Summary
Dauer formation, a major nematode survival strategy, represents a model for small-molecule regulation of metazoan development [1–10]. Free-living nematodes excrete dauer-inducing pheromones that have been assumed to target conspecifics of the same genotype [9, 11]. However, recent studies in Pristionchus pacificus revealed that the dauer pheromone of some strains affects conspecifics of other genotypes more strongly than individuals of the same genotype [12]. To elucidate the mechanistic basis for this intriguing cross-preference, we compared six P. pacificus wild isolates to determine the chemical composition of their dauer-inducing metabolomes and responses to individual pheromone components. We found that these isolates produce dauer pheromone blends of different composition and respond differently to individual pheromone components. Strikingly, there is no correlation between production of and dauer response to a specific compound in individual strains. Specifically, pheromone components that are abundantly produced by one genotype induce dauer formation in other genotypes, but not necessarily in the abundant producer. Furthermore, some genotypes respond to pheromone components they do not produce themselves. These results support a model of intraspecific competition in nematode dauer formation. Indeed, we observed intraspecific competition among sympatric strains in a novel experimental assay, suggesting a new role of small molecules in nematode ecology.
Results and Discussion
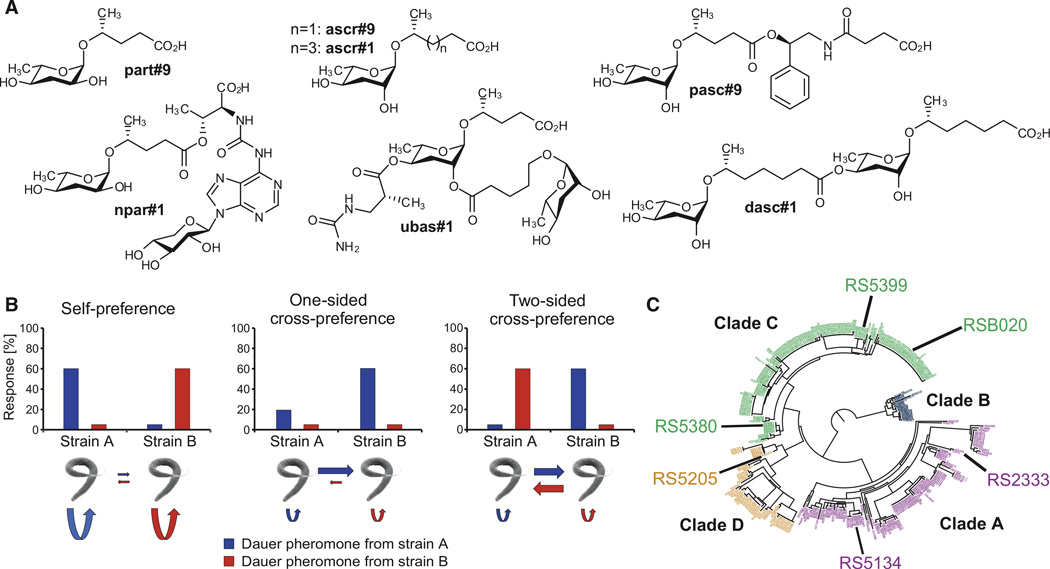
A modular library of small-molecule signals, the ascarosides and paratosides, which are derived from a combination of the dideoxysugars ascarylose or paratose with chemically diverse building blocks from all major primary metabolic pathways, induce the formation of dauer larvae in Caenorhabditis elegans and Pristionchus pacificus in a species-specific manner (Figure 1A; Figure S1 available online) [7, 8]. Dauer formation represents both a survival strategy and a dispersal strategy, enabling individuals of a population to endure and escape unfavorable conditions [10]. It has long been assumed that dauer pheromone excretion targets conspecifics of the same genotype [9, 11]. However, a recent study in P. pacificus showed that dauer pheromones often induce the highest rate of dauer formation in individuals of genotypes other than the strain the pheromone was obtained from (Figure 1B) [12]. This phenomenon has been called dauer pheromone “cross-preference” (Figure 1B). Although the mechanistic basis of cross-preference remains unknown, it could result from strain-specific differences in pheromone production, responsiveness to specific pheromone components, or both.
