Abstract
Fibroblast growth factors (Fgfs) mediate organ repair. Lung epithelial cell overexpression of Fgf10 postbleomycin injury is both protective and therapeutic, characterized by increased survival and attenuated fibrosis. Exogenous administration of FGF7 (palifermin) also showed prophylactic survival benefits in mice. The role of endogenous Fgfr2b ligands on bleomycin-induced lung fibrosis is still elusive. This study reports the expression of endogenous Fgfr2b ligands, receptors, and signaling targets in wild-type mice following bleomycin lung injury. In addition, the impact of attenuating endogenous Fgfr2b-ligands following bleomycin-induced fibrosis was tested by using a doxycycline (dox)-based inducible, soluble, dominant-negative form of the Fgfr2b receptor. Double-transgenic (DTG) Rosa26rtTA/+;tet(O)solFgfr2b mice were validated for the expression and activity of soluble Fgfr2b (failure to regenerate maxillary incisors, attenuated recombinant FGF7 signal in the lung). As previously reported, no defects in lung morphometry were detected in DTG (+dox) mice exposed from postnatal days (PN) 1 through PN105. Female single-transgenic (STG) and DTG mice were subjected to various levels of bleomycin injury (1.0, 2.0, and 3.0 U/kg). Fgfr2b ligands were attenuated either throughout injury (days 0–11; days 0–28) or during later stages (days 6–28 and 14–28). No significant changes in survival, weight, lung function, confluent areas of fibrosis, or hydroxyproline deposition were detected in DTG mice. These results indicate that endogenous Fgfr2b ligands do not significantly protect against bleomycin injury, nor do they expedite the resolution of bleomycin-induced lung injury in mice.
the interstitial lung disease idiopathic pulmonary fibrosis (IPF) occurs between the sixth and seventh decades of life at a rate of 2–4/10,000 (21). The 5-year survival rates approximate just 10–15% (21). The pathomechanism of IPF is not yet fully understood; however, it is thought to occur as a result of chronic epithelial injury or stress, resulting in the accumulation of myofibroblasts that express high levels of extracellular matrix (19). Genetic manipulation of lung development pathways in the context of bleomycin-lung injury, including Notch, transforming growth factor-β (Tgfβ), bone morphogenetic protein (Bmp), Sonic hedgehog (Shh), fibroblast growth factors (Fgfs), epidermal growth factor (Egf), and wingless-type MMTV integration site family (Wnt), combined with results based on studies using IPF patient materials, have led to the development of potential therapeutic treatments for IPF (31, 38).
Fgf7 and Fgf10 signal in a paracrine fashion via epithelially expressed Fgfr2b receptor (43). The signal results in a phosphorylation cascade, mediated by fibroblast growth factor receptor substrate (Frs2), which activates PI3K- and MAPK-signaling pathways and/or activation of phospholipase C-γ (Plc-γ). Depending on the cell type and context, Fgfr2b signaling culminates in survival, growth, and differentiation of epithelial cells. Fgf10/Fgfr2b signaling is critical for murine lung development whereas Fgf7 is dispensable (3, 16, 27). Fgfs have been reported to act upstream of Wnt signaling (23). Interestingly, bleomycin-injured mice with epithelial-specific deletion of β-catenin signaling, a downstream target of both Fgf- (23) and Wnt signaling (26), suffered increased fibrosis (37). Although Wnt signaling was previously thought to play an exclusively profibrotic role by mediating epithelial-to-mesenchymal transition (EMT), this report revealed the importance of β-catenin-mediated protection of epithelial cells against bleomycin injury.
In the bleomycin mouse model, past studies have focused primarily on the beneficial effect of prophylactic treatment with palifermin, a pharmacological agent composed of a truncated form of keratinocyte growth factor (KGF), also known as FGF7 (7, 36). Although palifermin demonstrated a protective, prophylactic effect, genetic Fgf10 expression postbleomycin injury (11) resulted in increased survival as well as prevention and accelerated resolution of lung fibrosis in mice. Although current therapies target tyrosine kinases for the treatment of IPF (2), whether endogenous FGF signaling plays a protective, pathogenic, or ambivalent role in IPF is still unknown. Given the beneficial effects of exogenous Fgfr2b ligands on lung repair, the authors hypothesized that endogenous Fgfr2b ligands play a critical role in repair following bleomycin injury. However, endogenous Fgfr2b ligands as well as Fgfr2b receptor expression, were decreased following bleomycin injury in wild-type mice. Thus, unsurprisingly, attenuating endogenous Fgfr2b ligands during bleomycin-induced lung injury did not lead to significantly increased fibrosis or decreased survival. In summary, although endogenous Fgfr2b ligand signaling failed to play a critical role in limiting fibrosis, these results do not negate the potential benefit of exogenously stimulating developmental pathways to protect against lung fibrosis.
METHODS
Animal care.
All experiments were performed in accordance with the National Institutes of Health Guidelines for the Use of Laboratory Animals. Animal experiments were approved by the Institutional Animal Care and Use Committee at Children's Hospital Los Angeles protocol 193-12 and the Federal Authorities for Animal Research of the Regierungspraesidium Giessen, Hessen, Germany, protocols 72/2012 and 73/2012.
Generation of mice.
CMV-Cre mice (33) were crossed with rtTAflox mice (4) to generate mice expressing rtTA under the ubiquitous Rosa26 promoter. This constitutive Rosa26rtTA/+ mouse line was then crossed with the tet(O)solFgfr2b/+ responder line to generate Rosa26rtTA/+;tet(O)sFgfr2b/+ double-heterozygous animals, allowing ubiquitous expression of dominant-negative soluble Fgfr2b (28). All mice were generated on a CD1 mixed background. Attenuation of Fgfr2b ligand activity was achieved by administration of doxycycline-containing food; normal rodent diet with 0.0625% doxycycline (Harlan Teklad). Tet(O)Cre [B6.Cg-Tg(tetO-cre)1Jaw/J] (30) and Tomatoflox/flox reporter mice [B6;129S6-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J] (24) were purchased from Jackson Laboratory. Mice were genotyped as described previously (4, 12, 34).
Bleomycin administration.
Female mice 10–14 wk old were anesthetized with a mixture of 0.6 μl/g ketamine 10% (100 mg/ml) and 0.3 μl/g Domitor 10% (0.5 mg/ml) dissolved in 0.7% saline. A microsprayer (PennCentury) was used to administer an intratracheal dose of either 0.7% saline or bleomycin (1.0–3.0 U/kg) (Hexal or Sigma-Aldrich). Weight, activity, respiration, and temperature was monitored daily and mice were euthanized if they showed a significant decline in health parameters.
Lung compliance measurement.
Mice were deeply anesthetized with a mixture of 1.2 μl/g ketamine 10% (100 mg/ml, Bela Pharm), 0.6 μl/g Domitor 10% (0.5 mg/ml; Orion), 1:4 parts heparin, and dissolved in saline. Lung function was measured by using the SCIREQ flexiVent forced-oscillation plethysmograph to give an overall readout of lung function. Mice were intubated transtracheally and ventilated at a rate of 150 breaths/min with a positive end-expiratory pressure (PEEP) between 1 and 3 cmH2O. PEEP was calculated automatically by flexiVent 7 software and was dependent on the weight of the animal. After stable ventilation was achieved (spontaneous breathing ceased as the heart continued to beat), a 3-s, weight-dependent, fixed-volume waveform was initiated every 15–20 s, eight times. During this perturbation, “snapshots” of respiratory compliance were taken and the average of eight measurements represented the value of one biological sample.
Left lobe perfusion and isolation.
The left lobe was perfused from 22–24 cm above the mouse for 1 min with PBS followed by 2 min with 4% paraformaldehyde (PFA). The trachea was tied off with a string, and the lung was removed and placed in 4% PFA for at least 24 h at room temperature or up to 1 wk at 4°C. Lungs were then embedded with a Leica embedding machine (EG 1150C). Paraffin blocks were kept cold and 3- to 4-μm sections were cut.
