Abstract
Alterations in extracellular matrix (ECM) have been implicated in the pathophysiology of pulmonary hypertension. Here, we have undertaken a compartment-specific study to elucidate the expression profile of collagens and their processing enzymes in donor and idiopathic pulmonary arterial hypertension (IPAH) pulmonary arteries. Predominant intimal, but also medial and perivascular, remodeling and reduced lumen diameter were detected in IPAH pulmonary arteries. Two-photon microscopy demonstrated accumulation of collagen fibers. Quantification of collagen in pulmonary arteries revealed collagen accumulation mainly in the intima of IPAH pulmonary arteries compared with donors. Laser capture-microdissected pulmonary artery profiles (intima+media and perivascular tissue) were analyzed by real-time PCR for ECM gene expression. In the intima+media of IPAH vessels, collagens (COL4A5, COL14A1, and COL18A1), matrix metalloproteinase (MMP) 19, and a disintegrin and metalloprotease (ADAM) 33 were higher expressed, whereas MMP10, ADAM17, TIMP1, and TIMP3 were less abundant. Localization of COLXVIII, its cleavage product endostatin, and MMP10, ADAM33, and TIMP1 was confirmed in pulmonary arteries by immunohistochemistry. ELISA for collagen XVIII/endostatin demonstrated significantly elevated plasma levels in IPAH patients compared with donors, whereas circulating MMP10, ADAM33, and TIMP1 levels were similar between the two groups. Endostatin levels were correlated with pulmonary arterial wedge pressure, and established prognostic markers of IPAH, right atrial pressure, cardiac index, 6-min walking distance, NH2-terminal pro-brain natriuretic peptide, and uric acid. Expression of unstudied collagens, MMPs, ADAMs, and TIMPs were found to be significantly altered in IPAH intima+media. Elevated levels of circulating collagen XVIII/endostatin are associated with markers of a poor prognosis.
Keywords: vascular remodeling, vascular fibrosis, laser microdissection, collagen XVIII, endostatin
idiopathic pulmonary arterial hypertension (IPAH) is a severe and fatal disease of unknown etiology. IPAH is characterized by vascular remodeling of all three layers of the intrapulmonary arterial wall, resulting in elevated pulmonary vascular resistance, right ventricular hypertrophy, and eventually right heart failure (49, 54). The reduction of the vessel lumen results from endothelial cell (intima), smooth muscle cell (media), and fibroblast (perivascular tissue) proliferation (58). Triggers for the vascular remodeling include injury, inflammation, abnormal growth factor expression, and/or exposure to hypoxia (53, 56). Furthermore, disturbances in extracellular matrix (ECM) production, deposition, and composition have been implicated (35, 43, 46, 62), and restoration of ECM balance has been suggested as a strategy to prevent or reverse vascular remodeling processes (8, 25).
The complex ECM network provides structural support for the cells in the vessel wall, enables intracellular communication, and promotes proliferation, migration, and apoptosis (61). The ECM in human pulmonary vessels predominantly consists of collagen I, elastins, and laminins as well as other ECM components such as fibronectin, tenascin C, and glucosaminoglycans (22). Turnover of the ECM is controlled by the balance of proteolytic enzymes such as a disintegrin and metalloproteases (ADAMs), matrix metalloproteinases (MMPs), and their endogenous inhibitors, tissue inhibitors of MMPs (TIMPs). In IPAH, accumulation of collagen (4), neutrophil-elastase (27), and MMPs and loss of their modulating counterparts TIMPs (31) leads to an imbalance of the ECM turnover and subsequently to a disturbance of the structural integrity of the vessel wall. Several studies using animal models of pulmonary hypertension (PH), namely monocrotaline (MCT) as well as hypoxia-induced PH, have implicated some vascular MMPs, collagens, elastin, and tenascin C in disease pathogenesis (6, 22, 43, 57). MMPs have been described to be elevated in lung homogenates, plasma, urine, and pulmonary artery smooth muscle cells from human PH samples (2, 31, 52). However, the differences in expression of factors responsible for ECM composition between pulmonary vessel walls of IPAH patients and healthy donors remain to be elucidated. Therefore, we hypothesized that delineating the changes in the expression and composition of the collagens and their processing enzymes in IPAH pulmonary arteries might lead to a better understanding of disease pathogenesis, earlier diagnosis, or more targeted and specific IPAH therapy.
Because intrapulmonary arteries represent only a minor portion of the total lung, compartment-specific gene expression can be masked (29). In the present study, we have overcome this limitation by utilizing a compartment-specific approach, segregating intima+media from perivascular tissue of pulmonary artery profiles by laser capture microdissection. Here, we reveal several so-far-unstudied collagens (especially collagen XVIII/endostatin), MMPs, ADAMs, as well as TIMPs as interesting new players in the pathophysiology of IPAH.
METHODS
Human lung tissue.
Human lung tissue samples were obtained from IPAH patients (n = 20) who underwent lung transplantation at the Department of Surgery, Division of Thoracic Surgery, Medical University of Vienna, Austria. The protocol and tissue usage were approved by the institutional ethics committee (976/2010), and patient consent was obtained before lung transplantation. Downsized nontumorous nontransplanted donor lungs served as controls (n = 22). For a detailed description on how the explanted lungs were sampled and stored, please refer to Ref. 20. Patient information including age, sex, mean pulmonary arterial pressure, pulmonary arterial wedge pressure (PAWP), right atrial pressure (RAP), cardiac output (CO), cardiac index (CI), 6-min walking distance (6MWD), NH2-terminal pro-brain natriuretic peptide (NT-proBNP), and uric acid are presented in Table 1 and 2.
Table 1.
Clinical characteristics of the donors and IPAH patients for quantification of vascular remodeling and real-time PCR
| Donors (n = 22) | IPAH (n = 20) | P Value | |
|---|---|---|---|
| Age, yr | 42 ± 15 | 30 ± 13 | 0.0076 |
| Sex, M/F | 13/22 | 4/20 | 0.0113 |
| HR, beats/min | 84 ± 9.7 | ||
| mPAP, mmHg | 79 ± 23 | ||
| PAWP, mmHg | 9.70 ± 3.74 | ||
| RA, mmHg | 6.72 ± 2.61 | ||
| CO, l/min | 3.25 ± 1.23 | ||
| CI, l·min−1·m−2 | 1.97 ± 0.72 | ||
| Po2, mmHg | 55.0 ± 27.8 | ||
| Pco2, mmHg | 34.4 ± 19.7 | ||
| PVR, dyn·s·cm−5 | 1710 ± 812 | ||
| 6MWD, m | 292 ± 168 | ||
| NT-proBNP, pg/ml | 3949 ± 2496 | ||
| CRP, mg/l | 0.39 ± 0.43 | ||
| Bilirubin, mg/dl | 1.87 ± 2.07 | ||
| Uric acid, mg/dl | 12.3 ± 9.1 |
IPAH, idiopathic pulmonary arterial hypertension; M, male; F, female; HR, heart rate; mPAP, mean pulmonary arterial pressure; PAWP, pulmonary arterial wedge pressure; RA, right atrial pressure; CO, cardiac output; CI, cardiac index; Po2, partial pressure of oxygen; Pco2, partial pressure of carbon dioxide; PVR, pulmonary vascular resistance; 6MWD, 6-min walking distance; NT-proBNP, NH2-terminal pro-brain natriuretic peptide; CRP, C-reactive protein.
Table 2.
Clinical characteristics of the donors and IPAH patients for ELISAs
| Donors (n = 40) | IPAH (n = 40) | P Value | |
|---|---|---|---|
| Age, yrs | 49 ± 2 | 52 ± 2 | 0.395 |
| Sex, M/F | 14/26 | 7/33 | 0.078 |
| HR, beats/min | 77.8 ± 13.8 | ||
| mPAP, mmHg | 49 ± 20 | ||
| PAWP, mmHg | 9.45 ± 3.73 | ||
| RA, mmHg | 6.49 ± 3.17 | ||
| CO, l/min | 4.32 ± 1.44 | ||
| CI, l·min−1·m−2 | 2.57 ± 1.14 | ||
| Po2, mmHg | 65.5 ± 11.6 | ||
| Pco2, mmHg | 35.5 ± 6.9 | ||
| PVR, dyn·s·cm−5 | 748 ± 589 | ||
| 6MWD, m | 351 ± 147 | ||
| NT-proBNP, pg/ml | 2780 ± 5117 | ||
| CRP, mg/l | 4.68 ± 6.68 | ||
| Bilirubin, mg/dl | 0.94 ± 0.81 | ||
| Uric acid, mg/dl | 7.73 ± 2.78 |
Quantification of vascular remodeling and collagen deposition.