Figure 1. Introduction to P. pacificus Small Molecules and Strains Used in This Study.
(A) Ascarosides and paratosides previously identified from P. pacificus. The shown compounds constitute the most-abundant members (across all strains in this study) of the known families of ascarosides and paratosides, including unmodified ascarosides (ascr#1 and ascr#9), unmodified paratosides (part#9), phenylethanolamine-containing ascarosides (pasc#9), dimeric ascarosides (dasc#1), ureido-isobutyrate ascarosides (ubas#1), and nucleoside-containing paratosides (npar#1).
(B) Potential theoretical outcomes of pheromone interactions. Nematodes show the strongest response either to their own pheromones (self-preference) or to pheromones from other strains (cross-preference).
(C) Phylogenetic structure of P. pacificus. The six strains are highlighted in the phylogenetic dendrogram from [13].
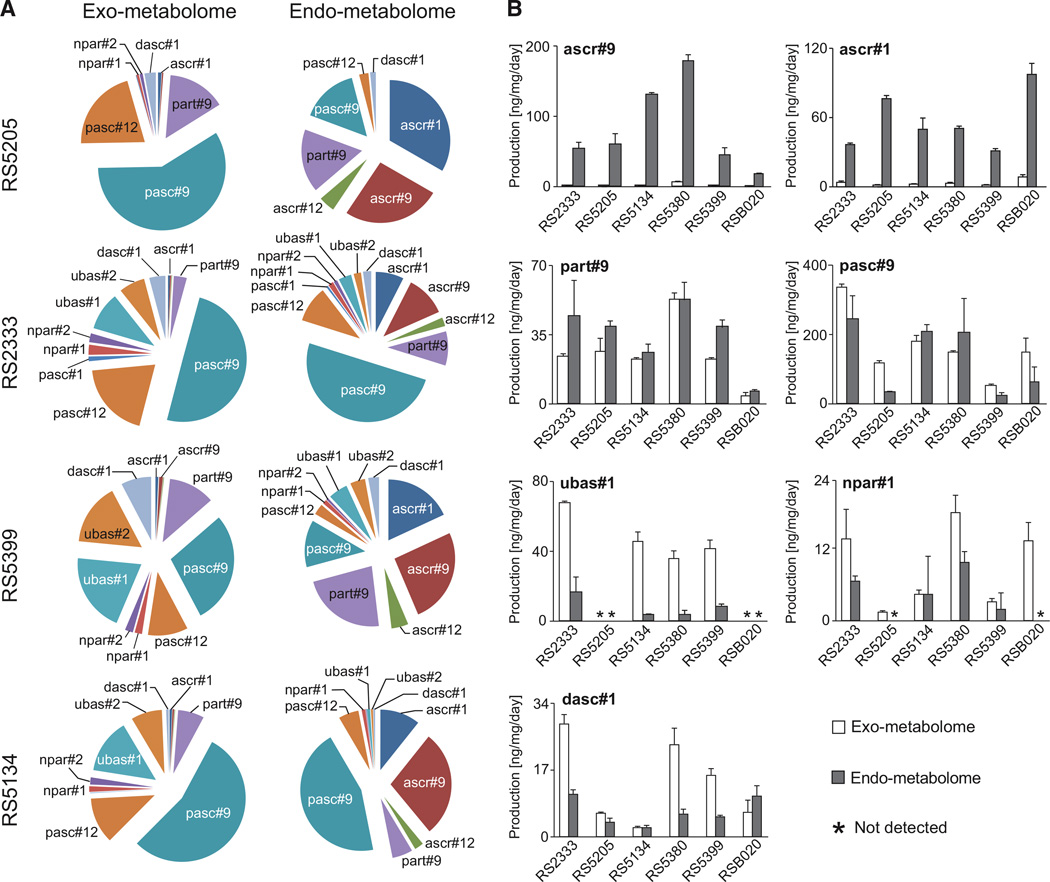
To identify and quantify ascaroside- and paratoside-based pheromones, we analyzed the exometabolomes (the entirety of all excreted small molecules) of six P. pacificus wild isolates by using targeted high-pressure liquid chromatography mass spectrometry (HPLC-MS) [7]. The six strains used in this study have diverse genetic backgrounds, covering three of the four P. pacificus clades (Figure 1C) [13]. They include the P. pacificus reference strain RS2333 from California, one strain from Ohio (RS5134), one strain from South Africa (RS5205), and three more closely related strains from La Réunion Island (RS5380, RS5399, and RSB020). Our results indicate both qualitative and quantitative differences in pheromone production among the six isolates, resulting in six characteristic, strain-specific blends of small molecules (Figure 2A; Figures S2A and S2B). Production of many compounds varied strongly among strains. For example, the phenylethanolamine-derived pasc#9 was found to represent a major constituent of the exometabolome in all six strains but was found to be 6.4-fold more abundant in RS2333 (highest producer) than in RS5399 (lowest producer). Furthermore, production of different pheromone components appears to be independently regulated. For example, RS2333, which produces the highest amounts of npar#1, ubas#1, and dasc#1, produces smaller amounts of two other compounds, ascr#9 and part#9, than RS5380. Similarly, pasc#9 production is strongly reduced in RS5399, which produces most other compounds in amounts similar to those in other strains (Figure 2). Thus, differences in small-molecule profiles do not result from simple variation in basal metabolic activity but from genetic differentiation among strains.
