Abstract
Background
Working memory problems have been targeted as core deficits in individuals with Fragile X syndrome (FXS); however, there have been few studies that have examined working memory in young boys with FXS, and even fewer studies that have studied the working memory performance of young boys with FXS across different degrees of complexity. The purpose of this study was to investigate the phonological loop and visual–spatial working memory in young boys with FXS, in comparison to mental age-matched typical boys, and to examine the impact of complexity of the working memory tasks on performance.
Methods
The performance of young boys (7 to 13-years-old) with FXS (n = 40) was compared with that of mental age and race matched typically developing boys (n = 40) on measures designed to test the phonological loop and the visuospatial sketchpad across low, moderate and high degrees of complexity. Multivariate analyses were used to examine group differences across the specific working memory systems and degrees of complexity.
Results
Results suggested that boys with FXS showed deficits in phonological loop and visual–spatial working memory tasks when compared with typically developing mental age-matched boys. For the boys with FXS, the phonological loop was significantly lower than the visual–spatial sketchpad; however, there was no significant difference in performance across the low, moderate and high degrees of complexity in the working memory tasks. Reverse tasks from both the phonological loop and visual–spatial sketchpad appeared to be the most challenging for both groups, but particularly for the boys with FXS.
Conclusions
These findings implicate a generalised deficit in working memory in young boys with FXS, with a specific disproportionate impairment in the phonological loop. Given the lack of differentiation on the low versus high complexity tasks, simple span tasks may provide an adequate estimate of working memory until greater involvement of the central executive is achieved.
Keywords: central executive, Fragile X syndrome (FXS), phonological loop, visual–spatial sketchpad, working memory, working memory complexity in FXS
Introduction
Working memory involves the short-term storage, retrieval and processing of information that is central to the performance of many cognitive tasks. Baddeley (1986, 1996, 2003, 2007) and Baddeley & Hitch (1974) have proposed that working memory reflects the operation of a central executive system and two ‘slave’ subsystems: the phonological loop and visuospatial sketchpad. An important aspect of this model is that the storage of visual–spatial and phonological loop information is largely dissociated and, in this way, separate systems are responsible for the online storage and maintenance of the different types of information. If there is disruption to any of these components, various deficits can arise, such as an intrusion on academic skills (Henry & Winfield 2010).
Working memory and intellectual disabilities
Certain intellectual and other developmental disabilities manifest deficits that can be attributed to a specific subsystem within the working memory model (Alloway et al. 2009; Carretti et al. 2010; Schuchardt et al. 2010). For example, the phonological loop has been shown to be disproportionately impaired in children with Down syndrome (Varnhagen et al. 1987; Kay-Raining Bird & Chapman 1994; Wang & Bellugi 1994; Das & Mishra 1995). Most recently, Lanfranchi et al. (2009b) reported that individuals with Down syndrome experienced deficits in both the central executive and phonological loop, and that these deficits were independent of general verbal deficits. In a study that further examined the working memory of children with Down syndrome, Lanfranchi et al. (2009a) noted that visual–spatial working memory was not entirely intact when different types of visual–spatial working memory were examined. These investigators reported that children with Down syndrome performed comparably to their mental age-matched peers on spatial–sequential working memory tasks, but were poorer on the spatial–simultaneous type tasks. Similarly, O’Hearn et al. (2009) studied the working memory functions in children with Williams syndrome and reported an overall working memory impairment in this population.
The level and pattern of working memory capabilities of individuals with other intellectual and developmental disabilities are not so clear. For example, work by Russell et al. (1996) and others (Ozonoff & Strayer 2001) has suggested children with autism have weaker central executive capacity than typically developing peers, but that their working memory abilities are relatively intact. Steele et al. (2007) challenged this notion of intact working memory in autism by suggesting that these findings may be impaired when working memory load exceeds the limited capacity of the individual, but may appear intact when the targeted task is within that working memory capacity. Cui et al. (2010) supported this assertion in their sample of children with Asperger’s syndrome. These investigators reported that children with Asperger’s syndrome performed better on tasks measuring the phonological loop, such as digit and word span tasks, but worse on tasks measuring the visual–spatial sketchpad, such as a block recall task. They also noted hat children with Asperger’s syndrome required more time to complete the n-back task, and that the larger and more complex the task load, the larger the group differences manifested. As these studies illustrate, the examination of working memory profiles in individuals with intellectual and other developmental disabilities will require ongoing investigation, but the complexity of the working memory tasks may hold a key element for these investigations.
Working memory and Fragile X syndrome
Fragile X syndrome (FXS) is a neurodevelopmental disorder that is the leading known inherited condition causing intellectual disabilities with a prevalence rate of 1:2500 (Crawford et al. 2002; Hagerman 2008). Considerable variation exists in the expression of FXS in men and women, but men tend to have moderate to severe intellectual disabilities and women commonly have specific learning and attention difficulties (Freund et al. 1993; Mazzocco et al. 1993; Mazzocco 2000). With respect to working memory, some studies of women with FXS have reported relatively poorer performances in the phonological loop (Mazzocco et al. 1993; Keenan & Simon 2004), while others (Kwon et al. 2001) have reported significant deficits in the visuospatial sketchpad.