Quantification of vascular remodeling and fibrosis was performed with Visiopharm integrated software VIS (Visiopharm, Hoersholm, Denmark) on Masson's trichrome-stained lung sections. For analyses of vascular remodeling and collagen quantification n = 20 donors and n = 17 IPAH were used. Ten of the 20 donors have been reported elsewhere (20). The patients' characteristics are reported in Table 1. To avoid selection bias, four random sections/blocks per patient representing different areas of the lung were taken for analysis. Four to 22 vessels per block were analyzed. For a detailed description on how analysis of vascular remodeling was performed, please refer to Ref. 20. Briefly, the diameter of the following four vessel wall components perivascular tissue, media, intima, and lumen (a); media, intima, and lumen (b); intima and lumen (c); and lumen (d) was calculated by using the following formula: diameter = 2×[]. The diameter of individual vessel wall layers was calculated as follows: perivascular tissue (a − b); media (b − c); intima (c − d); lumen (d). Vessels were subgrouped according to their size (diameter 20–200 and 200–500 μm) and presented as dots (patient mean), for both quantification of vascular remodeling and collagen deposition. Vessel size was defined by the external elastic lamina. Plexiform lesions were not included in the analysis. Vascular collagen was determined for each vessel by dividing the area of collagen (“blue”) by total analyzed compartment area (intima, media, or perivascular tissue) and compared with the vessel diameter.
Human plasma.
For ELISA analyses n = 40 donors and n = 40 IPAH plasma samples were investigated. The patients' characteristics are reported in Table 2. Blood samples were prospectively taken from an IPAH cohort of patients who were treated at the Medical University of Graz (Graz, Austria) when undergoing diagnostic or follow-up right heart catheterization as well as from the end-stage transplant patients from the Department of Surgery, Division of Thoracic Surgery, Medical University of Vienna, Austria. The samples from the healthy controls were prospectively collected and stored in the Biobank of the Medical University of Graz between 2011 and 2014. Written, informed consent was obtained from all subjects. The studies were approved by the Institutional Review Board of the Medical University of Graz (23-408 ex 10/11) as well as the Institutional Review Board of the Medical University of Vienna (976/2010) in accordance with national law.
Two-photon microscopy.
Paraformaldehyde-fixed lung samples of 1 cm3 were cleared by the 3DISCO clearing method (14). Briefly, samples were incubated in increasing concentrations of tetrahydrofuran (50, 70, and 80% and three times in 100% for 30 min each). After incubation for 20 min with dichloromethane samples were transferred in dibenzyl ether (all chemicals from Sigma-Aldrich, Munich, Germany). The cleared lung samples were placed in dibenzyl ether filled chamber of 1-cm height made of Sylgard polymer (Dow Corning, Wiesbaden, Germany) and covered with a cover glass. Imaging was done with a TriM Scope II two-photon microscope (LaVision BioTec, Bielefeld, Germany) equipped with a XLUMPLFL ×20 W/0.95 Objective (Olympus, Hamburg, Germany), two MaiTai DeepSee Lasers (Newport Spectra-Physics, Darmstadt, Germany), and an InSight DeepSee Laser (Newport Spectra-Physics). Sequential line-by-line scans with excitation wavelengths of 740, 860, and 1,100 nm were performed. Emitted light for each excitation wavelength was detected by four wavelength-separated photomultiplier tubes (<435, 453–495, 495–560, and >560 nm). Image processing was done with Imaris Software (Bitplane, Zurich, Switzerland).
Laser capture microdissection and RNA extraction.
The intima+media or perivascular tissue was laser capture microdissected by use of an Arcturus LCM System, and 100 vessel profiles (50–300 μm diameter) per patient were collected (plexiform lesions were not included). For more details please refer to Ref. 20.
Real-time PCR.
The PCR reactions were set up by using a QuantiFast SYBR PCR kit (Qiagen, Hilden, Germany). Cycling conditions were as follows: 5 min at 95°C, [5 s at 95°C, 5 s at 60°C, and 10 s at 72°C] × 45. Because of the nonselective double-strand DNA binding of the SYBRGreen I dye, melting curve analysis and gel electrophoresis were performed to confirm the specific amplification of the expected PCR product. For real-time PCR analyses, samples from n = 8 donors and n = 6–10 IPAH were investigated. Three of 10 utilized IPAH lungs have been previously reported (28). These patients' characteristics are reported in Table 1. The threshold cycle (Ct) values were normalized to internal control beta-2-macroglobulin (B2M) by using the following formula: ΔCt = Ct reference gene − Ct gene of interest. Primer sequences are provided in Table 3.
Table 3.
Sequences of primers applied in this study
| Gene | Accession Number | Forward Primer (5′-3′) | Reverse Primer (5′3′) | Product Length, base pairs |
|---|---|---|---|---|
| COL1A1 | NM_000088.3 | ACATGTTCAGCTTTGTGGACC | TGTACGCAGGTGATTGGTGG | 127 |
| COL1A2 | NM_000089.3 | GGCTCTGCGACACAAGGAGT | CGGCTGGGCCCTTTCTTACA | 147 |
| COL3A1 | NM_000090.3 | GGTGTCCCAGGGAAAGATGG | CTCTCTCACCAGGGCTACCA | 145 |
| COL4A5 | NM_000495.4 | GGCCCCAAGGTCCTCCT | CTCCTTTCAAACCAGGCAAGC | 156 |
| COL5A1 | NM_000093.4 | ATCTTCCAAAGGCCCGGATG | AAATGCAGACGCAGGGTACA | 91 |
| COL6A3 | NM_057164.4 | AGGTTTGCTCAGGGGTTCATA | AGCCGCACCATTTTTGACAT | 155 |
| COL9A2 | NM_001852.3 | ATATAAAGGCATGGTGGGCG | CCTGGGGTCCACGAATACC | 124 |
| COL11A1 | NM_001854.3 | TAGCACAGACGGAGGCAAAC | GCCTAGGAGCTTCTGTCTGG | 86 |
| COL14A1 | NM_021110.2 | GTCGGGACCACACTTGACAG | TACCCGCAGGCTAGAAGTAGT | 135 |
| COL18A1 | NM_030582.3 | TCAGTGCCACCACCATCTTCA | GCATCTGGCCCAAAGACGTAG | 136 |
| MMP1 | NM_002421.3 | AGTCCAGAAATACCTGGAAAAATAC | TTTTTCAACCACTGGGCCAC | 186 |
| MMP2 | NM_004530.4 | CCGCAGTGACGGAAAGATGT | GCCACGAGGAACAGGCTGTA | 102 |
| MMP7 | NM_002423.3 | GAACGCTGGACGGATGGTAG | CAGAGGAATGTCCCATACCCA | 93 |
| MMP10 | NM_002425.2 | TCCAGGAGTTGAGCCTAAGGT | CGCCTAGCAATGTAACCAGC | 148 |
| MMP19 | NM_002429.5 | GCCCGTGGACTACCTGTCAC | TGTGGCATCATCCAGCTGAC | 148 |
| ADAM10 | NM_001110.3 | TGCCCAGATATCCAGTCATGTTA | ACGGAAAGGATTTGTAGGGTCC | 145 |
| ADAM17 | NM_003183.4 | CGTTGGGTCTGTCCTGGTTT | GCATTTCGACGTTACTGGGG | 131 |
| ADAM33 | NM_025220.3 | TGCTTGAGCTGGAGAAGAACC | GTGGCAATGATCCGTGTGGT | 113 |
| ADAMTS1 | NM_006988.3 | AAAGCGGAGACCGAAGACGA | GCGGTGACTGGACACAAATC | 147 |
| ADAMTS13 | NM_139025.4 | AGTTACCCCCAACCTGACCA | CATCTTCCCAGCCACGACAT | 126 |
| TIMP1 | NM_003254.2 | GCCTTCTGCAATTCCGACCT | TTGGAACCCTTTATACATCTTGGT | 120 |
| TIMP2 | NM_003255.4 | ATGCAGATGTAGTGATCAGGGC | TCTATATCCTTCTCAGGCCCTTTG | 142 |
| TIMP3 | NM_000362.4 | CTCCGACATCGTGATCCGGG | TGGATGTACTGCACATGGGG | 138 |
| TIMP4 | NM_003256.3 | TTTGACTCTTCCCTCTGTGGTG | TCCTCCCAGGGCTCGATGTA | 128 |
| β2M | NM_004048.2 | TGGAGGCTATCCAGCGTACT | TGTCGGATGGATGAAACCCA | 110 |
Immunohistochemistry and staining.
