Abstract
Purpose
Use of enzalutamide has produced a revolutionary change in the treatment of advanced prostate cancer. However, clinical resistance to enzalutamide can develop swiftly in initial responders. This study aimed to test whether overexpression of IL-6 and constitutive activation of Stat3 in prostate cancer cells increase resistance to enzalutamide.
Experimental design
Sensitivity of prostate cancer cells to enzalutamide was tested using cell growth assays and clonogenic assays. Quantitative reverse transcription-PCR, ELISA and Western blotting were performed to detect expression levels of IL-6, c-Myc, survivin and AR. Expression of Stat3 was downregulated using siRNA specific to Stat3. ChIP assay was performed to examine recruitment of AR to the PSA promoter.
Results
Prostate cancer cells expressing autocrine IL-6 are resistant to enzalutamide and autocrine IL-6 leads to constitutive activation of Stat3 and its target genes. Down regulation of Stat3 led to an increase in sensitivity of prostate cancer cells to enzalutamide. Overexpression of constitutively active Stat3 in prostate cancer cells induced resistance to enzalutamide treatment. Constitutively active Stat3 also enhanced the recruitment of AR to PSA promoter which could not be disrupted by enzalutamide. The Stat3 inhibitor AG490 reversed enzalutamide resistance in prostate cancer cells, while combination treatment with enzalutamide and AG490 significantly inhibited cell growth and induced cell apoptosis.
Conclusions
This study demonstrates that the autocrine IL-6 pathway induces enzalutamide resistance in prostate cancer cells via the constitutive activation of Stat3. Co-targeting IL6-Stat3 pathway with enzalutamide may be utilized for treatment of advanced prostate cancer.
Keywords: Prostate cancer, Interleukin-6, Enzalutamide, Stat3
Introduction
Prior to the approval of the second generation androgen antagonist Enzalutamide (MDV3100), few treatment choices existed after the progression of many patients to castration resistant prostate cancer (CRPC). Enzalutamide created a revolutionary change in the treatment of advanced prostate cancer, whereby it not only prolonged the survival of men with metastatic CRPC after chemotherapy, but also significantly improved their quality of life (1). Unfortunately, on the other hand, resistance to enzalutamide can develop in the initial responders (2). There is an urgent need to understand the mechanisms of resistance to enzalutamide which would provide more information to clinical investigators applying better intervention strategies to those patients.
Interleukin-6 (IL-6) is a pleiotropic cytokine that plays a central role in host defense mechanisms. The biological activities of IL-6 are mediated by the IL6 receptor which is composed of an IL6-specific receptor subunit and a signal transducer, gp130. The binding of IL-6 to its receptor results in activation of several intracellular signaling cascades including JAK-Stat and MAPK pathways (3,4). IL-6 production correlates with tumor progression in many human cancers, such as ovarian and prostate cancers (5–9). The serum levels of IL-6 are significantly elevated in many men with advanced, hormone-refractory prostate cancer and are associated with progression and poor prognosis (10–12). IL-6 also regulates the expression of genes encoding many steroidogenic enzymes involved in androgen synthesis (13). Autocrine IL-6 promotes androgen independent growth in prostate cancer cells (14). Constitutively active Stat3 is a part of the positive autocrine IL-6 loop and Stat3 activation in human tumors is often observed at the invasive front of tumors adjacent to inflammatory cells, which suggests that Stat3-dependent tumorigenesis is mediated by IL-6 (15). In the current study, we demonstrate that autocrine IL-6 in prostate cancer cells induces resistance to enzalutamide and that constitutive activation of Stat3 maybe one of the underlying mechanisms. Suppression of constitutively active Stat3 by Stat3 siRNA or a Stat3 inhibitor-AG490, could overcome enzalutamide resistance in prostate cancer cells. The IL-6-Stat3 feed forward axis in prostate cancer patient maybe one of the critical mechanisms of enzalutamide resistance.
Materials and Methods
Reagents and Cell Culture
LNCaP cells were maintained in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 100 units/ml penicillin and 0.1 mg/ml streptomycin. LNCaP-neo (LNCaP cells stably expressing pcDNA3.1 control vector), LNCaP-s17 (LNCaP cells stably expressing IL-6) (16), and LNCaP-Stat3C cells (LNCaP cells stably expressing constitutively active Stat3) (17) were described previously. The cells were maintained at 37°C in a humidified incubator with 5% carbon dioxide. Human recombinant IL-6 was obtained from R&D Systems (Minneapolis, MN. Cat. NO.: 206-IL-050). AG490 was purchased from Sigma-Aldrich, St. Louis, MO (Cat. NO.: T3454) and dissolved in DMSO.
Plasmids and cell transfection
For small interfering RNA (siRNA) transfection, cells were seeded at a density of 1×105 cells per well in 12-well plates and transfected with siRNA (Cell Signaling #6582) targeting Stat3 and a control siRNA targeting the luciferase (Luc) gene, using Lipofectamine-RNAiMAX (Invitrogen). Cells were transiently transfected with the indicated expression plasmids using Attractene (QIAGEN).
Chromatin immunoprecipitation assay
LNCaP-neo, LNCaP-s17 or LNCaP-Stat3C cells were treated with DMSO or 20 µM enzalutamide overnight. DNA-AR protein complexes were cross-linked inside the cells by the addition of 1% formaldehyde. Whole-cell extracts were prepared by sonication, and an aliquot of the cross-linked DNA-protein complexes was immunoprecipitated by incubation with the AR-specific antibody (AR-C19; Santa Cruz Biotechnology) overnight at 4°C with rotation. Chromatin-antibody complexes were isolated from solution by incubation with protein A/G agarose beads for 1 hour at 4°C with rotation. The bound DNA-protein complexes were washed and eluted from beads with elution buffer (1% SDS and 0.1 mol/L NaHCO3), cross links were reversed, and DNA was extracted. The resulting chromatin preparations were analyzed by PCR using primers spanning either the proximal or the distal enhancer AREs of the PSA promoter. Primers used for ChIP assay were: AREI/II: 5’-CCTAGATGAAGTCTCCATGAGCTACA, AREI/II -3’-GGGAGGGAGAGCTAGCACTTG (Proximal); ARE-III: 5’-CATGTTCACATT AGTACACCTTGCC, ARE-III: 3’-TCTCAGAT CCAGGCTTGCTTACTGTC (Distal). Isotype-matched IgG was used as control.