For men, earlier work indicated deficits in both working memory subsystems (Freund & Reiss 1991). Shapiro et al. (1995) found that the performance of men with FXS was similar to that of a comparison group with Down syndrome on a forward digit span task; however, on object and block tapping tasks, men with FXS showed significantly more impairment, suggesting a selective impairment in the visuospatial sketchpad as opposed to the phonological loop. A similar pattern of deficits has been reported for young boys with FXS (Freund & Reiss 1991; Shapiro et al. 1995; Turk 1998; Cornish et al. 1999), although generalised deficits in working memory also have been described (Munir et al. 2000; Ornstein et al. 2008).
Munir et al. (2000) failed to replicate the specific findings of disproportionate weakness in the visuospatial sketchpad in their examination of working memory in children with FXS. Using several key comparison groups, a chronological age matched group, a mental age-matched group and a group with Down syndrome, Munir et al. (2000) reported generalised deficits on all working memory tasks relative to the two groups of typically developing controls. Munir et al. (2000) concluded that children with FXS have a global deficit in working memory that may be attributed to the complexity of the task and how much attention resource it requires. In this regard, Lanfranchi et al. (2009c) directly addressed this issue and reported that children with FXS demonstrated a performance equal to controls on low and medium levels of attention demands across both phonological loop and visuospatial working memory tasks; however, significant deficits were apparent only when high degrees of attention control were required. Further research is necessary to explore the discrepant results between Lanfranchi et al. (2009c), Munir et al. (2000) and past studies that have indicated impairment in visual–spatial functions (e.g. Freund & Reiss 1991; Turk 1998; Cornish et al. 1999), with the issue of task complexity and its operationalisation continuing to be of keen interest.
The current investigation
The present study was designed to examine the working memory of young boys with FXS as compared with a mental age-matched typical group using both phonological loop and visual–spatial working memory tasks. Additionally, we were interested in determining whether task complexity, across a gradient of relative difficulty, would affect performance in the working memory subsystems. It was hypothesised that young boys with FXS would show overall impairment on all working memory tasks when compared with mental age-matched typical controls, with no significant differences noted between the phonological loop and visual–spatial sketchpad. Further, in accordance with recent findings by Lanfranchi et al. (2009c), it was suspected that the FXS group would show significantly better performance on tasks of low complexity than on tasks of higher complexity across working memory subsystems.
Method
Participants
Forty boys diagnosed with full mutation FXS on the basis of DNA analyses participated in the study. Their chronological ages ranged between of 7.97 and 13.22 years of age (M = 10.68, SD = 1.55), and they had associated mental ages that ranged from 4.08 to 6.67 years (M = 5.26, SD = 0.68). Approximately 87.5% of participants were European American, 10% were African American, and 2.5% were Hispanic. About 68% of the mothers had a partial college education or a four-year college degree and 19% had a graduate degree. Of the 40 participants with FXS, 75% were taking some medication at the time of the testing, and about 27.5% met screening criteria for autism on the Childhood Autism Rating Scale.
The 40 typically developing boys were recruited from a variety of childcare centres and elementary schools in the areas surrounding the university. This group comprised children whose mental age (MA), based on the Leiter-R Brief IQ Screener Age Equivalent, was within 2 months of a child in the FXS group of the same race and gender. Only typically developing children with IQ estimates within one standard deviation of the norm ± 5 points (i.e. between 80 and 120) were included in the study. This group ranged in chronological age from 2.63 to 7.48 years, with an average age of 5.20 years (SD = 0.90), while the mean MA was 4.80 years (SD = 0.96). Approximately 87.5% of participants were European American, 10% were African American and 2.5% were Hispanic, and 56% of the mothers had a partial college education or a four-year college degree and 5% had a graduate degree. Boys with psychiatric disorders, learning disabilities, traumatic brain injuries, seizures, attention deficit disorder or other disabilities were excluded from the typical group.
Measures
In accordance with Baddeley’s model, three tasks were selected to tap each ‘slave’ subsystem, with each task representing an increasing level of difficulty across three levels of complexity (i.e. degree of demand on the central executive). In general, the tasks selected for the lowest levels of complexity required the child to repeat or reproduce a series of words or spatial locations. For the medium levels of complexity, the child needed to conduct an operation on the stimuli (i.e. reversing numbers and spatial sequences) in order to perform the task. Finally, for the highest levels of complexity, the child needed to engage in multiple operations in order to perform the task adequately (i.e. recalling pictures that were increasing further back in a sequence, remembering the order of both letters and objects presented in a random fashion). For this study, the level of difficulty of a task was determined by: (1) the number of processes involved to complete the task successfully (Turner & Engle 1989; Morris et al. 1990; Salthouse & Babcock 1991); and (2), given the nature of our sample, we also used a statistical standard examining the test floor for each of the tasks such that the higher the floor, the more difficult the working memory task. All working memory tasks were part of a comprehensive neuropsychological battery administered over a 2- or 3-day period, and all tasks were blocked and counterbalanced across the testing sessions to reduce any order effects. Given the young age of many of our participants, particularly the children in our mental age-matched typical group, examiners worked to ensure that all of the tasks were understandable by the children. If a child could not follow the directions of a task after several attempts, or was unable to perform the task after the teaching items on several of the tasks (e.g. Leiter-R Reverse Memory), it was not administered and counted as missing data.
Phonological loop working memory
Phonological loop measures included three subtests from the Woodcock–Johnson Test of Cognitive Abilities-III (WJ-III; Woodcock & Johnson 2001): Memory for Words (low complexity with the least demands on the central executive), Numbers Reversed (moderate complexity) and Auditory Working Memory (high complexity with the most demands on the central executive). All tasks were administered and scored in accordance with standardised procedures.