Immunohistochemistry was performed on 2-μm-thick paraffin-embedded lung sections. Sections were deparaffinized in xylene for 3 × 10 min and rehydrated in 100% ethanol for 2 × 5 min, 95% ethanol for 1 × 5 min, 70% ethanol for 1 × 5 min, and PBS for 2 × 5 min. Antigen retrieval was performed with pH 6 citrate buffer. Only for MMP10 (1:300 dilution, Abcam, Cambridge, UK) a pH 9 buffer solution was utilized. ADAM33 (1:1,400 dilution, Abcam), COL18A1 (1:50 dilution, Santa Cruz Biotechnology, Dallas, TX), TIMP1 (1:50 dilution, Abcam), endostatin (1:300 dilution, Abcam), and MMP10 were stained by using the ImmPACT NovaRED Peroxidase (HRP) Substrate Kit (Vector Laboratories, Burlingame, CA). For α-SMA staining, ZytoChem Plus AP Polymer anti-rabbit (ZYTOMED Systems, Berlin, Germany) was used. For von Willebrand factor (vWF)/α-smooth muscle actin (α-SMA) double stainings, antigen retrieval was performed with 0.05% trypsin (Zymed Laboratories, Invitrogen, Carlsbad, CA) for 10 min at 37°C. Double immunohistochemistry staining against α-SMA (1:200 dilution, Everest Biotech, Upper Heyford, UK) and vWF (1:900 dilution, Dako, Glostrup, Denmark) was performed by using a ImmPRESS α-Goat Ig (peroxidase) Polymer Detection Kit together with the VECTOR VIP substrate kit and the ImmPRESS α-rabbit Ig (peroxidase) with 3,3′-diaminobenzidine (all from Vector Laboratories). Methyl green counterstaining was performed. Negative controls were performed omitting the primary antibody. Positive controls for each antibody are provided for tissues suggested by the manufacturers. Slides were scanned with an Aperio slide scanner microscope and images were captured with Image Scope software (Aperio, Vista). White balance was adjusted for all images.
ELISA.
For quantitative measurement of collagen XVIII/endostatin (R & D Systems, Minneapolis, MN), MMP10 (R & D Systems), ADAM33 (USCN, Wuhan, China), and TIMP1 (R & D Systems) in human plasma, ELISAs were performed according to the manufacturers' instructions.
Statistical analysis.
Statistical analysis was performed in GraphPad Prism 5 software. Results of vascular remodeling and collagen deposition are presented as individual patients and means as a line with SE. Data of all real-time PCR experiments are presented as individual patients and the median as a line. For comparison of donor and IPAH a nonparametric Mann-Whitney test was carried out. A P value lower than 0.05 with nonparametric Mann-Whitney test was considered significant. Significance is indicated by an asterisk on the graphs. Correlation analysis for endostatin and clinical parameters was performed by using a Spearman R test. Age correction was performed by analysis of covariance (ANCOVA).
RESULTS
Vascular remodeling in human IPAH pulmonary arteries.
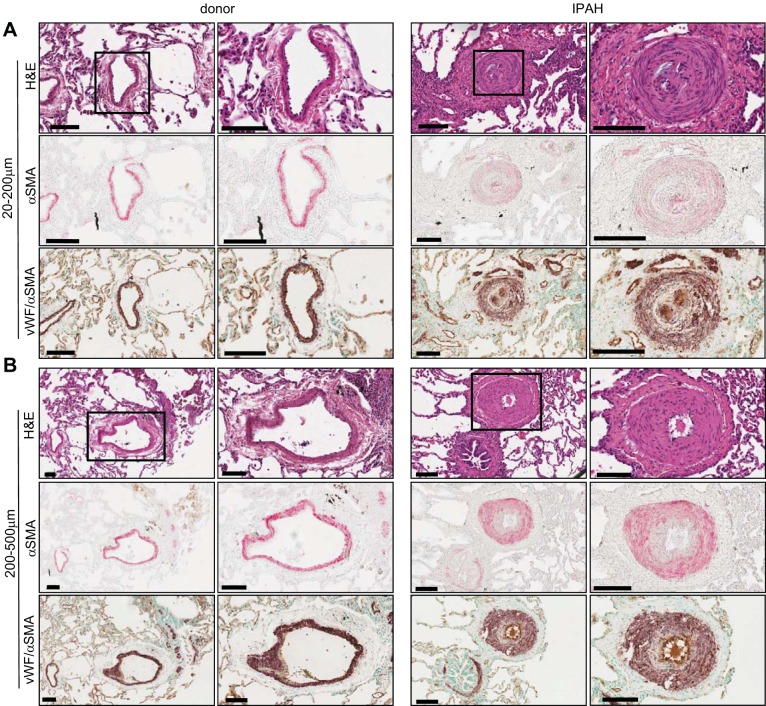
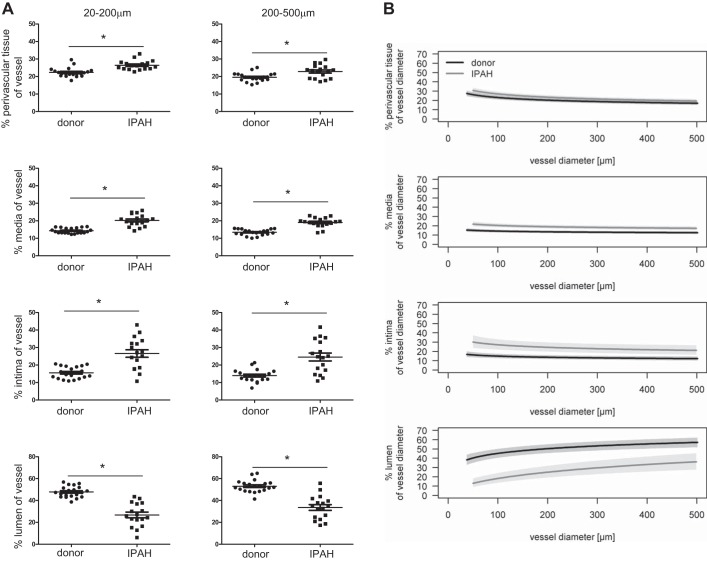
Clinically well-characterized IPAH explants and nontumorous donor lungs were included in this study. Representative hematoxylin and eosin, α-SMA (pink), and vWF/α-SMA stainings (purple/light brown) demonstrate healthy unremodeled pulmonary arteries of 20–200 μm and 200–500 μm diameter in donor lungs and remodeled arteries in IPAH lungs (Fig. 1). Plexiform lesions (PLs) adjacent to remodeled arteries were found in 11 of our 17 IPAH samples. Quantification of the remodeling present in pulmonary vessels (20–200 and 200–500 μm) of IPAH patients revealed a significant but modest thickening of perivascular tissue, higher involvement of media, and the most prominent thickening of intimal layer. This was concomitant with reduction in lumen diameter (Fig. 2A). A shift in the regression line demonstrates that remodeling of the vessel layers occurs in all vessel sizes (Fig. 2B).
Fig. 1.
Histological characterization of our idiopathic pulmonary arterial hypertension (IPAH) patient cohort. Representative images and zoom of vessels 20–200 μm (A) and 200–500 μm (B) in diameter from donors (left) and IPAH patients (right). Hematoxylin and eosin (H&E), α-smooth muscle actin (SMA, pink), double SMA (purple)/von Willebrand Factor (vWF, light brown) staining. Scale bar: 100 μm.
Fig. 2.
