Abstract
Tumor cells must overcome apoptosis to survive throughout metastatic dissemination and distal organ colonization. Here we show in the Polyoma Middle T mammary tumor model that N-cadherin expression causes Slug upregulation, which in turn promotes carcinoma cell survival. Slug was dramatically upregulated in metastases relative to primary tumors. Consistent with a role in metastasis, Slug knockdown in carcinoma cells suppressed lung colonization by decreasing cell survival at metastatic sites, but had no effect on tumor cell invasion or extravasation. In support of this idea, Slug inhibition by shRNA, sensitized tumor cells to apoptosis by DNA damage, resulting in caspase-3 and PARP cleavage. The pro-survival effect of Slug was found to be caused by direct repression of the pro-apoptotic gene, Puma, by Slug. Consistent with a pivotal role for a Slug-Puma axis in metastasis, inhibition of Puma by RNA interference in Slug-knockdown cells rescued lung colonization, whereas Puma overexpression in control tumor cells suppressed lung metastasis. The survival function of the Slug-Puma axis was confirmed in human breast cancer cells, where Slug knockdown increased Puma expression and inhibited lung colonization. This study demonstrates a pivotal role for Slug in carcinoma cell survival, implying that disruption of the Slug-Puma axis may impinge on the survival of metastatic cells.
Keywords: N-cadherin, FGFR, Slug, Puma, apoptosis, metastasis
Introduction
Metastasis is a well-orchestrated cascade of events involving the detachment and exit of carcinoma cells from the primary tumor, survival in the circulation, and dissemination to distant organs to build metastatic residence (1). Circulating tumor cells must overcome apoptotic stresses caused by anoikis, mechanical shearing in the bloodstream, or collisions with circulating host cells (2). Distal organ colonization is a late but rate-limiting step, which in the lungs involves sieving of tumor cells through the capillary bed, formation of intravascular emboli, extravasation, seeding, growth, and survival in the lung parenchyma (1). Although tumor cells are able to rapidly arrest in the lung capillaries, most are cleared by apoptosis, leaving less than one percent to undergo extravasation, seeding and growth in the lungs (1, 3), underscoring the fact that metastasis is a vastly inefficient process. To overcome apoptosis, disseminated tumor cells must turn on genes to ensure survival in the circulation or in distant organs, while maintaining migratory or invasive capability. Carcinoma cells undergo epithelial to mesenchymal transition (EMT), hence facilitating invasion away from the primary tumor, entry into the vasculature and breaching of basement membranes at distant organs (4-7). The EMT process is mediated by EMT-inducing transcription factors, involving Slug as a prominent player (4-7). Slug (SNAI2), is a zinc finger transcriptional repressor of the Slug/Snail family that promotes carcinoma cell invasion, stemness, and survival (8-10). Clinical evidence supports a role of Slug in advanced breast malignancies; high Slug levels are associated with poor prognosis, recurrence, and metastasis (11-13). Slug/Snail are known to inhibit the transcription of E-cadherin, cytokeratins, claudins, and plakoglobin, among others to attenuate the epithelial phenotype and promote malignancy (14-17). However, clinical data showed that high levels of Slug and E-cadherin were found in invasive duct carcinomas, suggesting that Slug expression does not necessarily preclude E-cadherin expression (13).
It is thought that disseminated cancer cells may revert from EMT to MET at sites of metastasis to establish growth and survival of new colonies (18). In support of this notion, Slug was found to promote cell survival, and not EMT, during kidney tubulogenesis, as shown by persistent E-cadherin expression (19). Similarly, in hematopoietic progenitor cells, where EMT is not involved, Slug was found to promote survival by blocking apoptosis in response to DNA damage (20, 21). Namely, Slug knockout mice succumbed to γ-irradiation, due to apoptosis of hematopoietic progenitor cells, thus impairing regenerative potential. In this case, Slug induced apoptosis by repressing the p53 pro-apoptotic target gene, Puma (21). Puma (BBC3), or p53-upregulated modulator of apoptosis, is a BH3-only member of the Bcl-2 family and a target of p53-mediated apoptosis (22, 23). It activates an apoptotic cascade by facilitating Bax activation, causing cytochrome C release from the mitochondria, caspase-3 activation and DNA fragmentation (24, 25).
Here we show that Slug is dramatically upregulated during metastasis in the PyMT-N-cadherin mouse which exhibits enhanced lung metastasis as compared to the PyMT mouse (26). Slug expression was increased in PyMT-N-cadherin mammary tumors and metastases relative to PyMT controls, and was further increased in distal metastases relative to primary tumors. Slug knockdown in metastatic tumor cells did not inhibit invasion, arrest or extravasation in the lungs, but greatly reduced colonization. Consistent with FGFR potentiation by N-cadherin (27), inhibition of FGFR, suppressed Slug expression and stimulated apoptosis. Moreover, Slug knockdown sensitized cells to apoptosis, effects that were reversed by Slug re-expression. Consistent with inhibition of Puma by Slug, Slug knockdown in PyMT-N-cad cells caused increased Puma and Bax expression, whereas silencing Puma in Slug-knockdown cells inhibited apoptosis and rescued lung colonization. Conversely, overexpression of Puma in PyMT-N-cad cells suppressed metastasis. The pro-survival function of Slug-Puma was also confirmed in human breast cancer cells. Thus, our study demonstrates that Slug-Puma promotes tumor cell survival leading to distal organ colonization.
Materials and Methods
Animals
FVB female mice and athymic nude mice were obtained from Taconic (Hudson, NY). Animal protocols of this study were approved by Institute for Animal Studies at Albert Einstein College of Medicine.
Cell lines
The PyMT and PyMT-N-cadherin mammary tumor cell lines were generated and characterized in 2007 as described (26). Briefly, primary mammary and metastatic tumor cell lines were derived from PyMT and PyMT-N-cad tumors or lung foci at 7 weeks post tumor onset by collagenase digestion, and plated in culture till they underwent crisis as detailed in (26, 28). The MDA-MB-231 metastatic subline 3475 was obtained in 2009 from Dr. Joan Massague (Memorial Sloan-Kettering Cancer Center, NY) and was tested for lung colonizing activity. The BT549 cell line was obtained in 2012 from the American Type Culture Collection and was characterized as triple negative by the lack of ER, PR and HER2 expression. All cell lines were tested and found negative for mycoplasma.
Antibodies and reagents
The antibodies used are against N-cadherin, E-cadherin, plakoglobin (BD Biosciences; San Jose, California); fibronectin, cytokeratin 18, β-actin (Sigma; St. Louis, MO); Slug, vimentin, p-ERK, p-Akt, p-p53, Akt, Bcl-2, Bcl-xL, Bax, Bim, Puma, cleaved caspase-3 and PARP (Cell Signaling; Danvers, MA); Erk, Bax, Noxa and FGFR1 (Santa Cruz; Santa Cruz, CA). Drugs used are PD173074 and PD0325901 (Pfizer; Groton, CT), Iressa or ZD1839 (AstraZeneca; Wilmington, DE), MK2206 (Tocris; Bristol, United Kingdom).
Immunoblotting Analysis
Cells were solubilized with RIPA lysis buffer, resolved by SDS-PAGE, and transferred to PVDF membrane. Blots were probed with indicated antibodies and developed by Pierce chemiluminescence substrate (Thermo scientific, Rockford, IL).
Slug, Puma, and N-cadherin shRNA and expression constructs
Two mouse Slug shRNA clones TRCN0000096227 (mature antisense: TTTACATCAGAGTGGGTCTGC), or TRCN0000096228 (mature antisense: TTGGTATGACAGGTATAGGGT) and non-silencing control shRNA in the pLKO.1 lentiviral vector (Open Biosystems; Huntsville, AL, USA) were used to knock down Slug. To generate viruses, lentiviral vectors were transfected into 293T cells with Tat, Rev, Gag/Pol and VSV-G vectors. Two mouse Puma shRNA clones, V3LHS_342433 (Sense sequence: CGGATGGCGGACGACCTCA) and V3LHS_342436 (Sense: AGTACGAGCGGCGGAGACA). Two human Slug shRNA clones and a non-silencing control shRNA in pLKO.1 lentiviral vectors were from Dr. Guo (AECOM). On-TARTGET plus mouse Puma siRNA (J-050032-08) and non-targeting siRNA (D-001810-01-05) were from Dharmacon (Chicago, IL). Mouse N-cad siRNA (sc-35999) and control siRNA were obtained from Santa Cruz (Santa Cruz, CA). Mouse Slug cDNA was amplified by PCR and subcloned into a pLXSN retroviral vector (Clontech, Palo Alto, CA). For expression of Puma protein into control-sh/PyMT-N-cad cells, mouse Puma cDNA (Clone ID: 5133742) was from Thermo Scientific (Rockford, IL).