Western blot analysis
Whole cell protein extracts were resolved on SDS–PAGE and proteins were transferred to nitrocellulose membranes. After blocking for 1 hour at room temperature in 5% milk in PBS/0.1% Tween-20, membranes were incubated overnight at 4°C with the indicated primary antibodies. [p-Stat3(Tyr705) (sc-7993-R, rabbit polyclonal antibody, 1:1000 dilution, Santa Cruz Biotechnology, Santa Cruz, CA); stat3 (C-20, sc-482, rabbit polyclonal antibody, 1:1000 dilution, Santa Cruz Biotechnology, Santa Cruz, CA); AR (441, sc-7305, Mouse monoclonal antibody, 1:1000 dilition, Santa Cruz Biotechnology, Santa Cruz, CA); c-myc (sc-764, rabbit polyclonal antibody, 1:1000 dilution, Santa Cruz Biotechnology, Santa Cruz, CA); Survivin (FL-142, rabbit polyclonal antibody, 1:1000 dilution, Sigma-Aldrich, St. Louis, MO) Bcl-2 (human specific) (#2872, rabbit polyclonal antibody, 1:1000 dilution, Cell Signaling Technology); Tubulin (T5168, Monoclonal Anti-α-Tubulin antibody, 1:5000 dilution, Sigma-Aldrich, St. Louis, MO)]. Tubulin was used to monitor the amounts of samples applied. Following secondary antibody incubation, immunoreactive proteins were visualized with an enhanced chemiluminescence detection system (Millipore, Billerica, MA), the western blot was quantified by Image J software.
Cell growth assay
LNCaP neo, LNCaPs17, LNCaP-IL6+ or LNCaP-Stat3C cells were seeded on 12-well plates at a density of 1×105 cells/well in RPMI 1640 media containing 10% FBS. LNCap-neo and LNCaP-s17 cells were treated with different concentration of enzalutamide (0 µM, 5 µM, 10 µM, 20 µM or 40 µM) for 48 hours; LNCaP and LNCaP-IL6+ cells or LNCaP-neo and LNCaP-Stat3C cells were treated with 0 µM, 10 µM or 20 µM enzalutamide for 48 hours; LNCaP-s17 cells were treated with 10µM AG490 with or without 20 µM enzalutamide for 48 hours, total cell numbers were counted and the cell survival rate (%) was calculated. Cell survival rate (%) = (Treatment group cell number / Control group cell number) ×100%.
Cell death ELISA
LNCaP-neo or LNCaP-s17 cells were seeded on 12-well plates (1 × 105 cells/well) in RPMI 1640 media containing 10% FBS and treated with 10µM AG490 with or without 20 µM enzalutamide for 48 hours. Mono- and oligonucleosomes in the cytoplasmic fraction were measured by the Cell Death Detection ELISA kit (Roche, Cat. NO. 11544675001) according to the manufacturer’s instructions. Briefly, floating and attached cells were collected and homogenized in 400 µL of incubation buffer. The wells were coated with antihistone antibodies and incubated with the lysates, horseradish peroxidase–conjugated anti-DNA antibodies, and the substrate, and absorbance was read at 405 nm.
Clonogenic Assay
LNCaP-neo cells or LNCaP-s17 stable clone cells were treated with DMSO or 20µM enzalutamide in media containing 10% complete FBS. Cells were plated at equal density (1000 cells/dish) in 100 mm dishes for 14 days; the medium was changed every 7 days. The colonies were rinsed with PBS before staining with 0.5% crystal violet/4% formaldehyde for 30 min and the number of colonies was counted.
Real-Time quantitative RT-PCR
Total RNAs were extracted using TriZOL reagent (Invitrogen). cDNAs were prepared after digestion with RNase-free RQ1 DNase (Promega). The cDNAs were subjected to real-time reverse transcription-PCR (RT-PCR) using Sso Fast Eva Green Supermix (Bio- Rad) according to the manufacturer's instructions and as described previously (18). Primers used for Real-time PCR were: IL6: 5’-AAGCCAGAGCTGTGCAGATGA, 3’-TGTCCTGCAGCCACTGGTTC; c-Myc: 5’-TGAGGAGACACCGCCCAC, 3’-CAACATCGATTTCTTCTTCAT; Survivin: 5’-AGAACTGGCCCTTCTTGGAGG, 3’-GTTTTTATGTTCCTCTATGGG; Actin: 5’-CCCAGCCATGTACGTTGCTA, 3’-AGGGCATACCCCTCGTAGATG. Each reaction was normalized by co-amplification of actin. Triplicates of samples were run on default settings of Bio-Rad CFX-96 real-time cycler.
Measurement of IL6
Secretion of IL-6 by LNCaP-neo and LNCaP-s17 cells was determined by Human IL6 ELISA Ready-SET-Go (eBioscience, San Diego, CA. Cat.NO. 88-7066) according to the manufacturer’s protocol. Briefly, LNCaP neo and LNCaP-s17 cells were cultured in RPMI 1640 media containing 10% FBS for 2 days, 50 µl of cell culture supernatants were collected to determine levels of IL-6 secretion, The wells were coated with capture antibody and incubated with the standard or supernatants samples, Avidin-HRP and the substrate, and absorbance was read at 450 nm.
