Abstract
Proanthocyanidins (PACs) are secondary plant metabolites that mediate non-enzymatic collagen cross-linking and enhance the properties of collagen based tissue, such as dentin. The extent and nature of cross-linking is influenced by the composition and specific chemical structure of the bioactive compounds present in certain PAC-rich extracts. This study investigated the effect of the molecular weight and stereochemistry of polyphenol compounds on two important properties of dentin, biomechanics and biostability. For that, purified phenols, a phenolic acid and some of its derivatives were selected: PACs dimers (A1, A2, B1 and B2) and a trimer (C1), gallic acid (Ga), its esters methyl gallate (MGa) and propyl gallate (PGa), and a pentagalloyl ester of glucose (PGG). Synergism was assessed by combination of the most active PAC and gallic acid derivative. Mechanical properties of dentin organic matrix were determined by the modulus of elasticity obtained in a flexural test. Biostability was evaluated by resistance to collagenase degradation. PACs significantly enhanced dentin mechanical properties and decreased collagen digestion. Among the gallic acid derivatives, only PGG had a significant enhancing effect. The lack of observed C1:PGG synergy indicates that both compounds have similar mechanisms of interaction with the dentin matrix. These findings reveal that the molecular weight of polyphenols have a determinant effect on their interaction with type I collagen and modulate the mechanism of cross-linking at the molecular, inter-molecular, and inter-micro-fibrillar levels.
Introduction
Dentin is a calcified extracellular matrix that forms the bulk of the tooth. It is a highly organized biological composite consisting of a type I collagen-rich organic phase (20 %w) and a mineral phase formed mainly by hydroxyapatite crystals (70 %w).1,2 Dentin is fundamentally involved in restorative and reparative therapies of missing tooth structure, which is based on an interaction of its organic matrix with polymer based biomaterials. However, dentin collagen biodegradation has been linked to poor clinical performance of resin-based restorations and progression of caries.3,4
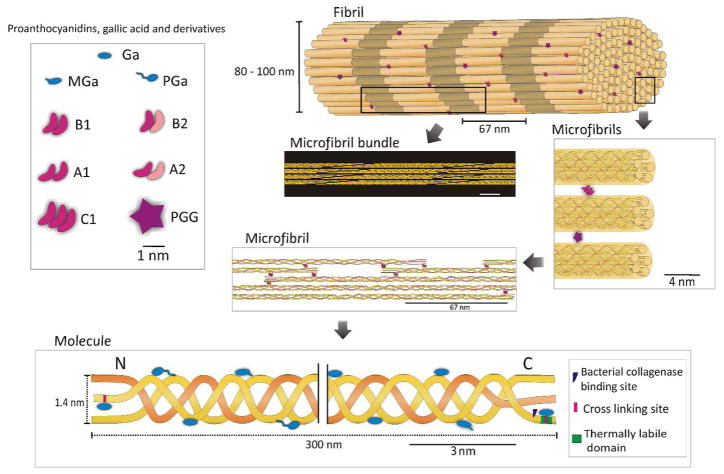
The collagen fibril is formed by bundles of cross-linked microfibrils arranged by the staggering of collagen molecules (Figure 1).5–10 Stability and tensile strength of the collagen molecule is primarily achieved by lysine-hydroxylysine cross-links between the C- and N-terminal telopeptides.11 Mimicking the physiological cross-linking mechanism in dentin can provide new insights into the development of biologically inspired biomaterials for tissue repair. This is specifically relevant for dentin since it has limited regenerative ability; therefore non-cellular based strategies are necessary for enhancing mechanically and enzymatically the existing substrate.
Figure 1.
Collagen fibril hierarchical structure and possible dentin biomodification mechanisms. PACs, gallic acid and its derivatives were scaled to the dimensions of tissue. The collagen fibril shows the 67 nm periodicity, due to the staggering of the collagen molecules in the axial direction. One single collagen fibril is formed by smaller entities grouped in bundles, known as microfibrilar bundles5, with about 10 – 25 nm diameter.6 The microfibrils are 4 – 5 nm diameter structures5, spaced 3 – 4.5 nm from each other.7 Thus, inter-microfibrillar cross-linking is likely to occur mediated by C1 and PGG. The inter-microfibrillar cross-links may significantly affect the mechanical properties. Each microfibril contains five 1D staggered, rope-like right hand twisted collagen molecule.6 The distance between the collagen molecules (1.26 – 1.33 nm)5 allows inter-molecular cross-link by A1, A2, B1 and B2 compounds. On the molecular scale, the collagen molecule is formed by one N and one C-terminus along with a triple helix with 1014 amino acid residues.8 Available cross-linking sites are found in both N and C-terminus that allows for intra-molecular cross-link by smaller compounds. A hydroxyproline-depleted area constitutes the thermally labile domain in the C-terminus.9 Undertwisted regions of the triple helix and a collagenase binding site on the C-terminus are primary sites for bacterial collagenase binding.10 A1: procyanidin A1; A2: procyanidin A2; B1: procyanidin B1; B2: procyanidin B2; C1: procyanidin C1. Ga: gallic acid; MGa: methyl-gallate; PGa: propyl-gallate; PGG: penta-O-galloyl-β-D-glucose.