Figure 2. Ascaroside and Paratoside Profiles of P. pacificus Isolates Used in This Study.
(A) Relative abundances of ascarosides and paratosides in the exometabolomes and endometabolomes of exemplary P. pacificus wild isolates derived from HPLC-MS analysis (Figures 1A and S1) [7].
(B) Comparison of abundances of seven major ascarosides and paratosides in the exometabolomes and endometabolomes of six different P. pacificus wild isolates, represented in ng/day and normalized by worm pellet dry weight (see Supplemental Experimental Procedures). Error bars show SD.
Although most of the detected ascarosides and paratosides were present in all strains, the two compounds ubas#1 and ubas#2 were not detected in the exometabolomes of RS5205 and RSB020 (Figures 2 and S2A–S2C). To exclude the possibility that the absence of ubas#1 and ubas#2 from the exometabolomes of these two strains was due to deficiencies in pheromone excretion, we also analyzed the endometabolomes (the entirety of small molecules extractable from worm pellets) of all six P. pacificus isolates. We found that the composition of the endometabolome differs significantly from that of the exometabolome (Figure 2A). Specifically, the endometabolome contained large amounts of the simple ascarosides, namely ascr#1, ascr#9, and ascr#12, which only represent minor components of the exometabolome (Figures 2, S2A, and S2B). In contrast, structurally more-elaborate ascarosides and paratosides, such as ubas#1, dasc#1, and npar#1, were much more abundant in the exometabolome than in the endometabolome (Figure 2B). This suggests that the simple ascarosides may act as precursors or storage forms for the biosynthesis of the structurally more-complex molecules, all of which play a role in dauer formation or in determining the mouth form and are preferentially excreted. Furthermore, our results indicate that ascaroside and paratoside excretion is tightly regulated, and even excretion of chemically very similar compounds appears to be highly selective. For example, ascr#9 is almost entirely retained in the worm body, whereas part#9, differing from ascr#9 merely in the stereochemistry of one hydroxyl group (Figure 1A), is similarly abundant in the exometabolomes and endometabolomes of all strains (Figures 2 and S2D). Notably, the two compounds that were not detected in the exometabolomes of RS5205 and RSB020, ubas#1 and ubas#2, were also not present in the corresponding endometabolomes, suggesting that these two strains may not be capable of synthesizing these compounds. Thus, significant qualitative and quantitative differences were observed in ascaroside and paratoside production for all six analyzed strains.