Vascular remodeling of pulmonary arteries of IPAH patients. A: mean percentage of perivascular tissue, media, intima and lumen for each patient presented for vessels 20–200 and 200–500 μm; n = 17–20 patients each, means with SE, *P < 0.05; each dot represents 16–88 vessel profiles. B: overlay of regression lines with 95% confidence bands showing the proportion of each respective compartment according to vessel diameter.
Vascular fibrosis in human IPAH pulmonary arteries.
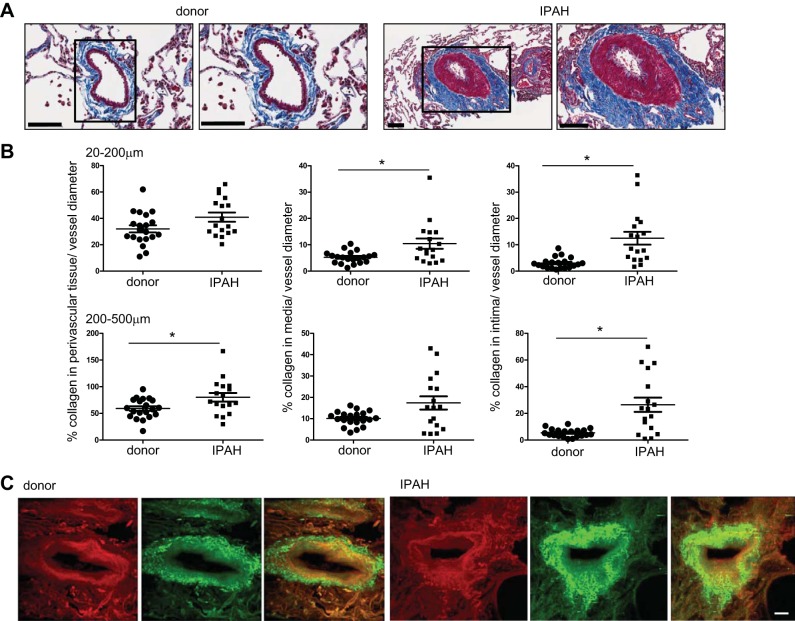
Representative images of Masson's trichrome stainings display collagen (blue) deposition in IPAH pulmonary arteries (Fig. 3A). Analysis of the total collagen (blue) within a vessel wall layer relative to vessel diameter revealed the highest collagen deposition in intima (in both vessel sizes) followed by media and perivascular tissue when comparing IPAH to donor pulmonary arteries (Fig. 3B). Visualization of total collagen by two-photon microscopy in cleared samples from IPAH and donor lungs showed presence of collagen fibers in the peri- as well as intravascular compartment on representative images (Fig. 3C).
Fig. 3.
Collagen deposition in IPAH pulmonary arteries. A: representative images and zoom of donor (left) and IPAH pulmonary artery (right) of Masson's trichrome staining (collagen: blue). Scale bar: 100 μm. B: quantification of percentage of absolute collagen as percentage of vessel diameter in perivascular tissue, media, and intima; n = 20 donors and n = 17 IPAH. Means with SE; *P < 0.05. One dot represents 16–88 vessel profiles. C: visualization of perivascular and vascular collagen by autofluorescence 2-photon microscopy. Maximum intensity projections of 1 mm2 × 80 μm cuboits. Left: donor. Right: IPAH. Each side shows connective tissue in red, channel 495–560 nm at 860-nm excitation wavelength (left); images of collagen in green, channel 495–560 nm at 1,100-nm excitation (middle); and overlay (right). Scale bar: 50 μm.
Compartment-specific expression of collagens in IPAH pulmonary arteries profiles.
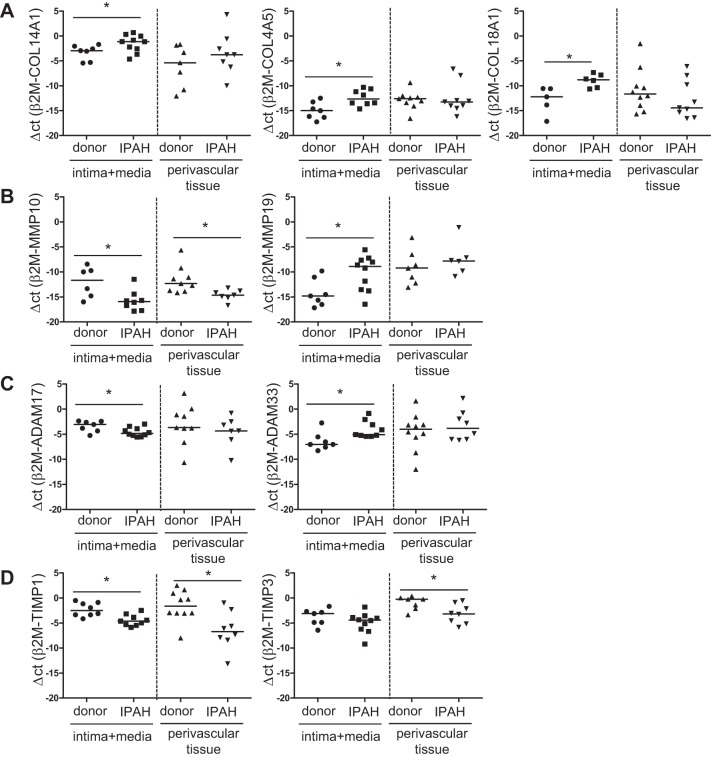
To identify the collagens that contribute to vascular fibrosis we performed compartment-specific real-time PCR analyses. Subsequently, broad-spectrum collagens expression analysis was performed. The fibril-forming collagens COL1A1, COL1A2, COL3A1, COL5A1, and COL11A1 were not differentially expressed in our IPAH cohort. Similarly, connecting collagen COL6A3 displayed no differential expression (data not shown). However, the fibril-associated collagen with interrupted triple helices (FACIT) COL14A1 showed significant upregulation in intima+media of IPAH (Fig. 4A) while another family member, COL9A2, was not regulated (data not shown). Both the network-forming collagen COL4A5 and the endostatin-producing collagen COL18A1 were significantly increased in vessel profiles of IPAH patients (Fig. 4A). Interestingly, all significant changes in collagen expression were only detected in the intimal/medial but not the perivascular tissue layer of pulmonary arteries from IPAH patients.
Fig. 4.
Expression of collagens, matrix metalloproteinases (MMPs), a disintegrin and metalloproteases (ADAMs), and tissue inhibitors of MMPs (TIMPs) in intima+media and perivascular tissue of donor and IPAH pulmonary arteries. Expression of significantly differentially regulated collagens (A), MMPs (B), ADAMs (C), and TIMPs (D); n = 6–10 each, median, *P < 0.05.
Compartment-specific expression of MMPs in IPAH pulmonary arteries profiles.
In addition to collagens, collagen-processing MMPs have been described to play a crucial role in ECM transformation in diseases (2, 6). MMP10 (stromelysin family member) revealed significantly decreased expression in intima+media as well as perivascular tissue (Fig. 4B), whereas MMP19 expression was significantly elevated only in intima+media of IPAH compared with donor vessels (Fig. 4B). Expression of other tested MMPs (MMP 1, 2, and 7) was not changed (data not shown).
Compartment-specific expression of ADAMs in IPAH pulmonary artery profiles.
The balance between collagen degradation and deposition is controlled not only by MMPs but also by ADAMs (11). When analyzing expression of ADAMs and ADAMs with thrombospondin motifs (ADAMTS), significant downregulation of ADAM17 and upregulation of ADAM33 (Fig. 4C) in IPAH intima+media was detected whereas no differential regulation was measured for ADAM10, ADAMTS1, and ADAMTS13 (data not shown).
Compartment-specific expression of TIMPs in IPAH pulmonary artery profiles.
MMPs and ADAMs are inhibited by TIMPs (36). From all analyzed TIMPs (TIMP1-4), TIMP1 showed downregulation in both analyzed compartments in pulmonary artery profiles from IPAH lungs (Fig. 4D). TIMP3 expression was significantly lower in perivascular tissue of IPAH patients (Fig. 4D). Both TIMP2 and TIMP4 were not differentially expressed in our samples (data not shown).
Tissue localization of collagen XVIII, endostatin, MMP10, ADAM33, and TIMP1.