TaqMan qRT-PCR
Total RNA was isolated using RNeasy Mini Kit and RNase-free DNase set (Qiagen, Valencia, CA). Real-time RT-PCR was carried out using TaqMan RNA-to-Ct 1-Step Kit (Applied Biosystems, Carlsbad, CA) and gene-specific TaqMan probes (Applied Biosystems, Carlsbad, CA) in StepOnePlus Real-time PCR system. Gapdh mRNA was used for loading normalization and a specified reference control was used for analyzing relative mRNA expression. Comparative CT (ΔΔCT) real-time analysis was performed using StepOne Software. The following are Gene Expression Assay IDs (Applied Biosystems, Carlsbad, CA) for each gene probe. Gapdh (Mm99999915_g1), Snail (Mm00441533_g1), Slug (Mm00441531_m1), Twist1 (Mm00442036_m1), Zeb1 (Mm00495564_m1), Sip1 (Mm00497193_m1), Foxc2 (Mm00546194_s1), Gsc (Mm00650681_g1), Vimentin (Mm00449208_m1), PyMT (Mm04214513_u1), Mmp2 (Mm00439498_m1), Mmp9 (Mm00442991_m1), Mmp14 (Mm00485054_m1), Puma (Mm00519268_m1).
Chromatin immunoprecipitation
Cells were fixed with 1% paraformaldehyde in PBS for 10 min, and pelleted nuclei were lysed in SDS lysis buffer supplemented with protease inhibitor cocktail (Roche, Indianapolis, IN) and 2 mM PMSF. The chromatin was sheared by sonication using a Branson 250 sonifier with a 2 mm microtip to generate 300~1000 bp fragments. Equal amounts of solubilized chromatin samples were incubated with either 1.5 μg of Rabbit anti-Slug antibody or rabbit IgG for 24 min in an ultrasonic bath at 4°C. Antigen-antibody complexes were collected with protein G conjugated Dynabeads (Invitrogen, Carlsbad, CA) at 4°C for 45 min. The beads were extensively washed in low and high salt washing buffers and the immunoprecipitated DNA samples were eluted and de-crosslinked by boiling the Dynabeads for 15 minutes in presence of Chelex-100 resin (Bio-Rad, Hercules, CA). Purified DNA samples were subjected to quantitative PCR using SYBR Green in a StepOnePlus Real Time PCR system to specifically amplify Slug binding region in Intron 1 of the Puma gene and also a negative control region with no Slug binding sequences located 9.4 kbs downstream from the Puma gene on chromosome 7. The primer sets used for the qPCR assays were: Puma Intron 1; Sense 5’-ACTAAGGCTGGGCCAGGCGG and Antisense 5’-GCGAGCCCGAACCCCATTGT, Negative control region: Sense 5’-AGCCACTGTTTCAAGAGCTTGAGTT and Antisense 5’- GCTCCAGGTCCCTTTCCAGCC. Specific enrichment was calculated and expressed as the percent recovery of input. Data were shown as Mean ± SEM. Unpaired t-test was used to determine P<0.05.
Migration/invasion
Cell migration and invasion assays were performed in a Boyden chamber using 8 μm Transwell filters as described (26).
Extravasation
Trans-endothelial migration was performed as described (29). Fluorescently labelled tumor cells were cultured on top of a 3B-11 endothelial monolayer on transwell filters. Bone marrow macrophages were plated on the reverse side of the transwell. After 36 hrs, transmigrated cells were counted and shown as Mean ± SEM (N=12); Unpaired t-test, P < 0.05.
Lung colonization
Half million cells were injected via tail vein into 6 weeks old female FVB mice, and mice were incubated for 24, 48, 96 hours, and 2 weeks or else as indicated. Lung metastases were quantified as described (26). Briefly, formalin-fixed/paraffin-embedded lungs were cut at 5 μm thickness in five sets with 50 μm intervals between each set. Sections were hematoxylin and eosin (H&E), stained and lung foci were counted. To visualize small cluster of cancer cells in the lung tissue, sections were further immunostained with N-cadherin cytosolic tail antibody or pancytokeratin antibody. To quantify arrest of tumor cells in the lungs post intravenous injection, we applied qPCR analysis as described (30). Briefly, 1 × 105 PyMT-N-cad cells expressing either non-silencing shRNA or slug-shRNA were injected via tail vein of 6 weeks old female mice. At 5 min post injection, lungs were harvested, minced, and digested with proteinase K overnight. Total DNA were isolated using DNeasy Kit (Qiagen, Valencia, CA). PyMT transgene from each DNA was quantified using TaqMan qPCR reagent in in StepOnePlus Real-time PCR system.
TUNEL and immunostaining
TUNEL assay was performed using the In Situ Cell Death Detection Kit (Roche, Indianapolis, IN) followed by co-immunostaining with N-cadherin antibody (26).
Gelatin zymography was performed as described (27).
Results
N-cadherin causes Slug upregulation in the PyMT model of mammary tumorigenesis
N-cadherin (N-cad) upregulation in breast cancer cells is associated with increased tumor invasion and metastasis, a function that is dependent on FGFR potentiation by N-cadherin (31-33). To study the effect of N-cadherin on metastasis, we generated a bitransgenic mouse model, which expresses N-cadherin and PyMT in the mammary epithelium (PyMT-N-cad). The PyMT-N-cad mouse exhibited enhanced lung metastasis as compared to the PyMT mouse (26). To determine whether N-cad influences epithelial to mesenchymal transition (EMT), we compared the mRNA levels of Twist, Snail, and Slug in two cell lines each derived from PyMT-N-cad or PyMT mammary tumors. We found a 3 and 13.7 fold increase in Slug mRNA in both PyMT-N-cad cell lines relative to PyMT control lines (Fig. 1A). Snail mRNA was increased by 5 fold in one of the PyMT-N-cad cell lines, whereas Twist mRNA was unchanged (Fig. 1A). We also compared Slug expression in cell lines derived from pulmonary metastases to those from primary tumors to determine if Slug is upregulated in metastases. Interestingly, Slug mRNA was increased by 7.6, and 61 fold in lung metastatic cell lines as compared to one of the PyMT-N-cad primary tumor cell lines (Fig. 1B). By contrast, Twist, Snail, or vimentin mRNAs were unchanged (Fig. 1B). The apparent variation in the extent of Slug mRNA upregulation in the individual PyMT-N-cad cell lines may reflect tumor cell heterogeneity that is inherent to breast tumors (34). We further tested for potential changes in other EMT factors including Zeb1, Sip1, FOXC2 and Goosecoid in PyMT-N-cad cells (12). Except for FOXC2 mRNA, which was increased by 5 fold in PyMT-N-cad metastatic cells, all other factors were unchanged (Fig. 1C). Slug was localized to the nucleus, and N-cad to cell-cell junctions in PyMT-N-cad cells (Fig. 1D), while E-cadherin remained expressed in these cells (Fig. 1D).
Fig. 1. Slug is upregulated in the PyMT-N-cadherin mammary tumor model.
