SUMMARY
Organ wasting, related to changes in nutrition and metabolic activity of cells and tissues, is observed under conditions of starvation and in the context of diseases, including cancers. We have developed a model for organ wasting in adult Drosophila, whereby overproliferation induced by activation of Yorkie, the Yap1 oncogene ortholog, in intestinal stem cells leads to wasting of the ovary, fat body, and muscle. These organ-wasting phenotypes are associated with a reduction in systemic insulin/IGF signaling due to increased expression of the secreted insulin/IGF antagonist ImpL2 from the overproliferating gut. Strikingly, expression of rate-limiting glycolytic enzymes and central components of the insulin/IGF pathway is upregulated with activation of Yorkie in the gut, which may provide a mechanism for this overproliferating tissue to evade the effect of ImpL2. Altogether, our study provides insights into the mechanisms underlying organ-wasting phenotypes in Drosophila and how overproliferating tissues adapt to global changes in metabolism.
INTRODUCTION
Wasting is the process characterized by an involuntary loss of body mass manifested in particular by degeneration of skeletal muscles and adipose tissues. Wasting is not only a physiological condition responding to extremely low energy intake and infection but also part of a complex systemic disorder associated with many diseases, including cancers, chronic obstructive lung disease, congestive heart failure, chronic kidney disease, and other chronic diseases (Deboer, 2009; Delano and Moldawer, 2006; Planté-Bordeneuve and Said, 2011; Tisdale, 1997). In particular, 50% of advanced cancer patients are affected by wasting syndrome, which accounts for approximately 20% of cancer death (Fearon et al., 2013; Penna et al., 2010). A number of studies have implicated proinflammatory cytokines, such as tumor necrosis factor α and interleukin 1 and 6, as secreted factors involved in wasting associated with various conditions (Fearon et al., 2013; Kir et al., 2014; Penna et al., 2010; Tisdale, 2009).Additionally, insulin-like growth factor 1 IGF-1) signaling is a critical regulator of muscle mass maintenance (Bodine et al., 2001; Rommel et al., 2001; Sandri et al., 2004). Downregulation of IGF-1 signaling in skeletal muscles decreases Akt activity and in tum increases Foxo activity, which induces muscle protein degradation through the ubiquitin-proteasome system and autophagy (Han et al., 2013). Moreover, the transforming growth factor β family members myostatin and activin have been identified as additional secreted factors regulating organ wasting (Fearon et al., 2012; Han et al., 2013). Stimulation of myostatin/activin signaling in skeletal muscles activates Smad2/3 signaling and inhibits Akt signaling, which increases catabolism of musole proteins (Fearon et al., 2012; Han et al., 2013).
The "bloating syndrome," observed in flies transplanted with imaginal discs mutant for the tumor suppressor lethal (2) giant larvae (l(2)gl) (Gateff and Schneiderman, 1974), is a systemic phenotype relevant to the wasting syndrome. Whereas a wild-type imaginal disc transplanted into the abdomen of an adult fly only grows until it reaches its normal size, a transplanted l(2)gl mutant disc undergoes neoplastic growth and eventually kills the fly. However, beforethey die, these flies develop the bloating syndrome, whereby the abdomen becomes swollen and translucent and the fat body and ovaries are almost completely degenerated (Gateff and Schneiderman, 1974). This degeneration of the fat body and ovaries is reminiscent of the wasting of adipose tissue and skeletal muscles in mammals, because the fat body and ovaries are the organs preserving energy in the forms of lipids and proteins in Drosophila. Strikingly, although the bloating syndrome is a robust phenotype, it has not been characterized in detail. In particular, it is not known whether the wasting process affects metabolism and how neoplastic l(2)gl mutant discs induce degeneration of ovaries and the fat body.
The transcriptional coactivator yorkie (yki) regulates growth, repair, and regeneration by inducing a transcriptional program required for cell proliferation and survival (Halder and Johnson, 2011; Harvey and Hariharan, 2012; Pan, 2010; Staley and Irvine, 2012; Yang and Xu, 2011). In particular, studies in adult Drosophila have identified a crucial role of yki in the regulation of intestinal stem cell ISC) proliferation during tissue homeostasis and damage (Karpowicz et al., 2010; Ren et al., 2010; Shaw et al., 2010). Furthermore, these studies have shown that activation of Yki in the midgut induces massive cell proliferation, which conceivably affects the physiology of the proliferating tissue as well as the homeostasis of distant tissues and the whole organism. However, it is not known whether and how localized cell proliferation in the midgut driven by activation of Yki perturbs the physiology and function of distant organs and the whole organism.
Here we show that induction of aberrant cell proliferation in the midgut by activation of Yki causes the bloating syndrome, which is associated with degeneration of the ovary, fat body, and muscle. We characterize in detail the systemic wasting phenotypes associated with the proliferating midgut using genomic, metabolomic, and physiological analyses. Finally, we show that the secreted insulin/IGF antagonist ImpL2 is involved in the wasting process by decreasing systemic insulin/IGF signaling.
RESULTS
Localized Aberrant Cell Proliferation Induced by Activation of Yki in ISCs Causes Systemic Organ Wasting
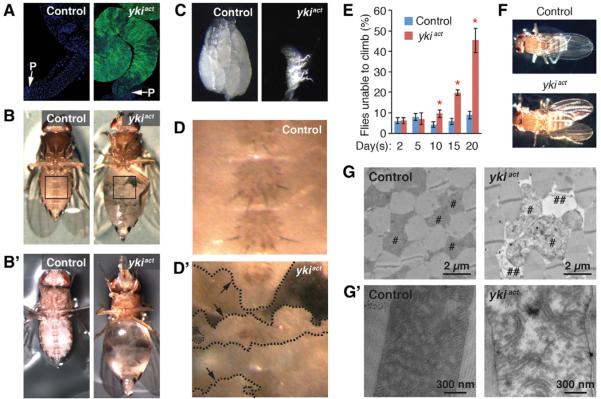
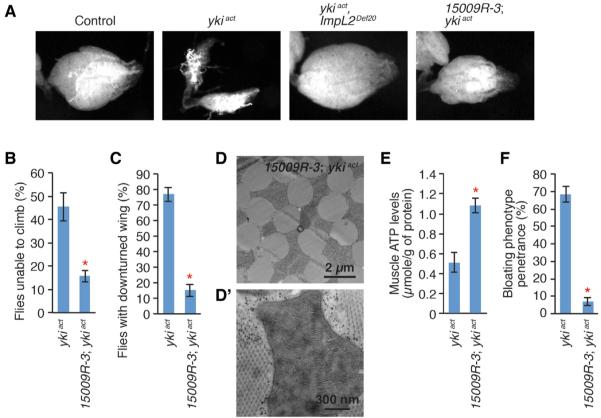
To address how localized aberrant cell proliferation alters organismal homeostasis, we expressed an active form of yki (ykiact) (Oh and Irvine, 2009) in adult midgut ISCs using the conditional GAL4 driver esgts (esg-GAL4, tub-GAL80ts, UAS-GFP/+) (hereafter referred to as esgts>ykiact). Consistent with the proposed role of yki in the midgut (Karpowicz et al., 2010; Ren et al., 2010; Shaw et al., 2010), expression of ykiact resulted in massive cell proliferation as detected by an increase in GFP signal and aberrant shape of the gut (Figure 1A). Strikingly, over time, these flies developed the bloating syndrome phenotype (Figures 1B and B′), originally described in adult flies with transplanted imaginal discs harboring a mutation in the tumor suppressor l(2)gl in the abdomen (Gateff and Schneiderman, 1974). esgts>ykiact flies exhibit this bloating phenotype as ear1y as 5 days after induction of ykiact. Both the penetrance and severity of the phenotype progressively increase, with ~70% of flies showing the phenotype at 6 days (Figure 1B) and ~95% at 12 days (Figure 1B′). Additionally, ovaries and fat bodies of esgts>ykiact females degenerate progressively with time (Figures 1C and 1D). In adult flies, the fat body does not form as a discrete structure but fills the abdominal space, resulting in a continuous light yellow mass. Whereas fat bodies in controls were not affected (Figures 1B and 1D), the fat bodies in esgts>ykiact flies were observed as discontinuous patches at 6 days (Figures 1B and 1D′) and were almost completely degenerated at 12 days of ykiact induction (Figure 1B′; magnified images are not shown). Furthermore, we observed accelerated decline of muscle function in esgts>ykiact flies. esgts>ykiact flies showed progressive climbing defects (Figure 1E) and downturned wings (Figure 1F), general indicators of muscle weakening/degeneration (Demontis and Perrimon, 2010; Greene et al., 2003). To characterize the underlying cellular defects associated with muscle weakening/degeneration in esgts>ykiact, we examined the structures of esgts>ykiact muscles by electron microscopy. Mitochondria of esgts>ykiact muscles were swollen and often associated with empty space (Figure 1G). Moreover, cristae were fragmented, and inner mitochondrial space was filled with low-electron-dense sectors (Figure 1G′), indicative of mitochondrial degeneration (Greene et al., 2003). Altogether, these findings reveal that aberrant cell proliferation induced by activation of Yki in ISCs causes systemic organ-wasting phenotypes affecting ovaries, fat body, and muscle.