Memory for Words is a word span measure that requires participants to repeat a series of unrelated words of increasing length in the same order in which the items are presented. The task begins with a single word, and increases in span with each item. It is discontinued when the highest three items are missed. The Memory for Words subtest has a median internal consistency reliability coefficient of 0.80. Raw scores range from 0 to 24.
As a measure of moderate complexity of phonological working memory, the WJ-III Numbers Reversed Subtest was utilised. Here, the participant must recall increasingly longer sequences of numbers and then repeat them back to the examiner in reverse order. The participant receives one point for each correct sequence, and the task is completed when three consecutive sequences are incorrect. The Numbers Reverse Subtest has a median internal consistency reliability coefficient of 0.80. Raw scores range from 0 to 30.
The Auditory Working Memory task requires the participant to listen to numbers and object names in a mixed-up order, and to repeat back the objects first and then the numbers in their respective orders. One point is given for the correct sequence of objects and one point for digits, for a possible raw score of 2 points on each trial. The task begins with a single number and a single object and increases to 4 numbers and 4 objects over 12 trials, and terminates when incorrect responses for both numbers and objects are provided. Median internal consistency reliability is 0.88. Raw scores range from 0 to 42.
Visual–spatial working memory tasks
Visual–spatial working memory measures included two subtests from the Leiter-R (Roid & Miller 1997), Spatial Memory (low complexity with the least demands on the central executive) and Reverse Memory (moderate complexity), and the n-Back Task (high complexity with the most demands on the central executive) (Cohen et al. 1994).
As a measure of visual working memory with low complexity, the Leiter-R Spatial Memory Subtest was used. For this subtest, a display of pictures in a matrix is shown for 10 s and then removed, after which the participant is asked to place cards of the pictured objects in the correct locations on a blank matrix. The task begins with a single picture in a two-box matrix and ends with eight pictures in a 12-box matrix. To receive credit on a given trial, the child must place correctly all pictures presented, and the task is discontinued after six errors in a row. Median internal consistency reliability is 0.85. Raw scores range from 0 to 20.
For moderate complexity in visual–spatial working memory, we used the Leiter-R Reverse Memory Subtest. For this task, the examiner points to pictures at the rate of one per second, and then the participant must point to the same pictures in reverse order. Items begin with one picture, and then the number of pictures increases, with the task terminating when six cumulative sequences have been failed. Median internal consistency reliability is 0.87. Raw scores range from 0 to 21.
The n-Back Task was adapted for use with the Fragile X and mental age-matched typical samples and served as our visual–spatial working measure of high complexity. Pictures of familiar objects (e.g. hand, spoon) were shown to the child for 2 s and then inserted into one of three folders placed side by side on the table. Once the picture was placed in the folder, the subject was asked to remember what picture was shown ‘n’ cues back, where ‘n’ is 0, 1, or 2 cues back. Ten trials for each condition were presented. A weighted score was created that gave credit for every correct item: one point was given for each correct response at 0-back, 2-points were given for each correct response at 1-back, and 3 points were given for each correct response at 2-back, for a maximum raw score range of 0 to 60. The total score comprised the overall weighted score across conditions.
Other measures
Two other measures were used to address issues of mental age matching and the number of autism symptoms. These measures included the Brief IQ from the Leiter-R (Roid & Miller 1997), which utilised different subtests that those used for the visual–spatial sketchpad, and the Childhood Autism Rating Scale (CARS; Schopler et al. 1988), which provided an examiner rated estimate of the number of autism symptoms.
Data analyses
This was a cross-sectional, repeated measures design as the participants were tested individually on several measures of working memory. A repeated measures multivariate analysis of variance (MANOVA) was used to test for main and interaction effects across the two groups (FXS and Typicals), the two types of working memory (phonological loop, visual–spatial sketchpad) and the three levels of complexity (low, moderate, high). For post hoc analyses, a Bonferroni adjustment was used.
Results
Preliminary analyses
First, in an initial review of the descriptive statistics for the tasks, it was apparent that the children’s performance on two of the tasks (WJ-III Numbers Reversed and Leiter-R Reverse Memory) was characterised by floor effects, and hence the scores were not normally distributed. These skewed distributions of scores represented violations of the assumptions of the planned statistical analysis, so these two tasks were removed from the full model analyses and examined independently. Scores from the four remaining dependent variables were normally distributed, although one outlier was identified and subsequently removed from the FXS group.
Second, raw scores on all dependent variables were transformed into standardised z-scores based on the mean and standard deviation of the entire subject pool (n = 80). Standardising in this way allowed for comparisons across groups and tasks given the different metrics used to score each task. Further, in the event of group differences on the working memory measures, this conversion provided a conservative strategy for examining the data for interactions across the two groups (FXS and Typicals), the two types of working memory (phonological loop, visual–spatial sketchpad), and the different levels of task complexity. Finally, for this study we were interested in the comparison of the FXS group to the mental age-matched typical group as opposed to a comparison to chronological age based normative data, and the use of our overall sample to generate the z-scores for comparative purposes provided that opportunity.