Plant-derived polyphenols are a complex group of secondary metabolites that include PACs. PACs are molecules that contain hydroxyl (mainly phenolic), ether and ester groups. They are formed by monomeric flavan-3-ol units that can be condensed to form oligomers and polymers characterized by different monomers linked by either an additional ether bond (C – O) (A-type PACs) or one or more C – C bonds (B-type PACs). Monomers can also conjugate with sugars, forming O- or C-glycosides, and with phenolic acids such as gallic acid, forming gallates.12
The ability of PACs to cross-link collagen has been attributed to their potential to induce linkages at the molecular, micro-fibrillar and fibrillar hierarchy of collagen.13 The presence and density of cross-links significantly affect the deformation behavior of collagen and stiffening can be promoted when they reach an extreme density14, such as cross-links promoted by polyphenols. Considerable evidence indicates that PAC-rich extracts enhance the mechanical properties and reduce biodegradation of the dentin organic matrix.15–18 However, the interactions are limited and, therefore, lower than what is reported for mixtures of mid and high-molecular weight forms present in PAC-rich extracts.18 Moreover, the variability in chemical structure, along with the presence of chiral centers, dramatically affects the arrangement of PAC molecules12 and therefore their bioactivities. This is supported by a recent observation of a strong correlation between interactions of galloylated monomeric PACs and the dentin matrix.19
Due to the presence of numerous hydroxyl groups, PACs could bind to hydroxyl, carboxyl, amino, and amide groups of collagen and mediate cross-links through hydrogen bonds.13,20,21 However, hydrophobic association between the aromatic nuclei of polyphenols and the pyrrolidine rings of protein can occur.22,23 The polarity of the polyphenols could also determine their interaction with proteins.24 Most of the studies aiming to characterize such mechanisms of interaction evaluate the binding of small peptides and phenol molecules, but PACs interaction with dentin might be different due to the structural and composition complexity of its organic matrix. Moreover, the presence, position and orientation of hydroxyl groups in polyphenols likely influences their ability to mediate cross-linking at the highly structured fibrillar, subfibrillar and microfibrillar hierarchical levels of collagen.
Using purified compounds, the present study correlates the chemical structure of plant-derived polyphenols and their interactions with dentin organic matrix. Molecules with different numbers and position of phenolic groups were selected to test the effect of phenols or phenolic acid components, molecular weight, and stereochemistry of the polyphenol compounds on their interactions with the dentin organic matrix. More specifically, A- and B-type PACs with different degrees of oligomerization and stereochemistry, a phenolic acid, its derivatives and an ester of glucose were investigated.
Experimental Section
Preparation of dentin specimens
Thirty intact extracted healthy human molars kept at −20 °C were used following approval by the Institutional Review Board of the University of Illinois at Chicago (#2011-0312). Crowns were cut using a slow speed diamond saw (Isomet, Buehler, Lake Bluff, IL) under water lubrication to obtain dentin beams of width 1.7 × thickness 0.5 × length 6.0 mm.16 Dentin specimens were demineralized in 10% phosphoric acid (Ricca Chemical Company, Arlington, TX, USA) for 5 hours16 to expose the organic matrix and randomly divided into the groups described below.