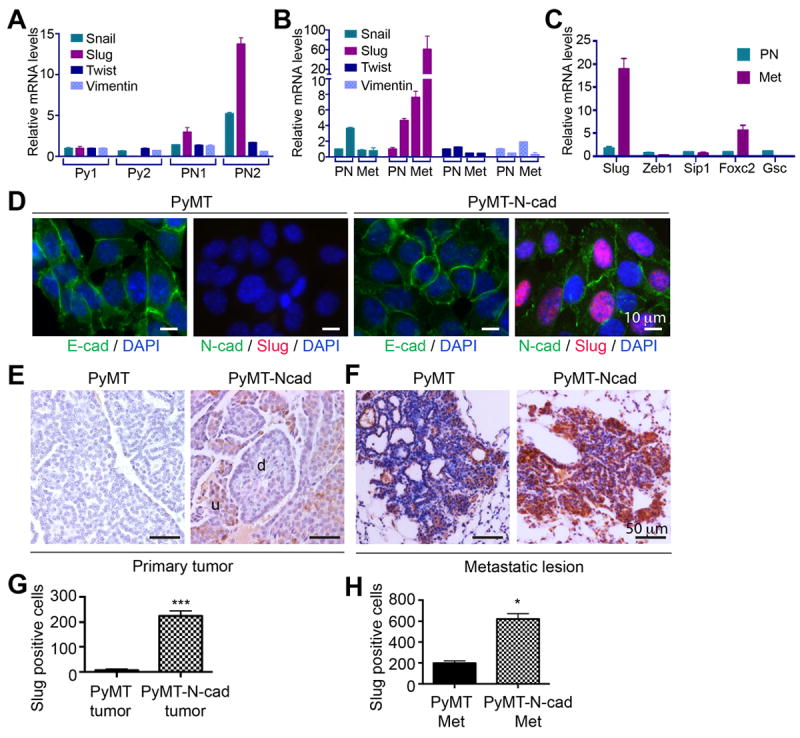
(A) mRNA levels for Snail, Slug, Twist, and vimentin in two primary mammary tumor cell lines from PyMT (Py1 and Py2) and PyMT-N-cad (PN1 and PN2) mice were analyzed by TaqMan qRT-PCR. One of the PyMT cancer cell lines (Py1) was used as a reference to compare relative mRNA expression. (B) Snail, Slug, Twist, and vimentin mRNA levels in primary (PN) and metastatic (Met) PyMT-N-cad cell lines were analyzed by TaqMan qRT-PCR. One of the primary PN cell lines was used as a reference. (C) Relative mRNA of Slug, Zeb1, Sip1, Foxc2, and Goosecoid (Gsc) in the PyMT-N-cad metastatic cell line (Met) was compared to mRNA levels in the PyMT-N-cad primary tumor cell line (PN) by qRT-PCR. Error bars for A-C show Mean ± SEM (N=3). (D) E-cad, N-cad, Slug, and nuclei (DAPI) were visualized by immunostaining of PyMT or PyMT-N-cad primary tumor cell lines. (E and F) Images of Slug IHC in PyMT and PyMT-N-cad primary tumors (E) and matching metastases (F) are shown (d; differentiated, u; undifferentiated tumor area). (G) Slug staining in primary PyMT and PyMT-N-cad tumors (Fig. 1E) was quantified in 5 tumors. Data are Mean ± SEM, ***P=0.0003. (H) Slug staining in PyMT and PyMT-N-cad metastatic lesions (Fig. 1F) in 5 lungs is shown as Mean ± SEM (N=5). Unpaired t-test, *P=0.0167.
We compared Slug expression in primary tumors and lung metastases from PyMT-N-cad and PyMT mice by immunohistochemistry. Slug positive cells were found in undifferentiated tumor areas and were more abundant in PyMT-N-cad than in PyMT tumors (Fig. 1E and G). Moreover, Slug expression was greater in metastases relative to primary tumors from both genotypes (Fig. 1 E-F), and was further exacerbated in PyMT-N-cad relative to PyMT metastases (Fig. 1 F and H).
Slug knockdown has no effect on epithelial markers or cell migration and invasion
We next sought to determine whether Slug affects EMT or invasion. We knocked-down Slug in PyMT-N-cad metastatic cells using two lentiviral mRNA targeting sequences and a non-targeting sequence as control. In knockdown cell lines, Slug protein and mRNA expression was inhibited, as shown by immunostaining, immunoblotting (Fig. 2A and 2C), and qRT-PCR (Fig. 2B). Slug knockdown did not increase epithelial E-cadherin, cytokeratin-18 or plakoglobin expression, or diminished mesenchymal N-cadherin or fibronectin expression (Fig. 2C). The expression of FGFR or EGFR was unaffected by Slug (Fig. 2C). These data were confirmed in PyMT-N-cad tumors in vivo in five individual mice (Supplementary Fig. S1). Staining for pan-cytokeratin or E-cadherin showed similar expression in Slug-knockdown and control tumors, whereas vimentin was only present in stromal cells (Fig. 2D). Furthermore, Slug knockdown did not inhibit cell migration or invasion (Supplementary Fig. S2 A-B), and while it increased MMP-2 mRNA, it did not increase the level of active/cleaved MMP-2, as shown by zymography (Supplementary Fig. S2 C-D).
Fig. 2. Slug knockdown suppresses lung colonization.
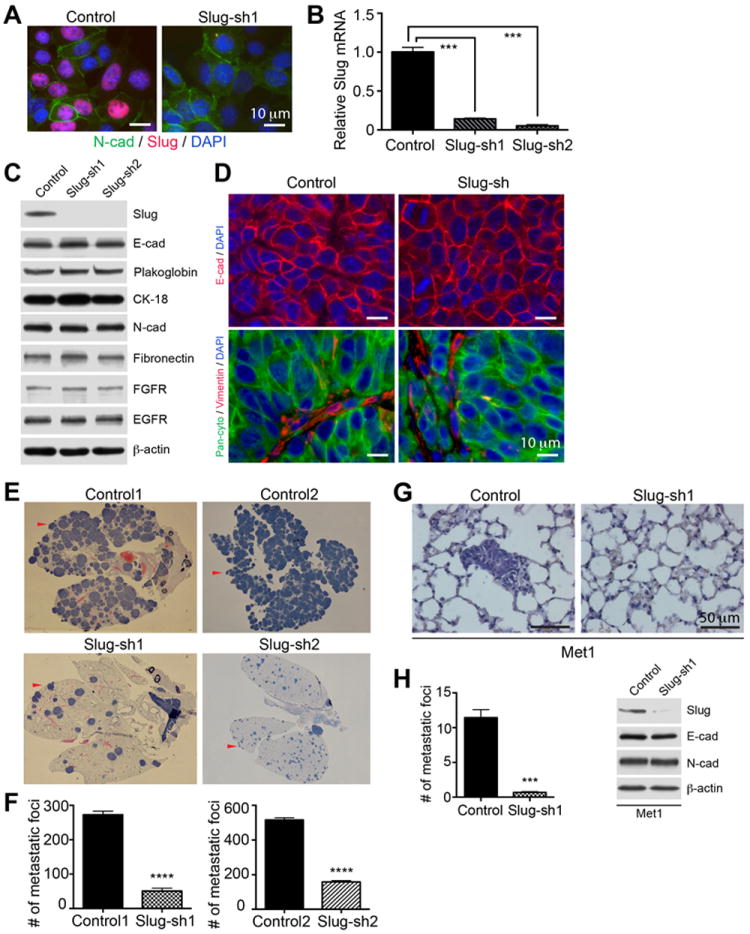
(A) metastatic PyMT-N-cad cells expressing control or Slug shRNA (sh1) were co-immunostained with anti-Slug, N-cad, and DAPI. (B) Slug mRNA levels in Slug-sh1 and sh2 cells relative to control shRNA cells. GAPDH mRNA was used as loading normalization and control shRNA cells as reference. Data are shown as Mean ± SEM (N=3). Unpaired t-test, ***P<0.001. (C) Immunoblotting of Slug-sh1, Slug-sh2 and control shRNA PyMT-N-cad cells for indicated proteins. (D) Mammary tumor sections from control and Slug-shRNA/PyMT-N-cad tumors were immunostained with anti-E-cad and DAPI. Sections were co-immunostained with pan-cytokeratin and vimentin. (E) Lung colonization of control vs Slug-sh1 and Slug-sh2 cells was determined at 2 weeks post tail-vein injection. H&E stained whole lung scans show metastatic foci and (F) shows quantification of foci as Mean ± SEM (N=5). Unpaired t-test, ****P<0.0001. (G) Lung colonization of control or Slug-sh1/Met-1 cells at 2 weeks post intravenous injection and (H) number of foci displayed as Mean ± SEM (N=5). Unpaired t-test, ***P<0.001. Control and Slug-sh1 expressing Met-1 cell lysates were immunoblotted for Slug, E-cad, N-cad, or β-actin.
Slug knockdown suppresses metastatic colonization
To examine the role of Slug in metastasis, we used tail vein injection of carcinoma cells into syngeneic female mice, which measures lung colonization, a late but rate-limiting step in metastasis (1). Distal organ colonization is thought to measure tumor cell extravasation and survival, two hallmarks of metastatic potency (1). To test the effect of Slug on lung colonization, PyMT-N-cad cells expressing a non-targeting sequence (control), or two Slug shRNA sequences (Slug-sh) were injected into the tail vein of syngeneic female mice, and the number of metastatic foci was determined in the lungs, two weeks post inoculation. We found a dramatic inhibition (70%) of lung colonization as a result of Slug knockdown in two PyMT-N-cad cell lines (Fig. 2 E-F). Similarly, Slug inhibition in the PyMT lung metastatic cell line, Met-1 (35), which expresses Slug and N-cad, also suppressed colonization (Fig. 2 G-H), without however increasing the levels of E-cad (Fig. 2H).