Group differences on working memory measures
Mean raw scores for all of the dependent variables from both groups, including the WJ-III Numbers Reversed and Leiter-R Reverse Memory tasks, are represented in Table 1. Table 2 shows the representative z-scores for all of the working memory tasks used in the data analyses. It was suspected that the performance patterns on working memory measures would differ significantly for the boys with FXS when compared with the typically developing comparison group. To address this hypothesis, a Group (FXS, TYP) X Type (phonological loop, visual–spatial) X Complexity (low complexity vs. high complexity) repeated measures MANOVA was used to test for main effects and interactions Results of the MANOVA revealed that there was a significant difference for the group variable (F1,66 = 29.05, P < 0.001). Examination of the group mean raw scores for each of the tasks listed in Table 1 showed the scores of the FXS group to be lower than those of the typical group on all of the working memory tasks.
Table 1.
Mean raw scores for phonological loop and visual–spatial sketchpad working memory tasks in boys with Fragile X syndrome (FXS) and mental age-matched typically developing boys
| Task | FXS group
|
Typical group
|
||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Phonological loop tasks | ||||
| WJ-III Memory for Words | 5.95 | 3.00 | 12.10 | 3.00 |
| WJ-III Numbers Reversed* | 0.83 | 1.89 | 4.60 | 3.14 |
| WJ-III Auditory Working Memory | 2.71 | 2.98 | 6.70 | 4.57 |
| Visual–spatial sketchpad tasks | ||||
| Leiter-R Spatial Memory | 2.41 | 1.29 | 3.98 | 1.75 |
| Leiter-R Reverse Memory* | 1.41 | 1.58 | 3.33 | 2.95 |
| n-Back | 23.58 | 7.38 | 26.40 | 7.62 |
These tasks showed skewed distributions in both populations and consequently were not used in the multivariate analyses.
WJ-III, Woodcock-Johnson Test of Cognitive Abilities-III.
Table 2.
Mean z-scores for phonological loop and visual–spatial sketchpad working memory tasks in boys with Fragile X syndrome (FXS) and mental age-matched typically developing boys
| Task | FXS group
|
Typical group
|
||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Phonological loop tasks | ||||
| WJ-III Memory for Words | −0.56 | 0.64 | 0.76 | 0.61 |
| WJ-III Numbers Reversed* | −0.61 | 0.59 | 0.55 | 0.98 |
| WJ-III Auditory Working Memory | −0.43 | 0.70 | 0.47 | 1.03 |
| Visual–spatial sketchpad tasks | ||||
| Leiter-R Spatial Memory | −0.33 | 0.80 | 0.54 | 0.96 |
| Leiter-R Reverse Memory* | −0.38 | 0.62 | 0.37 | 1.16 |
| n-Back | −0.13 | 0.17 | 0.17 | 1.00 |
These tasks showed skewed distributions in both populations and consequently were not used in the multivariate analyses.
WJ-III, Woodcock-Johnson Test of Cognitive Abilities-III.
Given that approximately one-quarter of our sample of boys with FXS expressed a significant number of autism symptoms, we re-examined the data to determine the impact of autism symptoms on our findings: (1) by correlating the CARS score to the working memory outcomes; and (2) by removing these 11 cases and re-running our main data analyses. First, it is important to note that the number of autism symptoms on the CARS did not correlated with the various working memory outcomes. Second, when the data were re-analysed without the 11 cases with autism, the same level and pattern of findings were present in the group comparisons. Taken together, these secondary analyses revealed that the inclusion of boys with both FXS and autism did not skew the analyses towards more impairment for the FXS group; however, given that individuals with FXS and autism do tend to be more impaired (e.g. Lewis et al. 2006), a larger number of such cases may have magnified the group differences achieved.
Type of working memory
Group differences on the two types of working memory (i.e. phonological loop vs. visual–spatial sketchpad) were examined. Using the standardised z-scores, there was a significant difference between the FXS and typical groups in performance on the two phonological loop tasks versus the two visual–spatial tasks. There was a significant group–type interaction (F1,66 = 10.58, P < 0.002), indicating that the pattern of performance between groups was different. Here, the FXS group performed significantly higher on visual–spatial sketchpad tasks than phonological loop tasks relative to the group mean (F1,29 = 4.51, P < 0.05), with moderate effect sizes being present (ηp2 = 0.14). In contrast, performance within the typical group revealed higher performance on the phonological loop tasks than the visual–spatial tasks relative to the group mean (F1,37 = 6.22, P < 0.05), with the effect size being moderate (ηp2 = 0.15).
Low versus high task complexity
Next, the relationship between low complexity (i.e. WJ-III Memory for Words, Leiter-R Spatial Memory) versus high complexity (i.e. WJ-III Auditory Working Memory, n-Back Task) tasks was examined. It was suspected that boys with FXS would exhibit a pervasive deficit across all tasks, while the typical boys would show significantly higher performance on the lower level working memory tasks and a decreased performance as central executive demands increased. Findings showed a significant group–complexity interaction (F1,66 = 9.61, P < 0.003), suggesting that the pattern of performance across groups was different for the low versus high complexity tasks. A post hoc repeated measures analysis examining main effects for the four tests between groups indicated significant group differences for Memory for Words (F1,67 = 79.27, P < 0.001; ηp2 = 0.55); Auditory Working Memory (F1,67 = 18.17, P < 0.001; ηp2 = 0.22); and Spatial Memory (F1,67 = 24.14, P < 0.001; ηp2 = 0.27), with the FXS group being lower on each of these tasks and effects sizes being large in magnitude. Group differences for the n-Back Test were not significant (F1,67 = 1.61, ns).