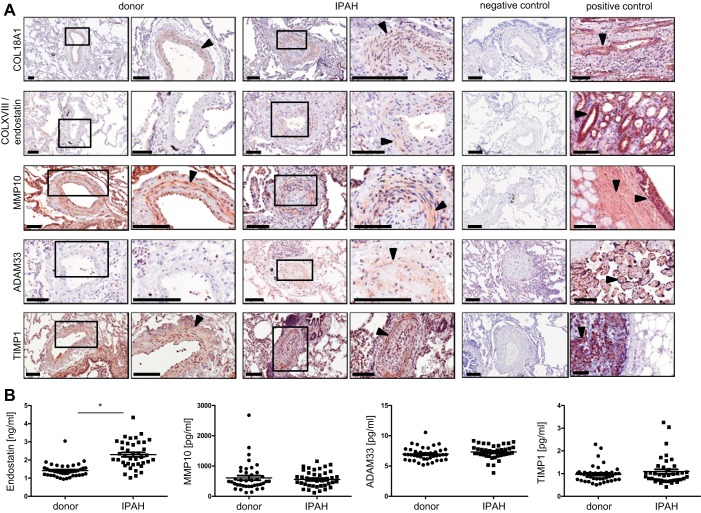
From the significantly regulated genes, localization within the pulmonary vasculature of one protein per group was determined by immunohistochemical stainings. Collagen XVIII (light brown) and its cleavage product endostatin (light brown dots), MMP10 (light brown), ADAM33 (light brown), and TIMP1 (light brown) were confirmed to be localized to intima or media of pulmonary arteries of donors and IPAH patients (Fig. 5A). To illustrate antibody specificities, respective negative and positive controls are provided.
Fig. 5.
Localization of collagens and collagen-processing enzymes in pulmonary vessels and plasma levels in donors and IPAH patients. A: immunohistochemical staining of COL18A1, COLXVIII/endostatin, MMP10, ADAM33, TIMP1. Right: donors. Left: IPAH. Black arrowheads point toward positive staining (light brown). Negative and positive controls (COL18A1: human kidney, COLXVIII/endostatin: human kidney, MMP10: human trachea, ADAM33: human placenta, TIMP1: human breast carcinoma) are provided. Scale bar: 100 μm. B: detection of endostatin, MMP10, ADAM33, and TIMP1 in donor and IPAH plasma by ELISA, n = 40 each, *P < 0.05.
Plasma concentrations of collagen XVIII/endostatin, MMP10, ADAM33, and TIMP1.
To determine potential prognostic relevance of regulated collagens and collagen-processing enzymes, circulating levels of soluble collagen XVIII/endostatin, MMP10, ADAM33, and TIMP1 were compared in samples from donors and IPAH patients (n = 40 each, Table 2). Although MMP10, ADAM33, and TIMP10 showed similar levels in donor and IPAH plasma samples, the soluble product of collagen XVIII, endostatin, was significantly elevated in IPAH plasma (Fig. 5B).
Endostatin levels correlated with clinical parameters in IPAH patients.
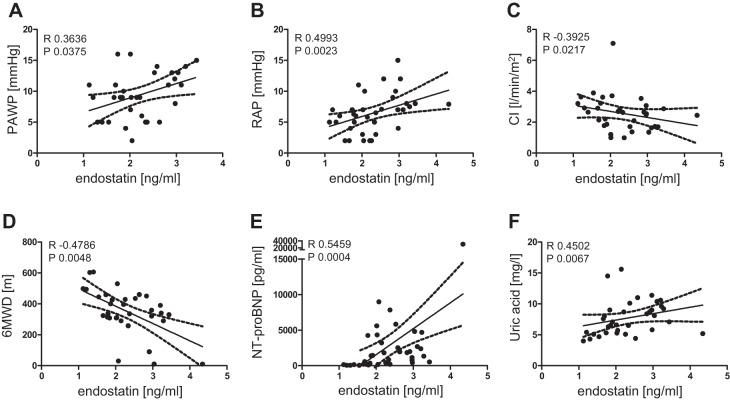
Because endostatin levels were elevated in the plasma of our IPAH patient cohort, correlation analyses were performed with the corresponding clinical patient data. To exclude a possible age dependency of endostatin levels, data were recalculated with ANCOVA. After age correction, endostatin was still significantly elevated. In IPAH patients, endostatin levels significantly positively correlated with PAWP (A), RAP (B), NT-proBNP (E), and uric acid (F). Significant negative correlations of endostatin levels were found with CI (C) and 6MWD (D) (Fig. 6 and Table 4).
Fig. 6.
Correlation of endostatin levels with clinical parameters of IPAH patients. Significant positive correlation of endostatin level and pulmonary arterial wedge pressure (PAWP; A), right atrial pressure (RAP; B), NH2-terminal pro-brain natriuretic peptide (NT-proBNP; E), and uric acid (F) and negative correlation with cardiac index (CI; C) and 6-min walking distance (6MWD; D). R, Spearman R; P, significance level.
Table 4.
Correlation of endostatin levels with clinical parameters of IPAH patients
| Clinical Parameters | R | P |
|---|---|---|
| Age | 0.29 | 0.07 |
| HR | 0.04 | 0.82 |
| mPAP | 0.10 | 0.55 |
| PAWP | 0.36 | 0.04 |
| RAP | 0.50 | 0.002 |
| CO | −0.24 | 0.17 |
| CI | −0.39 | 0.02 |
| Po2 | 0.01 | 0.95 |
| Pco2 | −0.02 | 0.92 |
| PVR | 0.14 | 0.47 |
| 6MWD | −0.48 | 0.005 |
| NT-proBNP | 0.55 | 0.0004 |
| CRP | 0.21 | 0.20 |
| Bilirubin | 0.12 | 0.48 |
| Uric acid | 0.45 | 0.01 |
DISCUSSION
Previous studies have implicated that a disturbed balance of ECM components can regulate vascular remodeling during the pathogenesis of PH (35, 46). Increased deposition of collagens in the medial layer of pulmonary arteries leads to thickening of the vessel wall and increases arterial stiffening (12). In diseases such as IPAH, local changes in ECM composition not only lead to vascular remodeling but also guide cellular behavior (35). The expression levels of collagens, MMPs, ADAMs, and TIMPs determine ECM turnover in health and disease and could therefore provide novel insights into ECM-related IPAH pathophysiology.
PLs are hallmark of IPAH. PLs result from a disorganized growth of endothelial cells (7, 59). Alternatively, the low expression of angiogenic-associated markers in the arteries from which PLs arise suggested rather a regenerative process (23). Since no correlations were reported between the average number of PL profiles and intima and media thickness (56), we have solely concentrated on the analysis of remodeled vessels. Similar to others, we showed that the most prominent remodeling occurred in the intima, followed by media, with weak involvement of the perivascular tissue (adventitia) (42, 56). Quantitative analysis demonstrated different degrees of collagen deposition with the highest accumulation in the intimal layer of IPAH pulmonary arteries. Therefore, we analyzed expression of ECM components in the intima+media and perivascular tissue separately.
To date most studies investigating the involvement of ECM in PH have focused on single MMPs or the classical collagens such as collagen I and III, while neglecting the other members of the collagen and collagen-processing superfamily. Exemplary, active MMP2 has been shown to promote the proliferation and migration of smooth muscle cells (60) and thereby contribute to media thickening in pulmonary arteries of IPAH patients (31). Modulation of MMPs in vascular cells has been suggested to orchestrate the pathological events involved in the development of vascular lesions in PAH (34).
Collagens represent the major component of the “interstitial” matrix as well as the basement membranes (BM). They not only provide structural support to the vessel wall but also contribute to the entrapment, local storage and delivery of growth factors and cytokines and, by release of noncollagenous fragments, influence angiogenesis (15). Fibril-forming collagens assemble to build a microfibril and, when stabilized by cross-linking, provide structure and strength to the vessel wall. The structural integrity of the vessel wall is further strengthened by FACITs, which facilitate the interactions of adjacent collagen fibrils such as collagen I (24). Interestingly, the fibril-forming collagens (COL1A1, COL1A2, COL3A1, COL5A1, and COL11A1) showed no differences in mRNA expression in our samples. This is in accordance with other studies that indicated accumulation of type 1 collagen mainly in the early time points of hypoxia-induced PH in mice (3). Since our study was performed on pulmonary arteries of end-stage transplant lungs, expression of classic fibril-forming collagens might not be increased at this stage. On the other hand, the fibril-associated collagen COL14A1 was significantly elevated in IPAH intima+media, indicating increased vessel stiffness by stabilizing of existing collagen fibers.