Selection of Purified Polyphenolic Compounds
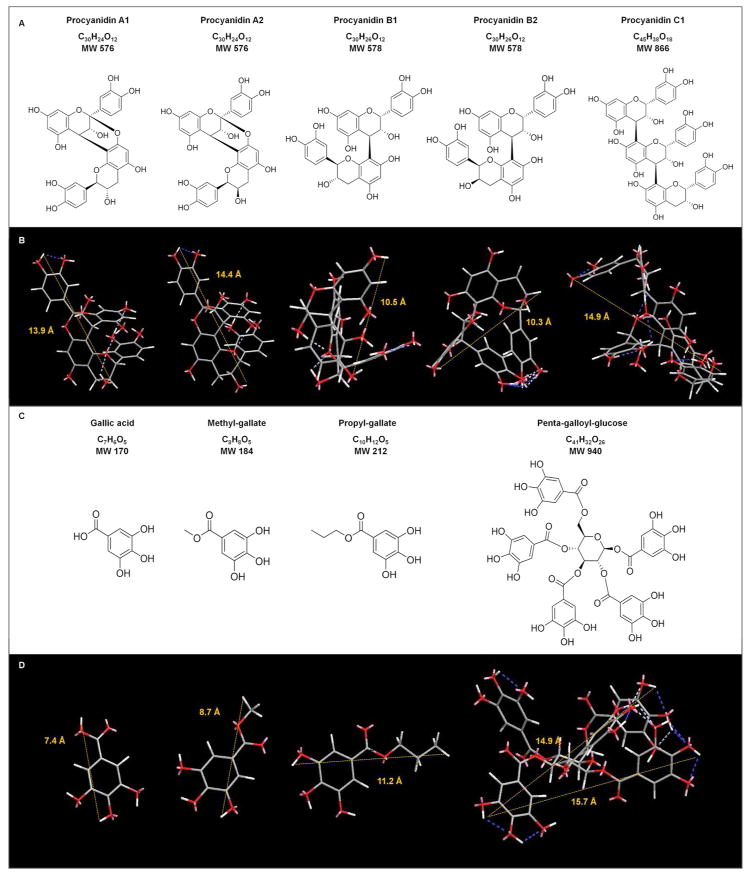
PACs, gallic acid, its derivatives and ester of glucose were chosen based on the diversity of their chemical structure. Commercially available purified A- and B-type PACs dimers and a trimer were selected: PACs A1, A2, B1, B2 and C1. Their chemical structure and molecular weight are presented in Figure 1. The compounds were obtained from ChromaDex® (Irvine, CA, USA) and had from 75 – 99.7% adjusted purities verified by high performance liquid chromatography (HPLC).
To determine the interaction of collagen with gallic acid derivatives, different alkyl esters of gallic acid and penta-galloyl-glucose were included (Figure 1). Gallic acid (Ga), methyl-gallate (MGa), propyl-gallate (PGa) and penta-galloyl-glucose (PGG) were obtained from Sigma-Aldrich (St. Louis, MO, USA), with adjusted purities (> 97%) verified by HPLC.
Potential synergy resulting from the association of the most active PAC and gallic acid and its derivatives were investigated at different ratios: 100% C1, 75% C1/25% PGG, 50% C1/50% PGG, 25% C1/75% PGG and 100% PGG.
Solutions of each compound were prepared at 0.65% (w/v) in 20 mM HEPES buffer at pH 7.2. Demineralized dentin beams were treated for 1 hour at room temperature.15 A control group was incubated with buffer under the same experimental conditions.
Biomechanical assay - Apparent modulus of elasticity
Modulus of elasticity (E) of the demineralized dentin beams was assessed by 3-point bending flexural test as previously described.19 The test was performed on a universal testing machine (EZ Graph, Shimadzu, Kyoto, Japan) using a 1 N load cell at crosshead speed of 0.5 mm/min. Beams were placed in an apparatus consisting of 3 mm span supports and tested immersed in distilled water at baseline and after incubation with the experimental solutions.
Biostability assay – Resistance to Enzymatic Biodegradation
Following the biomechanical assay, the same dentin specimens were incubated in collagenase to determine the dentin matrix biostability. Briefly, dentin beams were digested with type I bacterial collagenase (100 μg/ml Clostridium histolyticum) (Sigma-Aldrich, St. Louis, MO, USA) in 0.2 M ammonium bicarbonate pH 7.9 at 37 °C for 24 hours.17 Dentin biodegradation rates (R) were determined by the following formula: R (%) = 100 − (M2×100)/M1; where M1 is the dry mass before incubation and M2 is the dry mass after bacterial collagenase digestion.
Statistical analysis
The effects of PACs, gallic acid and its derivatives, and different C1/PGG ratios were statistically evaluated by two-way and one-way ANOVA followed by Tukey and Games-Howell post hoc tests (α = 0.05). Modulus of elasticity after treatment and biodegradation rates were correlated with molecular weight and number of hydroxyl groups of all phenols and phenolic acid compounds using Pearson’s correlation factor (α = 0.05).
