Abstract
Conventional culture methods to detect methicillin-resistant Staphylococcus aureus (MRSA) take a few days, and their sensitivity and usefulness also need to be improved. In this study, active screening was performed using the polymerase chain reaction (PCR) for colonization with MRSA on admission and follow-up surveillance after admission to an emergency department between June 2012 and August 2012, and the backgrounds of PCR and/or culture-method-positive patients were compared. Among 95 patients, 15 (15.8%) patients were positive for MRSA on PCR and/or culture; 6.3% (6/95) of patients were positive on admission, and 9.5% (9/95) became positive during the stay after admission. The major primary diagnoses in MRSA-positive patients were trauma and cerebrovascular diseases. Nine (60%) of 15 patients were MRSA-positive on both PCR and culture, compared with three (20%) of 15 who were PCR-positive but culture-negative. The other three (20%) of 15 patients were PCR-negative but culture-positive. Furthermore, there was a tendency for younger age and shorter stay to be associated with PCR-positive but culture-negative results. These findings suggest that active surveillance with PCR may be highly sensitive and useful for the early diagnosis of MRSA colonization to prevent nosocomial transmission from the emergency department to the regular inpatient wards of the hospital.
Keywords: active surveillance, length of stay, nosocomial transmission, BD GeneOhm MRSA assay, sensitivity, specificity
Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) has become one of the leading causes of infections in hospitals, and mortality associated with MRSA infection is still high in Japan.1 MRSA bacteremia is a serious problem among infectious diseases, not only because of its increasing frequency, but also because of its difficulties with treatment and high mortality.1,2 In our previous study, the mortality of MRSA bacteremia in our hospital was 40%, but the survivors had been referred significantly more to the Infection Control Team than non-survivors.1 Therefore, early diagnosis and rapid, appropriate prevention are very important for improving patient outcomes.
Screening for MRSA is one of the most important components of successful infection control. Active surveillance culture and subsequent isolation of colonized patients are effective and usually used as a strategy for reducing the transmission of MRSA in emergency care departments. Active surveillance culture for MRSA in the nares of at-risk patients is recommended by the Society for Healthcare Epidemiology of America for control of nosocomial transmission of MRSA.3,4 However, conventional culture methods to detect MRSA may be time-consuming and often lead to delayed precautions.5
Recently, rapid detection polymerase chain reaction (PCR) assays for detection of MRSA directly from screening swabs, which take a few hours, have been developed, and Taguchi et al reported the usefulness of active screening for colonization with MRSA in tertiary care centers.6 Their results suggested higher sensitivity of PCR-based active surveillance compared with conventional culture methods, but they are expensive, and their sensitivity/specificity and efficiency for prevention of MRSA transmission and their cost-effectiveness have not been convincingly established.2,7,8
In this study, the prevalence of MRSA infection and the characteristics of the patients who were positive on either or both of PCR screening and culture in an emergency department in Osaka, Japan, were investigated.
Materials and methods
Hospital and patient setting
Our hospital is a 1,076-bed university hospital located in Osaka, Japan. Surveillance of hospital-acquired infections using data from June 2012 to August 2012 in a 20-bed emergency department was performed. Emergency departments in Japan are different from those in the United States; they are closer to an outpatient setting with access to inpatient facilities, and they include a critical care unit. Informed consent was obtained from the patients in accordance with the requirements of our hospital’s Institutional Review Board. This study was approved by the Research Ethics Committee of Osaka University and assigned accession number 11159-6. Patients who died within 24 hours and those who were unwilling to participate in this study were excluded.
Microbiological procedures
All patients underwent MRSA screening culture of nasal swabs and PCR using the GeneOhm MRSA assay (Becton Dickinson, Franklin Lakes, NJ, USA) on admission. Unilateral anterior nares swabs (Culture Swab Liquid Stuart Single Swab; Becton Dickinson) were used. Samples of nasal swabs for MRSA culture and PCR were taken on admission and every 7 days thereafter. Therefore, “imported” means that MRSA was isolated on admission (usually within 48 hours), and “acquired” means that MRSA was not isolated on admission, but isolated at 48 hours after admission (usually 7 days later). In addition, “infection” means that MRSA was considered to be a pathogen, and “colonization” means that MRSA was just isolated without any related inflammation and diseases.