Given the above, post hoc analyses were needed to explore the main effects within the FXS group. Within this group, there was no significant main effect of test (F1,29 = 3.29, P < 0.08), indicating that the FXS group performed similarly on low and high complexity tasks in relation to the group mean across both phonological loop and visual–spatial tasks.
Numbers reversed and reverse memory tasks
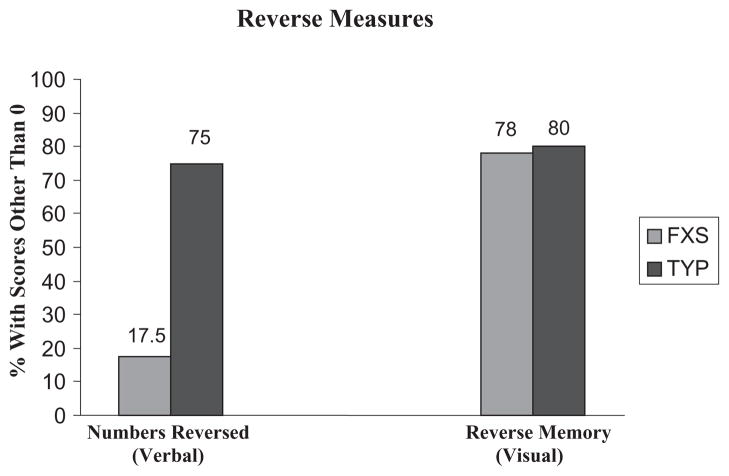
As noted earlier, two tasks involving reversal operations, WJ-III Numbers Reversed and Leiter-R Reverse Memory, were not normally distributed and violated statistical assumptions for inclusion in the multivariate analyses. In further examination of the data, it appeared that many of the participants were unable to do the tasks or obtained a score of 0 or 1; therefore, the distributions were skewed because of floor effects. Figure 1 shows the percentage of children in each group that could complete any items on the two tasks (i.e. a raw score > 0). On WJ-III Numbers Reversed, only 17.5% of the FXS group scored above a 0, as opposed to 75% of the typical group. Differences between groups on the Leiter-R Reverse Memory were not as striking, with 77.5% of the FXS group scoring more than a 0% and 80% of the typical group, with the data being skewed for both groups.
Figure 1.
Percentage of participants that obtained a score of 0 on WJ-III Numbers Reverse (verbal) versus Leiter-R Reverse Memory (visual) tasks. WJ-III, Woodcock-Johnson Test of Cognitive Abilities-III.
Discussion
The purpose of this study was to investigate the pattern and complexity of working memory in young boys with FXS. It was hypothesised that young boys with FXS would show global working memory deficits when compared with mental age-matched typical controls, and that there would be no differences across working memory subsystems. In contrast, we suspected that we would find a better performance on low versus high complexity tasks for the FXS group. The results of this study support the notion that young boys with FXS exhibit global working memory deficits when compared with mental age-matched controls but, in contrast to one of our hypotheses, the FXS group exhibited significantly lower phonological working memory than visual–spatial working memory. Additionally, in contrast to our hypothesis on task complexity, the FXS group did not show any significant differences across task complexity – even after re-examining the data for the additive negative effects from autistic behaviours. More generally, the significantly lower performance of young boys with FXS on the working memory tasks when compared with the mental age-matched typical group, and the lack of impact of task complexity at this developmental time point, suggested a deficit hypothesis for FXS as opposed to a delay in the development of these functions.
The findings showing greater deficits on the phonological loop than on the visual–spatial sketchpad in the boys with FXS were in contrast to earlier studies that suggested specific deficits in visual–spatial working memory tasks (Kemper et al. 1988; Crowe & Hay 1990); however, they were consistent with work showing phonological loop problems in women with FXS (Keenan & Simon 2004). If replicated, these findings suggest that the phonological loop may be critical in understanding many of the language-based deficits exhibited by young boys with FXS.
There also were greater differences between groups on low complexity tasks, assumed to require minimal involvement of the central executive, rather than on high complexity tasks, assumed to place relatively higher demands on the central executive; however, the results of this study did not support the notion that the working memory of boys with FXS would be higher on less complex tasks (e.g. forward span tasks) and lower on more complex tasks. These findings were not consistent with the earlier assertions advanced by Kaufmann et al. (1990), who found that boys with FXS may show greater impairment with increased task complexity; or with the findings of Munir et al. (2000), who suggested that increased attention demands may compromise working memory functions; or the more recent work of Lanfranchi et al. (2009c), who did find differential effects of different working memory loads. One speculation here is that, despite our efforts to ground our complexity rankings in test-based data, perhaps our relative ranking of the working memory tasks was faulty in some way, and/or that the range of complexity of the tasks was more limited that we anticipated. For the latter assertion, there is some sense that this could have happened given the excessive difficulty experienced by the FXS group on the moderately complex tasks (i.e. reverse memory) as well as on one of the high complexity working memory tasks (i.e. Auditory Working Memory). In contrast, the groups were not different on the n-Back task, which was initially considered to be a working memory task of high complexity. This latter task also may have been compromised by our modifications which may have lessened the visuospatial nature of this task such that children in both groups could use both visual and verbal strategies to recall an item, thus lessening its alignment with the visual–spatial sketchpad as well as its degree of complexity.