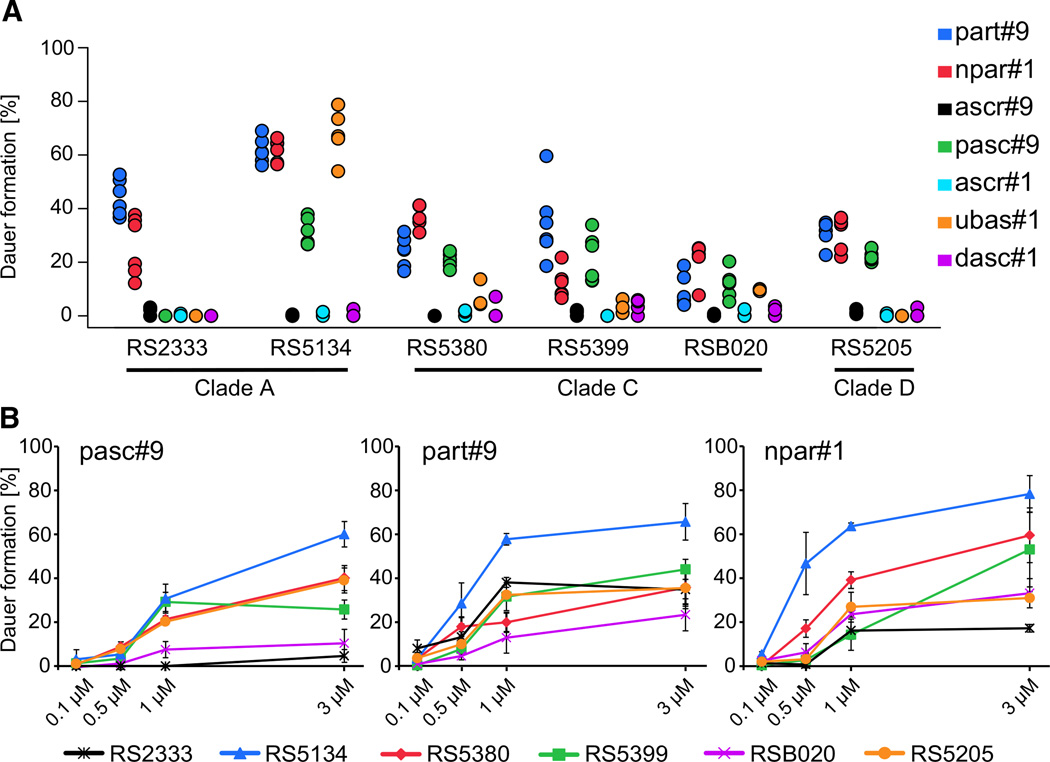
Next, we tested whether these six strains also differ in their response to ascarosides and paratosides in dauer formation assays. For this purpose, synthetic samples of seven ascarosides and paratosides [1, 7, 8], including the most-abundant members that have been identified from P. pacificus so far, were tested on all six strains (Figure 3A). To minimize the effects of pheromone production of the worms during the experiment, we developed a novel assay that results in dauer formation within one generation. We used a concentration of 1 µM, which is similar to ascaroside and paratoside concentrations in P. pacificus liquid cultures [7]. Our results show that all six strains responded to the tested compounds in a strain-specific manner and with compound-specific patterns (Figure 3A; ANOVA [generalized linear model, or GLM] F6,5 = 26.44, p < 0.001; Table S1). The two compounds part#9 and npar#1 induce dauer formation in all six strains to varying extents. In contrast, the phenylethanolamine-derived ascaroside pasc#9 induces dauer formation in all strains except in RS2333 (Figure 3A), although the compound is produced by all strains. Most strikingly, the dimeric ascaroside ubas#1 induced the highest dauer formation in RS5134, resulting in about 70% dauers, whereas this compound either was inactive or induced less than 10% dauers in all other strains (Figure 3A). When we tested the three most broadly active dauer-inducing compounds, part#9, npar#1, and pasc#9, over a wider concentration range, the resulting concentration curves revealed additional variation in dauer response (ANOVA [GLM] F2,5 = 10.38, p < 0.001) (Figure 3B). However, we never observed any dauer response to concentrations lower than 0.1 µM. Together, these response patterns are sufficient to distinguish the strains from one another.