Statistical Analysis
All data are presented as means ± standard deviation of the mean (SD). Statistical analyses were performed with Microsoft Excel analysis tools. Differences between individual groups were analyzed by one-way analysis of variance (ANOVA) followed by the Scheffé procedure for comparison of means. P < 0.05 was considered statistically significant.
Results
Overexpression of IL-6 increases LNCaP cell resistance to enzalutamide
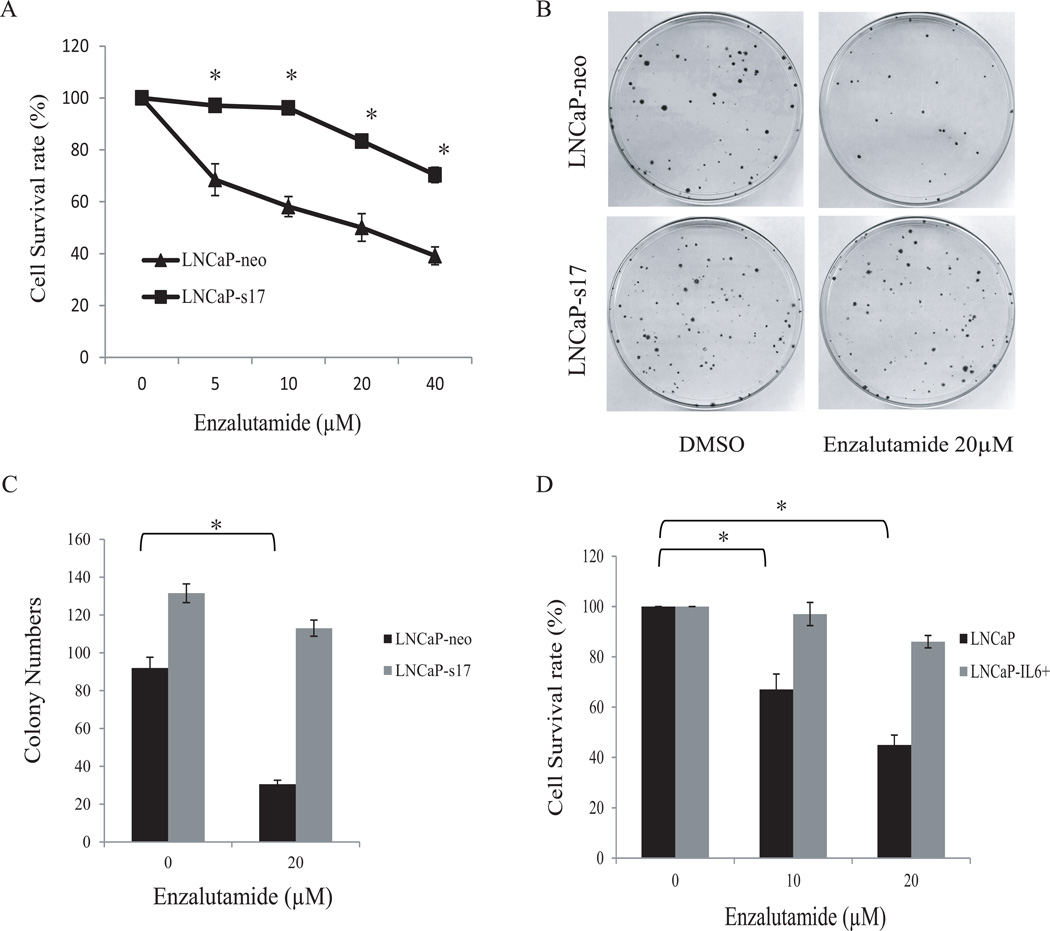
Our previous data demonstrated that autocrine expression of IL-6 in LNCaP (LNCaP-s17) cells promotes cell growth and increases resistance to bicalutamide treatment (14,19). To test whether expression of IL-6 affects the response of prostate cancer cells to enzalutamide, LNCaP-s17 cells were treated with increasing doses of enzalutamide and cell numbers were counted. As shown in Fig.1A, LNCaP-neo cells were highly sensitive to enzalutamide treatment compared to LNCaP-s17 cells. Enzalutamide at a concentration of 5 µM reduced the growth of LNCaP-neo cells by more than 30%, while it had almost no effect on the growth of LNCaP-s17 cells. Even at a higher concentration of enzalutamide (40 µM), the growth of LNCaP-s17 cells was only reduced by about 30% compared to almost 60% reduction in LNCaP-neo cells. To further confirm these results, clonogenic assay was performed. LNCaP-neo cells and LNCaP-s17 cells were treated with 20 µM enzalutamide and clonogenic ability was determined. As shown in Fig.1B and 1C, the colony formation ability was significantly inhibited in LNCaP-neo cells treated with 20 µM enzalutamide, while LNCaP-s17 cells continued to grow and form colonies. To further confirm that overexpression of IL-6 is involved in enzalutamide resistance, LNCaP-IL6+ cells, LNCaP cells expressing IL-6 by long-term culture of LNCaP cells in media containing IL-6 (20), were treated with 10 µM and 20 µM enzalutamide in media containing complete FBS for 48 hours, As shown in Fig.1D, enzalutamide significantly inhibited growth of LNCaP cells. In contrast, enzalutamide had little effect on the growth of LNCaP-IL6+ cells. Collectively, these data suggest that overexpression of IL-6 in prostate cancer cells is associated with enzalutamide resistance.
Figure 1. Overexpression of IL-6 increases LNCaP cell resistance to enzalutamide.
