Summary
Research in human induced pluripotent stem cells (hiPSCs) is rapidly developing and there are expectations that this research may obviate the need to use human embryonic stem cells (hESCs), the ethics of which has been a subject of controversy for more than 15 years. In this study, we investigated approximately 3,400 original research papers that reported an experimental use of these types of human pluripotent stem cells (hPSCs) and were published from 2008 to 2013. We found that research into both cell types was conducted independently and further expanded, accompanied by a growing intersection of both research fields. Moreover, an in-depth analysis of papers that reported the use of both cell types indicates that hESCs are still being used as a “gold standard,” but in a declining proportion of publications. Instead, the expanding research field is diversifying and hESC and hiPSC lines are increasingly being used in more independent research and application areas.
Highlights
-
•
Research in hESCs and hiPSCs has recently expanded, but to different extents
-
•
Research in hESCs and hiPSCs partially overlaps, but is also diversifying
-
•
The “gold standard” use of hESCs is relatively declining
-
•
Only a few hESC lines are predominantly used as a benchmark in hiPSC research
In this article, Löser, Kobold, and colleagues investigate recent trends in research involving human embryonic stem cells (hESCs) and induced pluripotent stem cells (hiPSCs). They show that research has expanded during the past 6 years, and that research in hESCs and hiPSCs partially overlaps. However, they show that the two cell types dominate different fields of research, and a predominant use of hESCs as a “gold standard” in hiPSC research was not observed.
Introduction
With the first reports on generating human induced pluripotent stem cells (hiPSCs) from human cells (Takahashi et al., 2007; Yu et al., 2007), the controversy regarding the ethics of research involving human embryonic stem cells (hESCs) (Thomson et al., 1998) has arisen once again (Holm, 2008). Opponents of hESC research have been quick to argue that, considering the availability of an alternative source of human PSCs (hPSCs), research in hESCs is no longer needed to realize the promise of hPSCs. However, even before the derivation of hiPSCs was first reported, leading scientists in the field of hPSC research emphasized the need to continue research in ESCs in case hiPSCs became available (Hyun et al., 2007).
Several arguments have been put forward to support the continuation or even an extension of hESC research. For example, it has been reasoned that hESCs have advantages over hiPSCs for regenerative therapies because the latter may contain somatic mutations or reprogramming-induced epigenetic defects. Indeed, there are currently 11 clinical trials registered with the FDA in which hESC-derived cells are being used, mainly to establish treatments for different forms of macular degeneration, but also for neurological, cardiac, and pancreatic disorders (NIH, clinicaltrials.gov; https://clinicaltrials.gov/). Although the first results from one of the studies on macular degeneration have been reported (Schwartz et al., 2015), the vast majority of these trials started very recently, at a time when hiPSCs have already been available for years. Currently, hiPSC-derived cells are being used in one clinical trial in Japan (UMIN Clinical Trial Registry, ID UMIN000011929; http://www.umin.ac.jp/ctr/). Another argument in favor of continuing the use of hESCs is their utility for basic research (e.g., to gain a better understanding of human ground-state pluripotency) (Gafni et al., 2013), for studies of early human development (Niakan et al., 2012), or as cells that are unimpeded by epigenetic or environmental disturbances that are likely present in hiPSCs (e.g., to study gene function in a rather naive cell).
One of the most widely used arguments to justify hESC research is that these cells are still needed as the “gold standard” for human pluripotency to characterize and qualify hiPSC lines and gain a deeper understanding of the reprogramming process. This argument is frequently used in the political debate among stem cell researchers and proponents of hESC research, and has become a central point in the attempt to justify continued support for this research, for example, by the European Union. Thinking this argument through implies that research into hESCs would mainly lead to a more complete understanding of induced pluripotency and would become more and more dispensable with increasing progress in hiPSC research. Indeed, although novel and less invasive methods for reprogramming somatic cells to pluripotency have been developed in recent years, and some difficulties in the reprogramming procedure have been overcome (Anokye-Danso et al., 2011; Kim et al., 2009; Warren et al., 2010; Yoshioka et al., 2013; Yu et al., 2009), many controversial studies have reported differences between the two types of hPSCs on both genetic and epigenetic levels (Liang and Zhang, 2013; Ma et al., 2014) that may, for example, result in deviant behaviors in specific differentiation settings (Bar-Nur et al., 2011; Hu et al., 2010; Mills et al., 2013). Thus, it is currently unequivocally crucial to use hESCs as a reference material to gain a deeper understanding of hiPSC biology and to improve reprogramming strategies.
However, at present, the degree to which studies of hESCs and hiPSCs overlap, whether hESCs are being increasingly replaced by hiPSCs, and the purposes for which hESCs are used in iPSC research remain unknown. Six years after the onset of research into hiPSCs, scientific projects that were planned and started after hiPSCs became available should now be completed and published, and a meta-analysis of the relevant papers can be performed to indicate trends with respect to the relationship between hESC and hiPSC research. For example, if hESC research were just a transient technology and hESCs were mainly used as a “gold standard” in hiPSC research, one would expect the extent of independent hESC research to have declined in recent years and the cells to be mainly used in the context of comparative studies with hiPSCs.
In this study, we aimed to address these issues and provide a substantiated and validated database to facilitate further discussion. We analyzed all original research papers involving the experimental use of hPSCs that were published after the onset of hiPSC research. This analysis revealed that although research in hESCs and hiPSCs co-exists, both areas are growing into independent and autonomous research fields that increasingly intersect. About one-quarter of studies involving hESCs were found to also involve hiPSCs. Furthermore, a close inspection of the overlap of hESC and hiPSC studies showed that in the majority of these studies, hESCs were not used as a mere “gold standard” to qualify and better understand hiPSCs, and that this role of hESCs is declining while their use is diversifying and increasing in other areas.
Results
Database Searches and Paper Selection
We performed extensive searches of the PubMed database for studies that reported an experimental use of hESCs or hiPSCs and were published from 2008 to 2013. Our searches resulted in 11,137 hits for hESC-related studies and 6,291 hits for hiPSC-related studies. Of the identified studies, we excluded 3,313 and 2,444 studies, respectively, that were categorized by PubMed as non-original research (e.g., comments, editorials, and reviews). In addition, we excluded 473 hESC and 227 iPSC papers because they appeared in journals that do not publish original experimental research. To identify studies reporting original research in hPSCs, we manually inspected abstracts and/or full texts of the remaining 7,351 hESC and 3,620 hiPSC papers for the use of hESCs and/or hiPSCs, and excluded articles of no relevance for our analysis (e.g., studies reporting on research in mouse or non-human primate stem cells, somatic human stem cells, or political or ethical aspects of research).