Results and Discussion
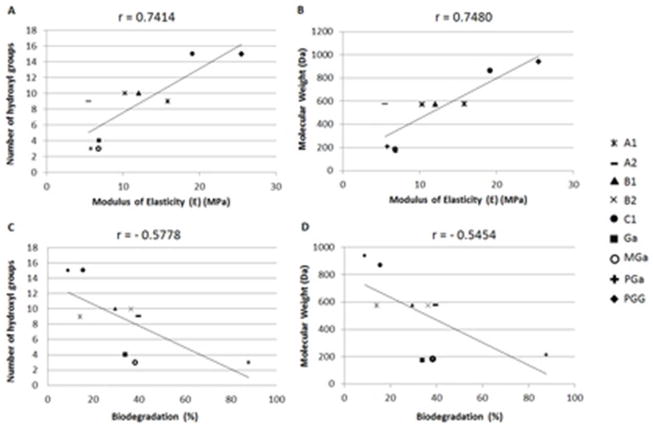
All PACs significantly enhanced dentin mechanical properties (Figure 3A), except for A2 which showed no significant difference when compared to the control (p < 0.05). The stereochemistry of the compounds exerted a secondary role in their interactions with dentin matrix. A higher bioactivity was observed for A1 when compared to A2. On the other hand, while B1 and B2 enhanced dentin mechanical properties and collagen stability significantly when compared to control (Figure 3A and 3D), they still presented similar bioactivity (p = 0.851). So a trend was seen, but no significant differences were observed arising from variations of the interflavane linkages of A- and B-type PACs. However, the molecular weight of the compounds played a major role in the enhancement of dentin mechanical properties. PAC C1 significantly increased dentin mechanical properties and determined the highest increase in E (Figure 3A). The highest protective effect against collagenase degradation was observed for C1 and A1.
Figure 3.
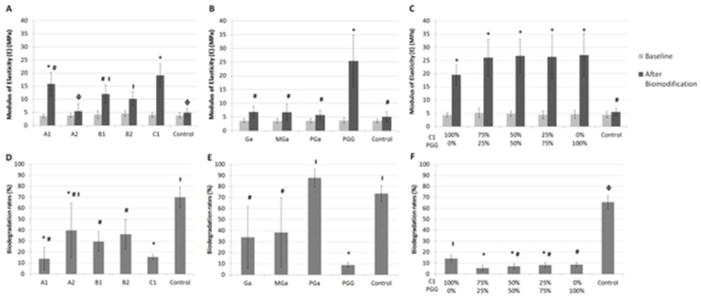
Mean (standard deviation) of modulus of elasticity and biodegradation rates of dentin beams treated with different PACs, gallic acid and its derivatives and association of C1 and PGG. Different symbols represent statistical significant difference (p < 0.05). No statistically significant difference was observed between groups at baseline (p > 0.05). A1: procyanidin A1; A2: procyanidin A2; B1: procyanidin B1; B2: procyanidin B2; C1: procyanidin C1. Ga: gallic acid; MGa: methyl-gallate; PGa: propyl-gallate; PGG: penta-O-galloyl-β-D-glucose.
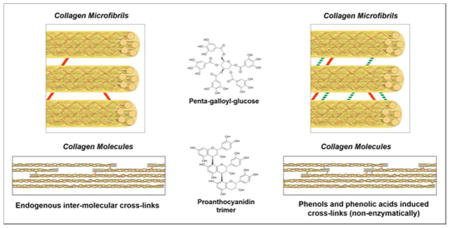
Oligomeric PACs improve collagen stability, but only selective compounds induce mechanical changes on dentin, as also observed with monomeric PACs.19 This is confirmed by the significant correlation between the molecular weight of the polyphenols and the improvement of dentin mechanical properties (p < 0.001) (Figure 4B and 4D). This effect can be explained by the role of collagen cross-links at different hierarchical organization levels of the fibril, specifically inter-molecular and inter-fibrillar spaces in type I collagen fibril (Figure 1). Intra-molecular cross-links provide primarily biostability to the collagen molecule, while inter-molecular and inter-microfibrillar cross-links enhance mechanical properties, in addition to fibril biostability. Based on an expected round or elongate molecular shape in solution, the molecular size of the compounds was calculated by the longest distance between atoms for each molecule (Figure 2B and 2D). Dynamic light scattering are needed to confirm the size profile of these compounds.25 Mid molecular weight PACs A1, B1, B2, and C1 can diffuse into the collagen fibril and mimic higher-scale collagen cross-links (inter-molecular and inter-microfibrillar cross-links) (Figure 1). Neighboring microfibrils are 3 – 4.5 nm spaced apart7, which can explain the inter-microfibrillar cross-links mediated by C1 (Figure 1). Except for A2, all PACs significantly reduced biodegradation rates of collagen (Figure 3D). The higher biostability promoted by PACs is also associated to their ability to inhibit bacterial collagenase26 and also matrix metalloproteinases (MMPs).27 It should be mentioned that bacterial collagenase and MMPs have different mechanism of protein degradation. While MMPs cleavage site is a specific Gly-Ile/Leu bond28, yielding three-quarter and one-quarter length fragments, bacterial collagenase can cleave collagen at multiple sites.29 Moreover, structural changes of non-enzymatic collagen cross-links induced by PACs may hide cleavage sites for enzyme docking and therefore reduce or impair collagen degradation. Different than bacterial collagenase, MMPs bind to type I collagen at a specific cleavage site, and the surrounding amino acid residues as well as triple helix dissociation are important for enzyme binding to collagen and further catalysis.30 Collagen cross-linking by PACs might also change dissociation of collagen triple helix, preventing MMPs binding to their cleavage site.