A: LNCaP cells stably expressing IL6 (LNCaP-s17) and control LNCaP cells (LNCaP-neo) were treated with different doses of enzalutamide in media containing complete FBS. Total cell numbers were counted after 48 h and cell survival rates were calculated as described in the methods. Results are presented as means ± SD of 3 experiments performed in duplicate. B: LNCaP-neo and LNCaP-s17 cells were treated with 0 or 20 µM enzalutamide and clonogenic assays were performed. C: Colonies were counted and results are presented as means ± SD of 2 experiments performed in duplicate. LNCaP-s17 cells formed higher numbers of colonies compared to LNCaP-neo cells when treated with enzalutamide. D: LNCaP-IL6+ cells (continuous culture in medium containing 10% FBS and 5 ng/mL IL-6) and control LNCaP cells were treated with 0, 10 or 20 µM enzalutamide in media containing complete FBS and cell numbers were counted after 48 h. Results are presented as means ± SD of 3 experiments performed in duplicate. * P <0.05.
Autocrine IL-6 constitutively activates Stat3 pathway and enhances androgen receptor transactivation in prostate cancer cells
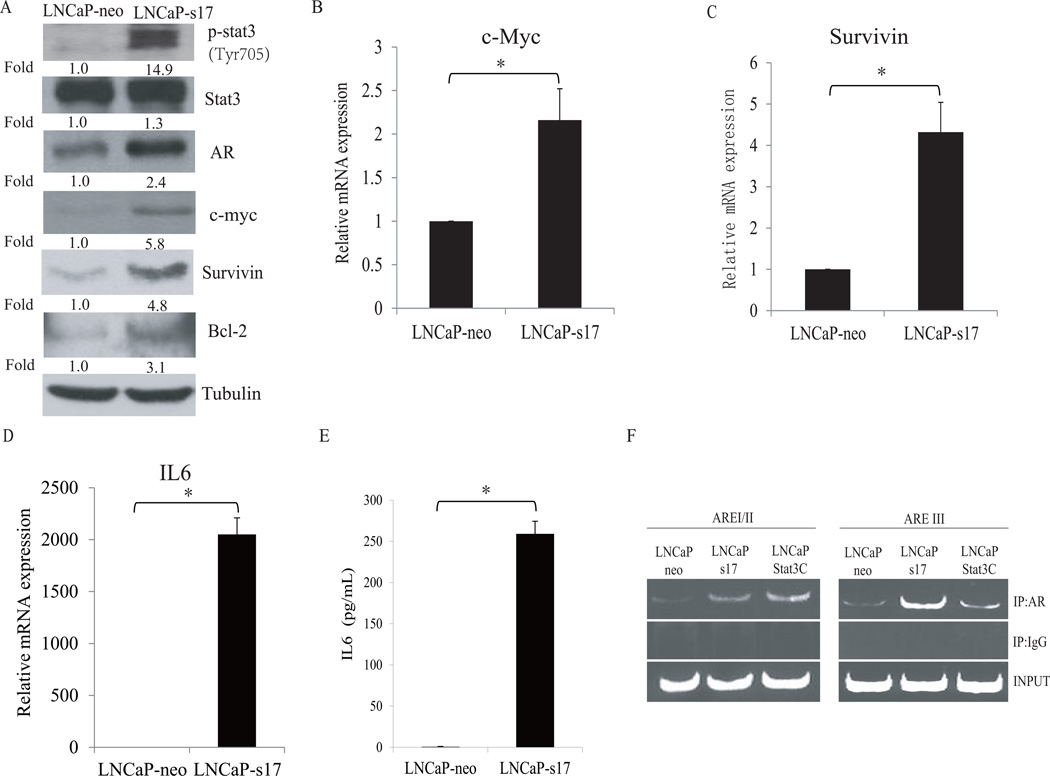
Numerous reports have demonstrated that constitutive Stat3 activation is oncogenic and contributes to tumor progression and metastasis (21–23). Previous studies showed that Stat3 is constitutively activated in LNCaP-s17 cells (14). To test whether LNCaP-s17 cells exhibit elevated Stat3 signaling, we examined the levels of expression of several Stat3 target genes including c-Myc, survivin, and Bcl-2. As shown in Fig.2A, LNCaP-s17 cells express constitutively activated Stat3 (Stat3 phosphorylated at Tyr705) and express higher levels of AR, c-Myc, survivin, and Bcl-2 proteins than LNCaP-neo cells. Consistent with the protein levels, LNCaP-s17 cells express higher levels of c-Myc and survivin mRNA than LNCaP-neo cells (Fig. 2B and 2C). We also confirmed that LNCaP-s17 cells expressed higher levels of IL-6 mRNA and protein than LNCaP-neo cells (Fig.2D and 2E). In our previous study, we showed that over expression of IL-6 enhances AR-ARE DNA binding activity in LNCaP cells (14). To determine whether constitutively active Stat3 increases the recruitment of AR to the ARE sites, ChIP assay was performed in LNCaP, LNCaP-s17 and LNCaP-Stat3C cells. As shown in Fig.2F, both LNCaP-s17 cells and LNCaP-Stat3C cells showed enhanced recruitment of AR to both the proximal binding site (AREI/II) and the distal enhancer binding site (AREIII) of the PSA promoter compared to LNCaP-neo cells. Collectively, these data demonstrate that overexpression of IL-6 activates Stat3 and AR signaling pathways in prostate cancer cells.
Figure 2. Autocrine IL-6 constitutively activates Stat3 pathway and enhances androgen receptor transactivation in prostate cancer cells.