This paper-selection procedure finally resulted in a pool of 2,922 studies reporting on experimental use of hESCs (38.4% of the studies inspected manually) and 1,376 studies reporting on experimental use of hiPSCs (36.2% of studies inspected manually). These publications were used for subsequent analyses, and the full texts of these papers were also investigated to identify the specific hESC and/or hiPSC lines used in the studies.
Research Involving hESCs
In the course of our analysis of the 2,922 studies involving experimental use of hESCs, we first identified papers in which hESCs were solely used for comparison with hiPSCs (as a “gold standard,” e.g., to compare novel hiPSC lines with hESCs with respect to pluripotency marker gene expression; see Experimental Procedures for criteria). We identified 401 papers that used hESCs only for comparative reasons and thus provided no inherent contribution to hESC research. Therefore, these papers were excluded from the hESC paper pool for the analyses of hESC research trends. However, these 401 papers were included again for the subsequent analysis of research involving both hESCs and hiPSCs (see below).
The distribution of the remaining 2,521 (not “gold standard”) hESC research papers on a timeline ranging from 2008 through the end of 2013 is shown in Figure 1A. To illustrate long-term trends during the whole period of hESC research, we also included the respective data for the years before 2008 that were derived in a previous study (Löser et al., 2008). Altogether, the number of hESC papers published per year steadily increased throughout the whole era of hESC research, with a minor decline in 2011. Although there apparently has been a slower increase from 2010 on, the result clearly indicates a sustained high interest in hESCs despite the worldwide availability of hiPSCs.
Figure 1.
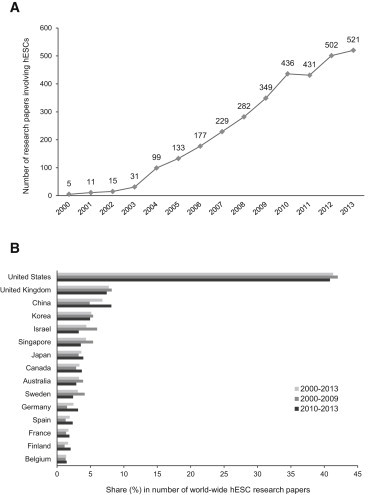
Worldwide Research in hESCs
(A) Number of hESC research papers published worldwide from 2000 to 2013. Note that research papers in which hESCs were solely used for comparison with hiPSCs (“gold standard”) were not included.
(B) Allocation of hESC research papers to individual nations according to the affiliation of the corresponding author. Shown is the share (in percent) of papers from a given nation in relation to the total number of papers for the indicated period. Nations with more than 40 original publications in the hESC field were included.
We next investigated the regional distribution of hESC research. By the end of 2013, research groups located in 38 nations reported results of experimental hESC research in international scientific journals. To determine the contribution of individual nations to worldwide hESC research, we allocated each paper to a specific country according to the affiliation of the corresponding author. This approach is justified since about 73% of the hESC research papers published so far resulted from national research with scientists from only one country mentioned in the authors list in the respective paper. The results of our analysis are presented in Figure 1B for the complete era of hESC research (2000–2013) and for the years 2000–2009 and 2010–2013. Our results confirm the unchallenged leadership in hESC research by groups located in the United States, which continuously contributed about 40% of publications to international hESC research for the past one and a half decades. The contribution of United States-based researchers remained nearly unaltered during the two periods shown. This is interesting because these results reflect the research output under the two fundamentally different stem cell policies of the Bush and Obama administrations (2000–2009 and 2010–2013, respectively). In contrast to the United States, the relative contribution to worldwide research from some other nations that entered the hESC field very early, such as Israel, Sweden, and Singapore, markedly declined in the second period, whereas the research output from countries that entered the hESC research field after a delay, such as Germany, France, and Spain, increased to some degree. Notably, the share of research from China (including Hong Kong and Taiwan) in international hESC research increased from less than 5% in the 2000–2009 period to more than 8% in the second period (2010–2013). We also related the number of hESC papers published in the 2008–2013 period to the overall number of publications in the life and health science fields. As shown in Figure S2A, the share of hESC research papers in the overall publication number slightly increased from 2008 to 2013, with some regional differences.
To exclude distortion of this comparison, the number of hESC research papers was related to the overall research output of the respective country in the fields of life and health science (Figure S2A). For example, it became apparent that Singapore, Israel, and Finland overproportionally published research in the hESC field, whereas there was no major imbalance toward this field in other countries.
Research Involving hiPSCs
We next determined the extent of research involving hiPSCs through the end of 2013. As shown in Figure 2A, the number of papers reporting on experimental use of hiPSCs substantially increased from 2007 (the year of the first publication regarding hiPSCs, with only two original research papers in the field) to 2013. We divided the hiPSC era into two periods: 2008–2010 and 2011–2013. While only 267 hiPSC research papers were published in the first 3 years, the number more than quadrupled to 1,109 papers in the following 3-year period. This seems to be a usual phenomenon after the establishment of a novel research field: in the case of hESC research, the output of research even increased more than 8-fold in the second 3-year period of hESC research (2003–2006) compared with the first 3-year period after the first derivation of hESCs (2000–2002). Notably, in 2013 the output from both fields of hPSC research was at a comparable level (about 500 papers each).
Figure 2.
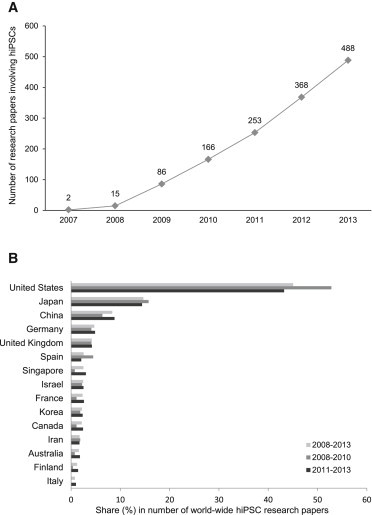
Worldwide Research in hiPSCs
(A) Number of original research papers involving hiPSCs and published worldwide from 2008 to 2013.