Figure 3. Natural Variation in Dauer Formation.
(A) Dauer formation of six P. pacificus strains in response to synthetic standards at 1 µM concentration (three to six independent biological replicates). The significance of the variation among strains and small molecules tested was calculated by using an ANOVA (GLM; strain: p = < 0.001, F = 12.51; compound: p = < 0.001, F = 20.73). Each dot represents one replicate. If fewer than three dots are visible, these dots are hidden behind the ones displayed.
(B) Concentration dependencies for the three most-potent dauer-inducing small molecules: pasc#9, part#9, and npar#1 (three independent biological replicates). The mean dauer formation of three replicates is displayed for each of the six wild isolates tested. Error bars show exact binomial 95% confidence intervals.
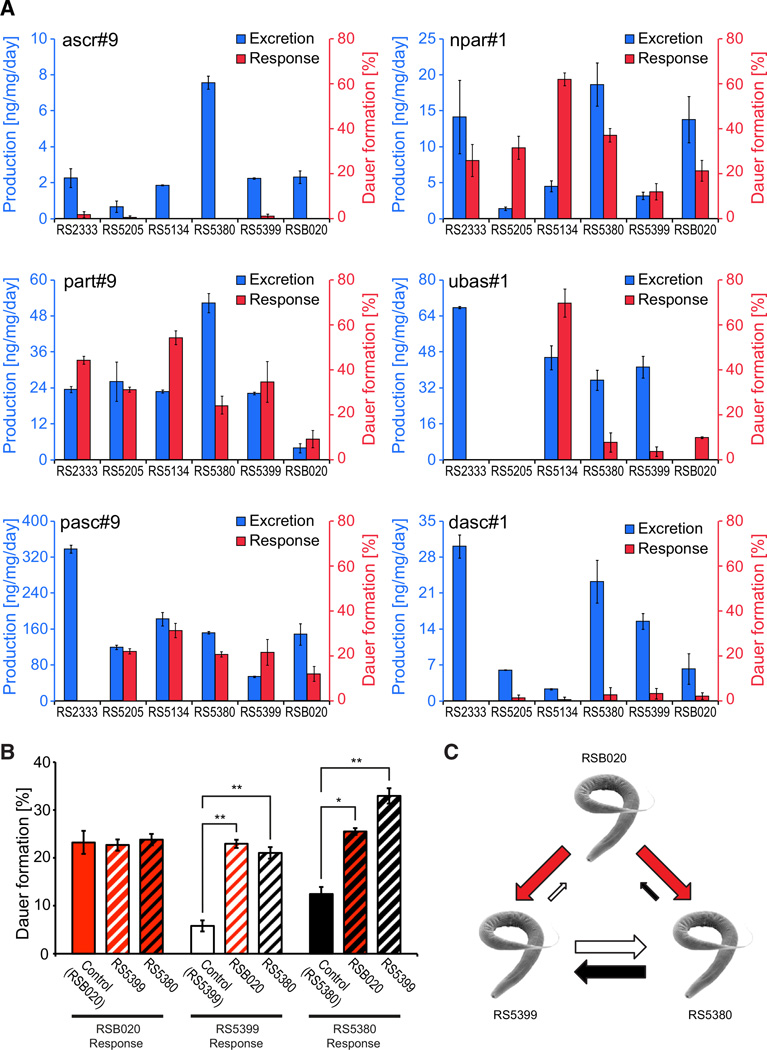
The quantitative and qualitative differences observed for both compound production and dauer response suggest that dauer pheromone may play a role in intraspecific competition among P. pacificus genotypes. Therefore, we further analyzed our data and identified two properties that support the idea that the observed genetic differences might indicate competitive interactions. Because small molecules can have multiple functions in the worm, the examples listed below were specifically chosen to focus on strict presence versus absence of compounds when comparing the dauer response of different strains. First, pasc#9 has no dauer-inducing activity in RS2333, although RS2333 produces the highest amounts of pasc#9 (Figure 4A). This finding indicates that a strain does not necessarily respond to its own excreted small molecule in dauer formation. Second, ubas#1 represents the opposite scenario to pasc#9. RSB020 showed 10% dauer formation in response to ubas#1 (Fisher’s exact test, p < 0.05, comparing treatment to control conditions), although ubas#1 was not detectable in its metabolome (Figure 2B). Furthermore, some of the strongest producers of ubas#1 either do not respond at all (RS2333) or, in contrast, respond very strongly (70% dauers in RS5134). Thus, in the case of ubas#1, a strain can respond to a compound it does not produce, whereas a strong producer of ubas#1 does not show any dauer response. More generally, there is no strict correlation between production of and dauer response to a specific pheromone component in individual strains (Figure 4A). For example, RS5134 is the strongest responder to part#9 and npar#1, resulting in 62% and 63% dauers. However, it produces less npar#1 and part#9 than most other strains, suggesting that small-molecule production and response are largely uncoupled (Figure 4A). Taken together, these results show that strains can respond to compounds they do not synthesize themselves and can synthesize compounds that induce dauer formation only in other strains.
Figure 4. Comparison of Production of and Dauer Response to Ascarosides and Paratosides Supporting Intraspecific Competition in P. pacificus Wild Isolates.
(A) Comparative differences between production of and response to major ascarosides and paratosides in P. pacificus isolates used in this study.
