Abstract
Chronic high-fat feeding is associated with functional dyspepsia and delayed gastric emptying. We hypothesize that high-fat feeding upregulates gastric neuronal nitric oxide synthase (nNOS) expression, resulting in delayed gastric emptying. We propose this is mediated by increased bile acid action on bile acid receptor 1 (TGR5) located on nNOS gastric neurons. To test this hypothesis, rats were fed regular chow or a high-fat diet for 2 wk. Rats fed the high-fat diet were subjected to concurrent feeding with oral cholestyramine or terminal ileum resection. TGR5 and nNOS expression in gastric tissue was measured by immunohistochemistry, PCR, and Western blot. Gastric motility was assessed by organ bath and solid-phase gastric emptying studies. The 2-wk high-fat diet caused a significant increase in neurons coexpressing nNOS and TGR5 in the gastric myenteric plexus and an increase in nNOS and TGR5 gene expression, 67 and 111%, respectively. Enhanced nonadrenergic, noncholinergic (NANC) relaxation, deoxycholic acid (DCA)-induced inhibition in fundic tissue, and a 26% delay in gastric emptying accompanied these changes. A 24-h incubation of whole-mount gastric fundus with DCA resulted in increased nNOS and TGR5 protein expression, 41 and 37%, respectively. Oral cholestyramine and terminal ileum resection restored the enhanced gastric relaxation, as well as the elevated nNOS and TGR5 expression evoked by high-fat feeding. Cholestyramine also prevented the delay in gastric emptying. We conclude that increased levels of circulatory bile acids induced by high-fat feeding upregulate nNOS and TGR5 expression in the gastric myenteric plexus, resulting in enhanced NANC relaxation and delayed gastric emptying.
Keywords: bile acid receptor 1 (TGR5), enteric nervous system, gastric emptying, high-fat diet, nNOS
high-fat feeding is associated with functional dyspepsia (34) and delayed gastric emptying (39). Patients with these conditions often complain of bloating, fullness, and nausea. The consumption of a high-fat diet is known to increase the postprandial plasma CCK level, which slows gastric emptying (15) and suppresses energy intake (6). We have observed that chronic exposure to a high-fat diet causes delayed gastric emptying in rats. This abnormality is not reversed by a CCK antagonist, suggesting that CCK-independent mechanisms may be responsible.
Gastric motility is regulated primarily by the enteric nervous system. The neurotransmitter nitric oxide (NO), which is produced by myenteric neurons containing nitric oxide synthase (NOS), mediates smooth muscle relaxation and regulates the accommodation reflex of the fundus (9, 42). Impaired neuronal NOS (nNOS) expression in gastric neurons results in abnormal gastric motility (41). Currently, little is known about the impact of chronic high-fat feeding on the enteric nervous system.
High-fat feeding leads to an increase in circulating bile acids. The primary bile acids, cholic acid and chenodeoxycholic acid, are synthesized from cholesterol in hepatocytes and stored in the gallbladder. They are secreted postprandially into the intestine, more than 95% being actively absorbed in the terminal ileum (14). Small quantities of primary bile acids reach the colon and are metabolized by colonic bacteria to form the secondary bile acids deoxycholic acid (DCA) and lithocholic acid (7). These are absorbed and enter the enterohepatic circulation, and, together with the primary bile acids, form the bile acid pool. Both primary and secondary bile acids exert their effects by activating nuclear (45) and plasma membrane receptors (17, 30). Among these receptors, TGR5 is a bile acid plasma membrane receptor that is widely distributed in hepatocytes, epithelial cells, endocrine cells, adipocytes, smooth muscle cells, and immune cells (17–19). TGR5 regulates bile acid synthesis (5), intestinal secretion (47), glucose homeostasis (43), energy expenditure (48), gastrointestinal motility (21, 33, 44), and inflammation (30). TGR5 immunoreactivity and mRNA have been detected in the enteric nervous system of the mouse stomach, as well as the small and large intestines (35).
Immunocytochemistry studies have shown that TGR5 is colocalized to 80% of inhibitory motor neurons expressing NOS, and stimulation of TGR5 causes a sustained inhibition of the phasic activity of the ileum and colon by way of a nitrergic mechanism (35). It is therefore conceivable that high-fat feeding induces upregulation of TGR5 and nNOS in the gastric myenteric plexus, resulting in delayed gastric emptying. To test this hypothesis, we used in vivo and in vitro approaches to investigate the effects of chronic high-fat feeding on the expression of nNOS and TGR5 in the gastric myenteric plexus, and we assessed the functional significance of these effects on gastric nonadrenergic, noncholinergic (NANC) relaxation and stomach gastric emptying. The bile acid binder cholestyramine and terminal ileum resection to interrupt the enterohepatic circulation were used to identify the role of circulatory bile acids in mediating these events.
MATERIALS AND METHODS
Materials
The following materials were used: atropine sulfate, carbachol, guanethidine, TTX, devazepide, cholestyramine, deoxycholic acid, cholic acid, oleanolic acid, GW4064, SQ 22,536, BAPTA (Sigma-Aldrich, St. Louis, MO); NG-nitro-l-arginine methyl ester (l-NAME, Research Biochemicals International, Natick, MA); Rp-cAMP (Santa Cruz Biotechnology, Dallas, TX); TRIzol [Life Sciences Solutions (Thermo Fisher Scientific), Carlsbad, CA]. iScript cDNA Synthesis Kit and iQ SYBR Green Supermix were purchased from Bio-Rad Laboratories (Philadelphia, PA). RT-qPCR primer assays for GAPDH (330001 PPR06557A), NOS1 (330001 PPR44930E), and GPBAR1 (TGR5, 330001 PPR49442A) were purchased from QIAGEN (Valencia, CA). Fungizone antimycotic (15290-018) and penicillin-streptomycin (15070-063) were purchased from GIBCO, Life Sciences Solutions.
Ethical Approval
All procedures were performed in accordance with National Institutes of Health guidelines and were approved by the University of Michigan Committee on Use and Care of Animals.
Animal Preparation
Experiments were performed on male Sprague-Dawley rats (100–150 g, Charles River Laboratories, Wilmington, MA). The rats were housed three per cage in a pathogen-free facility maintained on a 12-h light-dark cycle. They were given food and water ad libitum and allowed to acclimate in the facility for 2 days before being randomly assigned to dietary treatments.
Rats were fed one of three diets for 2 consecutive wk: a control diet of regular chow (11.3% kcal fat), a high-fat diet (58% kcal fat, D12330; Research Diets, New Brunswick, NJ), or a high-fat diet mixed with 6% (wt/wt) cholestyramine. At the end of the 2-wk period, the rats were deprived of food overnight. Serum was collected and stored at −80°C for analysis of total bile acid concentration. Gastric tissue was harvested for immunochemistry, RT-PCR, and organ bath studies. Solid-phase gastric emptying studies were performed in a separate group of rats.
Surgical Procedure
To show that increased nNOS expression and enhanced gastric NANC relaxation following a 2-wk high-fat diet are mediated by increased levels of bile acids, we performed terminal ileum resection to interrupt the enterohepatic circulation of bile acids. Bile acids are secreted into the duodenum through the bile duct and largely reabsorbed via the ileal Na1-dependent bile acid transporter ASBT, which is localized at the terminal ileum (16). To eliminate the ileal bile acid transporter completely from the intestine and interrupt the enterohepatic circulation of bile acids, we resected the last 20 cm of the terminal ileum, as previously described (16).
Rats were anesthetized by an intraperitoneal injection of a mixture of ketamine (60 mg/kg) and xylazine (10 mg/kg). The terminal ileum-resection group (n = 12) underwent laparotomy and resection of 20 cm of the ileum proximal to the ileocecal valve. All bowel anastomoses were performed with a single layer of interrupted 6-0 silk sutures. Postoperatively, rats were housed individually in cages and kept warm. They were allowed water ad libitum on the first postoperative day and chow at the beginning of the third day. The terminal ileum-resection rats were randomly assigned to one of two dietary treatments, high-fat diet or regular chow, for 2 wk (each group, n = 6). On the morning of day 14, after an overnight fast, all animals were euthanized by CO2 asphyxiation, and the gastric tissue was removed rapidly for in vitro studies.