MRSA-PCR was performed universally in the emergency department, and collecting nasal swabs was discontinued when the patients moved to general wards.
Bacterial culture methods
After collecting the samples, nasal swabs were transported to the laboratory immediately and plated directly onto mannitol salt agar, incubated at 35°C with 5% CO2 for 48 hours, and then maintained at 25°C for 5 days. Colonies that were mannitol-fermenting, catalase-positive, and coagulase-positive were screened for methicillin resistance on Mueller–Hinton agar supplemented with sodium chloride cations and oxacillin at 4 μg/mL according to the Clinical Laboratory Standards Institute guidelines. Antimicrobial susceptibility testing for agents was measured by the broth microdilution method and automated equipment (MicroScan WalkAway, Siemens, Munich, Germany).8
PCR assay
The swabs were also used for the real-time PCR-based BD GeneOhm MRSA assay (Becton Dickinson Diagnostics) in accordance with the manufacturer’s instructions.
In brief, the nasal swab was placed in a tube with 7% NaCl buffer, and the resulting suspension was transferred to a lysis tube for DNA extraction. Subsequent addition of the kit’s molecular reagents was followed by real-time PCR. The lysis buffer without the DNA template was used as a negative control, and the kit supplied the template DNA as the positive control. The cycling conditions included a total of 45 cycles for annealing, denaturation, and extension. The entire process run-time was about 1 hour. In the GeneOhm assay, the use of primer sequences for the SCCmec and orf X regions generates MRSA-specific products.
Data analysis
GeneOhm MRSA results were compared with those obtained from bacterial cultures. Samples that were positive on both the GeneOhm assay and culture were reported as PCR-positive and culture-positive (PCR+Culture+), and those that were positive on the GeneOhm assay and negative on culture were reported as PCR-positive and culture-negative (PCR+Culture−). PCR−Culture+ means PCR negative but culture positive.
Data regarding patient characteristics and culture results were obtained by prospective chart review. Data are presented as means ± SEM (standard error of the mean).
Statistical analysis
The non-parametric Kruskal–Wallis test was used to compare categorical variables among the groups, including the PCR+Culture+, PCR+Culture−, and PCR−Culture+ groups. All tests of significance were two-tailed, and values of P<0.05 were considered significant.
Results
Study patients
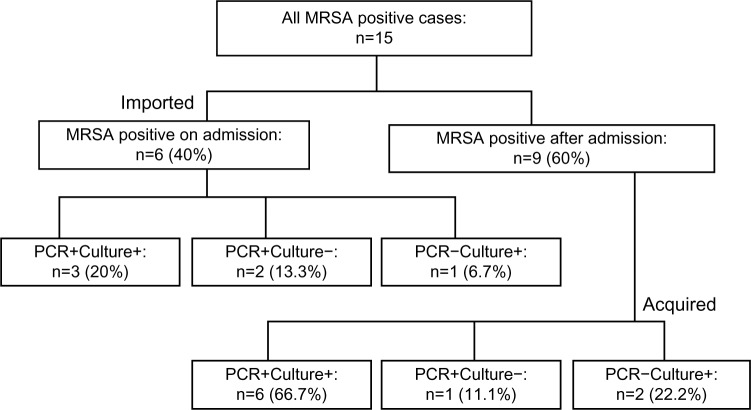
A total of 95 samples were collected from 95 patients on admission during the period; 15 patients (15.7%) were positive on PCR, culture, or both. Of these, six (6.3%) patients were positive on admission, whereas nine (9.5%) became positive during their emergency department stay (Figure 1). On admission, three of six patients were positive on both PCR and culture (PCR+Culture+), two on PCR only (PCR+Culture−), and one on culture only (PCR−Culture+). After admission, six of nine patients were positive on both PCR and culture (PCR+Culture+), one on PCR only (PCR+Culture−), and two on culture only (PCR−Culture+) (Figure 2).
Figure 1.