Figure 1. Degeneration of Ovary, Fat Body, and Muscle in esgts>ykiact Flies.
(A) Midgut images. GFP is driven by esgts (esg-GAL4, tub-GALBOts, UAS-GFP/+). The arrows indicate the posterior (P) end of the midgut.
(B and B′) Fly images. Transgenes were induced for 6 days (B) and 12 days (B′) with esgts
(C) Ovary images. Transgenes were induced for 8 days.
(D and D′) Magnified views of the insets in (B). The dashed lines and arrows indicate the boundary of the fat body.
(E) Quantification of climbing delects (mean ± SEMs). *p ≤ 0.05, Student's t test.
(F) Downturned wing phenotype in esgts>ykisct flies.
(G) Electron microscopic images of the transverse section of indirect flight muscles. #, mitochondria; ##, empty spaces.
(G′) Images of mitochondrion. In all muscle experiments, transgenes were induced for 20 days in male flies unless otherwise indicated. The genotype of control is esg-GAL4, tub-GAL80ts, UAS-GFP/+ and ykiact is esg-GAL4, tub-GAL80ts, UAS-GFP/+; UAS-ykiact/+.
Expression of ykiact in Midgut Causes Repression of Genes Involved in Energy Metabolism in Muscle
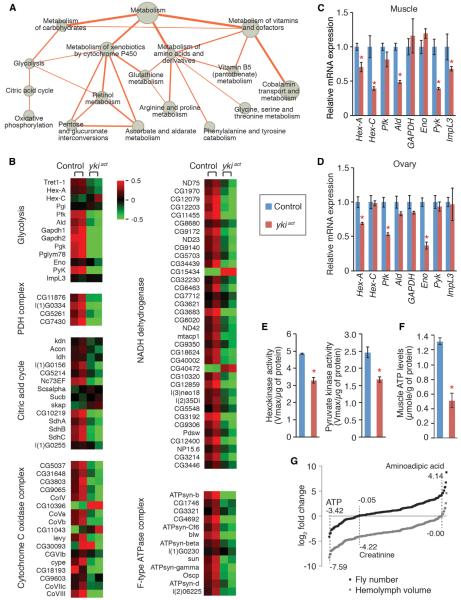
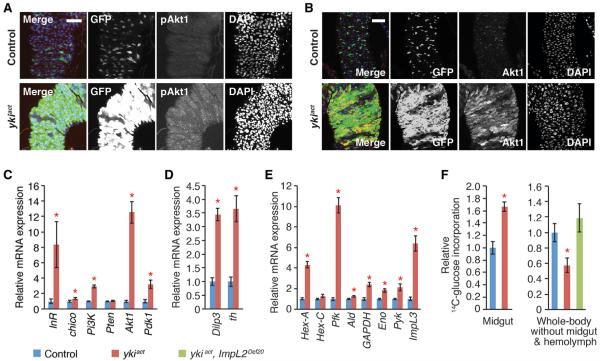
To gain insight into the systemic phenotypes induced by esgts>ykiact, we performed a transcriptomic analysis of thoracic muscles (Table S1), which play an important role in the regulation of organismal physiology and aging by affecting systemic insulin/IGF signaling and trehalose/glucose metabolism (Demontis and Penimon, 2010). Interestingly, gene list enrichment analysis of the downregulated muscle transcriptome revealed a striking enrichment of multiple metabolic processes impinging on carbohydrate metabolism (p = 0.0018), amino acid metabolism (p = 1.32 × 10 7), metabolism of vitamins and cofactors (p = 1.32 × 10−7), and metabolism of xenobiotics by cytochrome P450 (p = 1.45 × 10−4) (Figure 2A; Tables S2 and S3). Furthermore, we found a systematic repression of genes involved in energy metabolism (p = 0.000, gene set enrichment analysis; GSEA), including glycolysis (p = 0.018, GSEA), pyruvate metabolism and the citric acid cycle (p = 0.0252, GSEA), and oxidative phosphorylation (p = 0.000, GSEA)(Figures 2A and 2B; Figure S1; Table S4). We further confirmed by quantitative (q)PCR that the expression of multiple genes involved in glycolysis was decreased not only in muscles but also in ovaries of esgts>ykiact flies (Figures 2C and 2D). Moreover, the activities ofthetwo rate-limiting glycolytic enzymes Hexokinase (Hex-A and Hex-C) and Phosphofructokinase (Pfk) are reduced by approximately 30% in esgts>ykiact muscle (Figure 2E). Accordingly, ATP levels in muscles were significantly decreased in esgts>ykiact flies as compared to controls (Figure 2F). Moreover, metabolomic analyses revealed a decrease of ATP, NADH, and NADPH levels in the hemolymph of esgts>ykiact flies (Figure 2G; Figure S2; Table S5), which are the main products of energy metabolism. Altogether, these results suggest that esgts>ykiact alters the metabolic gene expression program of distant tissues, which is manifested by downregulation of genes involved in glycolysis in muscle and ovaries.
Figure 2. Repression of Energy Metabolism in esgts>ykiact Muscles.
(A) Network presentation of gene list enrichment analysis results focusing on metabolism. All presented metabolic processes are identified to be significantly downregulated inmuscles of esgts>ykiact flies (Tables S2 and S3).Node size indicates enrichment (−log100 p value), and edge thickness represents the number of common genes between two gene sets. The citric acid cycle and pyruvate metabolism (p = 0.0253, GSEA) and oxidative phosphorylation (p = 0.000, GSEA) are identified by GSEA analysis (Subramanian et al., 2005).
(B) Representative downregulated processes and complexes involved in energy metabolism. Heat map signal indicates log2 fold-change values relative to the mean expression level within the group. Red signal denotes higher expression and green signal denotes lower expression relative to the mean expression level within the group. Related GSEA plots are shown in Figure S1.
(C and D) Relative mRNA expression of glycolytic enzyme in muscle (C) and ovaries (D). Measurements shown are mean ± SDs.
(E) Activities of Hexokinase) (left) and Pyruvate kinase (right) in muscle at 8 days of induction (mean ± SEMs).
(F) ATP levels in muscle at 20 days of transgene induction.
(G) Metabolomic analysis of hemolymph metabolites. The metabolite counts are normalized to fly number (black) or extracted hemolymph volume (gray). Log2 fold-change values of the metabolites in hemolymph of esgts>ykiact flies relative to control are presented.
