Abstract
The blue swimming crab Portunus pelagicus is an important catch species for many coastal villages along the Java Sea coastline, but little is known regarding its reproductive biology or stock status. We examined the batch fecundity of female crabs that were collected monthly at landing sites from June 2011 to May 2012, calculated the relationships with body size, egg mass and month of the year, and determined the size at which females became potentially reproductive in the population inhabiting East Lampung waters (western Java Sea). Fecundity values ranged from 229,468 to 2,236,355 (mean = 926,638±30,975 [±SE]). The fecundity was positively and linearly correlated with carapace width (CW), but the relationships with body weight and egg mass were best described by logarithmic regression. A peaked, temporally cyclical pattern in fecundity was observed, with a peak period that was significantly different (F = 226.36; df = 22, p<0.05) from March to May 2012. Reproductive females were within the 111.0–155.9 mm CW size range; significantly higher reproductive potentials (F = 14.59; df = 30, p<0.05) were found in females within the 126.0–130.9 mm CW size group. The current minimum legal size (MLS = 100 mm CW) is not an appropriate limit reference point, and a precautionary approach is needed for a sustainable harvesting strategy. Resetting the MLS to 115 mm CW would potentially provide adequate protection for spawning females and increase total egg production, thereby maintaining population productivity and enhancing resilience in the face of current fishing pressures.
Keywords: Blue Swimming Crab, Fecundity, Reproductive Potential, Minimum Legal Size, East Lampung Waters
Abstract
Ketam renjong Portunus pelagicus merupakan spesies penting untuk banyak perkampungan persisiran pantai di perairan Laut Jawa, tetapi status pembiakan dan stok spesies tersebut kurang diketahui. Kami telah mengkaji fekunditi ketam betina yang telah dikutip setiap bulan dari tapak pendaratan dari Jun 2011 hingga Mei 2012, telah mengira perhubungan dengan saiz badan, jisim telur dan bulan dalam tahun, dan telah mengetahui saiz betina yang berpotensi untuk membiak dalam populasi yang menghuni di perairan Timur Lampung (barat Laut Jawa). Nilai-nilai fekunditi ialah daripada 229,468 hingga 2,236,355 (min = 926,638±30,975 [±SE]). Nilai ini positif dan berkorelasi secara linear dengan lebar karapas (CW), tetapi perhubungan dengan berat badan (BW) dan jisim telur diterangkan terbaik menggunakan regresi logaritma. Satu corak puncak, yang berkitaran sementara dalam fekunditi dilihat, dengan tempoh puncak yang berbeza secara signifikan (F = 226.36; df = 22, p<0.05) daripada Mac hingga Mei 2012. Betina yang boleh membiak berada dalam julat CW sebanyak 111.0–155.9 mm; potensi pembiakan yang tinggi secara signifikan (F = 14.59; df = 30, p<0.05) dijumpai dalam kumpulan betina dengan CW sebanyak 126.0–130.9 mm. Saiz minimum sah (MLS = 100 mm CW) masa kini tidak sesuai sebagai titik rujukan limitasi, dan satu langkah berjaga-jaga diperlukan untuk strategi penangkapan yang lestari. Pengesetan semula MLS kepada CW 115 mm berpotensi untuk memberi perlindungan secukupnya kepada betina yang membiak, dan meningkatkan produksi total telur, maka mengekalkan produktiviti populasi dan meningkatkan kebingkasan dalam pada menghadapi tekanan perikanan masa kini.
Keywords: Ketam Renjong, Fekunditi, Potensi Pembiakan, Saiz Sah Minimum, Perairan Timur Lampung
INTRODUCTION
The blue swimming crab Portunus pelagicus is abundant in shallow coastal and estuarine waters of tropical and temperate regions in the Indo-West Pacific. Demand for this species is increasing, which, coupled with its abundance, makes the crab a valuable target in the fishery sector (Ng 1998; Lai et al. 2010). In Indonesia, P. pelagicus is exploited year-round by small-scale fishery enterprises in most of the nation’s coastal waters. This resource is of considerable socioeconomic importance to coastal villages bordering the Java Sea, where the catch is used mainly in the crab meat industry and is also exported. However, the catch-per-trip and the sizes of captured crabs have been declining for several years. Accordingly, the P. pelagicus fishery has been placed under a recent management directive whereby the minimum legal size (MLS) of caught crabs has been set to 100 mm carapace width (CW, i.e., the distance between the tips of the 9th anterolateral spine; Ministry of Marine Affairs and Fisheries [MMAF] 2014). This directive was put in place without comprehensive consideration of the crab’s reproductive biology, which is poorly understood. Furthermore, no adequate stock assessments have been made for this species in most Indonesian waters.
Crab size at sexual maturity has been studied off the northern coast of Java (Hermanto 2004; Sunarto 2012) and in East Lampung waters (Zairion et al. unpublished data). These previous studies reported the mean size at which 50% of females reached physiological maturity (Lm50; judged by gonad condition) to be 105, 101 and 103 mm CW, respectively. Only Hermanto (2004) has estimated batch fecundity (the number of viable eggs released by a serial spawner in a pulse of spawning) in P. pelagicus, but he did not measure other key traits of reproductive biology (i.e., egg mass size and reproductive potential) that contribute to a better understanding of population dynamics and aid in stock assessment analyses. In addition, the reproductive biology of P. pelagicus is highly variable across its distribution area, which adds further complexity to the design of management strategies.
Measures of fecundity are key components of studies of the reproductive biology and population dynamics of all crustacean species. Fecundity estimates have increasingly become the preferred measures of stock reproductive potential and may be incorporated into scientific recommendations that provide biological reference points (BRPs) for sustainable fisheries management (Campbell & Robinson 1983; Goni et al. 2003; Tallack 2007; Cooper et al. 2013).
Reproductive potential may be determined as the largest egg contribution by a particular number of spawning females in the population (Kanciruk & Herrnkind 1976; Chang et al. 2007). Batch fecundity has been estimated as a measure of reproductive potential in P. pelagicus females off the coasts of south-western India (Sukumaran & Neelakantan 1997) and southeastern Australia (Johnson et al. 2010), where the highest reproductive output was from crabs in the 130–140 mm CW and 65–69 mm carapace length (CL) ranges, respectively. Estimates varied geographically in both studies, and it would therefore be inappropriate to make extrapolations from India or Australia to populations located in Indonesian coastal waters. Thus, local data on the reproductive biology of P. pelagicus in East Lampung waters are required to calculate an appropriate size-based reference point and for the development of a precautionary approach (PA) to sustainable crab harvesting.
The specific aims of this study were to measure: (1) batch fecundity and its relationships with body size, egg mass and time of year; and (2) the size of potentially reproductive females in the population of P. pelagicus in East Lampung waters, Indonesia.
