ester stimulation of RasGRP1 regulates the sodium-chloride cotransporter by a PKC-independent pathway. Proc Natl Acad Sci USA 104: 20120–20125, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ko B, Kamsteeg EJ, Cooke LL, Moddes LN, Deen PM, Hoover RS. RasGRP1 stimulation enhances ubiquitination and endocytosis of the sodium-chloride cotransporter. Am J Physiol Renal Physiol 299: F300–F309, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai L, Feng X, Liu D, Chen J, Zhang Y, Niu B, Gu Y, Cai H. Dietary salt modulates the sodium chloride cotransporter expression likely through an aldosterone-mediated WNK4-ERK1/2 signaling pathway. Pflügers Arch 463: 477–485, 2012. [DOI] [PubMed] [Google Scholar]
- 25.Louis-Dit-Picard H, Barc J, Trujillano D, Miserey-Lenkei S, Bouatia-Naji N, Pylypenko O, Beaurain G, Bonnefond A, Sand O, Simian C, Vidal-Petiot E, Soukaseum C, Mandet C, Broux F, Chabre O, Delahousse M, Esnault V, Fiquet B, Houillier P, Bagnis CI, Koenig J, Konrad M, Landais P, Mourani C, Niaudet P, Probst V, Thauvin C, Unwin RJ, Soroka SD, Ehret G, Ossowski S, Caulfield M, Bruneval P, Estivill X, Froguel P, Hadchouel J, Schott JJ, Jeunemaitre X. KLHL3 mutations cause familial hyperkalemic hypertension by impairing ion transport in the distal nephron. Nat Genet 44: 456–S3, 2012. [DOI] [PubMed] [Google Scholar]
- 26.Mark JK, Smith S, Hefford MA. Overexpression and refolding of MAP kinase phosphatase 3. Protein Expr Purif 54: 253–260, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Molina G, Vogt A, Bakan A, Dai W, Queiroz de OP, Znosko W, Smithgall TE, Bahar I, Lazo JS, Day BW, Tsang M. Zebrafish chemical screening reveals an inhibitor of Dusp6 that expands cardiac cell lineages. Nat Chem Biol 5: 680–687, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niisato N, Ohta M, Eaton DC, Marunaka Y. Hypotonic stress upregulates β- and γ-ENaC expression through suppression of ERK by inducing MKP-1. Am J Physiol Renal Physiol 303: F240–F252, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pedersen NB, Hofmeister MV, Rosenbaek LL, Nielsen J, Fenton RA. Vasopressin induces phosphorylation of the thiazide-sensitive sodium chloride cotransporter in the distal convoluted tubule. Kidney Int 78: 160–169, 2010. [DOI] [PubMed] [Google Scholar]
- 30.Picard N, Trompf K, Yang CL, Miller RL, Carrel M, Loffing-Cueni D, Fenton RA, Ellison DH, Loffing J. Protein phosphatase 1 inhibitor-1 deficiency reduces phosphorylation of renal NaCl cotransporter and causes arterial hypotension. J Am Soc Nephrol 25: 511–522, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rozansky DJ, Cornwall T, Subramanya AR, Rogers S, Yang YF, David LL, Zhu X, Yang CL, Ellison DH. Aldosterone mediates activation of the thiazide-sensitive Na-Cl cotransporter through an SGK1 and WNK4 signaling pathway. J Clin Invest 119: 2601–2612, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.San-Cristobal P, Pacheco-Alvarez D, Richardson C, Ring AM, Vazquez N, Rafiqi FH, Chari D, Kahle KT, Leng Q, Bobadilla NA, Hebert SC, Alessi DR, Lifton RP, Gamba G. Angiotensin II signaling increases activity of the renal Na-Cl cotransporter through a WNK4-SPAK-dependent pathway. Proc Natl Acad Sci USA 106: 4384–4389, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sandberg MB, Maunsbach AB, McDonough AA. Redistribution of distal tubule Na+-Cl− cotransporter (NCC) in response to a high-salt diet. Am J Physiol Renal Physiol 291: F503–F508, 2006. [DOI] [PubMed] [Google Scholar]
- 34.van der LN, Lim CH, Meima ME, van VR, Rosenbaek LL, Mutig K, Danser AH, Fenton RA, Zietse R, Hoorn EJ. Aldosterone does not require angiotensin II to activate NCC through a WNK4-SPAK-dependent pathway. Pflügers Arch 463: 853–863, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verlander JW, Hong S, Pech V, Bailey JL, Agazatian D, Matthews SW, Coffman TM, Le T, Inagami T, Whitehill FM, Weiner ID, Farley DB, Kim YH, Wall SM. Angiotensin II acts through the angiotensin 1a receptor to upregulate pendrin. Am J Physiol Renal Physiol 301: F1314–F1325, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wallace BK, Jelks KA, O'Donnell ME. Ischemia-induced stimulation of cerebral microvascular endothelial cell Na-K-Cl cotransport involves p38 and JNK MAP kinases. Am J Physiol Cell Physiol 302: C505–C517, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Z, Bildin VN, Yang H, Capo-Aponte JE, Yang Y, Reinach PS. Dependence of corneal epithelial cell proliferation on modulation of interactions between ERK1/2 and NKCC1. Cell Physiol Biochem 28: 703–714, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang LE, Sandberg MB, Can AD, Pihakaski-Maunsbach K, McDonough AA. Effects of dietary salt on renal Na+ transporter subcellular distribution, abundance, and phosphorylation status. Am J Physiol Renal Physiol 295: F1003–F1016, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou B, Wang D, Feng X, Zhang Y, Wang Y, Zhuang J, Zhang X, Chen G, Delpire E, Gu D, Cai H. WNK4 inhibits NCC protein expression through MAPK ERK1/2 signaling pathway. Am J Physiol Renal Physiol 302: F533–F539, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou B, Zhuang J, Gu D, Wang H, Cebotaru L, Guggino WB, Cai H. WNK4 enhances the degradation of NCC through a sortilin-mediated lysosomal pathway. J Am Soc Nephrol 21: 82–92, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]