Figure 4.
Pearson correlation between modulus of elasticity (E) (MPa) after treatment and biodegradation rates (%) with number of hydroxyl groups (A and C) and molecular weight (Da) (B and D) of PACs and gallic acid and its derivatives.
Figure 2.
Chemical structure, formulae, and molecular weights of naturally occurring phenolic compounds used in this study. Two and three dimensional models for PACs (A and B) and gallic acid and its derivatives (C and D) were performed using Chem3D Pro of ChemBioDraw® package (ver. 13.0.2, PerkinElmer, Waltham, MA, USA). The distance between two hydrogen atoms was determined with MM2 function during the molecular dynamics calculation for each molecule in angstroms (Å). The dynamic ranges for the compounds were: between 13.5 and 14.1 Å for A1, 13.9 and 15.1 Å for A2, 9.5 and 10.9 Å for B1, 10.0 and 11.2 Å for B2, 12.8 and 18.2 Å for C1, 7.1 and 7.7 Å for Ga, 8.1 and 8.9 Å for MGa, 10.8 and 11.4 Å for PGa, and 14.9 and 18.0 Å for PGG (β-D-glucose conformation confirmed by NMR). The mean distances for each compound are shown in B and D. The minimize Energy for each molecule were −17.32 Kcal/mol for A1, −15.40 Kcal/mol for A2, −28.66 kcal/mol for B1, −33.69 Kcal/mol for B2, −43.66 Kcal/mol for C1, −10.76 Kcal/mol for Ga, −1.88 Kcal/mol for MGa, −0.68 Kcal/mol for PGa, and −25.01 Kcal/mol for PGG. Calculations were done using the following parameters: intervals of step and frame were set as 2.0 fs and 10 fs, respectively; iteration was stopped after 10000 steps, rate of heating/cooling was set as 0.1 Kcal/atom/ps; and target temperature of 300 Kelvin. Blue and white dashed lines indicate intra-molecular hydrogen bonds. The high intensity in blue color represents less than ideal geometry.
Among the gallic acid and its derivatives, PGG was the only compound to enhance the mechanical properties of the dentin matrix (Figure 3B). The increase in lengths of the alkyl chain of gallic acid derivatives was not able to improve dentin mechanical properties as no significant differences were observed for Ga, MGa or PGa (Figure 3B). These results indicate that bioactivity of these compounds is dependent of the galloyl moieties to be linked to a core structure as seen for PGG and also reported for monomeric PACs and other galloylglucoses.19,31 As with PACs, the molecular weights of the gallic acid and its derivatives were the primary determinants of their interaction with dentin matrix (Figure 1). Ga, MGa, and PGa are at the bottom of an ascending size scale. While they have the potential to bind intra-molecularly to collagen, they have limited if any ability to cross-link dentinal collagen.
On the other hand, significant lower collagen degradation was observed for PGG, Ga, and MGa (Figure 3E). Only PGa did not affect collagen biodegradation rates when compared to the control group (p = 0.348). Type I collagen fibrilar structure limits the accessibility of proteolytic enzymes to their cleavage site at the fibril surface, regulating the collagenolysis.30 These compounds might exert a similar mechanism, since they might have the ability to bind collagen molecules in or near the cleavage sites (Figure 1), without mediating cross-links, which explains the observed protective effect against degradation by collagenase in Ga- and MGa-treated dentin (Figure 3E). They could prevent proteolysis by coating the collagen molecule, which is supported by their low or no potential to inhibit the activity of this enzyme.32
All evaluated ratios of C1:PGG significantly enhanced dentin biomechanical properties (Figure 3C). Similarly, neat C1 and PGG or a mixture of both increased collagen biostability regardless of the ratio of compounds (Figure 3F), indicating no synergistic potential. The absence of synergy observed at different ratios of PGG to C1 is likely due to similar cross-linking mechanism. Considering their molecular size, PGG and C1 are likely to induce primarily inter-microfibrillar cross-links. Moreover, the diffusion rates of the compounds may be a determinant factor of the presence of PGG- or C1-mediated inter-micro-fibrillar cross-links.