MATERIALS AND METHODS
Sample Collection and Environmental Regime
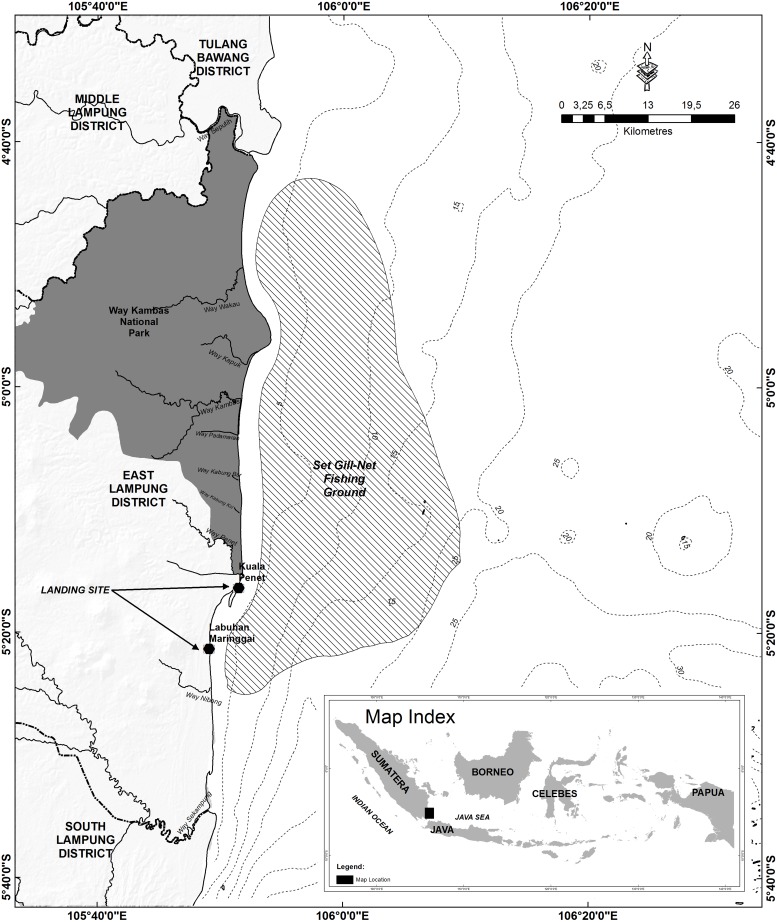
Samples of P. pelagicus were collected by stratified random sampling each month from June 2011 to May 2012, at two main landing sites: Kuala Penet (5°15′22.53″S, 105°51′41.52″E) and Labuhan Maringgai (5°21′32.98″S, 105°49′06.52″E) in the East Lampung District, Lampung Province, Indonesia (Fig. 1). Crabs brought ashore at these landing sites were captured by local fishers using set gill-nets as their main fishing gear; nets had mesh sizes of 3.0–4.5 in (7.6–11.4 cm). Each boat set three to five nets (Wardiatno & Zairion 2011). During the sampling year, the proportion of landed crabs (males, ovigerous females and non-ovigerous females) from selected fishing boats was recorded. Measurements of individual CW and body weight (BW) were conducted at different landing sites and fishing grounds. CW was measured to the nearest 0.01 mm using a digital calliper (PT Intralab Ekatama, Bogor, Indonesia), and BW was measured to the nearest 0.1 g using a digital balance (PT Intralab Ekatama, Bogor, Indonesia) (Potter at al. 2001, de Lestang et al. 2003). Ovigerous females (n = 22±2 [mean±SD]) of representative sizes and proportions were selected each month and were used to determine batch fecundity.
Figure 1:
Map of the coastal area and marine waters off East Lampung showing P. pelagicus fishing grounds using set gill-nets; the two main landing sites are indicated.
Based on 2003–2012 data from the Maritime Climatologic Station in Lampung, there are two main seasons in the East Lampung area; the wet season (west and northwest monsoon) takes place from December to May, and the rest is the dry season (east and southeast monsoon). However, a transition period occurs between both seasons, with the first transition occurring in April to May and the second in October to November. Mean monthly air temperature for the year was 24°C–34°C, and the mean annual rainfall ranged from 1295–2493 mm, whereas humidity was in the range of 76.8%–81.0%.
According to Wardiatno and Zairion (2011), the coastal land area of East Lampung is covered by approximately 60% lowland forest and is protected as Way Kambas National Park (WKNP). There is no seagrass bed found in the shallow waters, but most of the coastal waters up to ∼15 NM from the shoreline are P. pelagicus habitat and fishing grounds. Based on water quality monitoring at the fishing grounds in August 2011 and February 2012 (representative of the dry and wet seasons, respectively), there was not a high degree of variability in sea surface temperature (i.e., 28°C–32°C) for both seasons; temperatures near the bottom ranged from 28.0°C–29.5°C. Salinity at the sea surface and bottom were within 10–25 PSU and 25–29 PSU, respectively, from near shore to offshore in the northern sector in February, whereas values ranged from 29–30 PSU and 30–32 PSU, respectively, in the southern sector, close to Labuhan Maringgai. The low salinity in the northern sector was influenced by the high supply of freshwater from the Way Seputih River. In contrast, the salinity of the surface and bottom water during the dry season were 17–27 PSU and 27–30 PSU, respectively, in the northern sector, whereas the ranges were 28–32 PSU and 29–32 PSU, respectively, in the southern sector.
Fecundity and Egg Mass Estimation
Fecundity, defined as the number of eggs per batch produced by a female, and the weight of the spawning egg (brood) mass attached to the pleopods during embryonic development were estimated. Embryonic stages were categorized by the development of colour (Pinheiro & Fransozo 2002; Ikhwanuddin et al. 2012; Soundarapandian et al. 2013): (1) initial or 1st stage, which is yellow or orange-yellowish; (2) intermediate or 2nd stage, which is brown; and (3) final or 3rd stage, which is black-grey or black. The masses at each stage were measured following the procedure described by Johnson et al. (2010) before removing each egg batch. Egg-bearing pleopods were removed carefully; the wet weight of the whole egg mass (eggs+pleopods) was measured to the nearest 0.001 g using an electronic balance (PT Intralab Ekatama, Bogor, Indonesia). Prior to the removal of the eggs, the egg-bearing pleopods were immersed in 400 ml of 1M KOH for 12 h. The separation process was completed by scraping the pleopods clean and removing all setae. These organs were then weighed, and their combined weight was subtracted from the whole egg mass to give the mass of the eggs alone. Three replicate subsamples of ∼0.2 g were randomly taken from each wet egg mass, and the number of eggs in each subsample was counted under a stereomicroscope (PT Intralab Ekatama, Bogor, Indonesia) mounted over a counting tray. The mean number of eggs per unit subsample weight was counted; this value was then scaled up to estimate the total number of eggs per individual egg batch.
Data Analysis
Fecundity relationship
Regression analyses, either linear or logarithmic, and power functions were performed to establish the relationships between fecundity at all stages of spawning, egg development, body size and egg mass. An appropriate relationship was chosen based on the highest correlation coefficient (r). Differences in the regression slopes for the relationships between fecundity and body size were analysed statistically using a t-test (Fowler & Cohen 1992). Temporal patterns of fecundity were examined by comparing the relative mean fecundities across months (adapted from Pinheiro & Terceiro 2000), and one-way ANOVA was used to detect significant differences among the means.