Despite these findings, the importance of task complexity and attentional demands should not be dismissed, particularly for the population of children with FXS. Indeed, Lanfranchi et al. (2009c) documented problems at the highest levels of complexity using a four-level model as compared with the three-level model used in this study. Although our verbal and visual–spatial working memory tasks were similar in many respects to those used by Lanfranchi et al. (2009c), and the levels of complexity were roughly similar for the first three levels, our study did not have a fourth level where the highest attentional load would expected to manifest. Not having this level of differentiation in our task complexity hierarchy also may have compromised our findings. Further, it is important to note that, for the most part, we selected standardised tasks for inclusion in this study. While this strategy afforded the possibility of clinical utility and, perhaps, utilisation of normative data, it clearly compromised the comparability of tasks across the two working memory slave systems. This lack of comparability may have contributed to unknown error variance in our findings, thus influencing our interpretation of the data, and it remains an important variable to address in future investigations. It also may be the case, as uncovered in Lanfranchi et al. (2009c), that different types of working memory within a single slave system also may produce variable results, and this also will be an area for further investigation in individuals with FXS.
Interpretation of these findings also must take into account previous studies that have found that the central executive becomes increasingly more proficient with age, with adolescence being a critical time period in this regard (Gathercole & Baddeley 1990; Welsh et al. 1991), and this assertion would be consistent with work by Cornish et al. (2009) using an adult male premutation population and other investigative groups (Johnson-Glenberg 2008). Therefore, the FXS group in this study may have performed at a level comparable to the typical group given that the central executive has not yet developed fully in either group. If this is the case, then we would expect such differences to emerge with increasing development and involvement of the central executive.
Finally, interpretation of these findings must consider the procedures used to match groups; that is, the Brief IQ of the Leiter-R, a non-verbal measure of intelligence. The argument could be made that the use of a non-verbal instrument may have ‘levelled the playing field’ between groups in terms of visual spatial-working memory, thus contributing to a greater difference between groups on verbal measures and minimising differences on the visual–spatial measures. In this regard, it is important to note that the Leiter-R subtests of Reverse Memory and Spatial Memory were not part of the Leiter-R Brief IQ measure and, consequently, did not contribute to the matching strategy. Additionally, when the correlations between the visual spatial working memory measures from the Leiter-R and the Brief IQ score were examined, statistically significant (P < 0.05), but weak relationships were present (r = 0.29 to 0.39) that accounted for approximately 8–15% of the variance. These latter observations suggest that the performance on the visual working memory tasks was not necessarily neutralised by use of a non-verbal IQ estimate, but this could be tested directly by examining how the pattern of findings might change if a verbal ability estimate or other type of mental age matching strategy was utilised (Jarrold & Brock 2004).
In summary, this study represents one of the few studies examining the working memory capabilities of young boys with FXS. The results of this study provide further support for generalised working memory deficits in young boys with FXS, and suggest that task complexity has minimal effect on performance at this developmental epoch. Practically speaking, at this developmental time point simple span tasks, such as the WJ-III Memory for Words, or spatial recall tasks (e.g. Leiter-R Spatial Memory), may more than adequately assess the working memory capabilities of young boys with FXS; however, this will need to be examined further as children age and the central executive becomes more active in information processing. Our findings did evidence a disproportionate impairment in the phonological loop as compared with the visuospatial sketchpad in boys with FXS and, although inconsistent with some of the literature, these findings may hold important implications for boys with FXS, particularly as these deficits may persist and affect language, communication, learning and social skills. In addition, such findings lend support to the notion of executive deficits more generally (Hooper et al. 2008; Wilding et al., 2002), and working memory deficits more specifically, being core features of a cognitive phenotype for boys with FXS.
References
- Alloway TP, Rajendran G, Archibald LM. Working memory in children with developmental disorders. Journal of Learning Disabilities. 2009;42:372–82. doi: 10.1177/0022219409335214. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working Memory. Oxford University Press; Oxford: 1986. [Google Scholar]