Hematoxylin and eosin.
Three- to 4-μm sections were deparaffinized, dipped in water, and stained in Mayer's hematoxylin solution for 1–3 min and washed under running tap water for up to 10 min. Slides were monitored under the microscope for staining progression. Slides were then incubated for 2 min in eosin dye and brought back through increasing gradients of ethanol and xylene, then coverslipped with Pertex mounting medium.
Masson's trichrome stain.
Three- to 4-μm sections were deparaffinized and stained with Gomori's Green Trichrome Stain Kit (Dako AR166) according to manufacturer's protocol.
Lung morphometry.
For alveolar morphometry, lungs were flushed with PBS at a vascular pressure of 20 cmH2O. Then PBS was infused via the trachea at a pressure of 20 cmH2O and fixed with 4% paraformaldehyde in PBS (pH 7.0) via the trachea at a pressure of 20 cmH2O. Investigations were performed with 5-μm sections of paraffin-embedded left lobe of the lungs. The mean linear intercept, mean air space, and mean septal wall thickness were measured after staining with hematoxylin and eosin (H/E). Total scans from the left lobe were analyzed by using a Leica DM6000B microscope with an automated stage according to the procedure previously described (25, 42) which was implemented into the Qwin V3 software (Leica, Wetzlar, Germany). Horizontal lines (distance 40 μm) were placed across each lung section. The number of times the lines cross alveolar walls was calculated by multiplying the length of the horizontal lines and the number of lines per section then dividing by the number of intercepts. Bronchi and vessels above 50 μm in diameter were excluded prior to the computerized measurement. The air space was determined as the nonparenchyma, nonstained area. The septal wall thickness was measured as the length of the line perpendicularly crossing a septum. From the respective measurements, mean values were calculated.
Fibrosis quantification on histological sections.
Ashcroft scoring was performed blinded by using a modified Ashcroft scoring protocol (as described in Ref. 14) on H/E-stained sections of murine left lobes. Traditionally, multiple ×20 images are scored; however, we imaged stained left lobes with light microscopy at the lowest objective (×1.25), which allowed for visualization of the entire section. ImageJ software was used to measure the area of the lung that was covered in confluent fibrotic mass (data are presented as % confluent fibrosis per total area of the section). Sections were measured blindly, a total of three times, and scores were averaged.
Hydroxyproline assay.
QuickZyme total collagen assay was performed according to manufacturer's instructions. Briefly, either cranial and accessory or caudal and medial lobes were extracted, rinsed briefly in PBS, and dried overnight in a ventilated hood. Lungs were weighed and 6 M HCl was added for a final concentration of 50 mg tissue/ml. Lungs and collagen standards were then incubated for 20 h overnight at 95°C and cooled to room temperature. Next, tubes were centrifuged at 13,000 g for 10 min. Hydrolyzed supernatant was diluted 10-fold with 4 M HCl and used for the assay. A microplate reader (Tecan Infinite 200 PRO) was used for color detection. A standard curve was calculated from the collagen standards and the total hydroxyproline content was assessed.
RNA extraction.
After lung function measurements were taken, the right bronchus was clamped and either cranial and accessory or caudal and medial lobes were removed, placed in TRIzol, homogenized in GentleMACs, and frozen in liquid nitrogen for RNA extraction. Next, transcardiac perfusion of the left lobe was performed with a 20-G needle and 15 ml PBS.
Western blot.
Loading buffer was added to protein samples from cell extracts (5% SDS in bromophenol blue and β-mercaptoethanol) denatured for 5 min at 95°C and cooled on ice. At least 10 μg of sample was loaded on a 10% polyacrylamide gel and run at 25 mA per gel for ∼2 h. Samples were then electrically transferred to a polyvinylidene fluoride membrane (Amersham) by semidry electroblotting (70 mA per gel; gel size 7 × 9 cm) for 90 min. The membrane was blocked with 5% milk in Tris-buffered saline (TBS) blocking buffer at room temperature on shaker for 1 h followed by incubation with primary antibody: COL1a1 (Meridian no. T47770R), FGF1 (Abcam no. ab9588 1:2,000), FGF7 (Santa Cruz no. sc27126 1:200), FGF10 (Abcam no. ab71794, 1:200), Col1a1 (Meridian no. T40777R, 1:1,000, 8% gel), SPRY2 (Santa Cruz no. sc10082, 1:200), SPRY4 (Santa Cruz no. sc30051, 1:200), FGFR1 (Santa Cruz no. sc-121, 1:200), FGFR2 (Santa Cruz no. sc-122, 1:200), p-ERK1/2 (Cell Signaling no. 4370S; 1:1,000), total ERK1/2 (Cell Signaling no. 9102S; 1:1,000), p-Akt total (Cell Signaling no. 4060S, 1:1,000), total-Akt (Cell Signaling no. 4691S, 1:3,000), β-actin (Abcam no. ab8227; 1:30,000), and GAPDH (Cell Signaling no. cs2118, 1:1,000) overnight at 4°C. After washing with 1× TBS and Tween 20 (TBS-T) four times for 15 min each, the membrane was incubated with swine anti-rabbit horseradish peroxidase (Dako no. P0217) secondary antibody (dilution 1:2,000) at room temperature for 1 h followed by four times washing with 1× TBS-T buffer for 15 min each. The protein bands were detected by ECL (Enhanced Chemiluminescence, Amersham) treatment, followed by exposure of the membrane.
Quantitative PCR.
RNA was reverse transcribed (Qiagen QuantiTect Reverse Transcription Kit, no. 205313). cDNA was diluted to a concentration between 20 ng/μl. Primers were designed by use of Roche Applied Sciences online Assay Design Tool. All primers were designed to span introns and were blasted by using NCBI software for specificity. SYBRGreen Master Mix (Applied Biosciences 4309155) was used for RT-PCR with a Roche LightCycler 480 machine. Samples were run in triplicate with Hprt as reference genes for mouse samples.
FACS.
Accessory and caudal lobes were isolated in ice-cold Hanks' balanced salt solution (HBSS). Next, lobes were chopped finely using sterile razor blades and transferred to a 10-ml solution of 0.5% collagenase in HBSS. Then solution was heated to 37°C on a hot plate and stirred on high for 60 min. Next the dissociated homogenate was passed through 18-G, 20-G, 24-G needles, respectively, then filtered through 70-μm and 40-μm filters. One volume HBSS was added to dilute collagenase and homogenates were centrifuged at 1,500 rpm for 5 min to remove the enzyme solution. Cells were then resuspended in 500 μl 0.5% FCS in PBS and stained with anti-red fluorescent protein (RFP) pAb rabbit, Life Technologies, R10367 (1:200) for 20 min at 4°C, followed by washing and flow cytometric analysis with LSR Fortessa equipped with FACSDiva software (BD Bioscience).
Statistical analyses.
One-way ANOVA was performed on densitometry plots of Western blots followed by a Dunnett's test of significance. A Student's t-test was performed on the log-transformed value of the qPCR fold changes as well as compliance, hydroxyproline, and confluent areas of fibrosis measurements. For FACS analyses, t-tests were performed on the probit values. A binomial significance test was used to determine the statistical significance of soluble Fgfr2b detection. Conformity of the data with the assumptions of the tests was checked with residual analysis.
RESULTS
Modest recruitment of the Fgf-signaling pathway during spontaneous repair initiated by bleomycin-induced lung injury in mice.