Furthermore, the BM collagen COL4A5 was upregulated in the intima+media in our IPAH patient cohort. This is in line with previous studies that suggested BM components to be involved in the pathogenesis of PH. In healthy subjects, the BM is a thin sheet of fibers underlying the endothelial cell layer, formed by specific interactions between ECM components, such as laminins, type IV collagens, proteoglycans, glycoproteins, and different integrin and nonintegrin receptors (13), and provides structural and organizational stability. In IPAH, BM can be thickened and leaky for larger proteins (such as albumin and lipoproteins) and inflammatory cells (17), which secrete mediators such as interleukin 6, being involved in remodeling (44). Col4A5 is degraded by MMP10, which was lower expressed in intima+media and perivascular tissue of IPAH pulmonary arteries. MMP10 has been described to be angiogenic (19) and profibrinolytic (40) and its substrates include not only COLIV but also laminin, fibronectin, and elastin (39). The observed spatially coordinated changes in expression of MMP10 and its substrate COLIV suggest the presence of upstream common regulator(s) driving the balance toward collagen accumulation.
Contrary to MMP10, MMP19 displayed a significantly higher expression in intima+media of IPAH lung vessels. In line with our findings, MMP-19 has been described to be elevated in samples from idiopathic pulmonary fibrosis patients (63). MMP19 is inhibited strongly by TIMP3 and less efficiently by TIMP1. Both TIMP1 and TIMP3 were downregulated in our analysis, pointing toward activation of preexisting collagenases, which could contribute to the endothelial cell invasion through the BM and interstitial matrix (30). Among all TIMPs, TIMP3 has been identified to play a critical role in vascular remodeling as well as collagen and well as elastin deposition in response to angiotensin II-induced hypertension (1).
Additionally, we investigated the expression of ADAMs, which are proteinases closely related to MMPs. They display a wide spectrum of biological effects such cell adhesion, “shedding process,” cleavage of various substrates from the extracellular matrix, growth factors, or cytokines (41). ADAM33 has been reported to increase vascular smooth muscle migration through the BM (21), and its metalloprotease domain has been shown to exert a proangiogenic function (45). Therefore, the upregulation of ADAM33 in intima+media and its localization to the smooth muscle cell layer suggests that ADAM33 could be involved in the formation of the neointima.
Remodeling of the BM is further supported by increased expression of COL18A1 in the intima+media of IPAH pulmonary artery profiles. COL18A1 is classified as an endostatin-producing collagen and, similar to COL4A5, localizes predominantly to the BM (50). Proteolytic cleavage within the COOH-terminal domain of COL XVIII releases endostatin (5), a fragment that has been reported to exhibit antiangiogenic effects (33). In vitro immobilized endostatin supports endothelial cell survival and migration, whereas soluble endostatin inhibits endothelial cell functions, such as cell migration in an integrin-dependent fashion (47). Furthermore, in vivo soluble endostatin induces endothelial cell apoptosis (10) and inhibits vascular morphogenesis and perivascular cell recruitment (55). In preterm infants with PH due to bronchopulmonary dysplasia, an increased serum endostatin-to-angiopoietin-1 ratio was suggested to reflect impaired angiogenesis (26).
Circulating markers of collagen metabolism such as NH2-terminal propeptide of type III procollagen have been shown to serve as indicator of disease severity in IPAH (51). Also, higher levels of circulating TIMP1 have been reported in PAH (51), which could not be confirmed in our patient cohort. In general, these studies together with our data imply a possibility to use circulating levels of collagen cleavage products and collagen-processing enzymes for the detection of IPAH. The significant correlation of endostatin concentration with established prognostic markers such as RAP, CI, NT-proBNP, 6MWD, and uric acid in our IPAH patient cohort implies that endostatin itself could be a prognostic marker and predict heart failure in severe IPAH. This is supported by the association of circulating endostatin with mortality in patients with PAH (9) and chronic heart failure (16). In addition to its potential as a prognostic tool, therapeutic applicability of endostatin has also been demonstrated (48).
Here, we have described compartment-specific expression of different collagens, MMPs, ADAMs, and TIMPs in pulmonary arteries of IPAH patients. Vascular collagens, which have been described to be localized to the BM, showed elevated expression in intima+media, suggesting importance of this compartment in the pathophysiology of IPAH. The imbalance of ECM components leads to vascular leakage (18, 32, 38), enabling infiltration of inflammatory and structural cells and to release of growth factors (37), further aggravating cell proliferation and remodeling in this compartment. Particularly, COL XVIII and its cleavage product endostatin might contribute to the development of IPAH and serve as a marker for disease progression.
However, limitations of this study include incomplete collagen profile, the usage of end-stage lungs, and the descriptive nature of this article. Further studies are needed to identify the exact mechanisms and potential of dysregulated collagens and their processing products as possible biomarkers or targets for therapy.
GRANTS
The study was funded by the Ludwig Boltzmann Institute for Lung Vascular Research, Graz, Austria and by the Jubilee Foundation of the Austrian National Bank [Grants 16187 (G. Kwapiszewska)].
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.H., B.G., H.O., A.O., and G. Kwapiszewska conception and design of research; J.H., L.M.M., M.P., E.S., and G. Kwapiszewska performed experiments; J.H., L.M.M., M.P., E.S., G. Kovacs, and G. Kwapiszewska analyzed data; J.H., L.M.M., E.S., B.G., G. Kovacs, H.O., A.O., and G. Kwapiszewska interpreted results of experiments; J.H., L.M.M., and G. Kwapiszewska prepared figures; J.H., P.K., A.O., and G. Kwapiszewska drafted manuscript; J.H., L.M.M., E.S., B.G., G. Kovacs, P.K., H.W., H.M.H., G.H., W.K., H.O., A.O., and G. Kwapiszewska edited and revised manuscript; J.H., L.M.M., M.P., E.S., B.G., G. Kovacs, P.K., H.W., H.M.H., G.H., W.K., H.O., A.O., and G. Kwapiszewska approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Julia Schittl and Sabrina Reinisch for excellent technical support and Alexander Avian for statistical help. The plasma samples were stored and kindly provided by the Biobank of the Medical University of Graz. L. M. March, H. M. Haitchi, and G. Kwapiszewska are part of the European Cooperation in Science and Technology Action BM1201, Developmental Origins of Chronic Lung Disease.