Reduced 55 metabolites in hemolymph of esgts>ykiact flies are shown in Figure S2. *p ≥ 0.05 (Student's t test) compared to control. Genotypes are asshown in Figure 1.
Overproliferating Midgut due to Activation of Yki Causes Hyperglycemia
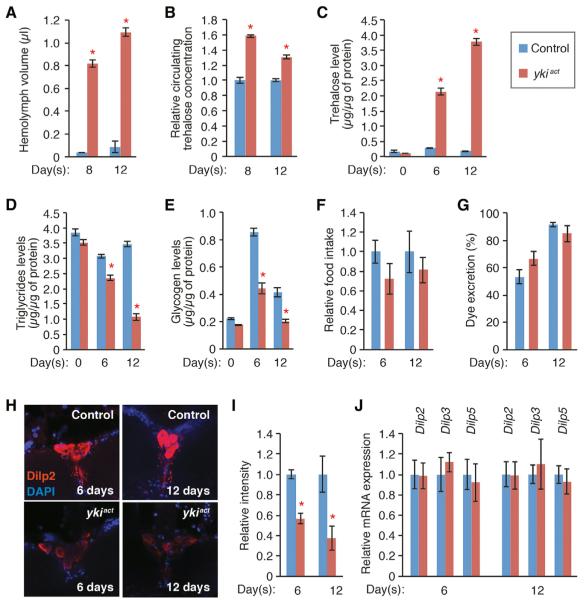
Because we observed that gene expression of glycolytic enzymes in muscles and ovaries was downregulated in esgts>ykiact flies, we investigated whether there were any changes in glucose metabolism in these flies. As expected from the bloating phenotype, the volume of extractable hemolymph from esgts>ykiact flies was greatly increased as compared to controls (Figure 3A). Nevertheless, the concentration of trehalose, the primary circulating sugar composed of two alpha-glucoses, was significantly increased in the hemolymph of esgts>ykiact flies (Figure 3B). Accordingly, whole-body trehalose levels were increased in esgts>ykiact flies (Figure 3C). Additionally, consistent with the degeneration of the fat body that stores triglycerides and glycogen in the adult, whole-body triglycerides and glycogen levels were reduced in esgts>ykact flies, as compared to controls (Figures 3D and 3E).
Figure 3. esgts>ykiact Causes Hyperglycemia and Depletion of Triglycerides and Glycogen.
(A) Volumes of extractable hemolymph per fly after 8 and 12 days of induction with esgts.
(B) Relative concentration of circulating trehalose normalized to control at 8 and 12 days of induction.
(C) Trehalose levels in whole flies normalized to extracted protein amounts at different induction times.
(D) Triglyceride levels in whole flies. Triglyceride levels were normalized to protein amounts.
(E) Glycogen levels inwhole flies. Glycogen levels were normalized to protein amounts.
(F) Food intake measured by CAFE assay (Demontis and Perrimon, 2010; Ja et al., 2007) after 6 and 12 days of transgene induction with esgts. Values relative to controls are presented.
(G) Food excretion rate. Transgenes are induced for 6 and 12 days. The percentages of excreted dye amount to total intake are shown.
(H) Dilp2 staining in the IPCs in the brain. Images are captured with the same confocal setting.
(I) Quantification of Dilp2 signal intensity in IPCs. Dilp2 signals are normalized to background signals, and values relative to controls are presented.
(J) mRNA levels inthe heads measured by qPCR. The results shown in (A)–(E) are mean ± SEMs. The values in (F), (G), (I), and (J) are mean ± SDs. *p ≤ 0.05 (Student's t test) compared to control. Genotypes are as shown in Figure 1.
Starvation causes a reduction in whole-body trehalose levels (Figure S3B), suggesting that the increase of trehalose levels in esgts>ykiact flies is not due to the effect of starvation (Figures 3B and 3C). Nevertheless, starvation affects storage of triglycerides and glycogen (Figures S3C and S3D). Thus, because the presence of cell overproliferation in the midgut could in principle perturb gut functions and mimic starvation, we addressed whether esgts>ykiact altered food intake and absorption. Measurements of food intake and excretion did not appear to be significantly affected in esgts>ykiact flies (Figures 3F and 3G). To further test whether esgts>ykiact flies are starved, we examined Drosophila insulin-like peptide 2 (Dilp2) levels in Dilp-producing cells QPCs) in the brain, because starvation causes accumulation of Dilp2, presumably due to a reduction in secretion (Demontis and Perrimon, 2010; Géminard et al., 2009; Ikeya et al., 2002). Surprisingly, the Dilp2 signal in the IPCs of esgts>ykiact flies was significantly decreased (Figures 3H and 3I). This decrease of Dilp2 signal is not due to a reduction in Dilp2 mRNA, as we observed that the levels of Dilp mRNAs in the heads remained unaffected in esgts>ykiact flies (Figure 3J). Moreover, we tested a starvation marker, Pepck, which is regulated by both sugar and glycine (Zinke et al., 1999), and found that Pepck mRNA expression was increased so-fold during starvation (Figure S3F). Conversely, Pepck mRNA expression remained unaltered at 6 days of ykiact induction and increased ~3-fold at 12 days (Figure S3F), suggesting that esgts>ykiact flies were not severely starved. Altogether, these findings indicate that aberrant cell proliferation, induced by activation of yki, causes systemic abnormality in trehalose/glucose metabolism, which resembles hyperglycemia. Further, because Dilp2 is not accumulated in the IPCs and Pepck expression is not greatly affected, it is unlikely that starvation is the main cause of the phenotypes associated with esgts>ykiact. However, we cannot rule out that other aspects of gut function are perturbed, contributing to the organ-wasting and bloating phenotypes (see Discussion).
Depletion of ImpL2 from esgts>ykiact Midguts Rescues Systemic Reduction of Akt1 Phosphorylation and Hyperglycemia
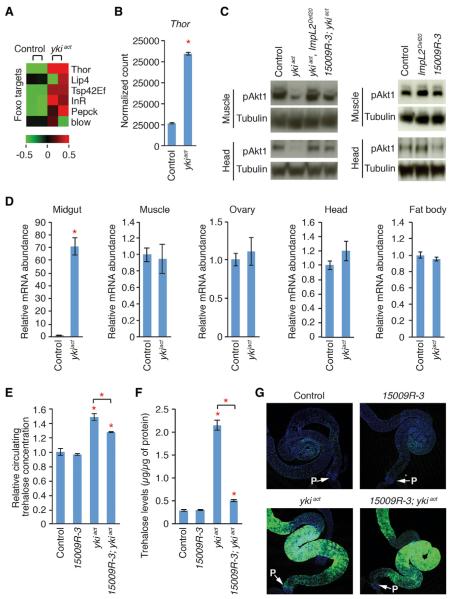
To identify the signaling factor(s) impinging on systemic phenotypes in esgts>ykiact flies, we interrogated the muscle transcriptome of esgts>ykiact. Interestingly, target genes of Foxo, a transcript ionfactor inhibited by insulin/IGF signaling, areenriched in the upregulated muscle transcriptome of esgts>ykiact flies (p = 0.039; Figure 4A). In particular, Thor (human 4E-BP ortholog), a well-characterized target of Foxo, is significantly upregulated (Figure 4B). Consistent with the transcriptome analysis results, Akt phosphorylation is significantly reduced in muscles and heads of esgts>ykiact flies (Figure 4C). To characterize the mechanism by which esgts>ykiact reduces systemic insulin/IGF signaling, we examined whether the expression of ImpL2, a secreted protein that resembles IGFBP7 (Sloth Andersen et al., 2000) and that inhibits insulin/IGF signaling by forming a protein complex with circulating Oilps (Alic et al., 2011; Honegger et al., 2008), was affected in esgts>ykiact flies. Strikingly, ImpL2 mRNA expression was greatly elevated in esgts>ykiact midguts at 6 days of ykiact induction, whereas the expression of ImpL2 remained unchanged in muscles, ovaries, heads, and fat bodies (Figure 4D). Interestingly, starvation regulates ImpL2 expression (Honegger et al., 2008), and we further confirmed that starvation increased ImpL2 mRNA expression in heads, muscles, midguts, and fat bodies (Figure S3E). The absence of ImpL2 mRNA induction in the muscles, ovaries, heads, and fat bodies of esgts>ykiact flies further supports that these flies are not calorie deprived. Moreover, ImpL2 mRNA expression is increased ~70-fold in the esgts>ykiact midgut, which appears to greatly exceed the range of induction during starvation (Figure 4D; Figure S3E).