A: Prostate cancer cells with autocrine IL-6 exhibit constitutive activation of Stat3. LNCaP cells and LNCaP-s17 cells were cultured in RPMI 1640 media containing 10% FBS condition for 24 hours and the lysates were immunoblotted with the indicated antibodies. B: LNCaP cells and LNCaP-s17 cells were cultured in RPMI 1640 media containing 10% FBS condition for 24 hours, total RNAs were extracted and c-Myc mRNA level was analyzed by qRT-PCR. C: Survivin mRNA level of LNCaP-neo and LNCaP-s17 cells were analyzed by qRT-PCR. D: IL6 mRNA level of LNCaP-neo and LNCaP-s17 cells were analyzed by qRT-PCR. Each reaction was normalized by co-amplification of actin. E: LNCaP-neo and LNCaP-s17 cells were cultured in RPMI 1640 media containing 10% FBS condition for 24 hours, the supernatant were collected and subjected to IL6 ELISA detection. Results are presented as means ± SD of 3 experiments performed in duplicate. F: Recruitment of AR to AREs in PSA promoter was analyzed by ChIP assay, LNCaP-s17 and LNCaP-Stat3C cells enhanced recruitment of AR to PSA promoter compared to LNCaP-neo cells. * P<0.05
Knockdown of Stat3 expression restores the sensitivity of LNCaP-s17 cells to enzalutamide treatment
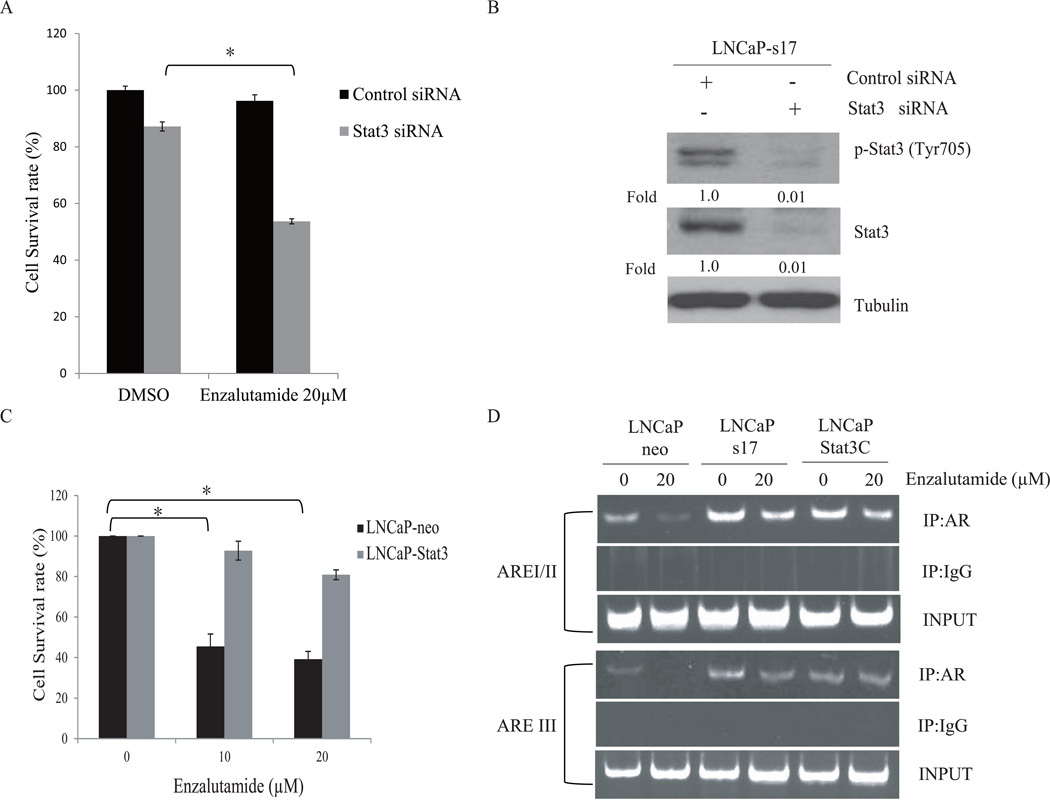
To determine whether Stat3 is involved in enzalutamide resistance in IL-6 expressing prostate cancer cells, LNCaP-neo and LNCaP-s17 cells were transfected with siRNA specific to Stat3. The cells were then treated with 20 µM enzalutamide for an additional 3 days and the cell numbers were determined. As shown in Fig 3A, knockdown of Stat3 expression in LNCaP-s17 cells resensitized the cells to enzalutamide treatment. The knockdown effects were confirmed by Western blotting using p-Stat3 (Tyr705) and Stat3 antibodies (Fig. 3B). In addition, LNCaP cells expressing constitutively active Stat3 (Stat3C) exhibited increased resistance to enzalutamide (Fig. 3C). These results suggest that stat3 activation is involved in enzalutamide resistance in prostate cancer cells.
Figure 3. Downregulation of Stat3 expression restores the sensitivity of LNCaP-s17 cells to enzalutamide treatment.
A: Down regulation of Stat3 expression increased sensitivity of LNCaP-s17 cells to enzalutamide. LNCaP-s17 cells were transfected with siRNAs specific to Stat3 or control siRNA and were treated with 0 or 20 µM enzalutamide. Cell numbers were counted and cell survival rates were calculated after 3 days. Results are presented as means ± SD of 3 experiments performed in duplicate. B: The knock down effects were examined by western blot; whole cell lysates were collected and subjected to Western blot. C: LNCaP-neo and LNCaP-Stat3C cells were treated with DMSO, 10 or 20 µM enzalutamide in media containing FBS for 48 hours, cell numbers were counted and cell survival rates were calculated. Results are presented as means ± SD of 3 experiments performed in duplicate. D: Recruitment of AR to AREs in PSA promoter was analyzed by ChIP assay, LNCaP-neo, LNCaP-s17 and LNCaP-Stat3C cells were cultured in FBS condition with or without 20 µM enzalutamide overnight, cross-linked with 1% formaldehydeand the resultant DNA-protein complexes were subjected to ChIP assay. * P <0.05
One of the mechanisms of action of enzalutamide is to inhibit the recruitment of the AR to AREs in promoters of target genes. To test whether Stat3-mediated enzalutamide resistance affects the recruitment of AR to AREs, ChIP assay was performed. As shown in Fig. 3D, enzalutamide significantly inhibited recruitment of the AR to AREs in the PSA promoter (both proximal binding site and distal binding site) in LNCaP-neo cells. In contrast, enzalutamide had little effect on the recruitment of the AR to the AREs in LNCaP-s17 cells and LNCaP-Stat3C cells. These results suggest that Stat3-mediated enzalutamide resistance may be involved in affecting the recruitment of the AR to the AREs in the PSA promoter.
