Abstract
Background
Candida albicans is an opportunistic fungal pathogen that induces strong proinflammatory responses, such as IL-1β production. Much less is known about the induction of immune modulatory cytokines, such as the IL-1 receptor antagonist (IL-1Ra) that is the main natural antagonist of IL-1, by C. albicans.
Methods
Peripheral blood mononuclear cells (PBMC) of healthy individuals were stimulated with C. albicans and different components of the fungal cell wall. The role of pathogen recognition receptors (PRRs) for the induction of IL-1β and IL-1Ra was investigated by using specific blockers or in PBMC from Dectin-1 deficient patients.
Results
C. albicans induced a strong IL-1Ra response, and this induction was primarily induced by the cell-wall component β-glucan. Blocking IL-1Ra significantly increased C. albicans β-glucan hyphae induced IL-1β and IL-6 production. Surprisingly, blocking the β-glucan receptor Dectin-1 or the downstream Syk or Raf-1 pathways only marginally reduced C. albicans-induced IL-1Ra production, while blocking of the complement receptor 3 (CR3), TLR2 or TLR4 had no effect. In line with this, blocking MAP kinases had little effect on Candida-induced IL-1Ra production. PBMC isolated from Dectin-1 deficient patients produced normal IL-1Ra amounts in response to C. albicans stimulation. Interestingly, the IL-1Ra synthesis induced by β-glucan was blocked by inhibitors of the Akt/PI3 K pathway.
Conclusions
β-glucan of C. albicans induces a strong IL-1Ra response, which is independent of the β-glucan receptors dectin-1 and CR3. These data strongly argue for the existence of an unknown β-glucan receptor that specifically induces an Akt/PI3 K-dependent anti-inflammatory IL-1Ra response upon recognition of C. albicans.
Keywords: β-Glucan, IL-1Ra, Candida albicans
1. Introduction
Candida albicans is a commensal fungus that colonizes the gastrointestinal tract, skin, and mucosa of more than 50% of healthy individuals. Colonization with Candida does not cause disease in healthy individuals, but in patients in whom the immune system is compromised Candida can cause severe mucosal and systemic infections, the latter with a mortality rate reaching up to 30–40% [1].
Several PRRs families mediate immune recognition of C. albicans, such as the Toll-like receptors (TLRs) (TLR2 [2] and TLR4 [3]), and the C-type lectin receptors (CLRs). Mannans on the Candida cell wall are recognized by the C-type lectin receptor macrophage mannose receptor (MMR) [4] and dectin-2 [5], while dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) recognizes both fucose and mannose/mannan residues [6]. The second major component of C. albicans cell wall, β-glucan, is recognized in monocytes and macrophages by dectin-1 [7,8], while in neutrophils complement receptor (CR) 3 plays a prominent role in its recognition [9].
These interactions between C. albicans and the immune system lead to phagocytosis of the fungus [10] and the induction of proinflammatory cytokines, further promoting clearance of the infection [11]. For example, Candida-stimulation of the MR, Dectin-1 and TLR2 leads to pro-IL-1β production, which in monocytes is immediately cleaved to active IL-1β by the constitutively active caspase-1 [12]. IL-1β plays an important role in inducing protective host responses during systemic Candida infections; mice deficient in the IL-1RI (the active IL-1 receptor) succumb to systemic Candida infections [13]. Additionally, IL-1β is a crucial cytokine in inducing the Th17 response [14], which is protective in mucosal host defense against C. albicans [15,16].
IL-1β is a very potent cytokine that can cause septic-like symptoms at concentrations as low as 1 ng/kg [17]. Therefore, the IL-1β systemic effects are counterbalanced by the naturally occurring interleukin-1 receptor antagonist (IL-1Ra). IL-1Ra competitively binds to the same receptor as IL-1α and IL-1β, but does not recruit the signaling accessory protein (IL-1RAcP), thereby decreasing responsiveness to IL-1β [18]. This represents a crucial mechanism for modulation of the inflammatory reaction during infection. Genetic defects in the production of IL-1Ra, also known as deficiency of IL-1Ra (DIRA), has been described to lead to a severe autoinflammatory syndrome characterized by severe systemic inflammation, sterile multifocal osteomyelitis, periostitis and pustulosis [19].
Since C. albicans induces a strong IL-1β response, and the effect of IL-1β must be balanced by IL-1Ra, we investigated the Candida-induced IL-1Ra response. We demonstrate that C. albicans induces a strong IL-RA response, which is specifically induced by C. albicans β-glucans. Surprisingly, this effect of C. albicans β-glucans was mediated through a recognition pathway distinct from the known β-glucan receptors dectin-1 and CR3.
2. Materials and methods
2.1. Healthy volunteers and Dectin-1−/− patients
PBMC were isolated from buffy coats isolated from healthy volunteers (Sanquin Bloodbank, Nijmegen, the Netherlands). In addition, PBMCs were isolated from three patients with Dectin-1 deficiency [20] (one patient was measured two times) and from four healthy controls. After informed consent was obtained, blood was collected by venipuncture from both patients and volunteers into 10-mL ethylenediaminetetraacetic acid (EDTA) tubes (Monoject, s-Hertogenbosch, The Netherlands). The study was approved by the Ethics Committee of Radboud University Nijmegen Medical Centre, and performed in accordance with the declaration of Helsinki.