Measurement of Total Bile Acid Concentration
All four groups of rats (regular chow, high-fat diet, high-fat diet mixed with cholestyramine, and high-fat diet plus terminal ileum resection) were food deprived for 6 h and then euthanized by CO2 asphyxiation. Fresh serum was collected to measure total bile acid concentration by using the Rat Total Bile Acids Assay Kit (Crystal Chem, Downers Grove, IL), which is based on enzymatic technology.
Solid-Phase Gastric Emptying Studies
Solid-phase gastric emptying studies were performed in four groups of rats after 2 wk of feeding with regular chow, a high-fat diet, a high-fat diet plus cholestyramine, or a high-fat diet plus the CCK-A receptor antagonist devazepide (100 μg/kg) administered 15 min before the initiation of gastric emptying studies (31, 36). The gastric emptying rate of a solid meal was measured, as described previously (11). After a 20- to 24-h fast, rats were given a known amount of rat chow with free access to water for 3 h. Food and water were then removed, and 4 h later, the gastric emptying rate of the ingested meal was assessed. Rats were euthanized by CO2 asphyxiation. The abdominal cavity was opened and the pylorus and cardia were clamped. The stomach was removed and the amount of food remaining in the stomach was quantified. The rate of gastric emptying during the 4-h period was calculated according to the following equation: gastric emptying (% in 4 h) = (1 − gastric content/food intake) × 100 (11).
Immunohistochemistry
Tissue preparation.
Whole-mount preparations were used for immunohistochemistry studies, as described previously (49). To obtain a whole-mount gastric preparation, the stomach was removed and opened along the greater and lesser curvatures. The gastric corpus was pinned flat in a Sylgard-coated petri dish filled with ice-cold Krebs solution. The mucosa of the gastric corpus was removed and the tissue was fixed for 4–6 h in Zamboni's solution containing 2% paraformaldehyde. The tissue was rinsed in PBS (0.1 M) and the whole-mount preparations were prepared by removal of the circular muscle layer.
Immunofluorescent labeling.
Double or triple immunohistochemical staining was performed against Hu, nNOS, and TGR5, as markers of enteric neurons, nNOS, and bile acid receptor, respectively. Whole-mount preparations containing the myenteric plexus were washed three times in PBS (5 min each time). After preincubation in a blocking solution containing 5% normal donkey serum and 0.3% Triton X-100 in PBS, the preparations were incubated overnight with the primary antibodies, at room temperature. The following antiserum and antibodies were used: rabbit anti-nNOS (1:200, SC-648, Santa Cruz Biotechnology), rabbit anti-TGR5 (1:250, ab 72608, Abcam, Cambridge, MA), and mouse anti-Hu (1:500, BD Biosciences, San Jose, CA). After incubation with the primary antibodies, the preparations were washed three times in PBS (5 min each time) and then incubated for 60 min at room temperature with species-specific fluorophore-conjugated secondary antibodies Cy3, aminomethylcoumarin acetate (Jackson ImmunoResearch Laboratories, West Grove, PA), Alexa Fluor 488 (Molecular Probes, Life Sciences Solutions), and Alexa Fluor 350 (donkey anti-rabbit IgG, Molecular Probes). Tissues were then washed three times in PBS (5 min each time). Whole-mount preparations were mounted on gelatin-coated glass slides and coverslipped with antifading aqueous mounting medium (Gel Mount, Sigma-Aldrich). For control experiments, either the primary or the secondary antibodies were omitted. Tissues were examined and photographed by use of a fluorescence microscope (Olympus BX-51, Tokyo, Japan).
Hu-, nNOS-, and TGR5-immunoreactive neurons were counted in each ganglion of the myenteric plexus by using the fluorescence microscope with ×20 or ×40 objectives. Only neurons within the border of the intact myenteric plexus were counted. Rats were fed a high-fat diet or regular chow (n = 4, each group). A similar number of gastric myenteric neurons in the stomach were examined in each rat.
Recording of Stomach Muscle Relaxation
In vitro organ bath studies were performed to measure electrical field stimulation (EFS)-induced NANC relaxation in fundic muscle strips. Rats fed regular chow (controls), high-fat diet, high-fat diet plus cholestyramine, or high-fat diet with ileum resection were fasted overnight and euthanized by CO2 asphyxiation. After laparotomy, the stomach was removed and circular muscle strips were obtained from the fundus. As previously described (40), muscle strips (10-mm long, 3-mm wide) were suspended with a load of 1 g between two platinum electrodes in an organ bath filled with Krebs-Henseleit buffer of the following composition (in mM): 118 NaCl, 4.8 KCl, 2.5 CaCl2, 25 NaHCO3, 1.2 KH2PO4, 1.2 MgSO4, and 11 glucose. The Krebs-Henseleit buffer was continuously gassed with 95% O2-5% CO2 and maintained at 37°C and pH 7.4. The muscle strips were allowed to equilibrate for at least 60 min before experimentation. Mechanical activity was recorded by means of isometric transducers. Dose-response curves for DCA (10−6 to 10−3 M), a proven TGR5 agonist, were constructed on each muscle strip, as previously described (35). DCA (10−4 M) was added to produce a sustained decrease in tone, followed by a further 10-min equilibration period.
In separate studies, EFS (60 V, 2 ms; 1, 2.5, 5, 10, 20 Hz, 30 s) was applied through two platinum wire electrodes. Before EFS, muscle strips were preincubated with various antagonists for 15 min. To determine the neural pathways responsible for NANC relaxation induced by EFS, atropine (10−6 M) and guanethidine (10−6 M) were added to the organ bath. We also examined the effects of the NO biosynthesis inhibitor l-NAME (10−4 M) on EFS-induced relaxations. The concentrations of these antagonists were determined with reference to our previous studies (40). The reduction of tone induced by EFS in the presence or absence of various antagonists or agonists was measured. In separate studies to determine whether a high-fat diet changes the intrinsic muscle properties of the gastric fundus, dose-response curves of sodium nitroprusside (10−7 M to 10−5 mol/l), an activator of soluble guanylate cyclase (29), were constructed on muscle strips obtained from rats fed regular chow or a high-fat diet.
Stomach Explant Culture
The gastric fundus from control and high-fat diet rats was washed in ice-cold oxygenated PBS, and the mucosa was removed. After a 5-min incubation in ice-cold PBS containing 1% Fungizone, tissues were placed in a six-well cluster with 10 ml DMEM containing 100 units/ml penicillin-streptomycin. Explants were exposed to DCA (10−4 M) or vehicle (0.1% ethanol) in the presence or absence of adenylyl cyclase inhibitor SQ 22,536 (10−4 M), PKA inhibitor Rp-cAMP (10−5 M), or calcium chelator BAPTA (10−5 M) and were incubated at 37°C under 95% O2-5% CO2. In separate studies, explants were exposed to oleanolic acid (10−4 M), a specific analog of TRG5 (37), or to GW4064 (5×10−6 M) (20, 28), a specific analog of the bile acid nuclear receptor FXR (farnesoid X receptor) (27). Tissues were collected 24 h after culture for Western blot.
Western Blot Analysis
Proteins extracted from stomach tissues were analyzed on Ready Gel Tris·HCl (Bio-Rad Laboratories). The tissue was homogenized in ice-cold lysis buffer, as previously described (32). The homogenate was centrifuged at 14,000 g for 10 min. Protein samples were run on Ready Gel 7.5% or 4–20% Tris·HCl for 1 h at 0.06 A and transferred to nitrocellulose Hybond-enhanced chemiluminescence membranes (GE Healthcare, Life Sciences, Pittsburgh, PA) for 1.5 h at 80 V. The membranes were blocked with StartBlock T20 blocking buffer (Thermo Scientific, Rockford, IL) for 1 h at room temperature, probed overnight with primary antibodies against nNOS (i.e., SC-648) at 1:400 dilution or with antibodies against TGR5 (i.e., ab 72608) at 1:300 dilution at 4°C overnight, and then washed in Tris-buffered saline for 1 h. The membranes were probed with corresponding horseradish peroxidase-conjugated secondary antibodies at 1:8,000 dilution for 1 h at room temperature, and the bands were visualized by electrochemiluminescence (PerkinElmer, Waltham, MA). Signals were quantified with use of Image J (National Institutes of Health, Bethesda, MD) and normalized by GAPDH.