AG490, a Stat3 inhibitor, reverses enzalutamide resistance in prostate cancer cells
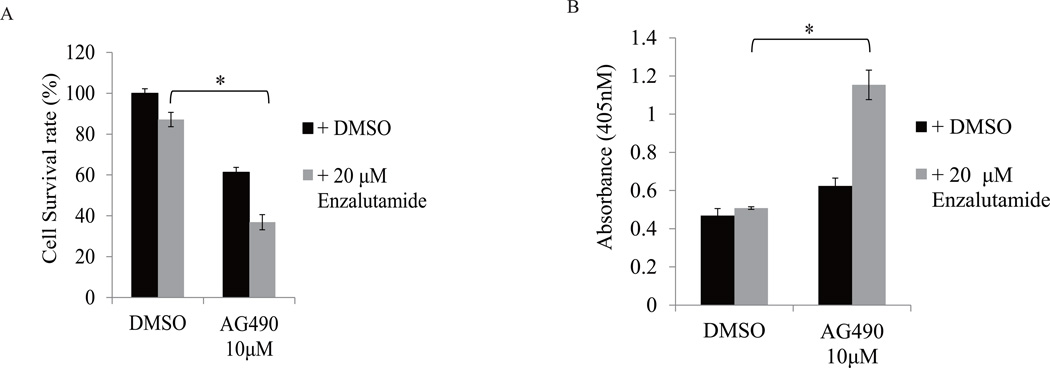
We next tested whether AG490, which has been known as a Jak2-Stat3 inhibitor, is able to reverse enzalutamide resistance in prostate cancer cells. As shown in Fig 4A, enzalutamide treatment at 20 µM had minimal effect on LNCaP-s17 cell growth. However, enzalutamide significantly inhibited cell growth in the presence of 10 µM AG490, possibly through induction of apoptotic cell death (Fig 4B). Collectively, these data demonstrate that using Jak2-stat3 inhibitor can overcome enzalutamide resistance though inhibition of Stat3 activation.
Figure 4. Stat3 inhibitor-AG490, reverses enzalutamide resistance in prostate cancer cells.
A: LNCaP-s17 cells were treated with 10 µM AG490 combined with or without 20 µM enzalutamide in media containing FBS, cell numbers were counted and cell survival rate was calculated after 48 h, the apoptosis was analyzed by Cell death ELISA (B). Results are presented as means ± SD of 3 experiments performed in duplicate. * P <0.05
Discussion
Enzalutamide is a second generation, non-sterodial AR antagonist rationally designed utilizing the AR crystal structure and cell based screening (24,25). It was approved by U.S. Food and Drug Administration (FDA) in September 2012 based on significant improvement in overall survival (18.4 vs. 13.6 months, p ≤0.001) in a phase III randomized study in men with progressive castration-resistant prostate cancer (CRPC) who were previously treated with docetaxel (1). In vitro, enzalutamide binds to the ligand binding domain of the AR with 5 fold higher affinity than bicalutamide and impairs AR nuclear translocation and DNA binding (25). However, despite its significant clinical effects and being the first choice after docetaxel failure in CRPC patients, resistance to enzalutamide would eventually occur in many patients (2). A better understanding of the therapeutic effects of enzalutamide and the mechanisms of resistance would be very important for patient treatment in the future. A recent study showed expression of AR variants driven by AR gene rearrangements can mediate resistance to therapies targeting full-length AR, including enzalutamide (26). But many questions remain to be answered, including the absence of expression of AR variants in many patients who may still develop resistance to enzalutamide. In the present study, we report that the axis between IL-6 and persistent Stat3 activation may be one of the critical mechanisms involved in enzalutamide resistance.
Our previous studies demonstrated that IL-6 overexpression enhances AR-ARE DNA binding activity and enhances AR nuclear translocation in LNCaP cells (14) and that IL-6 up regulates intraprostatic androgen synthesis in the absence of exogenous steroid precursors (13). Here we found that autocrine IL-6 in LNCaP cells activates Stat3 pathway and enhances resistance to enzalutamide, while downregulation of Stat3 in LNCaP-s17 cells dramatically resensitized LNCaP-s17 cells to enzalutamide. In addition, transfection of constitutively active Stat3 into LNCaP cells induced resistance to enzalutamide, which indicates that enzalutamide resistance may be driven by Stat3 activation in prostate cancer cells. Enzalutamide as a second generation androgen antagonist is superior to bicalutamide, in that enzalutamide does not promote translocation of AR to the nucleus, and in addition, it prevents binding of AR to DNA and AR to co-activator proteins, which abrogates agonist activity (25). However, our study showed that in prostate cancer cell lines expressing constitutively active Stat3, enzalutamide treatment only slightly reduced recruitment of AR to the PSA promoter compared with LNCaP parental cells, which suggests that persistent Stat3 activation may counteract enzalutamide action in blocking the recruitment of AR to target gene promoters.