REFERENCES
- 1.Basu R, Fan D, Kandalam V, Lee J, Das SK, Wang X, Baldwin TA, Oudit GY, Kassiri Z. Loss of Timp3 gene leads to abdominal aortic aneurysm formation in response to angiotensin II. J Biol Chem 287: 44083–44096, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benisty JI, Folkman J, Zurakowski D, Louis G, Rich S, Langleben D, Moses MA. Matrix metalloproteinases in the urine of patients with pulmonary arterial hypertension. Chest 128: 572S, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Berg JT, Breen EC, Fu Z, Mathieu-Costello O, West JB. Alveolar hypoxia increases gene expression of extracellular matrix proteins and platelet-derived growth factor-B in lung parenchyma. Am J Respir Crit Care Med 158: 1920–1928, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Botney MD, Liptay MJ, Kaiser LR, Cooper JD, Parks WC, Mecham RP. Active collagen synthesis by pulmonary arteries in human primary pulmonary hypertension. Am J Pathol 143: 121–129, 1993. [PMC free article] [PubMed] [Google Scholar]
- 5.Chang JH, Javier JA, Chang GY, Oliveira HB, Azar DT. Functional characterization of neostatins, the MMP-derived, enzymatic cleavage products of type XVIII collagen. FEBS Lett 579: 3601–3606, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Chelladurai P, Seeger W, Pullamsetti SS. Matrix metalloproteinases and their inhibitors in pulmonary hypertension. Eur Respir J 40: 766–782, 2012. [DOI] [PubMed] [Google Scholar]
- 7.Cool CD, Stewart JS, Werahera P, Miller GJ, Williams RL, Voelkel NF, Tuder RM. Three-dimensional reconstruction of pulmonary arteries in plexiform pulmonary hypertension using cell-specific markers. Evidence for a dynamic and heterogeneous process of pulmonary endothelial cell growth. Am J Pathol 155: 411–419, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowan KN, Heilbut A, Humpl T, Lam C, Ito S, Rabinovitch M. Complete reversal of fatal pulmonary hypertension in rats by a serine elastase inhibitor. Nat Med 6: 698–702, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Damico R, Kolb TM, Valera L, Wang L, Housten T, Tedford RJ, Kass DA, Rafaels N, Gao L, Barnes KC, Benza RL, Rand JL, Hamid R, Loyd JE, Robbins IM, Hemnes AR, Chung WK, Austin ED, Drummond MB, Mathai SC, Hassoun PM. Serum endostatin is a genetically determined predictor of survival in pulmonary arterial hypertension. Am J Respir Crit Care Med 191: 208–218, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhanabal M, Ramchandran R, Waterman MJ, Lu H, Knebelmann B, Segal M, Sukhatme VP. Endostatin induces endothelial cell apoptosis. J Biol Chem 274: 11721–11726, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Dijkstra A, Postma DS, Noordhoek JA, Lodewijk ME, Kauffman HF, ten Hacken NH, Timens W. Expression of ADAMs (“a disintegrin and metalloprotease”) in the human lung. Virchows Arch 454: 441–449, 2009. [DOI] [PubMed] [Google Scholar]
- 12.Dodson RB, Morgan M, Galambos C, Hunter KS, Abman SH. Chronic intrauterine pulmonary hypertension increases main pulmonary artery stiffness and adventitial remodeling in fetal sheep. Am J Physiol Lung Cell Mol Physiol 307: L822–L828, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erickson AC, Couchman JR. Still more complexity in mammalian basement membranes. J Histochem Cytochem 48: 1291–1306, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Erturk A, Becker K, Jahrling N, Mauch CP, Hojer CD, Egen JG, Hellal F, Bradke F, Sheng M, Dodt HU. Three-dimensional imaging of solvent-cleared organs using 3DISCO. Nat Protoc 7: 1983–1995, 2012. [DOI] [PubMed] [Google Scholar]
- 15.Gelse K, Poschl E, Aigner T. Collagens—structure, function, biosynthesis. Adv Drug Deliv Rev 55: 1531–1546, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Gouya G, Siller-Matula JM, Fritzer-Szekeres M, Neuhold S, Storka A, Neuhofer LM, Clodi M, Hulsmann M, Pacher R, Wolzt M. Association of endostatin with mortality in patients with chronic heart failure. Eur J Clin Invest 44: 125–135, 2014. [DOI] [PubMed] [Google Scholar]
- 17.Hayden MR, Sowers JR, Tyagi SC. The central role of vascular extracellular matrix and basement membrane remodeling in metabolic syndrome and type 2 diabetes: the matrix preloaded. Cardiovasc Diabetol 4: 9, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hendel A, Hsu I, Granville DJ. Granzyme B releases vascular endothelial growth factor from extracellular matrix and induces vascular permeability. Lab Invest 94: 716–725, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heo SH, Choi YJ, Ryoo HM, Cho JY. Expression profiling of ETS and MMP factors in VEGF-activated endothelial cells: role of MMP-10 in VEGF-induced angiogenesis. J Cell Physiol 224: 734–742, 2010. [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann J, Wilhelm J, Marsh LM, Ghanim B, Klepetko W, Kovacs G, Olschewski H, Olschewski A, Kwapiszewska G. Distinct differences in gene expression patterns in pulmonary arteries of patients with chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis with pulmonary hypertension. Am J Respir Crit Care Med 190: 98–111, 2014. [DOI] [PubMed] [Google Scholar]
- 21.Holloway JW, Laxton RC, Rose-Zerilli MJ, Holloway JA, Andrews AL, Riaz Z, Wilson SJ, Simpson IA, Ye S. ADAM33 expression in atherosclerotic lesions and relationship of ADAM33 gene variation with atherosclerosis. Atherosclerosis 211: 224–230, 2010. [DOI] [PubMed] [Google Scholar]
- 22.Jones PL, Cowan KN, Rabinovitch M. Tenascin-C, proliferation and subendothelial fibronectin in progressive pulmonary vascular disease. Am J Pathol 150: 1349–1360, 1997. [PMC free article] [PubMed] [Google Scholar]
- 23.Jonigk D, Golpon H, Bockmeyer CL, Maegel L, Hoeper MM, Gottlieb J, Nickel N, Hussein K, Maus U, Lehmann U, Janciauskiene S, Welte T, Haverich A, Rische J, Kreipe H, Laenger F. Plexiform lesions in pulmonary arterial hypertension composition, architecture, and microenvironment. Am J Pathol 179: 167–179, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kadler KE, Baldock C, Bella J, Boot-Handford RP. Collagens at a glance. J Cell Sci 120: 1955–1958, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Kerr JS, Ruppert CL, Tozzi CA, Neubauer JA, Frankel HM, Yu SY, Riley DJ. Reduction of chronic hypoxic pulmonary hypertension in the rat by an inhibitor of collagen production. Am Rev Respir Dis 135: 300–306, 1987. [DOI] [PubMed] [Google Scholar]
- 26.Kim DH, Kim HS. Serial changes of serum endostatin and angiopoietin-1 levels in preterm infants with severe bronchopulmonary dysplasia and subsequent pulmonary artery hypertension. Neonatology 106: 55–61, 2014. [DOI] [PubMed] [Google Scholar]
- 27.Kim YM, Haghighat L, Spiekerkoetter E, Sawada H, Alvira CM, Wang L, Acharya S, Rodriguez-Colon G, Orton A, Zhao M, Rabinovitch M. Neutrophil elastase is produced by pulmonary artery smooth muscle cells and is linked to neointimal lesions. Am J Pathol 179: 1560–1572, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwapiszewska G, Markart P, Dahal BK, Kojonazarov B, Marsh LM, Schermuly RT, Taube C, Meinhardt A, Ghofrani HA, Steinhoff M, Seeger W, Preissner KT, Olschewski A, Weissmann N, Wygrecka M. PAR-2 inhibition reverses experimental pulmonary hypertension. Circ Res 110: 1179–1191, 2012. [DOI] [PubMed] [Google Scholar]
- 29.Kwapiszewska G, Wilhelm J, Wolff S, Laumanns I, Koenig IR, Ziegler A, Seeger W, Bohle RM, Weissmann N, Fink L. Expression profiling of laser-microdissected intrapulmonary arteries in hypoxia-induced pulmonary hypertension. Respir Res 6: 109, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamoreaux WJ, Fitzgerald ME, Reiner A, Hasty KA, Charles ST. Vascular endothelial growth factor increases release of gelatinase A and decreases release of tissue inhibitor of metalloproteinases by microvascular endothelial cells in vitro. Microvasc Res 55: 29–42, 1998. [DOI] [PubMed] [Google Scholar]
