Abstract
The lethality of ovarian cancer stems from its propensity to involve the peritoneal cavity. However, the mechanisms that enable ovarian cancer cells to readily adapt to the peritoneal environment are not well understood. Here, we describe our recent studies in which we identified the mechanisms by which the transcription factor encoded by the patterning gene HOXA9 promotes the aggressive behavior of ovarian cancer. Firstly, we identified that HOXA9 promotes ovarian tumor growth and angiogenesis by activating the gene encoding transforming growth factor-β2 (TGF-β2), which in turn stimulates peritoneal fibroblasts and mesenchymal stem cells to acquire features of cancer-associated fibroblasts. Secondly, by inducing TGF-β2 and chemokine (C-C motif) ligand 2, HOXA9 stimulates peritoneal macrophages to acquire an immunosuppressive phenotype. Thirdly, HOXA9 stimulates attachment of ovarian cancer cells to peritoneal mesothelial cells by inducing expression of P-cadherin. By inducing P-cadherin, HOXA9 also enables floating cancer cells in the peritoneal cavity to form aggregates and escape anoikis. Together, our studies demonstrate that HOXA9 enables ovarian cancer cells to adapt to the peritoneal environment and ‘educates’ different types of stromal cells to become permissive for tumor growth. Our studies provide new insights into the regulation of tumor-stroma interactions in ovarian cancer and implicate several key effector molecules as candidate therapeutic targets.
Keywords: ovarian cancer, fibroblast, macrophage, mesothelial cell, homeobox gene
Epithelial ovarian cancer is often called the ‘silent killer’ because this disease frequently involves the peritoneal cavity in a rapid and asymptomatic manner. As a consequence, more than 60% of ovarian cancer patients present with advanced-stage disease at the time of initial diagnosis [1]. Patients with advanced-stage ovarian cancer are rarely cured by conventional platinum- and taxane- based chemotherapy and their 5-year survival rate is less than 30% [1]. Unlike many other types of tumors, ovarian cancer does not primarily metastasize via hematogenous routes. Typically, ovarian cancer cells are exfoliated from the primary tumor by the peritoneal fluid that then circulates cancer cells throughout the peritoneal cavity [2, 3]. The omentum and other peritoneal surfaces on to which ovarian cancer cells implant are lined by a monolayer of mesothelial cells that overlie connective and adipose tissues [3]. Peritoneal carcinomatosis is frequently associated with formation of ascites that is abundant in macrophages and other immune cells [4]. Investigating the mechanisms by which ovarian cancer cells interact with the cellular constituents of the peritoneal cavity is therefore important for understanding the biological behavior of this disease and for developing more effective therapies.
Epithelial ovarian cancer comprises several subtypes of tumors that are classified by their morphologic features [5]. In an earlier study, we identified that the major ovarian tumor subtypes are distinguished by their expression of members of the HOX family of homeobox genes that are normally expressed during development of the reproductive tract [6]. Homeobox genes encode transcription factors and were originally identified in Drosophila by their mutations that caused body segments to form in the wrong context [7]. Homeobox genes control tissue patterning and body plan specification, and are expressed in a tightly regulated temporal- and tissue- specific manner [8]. Using mouse i.p. xenograft models of ovarian cancer, we identified that the HOXA9, HOXA10 and HOXA11 genes induce morphologic features of the serous, endometrioid and mucinous tumor subtypes, respectively [6]. However, the role and mechanisms of HOX genes in the clinical behavior of ovarian cancers remained unknown.