Reproductive potential
To estimate reproductive potential, the relative proportions of non-ovigerous and ovigerous females in each 5 mm CW size class were determined. The procedures of Kanciruk and Herrnkind (1976) and Sukumaran and Neelakantan (1997) were used to calculate an index of relative reproductive potential (IRP) and of reproductive productivity, which allowed us to estimate the relative contribution of each size class to the total number of eggs produced. The IRP was calculated using the following expression:
where, Ai is the proportion of size class i in a group containing all females across size classes that contained ovigerous females; Bi is the proportion of ovigerous females in size class i; Ci is the mean fecundity in size class i; and D is a constant (31.942). The index for the 106.0–110.9 mm size class was assigned a standardized value of 100. Productivity was estimated by dividing Ci by Ai, and reproductive females were defined as having a productivity value ≥1.0. One-way ANOVA was used to detect significant differences in the IRP and productivity values across the size classes.
RESULTS
Fecundity and Its Relationships
A total of 267 ovigerous females were collected within the following size ranges: 91.58–168.00 mm CW (mean = 126.42±0.88 mm [±SE]) and 78.1–387.5 BW (mean = 166.3±3.82 g [±SE]). Within the total, 168 (62.55%), 26 (9.74%) and 74 individuals (27.72%) were in the 1st, 2nd and 3rd stages of egg development, respectively. The estimated batch fecundity range for the 1st stage of egg development was 229,468–2,236,355 (mean = 926,638±30,975 [±SE]). Table 1 lists the mean estimated fecundity, mean CW, net BW and egg mass wet weight for each size class. The estimated total mean fecundities (±SE) were within the range of 264,134±15,867–1,534,538±308,890 eggs across the size classes. Fecundity was linearly and positively correlated with CW (Fig. 2), but logarithmically related to net BW (Fig. 3) and egg mass weight (EMW; i.e., fecundity = 695.74*ln[EMW] − 1140.6; R2 = 0.7099). The logarithmic relationships were influenced by large variations in egg mass and fecundity, particularly in crabs exceeding 111 mm CW (coefficient of variation [CV] = 24%–38%). Moreover, estimated mean fecundity varied temporally within the 567,003±51,067–1,237,665±121,968 (mean±SE; CV = 6.23%–14.37%) range and showed a cyclical pattern (Fig. 4). Values differed significantly between months (F = 226.36; df = 22, p<0.05), with the lowest and the highest significantly different values occurring in November 2011 and from March to May 2012, respectively.
Table 1:
Mean CW, BW, egg batch weight and fecundity of ovigerous female P. pelagicus in each 5 mm CW size class (values are the mean±SE).
| CW size class (mm) | Number of individuals | Mean CW (mm) | Mean net body wet weight (g) | Mean egg mass wet weight (g) | Total number of eggs per individual |
|---|---|---|---|---|---|
| 91.00–95.99 | 2 | 93.73±2.15 | 81.80±3.70 | 8.70±0.70 | 264,134±15,867 |
| 96.00–100.99 | 4 | 99.11±0.55 | 84.68±2.56 | 9.35±1.24 | 384,806±54,384 |
| 101.00–105.99 | 6 | 104.08±0.47 | 89.60±2.02 | 10.19±0.40 | 415,811±33,197 |
| 106.00–110.99 | 12 | 109.40±0.34 | 104.12±2.23 | 13.02±1.06 | 494,895±40,831 |
| 111.00–116.99 | 16 | 113.28±0.36 | 111.28±1.77 | 15.07±0.92 | 681,589±43,776 |
| 116.00–120.99 | 20 | 118.84±0.30 | 127.47±2.91 | 17.52±1.25 | 712,593±42,296 |
| 121.00–125.99 | 21 | 123.25±0.30 | 146.18±2.41 | 18.92±1.13 | 825,907±40,902 |
| 126.00–130.99 | 22 | 128.45±0.31 | 164.71±3.63 | 20.69±1.73 | 970,608±55,473 |
| 131.00–135.99 | 19 | 133.10±0.30 | 190.91±4.63 | 26.50±2.53 | 1,107,402±76,177 |
| 136.00–140.99 | 13 | 138.82±0.43 | 213.63±3.99 | 28.59±3.05 | 1,258,908±102,362 |
| 141.00–145.99 | 10 | 143.02±0.41 | 244.29±3.90 | 29.05±4.75 | 1,225,732±129,916 |
| 146.00–150.99 | 8 | 148.51±0.42 | 257.95±4.02 | 30.25±4.45 | 1,237,420±101,290 |
| 151.00–155.99 | 6 | 153.54±0.72 | 294.95±5.87 | 40.03±5.16 | 1,487,686±186,448 |
| 156.00–160.99 | 4 | 158.14±0.39 | 335.65±10.26 | 31.33±5.69 | 1,420,511±150,184 |
| 161.00–165.99 | 3 | 163.69±1.24 | 331.63±13.71 | 43.75±16.21 | 1,368,550±197,558 |
| 166.00–170.99 | 2 | 167.45±0.10 | 386.35±1.15 | 43.65±13.15 | 1,534,538±308,890 |
|
| |||||
| Total | 168 | ||||
Figure 2:
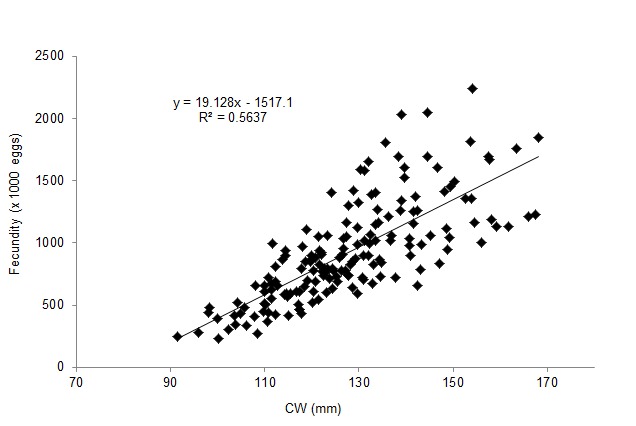
Linear relationship between the fecundity of P. pelagicus and CW.
Figure 3:
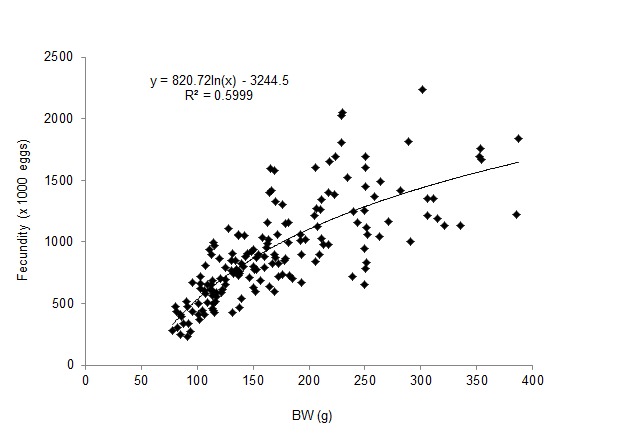
Logarithmic relationship between the fecundity of P. pelagicus and net BW.