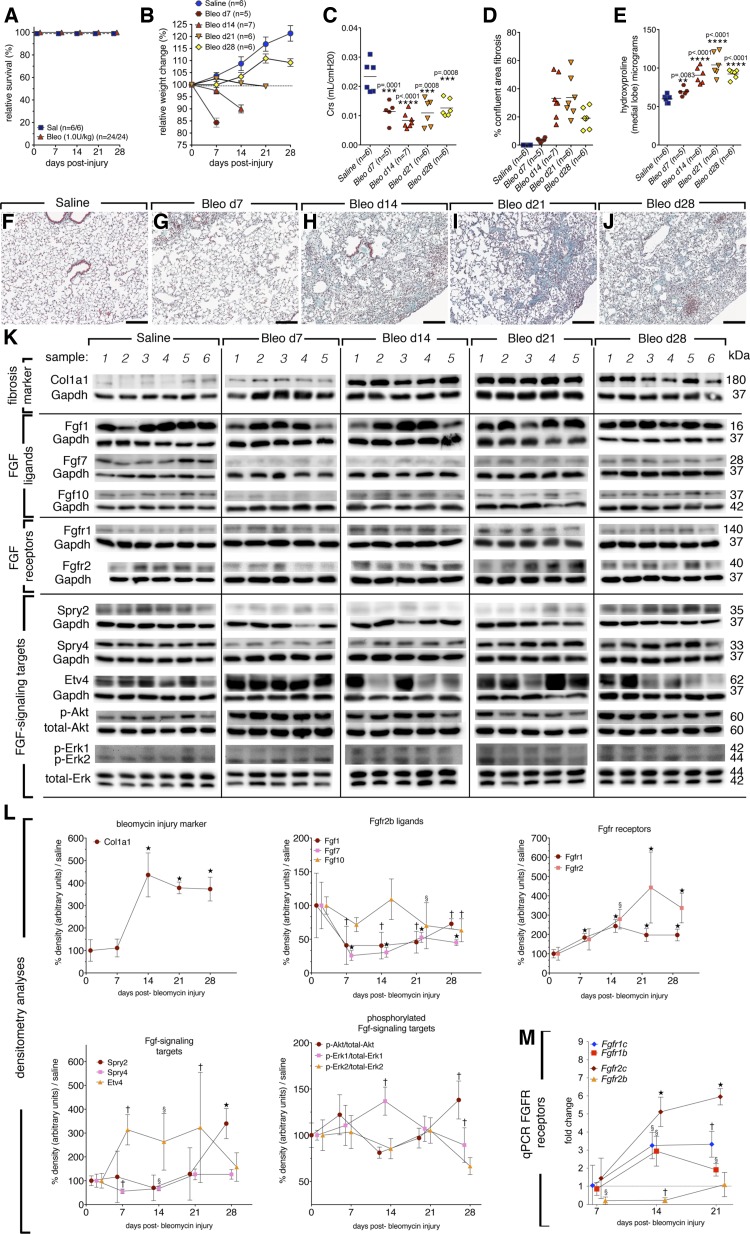
To investigate whether the endogenous Fgf-signaling pathway is recruited during bleomycin-induced lung injury, CD1 mice were given 1 U/kg bleomycin or saline intratracheally. This bleomycin dose generates robust fibrosis and gives a survival rate of 100% at 28 dpi in CD1 mice (Fig. 1A). Mice were euthanized at given time points according to weight loss to ensure maximal survival to 28 dpi (Fig. 1B). Injury was confirmed in each mouse by lung compliance measurements (Fig. 1C), calculation of confluent fibrotic areas of H/E-stained left lobes (Fig. 1D), hydroxyproline deposition (Fig. 1E), Masson's trichrome staining (Fig. 1, F–J), and Col1a1 Western blotting of whole lung homogenate (Fig. 1, K and L). In this model, bleomycin-induced lung injury peaked between 14 and 21 days postinjury (dpi). An additional model using historical controls (5 U/kg, C57bl/6 females) was also used to comparatively evaluate Fgf-signaling targets and mRNA expression, and similar results were obtained (data not shown).
Fig. 1.
Fibrosis injury (1 U/kg) peaked between 14 and 21 days postinjury (dpi) in CD1 female, wild-type mice, moderate recruitment of Fgfr2b signaling following injury (Fgfs). A: survival curve. B: relative weight change. C: compliance. D: quantification of confluent fibrotic areas in hematoxylin and eosin (H/E) stains of left lobes. E: hydroxyproline content of medial lobes. F–J: Masson's trichrome stain of representative time points following injury. K: Western blots for Fgf-signaling pathway members of saline or bleomycin-treated mice. L: quantification of blot densities normalized to saline controls (gene of interest divided by control gene) and represented as percent of saline expression. M: since the Western blot antibodies used do not distinguish between receptor isoforms, qPCR was performed on epithelial (b-isoforms) and mesenchymal c-isoforms of Fgfr1 and Fgfr2 receptor. Already at 7 dpi, Fgfr2b was significantly reduced and remained so until 21 dpi. At 14 dpi, c-isoforms of both receptors were increased, as well as Fgfr1b. The c-isoforms remained elevated at 21 dpi, while Fgfr1b returned to saline levels. One-way ANOVA was performed against control values and error bars represent 95% confidence intervals; §P < 0.05; †P < 0.005; ⋆P < 0.0001. Scale bars: 100 μm.
Next, Western blots were performed on Fgfr2b ligands, receptors, and downstream signaling targets on lung homogenate lysates isolated from animals 7, 14, 21, and 28 days after bleomycin injury or 2 wk after saline administration. Col1a1 expression was used as an indicator of fibrotic injury and most strongly expressed at 14 dpi and slightly reduced thereafter (Fig. 1, K and L). Fgf ligands were decreased for the most part following injury with the exception of Fgf10, which was slightly elevated compared with Fgf1 and Fgf7 at 14 dpi (Fig. 1, K and L). Fgfr1 and Fgfr2 expression increased significantly following injury. Importantly, qPCR analyses indicated that Fgfr11b, Fgfr2c, and Fgfr1c isoforms were increased although Fgfr2b remained decreased (Fig. 1M). Spry2, a negative regulator of MAPK signaling in the epithelium was decreased following injury, though expression was increased at 28 dpi (Fig. 1, K and L). Spry4, a negative regulator of MAPK signaling in the cells of mesenchymal origin (lung fibroblasts) was slightly elevated at 21 and 28 dpi (Fig. 1, K and L). Etv-4, a downstream target of Fgf10 signaling, also known as Pea-3, was strongly induced following injury (Fig. 1, K and L). Lastly, general MAPK-signaling targets, which are also targets of Fgf signaling were evaluated. At 7 dpi, p-Akt was slightly increased and again strongly activated at 28 dpi (Fig. 1, K and L). The marker p-Erk1 peaked at 14 dpi and decreased at 28 dpi (Fig. 1, K and L), whereas p-Erk2 was not significantly regulated (Fig. 1, K and L). Compared with mice injured with a higher dose of bleomycin (5 U/kg, data not shown), p-Akt signaling was similarly regulated. It was strongly induced 3–4 wk following injury (Fig. 1, K and L). In addition, p-Erk1 and p-Erk2 were moderately regulated in both models.
In summary, during spontaneous repair after bleomycin injury in wild-type mice, Fgf10 expression was moderately increased along with receptor Fgfr1b and downstream targets Spry2, Etv4, and p-Akt at 28 dpi. The endogenous epithelial-expressed Fgfr2b receptor was drastically reduced following bleomycin injury (Fig. 1M) and began to recover expression at 21 dpi, whereas c-isoform expression remained significantly elevated. The significance and contribution of the Fgf10/Fgfr1b-signaling axis to lung repair is still elusive and will require further investigation.
Validation of the Rosa26rtTA/+;tet(O)sFgfr2b/+ transgenic line.