2.2. Microorganisms
Candida yeast (UC820), were grown overnight in Sabouraud broth at 37 °C. Cells were harvested by centrifugation, washed twice, and resuspended in RPMI 1640 medium. C. albicans yeasts or hyphae were heat-killed for one hour at 100 °C.
2.3. Reagents
The following reagents were used: For cell isolation: Ficoll-Paque (GE Healthcare, Diegem, Belgium), RPMI 1640 Dutch modifications culture medium (Sigma–Aldrich, Zwijndrecht, the Netherlands). The RPMI 1640 medium was supplemented with 1% gentamicin, 1% L-glutamine and 1% pyruvate (Life Technologies, Nieuwerkerk, the Netherlands). For isolation of monocyte subsets we used the cluster of differentiation (CD)16 isolation kit (130-091-765, Miltenyi Biotec, Utrecht, the Netherlands), and CD14 isolation kit (130-050-201, Miltenyi Biotec). β-Glucan from C. albicans yeast and hyphae [21], chitin [22] and mannan [23] were prepared as previously described. Pam3Cys was purchased from EMC Microcollections (Tübingen, Germany). Syk inhibitor was purchased from Calbiochem (San Diego, CA, USA). TLR4 was blocked using Bartonella quintana LPS (obtained as described previously [24]). Anti-TLR2 blocking antibody and control IgG were purchased from eBioscience (Halle-Zoersel, Belgium). Laminarin, RAF-1-inhibitor, 3MA and p38 inhibitor were purchased from Sigma–Aldrich. Anti-CR3, anti-IL-1Ra and goat IgG were purchased from R&D systems (Abingdon, UK). Wortmannin was purchased from Invivogen (Toulouse, France). The inhibitors for ERK and JNK were purchased from Promega (Leiden, The Netherlands) and AG Scientific (San Diego, CA, USA), respectively.
2.4. Cell isolation
PBMCs were obtained by density centrifugation of diluted blood (1 part blood to 1 part pyrogen-free saline) over Ficoll-Paque (Pharmacia Biotech, Uppsala, Sweden). PBMCs were washed twice in saline and suspended in culture medium. The PBMC were counted in a Coulter counter (Coulter Electronics, Buckinghamshire, England) and their number was adjusted to 5 · 106/mL.
Lymphocytes and monocyte subsets were purified from freshly isolated PBMC using MACS microbeads, according to the instructions of the manufacturer (Miltenyi Biotec). In short, lymphocytes were negatively selected using CD14 beads. To isolate monocyte subsets, PBMC were depleted of granulocytes and natural killer cells using CD15 and CD56 microbeads. CD14+CD16+ monocytes were positively selected using CD16 microbeads. Subsequently, CD14++CD16− monocytes were positively selected from the CD16− population, using CD14 microbeads.
2.5. Cell stimulation
A total of 5 × 105 mononuclear cells, 1 × 105 monocytes or 4 × 105 lymphocytes, in a 100 μL volume of culture medium was added to 96-wells round-bottom plates (Greiner). The cells were stimulated with the various stimuli and blockers as described below. After 24 h supernatants were stored at −20 °C. IL-1β and IL-1Ra were measured in cell culture supernatants using enzyme-linked immunosorbent assay (ELISA) (R&D Systems, MN, USA and Sanquin, Amsterdam, The Netherlands).
2.6. Statistical analysis
The differences between groups were analyzed using the Wilcoxon signed rank test for paired data and the Mann–Whitney test for unpaired data (Fig. 3B and C). The IL-1Ra/IL-1β ratio was calculated at the individual level. When values where below the detection limit of the ELISA (only the case for IL-1β, unstimulated samples), the corresponding detection limit was used (39 ρg/mL). In the blocking experiments, cytokine production induced by HK C. albicans alone was set to a 100%, except for anti-CR3 and anti-TLR2, where cytokine production induced by the corresponding control antibody was set to a 100%. Data are presented as mean + standard error of the mean (SEM). Differences were considered statistically significant if p ≤ 0.05 (*), p ≤ 0.01 (**) or p ≤ 0.001 (***).
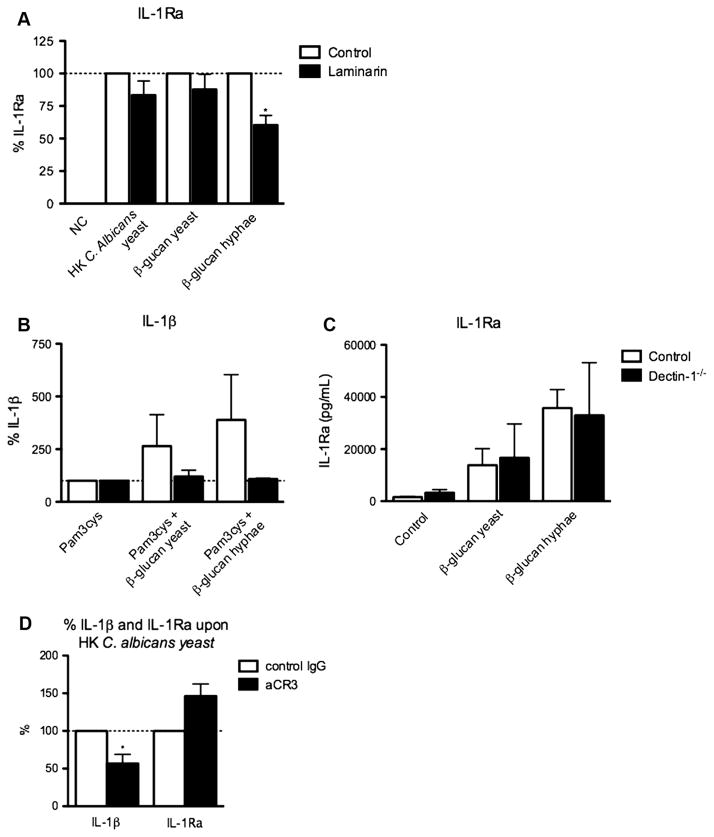
Fig. 3.