RT-PCR
Total RNAs were extracted from rat fundus and corpus tissue by use of Direct-zol. RNAs treated with DNase I were used to synthesize cDNA (iScript cDNA Synthesis Kit). The resultant cDNAs were used for qPCR, with primer sets targeting GPBAR1 (TGR5) and NOS1. GAPDH served as an endogenous housekeeping reference gene. RT-qPCRs were analyzed by using iQ SYBR Green Supermix according to the manufacturer's instructions.
Statistical Analysis
All results are shown as means ± SE for the number of rats indicated. For statistical analyses, the unpaired Student t-test, paired Student t-test, and Mann-Whitney U-test were used to compare the results from control and high-fat diet rats. P < 0.05 was considered significant. All data were analyzed with SPSS 16.0 software (Chicago, IL).
RESULTS
High-Fat Diet Increases the Level of Circulating Bile Acids and Enhances Expression of nNOS and TGR5 in the Myenteric Plexus
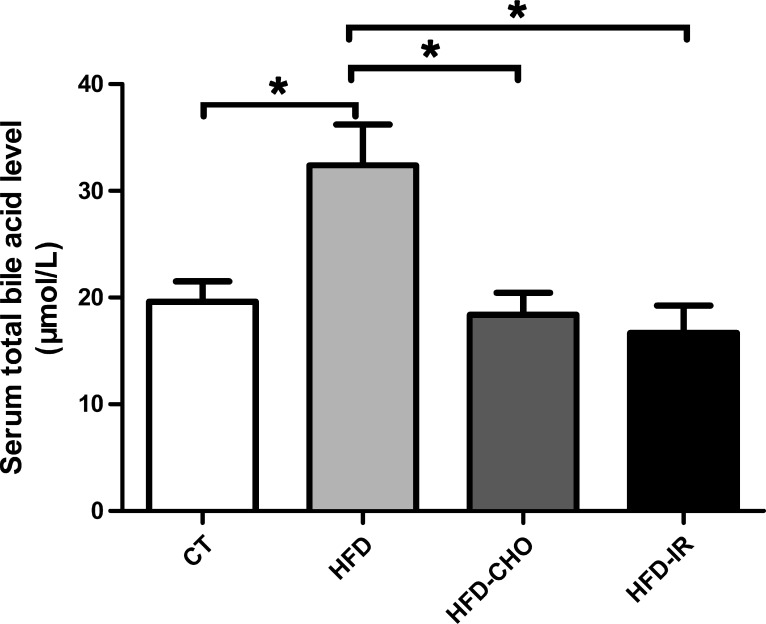
Rats fed a regular chow diet had a serum bile acid level of 19.61 ± 1.90 μmol/l after a 12-h fast (n = 9). A 2-wk high-fat diet increased the serum bile acid level to 32.40 ± 3.83 μmol/l, representing a 65% increase compared with control (n = 9, P < 0.05) (Fig. 1). This increase was prevented by concurrent administration of cholestyramine (18.38 ± 2.34 μmol/l, n = 8) or by terminal ileum resection (16.67 ± 2.59 μmol/l, n = 9) (Fig. 1).
Fig. 1.
Measurement of total bile acid concentration. A 2-wk high-fat diet (HFD) induced a 65% increase in the serum bile acid level, compared with a diet of regular chow (control, CT) (n = 9, *P < 0.05). This increase was prevented by concurrent administration of cholestyramine (CHO) (n = 8, *P < 0.05) or by terminal ileum resection (IR) (n = 9, *P < 0.05). Each bar represents means ± SE.
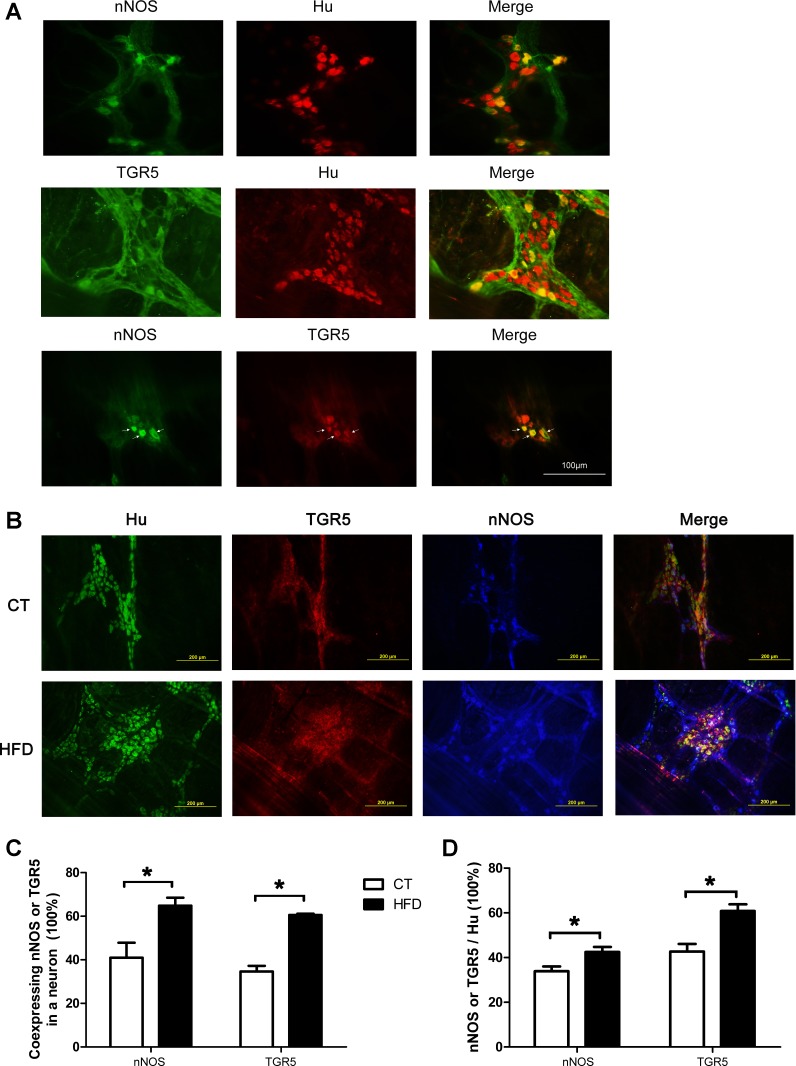
Immunocytochemistry studies showed significant quantities of nNOS- and TGR5-containing neurons in the gastric myenteric plexus (Fig. 2A). Triple immunostaining demonstrated colocalization of TGR5 and nNOS in Hu-expressing neurons. In rats fed regular chow, 35% of gastric neurons expressed nNOS and 43% expressed TGR5. Colocalization studies showed that 41% of nNOS-containing neurons expressed TGR5 and 35% of TGR5-containing neurons expressed nNOS (Fig. 2D). Two weeks of high-fat feeding resulted in a 9% increase in nNOS expression and an 18% increase in TGR5 expression in the gastric myenteric neurons (Fig. 2, B and C). Hence, there was a 24% increase in nNOS-containing neurons coexpressing TGR5 (P < 0.05) and a 26% increase in TGR5-containing neurons coexpressing nNOS (P < 0.05) (Fig. 2, B and D). There was, however, no increase in the number of Hu-expressing neurons in each ganglion in the corpus between the high-fat diet group and control (data not shown).
Fig. 2.
Activation of neuronal nitric oxide synthase (nNOS)- and TGR5-containing neurons in the gastric myenteric plexus following a 2-wk high-fat diet (HFD). A: nNOS- and TGR5-containing neurons were present in significant quantities in the gastric myenteric plexus. A and B: colocalization of TGR5 and nNOS in Hu-containing neurons. C: 35% gastric neurons expressed nNOS and 43% expressed TGR5. D: 41% nNOS-containing neurons also expressed TGR5 and 35% of TGR5-containing neurons also expressed nNOS in rats fed a regular chow diet (control, CT). A 2-wk HFD caused increases of 9% in nNOS expression and 18% in TGR5 expression (*P < 0.05, B and C), as well as significant increases in neurons coexpressing nNOS and TGR5 (*P < 0.05, B and D). Each bar represents means ± SE.