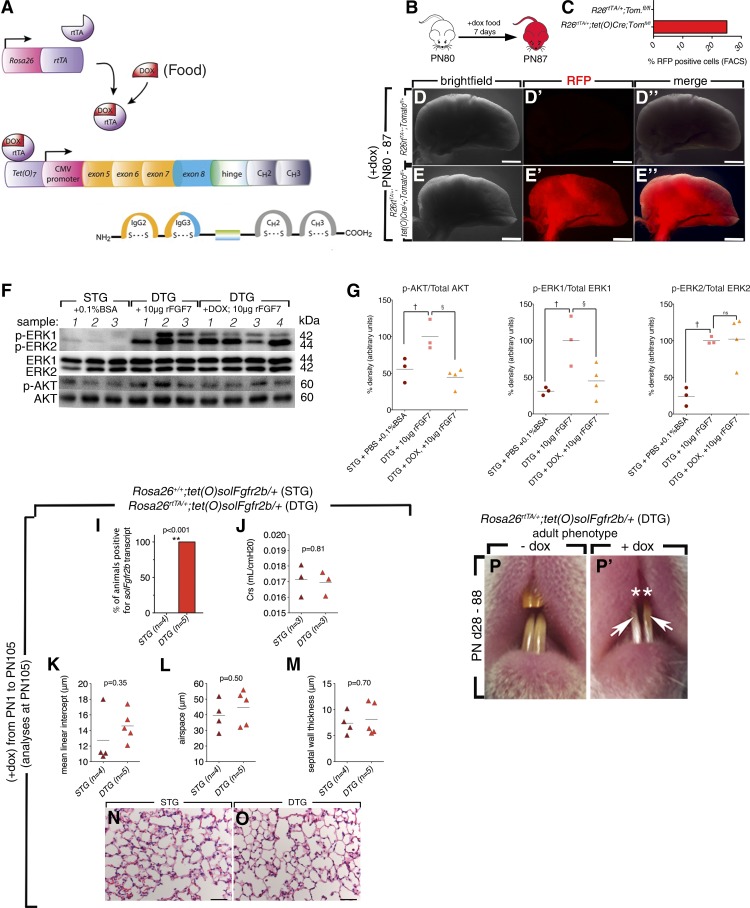
To test whether the attenuation of all Fgfr2b ligands would result in increased fibrosis, the Rosa26rtTA/+;tet(O)sFgfr2b/+; in this study referred to as the double-transgenic (DTG) mouse line was used (28) (Fig. 2A). The Rosa26 promoter drives ubiquitous expression of reverse tetracycline transactivator, which in the presence of doxycycline (dox) binds a tetracycline response sequence. Binding results in the activation of a cytomegalovirus (CMV) promoter, which drives expression of a chimeric transgene containing the extracellular binding domain of Fgfr2b fused with the heavy chain domain of IgG (Fig. 2A). The line was previously used in the context of naphthalene and hyperoxia lung injury to demonstrate the critical role of Fgfr2b ligands in the lung repair process (12, 13, 39). In addition, this line was also used to define the role of Fgfr2b ligands during early lung embryonic and late lung development (12), limb development (6), postnatal mammary gland development (29), incisor homeostasis (28), and gut homeostasis (1).
Fig. 2.
Validation of Rosa26rtTA/+;tet(O)sFgfr2b/+ [double-transgenic (DTG)] mice. The Rosa26 promoter drives ubiquitous expression of reverse tetracycline transactivator (rtTA), which in the presence of doxycycline (dox) binds a tetracycline response sequence. Binding results in the activation of a CMV promoter, which drives expression of a chimeric transgene containing the extracellular binding domain of Fgfr2b fused with the heavy chain domain of IgG (A). Lung-specific efficiency of Rosa26 promoter was tested in Rosa26rtTA/+;tet(O)Cre/+;Tomatofl/+ mice fed +dox-food for 7 days (B and C). Red fluorescent protein (RFP) expression was detected in ∼25% of total cells by FACS and in none of the cells in mice lacking the tet(O)Cre transgene as illustrated by fluorescence stereomicroscopy (D–E″). Single-transgenic (STG) mice (n = 3) received 50 μl of PBS with 0.1% BSA; DTG mice were fed normal food (n = 3) or +dox food (n = 4) for 1 wk and were given an intratracheal dose of 10 μg of FGF7. At 30 min later, the lysates were collected and blotted for p-Akt and p-Erk1/2 signals (F). +dox DTG mice blocked FGF7-mediated p-Akt (G) and p-Erk1 signals (H), p-ERK2 (H′) remained elevated (F–H′). †Significant; §very significant. The chimeric transcript was detected only in DTG mice (I) and lung compliance was not changed in DTG mice fed +dox food from postnatal day (PN) 1 to PN105 (J). **Very significant. K–O: morphometric analyses were performed on these mice, and in concurrence with previous studies no significant differences in mean linear intercept, air space, or septal wall thickness were observed in DTG mice. Adult DTG mice fed +dox food from PN28-88 failed to regenerate maxillary incisors (P–P′).
In this study, DTG littermates lacking the Rosa26rtTA/+ construct [Rosa26+/+;tet(O)sFgfr2b/+] were used as controls, and referred to as single transgenics (STG). To confirm the functionality of soluble Fgfr2b in our experimental conditions, validation was also performed in adult female animals. First, the lung-specific efficiency of the driver was tested in Rosa26rtTA/+;tet(O)Cre/+;Tomatofl/+ mice fed +dox food for 7 days (Fig. 2, E and F). RFP expression was detected in ∼25% of total cells by FACS and in none of the cells in mice lacking the tet(O)Cre transgene as illustrated by fluorescence stereomicroscopy (Fig. 2, B–E″).
To confirm the function of the chimeric receptor in adult lungs, a study was performed to test the ability of induced DTG lungs (+dox food ad libitum) to attenuate exogenous FGF7 signaling. Female STG mice were used as controls and received PBS with 0.1% BSA, the same solution in which the recombinant FGF7 was resuspended. DTG mice were fed either normal food or +dox food for 1 wk. Next, females from each group received an intratracheal dose of 10 μg of rFGF7. Then, 30 min later, the whole lung was collected, and lysates were blotted for p-Akt and p-Erk1/2 signals. Although +dox DTG mice blocked the FGF7-mediated elevation in p-Akt and p-Erk1-signals to levels of PBS-treated controls, p-Erk2 remained elevated. Failure to block p-Erk2 was possibly due to overabundance of FGF7 and resultant signaling via endogenous Fgfr2 receptors (Fig. 2, F–H′).
Morphometric analyses were performed on STG and DTG mice fed +dox food from postnatal days (PN) 1 to PN105 (Fig. 2, I–L). The chimeric transcript was detected only in DTG mice (Fig. 2I) and lung compliance remained unaffected (Fig. 2J). In concurrence with previous studies (12), morphometric analyses revealed no significant differences in mean linear intercept, air space, or septal wall thickness in DTG mice (Fig. 2, K–M). Although no lung defects were present, DTG mice fed +dox food from PN28 to PN88 showed characteristic inhibition of maxillary incisor regeneration (Fig. 2, P and P′) (28).
Attenuation of Fgfr2b ligands during fibrosis formation postbleomycin injury did not result in increased fibrosis.
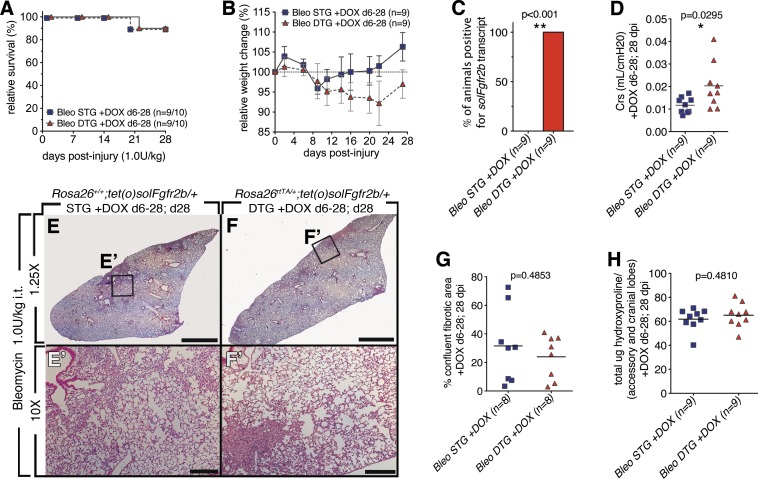
To determine whether endogenous Fgfr2b ligands expedite fibrosis resolution, a relatively low dose of bleomycin (1 U/kg it), which generated between 80 and 100% survival and mild fibrosis at 28 dpi, was used. First, Fgfr2b ligands were attenuated from 6 to 14 dpi (Fig. 3) and next during fibrotic resolution (14–28 dpi) (Fig. 4). Fgfr2b ligands attenuation had no impact on relative survival (Fig. 3A) though a trend toward delayed weight recovery in DTG mice was observed (Fig. 3B). Specific soluble Fgfr2b expression in DTG was confirmed (Fig. 3C). A slight increase in lung compliance was measured at 28 dpi in DTG vs. STG (Fig. 3D). Upon histological examination both STG and DTG lungs showed areas of fibrosis (Fig. 3, E and F). However, quantification of the confluent areas of fibrosis in the left lobe did not reveal any difference between DTG and STG (Fig. 3G). In agreement with these results, no differences were observed for total hydroxyproline (Fig. 3H). Together, these results indicate that attenuation of Fgfr2b ligands following bleomycin injury (6–28 dpi) had no impact on fibrosis resolution by 28 dpi.