- Baddeley A. The fractionation of working memory. Proceedings from the National Academy of Sciences USA. 1996;93:13468–72. doi: 10.1073/pnas.93.24.13468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A. Working memory and language: an overview. Journal of Communication Disorders. 2003;36:189–208. doi: 10.1016/s0021-9924(03)00019-4. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. Working Memory, Thought and Action. Oxford University Press; Oxford: 2007. [Google Scholar]
- Baddeley AD, Hitch G. Working memory. In: Bower GA, editor. Recent Advances in Learning and Motivation. Vol. 8. Academic Press; New York: 1974. pp. 47–89. [Google Scholar]
- Carretti B, Belacchi C, Cornoldi C. Difficulties in working memory updating in individuals with intellectual disability. Journal of Intellectual Disability Research. 2010;54:337–45. doi: 10.1111/j.1365-2788.2010.01267.x. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Forman SD, Braver TD, Casey BJ, Servan-Schreiber D, Noll DC. Activation of prefrontal cortex in a non-spatial working memory task with functional MRI. Human Brain Mapping. 1994;1:293–304. doi: 10.1002/hbm.460010407. [DOI] [PubMed] [Google Scholar]
- Cornish KM, Munir F, Cross G. Spatial cognition in males with Fragile X syndrome: evidence for a neuropsychological phenotype. Cortex. 1999;35:263–71. doi: 10.1016/s0010-9452(08)70799-8. [DOI] [PubMed] [Google Scholar]
- Cornish KM, Kogan CS, Li L, Turk J, Jacquemont S, Hagerman RJ. Lifespan changes in working memory in fragile X permutation males. Brain and Cognition. 2009;69:551–8. doi: 10.1016/j.bandc.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford DC, Meadows KL, Newman JL, Taft LF, Scott E, Leslie M, et al. Prevalence of the fragile X syndrome in African-Americans. American Journal of Medical Genetics. 2002;110:226–33. doi: 10.1002/ajmg.10427. [DOI] [PubMed] [Google Scholar]
- Crowe SF, Hay A. Neuropsychological dimensions of the fragile x syndrome: support for a non-dominant hemisphere dysfunction hypothesis. Neuropsychologia. 1990;28:9–16. doi: 10.1016/0028-3932(90)90082-y. [DOI] [PubMed] [Google Scholar]
- Cui J, Gao D, Chen Y, Zou X, Wang Y. Working memory in early-school-age children with Asperger’s syndrome. Journal of Autism and Developmental Disorders. 2010;40:958–67. doi: 10.1007/s10803-010-0943-9. [DOI] [PubMed] [Google Scholar]
- Das JP, Mishra RK. Assessment of cognitive decline associated with aging; A comparison of individuals with Down syndrome and other etiologies. Research in Developmental Disabilities. 1995;16:11–25. doi: 10.1016/0891-4222(94)00032-5. [DOI] [PubMed] [Google Scholar]
- Freund L, Reiss AL. Cognitive profiles associated with the fraX syndrome in males and females. American Journal of Medical Genetics. 1991;38:542–7. doi: 10.1002/ajmg.1320380409. [DOI] [PubMed] [Google Scholar]
- Freund LS, Reiss AL, Abrams MT. Psychiatric disorders associated with fragile X in the young female. Pediatrics. 1993;92:321–9. [PubMed] [Google Scholar]
- Gathercole SE, Baddeley AD. Phonological memory deficits in language disordered children: is there a causal connection? Journal of Memory and Language. 1990;29:336–60. [Google Scholar]
- Hagerman PJ. The fragile X prevalence paradox. American Journal of Medical Genetics. 2008;45:498–9. doi: 10.1136/jmg.2008.059055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry L, Winfield J. Working memory and educational achievement in children with intellectual disabilities. Journal of Intellectual Disability Research. 2010;54:354–65. doi: 10.1111/j.1365-2788.2010.01264.x. [DOI] [PubMed] [Google Scholar]
- Hooper SR, Hatton DD, Sideris J, Sullivan K, Hammer J, Schaaf J, et al. Executive functions in young males with Fragile X Syndrome in comparison to mental age-matched controls: baseline findings from a longitudinal study. Neuropsychology. 2008;22:36–47. doi: 10.1037/0894-4105.22.1.36. [DOI] [PubMed] [Google Scholar]
- Jarrold C, Brock J. To match or not to match? Methodological issues in Autism-related research. Journal of Autism and Developmental Disorders. 2004;34:81–6. doi: 10.1023/b:jadd.0000018078.82542.ab. [DOI] [PubMed] [Google Scholar]
- Johnson-Glenberg MC. Fragile X syndrome: neural network models of sequencing and memory. Cognitive Systems Research. 2008;9:274–92. doi: 10.1016/j.cogsys.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann PM, Leckman JF, Ort SI. Delayed response performance in males with fragile X syndrome. Journal of Clinical and Experimental Neuropsychology. 1990;12:69. [Google Scholar]
- Kay-Raining Bird E, Chapman RS. Sequential recall in individuals with Down syndrome. Journal of Speech and Hearing Research. 1994;37:1369–80. doi: 10.1044/jshr.3706.1369. [DOI] [PubMed] [Google Scholar]
- Keenan JM, Simon JA. Inference deficits in women with Fragile X Syndrome: a problem in working memory. Cognitive Neuropsychology. 2004;21:579–96. doi: 10.1080/02643290342000294. [DOI] [PubMed] [Google Scholar]
- Kemper MB, Hagerman RJ, Altshul-Stark D. Cognitive profiles of boys with the fragile X syndrome. American Journal of Medical Genetics. 1988;30:191–200. doi: 10.1002/ajmg.1320300118. [DOI] [PubMed] [Google Scholar]
- Kwon H, Menon V, Eliez S, Warsofsky IS, White CD, Dyer-Friedman J, et al. Functional neuroanatomy of visuospatial working memory in fragile X syndrome: relation to behavioral and molecular measures. The American Journal of Psychiatry. 2001;158:1040–51. doi: 10.1176/appi.ajp.158.7.1040. [DOI] [PubMed] [Google Scholar]