In the first of a recent series of studies, we found that high HOXA9 expression is strongly associated with reduced survival of ovarian cancer patients and promotes ovarian tumor growth in i.p. xenograft models [9]. However, HOXA9 had no effect on tumor cell growth in vitro, indicating that its growth-promoting ability depends on interactions with host cells. We identified that high HOXA9 expression in ovarian cancer cells increases the abundance of cancer-associated fibroblasts (CAFs) in xenografts, and is associated with increased expression of CAF markers such as α-smooth muscle actin (αSMA) in ovarian cancer clinical specimens [9]. Increasing evidence indicates that CAFs derive from several different types of cells (reviewed in [10]). For example, epithelial tumor cells that have undergone epithelial-to-mesenchymal transition (EMT) can be a source of CAFs [11]. However, we found that HOXA9 does not alter expression of EMT-inducing transcription factors in ovarian cancer cells [9]. Furthermore, we generated xenografts from green fluorescent protein (GFP)-transfected ovarian cancer cells and found that virtually all αSMA+ stromal cells did not express GFP [9]. These findings indicated that HOXA9 does not induce trans-differentiation of ovarian cancer cells into CAFs. Normal tissue-resident fibroblasts are an important source of CAFs [12]. We identified that expression of HOXA9 in ovarian cancer cells induces normal omental fibroblasts to express CAF markers [9]. Furthermore, we found that HOXA9 promotes growth of ovarian cancer cells and endothelial cells by inducing omental fibroblasts to express interleukin (IL)-6, chemokine (C-X-C motif) ligand 12 (CXCL12) and vascular endothelial growth factor-A (VEGF-A) [9]. Another important source of CAFs are mesenchymal stem cells (MSCs) [13]. Bone marrow is the most studied source of MSCs, but MSCs are abundant in white adipose tissues such as the omentum [14]. We also found that expression of HOXA9 in ovarian cancer cells induces normal adipose MSCs to acquire features of CAFs [9].
In our study, we identified that the stimulatory effects of HOXA9 on CAFs and ovarian tumor growth are largely mediated by its transcriptional activation of the TGFB2 gene that encodes transforming growth factor (TGF)-β2 [9]. Inhibition of TGF-β2 in HOXA9-expressing tumor cells substantially reduced the stimulatory effects of HOXA9 on CAFs and tumor growth. Conversely, the CAF-activating, tumor growth-promoting effect of HOXA9 was restored when TGF-β2 was reconstituted in tumor cells in which HOXA9 was inhibited [9]. In addition, the induction of tumor-derived TGF-β2 by HOXA9 stimulated stromal expression of TGF-β2 and TGF-β1 [9]. Increased levels of tumor-derived TGF-β2, coupled with increased stromal levels of TGF-β ligands, might therefore chronically stimulate CAFs in HOXA9-expressing tumors. Together, these findings support a model in which HOXA9 expression in ovarian cancer cells ‘educates’ normal peritoneal fibroblasts and adipose MSCs to become permissive for tumor growth.
In a second recent study, we identified that HOXA9 expression in ovarian cancer cells also ‘educates’ peritoneal macrophages to acquire an immunosuppressive phenotype. Macrophages are normally present in the peritoneal cavity of healthy women and are a major cellular constituent of ascites [4]. Phagocytic activity of macrophages is often defective in ovarian cancer patients [15]. Macrophages exhibit diverse phenotypes, of which the most studied are termed M1 and M2. M1 macrophages typically express inflammatory and immunostimulatory cytokines such as tumor necrosis factor-α and IL-12, whereas M2 macrophages express immunosuppressive cytokines and chemokines such as IL-10 and CCL17 [16]. Tumor-associated macrophages (TAMs) often exhibit features of M2 macrophages [16]. We identified that high HOXA9 levels in clinical specimens of ovarian cancer are associated with increased abundance of TAMs and with increased levels of IL-10 and CCL17 in ascites fluid [17]. Furthermore, we found that expression of HOXA9 in ovarian cancer cells stimulates chemotaxis of peritoneal macrophages and induces macrophages to express IL-10 and CCL17 in in vitro assays [17]. These effects of HOXA9 were found to be primarily due to its induction of tumor-derived TGF-β2 and CCL2 that act in a paracrine manner on macrophages. In addition to TGFB2, CCL2 was found to be a transcriptional target of HOXA9 [17]. T regulatory (Treg) cells suppress effector T cell activity and CCL17 has been reported to stimulate Treg recruitment to tumors [18]. Consistent with this previous report, we found that clinical specimens of HOXA9-high ovarian cancers have significantly higher abundance of Treg cells and lower abundance of CD8+ tumor-infiltrating lymphocytes than HOXA9-Low ovarian cancers [17]. A study by Sato and colleagues reported that a high CD8+/Treg cell ratio is associated with favorable prognosis in ovarian cancer [19]. HOXA9 might therefore contribute to poor outcomes in ovarian cancer patients by increasing the abundance of TAMs that in turn suppress anti-tumor immune responses.