Fig. 3.
Lack of endogenous Fgfr2b ligands signaling during injury (6–28 dpi) did not lead to increased bleomycin-induced lung injury (28 dpi). A: survival curve. B: relative weight change. C: all DTG mice tested positive for solFgfr2b transcript at 28 dpi. **Very significant. D: compliance. *Significant. E–F′: low and high magnification of H/E staining of STG control mice (E and E′) and DTGs fed doxycycline food from 6 to 28 dpi (F and F′). G: quantification of confluent fibrotic areas in H/E stains of left lobes. H: hydroxyproline content of accessory and medial lobes. Scale bars E and F: 2 mm; E′ and F′: 200 μm.
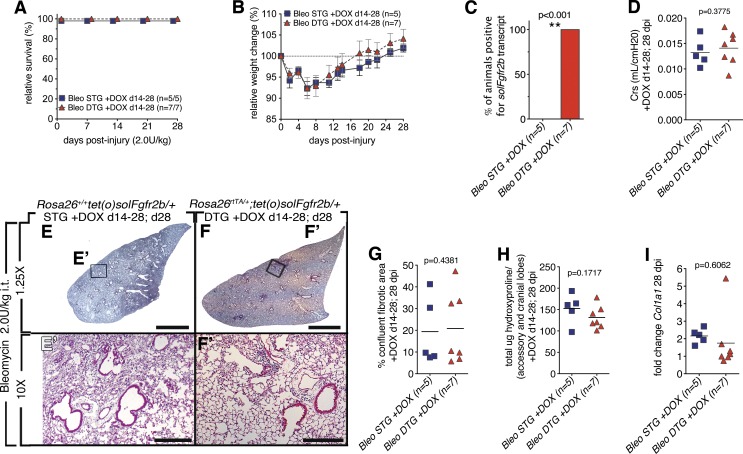
Fig. 4.
Lack of endogenous Fgfr2b ligands signaling during injury (14–28 dpi) did not lead to increased bleomycin-induced lung injury (28 dpi). A: survival curve. B: relative weight change. C: all DTG mice tested positive for solFgfr2b transcript at 28 dpi (C). D: compliance. E–F′: low and high magnification of H/E staining of STG control mice (E and E′) and DTGs fed doxycycline food from 14 to 28 dpi (F and F′). G: quantification of confluent fibrotic areas in H/E stains of left lobes. H: hydroxyproline content of accessory and medial lobes. I: Col1a1 expression. Scale bars E and F: 2 mm; E′ and F′: 200 μm.
Although downstream targets were just moderately engaged in wild-type mice injured with either 1.0 U/kg or 5.0 U/kg bleomycin doses, a slightly higher bleomycin dose (2 U/kg it) was administered to our genetically modified mice to test whether endogenous Fgfr2b ligands would play an important role in lung repair following more severe injury. To focus on the contribution of Fgfr2b ligands to the resolution phase, ligands were attenuated from 14 through 28 dpi. No difference in survival rate (Fig. 4A) was observed. The relative weight change between DTG and STG were similar (Fig. 4B). The expression of soluble Fgfr2b was detected only in DTG as previously reported (Fig. 4C). No difference in lung function was observed (Fig. 4D). No difference for hydroxyproline deposition between DTG and STG (Fig. 4H) was observed. In summary, attenuation of Fgfr2b ligands post-bleomycin injury following the peak of fibrosis (14–28 dpi) does not have any impact on the extent of fibrosis at 28 dpi.
Attenuation of Fgfr2b ligands immediately following bleomycin injury did not result in increased fibrosis.
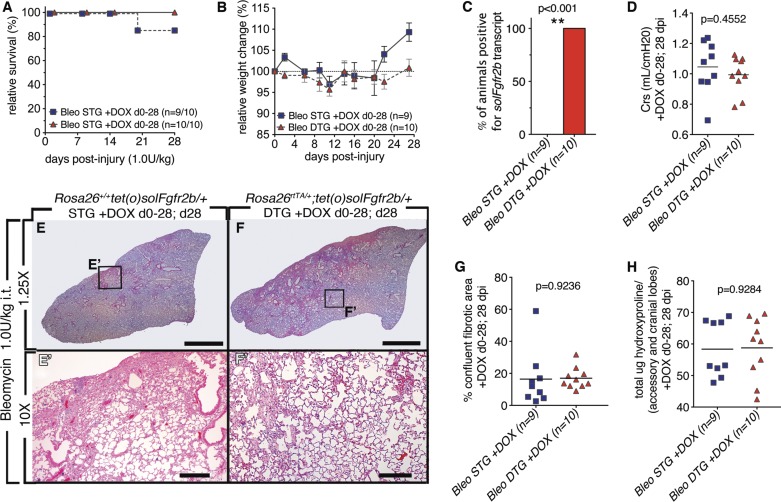
Attenuating Fgfr2b ligands at later stages following injury (6–28 and 14–28 dpi) had no effect on the level of bleomycin-induced lung injury incurred. Because Fgfr2b ligands are known to have a protective effect on lung epithelium, next it was tested whether attenuation of endogenous Fgfr2b ligands signaling immediately afterward and throughout injury impacts the extent of fibrosis incurred at 28 dpi. Fgfr2b ligands were attenuated throughout injury (0–28 dpi). With the exception of one STG animal, all animals survived until euthanized at 28 dpi (Fig. 5A). No significant differences in relative weight change were observed, although as in Fig. 5B, a trend toward delayed weight recovery in DTG mice was observed (Fig. 5B). Specific soluble Fgfr2b expression in DTG was confirmed (Fig. 5C). No significant change in lung compliance was measured at 28 dpi in DTG vs. STG (Fig. 5D). Upon histological examination both STG and DTG had areas of fibrosis (Fig. 5, E and F). However, quantification of the confluent fibrosis areas did not reveal any difference between DTG and STG lungs (Fig. 5G). In agreement with these results, no differences were observed for total hydroxyproline (Fig. 5H). Attenuation of Fgfr2b ligands in DTG mice for the duration of the injury did not lessen fibrosis incurred at 28 dpi.
Fig. 5.
Lack of endogenous Fgfr2b ligands signaling during injury (0–28 dpi) did not lead to increased bleomycin-induced lung injury (28 dpi). A: survival curve. B: relative weight change. C: all DTG mice tested positive for solFgfr2b transcript at 28 dpi. **, Very significant. D: compliance. E–F′: low and high magnification of H/E staining of STG control mice (E and E′) and DTGs fed doxycycline food from 0 to 28 dpi (F and F′). G: quantification of confluent fibrotic areas in H/E stains of left lobes. H: hydroxyproline content of accessory and medial lobes. Scale bars E and F: 2 mm; E′ and F′: 200 μm.