Slug knockdown disrupts metastatic seeding at an early phase of lung colonization
These data support a critical role for Slug in metastatic colonization. However, it was unclear which stage of tumor cell dissemination was regulated by Slug. We sought to determine whether Slug protected tumor cells from apoptosis before or after extravasation in the lungs. One possibility is that Slug-knockdown cells arrived to the lungs, extravasated, and then underwent apoptosis due to low Slug levels. Alternatively, cells might have died in the circulation prior to reaching the lungs. Arrest in the lungs is a rapid process that occurs within 5 minutes following intravenous injection (29, 30). However, the majority of arrested cells (99%) usually undergo apoptosis within 24 hours, leaving less than 1% to extravasate, seed and grow in the lungs (1, 3, 29, 30). To determine the effect of Slug on lung colonization, we measured the onset of metastatic foci in the lungs at 24, 48 and 96 hrs post tail vein injection of 5×105 PyMT-N-cad cells into syngeneic female mice. We followed tumor cell seeding in the lungs by tracking carcinoma cells with pan-cytokeratin (Fig. 3A) or N-cad immunostaining (Fig. 3C-D). About 25 colonies of 2-6 cell clusters of control PyMT-N-cad cells were observed in the lungs 48 hrs post injection, an effect which was sharply reduced in mice injected with Slug-knockdown cells (Fig. 3B). At 96 hrs, there were 4 times more foci in control lungs as compared to Slug-sh lungs (Fig. 3B). No carcinoma cells were detected in the lungs 24 hrs post injection, likely due to apoptosis and/or to that single cells are not easily detected by immunostaining (Fig. 3C) (3, 29, 30). To determine the effect of Slug knockdown on tumor cell apoptosis in vivo, we co-stained lungs for N-cad and TUNEL at 24, 48, 96 hrs and 2 weeks post injection (Fig. 3C-D). We were unable to detect TUNEL activity in the lungs at these time points, suggesting that apoptotic cells are rapidly cleared from the lungs (Fig. 3C). However, staining of metastases at two weeks, showed that foci derived from Slug-knockdown cells contained a higher proportion of TUNEL positive cells than control foci, suggesting a higher rate of apoptosis caused by Slug inhibition (Fig. 3D and 3E).
Fig. 3. Slug knockdown inhibits tumor cell survival following arrest in the lungs.
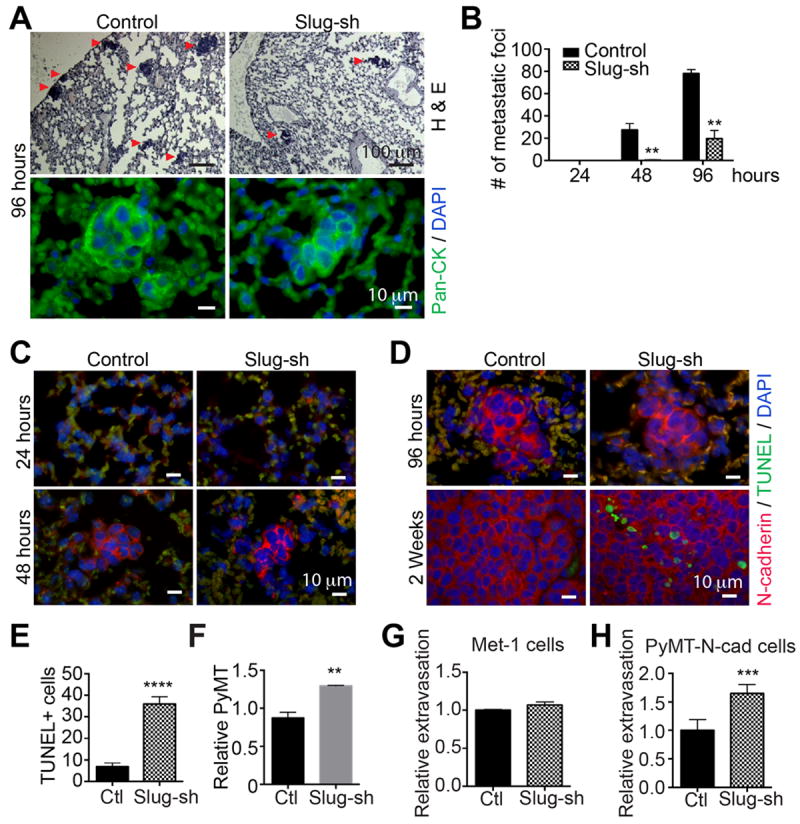
(A) Lung colonization of control or Slug-sh expressing PyMT-N-cad cells was determined at 24, 48, and 96 hours post tail vein injection. Images of H&E (top) or pan-cytokeratin (bottom) stained control or Slug-sh lungs at 96 hours are shown. (B) The number of foci in 5 animals at 24, 48 and 96 hours is shown as Mean ± SEM (N=5). Unpaired t-test, **P=0096 (48 hours), **P=0.002 (96 hours). (C and D) TUNEL and N-cad staining of PyMT-N-cad control vs Slug-sh cells in the lungs at (C) 24 and 48 hours, (D) 96 hours and two weeks post injection is shown. (E) TUNEL positive cells at 2 weeks post injection are shown as Mean ± SEM (N=5). Unpaired t-test, ****P<0.0001. (F) PyMT-N-cad control or Slug-sh1 cells were injected via tail vein (N=3); 5 minutes later, lung DNA was extracted and analyzed by qPCR using PyMT primers. The relative increase in PyMT DNA in Slug-sh1 compared to control-sh is shown as Mean ± SEM (N=3); **P<0.0049. (G and H) Fluorescently-labeled control or Slug-shRNA expressing Met-1 and PyMT-N-cad cells, were cultured onto an endothelial monolayer in the transwell. After 36 hours, extravasation of Slug-sh cells compared to control shRNA cells is shown as Mean ± SEM (N=12). Unpaired t-test, ***P<0.001.
To determine if Slug affects survival of tumor cells in the blood circulation prior to arrest in the lungs, we tracked PyMT-N-cad cells in the lungs 5 min post tail-vein injection using PyMT qPCR (30). We found that Slug-shRNA did not attenuate survival of PyMT-N-cad cells in the circulation, since the real-time PCR showed a slight increase (1.5 fold) in the number of Slug- knockdown cells arrested in the lungs compared to controls (Fig. 3F).
To rule out a potential defect in tumor cell extravasation in the lungs caused by Slug depletion, we performed a trans-endothelial cell migration assay. This assay measures tumor cell transmigration of an endothelial cell layer in response to cues from macrophages in the bottom chamber, which release VEGF that stimulate endothelial cell permeability, mimicking an in vivo setting in the lungs (29). Using Met-1 and PyMT-N-cad cells, we found in Met-1, no difference in transmigration between control and Slug-knockdown cells (Fig. 3G), whereas PyMT-N-cad/Slug-sh cells exhibited a 1.7 fold increase in extravasation relative to control cells (Fig. 3H), which corresponded to the extent of arrest in the lungs (Fig. 3F). These data argue that Slug knockdown does not inhibit arrest or extravasation, but inhibits the survival of carcinoma cells in the lungs post extravasation.
The FGF receptor regulates Slug expression in PyMT-N-cad lung metastatic cells
FGFR1 amplification was found to be one out of a 66-gene signature that is strongly associated with reduced breast cancer patient survival duration (36). We showed that FGFR1 interaction with N-cadherin stabilizes the FGFR to promote invasiveness (27). We confirmed that PyMT-N-cad cells expressed higher levels of FGFR1 than PyMT cells (Supplementary Fig. S3A). We tested whether FGFR regulates Slug expression, using an FGFR inhibitor, PD173074 (FGFRi), or an EGFR inhibitor, Iressa (EGFRi) as control (26). Treatment of PyMT-N-cad cells with 0.5-2.0 μM FGFRi inhibited the expression of Slug protein and mRNA (Supplementary Fig. S3B-C), whereas EGFRi had no significant effect (Supplementary Fig. S3B and D). Since ERK and Akt phosphorylation were attenuated by FGFRi, we tested whether these pathways regulate Slug expression. Inhibition of ERK by the MEK1 inhibitor PD0325901 (MEKi) or AKT by MK2206 (AKTi) (Supplementary Fig. S3E), did not appreciably inhibit Slug expression. Therefore, FGFR, and not EGFR, regulates Slug, independently of ERK or AKT activation. Consistent with an effect of N-cad on FGFR leading to Slug upregulation, N-cad knockdown in PyMT-N-cad cells attenuated Slug expression, without however affecting E-cad levels (Supplementary Fig. S3F).