Although our study demonstrated that HOXA9-induced tumor-derived factors directly stimulate M2 polarization of peritoneal macrophages, HOXA9 might also increase TAMs by indirect mechanisms. One mechanism is by increasing the abundance of CAFs that express TGF-β2 and other factors which in turn could stimulate TAMs. The abundance of TAMs could also be increased by stimulating Treg accumulation. Treg cells express cytokines that induce M2 polarization of macrophages [20]. CCL2, a target of HOXA9, can directly stimulate Treg recruitment [21]. HOXA9 increases tumor- and stromal-derived levels of TGF-β ligands which in turn could regulate FOXP3, a master regulator of Treg cell differentiation and function [22]. By inducing tumor-derived TGF-β2 and CCL2 levels, HOXA9 might promote an immunosuppressive microenvironment that is permissive for ovarian tumor growth by stimulating reciprocal interactions between fibroblasts, macrophages and Treg cells.
A key step in the progression of ovarian cancer is the implantation of floating tumor cells onto surfaces of the peritoneal cavity. The classical cadherins have well-characterized functions in facilitating cell adhesion via homophilic interactions [23]. P-cadherin has been found to be the predominant type of cadherin that is expressed in tumor cells in the peritoneal fluid of ovarian cancer patients [24]. P-cadherin is also the most predominant type of cadherin that is expressed in normal peritoneal tissues [25]. In a third recent study, we identified that P-cadherin facilitates the attachment of floating ovarian cancer cells to mesothelial cells that line peritoneal surfaces [26]. In a parallel fourth study, we identified that the expression of HOXA9 in ovarian cancer cells not only stimulates tumor growth but also promotes implantation of tumor cells on to peritoneal surfaces [27]. The ability of HOXA9 to promote tumor cell implantation was attributed to its induction of P-cadherin that is encoded by the CDH3 gene, a transcriptional target of HOXA9. Inhibition of P-cadherin in HOXA9-expressing ovarian tumor cells abrogated the stimulatory effect of HOXA9 on tumor-mesothelial cell interactions. Conversely, reconstituting P-cadherin in tumor cells in which HOXA9 was inhibited restored the attachment-promoting effect of HOXA9 [27].
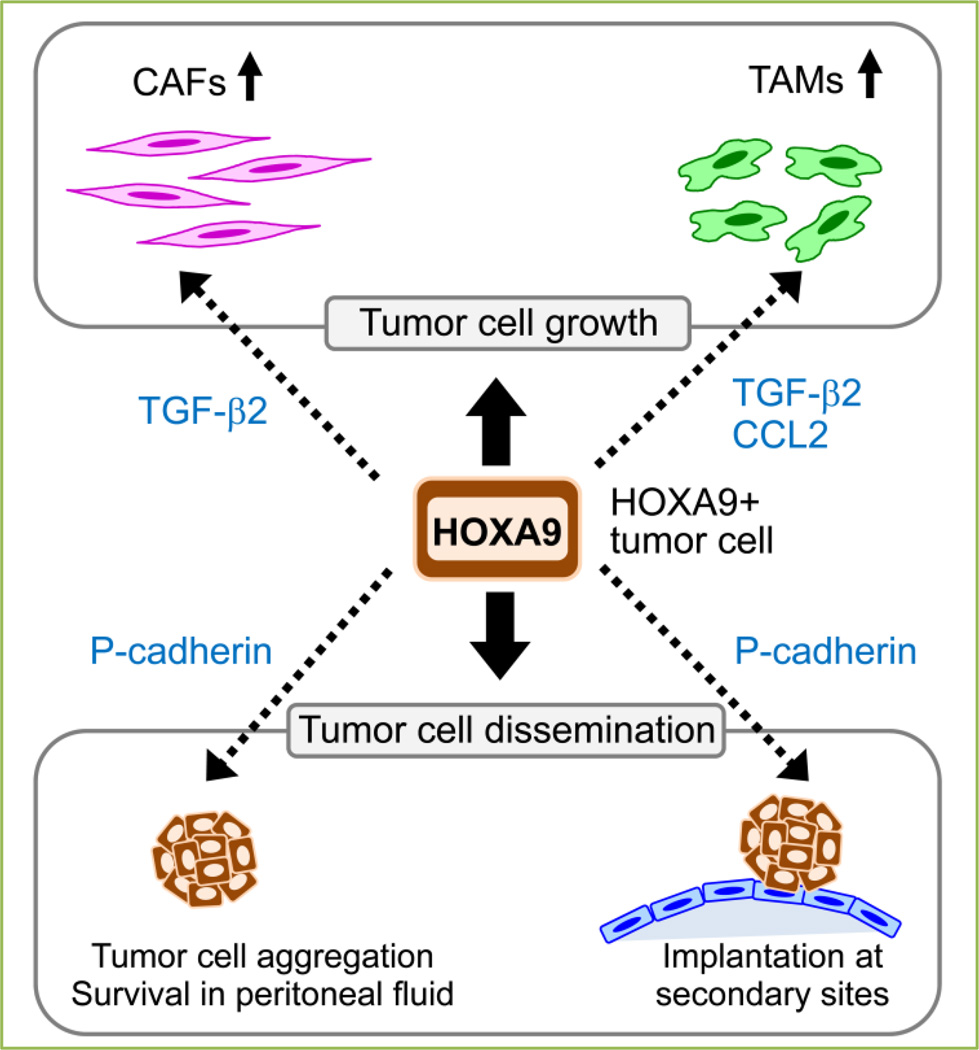
Another key rate-limiting step in the progression of ovarian cancer is the survival of exfoliated, floating tumor cells in the peritoneal fluid. Whereas P-cadherin is the predominant type of cadherin that is expressed in malignant peritoneal effusions of ovarian cancer patients, levels of E-cadherin and N-cadherin have been found to be decreased in floating ovarian cancer cells as compared to solid tumors [24, 28]. Using suspension cultures of ovarian cancer cells and i.p. xenograft models, we have identified that inhibition of P-cadherin prevents floating tumor cells from forming multi-cellular aggregates and