Blocking Dectin-1 has modest effects on IL-1Ra production. (A) Human PBMC of 6–12 healthy volunteers were stimulated for 24 h with HK C. albicans (1 × 105/mL) yeast or hyphae or β-glucan from Candida albicans yeast or hyphae (10 μg/mL), in the absence of presence of the dectin-1 blocker laminarin (50 μg/mL). The concentration of IL-1Ra was measured in cell culture supernatants using ELISA, and is expressed in percentage. (B) Adherent monocytes of two Dectin-1 deficient patients and of 2 healthy controls were stimulated with Pam3cys (10 μg/mL) in the absence of presence of β-glucan from Candida albicans yeast or hyphae (10 μg/mL). IL-1β was measured in cell culture supernatants using ELISA, and is expressed in percentage. (C) PBMCs of three Dectin-1 deficient patients and four healthy controls were stimulated with β-glucan from Candida albicans yeast or hyphae (10 μg/mL). IL-1Ra was measured in cell culture supernatants using ELISA. (D) Human PBMC of 6 healthy volunteers were stimulated for 24 h with 1 × 105/mL heat-killed Candida albicans yeast in the absence or presence of αCR3 (10 μg/mL). The concentration of IL-1Ra and IL-1β was measured in cell culture supernatants using ELISA and are expressed in percentages. Bars represent mean + SEM.
3. Results
3.1. C. albicans induces a strong IL-1Ra response
In order to investigate the Candida induced IL-1Ra response, human PBMC were stimulated for 24 h with live or heat-killed C. albicans yeast or hyphae, or E. coli LPS. All three forms of C. albicans but especially E. coli LPS induced a strong IL-1β response (Fig. 1A). Live C. albicans and HK hyphae were the main inducers of IL-1Ra, while HK yeast and E. coli LPS induced little IL-1Ra (Fig. 1B). Interestingly, although HK C. albicans yeast and E. coli LPS induced the highest IL-1β production, they induced relatively little IL-1Ra (Fig. 1C). In contrast, live C. albicans and HK C. albicans hyphae induced 38- and 27-fold more IL-1Ra than IL-1β, respectively.
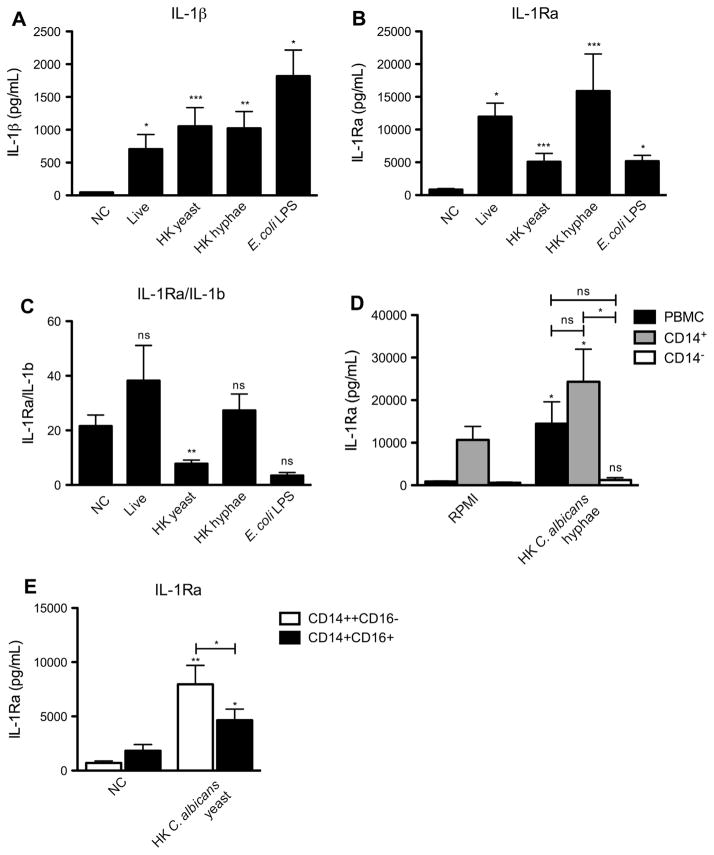
Fig. 1.
C. albicans induces a strong IL-1Ra response. Human PBMC of 4–17 healthy volunteers were stimulated for 24 h with live or HK Candida albicans yeast or hyphae (1 × 105/mL) or E. coli LPS (10 ηg/mL). The concentration of (A) IL-1β and (B) IL-1Ra was measured in cell culture supernatants using ELISA. (C) The IL-1Ra/IL-1β ration was calculated by dividing the amount of IL-1Ra by that of IL-1β. (D) Human PBMCs, CD14+ and CD14− cells of 6 healthy volunteers were stimulated for 24 h with HK Candida albicans hyphae. (E) Human CD14++CD16− and CD14+CD16+ monocytes of 8 healthy volunteers were stimulated for 24 h with HK Candida albicans yeast. (D and E) The concentration of IL-1Ra was measured in cell culture supernatants using ELISA. (A–E) Bars represent mean + SEM.