Slug inhibition primes tumor cells to apoptosis
Since lung colonization, and not extravasation, is regulated by Slug, which in turn is controlled by the FGFR, we tested whether Slug inhibition by shRNA, or by FGFR inhibition, affects cell survival. Treatment of PyMT-N-cad cells with FGFRi increased cleaved caspase-3 and PARP levels, especially at 1.0 μM, which was strongly inhibitory of Slug (Fig. 4A). Consistent with these effects, treatment of Slug-sh1 cells with FGFRi enhanced caspase-3 and PARP cleavage relative to control cells (Fig. 4A). Similar effects were obtained with Slug-sh2 cells (Supplementary Fig. S4A). In line with these data, Slug shRNA induced the expression of monomeric (23 kD) and dimeric (46 kD) form of the apoptotic effector, Bax (24, 25), which was further increased by FGFRi (Fig. 4A). By contrast, the levels of the p53 target gene Noxa, the non-p53 regulated Bim gene (Supplementary Fig. S4B), or the anti-apoptotic Bcl-2 and Bcl-xL proteins (37-39), were not affected by Slug shRNA (Fig. 4A). Thus, Slug protects PyMT-N-cad cells from apoptosis triggered by FGFR inhibition.
Fig. 4. Slug inhibition or knockdown leads to apoptotic cell death.
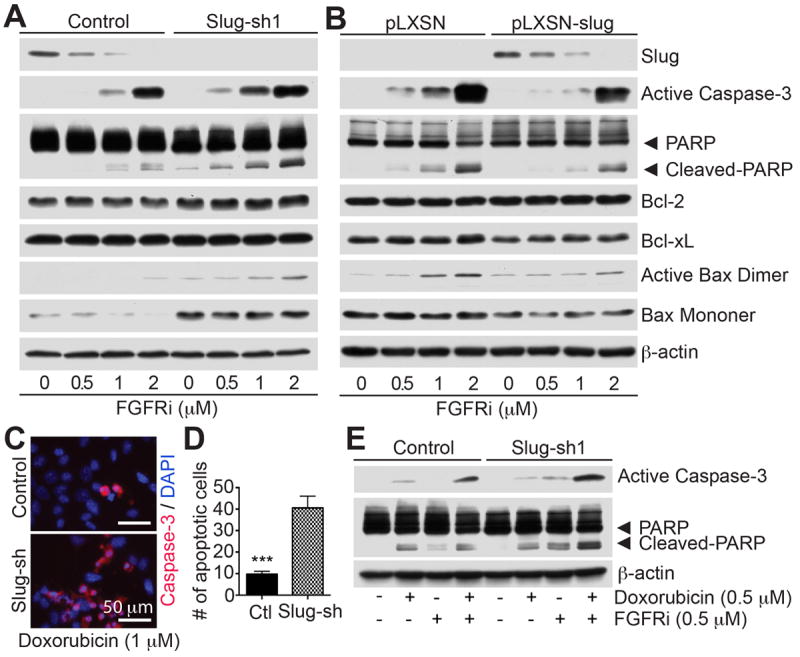
(A) Control or Slug-sh1 expressing PyMT-N-cad metastatic cells were treated with DMSO or PD173074 (FGFRi) at the indicated concentrations. Cell lysates were immunoblotted for the indicated proteins. (B) PyMT-N-cad cells expressing Slug-shRNA were further transduced with either empty vector (pLXSN) or mouse Slug (pLXSN-Slug) to rescue Slug expression. Cells were then treated with PD173074 and immunoblotted as in A. (C) Control and Slug-sh1 PyMT-N-cad cells were treated with DMSO or 1 μM doxorubicin for 16 hrs. Fixed cells were stained for active-caspase-3 and DAPI. (D) The number of caspase-3 positive cells is shown as Mean ± SEM (N=3). Unpaired t-test, ***P=0.0003. (E) Control or Slug-sh1 PyMT-N-cad cells were treated with DMSO, 0.5 μM doxorubicin or PD173074 (FGFRi), or both for 18 h. The levels of active-caspase-3 or cleaved-PARP were analyzed by immunoblotting.
To confirm the effect of Slug on cell survival, we re-expressed mouse Slug in Slug-knockdown cells using a retroviral vector (Fig. 4B). This resulted in rescuing the effect of FGFRi by reducing levels of cleaved caspase-3 or PARP and dimeric Bax (Fig. 4B), while having no effect on Bcl-2 or Bcl-xL expression (Fig. 4B).
To further validate the effect of Slug on resistance to apoptosis, we used doxorubicin, a drug used for treatment of breast cancer. Treatment of PyMT-N-cad cells with 1 μM doxorubicin, caused a 4-fold increase in active-caspase-3 levels in Slug-sh as compared to control PyMT-N-cad cells (Fig. 4C-D). Furthermore, a combination of doxorubicin and FGFRi enhanced the levels of apoptotic markers, especially in Slug-sh cells (Fig. 4E).
Slug promotes cell survival by repressing the cell death protein, Puma
Since Slug attenuation sensitized cells to apoptosis, we determined whether it affected the expression of the pro-apoptotic gene Puma, which was shown to be repressed by Slug (21). Treatment of PyMT-N-cad cells with FGFRi, which inhibits Slug expression, caused increased Puma protein and mRNA levels (Fig. 5A-B). In vivo, mammary tumors from Slug-sh cells also displayed higher Puma levels (Fig. 5C-D).
Fig. 5. Slug promotes tumor cell survival through Puma repression.
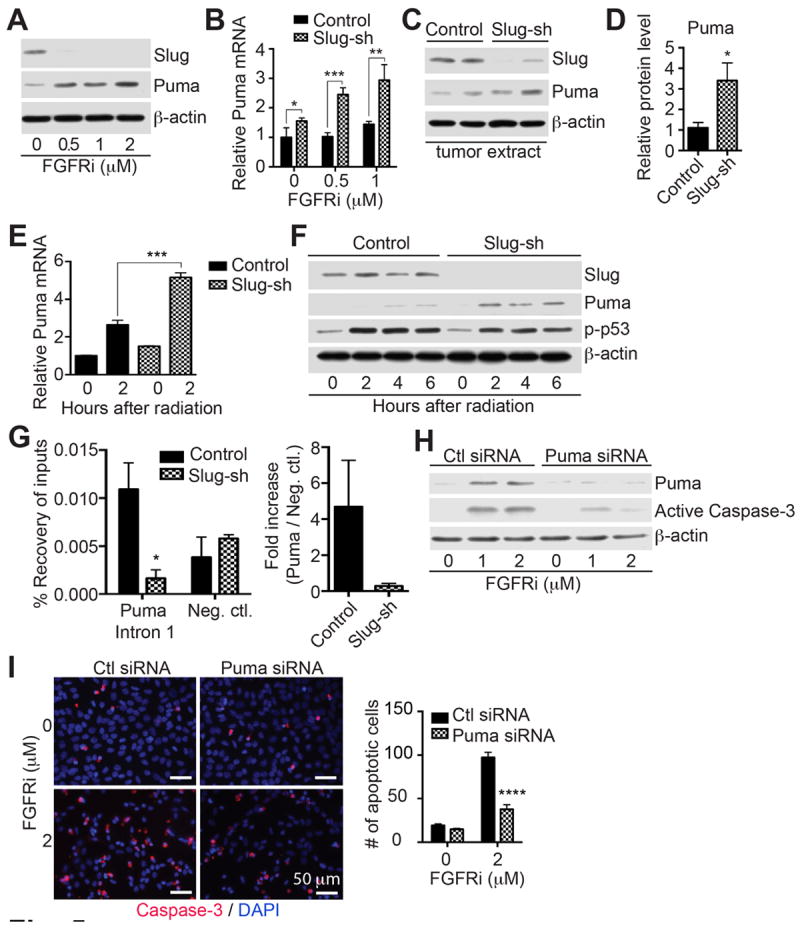
(A) PyMT-N-cad cells were treated with FGFRi and immunoblotted as indicated. (B) Control and Slug-sh PyMT-N-cad cells were treated with FGFRi, for 16 hrs, and relative Puma mRNA was quantified. Puma mRNA in untreated control shRNA cells was used as reference. Error bars show Mean ± SEM (N=3). Unpaired t-test, *P=0.0385, **P=0.0061, ***P=0.0006. (C) Slug, Puma, and β-actin were analyzed in Slug-sh mammary tumors by immunoblotting. (D) Relative Puma protein expression in four individual of control or Slug-knockdown PyMT-N-cad cells is shown as Mean ± SEM (N=4). Unpaired t-test, *P=0.0155. (E) Control or Slug-sh PyMT-N-cad tumor cells were γ-irradiated and Puma mRNA was quantified relative to Puma mRNA in un-irradiated control cells. Error bars show Mean ± SEM (N=3). Unpaired t-test, ***P=0.0002. (F) Cell lysates from indicated times post-irradiation were immunoblotted as shown. (G) Enrichment of Slug protein on Puma intron 1 or negative control region was analyzed by ChIP followed by real-time PCR in PyMT-N-cad metastatic tumor cells expressing control shRNA (Control) or -shRNA (Slug-sh). Error bars show Mean ± SEM (N=3). Unpaired t-test, *P=0.0327. Relative occupancy of Slug at the Puma intron 1 compared to the negative control region is shown as Mean ± SEM. (H) PyMT-N-cad/Slug-sh cells were transfected with either control siRNA or Puma siRNA and followed by FGFRi treatment for 16h. Cell lysates were immunoblotted as indicated. (I) PyMT-N-cad/Slug-sh cells were transfected with either control siRNA or Puma siRNA and followed by FGFRi addition for 16hrs. Cells were immunostained with anti-cleaved-caspase-3 and DAPI. The number of active-caspase-3 positive cells was quantified as Mean ± SEM (N=3). Unpaired t-test, ****P<0.0001.