Figure 4:
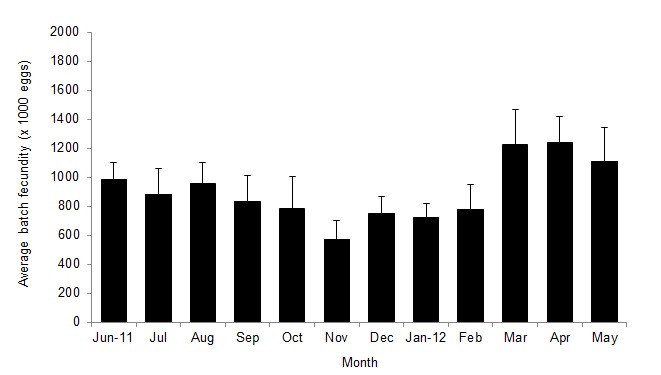
Mean fecundity (+95% confidence interval) of P. pelagicus in each month.
Estimated mean fecundity in the 2nd stage of egg development was lower than that in the 1st stage, and the value in the 3rd stage was lower than that in the 2nd. The relationships between stage and fecundity for the 2nd and 3rd stages were similar to the relationships in the 1st stage. The linear regression slopes for the 1st and 2nd stages of egg development were not significantly different (t = 0.02, df = 190, p<0.05); the slopes for the 2nd and 3rd stages (t = 0.178, df = 96, p<0.05) and for the 1st and 3rd stages of egg development (t = 0.188, df = 238, p<0.05) also were not significantly different. Because the regression slopes of the relationships between CW and fecundity were not significantly different in the egg developmental stages, only the fecundity of the 1st stage was used in further analyses.
Reproductive Potential
Among the data for all P. pelagicus females across the size classes that contained ovigerous individuals, estimated IRPs were within the range 1.00–384.02 (mean = 132.02); IRP values differed significantly (F = 14.59, df = 30, p<0.05) across the size groups, with the lowest and highest values found in the 91.0–95.9 mm and 126.0–130.9 mm size classes, respectively (Table 2). The productivity estimates exhibited a similar pattern, with a range from 0.01–1.86 (mean = 0.88) and values that differed significantly (F = 40.26, df = 30, p<0.05) across the size classes. Our findings revealed that newly spawning females (91.0–95.9 mm CW) represented ∼4.32% and ∼0.28% of non-ovigerous and ovigerous females, respectively, and produced only ∼0.05% of the estimated total egg production; this group of females had the lowest reproductive productivity (productivity estimate = 0.01) of all of the size classes (Table 2). The highest percentage of P. pelagicus females occurred in the 111–115.9 mm CW size class, but ovigerous females were most frequent in the 126–130.9 mm size class, which also had the highest IRP, producing ∼18.16% of the estimated total egg production (productivity estimate = 1.86). The reproductive P. pelagicus females were in the 111–155.9 mm CW size class (productivity estimate ≥1.0). Individuals below the current MLS of 100 mm CW (91–100 mm) comprised 9.85% of the total number of females and 1.41% of ovigerous females. These smaller individuals produced only 0.4% of the estimated total egg production. Ovigerous females of less than 115 mm CW accounted for ∼21.38% of the ovigerous female total and produced ∼17.45% of the estimated total egg production.
Table 2:
Indices of reproductive potential and reproductive productivity for female P. pelagicus in each 5 mm CW size class.
| Metric | Size class (mm CW) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| 91–95 | 96–100 | 101–105 | 106–110 | 111–115 | 116–120 | 121–125 | 126–130 | 131–135 | 136–140 | 141–145 | 146–150 | 151–155 | 156–160 | 161–165 | 166–170 | |
| A. Percentage of females caught | 4.32 | 5.54 | 8.09 | 9.18 | 10.29 | 9.64 | 9.77 | 9.77 | 7.50 | 6.43 | 5.60 | 3.64 | 2.01 | 1.70 | 0.87 | 0.70 |
| B. Percentage of ovigerous (OVI) females | 0.28 | 1.13 | 2.11 | 7.03 | 10.83 | 12.10 | 12.10 | 12.94 | 10.13 | 9.28 | 6.89 | 5.63 | 4.64 | 2.81 | 1.27 | 0.42 |
| C. Ratio of OVI females to adult females | 0.01 | 0.03 | 0.04 | 0.12 | 0.16 | 0.19 | 0.19 | 0.21 | 0.21 | 0.22 | 0.19 | 0.24 | 0.36 | 0.26 | 0.23 | 0.14 |
| D. Mean total potential fecundity (×100,000) | 2.64 | 3.85 | 4.16 | 4.95 | 6.82 | 7.13 | 8.26 | 9.71 | 11.07 | 12.59 | 12.26 | 12.37 | 14.88 | 14.21 | 13.69 | 15.35 |
| E. Index of reproductive potential standardised to the value of 100.00 in the 106–110 mm CW size class | 1.00 | 7.51 | 22.21 | 100.00 | 237.21 | 260.02 | 305.45 | 384.02 | 263.29 | 235.29 | 148.17 | 79.35 | 43.36 | 21.27 | 4.73 | 1.41 |
| F. Percentage of total egg production | 0.05 | 0.36 | 1.05 | 4.73 | 11.22 | 12.30 | 14.45 | 18.16 | 12.45 | 11.13 | 7.01 | 3.75 | 2.05 | 1.01 | 0.22 | 0.07 |
| G. Productivity (F/A) | 0.01 | 0.06 | 0.13 | 0.52 | 1.09 | 1.28 | 1.48 | 1.86 | 1.66 | 1.73 | 1.25 | 1.03 | 1.02 | 0.59 | 0.26 | 0.10 |
DISCUSSION
Fecundity and Its Relationships
Our estimate of P. pelagicus batch fecundity in the study site (range: 229,468–2,236,355) was high, similar to most species of portunid crabs (e.g., Batoy et al. 1987; Ravi et al. 2008; Johnson et al 2010; Ikhwanuddin et al. 2011). It is believed that these types of reproductive traits in crustaceans are associated with evolutionary mechanisms that compensate for the eggs lost during the incubation period and larval mortality during development (e.g., Muino 2002; Goes et al. 2005). Specifically, our estimated batch fecundity exceeded previous estimates for a population of a similar size off the coastal waters of Subang, West Java (81,000–1,343,000; Hermanto 2004) and in south-western India (56,000–1,070,000; Sukumaran & Neelakantan 1997); it also differed from all other estimates throughout the species distribution range (Table 3). Thus, the reproductive biology characteristics of P. pelagicus in our study area differed from those in all other populations that have been analysed.