(B) Allocation of hiPSC research papers to individual nations according to the affiliation of the corresponding author. Shown is the share (in percent) of papers from a given nation in relation to the total number of papers for the indicated period. Nations with more than ten original research papers involving hiPSCs were included.
By the end of 2013, research groups from 27 nations contributed to research into hiPSCs. As in the case of hESCs, the leadership of US-based researchers is unchallenged, with an overall share of 45% in hiPSC research published worldwide, although the contribution declined from more than 50% in the 2008–2010 period to about 43% in the more recent period (Figure 2B). As expected, when the number of hiPSC research papers was related to the overall publication numbers in life and health sciences from selected countries, we observed a strong increase in the share of hiPSC papers for all countries from 2008 to 2013 (Figure S2B). However, there are some regional differences. Although hiPSC research accounted for less than 0.1% of research from the United States published from 2011 to 2013, it represented more than 0.2% of research from Israel or Singapore in the same period.
Impact of Research in hPSCs
The number of studies originating from an individual country does not necessarily mirror the relevance of that country’s contribution to a research field. We therefore determined, as a measure of the impact of research, the average frequencies with which hESC and hiPSC research papers published from 2008 to 2012 were cited through the end of 2013. Since reliable and comparable citation frequencies are not yet available for papers published in 2013, those papers were not included in the analysis.
As shown in Figure 3A, papers from the hESC research field were cited at an average frequency of 9.1 per year, whereas papers reporting experimental work involving hiPSCs were cited more frequently (19.4 citations per paper and year). In the hESC field, research papers from the United States, The Netherlands, and Canada were cited more often than the average and far more than studies originating from countries such as China, Korea, Sweden, and Israel, confirming our previous data on the high impact of United States-based hESC research (Löser et al., 2012).
Figure 3.
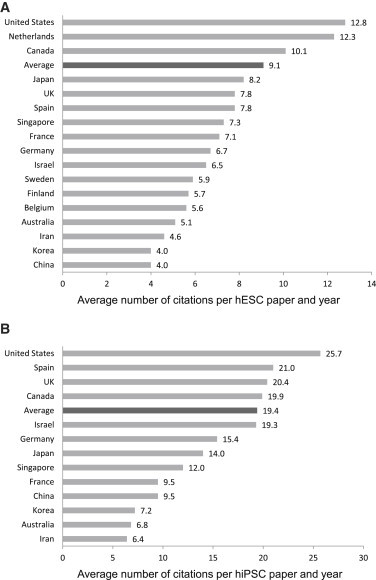
Impact of Research Papers Involving hPSCs from Selected Countries
Average number of citations per paper and year of original research papers involving hESCs (A) or hiPSCs (B). Papers published from 2008 to 2012 by research groups located in the indicated countries were included in the citation analysis. Please note that studies that involved hESCs solely for the purpose of comparison with hiPSCs were not considered.
Similarly, we found notable differences in the impact of hiPSC research from different nations (Figure 3B). Research from the US, Spain, the UK, and Canada overperformed with respect to citation frequency, whereas research papers from other countries, such as Australia, China, and Korea, were cited less frequently. The results are also in agreement with our previous study and confirm the surprising finding on an underperformance of Japanese hiPSC research with respect to impact.
It should be noted that the high average citation number of papers from the hiPSC field is mainly due to the high impact of early work in this field, although we did not include pioneering work from the Yamanaka and Thomson groups (Takahashi et al., 2007; Yu et al., 2007) in our citation analysis. While the average citation number per year only moderately decreased for hESC research papers published from 2008 to 2012, it sharply declined for hiPSC papers (from 92.2 citations per year for studies published in 2008 to 8.6 citations per year for studies published in 2012; Figure S3A). To determine whether the observed diversity in citation frequencies among papers from several nations may be due to extremely frequent citations of only a few highly popular studies, we grouped hESC and hiPSC research papers from selected nations according to their average citation frequency per year (Figure S3B). In the case of hESC research, the share of papers cited at a frequency of more than ∼150% of the average (15 citations per year) was more than 20% among studies from the United States, The Netherlands, and Canada, indicating that a rather broad range of influential hESC papers contributed to the high citation frequency of work from these countries. For hiPSC research, the proportion of papers cited at a frequency of more than 150% of the average (30 citations per year) is highest for work from the United States and The Netherlands (but only about 9%), indicating that the high average citation frequency of hiPSC papers may be partially the result of the high impact of an only moderate number of highly influential papers.
Research Involving hESCs and hiPSCs
In public discussions about the tenability of using hESCs despite the availability of hiPSCs, it is frequently reasoned that hESCs are still needed as a gold standard for the verification and qualification of hiPSCs. Accordingly, hESC research was predicted to be a transient technology that would lead to a complete understanding of hiPSC characteristics and would become dispensable with progress in understanding hiPSC biology. Thus, one would expect that (1) hiPSCs should increasingly replace hESCs; (2) consequently, the number of studies involving hESCs (and not hiPSCs) should decrease over time; and (3) the majority of studies involving hESCs should also involve hiPSCs, and hESCs should be used as a reference material (gold standard) for purposes of comparison only.
To test the validity of these hypotheses, we first analyzed the simultaneous use of hESCs and hiPSCs in experimental research. For this purpose, we scrutinized the full texts of all papers that reported an experimental use of hESCs and were published in 2008–2013 for the use of hiPSCs, and also examined the full texts of all papers that reported an experimental use of hiPSCs and were published in the same period for hESC utilization. The results are shown in Figure 4A. Of the more than 3,400 original research papers involving hPSCs that were published in 2008–2013, more than 2,000 involved the use of hESCs (but not hiPSCs) and almost 500 papers involved the use of hiPSCs (but not hESCs). Work reported in 890 research papers (26.1%) was based on both types of hPSCs. As indicated in Figure 4B, the number of papers based on either hiPSCs only or on both hPSC types markedly increased from 2008 to 2013, whereas the number of studies that were based solely on hESCs (but not on hiPSCs) remained stable at a high level.
Figure 4.