increases anoikis [26]. We also identified that HOXA9 promotes aggregation and inhibits anoikis in floating ovarian cancer cells via its induction of P-cadherin [27]. Although it is not possible to exclude the possibility that HOXA9 also promotes tumor cell aggregation by inducing expression of other adhesion molecules, we found that HOXA9 does not induce expression of E-cadherin or N-cadherin and the stimulatory effect of HOXA9 on tumor cell aggregation is abrogated when P-cadherin is inhibited [27]. Together, our studies indicate that HOXA9 via its induction of P-cadherin promotes homotypic and heterotypic cell interactions that enable floating ovarian cancer cells to survive in the peritoneal cavity and to implant onto peritoneal surfaces Figure 1).
Figure 1. HOXA9 promotes tumor-stroma interactions that stimulate ovarian tumor growth and dissemination.
HOXA9, via its transcriptional activation of the genes encoding TGF-β2, CCL2 and P-cadherin in ovarian cancer cells, ‘educates’ peritoneal fibroblasts and adipose MSCs to acquire features of CAFs, ‘educates’ peritoneal macrophages to acquire features of TAMs, and enables floating tumor cells to aggregate, evade anoikis and implant on mesothelium-lined peritoneal surfaces.
Intercellular interactions are essential for orchestrating tissue morphogenesis, repair and regeneration. Because transcription factors encoded by homeobox genes (termed ‘homeoproteins’) play essential functions in tissue patterning, it is perhaps not surprising that we identified that HOXA9 promotes ovarian tumor growth and dissemination by stimulating interactions between tumor cells and between tumor cells and a variety of stromal cells. A number of homeoproteins have been reported to be aberrantly expressed in a variety of malignancies (reviewed in [29]). The transcriptional targets of many of these homeoproteins have not been identified, but several targets control cell cycle progression [30–32]. The mechanisms that control HOXA9 expression in ovarian cancer are poorly understood but may likely involve epigenetic alterations. HOXA9 promoter methylation has been detected in ovarian cancer [33, 34], and occurs at lower frequency in advanced-stage ovarian cancers than in early-stage tumors [33]. Expression of HOX genes is tightly controlled during normal development by Polycomb group (PcG) protein complexes that alter chromatin structure by modifying specific residues in histone tails [35]. The AKT signaling pathway, which is often activated in high-grade, advanced-stage ovarian cancers, suppresses histone methyltransferase activity of the PcG protein EZH2 [36, 37]. We did not detect endogenous HOXA9 in the parental SKOV3 ovarian cancer cell line whereas its aggressive sub clone SKOV3ip was found to express HOXA9 [27]. SKOV3ip cells have higher ERBB2 levels than parental SKOV3 cells [38]. It is possible that HOXA9 expression in ovarian cancer might stem in part from ERBB2-mediated AKT activation.
