Abstract
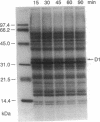
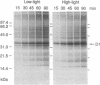
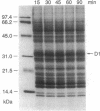
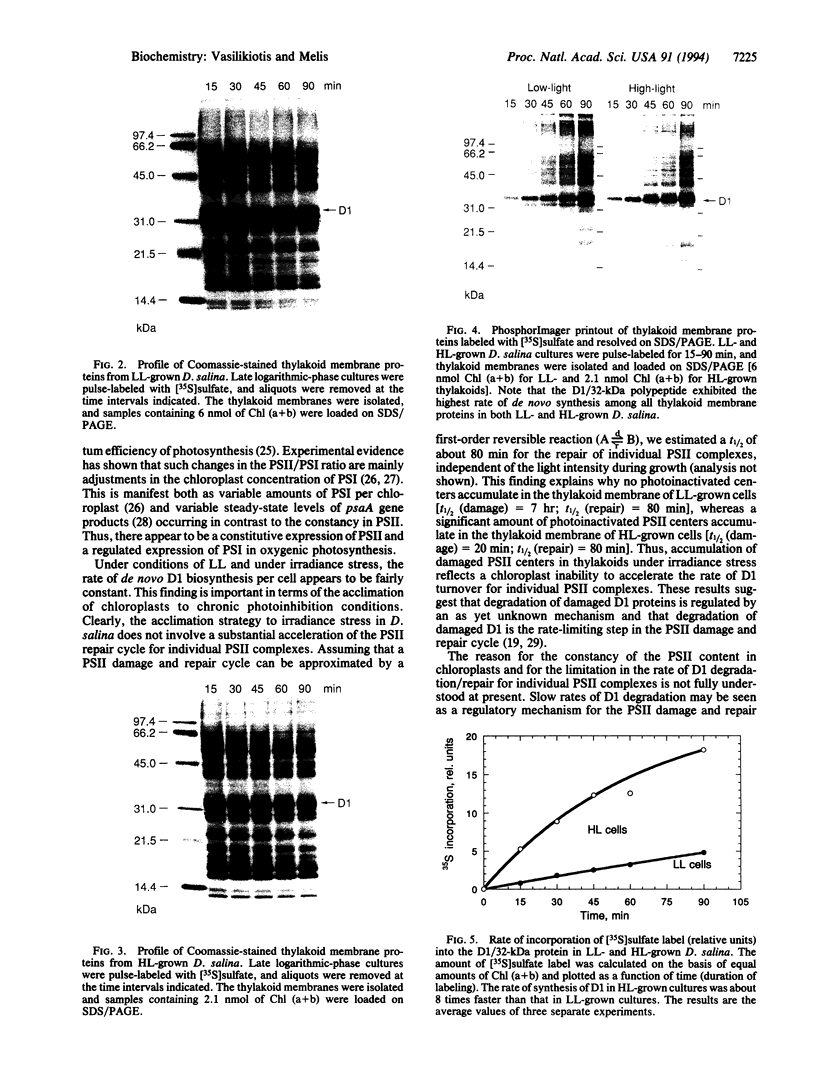
A daily occurrence in the life of a plant is the function of a photosystem II (PSII) damage and repair cycle in chloroplasts. This unique phenomenon involves the frequent turnover of D1, the 32-kDa reaction-center protein of PSII (chloroplast psbA gene product). In the model organism Dunaliella salina (a green alga), growth under low light (100 mol of photons per m2 per sec) entails damage, degradation, and replacement of D1 every 7 hr. Growth under irradiance stress (2200 micromol of photons per m2 per sec) entails damage to D1 every 20 min. The rate of de novo D1 biosynthesis under conditions of both low light and irradiance stress was found to be fairly constant on a per chloroplast or cell basis. The response of D. salina to the enhanced rate of damage entails an accumulation of photodamaged centers (80% of all PSII) and the formation of thylakoid membranes containing a smaller quantity of photosystem I (PSI) centers (about 10% of that in cells grown under low light). These changes contribute to a shift in the PSII/PSI ratio from 1.4:1 under low-light conditions to 15:1 under irradiance stress. The accumulation of photodamaged PSII under irradiance stress reflects a chloroplast inability to match the rate of D1 degradation or turnover with the rate of damage for individual PSII complexes. The altered thylakoid membrane organization ensures that a small fraction of PSII centers remains functional under irradiance stress and sustains electron flow from H2O to ferredoxin with rates sufficient for chloroplast photosynthesis and cell growth.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adir N., Shochat S., Ohad I. Light-dependent D1 protein synthesis and translocation is regulated by reaction center II. Reaction center II serves as an acceptor for the D1 precursor. J Biol Chem. 1990 Jul 25;265(21):12563–12568. [PubMed] [Google Scholar]
- Aro E. M., McCaffery S., Anderson J. M. Photoinhibition and D1 Protein Degradation in Peas Acclimated to Different Growth Irradiances. Plant Physiol. 1993 Nov;103(3):835–843. doi: 10.1104/pp.103.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aro E. M., Virgin I., Andersson B. Photoinhibition of Photosystem II. Inactivation, protein damage and turnover. Biochim Biophys Acta. 1993 Jul 5;1143(2):113–134. doi: 10.1016/0005-2728(93)90134-2. [DOI] [PubMed] [Google Scholar]
- Bottomley W., Spencer D., Whitfeld P. R. Protein synthesis in isolated spinach chloroplasts: comparison of light-driven and ATP-driven synthesis. Arch Biochem Biophys. 1974 Sep;164(1):106–117. doi: 10.1016/0003-9861(74)90012-5. [DOI] [PubMed] [Google Scholar]