In order to investigate which cell type was the main producer of IL-1Ra, lymphocytes and monocytes were compared with respect to their IL-1Ra producing capacity. Monocytes were the main producers of IL-1Ra after HK C. albicans hyphae stimulation, as CD14− cells only produced very low levels of IL-1Ra (Fig. 1D). CD14 + cells produced slightly more IL-1Ra upon stimulation with HK C. albicans hyphae compared to yeast, although this difference was not statistically significant. In contrast, IL-1β production was higher upon stimulation with HK C. albicans yeast compared to hyphae (data not shown).
In order to investigate which monocyte population was the main producer of IL-1Ra, CD14++CD16− and CD14+CD16++ monocytes were stimulated with HK C. albicans yeast. CD14++CD16− monocytes produced more IL-1Ra upon stimulation with HK C. albicans yeast (Fig. 1E), which may be in line with their slightly higher basal expression of the Dectin-1 receptor [25].
3.2. C. albicans β-glucan induces production of biologically active IL-1Ra
In order to find out which C. albicans structures induce IL-1Ra production, PBMC were stimulated with different components of the C. albicans cell wall. The IL-1β response induced by mannans and chitin was below the detection limit of the ELISA. In contrast, β-glucan isolated from C. albicans hyphae significantly increased IL-1β production (Fig. 2A, p < 0.001). Both β-glucan preparations isolated from yeast and hyphae significantly (p < 0.001 for both) increased the amount of IL-1Ra compared to unstimulated PBMC (Fig. 2B), and significantly increased the IL-1Ra/IL-1β ratio (Fig. 2C). Mannan and chitin did not induce IL-1Ra production (Fig. 2B).
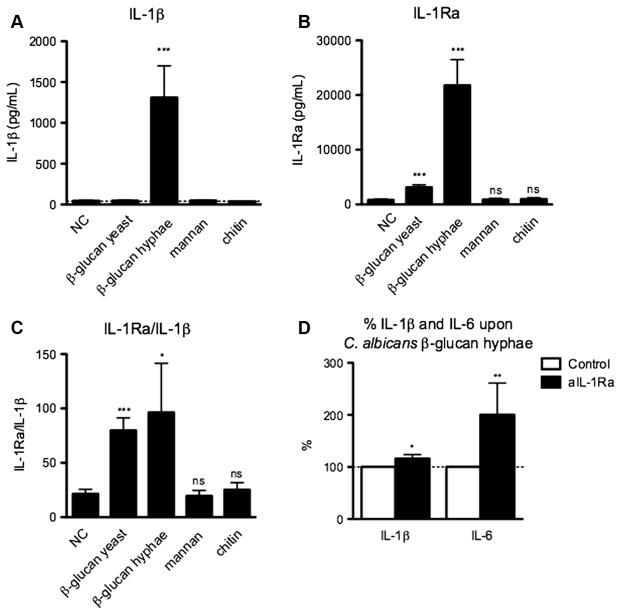
Fig. 2.
Candida albicans β-glucan induces a strong IL-1Ra response. (A–C) Human PBMC were stimulated for 24 h with β-glucan from heat-killed Candida albicans yeast or hyphae (10 μg/mL), mannan (100 μg/mL, or chitin (20 μg/mL). The concentration of (A) IL-1β and (B) IL-1Ra was measured in cell culture supernatants using ELISA. (C) The IL-1Ra/IL-1β ration was calculated by dividing the amount of IL-1Ra by that of IL-1β. Bars represent mean + SEM of 6–15 healthy volunteers. (D) Human PBMC were stimulated for 24 h with Candida albicans β-glucan hyphae (10 μg/mL), in the absence or presence of anti-IL-1Ra (1 μg/mL). The concentrations of IL-1β and IL-6 were measured in cell culture supernatants using ELISA and are expressed as percentages. Bars represent mean + SEM of nine healthy volunteers.
In order to assess whether the IL-1Ra released upon stimulation with β-glucan is biologically active, we blocked its activity using an anti-IL-1Ra antibody. Indeed, blocking IL-1Ra significantly increased β-glucan-induced IL-1β (p < 0.05) and IL-6 (p < 0.01) production (Fig. 2D), two cytokines that are inhibited by IL-1Ra.
3.3. The role of the β-glucan receptors Dectin-1 and CR3 for the C. albicans-induced IL-1Ra production
The next set of experiments investigated which pattern recognition receptor (PRR) is responsible for the C. albicans-induced IL-1Ra response. PBMCs were stimulated with HK C. albicans yeast or hyphae, or β-glucan isolated from C. albicans yeast or hyphae, in the absence or presence of blockers of the two known β-glucan receptors: dectin-1 or CR3. When Dectin-1 was blocked using laminarin, the IL-1Ra production decreased only partially (Fig. 3A). Similar data were obtained when we investigated the β-glucan-induced IL-1Ra response in Dectin-1 deficient patients [20]. β-Glucan did not synergistically boost Pam3cys-induced IL-1β production in PBMC from Dectin-1 deficient patients (Fig. 3B). In contrast, β-glucan isolated from HK C. albicans yeast and hyphae induced normal IL-1Ra production in PBMC from Dectin-1 deficient patients, similarly to healthy controls (Fig. 3C). Blocking CR3, another β-glucan receptor, did not influence the Candida or β-glucan-induced production of IL-1Ra either, while it significantly decreased IL-1 β release (Fig. 3D).