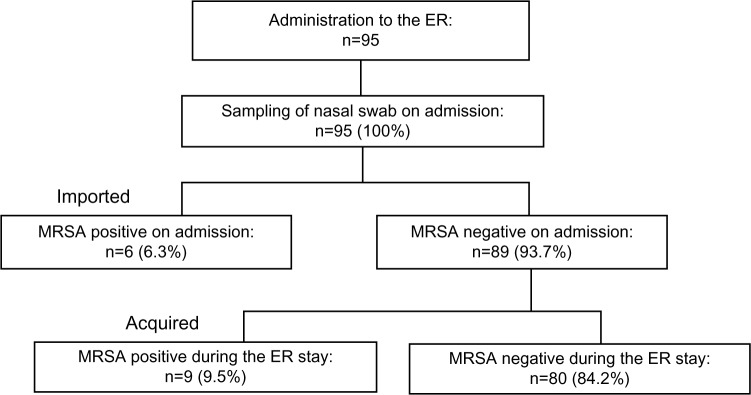
Distribution of the study population.
Notes: Data are numbers (%) of patients. Imported: MRSA was isolated on admission (within 48 hours). Acquired: MRSA was isolated after admission (48 hours later).
Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus; ER, emergency department.
Figure 2.
Distribution of the study population: polymerase chain reaction (PCR)-positive and culture-positive (PCR+Culture+), PCR-positive and culture-negative (PCR+Culture−), and PCR-negative and culture-positive (PCR−Culture+).
Notes: Imported: MRSA was isolated on admission (within 48 hours). Acquired: MRSA was isolated after admission (48 hours later).
Abbreviation: MRSA, methicillin-resistant Staphylococcus aureus.
Characteristics of MRSA-positive patients by detection method
The characteristics of the 15 MRSA-positive patients (eleven males and four females) are summarized in Table 1. No significant differences were seen in mean total Glasgow Coma Scale scores, number of definition items of SIRS (systemic inflammatory response syndrome), sepsis-related organ failure assessment scores, and APACHE (Acute Physiology and Chronic Health Evaluation) II scores among PCR+Culture+, PCR+Culture−, and PCR−Culture− patients on admission.
Table 1.
Backgrounds of MRSA-positive patients
| PCR+Culture+(n=9) | PCR+Culture−(n=3) | PCR−Culture+(n=3) | P-value | |
|---|---|---|---|---|
| Male (Female) | 7 (2) | 3 (0) | 1 (2) | 0.615 |
| Imported (Acquired) | 3 (6) | 2 (1) | 0 (3) | 0.624 |
| Colonization (Infection) | 6 (3) | 1 (2) | 0 (3) | 0.135 |
| Age, years (mean ± SD) | 56.4±24.9 | 18.9±7.5 | 43.7±14.4 | 0.067# |
| Glasgow Coma Scale (mean ± SD) | 7.3±5.3 | 3.0±0.0 | 4.3±1.9 | 0.263 |
| SIRS (mean ± SD) | 2.0±0.8 | 1.3±0.5 | 2.7±1.2 | 0.239 |
| SOFA score (mean ± SD) | 5.3±4.1 | 6.7±0.5 | 4.7±1.7 | 0.383 |
| APACHE II score (mean ± SD) | 20.8±9.1 | 20.0±1.4 | 22.0±2.1 | 0.64 |
| Anti-MRSA drugs use | 5 | 2 | 3 | 0.393 |
| Survivor (Non-survivor) | 7 (2) | 3 (0) | 2 (1) | 0.595 |
| LOS (mean ± SD) | 15.7±15.6 | 8.6±7.1 | 24.0±13.1 | 0.004** |
| Bacterial number (mean ± SD)* | 3.2±0.8 | 1.3±0.6 | 2.7±1.5 | 0.065# |
| Trauma | 4 | 0 | 0 | 0.183 |
| Cerebrovascular diseases | 3 | 3 | 1 | 0.103 |
| Infectious diseases | 1 | 0 | 1 | 0.579 |
| Post-resuscitation | 1 | 0 | 0 | 0.846 |
| Burn | 0 | 0 | 1 | 0.002 |
Notes:
Bacterial number: 1, <10 colonies/mL; 2, 10–100 colonies/mL; 3, 100–500 colonies/mL; and 4, >500 colonies/mL, respectively. MRSA detected from PCR+Culture− were collected within a week after first PCR/Culture.
P<0.1,
P<0.05.
Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus; PCR, polymerase chain reaction; SD, standard deviation; SIRS, systemic inflammatory response syndrome; SOFA, sepsis-related organ failure assessment; APACHE, Acute Physiology and Chronic Health Evaluation; LOS, length of stay.