(B) Dauer pheromone competition assay with RS5380, RS5399, and RSB020 grown in Ussing chambers (see also Figure S3A). In control experiments, both compartments of a chamber were filled with nematodes of the same strain (Control). In the competition experiments, one strain was grown in one compartment of an Ussing chamber, while a different strain was grown in the other compartment of the same chamber. For each strain, the mean dauer formation of eight samples (taken from two independent experiments) is shown in response to being cultured with nematodes of the same (Control) or of a different genotype. Error bars show exact binomial 95% confidence intervals.
(C) Model for intraspecific competition among RS5380, RS5399, and RSB020.
To directly investigate the possibility of dauer pheromone-mediated intraspecific competition, we developed a novel competition assay. Specifically, we tested the two allopatric strains RS2333 and RS5134 and the three sympatric strains RS5380, RS5399, and RSB020 from La Réunion Island for competition. In contrast to the dauer formation assays of individual strains in response to specific small molecules (Figure 3), this competition assay was designed to enable the observation of dauer formation in a more natural environment where a strain is exposed to its own pheromone blend plus that of another genotype. For this purpose, nematodes were grown in liquid culture in a commercially available Ussing chamber (http://phywe.com/461/pid/14188/ussing-chamber.htm) without adding any synthetic compounds (Figure S3A). An Ussing chamber consists of two compartments separated by a membrane through which the nematodes cannot pass but that is permeable to water and small molecules. Thus, this setup allows dauer formation of two strains to be studied in response to the blends of small molecules excreted by both strains, mimicking the natural habitat of P. pacificus. In control experiments, both compartments of a chamber were filled with worms of the same strain. For the allopatric strains, control chambers were filled with either RS2333 or RS5134 worms, leading to dauer formation rates of about 10% and 30% (Figure S3B). In the competition experiment of these strains, one compartment of the Ussing chamber contained RS2333 worms, and the other compartment contained RS5134 worms. Under these experimental conditions, RS2333 still formed 10% dauers, whereas the dauer formation of RS5134 was significantly increased to 41% (Fisher’s exact test, p < 0.05) (Figure S3B). In competition experiments of the three sympatric strains, we analyzed all three possible strain combinations: RSB020/RS5399, RSB020/RS5380, and RS5399/RS5380. We found that both RS5399 and RS5380 formed more dauers when grown in the same chamber with RSB020 as compared to control experiments (Fisher’s exact tests, p < 0.01 for RS5399 and p < 0.05 for RS5380) (Figure 4B). Most strikingly, the competition experiment between RS5380 and RS5399 revealed two-sided cross-preference, with both strains showing significantly increased dauer formation as compared to control experiments (Fisher’s exact tests, p < 0.01) (Figure 4B). Thus, all competition experiments revealed cross-preference of dauer pheromones, suggesting that intraspecific competition results in elevated dauer formation in animals of a different genotype.