3.4. TLRs are not involved in C. albicans-induced IL-1Ra production
In order to assess the role of TLR2 and TLR4, the two main TLRs involved in the recognition of C. albicans for the induction of IL-1Ra production upon challenge with C. albicans, PBMC were stimulated with HK C. albicans yeast in the absence or presence of blocking anti-TLR2 antibodies, or in the presence of the TLR4 antagonist B. quintana LPS. Blocking TLR2 or TLR4 did not inhibit the C. albicans-induced IL-1Ra production (Fig. 4A).
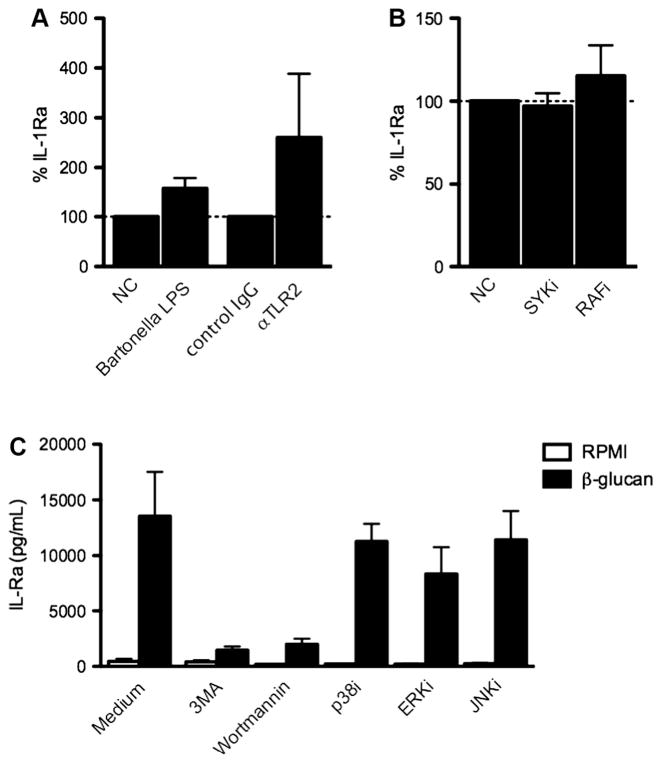
Fig. 4.
Pathways involved in C. albicans-induced IL-1Ra production. (A) Human PBMC from 4–6 healthy volunteers were stimulated for 24 h with 1 × 105/mL heat-killed Candida albicans yeast in the absence or presence of Bartonella LPS (1 μg/mL) or αTLR2 (10 μg/mL). (B) Human PBMC from 6 healthy volunteers were stimulated for 24 h with 1 × 105/mL heat-killed Candida albicans yeast in the absence or presence of SYKi (50 ηM) or RAFi (1 μM). (C) Human PBMC from 4 healthy volunteers were stimulated for 24 h with C. albicans β-glucan hyphae (10 μg/mL) in the absence or presence of 3MA (10 mM), Wortmannin (100 nM), p38i (1 μM), ERKi (10 μM) or JNKi (20 μM). (A–C) The concentration of IL-1Ra was measured in cell culture supernatants using ELISA. Bars represent mean + SEM.
3.5. The intracellular pathways involved in the induction of IL-1Ra by β-glucan
To get a hint for which type of receptor might be involved in β-glucan-induced IL-1RA production, we decided to block several intracellular signaling pathways. Blocking SYK and RAF1, two signaling molecules downstream of several C-type lectin receptors, did not influence C. albicans-induced IL-1Ra production (Fig. 4B). Interestingly, β-glucan-induced IL-1Ra production drastically decreased in the presence of inhibitors of Akt (3MA) and PI3K (Wortmannin). Blocking p38, ERK or JNK only marginally decreased β-glucan-induced IL-1Ra production (Fig. 4C).
4. Discussion
In the present study we investigated the induction of the anti-inflammatory cytokine IL-1Ra by C. albicans. While the production of proinflammatory cytokines by fungi in general and C. albicans in particular has been characterized in detail, much less is known about the pathways leading to the induction of IL-1Ra, the natural antagonist of IL-1. IL-1Ra is crucial for modulation of inflammation during infections, and lack of its production can lead to severe autoinflammatory reactions [19].
C. albicans induces a strong IL-1Ra response, mainly produced by CD14+ cells. Indeed Su et al. previously demonstrated that lymphocytes express low basal levels of IL-1Ra mRNA compared to monocytes [26]. β-Glucan is the main component of C. albicans responsible for the IL-1Ra induction, which is supported by the finding that mannan and chitin did not induce any cytokine production, even tough they were used in high concentrations. Furthermore, the HK C. albicans hyphae-induced IL-1Ra production was higher than the HK C. albicans yeast-induced IL-1Ra production. β-Glucan is shielded from recognition in C. albicans but becomes exposed when C. albicans forms hyphae [8], which rapidly happens when Candida is incubated at 37 °C and 5% CO2 [27]. β-Glucan also becomes exposed after heat killing [28], so the reason why the IL-1Ra induction is stronger by the live Candida compared to HK C. albicans yeast is puzzling, and additional studies need to address this. A possible explanation could be that the combination of different ligands in yeasts and hyphae act differently for the stimulation of IL-1beta and IL-1Ra. Also Poutsiaka et al. demonstrated that particulate β-glucan can induce a strong IL-1Ra response in monocytes, without inducing IL-1β production [29]. In addition, Luhm et al. demonstrated that β-1,3-D-glucan decreases the IL-1β/IL-1Ra ratio, without inducing any significant production of IL-1β, IL-6, TNF-α or IFNγ [30]. We and others have also previously shown that β-glucan stimulation of PBMCs alone does not induce a pro-inflammatory response, while it can synergistically enhance TLR2-induced cytokine and PGE2 production [28,31,32]. Thus, depending on the presence of co-stimulatory factors, β-glucan recognition can have either pro- or anti-inflammatory effects.