Figure 4. Depletion of ImpL2 from esgts>ykiact Midgut Rescues Systemic Reduction of Insulin/IGF Signaling and Hyperglycemia.
(A) Heat map showing expression of the Foxo target gene set. Foxo target genes are annotated from DrolD (Murali et al., 2011).
(B) Normalized expression levels of Thor (human 4E-BP ortholog). The values shown are mean ± SEMs. The asterisk denotes statistically significant difference from control (adjusted p = 1.239 × 10 −87; p values are adjusted with the Benjamini-Hochberg procedure, which controls the false discovery rate; Benjamini and Hochberg, 1995).
(C) Akt1 phosphorylation in muscle and head at 8 days of induction.
(D) Relative expression levels of ImpL2 mRNA in the midgut, muscle, ovary, head, and fat body. The active form of yki (ykiact) was expressed in the midgut with esgts for 6 days. The values are mean ± SDs. *p < 0.05, unpaired Student's t test.
(E) Circulating trehalose concentrations at 8 days of induction.
(F) Trehalose levels in the whole-body void at 6 days of induction. The measurements are normalized to total protein amounts.
The resultsshown in (E) and (F) are mean ± SEMs. *p ≥ 0.05 (Student's t test) compared to control other than when indicated by a bracket.
(G) Midgut morphology. GFP is driven by esgts and UAS-GFP. Nuclei are marked with 4′,6-diamidino-2-phenylindole (DAPI) (blue). The arrows indicate the posterior end of the midgut. The genotype of control is esg-GAL4, tub-GAL80ts, UAS-GFP/+, ykiact is esg-GAL4, tub-GAL80ts, UAS-GFP/+; UAS-ykiact/+, ykiact, ImpL2Def20 is esg-GAL4, tub-GAL80ts, UAS-GFP/+; UAS-ykiact, ImpL2Def20/ImpL2Def20, 15009R-3 is esg-GAL4, tub-GAL80ts, UAS-GFP/15009R-3 (UAS-ImpL2 RNAi from the National Institute of Genetics, Japan), 15009R-3; ykiact is esg-GAL4, tub-GAL80ts, UAS-GFP/15009R-3; UAS-ykiact/+, and ImpL2Def20 is esg-GAL4, tub-GAL80ts, UAS-GFP/+;ImpL2Def20/ImpL2Def20.
To address the role of ImpL2, we examined whether removal of ImpL2 activity could rescue the systemic phenotypes associated with esgts>ykiact The null allele ImpL2Def20 (Honegger et al., 2008) completely rescued the reduction of Akt1 phosphorylation in esgts>ykiact flies (Figure 4C) and restored circulating trehalose concentration and whole-body trehalose levels (Figures S4A and S4B). The rescue of the systemic phenotypes associated with esgts>ykiact by ImpL2Def20 is presumably due to a systemic increase of insulin/IGF signaling, as ImpL2Def20 alone caused a slight increase of Akt1 phosphorylation (Figure 4C) and reduction of circulating trehalose concentration (Figure S4A). Next, to examine the importance of ImpL2 induction in the midgut, we coexpressed an RNAi against ImpL2 (15009R-3) together with ykiact. The expression of ImpL2-RNAi efficiently suppressed the induction of ImpL2 in esgts>ykiact midgut (~80% knockdown efficiency), although the expression of ImpL2 remained 8-fold higher than in controls (Figure S4C). Notably, expression of ImpL2-RNAi alone using esgts did not significantly alter Akt1 phosphorylation and trehalose levels (Figures 4C, 4E, and 4F). Importantly, knockdown of ImpL2 with esgts restored Akt1 phosphorylation in muscles and heads significantly (Figure 4C) and reduced the trehalose levels (Figures 4E and 4F). This rescue is not due to suppression of cell proliferation, because the overall shape and GFP intensity of esgts>ykiact midguts remained unaltered after ImpL2-RNAi expression (Figure 4G). Additionally, similar experiments employing an additional ImpL2-RNAi line (30931) further confirmed the importance of ImpL2 induction in the midgut for the systemic phenotypes associated with esgts>ykiact (Figures S4O–S4F). In addition, ectopic expression of ImpL2 in enterocytes (ECs) in the midgut caused hyperglycemia, reduction of Akt1 phosphorylation in muscle, increase of hemolymph volume, and ovary atrophy (Figures S4G–S4K), suggesting that the increased ImpL2 expression was sufficient to cause some of the systemic phenotypes associated with esgts>ykiact. Altogether, our results indicate that induction of ImpL2 in esgts>ykiact midguts is a critical determinant for both the hyperglycemia and systemic reduction of insulin/IGF signaling in esgts>ykiact flies.
Depletion of ImpL2 in esgts>ykiact Midguts Rescues Ovary Wasting and Muscle Degeneration
Next, we addressed whether ImpL2 is involved in the organ-wasting process associated with esgts>ykiact. Interestingly, either ImpL2def20 or depletion of ImpL2 in the midgut with esgts suppressed wasting of ovaries caused by esgts>ykiact (Figure SA). In addition, expression of ImpL2 in the midgut is required for the muscle degeneration observed in esgts>ykiact flies. Depletion of ImpL2 in the midgut with esgts rescued the climbing defects and downturned wings in esgts>ykiact flies (Figures 1E, 1F, 5B, and 5C). Strikingly, expression of ImpL2-RNAi with esgts was sufficient to significantly rescue the mitochondrial defects (Figures 1G, 1G', SD, and SD') and reduced ATP levels (Figures 2F and 5E). Finally, we found that the bloating phenotype in esgts>ykiact flies was also dependent on ImpL2, because knockdown of ImpL2 in the midgut with esgts significantly rescued the bloating phenotype (Figure 5F). Altogether, these results indicate that degeneration of ovaries and muscle in esgts>ykiact flies is dependent on increased expression of ImpL2 in the midgut.
Figure 5. Depletion of ImpL2 with esgts Rescues Degeneration of Ovaries and Muscle Associated with esgts>ykiact.
(A) Images of ovaries from the indicated geno types. Transgenes are induced for 8 days with esgts
(B) Quantification of climbing defect.
(C) Penetrance of downturned wing phenotype.
(D) Electron microscopic image of transverse section of indirect flight muscles.
(D Image of mitochondrion.
(E) Muscle ATP levels normalized to protein levels. Inall muscle experiments, transgenes are induced for 20 days in male flies.
(F) Penetrance of the bloating syndrome at 8 days of transgene induction. Either control (esgts) or expression of 15009R-3 alone with esgts is not associated with the bloating syndrome phenotype.
All quantifications shown are mean ± SEMs. *p ≤ 0.05, Student's t test compared to ykiact. Genotypes are as shown in Figure 4.