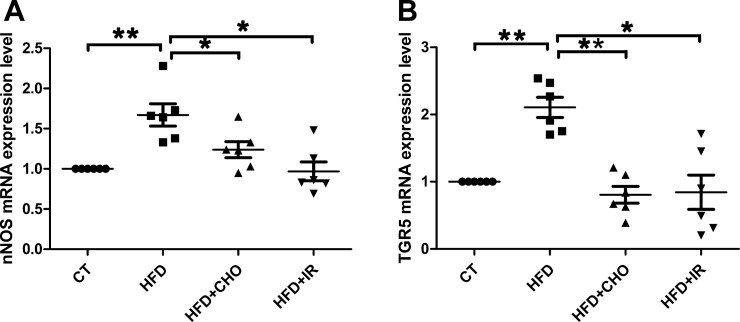
RT-PCR studies demonstrated that increases in nNOS and TGR5 immunoreactivities following high-fat feeding were accompanied by increases in gene expression of nNOS and TGR5 in the myenteric plexus of the gastric fundus; 67 and 111%, respectively (n = 6; both P < 0.01; Fig. 3, A and B). These increases in nNOS and TGR5 gene expression were prevented by concurrent oral feeding with cholestyramine (n = 6, P < 0.05) or by terminal ileum resection (n = 6, P < 0.05; Fig. 3, A and B). Similar increases in nNOS and TGR5 gene expression were observed in the corpus following 2 wk of high-fat feeding (data not shown).
Fig. 3.
Gene expression of nNOS and TGR5. Gene expression levels of nNOS and TGR5 increased in the gastric myenteric plexus in rats fed a high-fat diet (HFD); 67 and 111%, respectively (n = 6, both **P < 0.01; A and B). These increases in nNOS and TGR5 gene expression were restored to normal levels by concurrent oral feeding with cholestryamine (CHO) (n = 6, *P < 0.05) or by terminal ileum resection (IR) (n = 6, **P < 0.05) (A and B). All data are shown as means ± SE. CT, control.
High-Fat Diet Enhances NANC Relaxation of Gastric Muscle
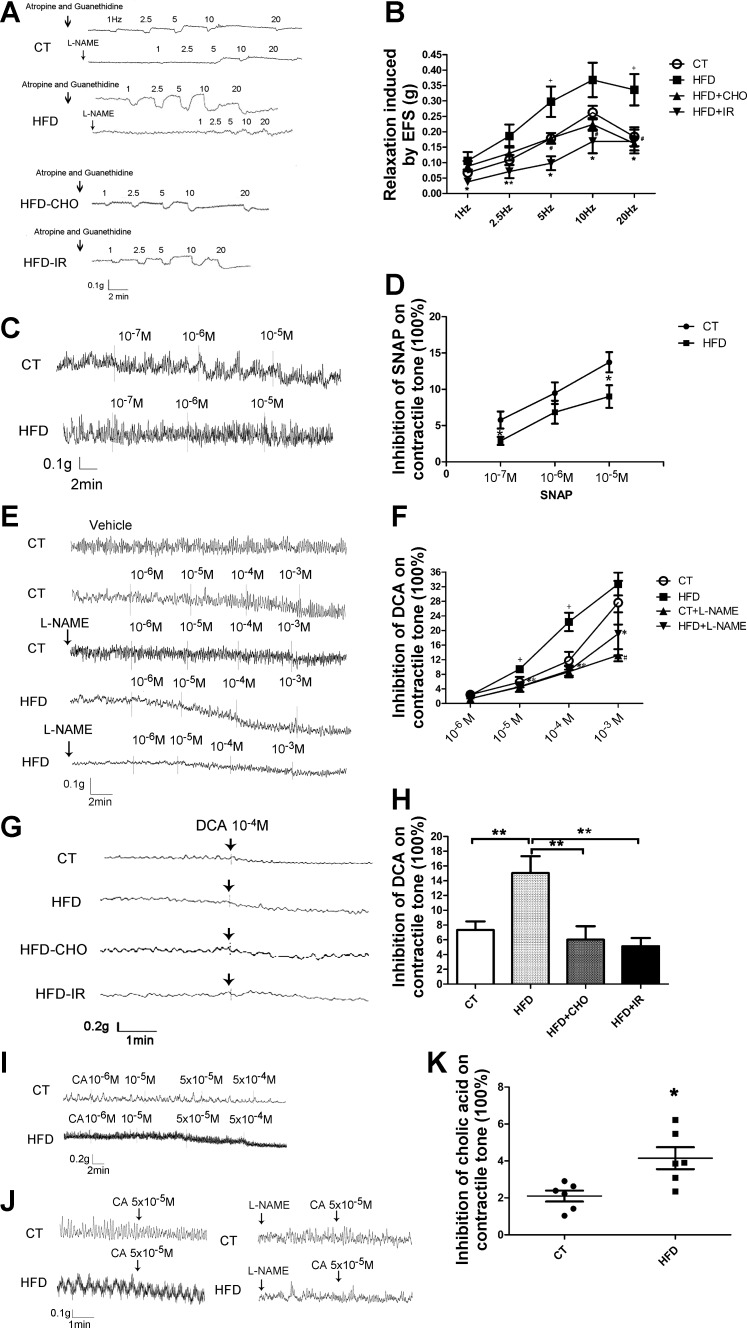
Transmural stimulation caused a frequency-dependent NANC relaxation in the presence of atropine and guanethidine in the muscle preparations from rats fed regular chow (Fig. 4, A and B). As previously described, NANC relaxation was markedly antagonized by l-NAME, which suggests mediation by neural release of NO from the gastric myenteric plexus (42). Transmural stimulation-evoked relaxation was significantly enhanced in gastric muscle strips of rats fed a high-fat diet. Similar to the muscle strips obtained from rats fed regular chow, l-NAME markedly decreased NANC relaxation induced by 1 and 2.5 Hz and partially reduced NANC relaxation evoked by 5, 10, and 20 Hz (Fig. 4, A and B).
Fig. 4.
A and B: nonadrenergic, noncholinergic (NANC) relaxations. Transmural stimulation caused a frequency-dependent NANC relaxation in muscle preparations from rats fed regular chow (control, CT) (A and B). This relaxation was markedly antagonized by NG-nitro-l-arginine methyl ester (l-NAME). Transmural stimulation (5 Hz, 20 Hz)-evoked relaxation was significantly enhanced in gastric muscle strips from rats fed a high-fat diet (HFD) (n = 10, both +P < 0.05; A and B). l-NAME markedly decreased the NANC relaxation induced by 1 and 2.5 Hz and partially reduced the NANC relaxation evoked by 5, 10, and 20 Hz (A). This enhanced relaxation was prevented by concurrent cholestyramine (CHO) (5 Hz, 10 Hz, 20 Hz: HFD-CHO vs. HFD, n = 8, #P < 0.05; A and B) or by terminal ileum resection (IR) at all levels of electric field stimulation (EFS) (1, 2.5, 5, 10, and 20 Hz: HFD-IR vs. HFD, n = 8–10, *P < 0.05 or **P < 0.01; A and B). All data are shown as means ± SE. C and D: sodium nitroprusside (SNAP) (10−5 mol/l) induced less relaxation in rats fed a high-fat diet compared with rats fed regular chow (control) (n = 7, both *P < 0.05, C and D). No significant difference in muscle relaxation was observed when lower concentrations of sodium nitroprusside (10−6 and 10−7 M) were used. All data are shown as means ± SE. E–K: deoxycholic acid (DCA) dose dependently inhibited gastric muscle tone in tissues obtained from CT rats and HFD rats (E and F). Enhanced relaxation was observed at 10−5 M and 10−4 M DCA in muscle strips obtained from HFD rats (HFD vs. CT, n = 6, both +P < 0.05; E and F). Pretreatment of the muscle strips with l-NAME abolished the inhibitory action of DCA at 10−4 M (CT + l-NAME vs. CT, n = 6, #P < 0.05; E and F) in muscle strips obtained from rat fed regular chow, and 10−5 M, 10−4 M, and 10−3 M in muscle strips from rats receiving a high-fat diet (HFD + l-NAME vs. HFD, n = 6, *P < 0.05 or **P < 0.01; E and F). DCA at 10−4 M induced enhanced relaxation of fundic muscle strips from rats fed a high-fat diet compared with the relaxation in rats fed regular chow (HFD vs. CT, n = 6, *P < 0.01; G and H). Concurrent cholestyramine feeding (HFD-CHO vs. HFD, n = 5 or 6, **P < 0.01; G and H) or terminal ileum resection (HFD-IR vs. HFD, n = 5 or 6, **P < 0.01; G and H) normalized the muscle response to DCA stimulation (G and H). Similar to the enhanced muscle response to DCA, fundic muscle strips from rats fed a 2-wk high-fat diet also demonstrated increased relaxation when stimulated by the primary bile acid, cholic acid (CA) (I–K). All data are shown as means ± SE.