Constitutive activation of Stat3 has been shown in many advanced cancers and is associated with poor prognosis (27). Constitutive activation of Stat3 is often accompanied by high levels of IL-6 expression, which implicates IL-6 as the main driver of persistent activation of Stat3(28). Recently, a group reported enzalutamide in prostate cancer patients might lead to unwanted side effects of enhanced macrophage infiltration/prostate cancer metastasis through modulation of CCL2-STAT3 signaling (29), which suggested inhibition of the IL-6-Stat3 axis appears to be a potent strategy to overcome enzalutamide resistance in prostate cancer cells. AG490 is a specific inhibitor of the Janus kinase 2 protein (JAK2) which is the upstream regulator of stat3 signaling. In the current study, we found that AG490 significantly reversed enzalutamide resistance in prostate cancer cells exhibiting constitutive activation of Stat3. Combination treatment with enzalutamide and AG490 not only inhibited cell proliferation but also induced apoptosis, co-targeting IL-6/Stat3 pathway using stat3 inhibitor and androgen signaling by enzalutamide may be logical for treatment of advanced prostate cancer.
In summary, our study demonstrates that the IL6-Stat3 axis is involved in the development of resistance to enzalutamide in prostate cancer. In addition, our results imply that targeting the IL-6-Stat3 Axis may be a potential therapeutic strategy in patients resistant to enzalutamide.
Acknowledgments
Grant Support: This work is supported in part by grants NIH/NCI CA140468, CA168601, DOD PCRP PC080538 (A.C. Gao), US Department of Veterans Affairs, Office of Research and Development VA Merits I01 BX000526 (A.C. Gao), and by resources from the VA Northern California Health Care System, Sacramento, California.
References
- 1.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, de Wit R, Mulders P, Chi KN, Shore ND, Armstrong AJ, Flaig TW, Flechon A, Mainwaring P, Fleming M, Hainsworth JD, Hirmand M, Selby B, Seely L, de Bono JS. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 367(13):1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 2.Scher HI, Beer TM, Higano CS, Anand A, Taplin ME, Efstathiou E, Rathkopf D, Shelkey J, Yu EY, Alumkal J, Hung D, Hirmand M, Seely L, Morris MJ, Danila DC, Humm J, Larson S, Fleisher M, Sawyers CL. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1–2 study. Lancet. 375(9724):1437–1446. doi: 10.1016/S0140-6736(10)60172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ben Jemaa A, Sallami S, Ramarli D, Colombatti M, Oueslati R. The Proinflammatory Cytokine, IL-6, and its Interference with bFGF Signaling and PSMA in Prostate Cancer Cells. Inflammation. doi: 10.1007/s10753-012-9586-7. [DOI] [PubMed] [Google Scholar]
- 4.Heinrich PC, Behrmann I, Muller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334(Pt 2):297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duffy SA, Teknos T, Taylor JM, Fowler KE, Islam M, Wolf GT, McLean S, Ghanem TA, Terrell JE. Health Behaviors Predict Higher Interleukin-6 Levels among Patients Newly Diagnosed with Head and Neck Squamous Cell Carcinoma. Cancer Epidemiol Biomarkers Prev. doi: 10.1158/1055-9965.EPI-12-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hao W, Zhu Y, Zhou H. Prognostic value of interleukin-6 and interleukin-8 in laryngeal squamous cell cancer. Med Oncol. 30(1):333. doi: 10.1007/s12032-012-0333-6. [DOI] [PubMed] [Google Scholar]
- 7.Yanaihara N, Anglesio MS, Ochiai K, Hirata Y, Saito M, Nagata C, Iida Y, Takakura S, Yamada K, Tanaka T, Okamoto A. Cytokine gene expression signature in ovarian clear cell carcinoma. Int J Oncol. 41(3):1094–1100. doi: 10.3892/ijo.2012.1533. [DOI] [PubMed] [Google Scholar]
- 8.Anglesio MS, George J, Kulbe H, Friedlander M, Rischin D, Lemech C, Power J, Coward J, Cowin PA, House CM, Chakravarty P, Gorringe KL, Campbell IG, Okamoto A, Birrer MJ, Huntsman DG, de Fazio A, Kalloger SE, Balkwill F, Gilks CB, Bowtell DD. IL6-STAT3-HIF signaling and therapeutic response to the angiogenesis inhibitor sunitinib in ovarian clear cell cancer. Clin Cancer Res. 17(8):2538–2548. doi: 10.1158/1078-0432.CCR-10-3314. [DOI] [PubMed] [Google Scholar]
- 9.Dossus L, Kaaks R, Canzian F, Albanes D, Berndt SI, Boeing H, Buring J, Chanock SJ, Clavel-Chapelon F, Feigelson HS, Gaziano JM, Giovannucci E, Gonzalez C, Haiman CA, Hallmans G, Hankinson SE, Hayes RB, Henderson BE, Hoover RN, Hunter DJ, Khaw KT, Kolonel LN, Kraft P, Ma J, Le Marchand L, Lund E, Peeters PH, Stampfer M, Stram DO, Thomas G, Thun MJ, Tjonneland A, Trichopoulos D, Tumino R, Riboli E, Virtamo J, Weinstein SJ, Yeager M, Ziegler RG, Cox DG. PTGS2 and IL6 genetic variation and risk of breast and prostate cancer: results from the Breast and Prostate Cancer Cohort Consortium (BPC3) Carcinogenesis. 31(3):455–461. doi: 10.1093/carcin/bgp307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iacopino F, Pinto F, Bertaccini A, Calarco A, Proietti G, Totaro A, Martorana G, Bassi P, Sica G. Soluble E-cadherin and IL-6 serum levels in patients affected by prostate cancer before and after prostatectomy. Oncol Rep. 28(1):370–374. doi: 10.3892/or.2012.1785. [DOI] [PubMed] [Google Scholar]