Expression of Insulin/IGF Pathway Components and Glycolytic Enzymes Is Upregulated inthe Proliferating Midgut due to Aberrant Activation of Yki
Although insulin/IGF signaling is indispensable for ykiact-mediated cell proliferation in the midgut (data not shown), esgts>ykiact midguts undergo cell proliferation irrespective of the induction of ImpL2. Strikingly, instead of observing a reduction of Akt1 phosphorylation, expression of ykiact increased the levels of both pAkt1 and Akt1 in the midgut (Figures 6A and 6B), similar to a previous observation in the wing disc (Ye et al., 2012). Moreover, overall Akt1 phosphorylation in the midgut was greatly increased as compared to control (Figure S5). Interestingly, we found that gene expression of insulin/IGF pathway components was systematically increased in esgts>ykiact midgut (InR, ~8-fold; Akt1, ~12-fold; Figure 6C). Conversely, the mRNA levels of these genes were not affected significantly in muscles and ovaries, with the exception of InR in muscles (Figure S6). Finally, expression of ykiact in the midgut elevated the transcript of Dilp3, which regulates insulin/IGF signaling in that tissue (O'Brien et al., 2011) (Figure 6D). Thus, our findings suggest that expression of ykiact in the midgut causes a disparity in the activity of insulin/IGF signaling between the midgut and other tissues. Interestingly, extracellular signal-regulated kinase activation was shown to enhance insulin/IGF signaling by increasing the expression of InR transcript (Zhang et al., 2011). Additionally, rasV12; csk −/− transformed cells increased InR expression through Wingless signaling to evade the insulin resistance induced by a high-sugar diet (Hirabayashi et al., 2013). Although Foxo is a well-characterized transcription factor regulating InR expression (Puig and Tjian, 2005), these observations and ours suggest that some mitogenic signals can enhance insulin/IGF signaling by increasing InR expression.
Figure 6. Upregulation of Insulin/IGF Signaling in esgts>ykiact Midguts.
(A and B) Both phosphorylation of Akt1 (pAkt1; red in merge; A) and expression of Akt1 (red in merge; B) are increased in esgts>ykiact midguts. Nuclear staining is shown with DAPI (blue in merge). The scale bars represent 5O µm. We characterized anti-Aki and anti-pAkt antibodies in the midgut (Figure S7).
(C) Relative mRNA expression of insulin/IGF pathway components in the midgut measured by qPCR at 6 days of induction.
(D) Relative expression of dilp3 mRNA in esgts>ykits midgut at 6 days of induction. thread (th) is a known transcriptional target of yki.
(E) Relative mRNA abundance of glycolytic enzymes in the midgut of esgts>ykiact flies at 8 days of induction.
(F) [U-14C]glucose incorporation in the midgut (left) and whole-body void of midgut and hemolymph (right).
All qPCR values are mean ± SDs, and other measurements are mean ± SEMs. Transgenes are induced with esgts by shifting to the nonpermissive temperature (29°C). *p ≤ 0.05, Student's t test compared to control. Genotypes are as shown in Figure 4.
This disparity of insulin/IGF signaling activities presumably leads to differential regulation of glucose metabolism between the midgut and other tissues. In contrast to the observations in muscles and ovaries (Figures 2C and 2D), the mRNA expression of glycolytic enzymes was systematically increased in esgts>ykiact midguts (Figure 6E). In particular, the mRNA levels of two key rate-limiting enzymes, Hex-A and Pfk, were increased by ~4- and ~10-fold, respectively, and the ortholog of lactate dehydrogenase, ImpL3, which is a major contributor to the Warburg effect (Vander Heiden et al., 2009; Warburg, 1956), was increased ~7-fold. Consistent with overproliferation in the midgut, we observed increased glucose incorporation in esgts>ykiact midgut as compared to controls (Figure 6F). Strikingly, in other parts of these flies, glucose incorporation was reduced by ~50% (Figure 6F), a process dependent on wild-type ImpL2 allele, because ImpL2def20 rescued the reduction of glucose incorporation in the whole-body void of midgut and hemolymph induced by esgts>ykiact (Figure 6F). Altogether, these observations suggest that activation of yki in the midgut causes a bias in glucose metabolism between the midgut and other tissues, and that ImpL2 is a genetic determinant of this phenomenon.
DISCUSSION
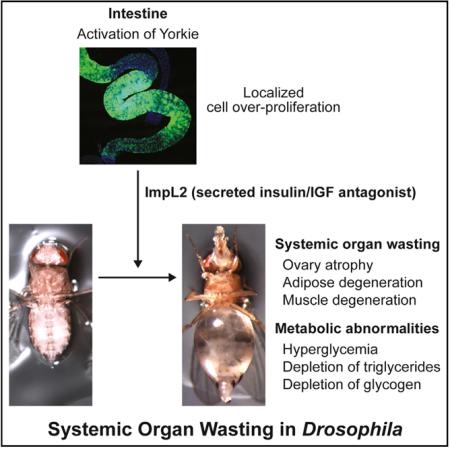
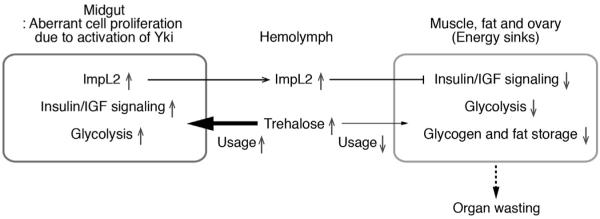
In this study, we describe the unexpected observation that the overproliferating midgut due to aberrant Yki activity in ISCs induces the bloating syndrome and systemic organ wasting. Additionally, the overproliferating midgut perturbs organismal metabolism, resulting in an increase of hemolymph trehalose and depletion of glycogen and triglyceride storage (Figure 7). Strikingly, we show that the accumulation of hemolymph trehalose and organ-wasting processes are dependent on the antagonist of insulin/IGF signaling, ImpL2, which is specifically upregulated in the proliferating midgut. Our study provides strong genetic evidence supporting that systemic organ wasting associated with the aberrant activation of Yki in ISCs cannot be explained solely by the perturbation of general gut function. Based on these findings, we propose that ImpL2 is a critical factor involved in systemic organ wasting in Drosophila.
Figure 7.
Model of the Tissue Autonomous and Systemic Changes Caused by Aberrant Activation of Yki in ISCs
In an accompanying paper in this issue of Developmental Cell, Figueroa-Clarevega and Bilder show that transplantation of scrib1/RasV12 disc tumors into wild-type flies induces the bloating syndrome phenotype and systemic organ wasting, affecting ovaries, fat bodies, and muscles (Figueroa-Clarevega and Bilder, 2015). Figueroa-Clarevega and Bilder also identify ImpL2 as a tumor-driven factor that plays a critical role in the organ-wasting process. These results are consistent with our findings and indicate that the bloating syndrome and organ-wasting phenotypes are not associated specifically with perturbation of gut function. Interestingly, Figueroa-Clarevega and Bilder observe that disc tumors derived by the expression of ykiS/A (an active form of yki that is less potent than ykiact used in this study) did not cause organ wasting, which can be explained by the low level of ImpL2 induction in the ykiS/A tumors as compared to scrib1/RasV12 tumors.
Our results do not rule out the existence of an additional factor(s) contributing to the bloating syndrome and organ-wasting phenotypes. Indeed, the partial rescue of the bloating syndrome and organ-wasting phenotypes by depletion of ImpL2 in esgts>ykiact midguts suggests the existence of an additional factor(s). Moreover, we observed that ectopic expression of ImpL2 in ECs was not sufficient to reduce whole-body triglyceride and glycogen levels (data not shown), although it caused hyperglycemia, reduction of Akt1 phosphorylation, and increase of hemolymph volume (Figures S4G-S4J). Thus, given the involvement of diverse factors in the wasting process in mammals, it is likely that in addition to ImpL2, another factor(s) contributes to systemic organ wasting in Drosophila.