Since Puma is a p53 response gene (21), we also examined whether Slug regulates Puma in response to DNA damage by γ-irradiation. Treatment of cells with 15 Gy γ-irradiation, stimulated Puma mRNA and protein expression in Slug-sh cells relative to PyMT-N-cad control cells (Fig. 5E-F). As control for induction of DNA damage, p53 phosphorylation was included (Fig. 5F).
Binding of Slug protein to the first intron of mouse Puma gene and subsequent transcriptional repression of Puma by this interaction was first elaborated in mouse hematopoietic progenitor cells (21). To verify the physical interaction of Slug to a known target regulatory region of the Puma gene, we performed Chromatin immunoprecipitation (ChIP) in PyMT-N-cad tumor cells with anti-Slug antibody or anti-rabbit IgG as control. Slug occupancy was significantly enriched at Puma intron 1 in control PyMT-N-cad cells as compared to Slug-knockdown cells (Fig. 5G). Enrichment of Slug at a negative control region with no Slug-binding sites, located 9.4 kbs downstream from Puma gene on chromosome 7, did not exhibit notable differences in control PyMT-N-cad cells as opposed to Slug-knockdown cells (Fig. 5G). These data demonstrate that Slug specifically binds to the first intron on the Puma gene, consequently suppressing Puma gene expression in PyMT-N-cad cells.
To verify whether Puma contributes to apoptosis in Slug-knockdown cells, Puma was silenced in PyMT-N-cad/Slug-sh cells using a pool of Puma siRNAs (Fig. 5H). Treatment with FGFRi decreased caspase-3 activation in Puma-siRNA treated cells relative to control cells (Fig. 5H), as well as reduced the number of active-caspase-3 positive cells by 60% (Fig. 5I). These findings suggest that Slug contributes to cell survival by inhibiting Puma.
To unequivocally prove the function of the Slug-Puma axis in metastatic colonization, we knocked down Puma in Slug-sh cells using two lentiviral Puma shRNA sequences as compared to a control sequence (Fig. 6A). Cells were injected intravenously into syngeneic FVB mice and metastatic foci were monitored in the lungs 96 hrs later by staining lungs for N-cad (Fig. 6C). We found a dramatic upregulation in the number of foci in the lungs of mice injected with Slug/Puma knockdown cells relative to control lungs (Fig. 6B). Conversely, transient expression of Puma in PyMT-N-cad cells (Fig. 6D), suppressed lung colonization (Fig. 6E-F). Thus, it appears that the Slug-Puma axis is a powerful axis that drives distal organ colonization.
Fig. 6. The Slug-Puma Axis regulates lung colonization.
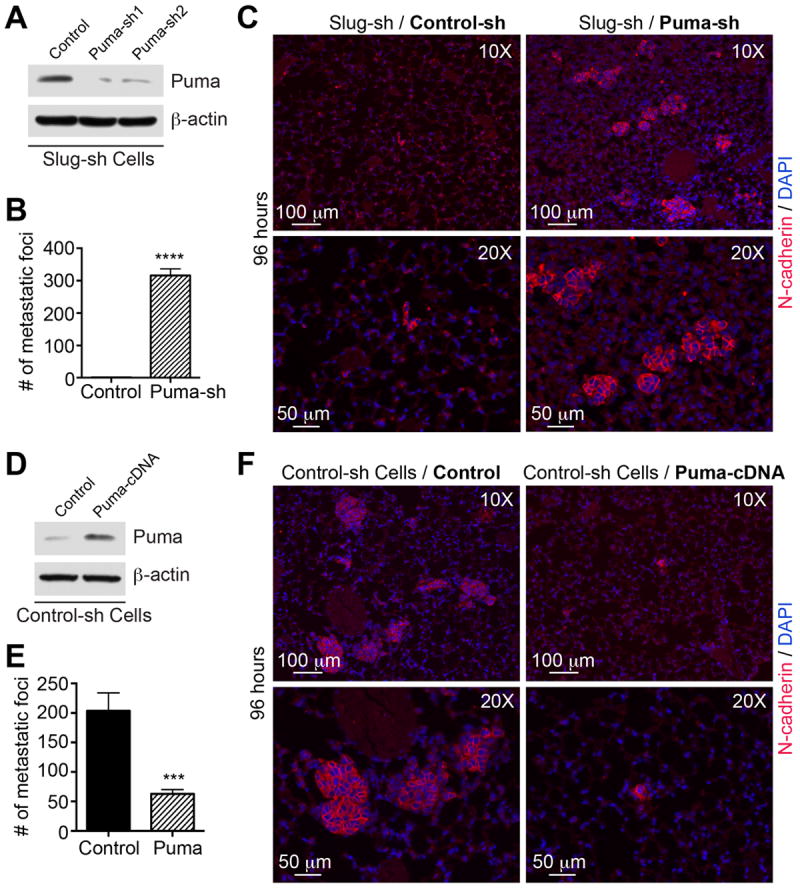
(A) Slug-knockdown PyMT-N-cad cells were further transduced with control-shRNA (Control) or two Puma-shRNAs (Puma-sh1 and Puma-sh2). Puma or β-actin levels are shown. (B) Slug- knockdown (control) or Slug/Puma double knockdown (Puma-sh) PyMT-N-cad cells were injected by tail-vein into FVB mice (N=3), and incubated for 96 hours. Five different sets of lung sections with 50μm interval between sets from each mouse were immunostained for N-cadherin and lung foci are shown as Mean ± SEM (N=3). Unpaired t-test, ****P<0.0001. (C) Images of foci of Slug-knockdown cells or Slug/Puma double knockdown PyMT-N-cad cells. Micrometastases were visualized by N-cad and DAPI staining. (D) PyMT-N-cad cells were transfected with control or mouse Puma cDNA. Puma and β-actin levels are shown; or (E) injected into tail-vein of FVB mice (N=3) and incubated for 96 hours. Five different sets of lung sections with 50 μm interval between sets were immunostained with anti-N-cad. Lung foci are shown as Mean ± SEM (N=3). Unpaired t-test, ***P=0.0003. (F) Metastases of control-sh (Control) or Puma-overexpressing PyMT-N-cad cells (Puma-cDNA) are shown.