- 11.Shariat SF, Andrews B, Kattan MW, Kim J, Wheeler TM, Slawin KM. Plasma levels of interleukin-6 and its soluble receptor are associated with prostate cancer progression and metastasis. Urology. 2001;58(6):1008–1015. doi: 10.1016/s0090-4295(01)01405-4. [DOI] [PubMed] [Google Scholar]
- 12.Nakashima J, Tachibana M, Horiguchi Y, Oya M, Ohigashi T, Asakura H, Murai M. Serum interleukin 6 as a prognostic factor in patients with prostate cancer. Clin Cancer Res. 2000;6(7):2702–2706. [PubMed] [Google Scholar]
- 13.Chun JY, Nadiminty N, Dutt S, Lou W, Yang JC, Kung HJ, Evans CP, Gao AC. Interleukin-6 regulates androgen synthesis in prostate cancer cells. Clin Cancer Res. 2009;15(15):4815–4822. doi: 10.1158/1078-0432.CCR-09-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee SO, Lou W, Hou M, de Miguel F, Gerber L, Gao AC. Interleukin-6 promotes androgen-independent growth in LNCaP human prostate cancer cells. Clin Cancer Res. 2003;9(1):370–376. [PubMed] [Google Scholar]
- 15.Bromberg J, Wang TC. Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell. 2009;15(2):79–80. doi: 10.1016/j.ccr.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lou W, Ni Z, Dyer K, Tweardy DJ, Gao AC. Interleukin-6 induces prostate cancer cell growth accompanied by activation of stat3 signaling pathway. Prostate. 2000;42(3):239–242. doi: 10.1002/(sici)1097-0045(20000215)42:3<239::aid-pros10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 17.DeMiguel F, Lee SO, Lou W, Xiao X, Pflug BR, Nelson JB, Gao AC. Stat3 enhances the growth of LNCaP human prostate cancer cells in intact and castrated male nude mice. Prostate. 2002;52(2):123–129. doi: 10.1002/pros.10110. [DOI] [PubMed] [Google Scholar]
- 18.Liu C, Nadiminty N, Tummala R, Chun JY, Lou W, Zhu Y, Sun M, Evans CP, Zhou Q, Gao AC. Andrographolide targets androgen receptor pathway in castration-resistant prostate cancer. Genes Cancer. 2(2):151–159. doi: 10.1177/1947601911409744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng S, Tang Q, Sun M, Chun JY, Evans CP, Gao AC. Interleukin-6 increases prostate cancer cells resistance to bicalutamide via TIF2. Mol Cancer Ther. 2009;8(3):665–671. doi: 10.1158/1535-7163.MCT-08-0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee SO, Chun JY, Nadiminty N, Lou W, Gao AC. Interleukin-6 undergoes transition from growth inhibitor associated with neuroendocrine differentiation to stimulator accompanied by androgen receptor activation during LNCaP prostate cancer cell progression. The Prostate. 2007;67(7):764–773. doi: 10.1002/pros.20553. [DOI] [PubMed] [Google Scholar]
- 21.Hedvat M, Huszar D, Herrmann A, Gozgit JM, Schroeder A, Sheehy A, Buettner R, Proia D, Kowolik CM, Xin H, Armstrong B, Bebernitz G, Weng S, Wang L, Ye M, McEachern K, Chen H, Morosini D, Bell K, Alimzhanov M, Ioannidis S, McCoon P, Cao ZA, Yu H, Jove R, Zinda M. The JAK2 inhibitor AZD1480 potently blocks Stat3 signaling and oncogenesis in solid tumors. Cancer Cell. 2009;16(6):487–497. doi: 10.1016/j.ccr.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7(1):41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 23.Abdulghani J, Gu L, Dagvadorj A, Lutz J, Leiby B, Bonuccelli G, Lisanti MP, Zellweger T, Alanen K, Mirtti T, Visakorpi T, Bubendorf L, Nevalainen MT. Stat3 promotes metastatic progression of prostate cancer. Am J Pathol. 2008;172(6):1717–1728. doi: 10.2353/ajpath.2008.071054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Payton S. Prostate cancer: MDV3100 has antitumor activity in castration-resistant disease. Nat Rev Urol. 7(6):300. doi: 10.1038/nrurol.2010.69. [DOI] [PubMed] [Google Scholar]
- 25.Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, Wongvipat J, Smith-Jones PM, Yoo D, Kwon A, Wasielewska T, Welsbie D, Chen CD, Higano CS, Beer TM, Hung DT, Scher HI, Jung ME, Sawyers CL. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324(5928):787–790. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Hwang TH, Oseth LA, Hauge A, Vessella RL, Schmechel SC, Hirsch B, Beckman KB, Silverstein KA, Dehm SM. AR intragenic deletions linked to androgen receptor splice variant expression and activity in models of prostate cancer progression. Oncogene. 2012;31(45):4759–4767. doi: 10.1038/onc.2011.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41(16):2502–2512. doi: 10.1016/j.ejca.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 28.Grivennikov S, Karin M. Autocrine IL-6 signaling: a key event in tumorigenesis? Cancer Cell. 2008;13(1):7–9. doi: 10.1016/j.ccr.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 29.Lin TH, Izumi K, Lee SO, Lin WJ, Yeh S, Chang C. Anti-androgen receptor ASC-J9 versus anti-androgens MDV3100 (Enzalutamide) or Casodex (Bicalutamide) leads to opposite effects on prostate cancer metastasis via differential modulation of macrophage infiltration and STAT3-CCL2 signaling. Cell Death Dis. 4:e764. doi: 10.1038/cddis.2013.270. [DOI] [PMC free article] [PubMed] [Google Scholar]