- 31.Lepetit H, Eddahibi S, Fadel E, Frisdal E, Munaut C, Noel A, Humbert M, Adnot S, D'Ortho MP, Lafuma C. Smooth muscle cell matrix metalloproteinases in idiopathic pulmonary arterial hypertension. Eur Respir J 25: 834–842, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Mammoto T, Jiang E, Jiang A, Lu Y, Juan AM, Chen J, Mammoto A. Twist1 controls lung vascular permeability and endotoxin-induced pulmonary edema by altering Tie2 expression. PloS One 8: e73407, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marneros AG, Olsen BR. Physiological role of collagen XVIII and endostatin. FASEB J 19: 716–728, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Matsui K, Takano Y, Yu ZX, Hi JE, Stetler-Stevenson WG, Travis WD, Ferrans VJ. Immunohistochemical study of endothelin-1 and matrix metalloproteinases in plexogenic pulmonary arteriopathy. Pathol Res Pract 198: 403–412, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Morrell NW, Adnot S, Archer SL, Dupuis J, Jones PL, MacLean MR, McMurtry IF, Stenmark KR, Thistlethwaite PA, Weissmann N, Yuan JX, Weir EK. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol 54: S20–S31, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murphy G. Tissue inhibitors of metalloproteinases. Genome Biol 12: 233, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nave AH, Mizikova I, Niess G, Steenbock H, Reichenberger F, Talavera ML, Veit F, Herold S, Mayer K, Vadasz I, Weissmann N, Seeger W, Brinckmann J, Morty RE. Lysyl oxidases play a causal role in vascular remodeling in clinical and experimental pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol 34: 1446–1458, 2014. [DOI] [PubMed] [Google Scholar]
- 38.Nichols WW. Clinical measurement of arterial stiffness obtained from noninvasive pressure waveforms. Am J Hypertens 18: 3S–10S, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Nicholson R, Murphy G, Breathnach R. Human and rat malignant-tumor-associated mRNAs encode stromelysin-like metalloproteinases. Biochemistry 28: 5195–5203, 1989. [DOI] [PubMed] [Google Scholar]
- 40.Orbe J, Barrenetxe J, Rodriguez JA, Vivien D, Orset C, Parks WC, Birkland TP, Serrano R, Purroy A, Martinez de Lizarrondo S, Angles-Cano E, Paramo JA. Matrix metalloproteinase-10 effectively reduces infarct size in experimental stroke by enhancing fibrinolysis via a thrombin-activatable fibrinolysis inhibitor-mediated mechanism. Circulation 124: 2909–2919, 2011. [DOI] [PubMed] [Google Scholar]
- 41.Paulissen G, Rocks N, Gueders MM, Crahay C, Quesada-Calvo F, Bekaert S, Hacha J, El Hour M, Foidart JM, Noel A, Cataldo DD. Role of ADAM and ADAMTS metalloproteinases in airway diseases. Respir Res 10: 127, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pietra GG, Edwards WD, Kay JM, Rich S, Kernis J, Schloo B, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, GOldring RM, Groves BM, Levy PS, Reid LM, Vreim CE, Williams GW. Histopathology of primary pulmonary hypertension. A qualitative and quantitative study of pulmonary blood vessels from 58 patients in the National Heart, Lung, and Blood Institute, Primary Pulmonary Hypertension Registry. Circulation 80: 1198–1206, 1989. [DOI] [PubMed] [Google Scholar]
- 43.Poiani GJ, Tozzi CA, Yohn SE, Pierce RA, Belsky SA, Berg RA, Yu SY, Deak SB, Riley DJ. Collagen and elastin metabolism in hypertensive pulmonary arteries of rats. Circ Res 66: 968–978, 1990. [DOI] [PubMed] [Google Scholar]
- 44.Price LC, Wort SJ, Perros F, Dorfmuller P, Huertas A, Montani D, Cohen-Kaminsky S, Humbert M. Inflammation in pulmonary arterial hypertension. Chest 141: 210–221, 2012. [DOI] [PubMed] [Google Scholar]
- 45.Puxeddu I, Pang YY, Harvey A, Haitchi HM, Nicholas B, Yoshisue H, Ribatti D, Clough G, Powell RM, Murphy G, Hanley NA, Wilson DI, Howarth PH, Holgate ST, Davies DE. The soluble form of a disintegrin and metalloprotease 33 promotes angiogenesis: implications for airway remodeling in asthma. J Allergy Clin Immunol 121: 1400–1406, 1406.e1–e4, 2008. [DOI] [PubMed] [Google Scholar]
- 46.Rabinovitch M. Pathobiology of pulmonary hypertension. Extracellular matrix. Clin Chest Med 22: 433–449, viii, 2001. [DOI] [PubMed] [Google Scholar]
- 47.Rehn M, Veikkola T, Kukk-Valdre E, Nakamura H, Ilmonen M, Lombardo C, Pihlajaniemi T, Alitalo K, Vuori K. Interaction of endostatin with integrins implicated in angiogenesis. Proc Natl Acad Sci USA 98: 1024–1029, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rong B, Yang S, Li W, Zhang W, Ming Z. Systematic review and meta-analysis of Endostar (rh-endostatin) combined with chemotherapy versus chemotherapy alone for treating advanced non-small cell lung cancer. World J Surg Oncol 10: 170, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rubin LJ. Primary pulmonary hypertension. N Engl J Med 336: 111–117, 1997. [DOI] [PubMed] [Google Scholar]
- 50.Saarela J, Rehn M, Oikarinen A, Autio-Harmainen H, Pihlajaniemi T. The short and long forms of type XVIII collagen show clear tissue specificities in their expression and location in basement membrane zones in humans. Am J Pathol 153: 611–626, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Safdar Z, Tamez E, Chan W, Arya B, Ge Y, Deswal A, Bozkurt B, Frost A, Entman M. Circulating collagen biomarkers as indicators of disease severity in pulmonary arterial hypertension. JACC Heart Fail 2: 412–421, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schumann C, Lepper PM, Frank H, Schneiderbauer R, Wibmer T, Kropf C, Stoiber KM, Rudiger S, Kruska L, Krahn T, Kramer F. Circulating biomarkers of tissue remodelling in pulmonary hypertension. Biomarkers 15: 523–532, 2010. [DOI] [PubMed] [Google Scholar]
- 53.Shimoda LA, Laurie SS. Vascular remodeling in pulmonary hypertension. J Mol Med 91: 297–309, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Krishna Kumar R, Landzberg M, Machado RF, Olschewski H, Robbins IM, Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 62: D34–D41, 2013. [DOI] [PubMed] [Google Scholar]
- 55.Skovseth DK, Veuger MJ, Sorensen DR, De Angelis PM, Haraldsen G. Endostatin dramatically inhibits endothelial cell migration, vascular morphogenesis, and perivascular cell recruitment in vivo. Blood 105: 1044–1051, 2005. [DOI] [PubMed] [Google Scholar]
- 56.Stacher E, Graham BB, Hunt JM, Gandjeva A, Groshong SD, McLaughlin VV, Jessup M, Grizzle WE, Aldred MA, Cool CD, Tuder RM. Modern age pathology of pulmonary arterial hypertension. Am J Respir Crit Care Med 186: 261–272, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Todorovich-Hunter L, Dodo H, Ye C, McCready L, Keeley FW, Rabinovitch M. Increased pulmonary artery elastolytic activity in adult rats with monocrotaline-induced progressive hypertensive pulmonary vascular disease compared with infant rats with nonprogressive disease. Am Rev Respir Dis 146: 213–223, 1992. [DOI] [PubMed] [Google Scholar]
- 58.Tuder RM, Archer SL, Dorfmuller P, Erzurum SC, Guignabert C, Michelakis E, Rabinovitch M, Schermuly R, Stenmark KR, Morrell NW. Relevant issues in the pathology and pathobiology of pulmonary hypertension. J Am Coll Cardiol 62: D4–D12, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tuder RM, Chacon M, Alger L, Wang J, Taraseviciene-Stewart L, Kasahara Y, Cool CD, Bishop AE, Geraci M, Semenza GL, Yacoub M, Polak JM, Voelkel NF. Expression of angiogenesis-related molecules in plexiform lesions in severe pulmonary hypertension: evidence for a process of disordered angiogenesis. J Pathol 195: 367–374, 2001. [DOI] [PubMed] [Google Scholar]
- 60.Uzui H, Lee JD, Shimizu H, Tsutani H, Ueda T. The role of protein-tyrosine phosphorylation and gelatinase production in the migration and proliferation of smooth muscle cells. Atherosclerosis 149: 51–59, 2000. [DOI] [PubMed] [Google Scholar]
- 61.Wagenseil JE, Mecham RP. Vascular extracellular matrix and arterial mechanics. Physiol Rev 89: 957–989, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Z, Lakes RS, Eickhoff JC, Chesler NC. Effects of collagen deposition on passive and active mechanical properties of large pulmonary arteries in hypoxic pulmonary hypertension. Biomech Model Mechanobiol 12: 1115–1125, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu G, Kovkarova-Naumovski E, Jara P, Parwani A, Kass D, Ruiz V, Lopez-Otin C, Rosas IO, Gibson KF, Cabrera S, Ramirez R, Yousem SA, Richards TJ, Chensny LJ, Selman M, Kaminski N, Pardo A. Matrix metalloproteinase-19 is a key regulator of lung fibrosis in mice and humans. Am J Respir Crit Care Med 186: 752–762, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]