In this study, we describe extreme natural variation in both dauer pheromone production and response among six wild isolates of P. pacificus, which is consistent with the previously observed cross-preference of 16 strains of this nematode [12]. Previously, little was known about the evolutionary and ecological circumstances of small-molecule signaling in dauer formation. We provide strong evidence for quantitative and qualitative natural variation in small-molecule production. Interestingly, variation in relative amounts can be very high not only in allopatric strains but also among sympatric isolates. Because it is obvious that several of the selected strains (e.g., from California and Ohio) would never meet in nature, we focused our experimental competition assay on the three sympatric strains from La Réunion (Figure 4C). Competitive interactions might be associated with evolutionary arms races, as suggested by theory [14], and probably occur when the nematodes exit the dauer stage to feed on a limited food source (e.g., the microbes that grow on a scarab beetle carcass). In nature, multiple P. pacificus haplotypes have been isolated from a single beetle [13], indicating their coexistence and potential competition for resources on the carcass. Natural variation in dauer formation probably represents an adaptive advantage for some strains over others. For example, if strain A produces a pheromone that induces high dauer formation in strain B, strain A would be able to force strain B into early dauer formation. Strain A would then gain the advantage of using a limited food source for a longer time, therefore producing a higher number of progeny than strain B. On the other hand, strain B might have evolved to respond strongly to the pheromone of strain A as a means of escaping overpopulation and potential starvation as early as possible. Therefore, different strains may have evolved different survival strategies, with strain A forcing other strains into early dauer formation and with strain B not competing with other strains but optimizing survival and dispersal. Thus, intraspecific competition might initiate adaptive processes that result in opposite survival strategies among strains of the same species.
Supplementary Material
Acknowledgments
This work was supported by the Max Planck Society (R.J.S.) and by the NIH (GM088290 and GM085285 to F.C.S.). N.B. is grateful to the Cornell/Rockefeller/Sloan-Kettering Tri-Institutional Training Program in Chemical Biology fellowship program. The authors thank R. Neher and A. McGaughran for their support with statistical analyses.
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, three figures, and one table and can be found with this article online at http://dx.doi.org/10.1016/j.cub.2014.05.045.
Author Contributions
N.B., J.M.M., F.C.S., and R.J.S. conceived the study and designed the experiments. A.O. prepared liquid cultures of the P. pacificus wild isolates. N.B. processed the biological samples and performed the HPLC-MS analysis. N.B. and F.C.S analyzed the HPLC-MS data. N.B. and J.J.Y. synthesized the small-molecule standards. J.M.M. performed the dauer assays. M.G.M. and J.M.M. performed the dauer pheromone competition experiments. J.M.M., J.J.Y., M.G.M., and G.V.M. analyzed the results from biological assays. N.B., J.M.M., J.J.Y., F.C.S., and R.J.S. wrote the manuscript.