- Chow W. S., Melis A., Anderson J. M. Adjustments of photosystem stoichiometry in chloroplasts improve the quantum efficiency of photosynthesis. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7502–7506. doi: 10.1073/pnas.87.19.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke A. K., Hurry V. M., Gustafsson P., Oquist G. Two functionally distinct forms of the photosystem II reaction-center protein D1 in the cyanobacterium Synechococcus sp. PCC 7942. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11985–11989. doi: 10.1073/pnas.90.24.11985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke A. K., Soitamo A., Gustafsson P., Oquist G. Rapid interchange between two distinct forms of cyanobacterial photosystem II reaction-center protein D1 in response to photoinhibition. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):9973–9977. doi: 10.1073/pnas.90.21.9973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick R. E., McCauley S. W., Gruissem W., Melis A. Light quality regulates expression of chloroplast genes and assembly of photosynthetic membrane complexes. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4287–4291. doi: 10.1073/pnas.83.12.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOK B. On the inhibition of photosynthesis by intense light. Biochim Biophys Acta. 1956 Aug;21(2):234–244. doi: 10.1016/0006-3002(56)90003-8. [DOI] [PubMed] [Google Scholar]
- Kim J. H., Nemson J. A., Melis A. Photosystem II Reaction Center Damage and Repair in Dunaliella salina (Green Alga) (Analysis under Physiological and Irradiance-Stress Conditions). Plant Physiol. 1993 Sep;103(1):181–189. doi: 10.1104/pp.103.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R. R., Mason H. S., Mullet J. E. Light-regulated translation of chloroplast proteins. I. Transcripts of psaA-psaB, psbA, and rbcL are associated with polysomes in dark-grown and illuminated barley seedlings. J Cell Biol. 1988 Feb;106(2):289–301. doi: 10.1083/jcb.106.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle D. J., Ohad I., Arntzen C. J. Membrane protein damage and repair: Selective loss of a quinone-protein function in chloroplast membranes. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4070–4074. doi: 10.1073/pnas.81.13.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mattoo A. K., Edelman M. Intramembrane translocation and posttranslational palmitoylation of the chloroplast 32-kDa herbicide-binding protein. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1497–1501. doi: 10.1073/pnas.84.6.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattoo A. K., Hoffman-Falk H., Marder J. B., Edelman M. Regulation of protein metabolism: Coupling of photosynthetic electron transport to in vivo degradation of the rapidly metabolized 32-kilodalton protein of the chloroplast membranes. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1380–1384. doi: 10.1073/pnas.81.5.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohad I., Kyle D. J., Arntzen C. J. Membrane protein damage and repair: removal and replacement of inactivated 32-kilodalton polypeptides in chloroplast membranes. J Cell Biol. 1984 Aug;99(2):481–485. doi: 10.1083/jcb.99.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick U., Karni L., Avron M. Determination of Ion Content and Ion Fluxes in the Halotolerant Alga Dunaliella salina. Plant Physiol. 1986 May;81(1):92–96. doi: 10.1104/pp.81.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. M., Morrissey P. J., Guenther J. E., Nemson J. A., Harrison M. A., Allen J. F., Melis A. Response of the Photosynthetic Apparatus in Dunaliella salina (Green Algae) to Irradiance Stress. Plant Physiol. 1990 Aug;93(4):1433–1440. doi: 10.1104/pp.93.4.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]