Among these patients, cases suspected to be imported/acquired, colonization/infection, anti-MRSA drug use, and survival rates were also similar, but a significantly shorter length of stay and a tendency for younger patients were suggested in PCR+Culture− cases, although PCR+Culture+ and PCR−Culture+ patients appeared older and stayed longer.
Trauma was the most common primary diagnosis in PCR+Culture+ patients, while there were no trauma patients in the PCR+Culture− and PCR−Culture+ patients. Cerebrovascular diseases were seen in three PCR+Culture+ and one PCR−Culture+ patient, and all three PCR+Culture− patients had cerebrovascular diseases as underlying diseases. One PCR+Culture+ and one PCR−Culture+ patient had infectious diseases, and post-resuscitation and a burn were seen in a PCR+Culture+ and a PCR−Culture+ patient, respectively.
MRSA findings by PCR assay and comparison with the bacterial culture method
Table 2 shows the results of the PCR assay and culture method with the 95 swab samples tested in this study. Of these, 12 (12.6%) nasal swabs were positive for MRSA colonization on PCR and/or culture. Among these, three of 12 (25.0%) were diagnosed by PCR alone, whereas three of 12 (25.0%) were diagnosed by culture alone. The sensitivity and specificity of PCR were 75.9% (9/12) and 96.4% (80/83), respectively.
Table 2.
Positive and negative results of PCR and culture for MRSA
| BD GeneOhm™ MRSA Assay (PCR) | Total | ||
|---|---|---|---|
| + | − | ||
| Culture | |||
| + | 9 | 3 | 12 |
| − | 3 | 80 | 83 |
| Total | 12 | 83 | 95 |
Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus; PCR, polymerase chain reaction.
Discussion
MRSA is one of the most important pathogens that cause infection among critically ill patients admitted to an emergency department. The report from the National Nosocomial Infection Surveillance System and the present data showed that approximately 60% of all S. aureus nosocomial infections in intensive care units were methicillin-resistant.1,9
In the present study, there was a relatively high rate of MRSA colonization in patients admitted to an emergency department in Japan. The colonization rate was 3.2% (3/95), but it increased to 6.3% (6/95) if the three PCR or culture alone-positive patients who were MRSA carriers on admission were included. This 6.3% prevalence rate at emergency department admission found in this study is lower than in some earlier studies in the endemic area of Europe, which ranged from 8% to 10%, but similar to the rates at admission in the studies in the United States, Canada, and France of 4% to 9%.3,10–13
The prevalence rate of MRSA colonization, detected by not only PCR, but also culture methods, in these studies of high-risk patients suggests the value of PCR screening in such patients. The positive proportion of patients in the present study on both methods, 6.3%, was relatively higher than the 4% on culture when all of the MRSA cases that had been imported from other wards within the hospital or had a history of visiting other hospitals were investigated.10 This result might be due to their restriction of screening to patients considered at high risk of MRSA carriage, those transferred from other wards with a known high prevalence rate of MRSA, those with a long stay in another ward before emergency department admission, and those with a history of admission in high-risk hospitals and units. Patients with these risk factors for MRSA carriage may be more likely to have an MRSA-positive clinical specimen at emergency department admission.12
In this study, all of those acquiring MRSA during admission to our emergency department had risk factors for MRSA colonization, such as trauma, cerebrovascular diseases, infectious diseases, post-resuscitation, and burns. It has been reported that risk factors for MRSA colonization include: hemodialysis; illicit intravenous drug use; previous exposure to antimicrobial agents; prolonged hospitalization; and severe underlying illness, such as dermatitis and diabetes mellitus. Furthermore, 25% of individuals who acquired MRSA colonization during admission subsequently developed MRSA infection.14 Detecting MRSA colonization at or after hospitalization may be a target high-risk population that may benefit from interventions to decrease the risk for MRSA infection subsequently.
In the present study, 20% of MRSA positive patients were PCR+Culture−. Lucet et al reported that 54% of MRSA colonized patients might have remained undetected at the time of admission in their study of intensive care unit patients.12 Although the use of nasal cultures alone for the detection of MRSA colonization shows 78%–85% sensitivity, the present results were similar and also suggest that active surveillance requires PCR, and that doing so must lead to an increase in the detection rate for MRSA.