To demonstrate that the enhanced gastric NANC relaxation following a 2-wk high-fat diet is mediated by increased levels of circulatory bile acids, we showed that concurrent cholestyramine feeding with a high-fat diet or terminal ileum resection restored normal gastric NANC relaxation (Fig. 4, A and B).
Sodium nitroprusside directly stimulates the production of soluble guanylate cyclase in smooth muscle cells, which then induces muscle relaxation. In contrast to transmural stimulation, exogenously applied sodium nitroprusside (10−5 mol/l) induced a lesser degree of relaxation in rats fed a high-fat diet compared with regular chow (Fig. 4, C and D). However, no significant difference in muscle relaxation was observed with lower concentrations of sodium nitroprusside (10−6 and 10−7 M). Therefore, after 2 wk of a high-fat diet, the sensitivity of the muscle cells to NO appeared to decrease, perhaps due to desensitization.
To demonstrate that bile acids affect smooth muscle contractile tone, we performed in vitro organ bath studies and showed that DCA (10−6 to 10−3 M) induced a concentration-dependent inhibition of the contractile tone of gastric fundic muscle (Fig. 4, E and F). Pretreatment of the muscle strips with l-NAME abolished the inhibitory action of DCA (Fig. 4, E and F), indicating mediation by NO.
Fundic muscle strips obtained from rats fed a 2-wk high-fat diet showed increased sensitivity to DCA stimulation. Enhanced relaxation was observed at 10−5 and 10−4 M (Fig. 4, E and F). Similar to muscle strips obtained from rats fed regular chow, l-NAME prevented relaxation induced by DCA in muscle strips from rats fed a high-fat diet (Fig. 4, E and F).
As shown in Fig. 4, E and F, DCA at 10−4 M caused a 15.0 ± 2.3% relaxation in fundic muscle strips from rats fed a high-fat diet compared with 7.3 ± 1.2% relaxation in fundic muscle strips from rats fed regular chow (Fig. 4, G and H). This represents a 105% increase in muscle relaxation compared with control. Concurrent cholestyramine feeding or terminal ileum resection normalized the muscle sensitivity to DCA stimulation (Fig. 4, G and H). Similar to enhanced muscle sensitivity to DCA, fundic muscle strips obtained from rats fed a 2-wk high-fat diet also demonstrated increased sensitivity to the primary bile acid, cholic acid (Fig. 4, I–K).
In Vitro Actions of Bile Acids on nNOS and TGR5 Protein Expression
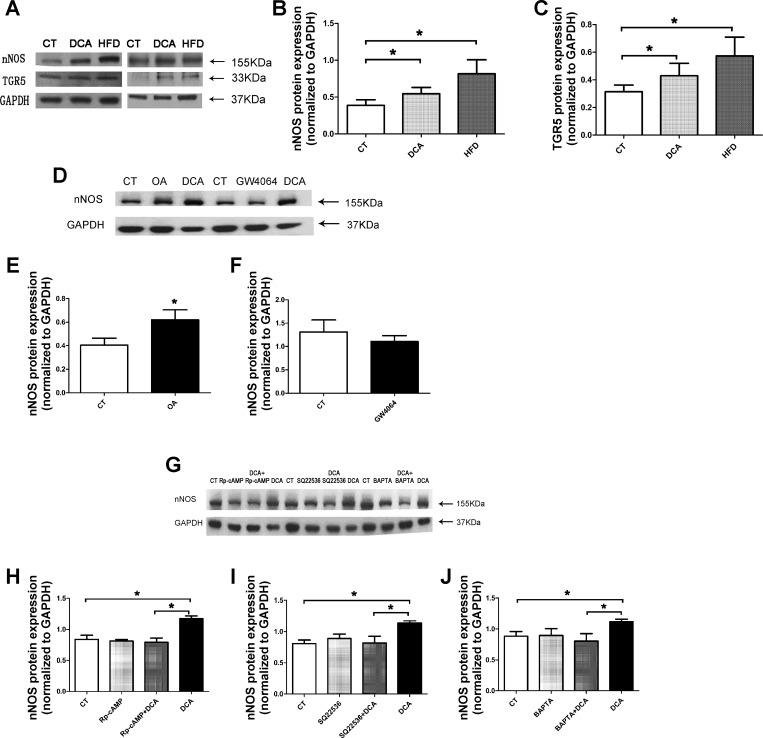
The density of nNOS and TGR5 immunoreactive bands at 155 and 35 kDa were clearly observed in the gastric tissue from rats fed regular chow. High-fat feeding caused respective increases of 110 and 82% in nNOS- and TGR5-immunoreactive bands (n = 7, P < 0.05) (Fig. 5, A–C). Incubation with 10−6 M DCA for 24 h evoked respective increases of 41 and 37% in nNOS and TGR5 immunoreactive bands (n = 7, P < 0.05) (Fig. 5, A–C). A similar increase in nNOS expression was observed when muscle strips were incubated with 10−4 M of oleanolic acid (specific activator of TGR5) (37), but not 5 × 10−6 M GW4064 (20, 28), which stimulates the bile acid nuclear receptor FXR (27). To determine the signal transduction pathway responsible for the upregulation of nNOS gene expression, we showed that stimulation of nNOS by DCA was markedly attenuated by adenylyl cyclase inhibitor SQ 22536 (10−4 M), PKA inhibitor Rp-cAMP (10−5 M), and calcium chelator BAPTA (10−5 M) (Fig. 5, E and H–J). The doses of these inhibitors were based on previous studies. This suggests bile acids stimulate nNOS expression via the TGR5-cAMP-PKA pathway, which increases the cytosolic Ca2+.
Fig. 5.
Protein expression of nNOS and TGR5. Protein bands at 155 and 35 kDa that corresponded to the molecular weights of nNOS protein and TGR5 protein were detected in the gastric tissue from rats fed regular chow (CT) (A). High-fat feeding (high-fat diet, HFD) caused respective increases of 110 and 82% in nNOS- and TGR5-immunoreactive bands (n = 7, *P < 0.05; A–C). Incubation with 10−6 M DCA for 24 h evoked respective increases of 41 and 37% in nNOS and TGR5 immunoreactive bands (n = 7, P < 0.05; A–C). A 60% increase was observed in nNOS band density in tissues cultured with oleanolic acid (OA) (n = 6, P < 0.05; D and E). No difference was found between GW4064- and vehicle-treated groups (n = 5, P > 0.05; D and F). Rp-cAMP, SQ 22,536, and BAPTA significantly inhibited nNOS expression evoked by 10−6 M DCA (n = 4–6, P < 0.05; G–J). All data are shown as means ± SE.