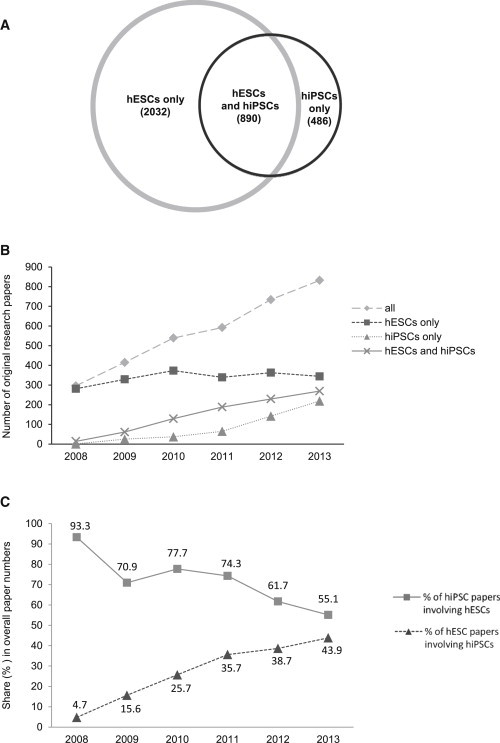
Use of hPSCs in Experimental Research from 2008 to 2013
(A) Number of original research papers involving hESCs, hiPSCs, or both.
(B) Number of original research papers that were published in the indicated years and were based on hESCs only (squares), hiPSCs only (triangles), or both cell types (crosses). The total numbers of research papers on hPSCs are represented by trapezoids.
(C) Simultaneous use of hESCs and hiPSCs in experimental research. Shown is the share of studies (in percent) that used hiPSCs and hESCs simultaneously in relation to the total number of hiPSC papers (squares) and hESC papers (triangles) published in the indicated years. Please note that for this analysis, all papers involving hESCs were used.
Notably, the relative share of hiPSC papers that also involved hESCs markedly declined within the period investigated. While nearly all hiPSC studies published in 2008 also involved hESCs (mainly for the purpose of comparison), this value decreased to about 55% in 2013 (Figure 4C), indicating that an increasing portion of hiPSC research is largely independent of hESCs. This may be partially due to the steadily growing number of papers that report on the derivation and use of hiPSCs in the course of establishing disease-specific cell lines, which usually do not involve ESCs. For example, we identified more than 250 original research papers that reported on the generation of at least one disease-specific hiPSC line. Conversely, the portion of hESC studies that also involved hiPSCs increased from less than 5% to more than 40% in the same period.
Altogether, these results indicate that although the two research fields increasingly intersect, they also exist independently. However, while most of the hESC research published in 2008–2013 did not involve hiPSCs, a large portion of even recent hiPSC research still involved hESCs.
We next analyzed the intersection of 890 papers that reported on experimental work in which both cell types were utilized. Assuming that hESCs are increasingly being used as a gold standard for hiPSC work, one would expect hESCs to be used mainly for the purpose of comparison in these studies. We therefore scrutinized these 890 papers for the specific use of hESCs and categorized them into two groups: one containing 401 papers in which hESCs were only used for purposes of comparison (see Experimental Proceduresfor criteria), and one containing 489 studies in which hESCs were rather autonomous research objects. The latter studies aimed to generate novel information about hESCs and hiPSCs, and were usually intended to gain insight into more general characteristics of hPSCs. Other studies that clustered in this second group were primarily performed with hESCs and results were just verified in hiPSCs to generalize the findings for a second type of hPSCs, or hiPSCs were solely used for the purpose of comparison. The results of this analysis with respect to time course are presented in Figure 5. At the onset of hiPSC research, hESCs were mainly used for mere comparison (about 92% of papers involved both cell types and were published in 2008). However, the share of this kind of paper declined to about 35% in 2013. In contrast, studies belonging to the second group formed the majority of such papers in more recent years.
Figure 5.
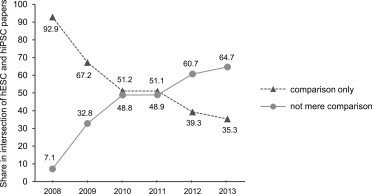
Type of Use of hESCs in Studies Reporting Experimental Application of Both hiPSCs and hESCs
Data are based on analysis of all papers reporting simultaneous experimental use of hESCs and hiPSCs, and published in 2008–2013 (n = 890, 100%). The share (in percent) was determined for papers in which hESCs were solely used for the purpose of comparison with hiPSCs (“gold standard” usage, triangles) or in which hESCs served as an autonomous research object (circles). Please note that the solid line (circles) also indicates studies in which hiPSCs played only a minor role.
Trends in Research with hPSCs
Our data indicate that hESCs are not mainly used as the gold standard for hiPSC research. We therefore wished to determine what kinds of scientific questions the different hPSC types were used to address. We roughly categorized papers with respect to the specific use of hESCs or hiPSCs. We restricted our analyses to the years 2011–2013 because during this period a sufficient number of hiPSC lines were available for investigating a broad range of scientific questions, whereas at the onset of hiPSC research (2008–2010), the majority of hiPSC papers focused mainly on the development of novel hiPSC lines.
Results of this analysis are shown in Table 1 (upper panels). Most studies involving hESCs were directed toward the analysis of developmental mechanisms in humans and the development and optimization of protocols to obtain pure populations of mature and functional human cells—mainly neural, cardiac, hematopoietic, and endothelial/vascular cells. A large portion of hESC research also aimed to analyze the cells’ specific characteristics, for example, to identify genes expressed specifically in these cells, to describe their genetic and epigenetic features, or to reveal their biochemical and metabolic peculiarities. A major part of the work was concentrated on uncovering the molecular mechanisms of pluripotency in human cells, optimizing culture protocols for hPSCs, and developing novel methods for their reliable characterization.
Table 1.