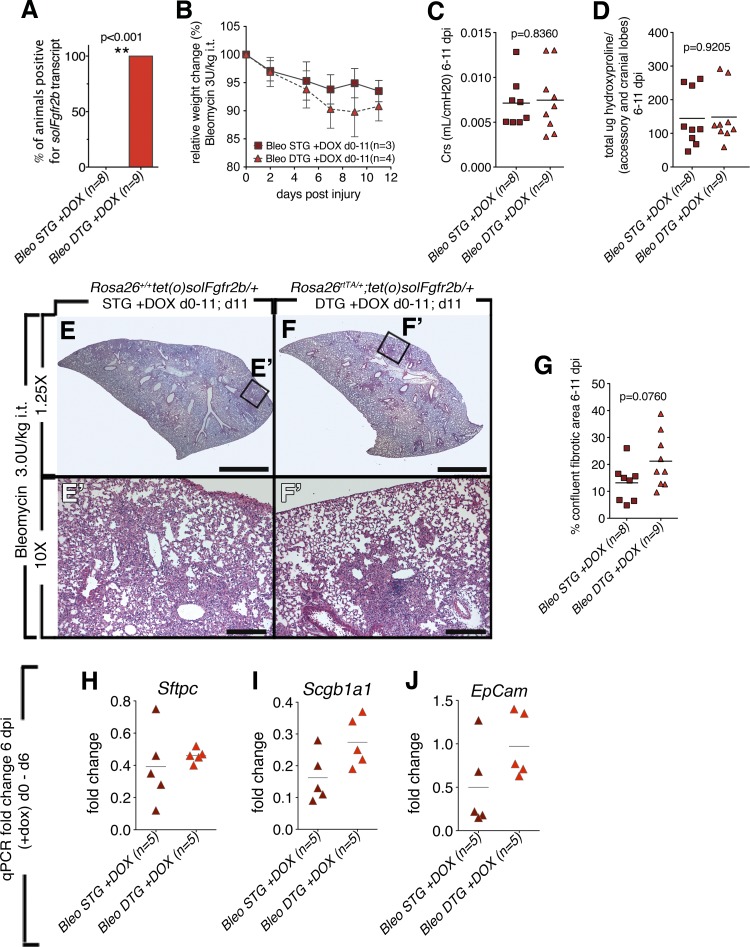
In an attempt to further engage endogenous repair mechanisms, 3.0 U/kg it of bleomycin was used in the final experiment (Fig. 6). The soluble receptor was induced from the day of bleomycin injury until the day of euthanasia; between 6 and 11 dpi (Fig. 6). Although the initial aim of this study was to analyze the extent of fibrosis at 28 dpi, 3.0 U/kg it of bleomycin was too severe for CD1 mice. Therefore mice were euthanized and analyzed at earlier time points. Five mice from both the STG and the DTG groups were removed at 6 dpi for analyses based on weight-loss criteria (< 20% of initial weight, data not shown). The other mice (STG; n = 3 and DTG; n = 4) were analyzed at 1 dpi. Induction of the soluble receptor was detected in DTG mice (Fig. 6A). A trend toward increased weight loss for DTG mice that survived to 1 dpi was observed (Fig. 6B). Although some variability in measurements occurred due to differences in the day of euthanasia (6–11 dpi), no differences between STGs and DTGs were detected at any time point in regards to compliance (Fig. 6C), hydroxyproline (Fig. 6D), and the confluent fibrotic areas (Fig. 6G). In wild-type mice, bleomycin injury compared with noninjured mice triggered decreased Sftpc expression at 7 dpi and Scgb1a1 at 14 dpi (data not shown). Therefore, expression of AECII cell-specific transcript Sftpc, Scgb1a1 for club cells, and the general epithelial marker EpCam was measured in animals euthanized at 6 dpi. However, no differences in marker expression were observed between DTG and STG lungs (Fig. 6, H–J).
Fig. 6.
Lack of endogenous Fgfr2b ligands signaling during injury (6–11 dpi) did not lead to increased bleomycin-induced lung injury (6–11 dpi). A: all DTG mice tested positive for solFgfr2b transcript at 28 dpi. **Very significant. B: relative weight change in mice that survived until 11 dpi (3/8 STG) and (4/9 DTG). C: compliance. D: hydroxyproline content of accessory and medial lobes. E–F′: low and high magnification of H/E staining of STG control mice at 11 dpi (E and E′) and DTGs fed doxycycline food from 0 to 11 dpi (F and F′). G: quantification of confluent fibrotic areas in H/E stains of left lobes. H–J: no decreases in epithelial marker expression were detected in injured 6 dpi DTG mice vs. STG; qPCR for Sftpc, Scgb1a1, EpCam; corresponding saline controls normalized to 1 (data not shown). Scale bars E and F: 2 mm; E′ and F′: 200 μm.
DISCUSSION
Fgfr2b ligand signaling is important for lung development and repair.
Fgfs play pleiotropic roles both during organogenesis and homeostasis (15). Fgfr2b ligands are part of a family of 22 identified members. Fgf10 is required for lung formation and loss of Fgf10 leads to lung agenesis, whereas loss of Fgf1 and Fgf7 during development does not result in a lung phenotype in mice. The cellular and molecular bases for differences between Fgf7 and Fgf10 signaling are still unclear. Recently, mass spectrometry-based proteomics revealed that Fgf7 and Fgf10 elicit distinct biochemical response downstream of the Fgfr2b receptor. In particular, Fgf7 leads to rapid but transient phosphorylation of Akt and Shc whereas Fgf10 leads to the progressive and sustained phosphorylation of these mediators. In addition, Fgf10 triggers phosphorylation of tyrosine 734 and the associated recruitment of SH3bp4, a relatively novel adaptor protein. It has been proposed that Fgf10 leads to increased recycling of Fgfr2b at the cell surface whereas Fgf7 results in transient signaling (8). Unlike Fgf7 and Fgf10, which act mostly through Fgfr2b, Fgf1 acts via all Fgf receptor isoforms.
Fgf10 appears to be critical not only for the survival and proliferation of the distal epithelial lung progenitors (35) but also for the repair of the bronchiolar epithelium following naphthalene exposure (39). Lung epithelial cell-specific overexpression of Fgf10 in mice during the first, second, or third week postbleomycin injury was both protective and therapeutic as characterized by increased survival and attenuated lung fibrosis (11). Administration of Fgf7 or palifermin, a pharmacological agent composed of a truncated form of Fgf7, also reduced fibrosis and increased survival in both rats and mice (7, 10, 22, 32). In humans, haploinsufficiency for FGF10 is associated with chronic obstructive pulmonary disease (COPD) (18). Likewise, in humans, SNPs in FGF7 correlate with increased risk for developing COPD (5).
In contrast to previous studies that used exogenous Fgfr2b ligands to attenuate bleomycin-induced lung injury, this study first assessed the activation of endogenous Fgfr2b ligands in injured wild-type mice and then attenuated Fgfr2b ligands following bleomycin injury to assess their contribution to lung repair. Given the significant reduction in Fgfr2b receptor expression following bleomycin injury, it was not surprising that attenuation of endogenous Fgfr2b ligands had no effect on fibrosis outcome.
DTG mice efficiently attenuate endogenous Fgfr2b ligands.
The Rosa26rtTA/+;tet(O)sFgfr2b/+ (DTG) mouse line was used to ubiquitously trap all Fgfr2b ligands during injury. Fgf1, in addition to ligands of the Fgf7 subfamily Fgf3, Fgf7, Fgf10, and Fgf22, binds most strongly to Fgfr2b (43). Although Fgf10 contains a nuclear localization signal and may in some contexts act in an intracrine fashion (20), the Rosa26rtTA/+;tet(O)sFgfr2b/+ model has been demonstrated to mimic the loss of Fgf10 expression during development (35), demonstrating that Fgf10’s activity in the lung is mainly via secreted, paracrine signaling. The impact of trapping Fgfr2b ligands during lung injury has been previously reported. Induction of soluble Fgfr2b under the SpC-rtTA/+ promoter in adult mice subjected to hyperoxia injury led to abnormal expression of surfactant during injury, which contributed to increased lethality (13). A similar result was obtained in Rosa26rtTA/+;tet(O)sFgfr2b/+ neonates exposed to hyperoxia (Chao and Bellusci, unpublished results). Furthermore, in the context of naphthalene injury (39) as well as H1N1 influenza virus infection (Quantius et al., unpublished results), lung injury was increased in DTG animals. Mice used in this study were thoroughly validated and demonstrated both the embryonic and adult phenotypes characteristic of this line.