Our study shows that the bloating syndrome caused by esgts>ykiact is associated with ImpL2, as depletion of ImpL2 from esgts>ykiact midguts significantly rescues the bloating phenotype. Given the observation that elevated expression of ImpL2 from esgts>ykiact midgut induces hyperglycemia, we speculate that the accumulation of trehalose in hemolymph is a factor involved in bloating, because a high concentration of trehalose can cause water influx to adjust hemolymph osmolarity to physiological levels. Interestingly, recent findings have shown that disruption of l(2)gl in discs activates yki (Grzeschik et al., 2010; Halder and Johnson, 2011; Menéndez et al., 2010; Staley and Irvine, 2012), suggesting that the bloating syndrome observed in flies with transplanted l(2)gl mutant discs may be due to aberrant yki activity.
Our findings are reminiscent of a previous study showing that in Drosophila, humoral infection with the bacterial pathogen Mycobacterium marinum, which isclosely related to Mycobacterium tuberculosis, causes a progressive loss of energy stores in the form of fat and glycogen–a wasting-like phenotype (Dionne et al., 2006). Similar to our observation, Dionne et al. found that infection with M. marinum caused a downregulation of Akt1 phosphorylation. Given our observation that ImpL2 produced from esgts>ykiact affects systemic insulin/IGF signaling, it will beof interest to test whether ImpL2 expression is increased upon infection with M. marinum and mediates the effect on the loss of fat and glycogen storage.
yki plays critical roles intissue growth, repair, and regeneration by inducing cell proliferation (Johnson and Halder, 2014; Pan, 2010; Staley and Irvine, 2012), a process requiring additional nutrientsto support rapid synthesis of macromolecules ineluding lipids, proteins, and nucleotides. In particular, increased aerobic glycolysis metabolizing glucose into lactate is a characteristic feature of many cancerous and normal proliferating cells (Vander Heiden et al., 2011). Interestingly, the aberrant activation of yki in ISCs caused a disparity in the gene expression of glycolytic enzymes and the activity of insulin/IGF signaling between the proliferating midgut and other tissues, such as muscle and ovaries (Figure 7). Thus, we speculate that this disparity favors Yki-induced cell proliferation by increasing the availability of trehalose/glucose to the proliferating midgut, which presumably requires high levels of trehalose/glucose (Figure 7). Additionally, it will be of interest to test whether activation of Yki during tissue growth, repair, and regeneration alters systemic metabolism in a similar manner.
EXPERIMENTAL PROCEDURES
Fly Stocks and Manipulation of Midgut Progenitor Cells
UAS-ykiact (w*;; UAS-yki.S111A.S168A.S250A.V5; 228817) (Oh and Irvine, 2009) was obtained from the Bloomington Drosophila Stock Center. esgts refers to tub-GAL8Ots, esg-GAL4, UAS-GFP (II) (Apidianakis et al., 2009). RNAi line 15009R-3 against ImpL2 was obtained from the National Institute ol Genetics, Japan. 30931 is an RNAi line against ImpL2 obtained from the Vienna Drosophila Resource Center, Austria. RNAi lines obtained from the Transgenic RNAi Project (http://www.flyrnai.org) are JF01482 (InR), JF01987 (Pten), and HM04007 (Akt1). Additionally, we used UAS-Pten (III), UAS-myr-Akt1 (III), and ImpL2Def20 (gift from Hugo Stocker).
To induce transgenes in midgut stem cells, we followed the experimental procedures described previously (Apidianakis et al., 2009). Briefly, crosses were set up with esgts at room temperature, and after 3 days of incubation at room temperature were transferred to 18°c to activate GAL80ts thus restricting the expression of the Gal4 induced transgenes. Zero- to 4-day-old adult progenies were collected and placed at 29°c to induce the transgenes. Progenies from a cross between esgts and w1118 were used as controls. During incubation at 29°c, flies were transferred onto fresh food xevery 2 days.
Measurement of Carbohydrate and Triglyceride Levels
We measured fly carbohydrates and triglycerides as described previously (Song et al., 2010, 2014; Teleman et al., 2005). To prepare fly lysates for metabolic assays, we homogenized six female adults from each group in 400 µl PBS supplemented with 0.2% Triton X-100, heated the homogenate at 70°c for 5 min, and collected the supernatant after centrifugation at 14,000 rpm for 10 min. Whole-body trehalose levels were measured from 10 µl of supernatant treated with 0.2 µl trehalase (Megazyme; E-TREH) at 37°C for 30 min using glucose assay reagent (Megazyme; K-GLUC) following the manufacturer's protocol. We subtracted the amount of free glucose from the measurement and then normalized the subtracted values to protein levels inthe supernatant. Whole-body glycogen levels were determined from 10 µl of supernatant preincubated with 1 µl amylogl ucosidase (Sigma-Aldrich; A7420) at 37°c for 30 min using glucose assay reagent (Megazyme; K-GLUC). Free glucose levels were subtracted from the measurements, and glycogen levels were normalized to total protein levels. To measure whole-body triglycerides, we processed 10 µl of supernatant using a Serum Triglyceride Determination kit (Sigma-Aldrich; TR0100). We subtracted the amount of free glycerol in the supernatant from the measurement and then normalized the subtracted values to protein levels in the supernatant.
To measure circulating trehalose concentrations, hemolymph was extracted from 10–20 decapitated female adults by centrifugation at 1,500 × g for 15 min. Half a microliter of the collected hemolymph was diluted in 40 µl of TBS buffer (5 mM Tris-HCI [pH 6.6], 137 mM NaCl, 2.7 mM KCI), heated at 70°c for 5 min, and centrifuged at 14,000 rpm for 10 min. The supernatant was treated with 02 µl trehalase (Megazyme; E-TREH) at 37°C for 30 min and then used to measure circulating trehalose levels with glucose assay reagent (Megazyme; K-GLUC). We subtracted the amount of free glucose in the supernatant from the measurement.
Hemolymph volumes were measured using either a micropipette P2.5 (Eppendorf) or P10 (Gilson) and normalized to the number of flies used for hemolymph extraction.
Glucose Incorporation Assay
Fifteen to 25 flies incubated at 29°c for 3 days were transferred onto fresh food with 2 µCi [U-14C]glucose (PerkinElmer; NEC042V). After 2 days of incubation at 29°c, the flies were transferred again onto fresh food with 2 µCi [U-14C] glucose and incubated for an additional 2 days. To remove the food in the gut, we placed the flies onto nonradioactive food for 7–8 hr prior to dissection. Then, seven midguts were dissected in PBS and collected in 250 µl RIPA buffer (SO mM Tris-HCI [pH 7.4], 150 mM NaCl, 1% sodium deoxycholate, 1 mM EDTA, 0.1% SDS, 1% NP-40) after rinsing them twice with PBS. To collect the whole-body void of midgut and hemolymph, we dissected out the midguts through a small incision in the abdomen. Dissected flies were placed in an Eppendorf tube with 1 ml PBS and then washed four times with 1 ml PBS by inverting three to five times. Three dissected flies were homogenized in 250 µl RIPA buffer. After homog enization, we added 300 µl water to the homogenates to increase the volume. Five hundred microliters of homogenate was mixed with 10 ml Ultima Gold liquid scintillation cocktail (PerkinElmer; 6013326) in 20-ml glass scintillation vials. Disintegrations per minute values were measured and normalized to control.