Because homeoproteins are transcription factors and share regions of homology, targeting HOXA9 is therapeutically challenging. However, inhibiting its downstream effectors (i.e. TGF-β2, CCL2, P-cadherin) may be a promising therapeutic strategy. Anti-TGF-β2 therapies have been developed to circumvent immunosuppression in cancer patients [39]. In addition, small molecule TGF-β receptor kinase inhibitors have been evaluated in animal models and in clinical trials [40, 41]. A monoclonal CCL2 antibody has recently undergone evaluation in a Phase I clinical trial of patients with various types of solid tumors including ovarian cancer [42]. Trabectedin is a marine natural product that inhibits CCL2 and IL-6 production and has been reported to exert selective toxicity for macrophages in xenograft models of ovarian cancer and other solid tumors [43]. A human monoclonal antibody to P-cadherin has been reported to inhibit metastasis in xenograft models of breast, colon and prostate cancer and is undergoing evaluation in clinical trials [44].
In conclusion, our recent studies support a model in which HOXA9, via its transcriptional control of distinct sets of target genes, enables ovarian cancer cells to adapt to the peritoneal environment and ‘educates’ different types of stromal cells to become permissive for tumor growth (Figure 1). Homeobox genes are normally expressed in a tightly regulated tissue-specific manner and their functions can differ depending on the context of their expression [8]. Further investigation of the mechanisms of these patterning regulators in tumors could therefore yield important insights into the unique clinical behavior of different types of tumors and the identification of effective focal points for therapeutic intervention.
Acknowledgments
Studies in the Naora laboratory are supported by Cancer & Prevention Research Institute of Texas grant RP120390 (H.N.) and US National Institutes of Health grant CA141078 (H.N.). The authors apologize for the inability to cite all contributing primary literature due to space constraints.
Footnotes
Conflict of Interest
The authors have declared that no conflicts of interest exist.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Naora H, Montell DJ. Ovarian cancer metastasis: integrating insights from disparate model organisms. Nat Rev Cancer. 2005;5:355–366. doi: 10.1038/nrc1611. [DOI] [PubMed] [Google Scholar]
- 3.Lengyel E. Ovarian cancer development and metastasis. Am J Pathol. 2010;177:1053–1064. doi: 10.2353/ajpath.2010.100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haskill S, Becker S, Fowler W, Walton L. Mononuclear-cell infiltration in ovarian cancer. I. Inflammatory-cell infiltrates from tumour and ascites material. Br J Cancer. 1982;45:728–736. doi: 10.1038/bjc.1982.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naora H. The heterogeneity of epithelial ovarian cancers: reconciling old and new paradigms. Expert Rev Mol Med. 2007;9:1–12. doi: 10.1017/S1462399407000324. [DOI] [PubMed] [Google Scholar]
- 6.Cheng W, Liu J, Yoshida H, Rosen D, Naora H. Lineage infidelity of epithelial ovarian cancers is controlled by HOX genes that specify regional identity in the reproductive tract. Nat Med. 2005;11:531–537. doi: 10.1038/nm1230. [DOI] [PubMed] [Google Scholar]
- 7.Gehring WJ, Hiromi Y. Homeotic genes and the homeobox. Annu Rev Genet. 1986;20:147–173. doi: 10.1146/annurev.ge.20.120186.001051. [DOI] [PubMed] [Google Scholar]
- 8.Pearson JC, Lemons D, McGinnis W. Modulating HOX gene functions during animal body patterning. Nat Rev Genet. 2005;6:893–904. doi: 10.1038/nrg1726. [DOI] [PubMed] [Google Scholar]