The main mechanism of interaction between polyphenols with collagen is not fully elucidated. It is believed that collagen cross-links induced by PACs are of hydrogen bond nature, and occur between the hydroxyl groups in their functional terminals with the side chains hydroxyl, carboxyl, amino and amide groups of collagen.13,21,24 The high positive correlation between the number of hydroxyl groups in the purified compounds and enhancing mechanical properties (p < 0.001) (Figure 4A and 4D) as well as the negative correlation between number of hydroxyl groups and biodegradation rates (p < 0.001) (Figure 4C) support the importance of hydrogen bonding in the non-enzymatic cross-linking induced by polyphenols. The stabilization of hydrogen bonds can be enhanced by the presence of hydrophobic pockets, believed to be unique for PACs.20 However, the possibility of additional hydrophobic interactions between collagen and PACs or gallic acid derivatives cannot be excluded. Such interaction was suggested as the main mechanism of polyphenols association with proline-rich peptides, in which prolyl residues have hydrophobic association with the aromatic phenolic rings of polyphenols.22,23 It is important to highlight that our model does not account for a possible influence of non-collagenous proteins in the interaction of polyphenols with collagen in dentin.
Similar to PAC-C1, PGG likely induces inter-microfibrillar cross-links (Figure 1). The higher increase in E following treatment with PGG as compared to PAC-C1 (6.99 and 4.76 fold increase, respectively) is likely related to the molecule backbone, as both compounds present the same number of hydroxyl moieties (15 –OH groups). PGG consists of D-glucose in which the five hydroxyl groups are esterified with gallic acids (Figure 2). This compound has no inter-galloyl covalent linkages, which provides flexibility to the galloyl groups; thus favoring interactions with collagen.33 Higher molecular flexibility is provided by the steric orientation of large size phenolic compounds (such as PGG), where a galloyl moiety interactions with proline is facilitated by another galloyl moiety-proline interaction.34 Moreover, PGG can form a hydrophobic coating around proteins24, which contributes to the presence of both hydrogen bonds and hydrophobic interactions with collagen.
Conclusions
In summary, the present study demonstrated that the improvement of dentin biomechanical and biostability are dependent on polyphenols molecular weight, which significantly affects their interaction with the dentin matrix. The enhancement of the mechanical properties is driven by both hydrogen and hydrophobic interactions of polyphenols, mediating collagen cross-linking at inter-molecular and inter-microfibrillar levels. However, the biostability is determined by distinct mechanisms and is also achieved by small molecular weight compounds due to their ability to bind/coat collagen intra- and extra-molecularly.