Topics of Research Involving hESCs and hiPSCs and Published from 2011 to 2013
| hESC Papers (2011–2013)a | ||
|---|---|---|
| Topicb | Paper Number | % of Papers |
| Development/optimization of differentiation protocols | 387 | 26.7 |
| Analysis of molecular mechanisms of development | 355 | 24.4 |
| Analysis of molecular characteristics of hESCs | 209 | 14.4 |
| Investigation of the molecular basis of pluripotency | 148 | 10.2 |
| Optimization of culture conditions/characterization methods | 132 | 9.1 |
| Provision of differentiated human cells for cell research | 93 | 6.4 |
| Use of hESC-derived cells in animal models of human diseases | 70 | 4.8 |
| Cell models for drug development/toxicity testing | 54 | 3.7 |
| Disease modeling | 30 | 2.1 |
| Development/optimization of methods for genetic manipulation | 24 | 1.7 |
| Derivation of novel hESC lines |
48 | 3.3 |
| hiPSC Papers (2011–2013) | ||
| Topicb | Paper Number | % of Papers |
| Generation of disease-specific cell lines | 227 | 20.5 |
| Disease modeling | 189 | 17.0 |
| Development/optimization of differentiation protocols | 161 | 14.5 |
| Development/optimization of methods for reprogramming | 150 | 13.5 |
| Analysis of molecular characteristics of hiPSCs | 116 | 10.5 |
| Molecular mechanisms of development | 81 | 7.3 |
| Molecular basis of reprogramming | 71 | 6.4 |
| Use of hiPSC-derived cells in animal models of human diseases | 61 | 5.5 |
| Optimization of culture conditions/characterization methods | 61 | 5.5 |
| Cell models for drug development/toxicity testing | 32 | 2.9 |
| Development/optimization of methods for genetic manipulation | 22 | 2.0 |
| Derivation of novel hiPSC lines | 562 | 50.7 |
| Research Papers Involving Any Type of hPSCs (2011–2013) | ||||
|---|---|---|---|---|
| Topicb | Paper Number | hESC (%) | hiPSC (%) | Both (%) |
| Development/optimization of differentiation protocols | 465 | 65.4 | 16.8 | 17.8 |
| Analysis of molecular mechanisms of development | 398 | 79.6 | 10.8 | 9.5 |
| Generation of disease-specific cell lines/disease modeling | 277 | 11.6 | 85.2 | 3.2 |
| Optimization of culture conditions/characterization methods | 156 | 60.9 | 15.4 | 23.7 |
| Use of hPSC-derived cells in animal models for human diseases | 133 | 46.6 | 47.4 | 6.0 |
| Drug development/toxicity testing | 81 | 60.5 | 33.3 | 6.2 |
| Development/optimization of methods for genetic manipulation | 36 | 38.9 | 33.3 | 27.8 |
Studies in which hESCs were used for mere comparison with hiPSCs were not considered.
Several topics can be the subject of the same paper.
In contrast, the relative majority of work that involved hiPSCs and was published from 2011 to 2013 aimed at the derivation of hiPSC lines from patients with specific diseases. In many studies, these cell lines were used as cell models for human diseases to reveal differentiation defects or functional deficiencies of the differentiated cells. A large portion of the work was focused on optimizing methods for improved reprogramming and identifying human cell types that are accessible for efficient reprogramming. In this context, the identification of molecules and signaling pathways involved in reprogramming was also of great interest. Work to develop and optimize differentiation procedures was frequently performed in conjunction with hESCs. The determination of characteristics specific to hiPSCs (or to hPSCs in general) and the verification of functional characteristics of hiPSC-derived cells in animal models of human diseases were also major topics of hiPSC research. In contrast to hESC research, which largely depended on previously derived cell lines, novel hiPSC lines were derived in more than half of the studies published from 2011 to 2013, indicating that there is a very large (and steadily growing) pool of hiPSC lines in the international research community (Soares et al., 2014).
We also wished to quantify the relative extent to which hESCs and hiPSCs were used to address questions within the same lines of research. Therefore, we determined the relative share of papers in which hESCs, hiPSCs, or both cell types were used in defined research fields, for example, to develop and optimize differentiation protocols or to establish disease models. As shown in Table 1 (lower panel), the vast majority of research that involved hPSCs and sought to uncover developmental and differentiation mechanisms in humans was done with hESCs only. Similarly, work directed toward the optimization of culture and differentiation protocols mainly involved hESCs. In contrast, the field of disease modeling in conjunction with the establishment of disease-specific cell lines was clearly dominated by hiPSCs. hiPSCs and hESCs were used to comparable extents to develop improved methods for the genetic manipulation of hPSCs or testing of hPSC-derived cells in animal models for human diseases.
Usage Pattern of hESC Lines in Comparative Studies
We previously reported that hESC research is dominated by only a few cell lines (Guhr et al., 2006; Löser et al., 2010) and that patterns of hESC line usage can be easily modeled as a cumulative advantage process (Schuldt et al., 2013). Others have proposed a policy-driven model to explain the preferential use of only a few hESC lines (Scott et al., 2009). Thus, we were interested in determining which hESC lines were the most frequently used for comparison with hiPSCs. To that end, we analyzed the 401 papers (“gold standard”) in which hESCs were used solely for the purpose of comparison. We found that 372 of these papers contained information about the specific hESC line(s) used. The results of our analysis are given in Table 2. Notably, in more than half of the papers (57.4%), the hESC H9 line was used for comparison, followed by the H1 line (29.8%), and frequently both lines were used in the same study. Altogether, the five oldest hESC lines (H1, H7, H9, H13, and H14), which were derived at WiCell as early as 1998 (Thomson et al., 1998), were used as the benchmark in about 74% of the studies to assess the integrity and characteristics of hiPSCs. The use especially of cell line H9 was significantly higher in comparative studies than in overall hESC research.
Table 2.
Use of hESC Lines for the Sole Purpose of Comparison in hiPSC Research: 2008–2013
| hESC Linea | Year of Publication | Provider | Use in Comparative Research (% of Studies)b | Use in Overall Research (% of Studies)b |
|---|---|---|---|---|
| H9 | 1998 | WiCell | 57.4 | 47.1 |
| H1 | 1998 | WiCell | 29.8 | 24.5 |
| H7 | 1998 | WiCell | 7.2 | 8.0 |
| HES-3 | 2000 | ES Cell International | 4.8 | 6.5 |
| KhES-1 | 2006 | Kyoto University | 4.8 | 3.1 |
| KhES-3 | 2006 | Kyoto University | 4.6 | 2.5 |
| HUES6 | 2004 | Harvard University | 4.0 | 1.7 |
| HUES9 | 2004 | Harvard University | 3.8 | 4.3 |
| BG01 | 2001 | BresaGen | 3.5 | 4.9 |
| HES-2 | 2000 | ES Cell International | 2.9 | 4.5 |
| H14 | 1998 | WiCell | 2.9 | 2.2 |
Sublines are grouped with the parental hESC line.
Note that in a subsection of papers, more than one hESC line was used.