High-Fat Diet Induces Delayed Gastric Emptying, Which Is Prevented by Cholestyramine, not by A CCK Antagonist
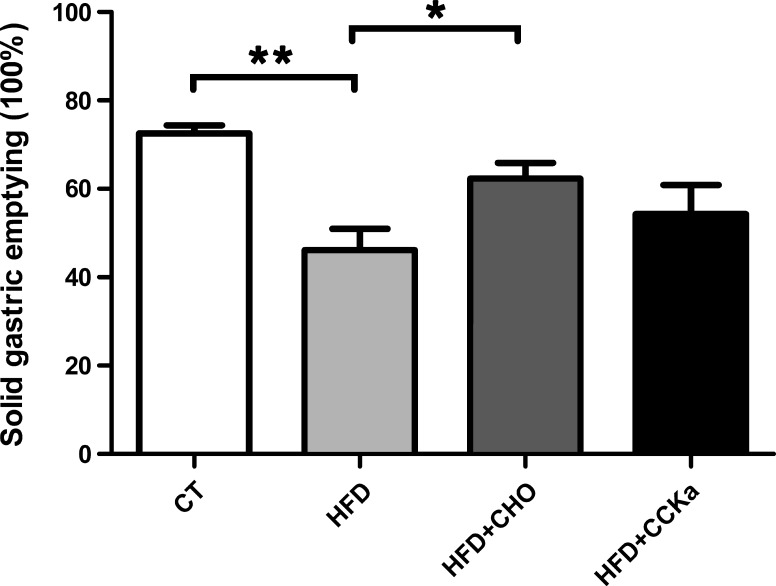
In control rats fed regular chow, 72 ± 2% of ingested food emptied into the intestine at 4 h after a test meal (regular chow). In rats fed a 2-wk high-fat diet, only 46 ± 5% of a regular chow meal emptied into the intestine in the same time period. This represents a 26% delay in gastric emptying (n = 9 in each group, P < 0.05) (Fig. 6). Concurrent cholestyramine feeding normalized gastric emptying in rats fed a high-fat diet (62 ± 4 vs. 46 ± 5%, n = 9, P < 0.05). In contrast, administration of the CCK-A antagonist devazepide 15 min before the gastric emptying studies failed to correct the delay in gastric emptying (54 ± 7 vs. 46 ± 5%, n = 6–9, P = 0.32) (Fig. 6).
Fig. 6.
Solid-phase gastric emptying. There was a 26% of delay in gastric emptying induced by a 2-wk high-fat diet (HFD), compared with a diet of regular chow (control, CT) (n = 9, **P < 0.01). This delay was prevented by the concurrent administration of cholestyramine (CHO) (n = 9, *P < 0.05), but not by the administration of devazepide (HFD-CCK-A receptor antagonist) (n = 6–9, P = 0.32). All data are shown as means ± SE.
DISCUSSION
Traditionally, bile acids are considered to be molecules discharged from the biliary system into the intestine to facilitate lipid digestion and absorption. With advances in the understanding of bile acid physiology, bile acids are now recognized as a complex family of hormones playing an important role in the regulation of gastrointestinal physiology and energy metabolism (14). In this study, we show for the first time that chronic high-fat feeding induces upregulation of nNOS and TGR5 expression in the gastric myenteric plexus, resulting in enhanced NANC relaxation and delayed gastric emptying. This appears to be mediated by increased levels of circulating bile acids stimulated by high-fat feeding, since the inhibitory effects of bile acids on gastric motor function were prevented by coadministration of cholestyramine and by terminal ileum resection to interrupt the enterohepatic circulation.
Baudry et al. (3) described the neuroprotective effects of diet-induced obesity on the murine antrum. This laboratory showed that feeding mice with a Western diet for 12 wk prevented age-associated decrease in antral nNOS-containing neurons. This was associated with a NO-dependent increase in gastric emptying. In comparison, our studies showed that high-fat feeding for 2 wk caused an upregulation of nNOS and TRG5 expression in the gastric (fundus and corpus) myenteric plexus. This was accompanied by enhanced NANC relaxation and delayed gastric emptying. In contrast to the studies by Baudry et al., we did not observe nNOS neuron loss following 2 wk of high-fat feeding. Instead, the upregulation of nNOS and TRG5 expression occurred in existing myenteric neurons in the stomach. Furthermore, we observed delayed, not accelerated, gastric emptying. These differences between the two studies may be due to duration of high-fat feeding, as well as to the sampling of tissue from different regions of the stomach (corpus vs. antrum).
It is well known that bile acids affect gastrointestinal motility (10, 21, 33, 44). Bile acid inhibits motility in the isolated perfused rabbit terminal ileum, independently of PYY release (2). In humans, luminal perfusion of bile acid at physiological concentrations delays intestinal transit (33). Serum bile acid levels peak about 90 min after a meal (1, 38) when the food bolus is passing through the small intestine. Thus it is conceivable that bile acids act as an “ileal brake,” slowing intestinal transit and allowing efficient digestion and absorption. Until recently, the mechanisms by which bile acids regulate intestinal motility were unclear. In 2010, Poole et al. (35) reported that the bile acid receptor TGR5 (GPBAR1) is expressed throughout the gastrointestinal tract, and predominantly in the myenteric and submucous plexuses. In their study of the intestinal myenteric plexus, TGR5 appeared to be localized to ∼50% of all neurons and NOS expression was evident in >80% of inhibitory motor neurons. These investigators further showed that DCA causes a rapid and sustained inhibition of spontaneous phasic contractions in isolated segments of mouse ileum by a neurogenic nitrergic mechanism. These observations provided a mechanism to explain the inhibitory action of bile acids on small intestinal motility.
Although Poole et al. (35) reported the presence of TGR5 in mouse gastric corpus and antrum, their investigation focused on small intestine and colon. Our study revealed that 35% of gastric myenteric neurons express nNOS and 43% express TGR5, significantly less than in mouse intestine, where TGR5 is expressed in >80% of inhibitory motor neurons (35). This may be related to species or organ differences.
The 2-wk high-fat diet caused a significant increase in neurons coexpressing nNOS and TRG5 in the myenteric plexus of the gastric corpus. Because of technical difficulties, we were not able to perform immunohistochemistry on whole mounts of the gastric fundus. However, we performed RT-PCR studies and showed that the gene expression of nNOS and TGR5 in the fundus and corpus were similarly elevated following high-fat feeding. This suggests that the upregulation of nNOS and TGR5 occurs in different regions of the stomach. We used Hu as a marker for enteric neurons and showed that there was no significant difference in the number of neurons in each ganglion in the corpus between the high-fat diet group and control group. This suggests that the increased expression of nNOS was not due to the proliferation of the new neurons but rather to neuroplasticity resulting in the expression of nNOS in a group of non-nNOS-expressing neurons.
We used gastric neuromuscular preparations from control rats fed regular chow to show a reproducible, dose-dependent relaxation in response to transmural stimulation in the presence of atropine and guanethidine (Fig. 4, A and B). This relaxation was markedly antagonized by pretreatment with l-NAME and completely abolished by TTX, indicating that gastric relaxation is mainly mediated by the neuronal release of NO. As shown in Fig. 4, l-NAME appears to be more effective in antagonizing relaxations evoked by low-frequency stimulation. This is not unexpected, since our studies of vascular isolated perfused rat stomach have shown that lower frequencies of stimulation (<2.5 Hz) mainly stimulate NO release, whereas higher frequencies (>5 Hz) evoke VIP release (42).
Two weeks of high-fat feeding caused a significant increase in NANC gastric muscle relaxation, 20–60% over controls. Similar to controls, the relaxations were markedly antagonized by l-NAME (Fig. 4, A and B), indicating mediation by NO release from the gastric myenteric neurons. Gastric inhibitory neurotransmission also likely involves purinergic neurons (13). However, purinergic transmission is unlikely to play a major role in mediating enhanced NANC relaxation following 2 wk of high-fat feeding, since the increased relaxation was markedly antagonized by l-NAME. High-fat feeding also induced upregulation of nNOS and TGR5 expression in the gastric myenteric plexus. This provides a plausible explanation for the increased relaxation of gastric muscle strips in response to transmural electrical stimulation after 2 wk of high-fat feeding. To rule out altered sensitivity of muscle cells to NO, we examined muscle response to exogenously applied sodium nitroprusside. NO relaxes the smooth muscle by a mechanism similar to that of sodium nitroprusside; i.e., by causing an increase in guanosine 3′,5′-cyclic monophosphate (29). As shown in Fig. 4, C and D, gastric relaxation induced by exogenously applied sodium nitroprusside (10−5 M) was less in preparations from rats fed a high-fat diet, suggesting reduced sensitivity of the muscle cells to NO following 2 wk of high-fat feeding. This may be due to desensitization of the muscle cells to the high dose of sodium nitroprusside. There was, however, no altered sensitivity of muscle cells to lower doses of sodium nitroprusside.