The contribution of SLUG-PUMA to the survival of human metastatic breast cancer cells
Slug expression was found to be inversely correlated with Puma gene expression in breast cancer genes data sets (40). To address the role of Slug in lung colonization by human breast cancer cells, we used the MDA-MB-231 metastatic 3475 subline, which has been selected for its ability to colonize the lungs (41). We silenced Slug using two shRNA lentiviral sequences and a control non-targeting sequence (Fig. 7A). This resulted in increased Puma expression, especially in FGFRi-treated 3475/Slug-knockdown cells (Fig. 7B). Moreover, Slug knockdown in BT549 breast cancer cells also caused increased Puma levels (Fig. 7C). Consistent with our data in the PyMT-N-cad model, Slug inhibition stimulated Puma without inducing E-cad expression in 3475 (Fig. 7A) or BT549 cells (Fig. 7C). This was independent of N-cad, which was present in BT549 and not in 3475 cells (Fig. 7A and C). Next, we tested lung colonization of 3475 control and Slug knockdown cells following tail vein injection in athymic nude mice. This led to a ~70% reduction in colonization for both Slug-knockdown cell lines compared to 3475 control cells (Fig. 7D). Thus, repression of Puma by Slug in human breast cancer cells contributes to survival during metastatic colonization.
Fig. 7. SLUG suppression in human breast cancer cells increases Puma expression and suppresses colonization.
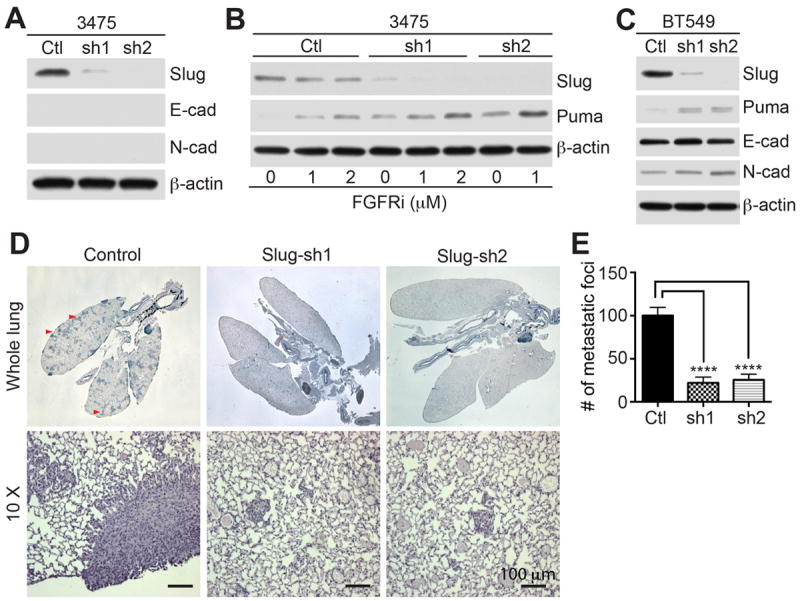
(A) Slug knockdown by two Slug shRNAs in metastatic 3475 cells was analyzed by immunoblotting. Control or Slug-shRNA (sh1 and sh2) cells were immunoblotted for Slug, E-cad, N-cad, β-actin. (B) Control 3475 cells and Slug-sh1 and sh2 cells were treated with PD173074 for 18 hrs, and immunoblotted for Slug, Puma, β-actin (right panels of A). (C) Control BT-549 or Slug shRNA (sh1 and sh2) cells were immunoblotted for Slug, Puma, E-cad, N-cad, β-actin. (D) Lung colonization of control 3475 and Slug-sh1 and Slug-sh2 cells was analyzed in 5 mice, 5 weeks post i.v injection. H&E stained lungs (top panels) show foci (arrowheads). Magnified control and 3475-Slug-sh mets are shown (bottom panels). (E) Quantification of metastatic foci was shown as Mean ± SEM. Unpaired t-test, ****P<0.0001.
Discussion
Slug is a transcriptional repressor, which promotes EMT, a process that accompanies cancer cell invasion and metastasis (6, 7). Slug upregulation was observed in high-grade invasive duct carcinomas, which exhibit high rate of recurrence, metastasis, and drug resistance (11, 13). Our studies showed that N-cadherin is expressed in aggressive breast cancers (42), and that it promotes EMT and metastasis by potentiating the FGFR (26, 27). Consistent with these findings, others have shown a strong association between N-cadherin (CDH2) and Slug (SNAI2) in breast cancer data sets (40). Moreover, FGFR1 amplification is part of a 66-gene signature in breast cancers that is strongly associated with reduced patient survival duration (36). Here we show that N-cadherin expression in PyMT tumor cells in vivo causes striking upregulation of Slug, at the exclusion of other EMT factors. However, Slug was affecting the behavior of disseminated cancer cells, not through EMT, but by promoting cell survival at distant organs. Namely, Slug inhibition did not alter the expression of epithelial or mesenchymal markers, nor promoted motility, invasion or extravasation, implying that the pro-metastatic function of Slug can be dissociated from its EMT-inducing effect (19). We believe that N-cad overexpression did not affect the ability of Slug to suppress E-cad in metastatic cells as Slug knockdown in the E-cad/N-cad positive BT549 or negative 3475 cell line did not increase or induce E-cad expression. It is believed that cancer cells undergo EMT to promote invasiveness but may also revert to MET at distant organs to resume growth and survival (18). We speculate that while Slug may be important for inducing EMT of cancer cells, especially at the primary tumor site, to facilitate detachment from the tumor mass and exit into the circulation, it may revert from promoting EMT to activating survival at metastatic sites. Although Slug expression is found to be regulated by the FGFR, it was not due to ERK or AKT activation downstream of FGFR. Interestingly, PyMT-N-cad tumor cell invasion was found to be attenuated by ERK inhibition, whereas cell migration was negatively regulated by the Akt3 isoform (26, 28). These findings suggest that Slug function in metastatic PyMT-N-cad cells is unlikely to be causing invasion or motility, and is most likely required for cell survival. While EMT and survival are not necessarily conflicting states, It is conceivable that the EMT and survival inducing functions of Slug may be mutually exclusive at distant sites, where survival may prevail over invasion.
Our findings demonstrate that Slug knockdown in PyMT-N-cad cells suppresses lung colonization. It was surprising that Slug, alone, would play such a dominant role. Given that lung colonization is a critical step in metastasis, which relies on tumor cell extravasation and survival in the lungs (1), we speculated that Slug promotes distal organ colonization by inhibiting apoptosis. We found that control and Slug-knockdown cells were efficiently arrested in the lungs 5 min post intravenous inoculation, but we were unable to detect them in the lungs prior to 48 hrs post injection, which may be due to apoptosis of tumor cells post extravasation and/or to low sensitivity of N-cad immunodetection of small tumor clusters in the lungs. Others have described this phenomenon as “metastatic insufficiency”, which is related to the poor survival of disseminated cancer cells due to apoptotic insults encountered throughout metastasis and especially at the stage following the arrest in the lungs (1, 3). In support of a role for Slug in suppressing apoptosis, Slug was found to protect tumor cells from apoptosis induced by radiation, which is reminiscent of the protective effect of Slug against DNA damage observed in hematopoietic progenitor cells (20, 21). In the latter, both Slug and Puma were shown to be targets of p53. Our results agree with these findings, showing that Slug targets Puma, and no other pro-apoptotic gene, including the p53-response gene, Noxa, or the non-p53 target gene Bim to suppress apoptosis. It implies that the dual control of Slug and Puma by p53 may constitute a built-in molecular module to ensure a swift transition from cell survival to cell death in response to DNA damage. The involvement of Puma in apoptosis is underscored by that Puma siRNA lowered the threshold for induction of apoptosis by doxorubicin. Importantly, the Slug-Puma axis was found to be operative in vivo as shown by that Puma shRNA in Slug-knockdown cells rescued lung colonization, whereas Puma overexpression in PyMT metastatic cells suppressed colonization. In sum, our study points to a pivotal function for Slug in metastasis, which allows tumor cells to overcome apoptosis, survive, and thrive throughout dissemination and colonization of distal organs. The later was achieved by repression of the pro-apoptotic gene Puma. Hence disrupting the Slug-Puma axis in cancer cells may impinge on the survival of metastatic cells.
Supplementary Material
Acknowledgments
We thank Dr. Peng Guo at the imaging facility at AECOM for expert microscopy.
Funding Source
This work was supported by grants from the National Cancer Institute (1R01 CA135061-01) and (1R01 CA136854-01) (R.B. H.) and the Breast Cancer Research Foundation (R.B.H. and L.N.).