β-glucans on the C. albicans cell-wall are known to be recognized by two PRRs: the C-type lectin receptor Dectin-1 which is the main receptor on monocytes, macrophages and DCs [33], and complement receptor 3 (CR3) which can recognize β-glucan mainly on neutrophils [34]. It has been previously demonstrated that cross-linking of the β-glucan receptor is required for IL-1Ra production, as demonstrated by the fact that monomeric β-glucan reduces particulate β-glucan-induced IL-1Ra production [29]. A previous study suggested that recognition of β-glucan by dectin-1 results in increased binding to the NFIL-6 and a NFAT site within the IL-1Ra promotor region, thereby increasing the transcription of IL-1RN [30]. However, blocking dectin-1 with laminarin had only a limited effect on the β-glucan hyphae-induced IL-1Ra response, while it had practically no effect on the yeast (β-glucan)-induced IL-1Ra. These data suggest that dectin-1 has only a secondary role, if any, in the stimulation of IL-1Ra production by C. albicans β-glucan. This hypothesis was confirmed by the normal production of IL-1Ra when PBMC isolated from patients with a complete defect of dectin-1 [20] were stimulated with β-glucan.
Several other PRRs recognize C. albicans in addition to dectin-1. Most importantly, CR3 recognize β-glucans from C. albicans [35], which can lead to the suppression of the proinflammatory cytokine TNF-α [36]. In the present study, we demonstrate that blocking CR3 leads to an increased IL-1β production, but has no effect on the IL-1Ra production induced by β-glucans. No effect of TLR2 or TLR4 for the induction of IL-1Ra by C. albicans has been demonstrated either, and blocking the downstream signaling molecules p38, JNK or ERK did not, or only partially reduced the IL-1Ra response. Other C-type lectin-1 receptors such as mannose receptor, dectin-2 or Mincle are practically excluded by the fact that their ligands, the Candida-derived mannan components, did not stimulate the production of IL-1Ra. Only blocking Akt and PI3 K drastically reduced C. albicans β-glucan hyphae-induced IL-1Ra production, suggesting that β-glucan might induce IL-1Ra production through another unknown pattern recognition receptor. While a TLR receptor (other than TLR2 or TLR4) cannot be excluded as this novel putative β-glucan receptor, considering that PI3 K and Akt kinases have been described to mediate signals downstream of several TLRs [37], another C-type lectin receptor is a more likely candidate due to the polysaccharide structure of β-glucan. For example, DC-SIGN has been demonstrated to signal through PI3 K (as evidenced by Akt phosphorylation) [38], although until now DC-SIGN has not been described in the recognition of β-glucan [39].
All together, these data suggest that the induction of IL-1Ra production by β-glucans is mediated by a novel Akt/PI3 K-dependent receptor pathway, independently of dectin-1 and CR3. The existence of dectin-1-independent pathways for recognition of β-glucans by macrophages has been suggested by earlier studies as well. In RAW264.7 RAW macrophages, β-glucan from Saccharomyces cerevisiae reduces LPS-induced decreases NO production, independently from Dectin-1 [40]. Moreover, we have recently demonstrated that β-glucans from C. albicans induces protection against Staphylococcus aureus sepsis through a dectin-1-independent pathway [41]; whether IL-1Ra is involved in this effect is not known. The induction of IL-1Ra as shown in this study, provides an easy parameter to be used for the identification of the novel β-glucan recognition pathway in the future.
Slight differences have been observed in the capacity to stimulate IL-1Ra between β-glucans isolated from C. albicans yeast and hyphae. β-Glucans from C. albicans hyphae induced a higher IL-1Ra response compared to β-glucan from C. albicans yeast. Blocking dectin-1 moderately reduced IL-1Ra production induced upon stimulation with β-glucan isolated from C. albicans hyphae, while it did not decrease IL-1Ra production induced by β-glucan isolated from C. albicans yeast. Van der Graaf et al. previously demonstrated that HK C. albicans hyphae induce less TNF-α and IFN-y production, but more IL-10 production, due to reduced TLR4 signaling, possibly indicating a mechanism to evade host immunity [42]. Similarly, Torosantucci et al. have demonstrated that C. albicans hyphae induce less MIP-1α, MIP-1β, IL-8 and MCP-1, compared to C. albicans yeast, and that this difference might be explained by the lower levels of β-1,6-glucan in the C. albicans hyphae cell wall [43]. Moreover, Lowman et al. recently demonstrated that there are important differences in the three-dimensional structure of β-glucan from yeast and hyphae [21], and this may also represent a potential source for the differences observed in IL-1Ra production.