- 9.Ko SY, Barengo N, Ladanyi A, Lee JS, Marini F, Lengyel E, et al. HOXA9 promotes ovarian cancer growth by stimulating cancer-associated fibroblasts. J Clin Invest. 2012;122:3603–3617. doi: 10.1172/JCI62229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marsh T, Pietras K, McAllister SS. Fibroblasts as architects of cancer pathogenesis. Biochim Biophys Acta. 2013;1832:1070–1078. doi: 10.1016/j.bbadis.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petersen OW, Nielsen HL, Gudjonsson T, Villadsen R, Rank F, Niebuhr E, et al. Epithelial to mesenchymal transition in human breast cancer can provide a nonmalignant stroma. Am J Pathol. 2003;162:391–402. doi: 10.1016/S0002-9440(10)63834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kojima Y, Acar A, Eaton EN, Mellody KT, Scheel C, Ben-Porath I, et al. Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc Natl Acad Sci USA. 2010;107:20009–20014. doi: 10.1073/pnas.1013805107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Direkze NC, Hodivala-Dilke K, Jeffery R, Hunt T, Poulsom R, Oukrif D, et al. Bone marrow contribution to tumor-associated myofibroblasts and fibroblasts. Cancer Res. 2004;64:8492–8495. doi: 10.1158/0008-5472.CAN-04-1708. [DOI] [PubMed] [Google Scholar]
- 14.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon IO, Freedman RS. Defective antitumor function of monocyte-derived macrophages from epithelial ovarian cancer patients. Clin Cancer Res. 2006;12:1515–1524. doi: 10.1158/1078-0432.CCR-05-2254. [DOI] [PubMed] [Google Scholar]
- 16.Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86:1065–1073. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- 17.Ko SY, Ladanyi A, Lengyel E, Naora H. Expression of the homeobox gene HOXA9 in ovarian cancer induces peritoneal macrophages to acquire an M2 tumor-promoting phenotype. Am J Pathol. 2014;184:271–281. doi: 10.1016/j.ajpath.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mizukami Y, Kono K, Kawaguchi Y, Akaike H, Kamimura K, Sugai H, et al. CCL17 and CCL22 chemokines within tumor microenvironment are related to accumulation of Foxp3+ regulatory T cells in gastric cancer. Int J Cancer. 2008;122:2286–2293. doi: 10.1002/ijc.23392. [DOI] [PubMed] [Google Scholar]
- 19.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci USA. 2007;104:19446–19451. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jordan JT, Sun W, Hussain SF, DeAngulo G, Prabhu SS, Heimberger AB. Preferential migration of regulatory T cells mediated by glioma-secreted chemokines can be blocked with chemotherapy. Cancer Immunol Immunother. 2008;57:123–131. doi: 10.1007/s00262-007-0336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, Tone M. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol. 2008;9:194–202. doi: 10.1038/ni1549. [DOI] [PubMed] [Google Scholar]
- 23.Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- 24.Patel IS, Madan P, Getsios S, Bertrand MA, MacCalman CD. Cadherin switching in ovarian cancer progression. Int J Cancer. 2003;106:172–177. doi: 10.1002/ijc.11086. [DOI] [PubMed] [Google Scholar]
- 25.Chen GT, Tai CT, Yeh LS, Yang TC, Tsai HD. Identification of the cadherin subtypes present in the human peritoneum and endometriotic lesions: potential role for P-cadherin in the development of endometriosis. Mol Reprod Dev. 2002;62:289–294. doi: 10.1002/mrd.10121. [DOI] [PubMed] [Google Scholar]
- 26.Usui A, Ko SY, Barengo N, Naora H. P-cadherin promotes ovarian cancer dissemination through tumor cell aggregation and tumor-peritoneum interactions. Mol Cancer Res. 2014;12:504–513. doi: 10.1158/1541-7786.MCR-13-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ko SY, Naora H. HOXA9 promotes homotypic and heterotypic cell interactions that facilitate ovarian cancer dissemination via its induction of P-cadherin. Mol Cancer. 2014;13:170. doi: 10.1186/1476-4598-13-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Veatch AL, Carson LF, Ramakrishnan S. Differential expression of the cell-cell adhesion molecule E-cadherin in ascites and solid human ovarian tumor cells. Int J Cancer. 1994;58:393–399. doi: 10.1002/ijc.2910580315. [DOI] [PubMed] [Google Scholar]
- 29.Haria D, Naora H. Homeobox gene deregulation: Impact on the hallmarks of cancer. Cancer Hallm. 2013;1:67–76. doi: 10.1166/ch.2013.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raman V, Martensen SA, Reisman D, Evron E, Odenwald WF, Jaffee E, et al. Compromised HOXA5 function can limit p53 expression in human breast tumours. Nature. 2000;405:974–978. doi: 10.1038/35016125. [DOI] [PubMed] [Google Scholar]