Attenuation of endogenous Fgfr2b ligands during bleomycin injury did not result in increased fibrosis.
Doxycycline-fed bleomycin-injured Rosa26rtTA/+;tet(O)sFgfr2b/+ (DTG) mice did not incur increased fibrosis compared with injured STG mice. There are several explanations for the lack of increased injury in DTG mice following bleomycin injury. First, the weak recruitment of Fgf ligands in this injury model indicated an insignificant contribution of endogenous Fgfr2b ligands to the repair process. Second, although solFgfr2b traps both Fgf7 and Fgf10 ligands, which have been shown to convey protective signals to the injured epithelium, it also traps Fgf1, which binds to all Fgfrs. Although its effects are not well characterized, Fgf1 signaling is potentially ambivalent in the context of bleomycin lung injury. As Fgf1 signals to cells of both epithelial and mesenchymal origins, blocking an Fgf1-mediated survival signal to fibroblasts following bleomycin injury, especially following 14 dpi, when fibroblasts are most abundant, may be beneficial. Moreover, in a study using a soluble, dominant-negative Fgfr2c-isoform construct, it was demonstrated that blocking mesenchymal Fgfr2c mediated signaling following bleomycin injury attenuated fibrosis (17). Third, although FACS analyses of Rosa26rtTA/+;Tomatofl/+ mice revealed that doxycycline induced rtTA expression in 25% of the lung cells, for maximal ligand attenuation during bleomycin injury a lung-specific driver may be required. Lastly, compensation by other endogenous repair pathways such as Wnt (37) may adequately compensate for the attenuation of Fgf signaling. In the future, lung cell type-specific models targeting specific ligands and receptors are needed to more accurately dissect the role of Fgf signaling in lung repair.
Tyrosine kinase inhibitors demonstrate therapeutic effects in bleomycin-treated mice, further suggesting endogenous Fgfr2b ligand signaling is dispensable for repair.
Tyrosine kinase receptors mediate a variety of growth factor signaling pathways. High levels of MAPK and ERK phosphorylation are associated with IPF. Three major tyrosine kinase pathways relevant for lung disease have been described: the VEGFR, PDGFR, and FGFR pathways. The tyrosine kinase inhibitor BIBF1120 blocks these pathways and has demonstrated a protective and therapeutic in the bleomycin model (40). In addition, phase III clinical trials demonstrated that BIBF1120 treatment both decreased the rate of decline in forced vital capacity and reduced the number of acute exacerbations in IPF patients (41). In mouse models, just as enhanced activation of the epithelial receptor tyrosine kinase Fgfr2b-isoform via exogenous Fgfr2b ligands leads to epithelial protection and lessens fibrosis, attenuation of Fgfr2c-isoform signaling also attenuates fibrosis. These results suggest that enhanced Fgfr2b-isoform signaling expedites lung protection and repair, whereas Fgfr2c-isoform ligand signaling (via Fgf1, Fgf2, Fgf9) may fuel the fibrosis fire by relaying survival signals to fibroblasts. In conclusion, although global tyrosine kinase inhibitors such as BIBF1120 likely inhibit FGFR2b signaling, the contribution of VEGF, PDGF, and FGFR2(c) to fibrosis formation may be far greater. Whether exogenously stimulating Fgfr2b signaling following treatment with tyrosine kinase inhibitors further attenuates bleomycin-induced fibrosis injury remains to be investigated.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
B.M. and W.S. conception and design of research; B.M., I.H., S. Hezel, D.A.A., E.E.A., C.-M.C., J.Q., M.J., K.G., and M.K. performed experiments; B.M., J.W., and A.A.R. analyzed data; B.M., I.H., S. Hezel, X.L., A.G., and S.B. interpreted results of experiments; B.M. prepared figures; B.M. drafted manuscript; B.M., D.A.A., E.E.A., M.J., M.K., A.G., and S.B. edited and revised manuscript; B.M., I.H., S. Hezel, D.A.A., E.E.A., C.-M.C., J.Q., J.W., M.J., K.G., X.L., W.S., M.K., S. Herold, A.A.R., A.G., and S.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Jonathan Branch, Clarence Wigfall, and Soula Danopolous for technical support at Childrens Hospital Los Angeles. S. Bellusci was supported by grants from the Deutsche Forschungsgemeinschaft (BE4443/4-1 and BE4443/6-1), Landes-Offensive zur Entwicklung wissenschaftlich-ökonomischer Exzellenz, and University Klinikum Giessen Marburg Centrum. A. A. Rizvanov and S. Bellusci acknowledge the support of the Russian Government Program of Competitive Growth of Kazan Federal University.
REFERENCES
- 1.Al Alam D, Danopoulos S, Schall K, Sala FG, Almohazey D, Fernandez GE, Frey MR, Ford HR, Grikscheit T. Fibroblast growth factor 10 alters the balance between goblet cells and Paneth cells in the adult mouse small intestine. Am J Physiol Gastrointest Liver Physiol 308: G678–G690, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antoniu SA. Nintedanib (BIBF 1120) for IPF: a tomorrow therapy? Multidiscip Respir Med 7: 41, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellusci S, Grindley J, Emoto H, Itoh N, Hogan B. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development 124: 4867–4878, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Belteki G, Haigh J, Kabacs N, Haigh K, Sison K, Costantini F, Whitsett J, Quaggin SE, Nagy A. Conditional and inducible transgene expression in mice through the combinatorial use of Cre-mediated recombination and tetracycline induction. Nucleic Acids Res 33: e51, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brehm JM, Hagiwara K, Tesfaigzi Y, Bruse S, Mariani TJ, Bhattacharya S, Boutaoui N, Ziniti JP, Soto-Quiros ME, Avila L, Cho MH, Himes B, Litonjua AA, Jacobson F, Bakke P, Gulsvik A, Anderson WH, Lomas DA, Forno E, Datta S, Silverman EK, Celedón JC. Identification of FGF7 as a novel susceptibility locus for chronic obstructive pulmonary disease. Thorax 66: 1085–1090, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danopoulos S, Parsa S, Al Alam D, Tabatabai R, Baptista S, Tiozzo C, Carraro G, Wheeler M, Barreto G, Braun T, Li X, Hajihosseini MK, Bellusci S. Transient inhibition of FGFR2b-ligands signaling leads to irreversible loss of cellular β-catenin organization and signaling in AER during mouse limb development. PLoS One 8: e76248, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deterding RR, Havill AM, Yano T, Middleton SC, Jacoby CR, Shannon JM, Simonet WS, Mason RJ. Prevention of bleomycin-induced lung injury in rats by keratinocyte growth factor. Proc Assoc Am Physicians 109: 254–268, 1997. [PubMed] [Google Scholar]
- 8.Francavilla C, Rigbolt KTG, Emdal KB, Carraro G, Vernet E, Bekker-Jensen DB, Streicher W, Wikström M, Sundström M, Bellusci S, Cavallaro U, Blagoev B, Olsen JV. Functional proteomics defines the molecular switch underlying FGF receptor trafficking and cellular outputs. Mol Cell 51: 707–722, 2013. [DOI] [PubMed] [Google Scholar]