- Lanfranchi S, Carretti B, Spanò G, Cornoldi C. A specific deficit in visuospatial simultaneous working memory in Down syndrome. Journal of Intellectual Disability Research. 2009a;53:474–83. doi: 10.1111/j.1365-2788.2009.01165.x. [DOI] [PubMed] [Google Scholar]
- Lanfranchi S, Jerman O, Vianello R. Working memory and cognitive skills in individuals with Down syndrome. Child Neuropsychology. 2009b;15:397–416. doi: 10.1080/09297040902740652. [DOI] [PubMed] [Google Scholar]
- Lanfranchi S, Cornoldi C, Drigo S, Vianello R. Working memory in individuals with fragile X syndrome. Child Neuropsychology. 2009c;15:105–19. doi: 10.1080/09297040802112564. [DOI] [PubMed] [Google Scholar]
- Lewis P, Abbeduto L, Murphy M, Richmond E, Giles N, Bruno L, et al. Cognitive, language and social-cognitive skills of individuals with fragile X syndrome with and without autism. Journal of Intellectual Disability Research. 2006;50:532–45. doi: 10.1111/j.1365-2788.2006.00803.x. [DOI] [PubMed] [Google Scholar]
- Mazzocco MM. Advances in research on the fragile X syndrome. Mental Retardation and Developmental Disabilities Research Reviews. 2000;6:96–106. doi: 10.1002/1098-2779(2000)6:2<96::AID-MRDD3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Mazzocco MM, Pennington BF, Hagerman RJ. The neurocognitive phenotype of female carriers of fragile X: additional evidence for specificity. Journal of Developmental and Behavioral Pediatrics. 1993;14:328–35. [PubMed] [Google Scholar]
- Morris R, Craik F, Gick ML. Age differences in working memory: the role of secondary memory and the central executive. Quarterly Journal of Experimental Psychology. 1990;42A:67–86. doi: 10.1080/14640749008401208. [DOI] [PubMed] [Google Scholar]
- Munir F, Cornish KM, Wilding J. Nature of the working memory deficit in fragile-X syndrome. Brain and Cognition. 2000;44:387–401. doi: 10.1006/brcg.1999.1200. [DOI] [PubMed] [Google Scholar]
- O’Hearn K, Courtney S, Street W, Landau B. Working memory impairment in people with Williams syndrome: effects of delay, task and stimuli. Brain and Cognition. 2009;69:495–503. doi: 10.1016/j.bandc.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornstein PA, Schaaf JM, Hooper SR, Hatton DD, Mirrett P, Bailey DB. Memory skills of boys with fragile X syndrome. American Journal on Mental Retardation. 2008;113:453–65. doi: 10.1352/2008.113:453-465. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Strayer DL. Further evidence of intact working memory in autism. Journal of Autism and Developmental Disorders. 2001;31:257–63. doi: 10.1023/a:1010794902139. [DOI] [PubMed] [Google Scholar]
- Roid GH, Miller LJ. Leiter International Performance Scale-Revised. Stoelting; Wood Dale, IL: 1997. [Google Scholar]
- Russell J, Jarrold C, Henry L. Working memory in children with autism and with moderate learning difficulties. Journal of Child Psychology and Psychiatry. 1996;37:673–86. doi: 10.1111/j.1469-7610.1996.tb01459.x. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Babcock R. Decomposing adult age-differences in working memory. Developmental Psychology. 1991;27:763–76. [Google Scholar]
- Schopler E, Reichler RJ, Renner BR. The Childhood Autism Rating Scale. Western Psychological Services; Los Angeles, CA: 1988. [Google Scholar]
- Schuchardt K, Gebhardt M, Mäehler C. Working memory functions in children with different degrees of intellectual disability. Journal of Intellectual Disability Research. 2010;54:346–53. doi: 10.1111/j.1365-2788.2010.01265.x. [DOI] [PubMed] [Google Scholar]
- Shapiro MB, Murphy DG, Hagerman RJ, Azari NP, Alexander GE, Miezejeski CM, et al. Adult fragile X syndrome: neuropsychology, brain anatomy and metabolism. American Journal of Genetics. 1995;60:480–93. doi: 10.1002/ajmg.1320600603. [DOI] [PubMed] [Google Scholar]
- Steele SD, Minshew NJ, Luna B, Sweeney JA. Spatial working memory deficits in autism. Journal of Autism and Developmental Disorders. 2007;37:605–12. doi: 10.1007/s10803-006-0202-2. [DOI] [PubMed] [Google Scholar]
- Turk J. Fragile X syndrome and attentional deficits. Journal of Applied Research in Intellectual Disabilities. 1998;11:175–91. [Google Scholar]
- Turner ML, Engle RW. Is working memory task dependent? Journal of Memory and Language. 1989;28:127–54. [Google Scholar]
- Varnhagen CK, Das JP, Varnhagen S. Auditory and visual memory span: cognitive processing by TMR individuals with Down syndrome or other etiologies. American Journal of Mental Deficiency. 1987;91:398–405. [PubMed] [Google Scholar]
- Wang PP, Bellugi U. Evidence from two genetic syndromes for a dissociation between verbal and visual-spatial short-term memory. Journal of Clinical and Experimental Neuropsychology. 1994;16:317–22. doi: 10.1080/01688639408402641. [DOI] [PubMed] [Google Scholar]
- Welsh MC, Pennington BF, Groisser DB. A normative-developmental study of executive function: a window on prefrontal function of children. Developmental Neuropsychology. 1991;7:131–49. [Google Scholar]
- Wilding J, Cornish K, Munir F. Further delineation of the executive deficit in males with fragile-X syndrome. Neuropsychologia. 2002;40:1343–9. doi: 10.1016/s0028-3932(01)00212-3. [DOI] [PubMed] [Google Scholar]
- Woodcock RW, Johnson MB. Woodcock-Johnson Tests of Cognitive Ability-III. DLM; Allen, TX: 2001. [Google Scholar]