Discussion
To identify global trends in the application of hESCs and hiPSCs in research, we established a curated database of published primary research conducted with these cells between 2008 and 2013, and performed a thorough analysis of studies involving only hESCs or only hiPSCs, as well as intersecting research. The results show that both the hESC and hiPSC research fields increased (hiPSC) or remained at a high level (hESC) with respect to impact and quantitative paper output. Research in which both hPSC types were applied in similar proportions included the development and optimization of cultivation and differentiation protocols, and research on animal models to develop cell-based therapies. Interestingly, we identified early segregation trends for the preferential research use of hESCs and hiPSCs in the recent past. For example, trends for the use of mostly hESCs include basic research on cell pluripotency and plasticity, and analysis of (early) developmental mechanisms. hiPSCs, on the other hand, clearly dominate the field of disease modeling, frequently in conjunction with the derivation of novel disease-specific hiPSC lines and the correction of genetic defects in vitro. Other topics of hiPSC research included the provision of cell models for drug development and toxicity testing, although rather surprisingly, a slight relative overweight of hESCs was found in this application field. This finding may have been influenced by our strict inclusion criteria, which only considered studies that directly used hPSCs, and excluded about 80 studies in which only commercially available hPSC-derived cardiomyocytes, hepatocytes, or neural cells were used. We also excluded other secondary studies that used hPSC-derived nucleic acids, proteins, or data. However, a more likely explanation is the relatively short time span of the research used in this analysis (years 2011–2013). Follow-up studies will be required to establish a trend in this specific area, especially in light of the recent establishment of large-scale hiPSC banking projects to meet the anticipated demand in this field (McKernan and Watt, 2013). It is intriguing that about 20% of the studies involving hiPSCs were focused on the establishment of disease-specific human cell lines, and frequently provided for the first time relevant human cell models for poorly understood, rare, and fatal human diseases (Cherry and Daley, 2013; Peitz et al., 2013). Notably, a large number of projects that aim to derive novel disease-specific hiPSC lines are currently registered with the NIH (ClinicalTrials.gov). While hESCs are a valuable resource for generating isogenic variants for specific diseases on a naive background, and therefore are also playing an increasing role in disease modeling, banking projects involving hESCs are mostly directed toward the distribution of highly characterized lines for comparable basic research and prospective clinical applications (Stacey et al., 2013). Moreover, in many cases, hESCs are used to provide a reliable source for differentiated or progenitor human cells such as neurons and cardiomyocytes, which are not readily accessible in other ways.
The trend for increased differentiation in the field is paralleled by a large and increasing proportion of papers in which both cell types are being used. To analyze the validity of the “gold standard” argument that is frequently used to justify the continued use of hESCs in research, we analyzed the intersection of research papers in which both cell types were applied for their specific uses. Although such intersecting research is increasing in absolute numbers, it includes only a minority (about 26%) of all papers involving hPSCs. Moreover, only a portion of these papers used hESCs solely as a gold standard for comparative research. In addition, the overall proportion of hiPSC studies that also use hESCs is steadily declining, likely because the “gold standard” aspect is less relevant if, for example, the research is focused on disease models or on differentiated progeny derived from hiPSCs. In the same period in which the proportion of hiPSC work involving hESCs declined, the number of hESC-only papers did not decline. These findings may indicate that the trend of field diversification and specification is at least partially due to the specific and differential applicability of these two cell types. These findings also show that hESCs are indeed useful as a gold standard and in general for standardization and benchmarking efforts in the field, but that this is not the major justification for their continued high level of use in research. In addition, the use of hESCs for standardization and comparative research is limited to a very small number of cell lines, which are already well characterized and available from established hESC banks. Hence, it appears that the “gold standard” itself is restricted to a small set: most studies used only a single hESC benchmark line (e.g., H9) rather than a larger, representative panel. The lack of generally accepted standard hPSC lines and insufficient knowledge about acceptable phenotypic tolerances may partly explain this restriction to the most commonly used lines and their quasi-standard status (Adewumi et al., 2007; Boulting et al., 2011; Loring and Rao, 2006; Martí et al., 2013; Nestor et al., 2013). The large hiPSC banks that are currently being established may help to define such benchmark standards.
In the controversial field of pluripotent stem cell research, it is vital to argue on the basis of reliable and solid data that best reflect the actual research situation and are carefully validated. However, available data on recent research activities in this field are often based on abstract searches and automatized algorithms, and not on manually verified data bank searches. For example, Pera and Trounson (2013) estimated the number of publications on hESC research to be nearly 2,000 per year for 2010 through 2012, since review articles were included in their data pool (Pera and Trounson, 2013). More strikingly, the recent European Union-funded Stem Cell Report, published in collaboration with Elsevier and Kyoto University (Barfoot et al., 2013), claimed that more than 500 papers on hESCs were published in 2008, and stated that in 2012, researchers from Germany published substantially more papers in the hESC field than groups from Japan, Korea, or Israel. However, a closer inspection of the data set used for this extensive and highly appreciated study revealed that, for example, the German hESC paper pool contained many publications in which hESCs were not used. Abstract statements such as “Despite their potential benefits, ethical and practical considerations limit the application of NSCs derived from hESCs or adult brain tissue. Thus, alternative sources are required” resulted in consideration of the respective paper as a contribution to hESC research. Altogether, nearly 50% of the alleged hESC papers from Germany used for this database did not report any research involving hESCs. Moreover, while our study was under review, Alberta et al. (2015) published an analysis assessing the impact of stem cell research funding programs in selected U.S. states. These authors searched the Web of Science for articles that contained the phrase “human embryonic stem cell” in the title, abstract, or key words, and were (co-)produced by authors with an affiliation in the United States. However, our analysis of this data pool (1,544 hESC-related papers from U.S. authors) revealed that more than 15% of the studies identified by Alberta et al. are not relevant because they did not report research involving hESCs. On the other hand, more than 550 relevant papers from the United States that involved hESCs and are present in our database were missed (including the one by Thomson et al. [1998]). The vast reduction of the initially high number of papers obtained through our selection process confirms that it may be essential for the assessment of research activities to initially generate broader publication-based data pools, and to manually validate each included paper. An analysis of papers only on the basis of meta-data provided by a search engine may result in a massive over- or underestimation of research output and may lead to misleading conclusions, which could potentially influence and misdirect political decision-making.
When compared with our previous studies (Guhr et al., 2006; Löser et al., 2008, 2010, 2012), the current analysis revealed some relevant changes in the number and ranking of countries involved in hESC research. In addition, we substantiated that a country’s quantitative output of papers in hPSC research does not necessarily correlate with the impact of this research. For example, our previous surprising finding that Japan is somewhat underperforming in the hiPSC field was confirmed for recent years with respect to impact per study. Moreover, although the number of hPSC research papers from China increased markedly over the past years, research from China clearly underperformed with respect to impact per study in both the hESC and hiPSC fields. However, it may be expected that this situation will change in the near future as Chinese research groups increasingly publish papers in highly influential, ranking international journals.