As expected, high-fat feeding for 2 wk resulted in a 65% increase in serum bile acid levels in the circulation. This provides a persistent source of stimulation of NOS-containing neurons in the gastric myenteric plexus and may be responsible for the enhanced relaxation of the gastric muscle following 2 wk of high-fat feeding. In separate studies, we demonstrated that physiological concentrations of bile acids can directly affect smooth muscle contractile tone (10−6 to 10−3 M) and that this action was markedly attenuated by l-NAME, indicating mediation by NO. Fundic muscle strips obtained from rats fed a 2-wk high-fat diet demonstrated a 105% increase in sensitivity to stimulation by DCA and cholic acid via a NO-dependent mechanism. These observations indicate that increased circulatory bile acid levels in response to high-fat feeding may serve as a persistent stimulus to activate gastric NOS-containing myenteric neurons, resulting in enhanced gastric relaxation. To confirm this possibility, we showed that concurrent cholestyramine feeding with a high-fat diet or terminal ileum resection to interrupt the enterohepatic circulation of bile acids prevented the increase in serum bile acid levels and restored normal NANC relaxation in rats fed a high-fat diet.
The mechanism by which bile acids activate NOS has not been clearly defined. TGR5 is coupled intracellularly to a heterotrimeric Gs protein and exerts it action through stimulation of cAMP and activation of protein kinase A (29). The resulting elevation of intracellular Ca2+ concentration may directly stimulate nNOS gene expression and nNOS synthesis. We observed an upregulation of nNOS and TGR5 expression following 2 wk of high-fat feeding, and this appears to be mediated by elevated levels of bile acids in the circulation. The upregulation of nNOS and TGR5 expression may result from transcriptional, translational, or posttranslational events. Western blot analysis of fundic neuromuscular preparations showed that incubation with 10−6 M DCA caused a significant increase in nNOS gene expression, suggesting that enhanced transcription at least partially contributes to the upregulation of nNOS. It is conceivable that bile acids may act via the nuclear receptor FXR (27) to regulate NOS synthesis. Research has shown that, on activation by bile acids, FXR induces the transcription of the nuclear receptor small heterodimer partner, which in turn blocks the transcription of CYP7A1, leading to a reduction in bile acid synthesis (8). FXR also plays an important role in regulating intestinal physiology, such as cell survival, epithelial barrier and transport, and innate immune response (23, 46), as well as nutrient metabolism (22). We showed that specific TGR5 activator oleanolic acid (37) but not GW4064, a specific activator of FXR, stimulated nNOS expression in gastric neuromuscular preparations, suggesting that TGR5 but not FXR mediates nNOS expression. As shown in Fig. 5, the upregulation of nNOS by DCA was antagonized by adenylyl cyclase inhibitor SQ 22,536 and PKA inhibitor Rp-cAMP, suggesting participation of the cAMP-PKA pathway in the upregulation of nNOS expression.
A high-fat diet may delay gastric emptying by different mechanisms. It has been demonstrated that fat in the distal ileum may release glucagon-like peptide 1 (GLP-1), which inhibits upper gut motility and secretion (12). In our study, we showed that the delayed gastric emptying following 2 wk of a high-fat diet could be prevented by concurrent cholestyramine administration. This argues against mediation by GLP-1. However, conclusive evidence excluding GLP-1 participation in the mediation of delayed gastric emptying following high-fat feeding would require pharmacological or genetic blockade of GLP-I action. CCK is another potential mediator to slow gastric emptying after ingesting a high-fat diet (26). However, the half-life of CCK in the circulation is less than several minutes (4). Previous studies have shown that plasma CCK is elevated for about 120 min after a meal (24, 25). Our gastric emptying studies were performed after a 20- to 24-h fast, at which time the plasma CCK level had returned to basal level. Hence, the delay in gastric emptying after 2 wk of a high-fat diet is not likely mediated by CCK. This was confirmed by our observation that the CCK-A receptor antagonist devazepide failed to reverse the prolonged gastric emptying observed in rats fed a 2-wk high-fat diet. Our observation that cholestyramine administration and terminal ileum resection prevented the delay in gastric emptying evoked by high-fat feeding indicate that the process is mediated by bile acids acting on gastric nNOS to induce gastric relaxation and decreased motility. Blocking TGR5 receptors would provide conclusive evidence that delayed gastric emptying after high-fat feeding is mediated by increased levels of bile acid. Unfortunately, reliable TGR5 antagonists are not currently available.
In conclusion, we have demonstrated that increased levels of circulatory bile acids induced by high-fat feeding upregulate nNOS and TGR5 expression in the gastric myenteric plexus, resulting in enhanced NANC relaxation and delayed gastric emptying. This provides a mechanism to explain functional dyspepsia and delayed gastric emptying following chronic ingestion of a high-fat diet.
GRANTS
The studies were supported by the National Institutes of Health Grants 2R01 DK058913 and P30 DK34933 and by the National Natural Science Foundation of China Grant 81100257.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
H.Z. and C.O. conception and design of research; H.Z., S.Z., J.G., G.Z., and Y.L. performed experiments; H.Z., S.Z., and C.O. analyzed data; H.Z., S.Z., and C.O. interpreted results of experiments; H.Z. prepared figures; H.Z. and C.O. drafted manuscript; H.Z. and C.O. edited and revised manuscript; H.Z. and C.O. approved final version of manuscript.
REFERENCES
- 1.Angelin B, Bjorkhem I. Postprandial serum bile acids in healthy man. Evidence for differences in absorptive pattern between individual bile acids. Gut 18: 606–609, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong DN, Krenz HK, Modlin IM, Ballantyne GH. Bile salt inhibition of motility in the isolated perfused rabbit terminal ileum. Gut 34: 483–488, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baudry C, Reichardt F, Marchix J, Bado A, Schemann M, des Varannes SB, Neunlist M, Moriez R. Diet-induced obesity has neuroprotective effects in murine gastric enteric nervous system: involvement of leptin and glial cell line-derived neurotrophic factor. J Physiol 590: 533–544, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berry H, Flower RJ. The assay of endogenous cholecystokinin and factors influencing its release in the dog and cat. Gastroenterology 60: 409–420, 1971. [PubMed] [Google Scholar]
- 5.Chiang JY. Bile acids: regulation of synthesis. J Lipid Res 50: 1955–1966, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Covasa M, Marcuson JK, Ritter RC. Diminished satiation in rats exposed to elevated levels of endogenous or exogenous cholecystokinin. Am J Physiol Regul Integr Comp Physiol 280: R331–R337, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Danielsson H, Sjovall J. Bile acid metabolism. Annu Rev Biochem 44: 233–253, 1975. [DOI] [PubMed] [Google Scholar]
- 8.Davis RA, Miyake JH, Hui TY, Spann NJ. Regulation of cholesterol-7alpha-hydroxylase: BAREly missing a SHP. J Lipid Res 43: 533–543, 2002. [PubMed] [Google Scholar]
- 9.Desai KM, Sessa WC, Vane JR. Involvement of nitric oxide in the reflex relaxation of the stomach to accommodate food or fluid. Nature 351: 477–479, 1991. [DOI] [PubMed] [Google Scholar]
- 10.Feldman S, Gibaldi M. Effect of bile salts on gastric emptying and intestinal transit in the rat. Gastroenterology 54: 918–921, 1968. [PubMed] [Google Scholar]
- 11.Gangula PR, Maner WL, Micci MA, Garfield RE, Pasricha PJ. Diabetes induces sex-dependent changes in neuronal nitric oxide synthase dimerization and function in the rat gastric antrum. Am J Physiol Gastrointest Liver Physiol 292: G725–G733, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gentilcore D, Chaikomin R, Jones KL, Russo A, Feinle-Bisset C, Wishart JM, Rayner CK, Horowitz M. Effects of fat on gastric emptying of and the glycemic, insulin, and incretin responses to a carbohydrate meal in type 2 diabetes. J Clin Endocrinol Metab 91: 2062–2067, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Grider JR, Cable MB, Said SI, Makhlouf GM. Vasoactive intestinal peptide as a neural mediator of gastric relaxation. Am J Physiol Gastrointest Liver Physiol 248: G73–G78, 1985. [DOI] [PubMed] [Google Scholar]