Footnotes
Conflict of interest: None
References
- 1.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–72. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 2.Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–95. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Wong CW, Lee A, Shientag L, Yu J, Dong Y, Kao G, et al. Apoptosis: an early event in metastatic inefficiency. Cancer Res. 2001;61:333–8. [PubMed] [Google Scholar]
- 4.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–8. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–54. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 6.Cobaleda C, Perez-Caro M, Vicente-Duenas C, Sanchez-Garcia I. Function of the zinc-finger transcription factor SNAI2 in cancer and development. Annu Rev Genet. 2007;41:41–61. doi: 10.1146/annurev.genet.41.110306.130146. [DOI] [PubMed] [Google Scholar]
- 7.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–28. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 8.Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol. 2002;3:155–66. doi: 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- 9.Savagner P, Yamada KM, Thiery JP. The zinc-finger protein slug causes desmosome dissociation, an initial and necessary step for growth factor-induced epithelial-mesenchymal transition. J Cell Biol. 1997;137:1403–19. doi: 10.1083/jcb.137.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo W, Keckesova Z, Donaher JL, Shibue T, Tischler V, Reinhardt F, et al. Slug and Sox9 cooperatively determine the mammary stem cell state. Cell. 2012;148:1015–28. doi: 10.1016/j.cell.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin TA, Goyal A, Watkins G, Jiang WG. Expression of the transcription factors snail, slug, and twist and their clinical significance in human breast cancer. Ann Surg Oncol. 2005;12:488–96. doi: 10.1245/ASO.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Taube JH, Herschkowitz JI, Komurov K, Zhou AY, Gupta S, Yang J, et al. Core epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypes. Proc Natl Acad Sci U S A. 2010;107:15449–54. doi: 10.1073/pnas.1004900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Come C, Magnino F, Bibeau F, De Santa Barbara P, Becker KF, Theillet C, et al. Snail and slug play distinct roles during breast carcinoma progression. Clin Cancer Res. 2006;12:5395–402. doi: 10.1158/1078-0432.CCR-06-0478. [DOI] [PubMed] [Google Scholar]
- 14.Tripathi MK, Misra S, Khedkar SV, Hamilton N, Irvin-Wilson C, Sharan C, et al. Regulation of BRCA2 gene expression by the SLUG repressor protein in human breast cells. J Biol Chem. 2005;280:17163–71. doi: 10.1074/jbc.M501375200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mittal MK, Singh K, Misra S, Chaudhuri G. SLUG-induced elevation of D1 cyclin in breast cancer cells through the inhibition of its ubiquitination. J Biol Chem. 2011;286:469–79. doi: 10.1074/jbc.M110.164384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mittal MK, Myers JN, Misra S, Bailey CK, Chaudhuri G. In vivo binding to and functional repression of the VDR gene promoter by SLUG in human breast cells. Biochem Biophys Res Commun. 2008;372:30–4. doi: 10.1016/j.bbrc.2008.04.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bailey CK, Mittal MK, Misra S, Chaudhuri G. High motility of triple-negative breast cancer cells is due to repression of plakoglobin gene by metastasis modulator protein SLUG. J Biol Chem. 2012;287:19472–86. doi: 10.1074/jbc.M112.345728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gunasinghe NP, Wells A, Thompson EW, Hugo HJ. Mesenchymal-epithelial transition (MET) as a mechanism for metastatic colonisation in breast cancer. Cancer Metastasis Rev. 2012;31:469–78. doi: 10.1007/s10555-012-9377-5. [DOI] [PubMed] [Google Scholar]
- 19.Leroy P, Mostov KE. Slug is required for cell survival during partial epithelial-mesenchymal transition of HGF-induced tubulogenesis. Mol Biol Cell. 2007;18:1943–52. doi: 10.1091/mbc.E06-09-0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perez-Losada J, Sanchez-Martin M, Perez-Caro M, Perez-Mancera PA, Sanchez-Garcia I. The radioresistance biological function of the SCF/kit signaling pathway is mediated by the zinc-finger transcription factor Slug. Oncogene. 2003;22:4205–11. doi: 10.1038/sj.onc.1206467. [DOI] [PubMed] [Google Scholar]
- 21.Wu WS, Heinrichs S, Xu D, Garrison SP, Zambetti GP, Adams JM, et al. Slug antagonizes p53-mediated apoptosis of hematopoietic progenitors by repressing puma. Cell. 2005;123:641–53. doi: 10.1016/j.cell.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 22.Han J, Flemington C, Houghton AB, Gu Z, Zambetti GP, Lutz RJ, et al. Expression of bbc3, a pro-apoptotic BH3-only gene, is regulated by diverse cell death and survival signals. Proc Natl Acad Sci U S A. 2001;98:11318–23. doi: 10.1073/pnas.201208798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu J, Wang Z, Kinzler KW, Vogelstein B, Zhang L. PUMA mediates the apoptotic response to p53 in colorectal cancer cells. Proc Natl Acad Sci U S A. 2003;100:1931–6. doi: 10.1073/pnas.2627984100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim H, Tu HC, Ren D, Takeuchi O, Jeffers JR, Zambetti GP, et al. Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis. Mol Cell. 2009;36:487–99. doi: 10.1016/j.molcel.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ren D, Tu HC, Kim H, Wang GX, Bean GR, Takeuchi O, et al. BID, BIM, and PUMA are essential for activation of the BAX- and BAK-dependent cell death program. Science. 2010;330:1390–3. doi: 10.1126/science.1190217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hulit J, Suyama K, Chung S, Keren R, Agiostratidou G, Shan W, et al. N-cadherin signaling potentiates mammary tumor metastasis via enhanced extracellular signal-regulated kinase activation. Cancer Res. 2007;67:3106–16. doi: 10.1158/0008-5472.CAN-06-3401. [DOI] [PubMed] [Google Scholar]
- 27.Suyama K, Shapiro I, Guttman M, Hazan RB. A signaling pathway leading to metastasis is controlled by N-cadherin and the FGF receptor. Cancer Cell. 2002;2:301–14. doi: 10.1016/s1535-6108(02)00150-2. [DOI] [PubMed] [Google Scholar]
- 28.Chung S, Yao J, Suyama K, Bajaj S, Qian X, Loudig OD, et al. N-cadherin regulates mammary tumor cell migration through Akt3 suppression. Oncogene. 2013;32:422–30. doi: 10.1038/onc.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–5. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qian B, Deng Y, Im JH, Muschel RJ, Zou Y, Li J, et al. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS One. 2009;4:e6562. doi: 10.1371/journal.pone.0006562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Islam S, Carey TE, Wolf GT, Wheelock MJ, Johnson KR. Expression of N-cadherin by human squamous carcinoma cells induces a scattered fibroblastic phenotype with disrupted cell-cell adhesion. J Cell Biol. 1996;135:1643–54. doi: 10.1083/jcb.135.6.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hazan RB, Phillips GR, Qiao RF, Norton L, Aaronson SA. Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. J Cell Biol. 2000;148:779–90. doi: 10.1083/jcb.148.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nieman MT, Prudoff RS, Johnson KR, Wheelock MJ. N-cadherin promotes motility in human breast cancer cells regardless of their E-cadherin expression. J Cell Biol. 1999;147:631–44. doi: 10.1083/jcb.147.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stingl J, Caldas C. Molecular heterogeneity of breast carcinomas and the cancer stem cell hypothesis. Nat Rev Cancer. 2007;7:791–9. doi: 10.1038/nrc2212. [DOI] [PubMed] [Google Scholar]
- 35.Borowsky AD, Namba R, Young LJ, Hunter KW, Hodgson JG, Tepper CG, et al. Syngeneic mouse mammary carcinoma cell lines: two closely related cell lines with divergent metastatic behavior. Clin Exp Metastasis. 2005;22:47–59. doi: 10.1007/s10585-005-2908-5. [DOI] [PubMed] [Google Scholar]
- 36.Chin K, DeVries S, Fridlyand J, Spellman PT, Roydasgupta R, Kuo WL, et al. Genomic and transcriptional aberrations linked to breast cancer pathophysiologies. Cancer Cell. 2006;10:529–41. doi: 10.1016/j.ccr.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 37.Oda E, Ohki R, Murasawa H, Nemoto J, Shibue T, Yamashita T, et al. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science. 2000;288:1053–8. doi: 10.1126/science.288.5468.1053. [DOI] [PubMed] [Google Scholar]
- 38.Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Kontgen F, et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–8. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 39.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–56. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 40.Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10:515–27. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–24. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagi C, Guttman M, Jaffer S, Qiao R, Keren R, Triana A, et al. N-cadherin expression in breast cancer: correlation with an aggressive histologic variant--invasive micropapillary carcinoma. Breast Cancer Res Treat. 2005;94:225–35. doi: 10.1007/s10549-005-7727-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