Table 3:
Ranges of fecundity values for P. pelagicus in Indonesian waters (1–2) and other parts of the species’ distribution range; CW = carapace width, CL = carapace length. Table values in brackets are average fecundities (±SE in two cases).
| No. | Location | Ovigerous female size (mm) | Fecundity (×1000) | Source |
|---|---|---|---|---|
| 1. | East Lampung, Indonesia (Western Java Sea) | 91.58–168.00 CW | 229–2236 (928±30.97) | Present study |
| 2. | Subang, West Java, Indonesia | 89–156 CW | 81–1343 (543.9) | Hermanto (2004) |
| 3. | Johor, Malaysia | 96.4–133.2 CW | 43–183.1 (105.4) | Ikhwanuddin et al. (2012) |
| 4. | Sarawak, Malaysia | 144–193 CW | 213.3–3376.7 (233±24) | Ikhwanuddin et al. (2011) |
| 5. | Port Dickson, Malaysia | 102.25–140.58 CW | 148–835 | Arshad et al. (2006) |
| 6. | Leyte and Bohol, Philippines | 41–71 CL | 420.98–1312.24 (894.284) | Batoy et al. (1987) |
| 7. | Mandapam, Tamil Nadu, India | 100–190 CW | 60–1976 | Josileen (2013) |
| 8. | Southwestern coast of India | 89–170 CW | 56–1070 | Sukumaran and Neelakantan (1997) |
| 9. | Khuzestan, Iran | 110–169 CW | 150.5–1106.2 (815.2) | Jazayeri et al. (2011) |
| 10. | Southeastern Australian estuary | 55–80 CL | 463–1781 | Johnson et al. (2010) |
| 11. | Cockburn, Southwestern Australia | 84–154 CW | 68.5–324.4 (196.4) | de Lestang et al. (2003) |
Note: The blue swimming crabs of the eastern India coastal waters have been recently assigned to Portunus reticulatus; in the western Indian Ocean (from Pakistan throughout the Arabian Sea, the Persian Gulf and off the coasts of Egypt and Tanzania) the crabs have been assigned to P. segnis; in the western, southern and southeastern waters of Australia, they have been assigned to P. armatus (Lai et al. 2010).
The fecundity of brachyuran crabs varies among individual females within the same area and among females of the same species in different areas within the same region and is influenced by several intrinsic and extrinsic factors. According to Ramirez-Llodra (2002), the major intrinsic factors contributing to differences in fecundity among females in the same population include variation in individual female size or maternal size, nutritional history related to food availability and quality, age and the age at sexual maturity or first reproduction, which influence the reproductive effort and residual reproductive value. The major extrinsic factors are both inter- and intra-specific competition. The production of eggs is energetically expensive, and optimal energy and energy costs are also required to maintain somatic growth. A trade-off between the energy spent on both types of growth is an important strategy and might affect fecundity. In addition, large females produce higher numbers of eggs than smaller ones, and female CW is the main factor contributing to fecundity variability (e.g., Muino 2002). This type of maternal effect is not related to genetics, but it significantly and positively affects fecundity (e.g., Fischer et al. 2009). This variation might also relate to physical constraints, e.g., large individual females have larger body cavities and thus larger pleopod capacities (Hines 1982). Reproductive effort and residual reproduction might also relate to the reproductive period or life stage of each female (e.g., Pinheiro et al. 2003; Arshad et al. 2006).
The presence of variability in the fecundity of the same species in dissimilar areas of the same region might be influenced by female growth rates, which are associated with variations in the environmental conditions of their habitat (e.g., habitat structure, environmental stress and pollution, food quantity and quality, population density and inter-specific competition). Environmental stress and toxic substances might also influence their body size, as might disparate biological and genetic compositions (Campbell & Eagles 1983; Ramirez-Llodra 2002; Litulo 2004; Linnane et al. 2008; Johnson et al. 2010) and parasitism (e.g., Shields & Wood 1993). Beyers and Goosen (1987) suggested that variations in fecundity might be used as an indicator of female growth rates associated with food supply. Environmental and climate factors (i.e., high temporal variation in temperature and photoperiodicity) may also influence the size of reproductive females, with a consequence of differing fecundity for the same species at varying latitudes (e.g., Pinheiro & Terceiro 2000). In the same way, salinity might also influence fecundity because fecundity tends to be stunted at low salinity (e.g., Batoy et al. 1987; de Lestang et al. 2003). However, both the temperature and salinity of the East Lampung waters did not vary widely by season during the study year, although sea surface salinity (10–25 PSU) was reduced in coastal waters close to the northern sector of the fishing ground during the month of February (wet season), when large volumes of freshwater were discharged by the Way Seputih River.
Despite the high fecundity of P. pelagicus, it was positively and linearly correlated with CW, and the greatest mean fecundity (1,534,538±308,890 [±SE]) was found for the 166.00–170.9 mm size class. The fecundity/size relationships in this study were consistent with earlier studies of portunid crabs (Prager et al. 1990; Arshad et al. 2006; Ravi et al. 2008; Johnson et al. 2010; Ikhwanuddin et al. 2011, 2012; Rodrigues et al. 2011; Safaie et al. 2013). In contrast, de Lestang et al. (2003) reported that fecundity peaked at 61 and 71 mm CL, and then declined in the next consecutive size. Kumar et al. (2003) also reported that fecundity increased by 83.9% when the CW increased from 105 mm to 125 mm and then decreased, but in other studies, the relationship was different and was best described by a power function (e.g., Sukumaran & Neelakantan 1997; Pinheiro & Terceiro 2000; Hamasaki et al. 2006; Rameshbabu et al. 2006; Rasheed & Mustaquim 2010; Josileen 2013). Such differences i n the relationships between fecundity and size might be related to (1) differences in the biology of species because P. pelagicus has a limited distribution area in the marine and coastal waters of southeast and east Asia (Lai et al. 2010; see note in Table 3) and (2) multiple (repeat) spawning of portunid crabs within the same season or in the same year (e.g., Sukumaran & Neelakantan 1997, 1999; Costa & Negreiros-Fransozo 1998; Santos & Negreiros-Fransozo 1999; Pinheiro & Fransozo 2002; de Lestang et al. 2003; Kumar et al. 2003; Johnson et al. 2010) because egg masses in larger female crabs tend to decrease in size over successive spawning during each season (e.g., Dickinson et al. 2006; Darnell et al. 2009), with a consequent reduction in egg production. Large females often produce eggs with large diameters, and the number of eggs decreases (e.g., Hines 1982; Kumar et al. 2003; Fischer et al. 2009; Przemysław & Marcello 2013), a phenomenon that may be related to the logarithmic relationship observed between fecundity and (1) net BW and (2) egg mass (brood size), which may be explained by large variations in egg mass and fecundity in the size classes exceeding 111 mm CW. However, the logarithmic functions we calculated differed from those reported in previous studies, which were described as positive linear correlations. Litulo (2004) reported that an increase in female body weight was not always followed by an increase in fecundity in Uca annulipes. Thus, data regarding the number of brachyuran eggs per brood are more important than information on brood size because of the large variation in fecundity with brood size; descriptions of the relationship between fecundity and BW are also crucial (Hines 1982; Muino 2002).
Seasonal changes in fecundity are also a significant factor for the reproductive traits of brachyuran crabs (Muino 2002). These variations might be related to environmental and biological factors (e.g., nutrition and energy allocated for reproduction) (Litulo 2004; de Arruda Leme 2006). Accordingly, oocyte development and yolk formation are primarily determined by the presence of sufficient quantities of highly nutritious food, and optimal energy is allocated to gonadic growth prior to the peak spawning season, which might influence both the quantity and quality of the eggs produced. Other factors might relate to successive breeding during the year (e.g., Dickinson et al. 2006; Darnell et al. 2009).