References
- 1.Marshall GW, Jr, Marshall SJ, Kinney JH, Balooch M. The Dentin Substrate: Structure and Properties Related to Bonding. J Dent. 1997;25:441–458. doi: 10.1016/s0300-5712(96)00065-6. [DOI] [PubMed] [Google Scholar]
- 2.Kinney JH, Marshall SJ, Marshall GW. The Mechanical Properties of Human Dentin: A Critical Review and Re-Evaluation of the Dental Literature. Crit Rev Oral Biol Med. 2003;14:13–29. doi: 10.1177/154411130301400103. [DOI] [PubMed] [Google Scholar]
- 3.Carrilho MR, Geraldeli S, Tay F, De Goes MF, Carvalho RM, Tjäderhane L, Reis AF, Hebling J, Mazzoni A, Breschi L, Pashley D. In vivo Preservation of the Hybrid Layer by Chlorhexidine. J Dent Res. 2007;86:529–533. doi: 10.1177/154405910708600608. [DOI] [PubMed] [Google Scholar]
- 4.Tjäderhane L, Larjava H, Sorsa T, Uitto VJ, Larmas M, Salo T. The Activation and Function of Host Matrix Metalloproteinases in Dentin Matrix Breakdown in Caries Lesions. J Dent Res. 1998;77:1622–1629. doi: 10.1177/00220345980770081001. [DOI] [PubMed] [Google Scholar]
- 5.Bertassoni LE, Orgel JPR, Antipova O, Swain MV. The Dentin Organic Matrix – Limitations of Restorative Dentistry Hidden on the Nanometer Scale. Acta Biomater. 2012;8:2419– 2433. doi: 10.1016/j.actbio.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orgel JPRO, Irving TC, Miller A, Wess TJ. Microfibrillar Structure of Type I CollagenIn Situ. Proc Natl Acad Sci USA. 2006;103:9001–9005. doi: 10.1073/pnas.0502718103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Habelitz S, Balooch M, Marshall SJ, Balooch G, Marshall GW., Jr In situ Atomic Force Microscopy of Partially Demineralized Human Dentin Collagen Fibrils. J Struct Biol. 2002;138:227–236. doi: 10.1016/s1047-8477(02)00029-1. [DOI] [PubMed] [Google Scholar]
- 8.Orgel JPRO, Miller A, Irving TC, Fischetti RF, Hammersley AP, Wess TJ. The in situ Supermolecular Structure of Type I Collagen. Structure. 2001;9:1061–1069. doi: 10.1016/s0969-2126(01)00669-4. [DOI] [PubMed] [Google Scholar]
- 9.Miles CA, Bailey AJ. Thermally Labile Domains in the Collagen Molecule. Micron. 2001;32:325–332. doi: 10.1016/s0968-4328(00)00034-2. [DOI] [PubMed] [Google Scholar]
- 10.Philominathan STL, Koide T, Hamada K, Yasui H, Seifert S, Matsushita O, Sakon J. Undirectional Binding of Clostridial Collagenase to Triple Helical Substrates. J Biol Chem. 2009;284:10868–10876. doi: 10.1074/jbc.M807684200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miguez PA, Pereira PNR, Atsawasuwan P, Yamauchi M. Collagen Cross-linking and Ultimate Tensile Strength in Dentin. J Dent Res. 2004;83:807–810. doi: 10.1177/154405910408301014. [DOI] [PubMed] [Google Scholar]
- 12.Bedran-Russo A, Pauli GF, Chen SN, McAlpine J, Castellan CS, Phansalkar RS, Aguiar TR, Vidal CMP, Napotilano JG, Nam JW, Leme AA. Dentin Biomodification: Strategies Renewable Resources and Clinical Applications. Dent Mater. 2014;30:62–76. doi: 10.1016/j.dental.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He L, Mu C, Shi J, Zhang Q, Shi B, Lin W. Modification of Collagen with a Natural Cross-linker, Procyanidin. Int J Biol Macromol. 2011;48:354–359. doi: 10.1016/j.ijbiomac.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 14.Buehler MJ. Nanomechanics of Collagen Fibrils Under Varying Cross-Link Denstities: Atomistic and Continuum Studies. J Mech Behav Biomed Mater. 2008;1:59–67. doi: 10.1016/j.jmbbm.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Bedran-Russo AKB, Pashley DH, Agee K, Drummond JL, Miescke KJ. Changes in Stiffness of Demineralized Dentin Following Application of Collagen Crosslinkers. J Biomed Mater Res Part B: Appl Biomater. 2008;86B:330–334. doi: 10.1002/jbm.b.31022. [DOI] [PubMed] [Google Scholar]