- 9.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA 89: 5547–5551, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo J, Yi ES, Havill AM, Sarosi I, Whitcomb L, Yin S, Middleton SC, Piguet P, Ulich TR. Intravenous keratinocyte growth factor protects against experimental pulmonary injury. Am J Physiol Lung Cell Mol Physiol 275: L800–L805, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Gupte VV, Ramasamy SK, Reddy R, Lee J, Weinreb PH, Violette SM, Guenther A, Warburton D, Driscoll B, Minoo P, Bellusci S. Overexpression of fibroblast growth factor-10 during both inflammatory and fibrotic phases attenuates bleomycin-induced pulmonary fibrosis in mice. Am J Respir Crit Care Med 180: 424–436, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hokuto I, Perl AKT, Whitsett JA. Prenatal, but not postnatal, inhibition of fibroblast growth factor receptor signaling causes emphysema. J Biol Chem 278: 415–421, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Hokuto I, Perl AKT, Whitsett JA. FGF signaling is required for pulmonary homeostasis following hyperoxia. Am J Physiol Lung Cell Mol Physiol 286: L580–L587, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Hübner RH, Gitter W, El Mokhtari NE, Mathiak M, Both M, Bolte H, Freitag-Wolf S, Bewig B. Standardized quantification of pulmonary fibrosis in histological samples. Biotechniques 44: 507–511, 514–517, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Itoh N, Ornitz DM. Fibroblast growth factors: from molecular evolution to roles in development, metabolism and disease. J Biochem 149: 121–130, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Izvolsky KI, Shoykhet D, Yang Y, Yu Q, Nugent MA, Cardoso WV. Heparan sulfate-FGF10 interactions during lung morphogenesis. Dev Biol 258: 185–200, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Ju W, Zhihong Y, Zhiyou Z, Qin H, Dingding W, Li S, Baowei Z, Xing W, Ying H, An H. Inhibition of α-SMA by the ectodomain of FGFR2c attenuates lung fibrosis. Mol Med 18: 992–1002, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klar J, Blomstrand P, Brunmark C, Badhai J, Håkansson HF, Brange CS, Bergendal B, Dahl N. Fibroblast growth factor 10 haploinsufficiency causes chronic obstructive pulmonary disease. J Med Genet 48: 705–709, 2011. [DOI] [PubMed] [Google Scholar]
- 19.Korfei M, Ruppert C, Mahavadi P, Henneke I, Markart P, Koch M, Lang G, Fink L, Bohle RM, Seeger W, Weaver TE, Guenther A. Epithelial endoplasmic reticulum stress and apoptosis in sporadic idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 178: 838–846, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kosman J, Carmean N, Leaf EM, Dyamenahalli K, Bassuk JA. Translocation of fibroblast growth factor-10 and its receptor into nuclei of human urothelial cells. J Cell Biochem 102: 769–785, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Lewis D, Scullion J. Palliative and end-of-life care for patients with idiopathic pulmonary fibrosis: challenges and dilemmas. Int J Palliat Nurs 18: 331–337, 2012. [DOI] [PubMed] [Google Scholar]
- 22.Liu CJ, Ha XQ, Jiang JJ, Lv TD, Wu C. Keratinocyte growth factor (KGF) gene therapy mediated by an attenuated form of Salmonella typhimurium ameliorates radiation induced pulmonary injury in rats. J Radiat Res (Tokyo) 52: 176–184, 2011. [DOI] [PubMed] [Google Scholar]
- 23.Lü J, Izvolsky KI, Qian J, Cardoso WV. Identification of FGF10 targets in the embryonic lung epithelium during bud morphogenesis. J Biol Chem 280: 4834–4841, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13: 133–140, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGrath-Morrow SA, Cho C, Soutiere S, Mitzner W, Tuder R. The effect of neonatal hyperoxia on the lung of p21Waf1/Cip1/Sdi1-deficient mice. Am J Respir Cell Mol Biol 30: 635–640, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science 303: 1483–1487, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohuchi H, Hori Y, Yamasaki M, Harada H, Sekine K, Kato S, Itoh N. FGF10 acts as a major ligand for FGF receptor 2 IIIb in mouse multi-organ development. Biochem Biophys Res Commun 277: 643–649, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Parsa S, Kuremoto KI, Seidel K, Tabatabai R, Mackenzie B, Yamaza T, Akiyama K, Branch J, Koh CJ, Al Alam D, Klein OD, Bellusci S. Signaling by FGFR2b controls the regenerative capacity of adult mouse incisors. Development 137: 3743–3752, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parsa S, Ramasamy SK, De Langhe S, Gupte VV, Haigh JJ, Medina D, Bellusci S. Terminal end bud maintenance in mammary gland is dependent upon FGFR2b signaling. Dev Biol 317: 121–131, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Perl AKT, Tichelaar JW, Whitsett JA. Conditional gene expression in the respiratory epithelium of the mouse. Transgenic Res 11: 21–29, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Rafii R, Juarez MM, Albertson TE, Chan AL. A review of current and novel therapies for idiopathic pulmonary fibrosis. J Thorac Dis 5: 48–73, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakamoto S, Yazawa T, Baba Y, Sato H, Kanegae Y, Hirai T, Saito I, Goto T, Kurahashi K. Keratinocyte growth factor gene transduction ameliorates pulmonary fibrosis induced by bleomycin in mice. Am J Respir Cell Mol Biol 45: 489–497, 2011. [DOI] [PubMed] [Google Scholar]
- 33.Sauer B. Inducible gene targeting in mice using the Cre/lox system. Methods 14: 381–392, 1998. [DOI] [PubMed] [Google Scholar]
- 34.Schwenk F, Baron U, Rajewsky K. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res 23: 5080–5081, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sekine K, Ohuchi H, Fujiwara M, Yamasaki M, Yoshizawa T, Sato T, Yagishita N, Matsui D, Koga Y, Itoh N, Kato S. Fgf10 is essential for limb and lung formation. Nat Genet 21: 138–141, 1999. [DOI] [PubMed] [Google Scholar]
- 36.Sugahara K, Iyama K, Kuroda MJ, Sano K. Double intratracheal instillation of keratinocyte growth factor prevents bleomycin-induced lung fibrosis in rats. J Pathol 186: 90–98, 1998. [DOI] [PubMed] [Google Scholar]
- 37.Tanjore H, Degryse AL, Crossno PF, Xu XC, McConaha ME, Jones BR, Polosukhin VV, Bryant AJ, Cheng DS, Newcomb DC, McMahon FB, Gleaves LA, Blackwell TS, Lawson WE. β-Catenin in the alveolar epithelium protects from lung fibrosis after intratracheal bleomycin. Am J Respir Crit Care Med 187: 630–639, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verkaar F, Zaman GJR. New avenues to target Wnt/β-catenin signaling. Drug Discov Today 16: 35–41, 2011. [DOI] [PubMed] [Google Scholar]
- 39.Volckaert T, Dill E, Campbell A, Tiozzo C, Majka S, Bellusci S, De Langhe SP. Parabronchial smooth muscle constitutes an airway epithelial stem cell niche in the mouse lung after injury. J Clin Invest 121: 4409–4419, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wollin L, Maillet I, Quesniaux V, Holweg A, Ryffel B. Anti-fibrotic and anti-inflammatory activity of the tyrosine kinase inhibitor, nintedanib, in experimental models of lung fibrosis. J Pharmacol Exp Ther 349: 209–220, 2014. [DOI] [PubMed] [Google Scholar]
- 41.Woodcock HV, Molyneaux PL, Maher TM. Reducing lung function decline in patients with idiopathic pulmonary fibrosis: potential of nintedanib. Drug Des Devel Ther 7: 503–510, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woyda K, Koebrich S, Reiss I, Rudloff S, Pullamsetti SS, Rühlmann A, Weissmann N, Ghofrani HA, Günther A, Seeger W, Grimminger F, Morty RE, Schermuly RT. Inhibition of phosphodiesterase 4 enhances lung alveolarisation in neonatal mice exposed to hyperoxia. Eur Respir J 33: 861–870, 2009. [DOI] [PubMed] [Google Scholar]
- 43.Zhang X, Ibrahimi OA, Olsen SK, Umemori H, Mohammadi M, Ornitz DM. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J Biol Chem 281: 15694–15700, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]