The estimated mean fecundity of P. pelagicus in East Lampung waters varied by month; it was highest from March–September, with a significantly different peak period (F = 226.36; df = 22, p<0.05) from March to May 2012 (the end of the wet season and the transition period between the wet and dry seasons, respectively). The crab P. pelagicus had two peak breeding seasons in East Lampung waters throughout the year: April–June and October–November, although the second peak was significantly weaker than the first (Zairion et al. unpublished data). The peak in fecundity coincided with the peak breeding seasons, and this result is consistent with several previous studies. Kumar et al. (2003) reported that fecundity increased from October to December (from spring to early summer) and then declined; while peak fecundity occurred simultaneously with the peak breeding season. Prager et al. (1990) also reported that the size-specific fecundity of Callinectes sapidus increased steeply during the peak breeding season, except when ovigerous females had a dissimilar density pattern with the season of a different year. Thus, a temporal fecundity trend may exist, linking reproductive strategy traits, spawning patterns and environmental conditions.
Most of the smaller ovigerous females (i.e., <105 mm CW) were collected from September–November; their relative fecundities were low (see Fig. 2). It is possible that the gonads of immature females will mature earlier as a consequence of slightly increasing temperatures during the dry season (i.e., mean bottom seawater temperature = 29.5°C), and they might spawn in the next consecutive month; crabs have been shown to mature earlier as temperatures increase (Campbell & Fielder 1986; Fisher 1999; de Lestang et al. 2003). It is also possible that the offspring produced in the first breeding season contributed substantially to recruitment.
Reproductive Potential and Its Relevance to Fisheries Management
The estimated IRPs differed significantly (F = 14.59, df = 30, p<0.05) across the size groups, with the lowest and highest values in the 91.0–95.9 mm and 126.0–130.9 mm size classes, respectively; the productivity estimates also differed significantly (F = 40.26, df = 30, p<0.05), with the lowest and the highest coinciding with the IRPs. Female crabs in the 91.0–95.9 mm CW size group were not the rarest in the population, but they had the lowest fecundity, reproductive potential and productivity. The largest females crabs (>156.0 mm CW) had the highest individual fecundity, but they were not abundant (i.e., ∼3.27% of the total population of females; see Table 3) and might therefore contribute little to total egg production in the population. The most abundant female crabs were in the 111.0–115.9 mm size range, but they did not have the highest reproductive potential (productivity estimated at ≥1.0, just above the mean value). The most fecund females were in the 126.0–130.9 mm CW categories. In contrast, Sukumaran and Neelakantan (1997) studied reproductive females in the 100–170 mm CW range and reported the highest fecundity and reproductive potential in the 90–100 mm class and the highest productivity in the 130–140 mm size class. Thus, reproductive potential depends on the quantity of eggs produced in certain size groups rather than on the mean size of the available females and on the overall mean individual fecundity in the population (Goni et al. 2003). The reproductive traits observed in P. pelagicus in East Lampung seem to be the consequences of interspecific variation and area-specific and regional differences in portunid crab reproductive biology.
Females below the current MLS of 100 mm CW (91–100 mm) accounted for ∼9.85% of the total population; few were ovigerous (1.41% of the total ovigerous population), and they contributed only 0.4% to the estimated total egg production. Although P. pelagicus is highly fecund and productive, these reproductive attributes are highly size-dependent, and fecundity seems to be difficult to be estimated. Therefore, the current MLS used in the harvesting strategy is not congruent with the limit reference point or a precautionary approach to crab fisheries management. Moreover, the current MLS is smaller than the mean size of females that have reached physiological maturity (Lm50; i.e., a CW of 103 mm) in East Lampung waters (Zairion et al. unpublished data). In addition, 103 mm CW does not seem to be appropriate for the MLS.
These results provide data on the fecundity and reproductive potential of the P. pelagicus population, and these two reproductive characteristics should contribute to the setting of a more appropriate MLS. It is therefore recommended that the MLS for the P. pelagicus artisanal fishery in East Lampung waters, including all parts of the western Java Sea, should be increased to 115 mm CW. The proportion of ovigerous females below this size in the population accounted for ∼21.38% of the ovigerous population and produced ∼17.45% of the total estimated egg production. This new MLS would allow an increase in total egg production, thereby maintaining population productivity and resilience under current fishing pressures, though this recommendation may reduce total catches and fishing revenues. The acquisition of more robust information regarding the spatiotemporal variability of the frequency, growth, mortality and spawner–recruitment relationships of ovigerous females will require further assessments of stock susceptibility and resilience.
CONCLUSION
The batch fecundity of female P. pelagicus was high at the study site; it varied among individual females in the population and appeared to be difficult to be estimated. The relationship between CW and fecundity was positive and linear, whereas the relationship between BW and egg mass was best described by a logarithmic fit. Fecundity varied temporally and peaked in the period of March–May 2012. Reproductive females were in the 111–155.9 mm CW size range. The highest reproductive potentials were measured in females within the 126–130.9 mm CW size range. The current MLS in the size-based harvesting strategy is not appropriate for sustainable use of the P. pelagicus resource in East Lampung waters. It should be reset to 115 mm CW for the appropriate protection of the population of spawning and breeding females and to increase total egg production.
Acknowledgments
This work was partially funded by the Directorate General of Higher Education, Ministry of Education and Culture of Indonesia and by the Indonesia Blue Swimming Crab Processing Association (APRI) in the form of collaborative research with the Department of Aquatic Resources Management, Faculty of Fisheries and Marine Sciences, Bogor Agricultural University. The authors are thankful to the anonymous reviewers for their constructive comments and to some graduate bachelor students and technicians for their assistance during field and laboratory work. We also thank David Harris and Daniel Johnson of Western Australian Fisheries and Marine Research Laboratories, Australia for their valuable literature support.