- 16.Castellan CS, Pereira PN, Grande RHM, Bedran-Russo AK. Mechanical Characterization of Proanthocyanidin–Dentin Matrix Interaction. Dent Mater. 2010;26:968–973. doi: 10.1016/j.dental.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bedran-Russo AKB, Castellan CS, Shinohara MS, Hassan L, Antunes A. Characterization of Biomodified Dentin Matrices for Potential Preventive and Reparative Therapies. Acta Biomater. 2011;7:1735–1741. doi: 10.1016/j.actbio.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aguiar TR, Vidal CMP, Phansalkar RS, Todorova I, Napolitano JG, McAlpine JB, Chen SN, Pauli GF, Bedran-Russo AK. Dentin Biomodification Potential Depends on Polyphenol Source. J Dent Res. 2014;93:417–422. doi: 10.1177/0022034514523783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vidal CMP, Aguiar TR, Phansalkar R, McAlpine JB, Napolitano JG, Chen SN, Araújo LSN, Pauli GF, Bedran-Russo A. Galloyl Moieties Enhance the Dentin Biomodification Potential of Plant-Derived Catechins. Acta Biomater. 2014;10:3288–3294. doi: 10.1016/j.actbio.2014.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han B, Jaurequi J, Tang BW, Nimni ME. Proanthocyanidin: A Natural Crosslinking Reagent for Stabilizing Collagen Matrices. J Biomed Mater Res. 2003;65A:118–124. doi: 10.1002/jbm.a.10460. [DOI] [PubMed] [Google Scholar]
- 21.Canon F, Giuliani A, Paté F, Sarni-Manchado P. Ability of a Salivary Intrinsically Unstructured Protein to Bind Different Tannin Targets Revealed by Mass Spectrometry. Anal Bioanal Chem. 2010;398:815–822. doi: 10.1007/s00216-010-3997-9. [DOI] [PubMed] [Google Scholar]
- 22.Luck G, Liao H, Murray NJ, Grimmer HR, Warminski EE, Williamson MP, Lilley TH, Haslam E. Polyphenols, Astringency and Proline-Rich Proteins. Phytochem. 1994;37:357–371. doi: 10.1016/0031-9422(94)85061-5. [DOI] [PubMed] [Google Scholar]
- 23.Baxter NJ, Lilley TH, Haslam E, Williamson MP. Multiple Interactions between Polyphenols and a Salivary Proline-Rich Protein Repeat Result in Complexation and Precipitation. Biochemistry. 1997;36:5566–5577. doi: 10.1021/bi9700328. [DOI] [PubMed] [Google Scholar]
- 24.Hagerman AE, Rice ME, Ritchard NT. Mechanisms of Protein Precipitation for Two Tannins, Pentagalloyl Glucose and Epicatechin16 (4f8) Catechin (Procyanidin) J Agric Food Chem. 1998;46:2590–2595. [Google Scholar]
- 25.Brás NF, Gonçalves R, Fernandes PA, Mateus N, Ramos MJ, Freitas V. Understanding the Binding of Procyanidins to Pancreatic Elastase by Experimental and Computational Methods. Biochemi. 2010;49:5097–5108. doi: 10.1021/bi100410q. [DOI] [PubMed] [Google Scholar]
- 26.Madhan B, Krishnamoorthy G, Rao JR, Nair BU. Role of Green Tea Polyphenols in the Inhibition of Collagenolytic Activity by Collagenase. Int J Biol Macromol. 2007;41:16–22. doi: 10.1016/j.ijbiomac.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 27.La VD, Howel AB, Grenier D. Cranberry Proanthocyanidins Inhibit MMP Production and Activity. J Dent Res. 2009;88:627–632. doi: 10.1177/0022034509339487. [DOI] [PubMed] [Google Scholar]
- 28.Visse R, Nagase H. Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases: Structure, Function, and Biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 29.Philominathan STL, Koide T, Matsushita O, Sakon J. Bacterial collagen-binding domain targets undertwisted regions of collagen. Prot Sci. 2012;21:1554–1565. doi: 10.1002/pro.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perumal S, Antipova O, Orgel JPRO. Collagen Fibril Architecture, Domain Organization, and Triple Helical Conformation Govern its Proteolysis. Proc Natl Acad Sci USA. 2008;105:2824– 2829. doi: 10.1073/pnas.0710588105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawamoto H, Nakatsubo N, Murakami K. Stoichiometric Studies of Tannin-Protein Co-Precipitation. Phytochem. 1996;41:1427–1431. doi: 10.1016/0031-9422(95)00728-8. [DOI] [PubMed] [Google Scholar]
- 32.Jackson JK, Zhao J, Wong W, Burton HM. The Inhibition of Collagenase Induced Degradation of Collagen by the Galloyl-Containing Polyphenols Tannic Acid, Epigallocatechin Gallate and Epicatechin Gallate. J Mater Sci: Mater Med. 2010;21:1435–1443. doi: 10.1007/s10856-010-4019-3. [DOI] [PubMed] [Google Scholar]
- 33.Tang HR, Covington AD, Hancock RA. Structure–Activity Relationships in the Hydrophobic Interactions of Polyphenols with Cellulose and Collagen. Biopolymers. 2003;70:403–413. doi: 10.1002/bip.10499. [DOI] [PubMed] [Google Scholar]
- 34.Cala O, Pinaud N, Simon C, Fouquet E, Laguerre M, Dufourc EJ, Pianet I. NMR and Molecular Modeling of Wine Tannins Binding to Saliva Proteins: Revisiting Astringency from Molecular and Colloidal Prospects. FASEB J. 2010;24:4281–4290. doi: 10.1096/fj.10-158741. [DOI] [PubMed] [Google Scholar]