RNA Sequencing Analysis of Muscle Transcriptome
To extract total RNAs for RNA sequencing (RNA-seq) experiments, we used ten thoraces dissected out from flies incubated for 8 days at 29°c. After assessing RNA quality with an Agilent Bioanalyzer, mRNAs were enriched by poly(A) pull-down. Then, sequencing libraries constructed with an Illumina TruSeq RNA preparation kit were sequenced using an lllumina HiSeq 2000 at the Columbia Genome Center (http://systemsbiology.columbia.edu/genome-center). We multiplexed samples in each lane, which yields a targeted number of single-end 100-bp reads for each sample, as a fraction of 180 million reads for the whole lane. Sequence reads were mapped back to the Drosophila genome (FlyBase genome annotation version r5.51) using TopHat (Trapnell et al., 2009). With the uniquely mapped reads, we quantified gene expression levels using Cufflinks (Trapnell et al., 2012) ragments per kb of exon per miIlion fragments mapped values). Next, we performed data normalization on the read counts and applied a negative binomial statistical framework using the Bioconductor package DESeq to quantify differential expression between experimental and control data. The RNA-seq data were deposited in the Gene Expression Omnibus (accession number GSE65325).
Metabolomics of Hemolymph Metabolites
To collect hemolymph, thoraces of approximately 200 flies were pierced with a tungsten needle. Next, the flies were placed in a perforated O.5-ml Eppendorf tube within a 1.5-ml Eppendorf tube and then centrifuged twice at 2,348 × g for 4 min at 4°c with a gentle mixing of the flies between centrifugations. Collected hemolymph was centrifuged again at 2,348 × g for 3 min to precipitate hemocytes and other debris. The supernatant was centrifuged at 14,000 × g for 15 min to remove the insoluble fraction. Processed hemolymph was flash-frozen on dry ice and kept at −80°C until metabolomic sample preparation.
Metabolomic samples were prepared essentially as dascribed previously (Yuan et al., 2012). Briefly, a hemolymph sample was diluted in −80°c methanol for a Iinal methanol concentration of 80%. Then, the sample was briefly vortexed and stored at −80°C overnight. The sample was then centrifuged at 14,000 × g for 10 min. The supernatant was dried in a SpeedVac and then frozen at −80°c. For liquid chromatography tandem mass spectrometry (LC-MS/MS), the resuspended sample in 20 µl of LC-MS-grade water was centrifuged and 10 µl was injected. Mass spectrometry was performed as described previously (Yuan et al., 2012) at the Beth Israel Deaconess Medical Center Mass Spectrometry Facility (http://www.bidmcmassspec.org). Briefly, selected reaction monitoring of 287 Q1/Q3transitionswas targeted inpositive/negative switching mode using a SSOO QTRAP hybrid triple quadrupole mass spectrometer (AB SCIEX). Amide HIUC chromatography (Waters) was used at high pH over a 20-min gradient. Integrated peak area values of each metab olite were normalized to hemolymph volume or fly number.
Supplementary Material
Highlights.
Localized Yki-induced overproliferation causes systemic organ wasting in Drosophila
The secreted insulin/IGF antagonist ImpL2 is a mediator of systemic organ wasting
ImpL2, secreted from overproliferating tissue, reduces systemic insulin/IGF signaling
Overproliferating tissue evades wasting via local elevation of insulin/IGF signaling
ACKNOWLEDGMENTS
We thank the Transgenic RNAi Project, National Institute of Genetics (Japan), and Bloomington Drosophila Stock Center for fly stocks, Dr. Hugo Stocker for ImpL2 stocks, and Drs. Stephanie Mohr, Richelle Sopko, Jonathan Zirin, Richard Binari, and Akhila Rajan for comments on the manuscript. We thank Alejandra Figueroa-Clarevega and David Bilder for exchange of information on the role of Impl2 before publication. We also thank Min Yuan for help with the mass spectrometry experiments. Y.K. was supported in part by the Damon Runyon Cancer Research Foundation. This work was supported in part by P01 CA120964 (J.M.A. and N.P.) and R01 DK088718 (N.P.). N.P. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
ACCESSION NUMBERS
The accession number for the RNA-seq data reported in this paper is GEO: GSE65325.
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures, seven figures, and five tables and can be found with this article online at http://dx.doi.org/10.1016/j.devcel.2015.02.012.
REFERENCES
- Alic N, Hoddinott MP, Vinti G, Partridge L. Lifespan extension by increased expression of the Drosophila homologue of the IGFBP7 tumour suppressor. Aging Cell. 2011;70:137–147. doi: 10.1111/j.1474-9726.2010.00653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apidianakis Y, Pitsouli C, Perrimon N, Rahme L. Synergy between bacterial infection and genetic predisposition in intestinal dysplasia. Proc. Natl. Ac ad. Sci. USA. 2009;106:20883–20888. doi: 10.1073/pnas.0911797106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B Stat. Methodol. 1995;57:289–300. [Google Scholar]
- Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hyper trophy and can prevent muscle atrophy invivo. Nat. Cell Biol. 2001;3:1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- Deboer MD. Animal models of anorexia and cachexia. Expert Opin. Drug Discov. 2009;4:1145–1155. doi: 10.1517/17460440903300842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delano MJ, Moldawer LL. The origins of cachexia in acute and chronic inflammatory diseases. Nutr. Clin. Pract. 2006;21:68–81. doi: 10.1177/011542650602100168. [DOI] [PubMed] [Google Scholar]
- Demontis F, Perrimon N. FOX0/4E-BP signaling in Drosophila muscles regulates organism-wide proteostasis during aging. Cell. 2010;143:813–825. doi: 10.1016/j.cell.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne MS, Pham LN, Shirasu-Hiza M, Schneider DS. Aki and FOXO dysregulation contribute to infection-induced wasting in Drosophila. Curr. Biol. 2006;76:1977–1985. doi: 10.1016/j.cub.2006.08.052. [DOI] [PubMed] [Google Scholar]
- Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. 2012;16:153–166. doi: 10.1016/j.cmet.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat. Rev. Clin. Oncol. 2013;70:90–99. doi: 10.1038/nrclinonc.2012.209. [DOI] [PubMed] [Google Scholar]
- Figueroa-Clarevega A, Bilder D. Malignant Drosophila tumors interrupt insulin signaling to induce cachexia like wasting. Dev. Cell. 2015;33:47–55. doi: 10.1016/j.devcel.2015.03.001. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gateff E, Schneiderman HA. Developmental capacities of benign and malignant neoplasms of Drosophila. Wilhelm Roux Arch. Entwickl. Mech. Org. 1974;176:23–65. doi: 10.1007/BF00577830. [DOI] [PubMed] [Google Scholar]
- Géminard C, Rulifson EJ, Leopold P. Remote control of insulin secretion by fat cells in Drosophila. Cell Metab. 2009;10:199–207. doi: 10.1016/j.cmet.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Greene JC, Whitworth AJ, Kuo I, Andrews LA, Feany MB, Pallanck LJ. Mitochondrial pathology and apoptotic muscle degener ation in Drosophila parkin mutants. Proc. Natl. Acad. Sci. USA. 2003;700:4078–4083. doi: 10.1073/pnas.0737556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzeschik NA, Paraons LM, Allott ML, Harvey KF, Richardson HE. Lgl, aPKC, and Crumbs regulate the Salvador/Warts/Hippo pathway through two distinct mechanisms. Curr. Biol. 2010;20:573–581. doi: 10.1016/j.cub.2010.01.055. [DOI] [PubMed] [Google Scholar]
- Halder G, Johnson RL. Hippo signaling: growth control and beyond. Development. 2011;138:9–22. doi: 10.1242/dev.045500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han HQ, Zhou X, Mitch WE, Goldberg AL. Myostatinlactivin pathway antagonism: molecular basis and therapeutic potential. Int. J. Biochem. Cell Biol. 2013;45:2333–2347. doi: 10.1016/j.biocel.2013.05.019. [DOI] [PubMed] [Google Scholar]