- 31.Xu J, Testa JR. DLX5 (distal-less homeobox 5) promotes tumor cell proliferation by transcriptionally regulating MYC. J Biol Chem. 2009;284:20593–20601. doi: 10.1074/jbc.M109.021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trinh BQ, Ko SY, Barengo N, Lin SY, Naora H. Dual functions of the homeoprotein DLX4 in modulating responsiveness of tumor cells to topoisomerase II-targeting drugs. Cancer Res. 2013;73:1000–1010. doi: 10.1158/0008-5472.CAN-12-3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu Q, Lothe RA, Ahlquist T, Silins I, Trope CG, Micci F, et al. DNA methylation profiling of ovarian carcinomas and their in vitro models identifies HOXA9, HOXB5, SCGB3A1, and CRABP1 as novel targets. Mol Cancer. 2007;6:45. doi: 10.1186/1476-4598-6-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montavon C, Gloss BS, Warton K, Barton CA, Statham AL, Scurry JP, et al. Prognostic and diagnostic significance of DNA methylation patterns in high grade serous ovarian cancer. Gynecol Oncol. 2012;124:582–588. doi: 10.1016/j.ygyno.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 35.Soshikova N. Hox genes regulation in vertebrates. Dev Dyn. 2014;243:49–58. doi: 10.1002/dvdy.24014. [DOI] [PubMed] [Google Scholar]
- 36.Yuan ZQ, Sun M, Feldman RI, Wang G, Ma X, Jiang C, et al. Frequent activation of AKT2 and induction of apoptosis by inhibition of phosphoinositide-3-OH kinase/Akt pathway in human ovarian cancer. Oncogene. 2000;19:2324–2330. doi: 10.1038/sj.onc.1203598. [DOI] [PubMed] [Google Scholar]
- 37.Cha TL, Zhou BP, Xia W, Wu Y, Yang CC, Chen CT, et al. Akt-mediated phosphorylation of EZH2 suppresses methylation of lysine 27 in histone H3. Science. 2005;310:306–310. doi: 10.1126/science.1118947. [DOI] [PubMed] [Google Scholar]
- 38.Yu D, Wolf JK, Scanlon M, Price JE, Hung MC. Enhanced c-erbB-2/neu expression in human ovarian cancer cells correlates with more severe malignancy that can be suppressed by E1A. Cancer Res. 1993;53:891–898. [PubMed] [Google Scholar]
- 39.Nemunaitis J, Dillman RO, Schwarzenberger PO, Senzer N, Cunningham C, Cutler J, et al. Phase II study of belagenpumatucel-L, a transforming growth factor beta-2 antisense gene-modified allogeneic tumor cell vaccine in non-small-cell lung cancer. J Clin Oncol. 2006;24:4721–4730. doi: 10.1200/JCO.2005.05.5335. [DOI] [PubMed] [Google Scholar]
- 40.Mohammad KS, Javelaud D, Fournier PG, Niewolna M, McKenna CR, Peng XH, et al. TGF-beta-RI kinase inhibitor SD-208 reduces the development and progression of melanoma bone metastases. Cancer Res. 2011;71:175–184. doi: 10.1158/0008-5472.CAN-10-2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Azaro A, Baselga J, Sepulveda JM, Seoane J, Ahnert JR, Brana I, et al. The oral transforming growth factor-beta (TGF-beta) receptor I kinase inhibitor LY2157299 plus lomustine in patients with treatment-refractory malignant glioma: The first human dose study. J Clin Oncol. 2012;30 (suppl: abstract 2042) [Google Scholar]
- 42.Sandhu SK, Papadopoulos K, Fong PC, Patnaik A, Messiou C, Olmos D, et al. A first-in-human, first-in-class, phase I study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 in patients with solid tumors. Cancer Chemother Pharmacol. 2013;71:1041–1050. doi: 10.1007/s00280-013-2099-8. [DOI] [PubMed] [Google Scholar]
- 43.Germano G, Frapolli R, Belgiovine C, Anselmo A, Pesce S, Liguori M, et al. Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell. 2013;23:249–262. doi: 10.1016/j.ccr.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 44.Zhang CC, Yan Z, Zhang Q, Kuszpit K, Zasadny K, Qiu M, et al. PF-03732010: a fully human monoclonal antibody against P-cadherin with antitumor and antimetastatic activity. Clin Cancer Res. 2010;16:5177–5188. doi: 10.1158/1078-0432.CCR-10-1343. [DOI] [PubMed] [Google Scholar]