REFERENCES
- Efrizal Arshad A, Kamarudin MS, Saad CR. Study on fecundity, embryology and larval development of blue swimming crab Portunus pelagicus (Linnaeus, 1758) under laboratory conditions. Research Journal of Fisheries and Hydrobiology. 2006;1(1):35–44. [Google Scholar]
- Batoy CB, Sarmago JF, Pilapil BC. Breeding season, sexual maturity and fecundity of the blue crab, Portunus pelagicus L. in selected coastal waters in Leyte and vicinity, Philippines. Annals of Tropical Research. 1987;93:157–177. [Google Scholar]
- Beyers CJdeB, Goosen PC. Variations in fecundity and size at sexual maturity of female rock lobster Jasus lalandii in the Benguela ecosystem. South African Journal of Marine Science. 1987;5(1):513–521. doi: 10.2989/025776187784522216. [DOI] [Google Scholar]
- Campbell A, Eagles M. Size at maturity and fecundity of rock crabs, Cancer irroratus, from the Bay of Fundy and southwestern Nova Scotia. Fishery Bulletin. 1983;81(2):357–362. [Google Scholar]
- Campbell A, Robinson DG. Reproductive potential of three American lobster (Homarus americanus) stocks in the Canadian Maritimes. Canadian Journal of Fisheries and Aquatic Science. 1983;40(11):1958–1967. doi: 10.1139/f83-225. [DOI] [Google Scholar]
- Campbell GR, Fielder DR. Size at sexual maturity and occurrence of ovigerous females in three species of commercially exploited portunid crabs in SE, Queensland. Proceedings of the Royal Society of Queensland. 1986;97:79–87. [Google Scholar]
- Chang Y, Sun C, Chen Y, Yeh S, Chiang W. Reproductive biology of the spiny lobster, Panulirus penicillatus, in the southeastern coastal waters of Taiwan. Marine Biology. 2007;151(2):553–564. doi: 10.1007/s00227-006-0488-9. [DOI] [Google Scholar]
- Cooper WT, Barbieri LR, Murphy MD, Lowerre-Barbieri SK. Assessing stock reproductive potential in species with indeterminate fecundity: Effects of age truncation and size-dependent reproductive timing. Fisheries Research. 2013;138:31–41. doi: 10.1016/j.fishres.2012.05.016. [DOI] [Google Scholar]
- Costa TM, Negreiros-Fransozo ML. The reproductive cycle of Callinectes denae Smith, 1969 (Decapoda: Portunidae) in Ubatuba Region, Brazil. Crustaceana. 1998;71(6):615–627. [Google Scholar]
- Darnell MZ, Rittschoff D, Darnell KM, McDowell RE. Lifetime reproductive potential of female blue crabs Callinectes sapidus in North Carolina, USA. Marine Ecology Progress Series. 2009;394:153–163. doi: 10.3354/meps08295. [DOI] [Google Scholar]
- de Arruda Leme MH. Seasonal changes in reproductive traits of the crab Sesarma rectum (Grapsoidea: Sesarmidae) on the northern coast of São Paulo State, Brazil. Journal of Crustacean Biology. 2006;26(2):141–147. doi: 10.1651/C-2621.1. [DOI] [Google Scholar]
- de Lestang S, Hall NG, Potter IC. Reproductive biology of the blue swimmer crab (Portunus pelagicus, Decapoda: Portunidae) in five bodies of water on the west coast of Australia. Fisheries Bulletins. 2003;101(4):745–757. [Google Scholar]
- Dickinson GH, Rittschof D, Latanich C. Spawning biology of the blue crab, Callinectes sapidus, in North Carolina. Bulletin of Marine Science. 2006;79(2):273–285. [Google Scholar]
- Fischer S, Thatje S, Brey T. Early egg traits in Cancer setosus (Decapoda, Brachyura): Effects of temperature and female size. Marine Ecology Progress Series (MEPS) 2009;337:192–202. doi: 10.3354/meps07845. [DOI] [Google Scholar]
- Fisher MR. Effect of temperature and salinity on size at maturity of female blue crabs. Transactions of the American Fisheries Society. 1999;128(3):499–506. doi: 10.1577/1548-8659(1999)128.0499. [DOI] [Google Scholar]
- Fowler J, Cohen L. Practical statistics for field biology. Chichester, England: John Wiley & Sons Ltd; 1992. [Google Scholar]
- Goes JM, Fransozo A, Fernandes-Goes LC. Fecundity of Eriphia gonagra (Fabricius, 1781) (Crustacea, Brachyura, Xanthidae) in the Ubatuba region, Sao Pauio, Brazil. Nauplius. 2005;13(2):127–136. [Google Scholar]
- Goni R, Quetglas A, Renones O. Size at maturity, fecundity and reproductive potential of protected population of the spiny lobster Palinurus elephas (Fabricius, 1787) from the western Mediterranean. Marine Biology. 2003;143(3):583–592. doi: 10.1007/s00227-003-1097-1095. [DOI] [Google Scholar]
- Hamasaki K, Fukunaga K, Kitada S. Batch fecundity of the swimming crab Portunus trituberculatus (Brachyura: Portunidae) Aquaculture. 2006;253(1–4):359–365. doi: 10.1016/j.aquaculture.2005.08.002. [DOI] [Google Scholar]
- Hermanto DT.2004Study on growth and some reproductive biology aspects of the blue swimming crab (Portunus pelagicus) in Mayangan waters, Subang, West JavaUndergraduate diss., Bogor Agricultural University [Google Scholar]
- Hines AH. Allometric constraints and variables of reproductive effort in brachyuran crabs. Marine Biology. 1982;69(3):309–320. [Google Scholar]
- Ikhwanuddin M, Azra MN, Siti-Aimuni H, Abol-Munafi AB. Fecundity, embryonic and ovarian development of blue swimming crab, Portunus pelagicus (Linnaeus 1758) in coastal waters of Johor, Malaysia. Pakistan Journal of Biological Science. 2012;15(15):720–728. doi: 10.3923/pjbs.2012.720.728. [DOI] [PubMed] [Google Scholar]
- Ikhwanuddin M, Shabdin ML, Abol-Munafi AB. Fecundity of blue swimming crab, Portunus pelagicus (Linnaeus 1758) from Sematan fishing district, Sarawak Coastal Waters of South China Sea. Borneo Journal of Science and Technology. 2011;1(1):46–51. [Google Scholar]
- Jazayeri A, Papan V, Savari A, Nejad TS. Biological investigation of Persian Gulf blue swimmer crab (Portunus pelagicus) in Khuzestan coasts. Journal of American Science. 2011;7(2):7–13. [Google Scholar]
- Johnson DD, Gray CA, William G, Macbeth WG. Reproductive biology of Portunus pelagicus in a South-East Australian Estuary. Journal of Crustacean Biology. 2010;30(2):200–205. doi: 10.1651/08-3076.1. [DOI] [Google Scholar]
- Josileen J. Fecundity of the blue swimmer crab, Portunus pelagicus (Linnaeus, 1758) (Decapoda, Brachyura, Portunidae) along the Coast of Mandapam, Tamil Nadu, India. Crustaceana. 2013;86(1):48–55. doi: 10.1163/15685403-00003139. [DOI] [Google Scholar]
- Kanciruk P, Herrnkind WF. Autumnal reproduction in Panulirus argus at Bimini, Bahamas. Bulletin of Marine Science. 1976;26(4):417–432. [Google Scholar]
- Kumar MS, Xiao Y, Venema S, Hooper G. Reproductive cycle of the blue swimmer crab, Portunus pelagicus off Southern Australia. Journal of the Marine Biological Association of the United Kingdom. 2003;83(5):983–994. [Google Scholar]
- Lai JCY, Ng PKL, Davie PJF. A revision of the Portunus pelagicus (Linnaeus, 1758) species complex (Crustacea: Brachyura: Portunidae), with the recognition of four species. The Raffles Bulletin of Zoology. 2010;58(2):199–237. [Google Scholar]