Our present study was based on a pool of stem cell publications that only included original research papers. It is a well-reasoned assumption that aspects concerning stem cell history, the prospect of using pluripotent stem cells in future therapies, and the ethical and legal aspects of research cannot reflect the extent of research activities in this field, and their inclusion in such an analysis is irrelevant. Our data on the extent of experimental research involving hPSCs show that both hESCs and hiPSCs supply a vital research field that has not yet reached maturity. The emerging trends of differentiation, diversification, and fusion with other research and technological fields indicate that both hESCs and hiPSCs will be essential and independent components of this research area.
Experimental Procedures
Paper pools were established by searches of the PubMed database, which is accessible through the NIH National Library of Medicine (NIH/NLM). Data bank searches were performed separately to identify hESC- and hiPSC-related publications using the search strings described earlier (Guhr et al., 2006; Müller et al., 2010) and modified as indicated in Supplemental Experimental Procedures. The complete procedures used to identify research papers with relevance for our analysis are described in the Supplemental Information and outlined schematically in Figure S1. Briefly, initial searches of the database resulted in about 17,400 hits (11,137 for hESC-related papers and 6,291 for hiPSC-related papers). We excluded articles that were categorized by PubMed as non-research papers, as well as studies that appeared in journals that do not publish original experimental research. Abstracts and/or full texts of the remaining ∼11,000 papers were inspected manually for the use of hESCs or hiPSCs before they were added to the paper repositories. Therefore, our paper pools only contain original research papers in which hESCs and/or hiPSCs were used experimentally.
To identify papers in which hESCs were merely used as a gold standard for iPSCs, we determined whether hESCs were solely used (1) to determine whether newly derived hiPSCs showed typical characteristics of hPSCs (usually with respect to cell morphology, presence of pluripotency-associated gene products, mRNA and miRNA gene-expression patterns, and/or DNA methylation patterns), (2) to verify that protocols developed for culture and differentiation of hiPSCs would also be applicable to hESCs, or (3) to investigate whether molecular characteristics initially identified in hiPSCs could also be found in hESCs. If hESCs were used for only these comparative purposes, the usage was assigned a “gold standard” application. These studies are not considered as original research in hESCs, and consequently the papers were not included in the analyses of hESC research.
Allocation of a paper to a country was done according to the corresponding author’s affiliation. Citation analysis was performed as described previously (Löser et al., 2012) using the Scopus database. Details are given in Supplemental Experimental Procedures.
Categorization of papers into topic groups was performed by manual inspection of abstracts/full texts, since the use of key words assigned by the publisher and provided by the PubMed and Scopus databases turned out to be an unreliable tool for grouping papers into scientific categories.
Author Contributions
S.K. and A.G. collected and assembled data and performed data analysis. A.K. was responsible for conception and design, data analysis and interpretation, and manuscript writing. P.L. was responsible for conception and design, collection and assembly of data, data analysis and interpretation, and manuscript writing.
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Contributor Information
Andreas Kurtz, Email: andreas.kurtz@charite.de.
Peter Löser, Email: loeserp@rki.de.
Supplemental Information
References
- Adewumi O., Aflatoonian B., Ahrlund-Richter L., Amit M., Andrews P.W., Beighton G., Bello P.A., Benvenisty N., Berry L.S., Bevan S., International Stem Cell Initiative Characterization of human embryonic stem cell lines by the International Stem Cell Initiative. Nat. Biotechnol. 2007;25:803–816. doi: 10.1038/nbt1318. [DOI] [PubMed] [Google Scholar]
- Alberta H.B., Cheng A., Jackson E.L., Pjecha M., Levine A.D. Assessing state stem cell programs in the United States: how has state funding affected publication trends? Cell Stem Cell. 2015;16:115–118. doi: 10.1016/j.stem.2015.01.007. [DOI] [PubMed] [Google Scholar]
- Anokye-Danso F., Trivedi C.M., Juhr D., Gupta M., Cui Z., Tian Y., Zhang Y., Yang W., Gruber P.J., Epstein J.A., Morrisey E.E. Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell. 2011;8:376–388. doi: 10.1016/j.stem.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Nur O., Russ H.A., Efrat S., Benvenisty N. Epigenetic memory and preferential lineage-specific differentiation in induced pluripotent stem cells derived from human pancreatic islet beta cells. Cell Stem Cell. 2011;9:17–23. doi: 10.1016/j.stem.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Barfoot, J., Kemp, E., Doherty, K., Blackburn, C., Sengoku, S., van Servellen, A., Gavai, A., and Karlsson, A. (2013). Stem cell research: trends and perspectives on the evolving international landscape. http://issuu.com/eurostemcell/docs/stem-cell-report-trends-and-perspec.