In conclusion, we demonstrate that C. albicans induces a strong IL-Ra response, which is specifically induced by C. albicans β-glucans. The C. albicans β-glucan-induced IL-1Ra production was mostly independent on recognition by dectin-1 or CR3. These data suggest for the existence of a novel Akt-PI3 K-dependent PRR recognizing β-glucans that can specifically induce the production of the anti-inflammatory cytokine IL-1Ra. Future studies are warranted for the identification of this novel recognition pathway.
Acknowledgments
M.G.N. was supported by an ERC Consolidator grant (ERC-310372). C.A.D. was supported by a NIH Grant (AI15614). The authors would like to thank Tania Azam for her assistance in the lab.
Abbreviations
- CD
cluster of differentiation
- CLR
C-type lectin receptor
- CR
complement receptor
- DC-SIGN
Dendritic Cell-Specific Intercellular adhesion molecule-3-Grabbing Non-integrin
- EDTA
ethylenediaminetetraacetic acid
- ELISA
enzyme-linked immunosorbent assay
- IL-1Ra
interleukin-1 receptor antagonist
- MMR
macrophage mannose receptor
- PBMC
peripheral blood mononuclear cell
- PRR
pattern recognition receptor
- SEM
standard error of the mean
- TLR
toll-like receptor
References
- 1.Wisplinghoff H, et al. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–17. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 2.Villamón E, et al. Toll-like receptor-2 is essential in murine defenses against Candida albicans infections. Microbes Infect. 2004;6:1–7. doi: 10.1016/j.micinf.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 3.Tada H, et al. Saccharomyces cerevisiae- and Candida albicans-derived mannan induced production of tumor necrosis factor alpha by human monocytes in a CD14- and Toll-like receptor 4-dependent manner. Microbiol Immun. 2002;46:503–12. doi: 10.1111/j.1348-0421.2002.tb02727.x. [DOI] [PubMed] [Google Scholar]
- 4.van de Veerdonk FL, et al. The macrophage mannose receptor induces IL-17 in response to Candida albicans. Cell Host Microbe. 2009;5:329–40. doi: 10.1016/j.chom.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Saijo S, et al. Dectin-2 recognition of α-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity. 2010;32:681–91. doi: 10.1016/j.immuni.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Appelmelk BJ, et al. Carbohydrate profiling identifies new pathogens that interact with dendritic cell-specific ICAM-3-grabbing nonintegrin on dendritic cells. doi: 10.4049/jimmunol.170.4.1635. [DOI] [PubMed] [Google Scholar]
- 7.Brown GD, Gordon S. Fungal β-glucans and mammalian immunity. Immunity. 2003;19:311–5. doi: 10.1016/s1074-7613(03)00233-4. [DOI] [PubMed] [Google Scholar]
- 8.Cheng S-C, et al. The dectin-1/inflammasome pathway is responsible for the induction of protective T-helper 17 responses that discriminate between yeasts and hyphae of Candida albicans. J Leukocyte Biol. 2011;90:357–66. doi: 10.1189/jlb.1210702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lavigne LM, Albina JE, Reichner JS. β-Glucan is a fungal determinant for adhesion-dependent human neutrophil functions. doi: 10.4049/jimmunol.177.12.8667. [DOI] [PubMed] [Google Scholar]
- 10.Heinsbroek SEM, et al. Stage-specific sampling by pattern recognition receptors during Candida albicans phagocytosis. PLoS Pathog. 2008;4:e1000218. doi: 10.1371/journal.ppat.1000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Netea MG, Brown GD, Kullberg BJ, Gow NAR. An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol. 2008;6:67–78. doi: 10.1038/nrmicro1815. [DOI] [PubMed] [Google Scholar]
- 12.van de Veerdonk FL, et al. Bypassing pathogen-induced inflammasome activation for the regulation of interleukin-1β production by the fungal pathogen Candida albicans. J Infect Dis. 2009;199:1087–96. doi: 10.1086/597274. [DOI] [PubMed] [Google Scholar]
- 13.Bellocchio S, et al. The contribution of the toll-like/IL-1 receptor superfamily to innate and adaptive immunity to fungal pathogens in vivo. doi: 10.4049/jimmunol.172.5.3059. [DOI] [PubMed] [Google Scholar]
- 14.Chung Y, et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–87. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conti HR, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kagami S, Rizzo HL, Kurtz SE, Miller LS, Blauvelt A. IL-23 and IL-17A, but not IL-12 and IL-22, are required for optimal skin host defense against Candida albicans. J Immunol. 2010;185:5453–62. doi: 10.4049/jimmunol.1001153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tewari A. Preliminary report: effects of interleukin-1 on platelet counts. Lancet. 1990;336:712–4. doi: 10.1016/0140-6736(90)92206-w. [DOI] [PubMed] [Google Scholar]
- 18.Dinarello CA. Biologic basis for interleukin-l in disease. Blood. 1996;87:2095–147. [PubMed] [Google Scholar]