References
- 1.Jeong PY, Jung M, Yim YH, Kim H, Park M, Hong E, Lee W, Kim YH, Kim K, Paik YK. Chemical structure and biological activity of the Caenorhabditis elegans dauer-inducing pheromone. Nature. 2005;433:541–545. doi: 10.1038/nature03201. [DOI] [PubMed] [Google Scholar]
- 2.Srinivasan J, Kaplan F, Ajredini R, Zachariah C, Alborn HT, Teal PEA, Malik RU, Edison AS, Sternberg PW, Schroeder FC. A blend of small molecules regulates both mating and development in Caenorhabditis elegans. Nature. 2008;454:1115–1118. doi: 10.1038/nature07168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pungaliya C, Srinivasan J, Fox BW, Malik RU, Ludewig AH, Sternberg PW, Schroeder FC. A shortcut to identifying small molecule signals that regulate behavior and development in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 2009;106:7708–7713. doi: 10.1073/pnas.0811918106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butcher RA, Ragains JR, Clardy J. An indole-containing dauer pheromone component with unusual dauer inhibitory activity at higher concentrations. Org. Lett. 2009;11:3100–3103. doi: 10.1021/ol901011c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butcher RA, Ragains JR, Kim E, Clardy J. A potent dauer pheromone component in Caenorhabditis elegans that acts synergistically with other components. Proc. Natl. Acad. Sci. USA. 2008;105:14288–14292. doi: 10.1073/pnas.0806676105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butcher RA, Fujita M, Schroeder FC, Clardy J. Small-molecule pheromones that control dauer development in Caenorhabditis elegans. Nat. Chem. Biol. 2007;3:420–422. doi: 10.1038/nchembio.2007.3. [DOI] [PubMed] [Google Scholar]
- 7.Bose N, Ogawa A, von Reuss SH, Yim JJ, Ragsdale EJ, Sommer RJ, Schroeder FC. Complex small-molecule architectures regulate phenotypic plasticity in a nematode. Angew. Chem. Int. Ed. Engl. 2012;51:12438–12443. doi: 10.1002/anie.201206797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Reuss SH, Bose N, Srinivasan J, Yim JJ, Judkins JC, Sternberg PW, Schroeder FC. Comparative metabolomics reveals biogenesis of ascarosides, a modular library of small-molecule signals in C. elegans. J. Am. Chem. Soc. 2012;134:1817–1824. doi: 10.1021/ja210202y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golden JW, Riddle DL. A pheromone influences larval development in the nematode Caenorhabditis elegans. Science. 1982;218:578–580. doi: 10.1126/science.6896933. [DOI] [PubMed] [Google Scholar]
- 10.Sommer RJ, Ogawa A. Hormone signaling and phenotypic plasticity in nematode development and evolution. Curr. Biol. 2011;21:R758–R766. doi: 10.1016/j.cub.2011.06.034. [DOI] [PubMed] [Google Scholar]
- 11.Viney ME, Gardner MP, Jackson JA. Variation in Caenorhabditis elegans dauer larva formation. Dev. Growth Differ. 2003;45:389–396. doi: 10.1046/j.1440-169x.2003.00703.x. [DOI] [PubMed] [Google Scholar]
- 12.Mayer MG, Sommer RJ. Natural variation in Pristionchus pacificus dauer formation reveals cross-preference rather than self-preference of nematode dauer pheromones. Proc. Biol. Sci. 2011;278:2784–2790. doi: 10.1098/rspb.2010.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morgan K, McGaughran A, Villate L, Herrmann M, Witte H, Bartelmes G, Rochat J, Sommer RJ. Multi locus analysis of Pristionchus pacificus on La Réunion Island reveals an evolutionary history shaped by multiple introductions, constrained dispersal events and rare out-crossing. Mol. Ecol. 2012;21:250–266. doi: 10.1111/j.1365-294X.2011.05382.x. [DOI] [PubMed] [Google Scholar]
- 14.Dawkins R, Krebs JR. Arms races between and within species. Proc. R. Soc. Lond. B Biol. Sci. 1979;205:489–511. doi: 10.1098/rspb.1979.0081. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.