- Boulting G.L., Kiskinis E., Croft G.F., Amoroso M.W., Oakley D.H., Wainger B.J., Williams D.J., Kahler D.J., Yamaki M., Davidow L. A functionally characterized test set of human induced pluripotent stem cells. Nat. Biotechnol. 2011;29:279–286. doi: 10.1038/nbt.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry A.B., Daley G.Q. Reprogrammed cells for disease modeling and regenerative medicine. Annu. Rev. Med. 2013;64:277–290. doi: 10.1146/annurev-med-050311-163324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafni O., Weinberger L., Mansour A.A., Manor Y.S., Chomsky E., Ben-Yosef D., Kalma Y., Viukov S., Maza I., Zviran A. Derivation of novel human ground state naive pluripotent stem cells. Nature. 2013;504:282–286. doi: 10.1038/nature12745. [DOI] [PubMed] [Google Scholar]
- Guhr A., Kurtz A., Friedgen K., Löser P. Current state of human embryonic stem cell research: an overview of cell lines and their use in experimental work. Stem Cells. 2006;24:2187–2191. doi: 10.1634/stemcells.2006-0053. [DOI] [PubMed] [Google Scholar]
- Holm S. Time to reconsider stem cell ethics—the importance of induced pluripotent cells. J. Med. Ethics. 2008;34:63–64. doi: 10.1136/jme.2007.023903. [DOI] [PubMed] [Google Scholar]
- Hu B.Y., Weick J.P., Yu J., Ma L.X., Zhang X.Q., Thomson J.A., Zhang S.C. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc. Natl. Acad. Sci. USA. 2010;107:4335–4340. doi: 10.1073/pnas.0910012107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun I., Hochedlinger K., Jaenisch R., Yamanaka S. New advances in iPS cell research do not obviate the need for human embryonic stem cells. Cell Stem Cell. 2007;1:367–368. doi: 10.1016/j.stem.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Kim D., Kim C.H., Moon J.I., Chung Y.G., Chang M.Y., Han B.S., Ko S., Yang E., Cha K.Y., Lanza R., Kim K.S. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4:472–476. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang G., Zhang Y. Genetic and epigenetic variations in iPSCs: potential causes and implications for application. Cell Stem Cell. 2013;13:149–159. doi: 10.1016/j.stem.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loring J.F., Rao M.S. Establishing standards for the characterization of human embryonic stem cell lines. Stem Cells. 2006;24:145–150. doi: 10.1634/stemcells.2005-0432. [DOI] [PubMed] [Google Scholar]
- Löser P., Guhr A., Kurtz A., Wobus A.M. Additional considerations relevant to meta-analyses of hESC publication data. Cell Stem Cell. 2008;3:129–130. doi: 10.1016/j.stem.2008.07.017. author reply 131. [DOI] [PubMed] [Google Scholar]
- Löser P., Schirm J., Guhr A., Wobus A.M., Kurtz A. Human embryonic stem cell lines and their use in international research. Stem Cells. 2010;28:240–246. doi: 10.1002/stem.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löser P., Kobold S., Guhr A., Müller F.J., Kurtz A. Scope and impact of international research in human pluripotent stem cells. Stem Cell Rev. 2012;8:1048–1055. doi: 10.1007/s12015-012-9409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H., Morey R., O’Neil R.C., He Y., Daughtry B., Schultz M.D., Hariharan M., Nery J.R., Castanon R., Sabatini K. Abnormalities in human pluripotent cells due to reprogramming mechanisms. Nature. 2014;511:177–183. doi: 10.1038/nature13551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martí M., Mulero L., Pardo C., Morera C., Carrió M., Laricchia-Robbio L., Esteban C.R., Izpisua Belmonte J.C. Characterization of pluripotent stem cells. Nat. Protoc. 2013;8:223–253. doi: 10.1038/nprot.2012.154. [DOI] [PubMed] [Google Scholar]
- McKernan R., Watt F.M. What is the point of large-scale collections of human induced pluripotent stem cells? Nat. Biotechnol. 2013;31:875–877. doi: 10.1038/nbt.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills J.A., Wang K., Paluru P., Ying L., Lu L., Galvão A.M., Xu D., Yao Y., Sullivan S.K., Sullivan L.M. Clonal genetic and hematopoietic heterogeneity among human-induced pluripotent stem cell lines. Blood. 2013;122:2047–2051. doi: 10.1182/blood-2013-02-484444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller F.J., Goldmann J., Löser P., Loring J.F. A call to standardize teratoma assays used to define human pluripotent cell lines. Cell Stem Cell. 2010;6:412–414. doi: 10.1016/j.stem.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Nestor M.W., Paull D., Jacob S., Sproul A.A., Alsaffar A., Campos B.A., Noggle S.A. Differentiation of serum-free embryoid bodies from human induced pluripotent stem cells into networks. Stem Cell Res. (Amst.) 2013;10:454–463. doi: 10.1016/j.scr.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Niakan K.K., Han J., Pedersen R.A., Simon C., Pera R.A. Human pre-implantation embryo development. Development. 2012;139:829–841. doi: 10.1242/dev.060426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peitz M., Jungverdorben J., Brüstle O. Disease-specific iPS cell models in neuroscience. Curr. Mol. Med. 2013;13:832–841. doi: 10.2174/1566524011313050014. [DOI] [PubMed] [Google Scholar]
- Pera M., Trounson A. Cloning debate: stem-cell researchers must stay engaged. Nature. 2013;498:159–161. doi: 10.1038/498159a. [DOI] [PubMed] [Google Scholar]
- Schuldt B.M., Guhr A., Lenz M., Kobold S., MacArthur B.D., Schuppert A., Löser P., Müller F.J. Power-laws and the use of pluripotent stem cell lines. PLoS ONE. 2013;8:e52068. doi: 10.1371/journal.pone.0052068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S.D., Regillo C.D., Lam B.L., Eliott D., Rosenfeld P.J., Gregori N.Z., Hubschman J.-P., Davis J.L., Heilwell G., Spirn M. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2015;385:509–516. doi: 10.1016/S0140-6736(14)61376-3. [DOI] [PubMed] [Google Scholar]
- Scott C.T., McCormick J.B., Owen-Smith J. And then there were two: use of hESC lines. Nat. Biotechnol. 2009;27:696–697. doi: 10.1038/nbt0809-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares F.A., Sheldon M., Rao M., Mummery C., Vallier L. International coordination of large-scale human induced pluripotent stem cell initiatives: Wellcome Trust and ISSCR workshops white paper. Stem Cell Rep. 2014;3:931–939. doi: 10.1016/j.stemcr.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey G.N., Crook J.M., Hei D., Ludwig T. Banking human induced pluripotent stem cells: lessons learned from embryonic stem cells? Cell Stem Cell. 2013;13:385–388. doi: 10.1016/j.stem.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Thomson J.A., Itskovitz-Eldor J., Shapiro S.S., Waknitz M.A., Swiergiel J.J., Marshall V.S., Jones J.M. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Warren L., Manos P.D., Ahfeldt T., Loh Y.H., Li H., Lau F., Ebina W., Mandal P.K., Smith Z.D., Meissner A. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka N., Gros E., Li H.R., Kumar S., Deacon D.C., Maron C., Muotri A.R., Chi N.C., Fu X.D., Yu B.D., Dowdy S.F. Efficient generation of human iPSCs by a synthetic self-replicative RNA. Cell Stem Cell. 2013;13:246–254. doi: 10.1016/j.stem.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Vodyanik M.A., Smuga-Otto K., Antosiewicz-Bourget J., Frane J.L., Tian S., Nie J., Jonsdottir G.A., Ruotti V., Stewart R. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Yu J., Hu K., Smuga-Otto K., Tian S., Stewart R., Slukvin I.I., Thomson J.A. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