- 14.Hofmann AF, Hagey LR. Bile acids: chemistry, pathochemistry, biology, pathobiology, therapeutics. Cell Mol Life Sci 65: 2461–2483, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holzer HH, Turkelson CM, Solomon TE, Raybould HE. Intestinal lipid inhibits gastric emptying via CCK and a vagal capsaicin-sensitive afferent pathway in rats. Am J Physiol Gastrointest Liver Physiol 267: G625–G629, 1994. [DOI] [PubMed] [Google Scholar]
- 16.Kanamoto R, Azuma N, Suda H, Saeki T, Tsuchihashi Y, Iwami K. Elimination of Na+-dependent bile acid transporter from small intestine by ileum resection increases [correction of increase] colonic tumorigenesis in the rat fed deoxycholic acid. Cancer Lett 145: 115–120, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, Fukusumi S, Habata Y, Itoh T, Shintani Y, Hinuma S, Fujisawa Y, Fujino M. A G protein-coupled receptor responsive to bile acids. J Biol Chem 278: 9435–9440, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Keitel V, Cupisti K, Ullmer C, Knoefel WT, Kubitz R, Haussinger D. The membrane-bound bile acid receptor TGR5 is localized in the epithelium of human gallbladders. Hepatology 50: 861–870, 2009. [DOI] [PubMed] [Google Scholar]
- 19.Keitel V, Donner M, Winandy S, Kubitz R, Haussinger D. Expression and function of the bile acid receptor TGR5 in Kupffer cells. Biochem Biophys Res Commun 372: 78–84, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Kim Y, Chang KO. Inhibitory effects of bile acids and synthetic farnesoid X receptor agonists on rotavirus replication. J Virol 85: 12570–12577, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirwan WO, Smith AN, Mitchell WD, Falconer JD, Eastwood MA. Bile acids and colonic motility in the rabbit and the human. Gut 16: 894–902, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuipers F, Stroeve JH, Caron S, Staels B. Bile acids, farnesoid X receptor, atherosclerosis and metabolic control. Curr Opin Lipidol 18: 289–297, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Lee FY, Lee H, Hubbert ML, Edwards PA, Zhang Y. FXR, a multipurpose nuclear receptor. Trends Biochem Sci 31: 572–580, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Liddle RA. Regulation of pancreatic secretion. In: Physiology of the Gastrointestinal Tract, edited by L. J. San Diego, CA: Elsevier Academic Press, 2012, p. 1425. [Google Scholar]
- 25.Liddle RA, Goldfine ID, Rosen MS, Taplitz RA, Williams JA. Cholecystokinin bioactivity in human plasma. Molecular forms, responses to feeding, and relationship to gallbladder contraction. J Clin Invest 75: 1144–1152, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lieverse RJ, Jansen JB, Masclee AA, Rovati LC, Lamers CB. Effect of a low dose of intraduodenal fat on satiety in humans: studies using the type A cholecystokinin receptor antagonist loxiglumide. Gut 35: 501–505, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. Identification of a nuclear receptor for bile acids. Science 284: 1362–1365, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Maloney PR, Parks DJ, Haffner CD, Fivush AM, Chandra G, Plunket KD, Creech KL, Moore LB, Wilson JG, Lewis MC, Jones SA, Willson TM. Identification of a chemical tool for the orphan nuclear receptor FXR. J Med Chem 43: 2971–2974, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Marletta MA. Nitric oxide synthase structure and mechanism. J Biol Chem 268: 12231–12234, 1993. [PubMed] [Google Scholar]
- 30.Maruyama T, Miyamoto Y, Nakamura T, Tamai Y, Okada H, Sugiyama E, Itadani H, Tanaka K. Identification of membrane-type receptor for bile acids (M-BAR). Biochem Biophys Res Commun 298: 714–719, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Moran TH, Ameglio PJ, Peyton HJ, Schwartz GJ, McHugh PR. Blockade of type A, but not type B, CCK receptors postpones satiety in rhesus monkeys. Am J Physiol Regul Integr Comp Physiol 265: R620–R624, 1993. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura K, Takahashi T, Taniuchi M, Hsu CX, Owyang C. Nicotinic receptor mediates nitric oxide synthase expression in the rat gastric myenteric plexus. J Clin Invest 101: 1479–1489, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Penagini R, Misiewicz JJ, Frost PG. Effect of jejunal infusion of bile acids on small bowel transit and fasting jejunal motility in man. Gut 29: 789–794, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pilichiewicz AN, Feltrin KL, Horowitz M, Holtmann G, Wishart JM, Jones KL, Talley NJ, Feinle-Bisset C. Functional dyspepsia is associated with a greater symptomatic response to fat but not carbohydrate, increased fasting and postprandial CCK, and diminished PYY. Am J Gastroenterol 103: 2613–2623, 2008. [DOI] [PubMed] [Google Scholar]
- 35.Poole DP, Godfrey C, Cattaruzza F, Cottrell GS, Kirkland JG, Pelayo JC, Bunnett NW, Corvera CU. Expression and function of the bile acid receptor GpBAR1 (TGR5) in the murine enteric nervous system. Neurogastroenterol Motil 22: 814–825, e227–e228, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raybould HE, Tabrizi Y, Meyer JH, Walsh JH. PYY immunoneutralization does not alter lipid-induced inhibition of gastric emptying in rats. Regul Pept 79: 125–130, 1999. [DOI] [PubMed] [Google Scholar]
- 37.Sato H, Genet C, Strehle A, Thomas C, Lobstein A, Wagner A, Mioskowski C, Auwerx J, Saladin R. Anti-hyperglycemic activity of a TGR5 agonist isolated from Olea europaea. Biochem Biophys Res Commun 362: 793–798, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Schalm SW, LaRusso NF, Hofmann AF, Hoffman NE, van Berge-Henegouwen GP, Korman MG. Diurnal serum levels of primary conjugated bile acids. Assessment by specific radioimmunoassays for conjugates of cholic and chenodeoxycholic acid. Gut 19: 1006–1014, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stacher G, Bergmann H, Gaupmann G, Schneider C, Kugi A, Hobart J, Binder A, Mittelbach-Steiner G. Fat preload delays gastric emptying: reversal by cisapride. Br J Clin Pharmacol 30: 839–845, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takahashi T, Kojima Y, Tsunoda Y, Beyer LA, Kamijo M, Sima AA, Owyang C. Impaired intracellular signal transduction in gastric smooth muscle of diabetic BB/W rats. Am J Physiol Gastrointest Liver Physiol 270: G411–G417, 1996. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi T, Nakamura K, Itoh H, Sima AA, Owyang C. Impaired expression of nitric oxide synthase in the gastric myenteric plexus of spontaneously diabetic rats. Gastroenterology 113: 1535–1544, 1997. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi T, Owyang C. Characterization of vagal pathways mediating gastric accommodation reflex in rats. J Physiol 504: 479–488, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski M, Pellicciari R, Auwerx J, Schoonjans K. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab 10: 167–177, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Ooteghem NA, Van Erpecum KJ, Van Berge-Henegouwen GP. Effects of ileal bile salts on fasting small intestinal and gallbladder motility. Neurogastroenterol Motil 14: 527–533, 2002. [DOI] [PubMed] [Google Scholar]
- 45.Wang H, Chen J, Hollister K, Sowers LC, Forman BM. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell 3: 543–553, 1999. [DOI] [PubMed] [Google Scholar]
- 46.Wang YD, Chen WD, Moore DD, Huang W. FXR: a metabolic regulator and cell protector. Cell Res 18: 1087–1095, 2008. [DOI] [PubMed] [Google Scholar]
- 47.Ward JB, Mroz MS, Keely SJ. The bile acid receptor, TGR5, regulates basal and cholinergic-induced secretory responses in rat colon. Neurogastroenterol Motil 25: 708–711, 2013. [DOI] [PubMed] [Google Scholar]
- 48.Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, Schoonjans K, Bianco AC, Auwerx J. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 439: 484–489, 2006. [DOI] [PubMed] [Google Scholar]
- 49.Zhou SY, Lu YX, Yao H, Owyang C. Spatial organization of neurons in the dorsal motor nucleus of the vagus synapsing with intragastric cholinergic and nitric oxide/VIP neurons in the rat. Am J Physiol Gastrointest Liver Physiol 294: G1201–G1209, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]