- 19.Aksentijevich I, et al. An autoinflammatory disease with deficiency of the interleukin-1-receptor antagonist. New Engl J Med. 2009;360:2426–37. doi: 10.1056/NEJMoa0807865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferwerda B, et al. Human dectin-1 deficiency and mucocutaneous fungal infections. New Engl J Med. 2009;361:1760–7. doi: 10.1056/NEJMoa0901053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lowman DW, et al. Novel structural features in candida albicans hyphal glucan provide a basis for differential innate immune recognition of hyphae versus yeast. J Biol Chem. 2014;289:3432–43. doi: 10.1074/jbc.M113.529131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mora-Montes HM, et al. Recognition and blocking of innate immunity cells by Candida albicans chitin. Infect Immun. 2011;79:1961–70. doi: 10.1128/IAI.01282-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowman DW, et al. Mannan structural complexity is decreased when Candida albicans is cultivated in blood or serum at physiological temperature. Carbohydr Res. 2011;346:2752–9. doi: 10.1016/j.carres.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Popa C, et al. Bartonella quintana lipopolysaccharide is a natural antagonist of toll-like receptor 4. Infect Immun. 2007;75:4831–7. doi: 10.1128/IAI.00237-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smeekens SP, et al. The classical CD14++ CD16− monocytes, but not the patrolling CD14+ CD16+ monocytes, promote Th17 responses to Candida albicans. Eur J Immunol. 2011;41:2915–24. doi: 10.1002/eji.201141418. [DOI] [PubMed] [Google Scholar]
- 26.Su AI, et al. A gene atlas of the mouse and human protein-encoding transcriptomes. PNAS. 2004;101:6062–7. doi: 10.1073/pnas.0400782101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sudbery P, Gow N, Berman J. The distinct morphogenic states of Candida albicans. Trends Microbiol. 2004;12:317–24. doi: 10.1016/j.tim.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 28.Netea MG, et al. Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and toll-like receptors. J Clin Invest. 2006;116:1642–50. doi: 10.1172/JCI27114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poutsiaka DD, Mengozzi M, Vannier E, Sinha B, Dinarello CA. Cross-linking of the beta-glucan receptor on human monocytes results in interleukin-1 receptor antagonist but not interleukin-1 production. [PubMed]
- 30.Luhm J, et al. B-(1→3)-D-glucan modulates DNA binding of nuclear factors κB, AT and IL-6 leading to an anti-inflammatory shift of the IL-1β/IL-1 receptor antagonist ratio. BMC Immunol. 2006;7:5. doi: 10.1186/1471-2172-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smeekens SP, et al. The Candida Th17 response is dependent on mannan- and beta-glucan-induced prostaglandin E2. Int Immunol. 2010;22:889–95. doi: 10.1093/intimm/dxq442. [DOI] [PubMed] [Google Scholar]
- 32.Goodridge HS, Underhill DM. Handbook of experimental pharmacology. Vol. 183. Berlin Heidelberg: Springer; 2008. pp. 87–109. [DOI] [PubMed] [Google Scholar]
- 33.Brown GD, Gordon S. Immune recognition. A new receptor for beta-glucans. Nature. 2001;413:36–7. doi: 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- 34.Van Bruggen R, et al. Complement receptor 3, not Dectin-1, is the major receptor on human neutrophils for β-glucan-bearing particles. Mol Immunol. 2009;47:575–81. doi: 10.1016/j.molimm.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 35.Diamond MS, Garcia-Aguilar J, Bickford JK, Corbi AL, Springer TA. The I domain is a major recognition site on the leukocyte integrin Mac-1 (CD11b/CD18) for four distinct adhesion ligands. J Cell Biol. 1993;120:1031. doi: 10.1083/jcb.120.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brandhorst TT, Wüthrich M, Finkel-Jimenez B, Warner T, Klein BS. Exploiting type 3 complement receptor for TNF-α suppression, immune evasion, and progressive pulmonary fungal infection. doi: 10.4049/jimmunol.173.12.7444. [DOI] [PubMed] [Google Scholar]
- 37.Brown J, Wang H, Hajishengallis GN, Martin M. TLR-signaling networks: an integration of adaptor molecules, kinases, and cross-talk. J Dent Res. 2011;90:417–27. doi: 10.1177/0022034510381264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caparrós E, et al. DC-SIGN ligation on dendritic cells results in ERK and PI3K activation and modulates cytokine production. doi: 10.1182/blood-2005-03-1252. [DOI] [PubMed] [Google Scholar]
- 39.Garcia-Vallejo JJ, van Kooyk Y. The physiological role of DC-SIGN: a tale of mice and men. Trends Immunol. 2013;34:482–6. doi: 10.1016/j.it.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 40.Xu X, Yasuda M, Mizuno M, Ashida H. β-Glucan from Saccharomyces cerevisiae reduces lipopolysaccharide-induced inflammatory responses in RAW264. 7 macrophages. Biochimica et Biophysica Acta (BBA) – General Subjects. 2012;1820:1656–63. doi: 10.1016/j.bbagen.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 41.Marakalala MJ, et al. Dectin-1 plays a redundant role in the immunomodulatory activities of β-glucan-rich ligands in vivo. Microbes Infect. 2013 doi: 10.1016/j.micinf.2013.03.002. http://dx.doi.org/10.1016/j.micinf.2013.03.002. [DOI] [PMC free article] [PubMed]
- 42.Van der Graaf CAA, Netea MG, Verschueren I, van der Meer JWM, Kullberg BJ. Differential cytokine production and toll-like receptor signaling pathways by Candida albicans blastoconidia and hyphae. Infect Immun. 2005;73:7458–64. doi: 10.1128/IAI.73.11.7458-7464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Torosantucci A, Chiani P, Cassone A. Differential chemokine response of human monocytes to yeast and hyphal forms of Candida albicans and its relation to the β-1,6 glucan of the fungal cell wall. [PubMed] [Google Scholar]