- Harvey KF, Hariharan IK. The Hippopathway. Cold Spring Harb. Perspect. Biol. 2012;4:a011288. doi: 10.1101/cshperspect.a011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirabayashi S, Baranski TJ, Cagan RL. Transformed Drosophila cells evade diet-mediated insulin resistance through Wingless signaling. Cell. 2013;154:664–675. doi: 10.1016/j.cell.2013.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honegger B, Galic M, Kohler K, Wittwer F, Brogiolo W, Hafen E, Stocker H. ImpL2, a putative homolog of vertebrate IGF-binding protein 7,counteracts insulin signaling in Drosophila and is essential for starvation resistance. J. Biol. 2008;7:10. doi: 10.1186/jbiol72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeya T, Galic M, Belawat P, Nairz K, Hafen E. Nutrient-dependent expression of insulin like peptides from neuroend ocrine cells in the CNS contributes to growth regulation in Drosophila. Curr. Biol. 2002;12:1293–1300. doi: 10.1016/s0960-9822(02)01043-6. [DOI] [PubMed] [Google Scholar]
- Ja WW, Carvalho GB, Mak EM, de la Rosa NN, Fang AY, Liong JC, Brummel T, Benzer S. Prandiology of Drosophila and the CAFE assay. Proc. Natl. Acad. Sci. USA. 2007;104:8253–8256. doi: 10.1073/pnas.0702726104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R, Halder G. The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat. Rev. Drug Discov. 2014;13:63–79. doi: 10.1038/nrd4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpowicz P, Perez J, Perrimon N. The Hippo tumor suppressor pathway regulates intestinal stem cell regeneration. Development. 2010;137:4135–4145. doi: 10.1242/dev.060483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kir S, White JP, Kleiner S, Kazak L, Cohen P, Baracos VE, Spiegelman BM. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature. 2014;513:100–104. doi: 10.1038/nature13528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menéndez J, Perez Garijo A, Calleja M, Morata G. A tumor-suppressing mechanism In Drosophila Involving cell competition and the Hippo pathway. Proc. Natl. Acad. Sci. USA. 2010;107:14651–14656. doi: 10.1073/pnas.1009376107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murali T, Pacifico S, Yu J, Guest S, Roberts GG, III, Finley RL., Jr. DrolD 2011: a comprehensive, Integrated resource for protein, transcriptlon factor, RNA and gene Interactions for Drosophila. Nucleic Acids Res. 2011;39:D736–D743. doi: 10.1093/nar/gkq1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien LE, Sollman SS, LI X, Biider D. Altered modes of stem cell division drive adaptive Intestinal growth. Cell. 2011;147:603–614. doi: 10.1016/j.cell.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H, Irvine KD. In vivo analysis of Yorkle phosphorylation sites. Oncogene. 2009;28:1916–1927. doi: 10.1038/onc.2009.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D. The Hippo signaling pathway In development and cancer. Dev. Cell. 2010;19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penna F, Minero VG, Costamagna D, Bonelli G, Baccino FM, Costelll P. Anti-cytokine strategies for the treatment of cancer-related anorexia and cachexla. Expert Opin. Biol.Ther. 2010;10:1241–1250. doi: 10.1517/14712598.2010.503773. [DOI] [PubMed] [Google Scholar]
- Planté-Bordeneuve V, Said G. Familial amylold polyneuropathy. Lancet Neurol. 2011;10:1086–1097. doi: 10.1016/S1474-4422(11)70246-0. [DOI] [PubMed] [Google Scholar]
- Puig O, Tjlan R. Transcriptional feedback controlof Insulin receptor by dFOXO/FOX01. Genes Dev. 2005;19:2435–2446. doi: 10.1101/gad.1340505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren F, Wang B, Yue T, Yun EY, Ip YT, Jiang J. Hippo signaling regulates Drosophila Intestine stem cell proliferation through multiple pathways. Proc. Natl. Acad. Sci. USA. 2010;107:21064–21069. doi: 10.1073/pnas.1012759107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ. Mediation of IGF-1-Induced skeletal myotube hypertrophy by Pl(3)K/Akt/mTOR and P(3)K/Akt/GSK3 pathways. Nat. Cell Biol. 2001;3:1009–1013. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- Sandri M, Sandri C, Giibert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Leeker SH, Goldberg AL. Foxo transcription factors Inducethe atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RL, Kohlmaler A, Polesello C, Veelken C, Edgar BA, Tapon N. The Hippo pathway regulates Intestinal stem cell proliferation during Drosophila adult mldgut regeneration. Development. 2010;137:4147–4158. doi: 10.1242/dev.052506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloth Andersen A, Hertz Hansen P, Schaffer L, Kristensen C. A new secreted insect protein belonging to the immunoglobulin superfamlly binds insulin and related peptides and inhibits their activities. J. Biol. Chem. 2000;275:16948–16953. doi: 10.1074/jbc.M001578200. [DOI] [PubMed] [Google Scholar]
- Song W, Ren D, LI W, Jiang L, Cho KW, Huang P, Fan C, Song Y, Liu Y, Rul L. SH2B regulation of growth, metabolism, and longevity In both Insects and mammals. Cell Metab. 2010;11:427–437. doi: 10.1016/j.cmet.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Veenstra JA, Perrlmon N. Control of lipid metabolism by tachykinin in Drosophila. Cell Rep. 2014;9:40–47. doi: 10.1016/j.celrep.2014.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley BK, Irvine KD. Hippo signaling In Drosophila: recent advances and Insights. Dev. Dyn. 2012;241:3–15. doi: 10.1002/dvdy.22723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovlch A, Pomeroy SL, Golub TR, Lander ES, Meslrov JP. Gene set enrichment analysis: a knowledge based approach for Interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teleman AA, Chen YW, Cohen SM. 4E BP functions as a metabolic brake used under stress conditions but not during normal growth. Genes Dev. 2005;19:1844–1848. doi: 10.1101/gad.341505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdale MJ. Biology of cachexla. J. Natl. Cancer Inst. 1997;89:1763–1773. doi: 10.1093/jnci/89.23.1763. [DOI] [PubMed] [Google Scholar]
- Tisdale MJ. Mechanisms of cancer cachexla. Physlol. Rev. 2009;89:381–410. doi: 10.1152/physrev.00016.2008. [DOI] [PubMed] [Google Scholar]
- Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-seq. Bioinformatlcs. 2009;25:1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012;7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell prollfer atlon. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, Lunt SY, Dayton TL, Fiske BP, Israelsen WJ, Mattaini KR, Vokes NI, Stephanopoulos G, Cantley LC, Metallo CM, Locasale JW. Metabolic pathway alterations that support cell proliferation. Cold Spring Harb. Symp. Quant. Biol. 2011;76:325–334. doi: 10.1101/sqb.2012.76.010900. [DOI] [PubMed] [Google Scholar]
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Yang X, Xu T. Molecular mechanism of size control In development and human diseases. Cell Res. 2011;21:715–729. doi: 10.1038/cr.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Deng Y, Lal ZC. Akt Is negatlwly regulated by Hippo signaling for growth Inhibition In Drosophila. Dev. Biol. 2012;369:115–123. doi: 10.1016/j.ydbio.2012.06.014. [DOI] [PubMed] [Google Scholar]
- Yuan M, Breltkopf SB, Yang X, Asara JM. A positive/negative Ion switching, targeted mass spectrometry based metabolomlcs platform for bodily fluids, cells, and fresh and fixed tissue. Nat. Protoc. 2012;7:872–881. doi: 10.1038/nprot.2012.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Thompson BJ, Hletakangas V, Cohen SM. MAPK/ERK signaling regulates Insulin sensitivity to control glucose metabolism In Drosophila. PLoS Genet. 2011;7:e1002429. doi: 10.1371/journal.pgen.1002429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinke I, Kirchner C, Chao LC, Tetzlaff MT, Pankratz MJ. Suppression of food Intake and growth by amino acids In Drosophila: the role of pumpless, a fat body expressed gene with homology to vertebrate glycine cleavage system. Development. 1999;126:5275–5284. doi: 10.1242/dev.126.23.5275. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.