- Linnane AJ, Penny SS, Ward TM. Contrasting fecundity, size at maturity and reproductive potential of southern rock lobster Jasus edwardsii in two South Australian fishing regions. Journal of the Marine Biological Association of the United Kingdom. 2008;88(3):583–589. doi: 10.1017/S0025315408001021. [DOI] [Google Scholar]
- Litulo C. Fecundity of the pantropical fiddler crab Uca annulipes (H. Milne Edwards, 1837) (Brachyura: Ocypodidae) at Costa do Sol Mangrove, Maputo Bay, Southern Mozambique (Short communication) Western Indian Ocean Journal of Marine Sciences. 2004;3(1):87–91. [Google Scholar]
- Ministry of Marine Affairs and Fisheries (MMAF). Surat edaran Direktur Jenderal Pengolahan dan Pemasaran Hasil Perikanan, Kementerian Kelautan dan Perikanan, nomor: SE. 1185/P2HP/HK.155/IV/2014 tentang persyaratan ukuran minimum ekspor rajungan (blue swimming crab – Portunus pelagicus) 10 centimeter, 30 April 2014. Jakarta: MMAF; 2014. [Google Scholar]
- Muino R. Fecundity of Liocarcinus depurator (Brachyura: Portunidae) in the Ría de Arousa (Galicia, north-west Spain) Journal of the Marine Biological Association of the United Kongdom. 2002;82(1):109–122. doi: 10.1017/S0025315402005222. [DOI] [Google Scholar]
- Ng PKL. Crabs. In: The living marine resources of the Western Central Pacific, vol. 2: Cephalopods, Crustaceans, Holothurians and sharks. In: Carpenter KE, Niem VA, editors. FAO species identification guide for fishery purposes. Rome: Food and Agriculture Organization (FAO); 1998. pp. 1045–1146. [Google Scholar]
- Pinheiro MAA, Baveloni MD, Terceiro OSL. Fecundity of the mangrove crab Ucides cordatus (Linnaeus, 1763) (Brachyura, Ocypodidae) Invertebrate Reproduction and Development. 2003;43(1):19. doi: 26.0168-8170103/$05.00. [Google Scholar]
- Pinheiro MAA, Fransozo A. Reproduction of the speckled swimming crab Arenaeus cribrarius (Brachyura: Portunidae) on the Brazilian Coast near 23°30′S. Journal of Crustacean Biology. 2002;22(2):416–428. [Google Scholar]
- Pinheiro MAA, Terceiro OSL. Fecundity and reproductive output of the speckled swimming crab Arenaeus cribrarius (Lamarck, 1818) (Brachyura, Portunidae) Crustaceana. 2000;73(9):1121–1137. [Google Scholar]
- Potter IC, de Lestang S, Melville-Smith R. The collection of biological data required for management of the blue swimmer crab fishery in the central and lower west coasts of Australia. Fisheries Research and Development Corporation Report. Perth: Centre for Fish and Fisheries Research, Murdoch University; 2001. FRDC project no. 97/137. [Google Scholar]
- Prager MH, McConaugha JR, Jones CM, Geer PJ. Fecundity of blue crab, Callinectes sapidus, in Chesapeake Bay: Biological, statistical and management considerations. Bulletin of Marine Science. 1990;46(1):170–179. [Google Scholar]
- Przemysław C, Marcello DG. Realized fecundity in the first brood and size of eggs of Chinese mitten crab (Eriocheir sinensis)-laboratory studies. International Research Journal of Biological Science. 2013;2(1):1–6. [Google Scholar]
- Rameshbabu KV, Benakappa S, Chandra-Mohan K, Ramachndra-Naik AT. Breeding biology of Charybdis (Charybdis) feriatus (Linnaeus) from Mangalore. Indian Journal of Fisheries. 2006;53(2):181–184. [Google Scholar]
- Ramirez-Llodra E. Fecundity and life-history strategies in marine invertebrates. Advances in Marine Biology. 2002;43:87–170. doi: 10.1016/S0065-2881(02)43004-0. [DOI] [PubMed] [Google Scholar]
- Rasheed S, Mustaquim J. Size at sexual maturity, breeding season and fecundity of three-spot swimming crab Portunus sanguinolentus (Herbst, 1783) (Decapoda, Brachyura, Portunidae) occurring in the coastal waters of Karachi, Pakistan. Fisheries Research. 2010;103(1–3):56–62. doi: 10.1016/j.fishres.2010.02.002. [DOI] [Google Scholar]
- Ravi R, Manisseri MK, Kuriakose S. Relationship between morphometric characteristics and fecundity of Portunus pelagicus (Linnaeus, 1758) Journal of the Marine Biological Association of India. 2008;50(2):217–220. [Google Scholar]
- Rodrigues MA, Heberle MF, D'incao F. Fecundity variation and abundance of female blue crabs Callinectes sapidus Rathbun, 1896 (Decapoda, Brachyura, Portunidae) in the Patos Lagoon Estuary, RS, Brazil. Atlântica, Rio Grande. 2011;33(2):141–148. doi: 10.5088/atl.2011.33.2.141. [DOI] [Google Scholar]
- Safaie M, Pazooki J, Kiabi B, Shokri MR. Reproductive biology of blue swimming crab, Portunus segnis (Forskal, 1775) in coastal waters of Persian Gulf and Oman Sea, Iran. Iranian Journal of Fisheries Sciences. 2013;12(2):430–444. [Google Scholar]
- Santos S, Negreiros-Fransozo ML. Reproductive cycle of the swimming crab Portunus spinimanus Latreille (Crustacea, Oecapoda, Brachyura) from Ubatuba, Sao Paulo, Brazil. Revista Brasileira de Zoologia. 1999;16(4):1183–1193. [Google Scholar]
- Shields JD, Wood FEI. Impact of parasites on the reproduction and fecundity of the blue sand crab P. pelagicus from Moreton Bay, Australia. Marine Ecology Progress Series. 1993;92:159–170. [Google Scholar]
- Soundarapandian P, Varadharajan D, Boopathi A. Reproductive biology of the commercially important portunid crab, Portunus sanguinolentus (Herbst). Journal of Marine Science: Research & Development. 2013;3(2):1–9. doi: 10.4172/2155-9910.1000124. [DOI] [Google Scholar]
- Sukumaran KK, Neelakantan B. Spawning biology of two marine portunid crabs, Portunus (Portunus) sanguinolentus (Herbst) and Portunus (Portunus) pelagicus (Linnaeus) from the Karnataka Coast. The Fourth Indian Fisheries Forum Proceeding; Kochi, India. 24–28 November 1996; Kochi, India: The Fourth Indian Fisheries Forum Proceeding; 1999. pp. 35–38. [Google Scholar]
- Sukumaran KK, Neelakantan B. Sex ratio, fecundity, reproductive potential of two marine portunid crabs, Portunus (Portunus) sanguinolentus (Herbst) and Portunus (Portunus) pelagicus (Linnaeus), along the Karnataka coast. Indian Journal of Marine Science. 1997;26(1):43–48. [Google Scholar]
- Sunarto 2012Bio-ecological characteristic of the blue swimming crab (Portunus pelagicus) in Brebes marine waters, Central JavaPhD diss., Bogor Agricultural University [Google Scholar]
- Tallack SML. Size–fecundity relationships for Cancer pagurus and Necora puber in the Shetland Islands, Scotland: How is reproductive capacity facilitated? Journal of Marine Biological Association of United Kingdom. 2007;87(2):507–515. doi: 10.1017/S0025315407054100. [DOI] [Google Scholar]
- Wardiatno Y, Zairion . Study on bioecology of the blue swimming crab and bioeconomic performance of crab fishery in order to propose of spawning ground protection. Bogor, Indonesia: The Indonesia Crab Processing Association (APRI) and Department of Aquatic Resources Management, Faculty of Fisheries and Marine Sciences, Bogor Agricultural University (ARM-FFMS-IPB); 2011. [Google Scholar]