The PCR assay in the present study was very effective for MRSA detection in critically ill patients. In fact, the PCR+Culture− patients stayed shorter and had a tendency to be younger, with a possibility of lower MRSA colonization, suggesting that they were low-risk carriers. Ghazal et al performed preemptive screening in emergency departments; specifically, they used PCR testing of nasal swab specimens collected from patients 1) who had been transferred from other hospitals, 2) who had a history of prior hospitalization in the last 6 months, or 3) who had a history of infection or colonization with MRSA to identify active carriers.15 Maximum precautions were performed for any patients who had positive PCR test results. They reported that the hospital-acquired-MRSA infection rate decreased significantly, from 0.17 cases per 1,000 patient-days in 2007 to 0.03 cases per 1,000 patient-days in 2009, and the rate of hospital-acquired-MRSA bloodstream infections decreased from 0.1 cases per 1,000 patient-days in 2007 to 0 cases per 1,000 patient-days in 2009.15 These results also strongly suggest that PCR-based surveillance might lead to early interventions by the Infection Control Team, and they could efficiently reduce nosocomial MRSA transmission and infection.
With regard to the BD GeneOhm MRSA assay, previous studies have confirmed that this assay is both specific and sensitive.6,16,17 The sensitivity of the BD GeneOhm MRSA assay in the present study was relatively low, although the specificity was similar to previous reports. These discrepant results might be due to the lower number of analyzed samples and cases, as when we previously investigated the clinical utility of the SeptiFast system, a multiplex pathogen detection system for bloodstream infection.7 Further investigation and detailed analysis are needed, but, interestingly, nine of the present patients who were MRSA-negative by both PCR and culture at admission subsequently became positive by both methods after admission; this suggested that “selective pressure” for MRSA was present. If so, this result may suggest that some of the present false-positive patients may have been true-positive. In fact, all five of nine (55.9%) patients were treated with antibiotics, including four with sulbactam/ampicillin and one with tazobactam/piperacillin. Furthermore, Senn et al reported that sensitivities of MRSA detection from nasal swabs alone were 48% and 62% by culture and by rapid PCR test, respectively. These percentages increased to 79% and 92% with the addition of groin swabs, and to 96% and 99% with the addition of groin and throat swabs.18 Collecting additional swabs including the groin and throat may increase the sensitivity of both PCR and conventional culture methods. We could use the PCR methods efficiently to perform effective prevention of MRSA transmission if we could collect samples from several sites.
In conclusion, PCR-based active surveillance was performed, and it showed a relatively high rate of MRSA colonization at the time of admission to the emergency department. PCR+Culture− patients showed a tendency of being younger and had a shorter stay, which suggested colonization with a relatively lower number of MRSA. These data suggest that PCR-based active surveillance was rapid and more sensitive than conventional culture methods, although the present study was performed in a single facility and the sample number was small. This PCR-based active surveillance system in combination with the implementation of contact precautions for patients with MRSA-positive results may contribute to further reductions of MRSA transmission in emergency departments of hospitals in the near future.
Footnotes
Disclosure
The authors have no conflicts of interest to disclose.
References
- 1.Isobe M, Uejima E, Seki M, et al. Methicillin-resistant Staphylococcus aureus bacteremia at a university hospital in Japan. J Infect Chemother. 2012;18(6):841–848. doi: 10.1007/s10156-012-0423-6. [DOI] [PubMed] [Google Scholar]
- 2.Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52:285–292. doi: 10.1093/cid/cir034. [DOI] [PubMed] [Google Scholar]
- 3.Salangsang JA HL, Brooks MM, Shutt KA, Saul MI, Muto CA. Patient-associated risk factors for acquisition of methicillin-resistant Staphylococcus aureus in a tertiary care hospital. Infect Control Hosp Epidemiol. 2010;31(11):1139–1147. doi: 10.1086/656595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muto CA, Jernigan JA, Ostrowsky BE, et al. SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and Enterococcus. Infect Control Hosp Epidemiol. 2003;24(5):362–386. doi: 10.1086/502213. [DOI] [PubMed] [Google Scholar]
- 5.Bühlmann M, Bögli-Stuber K, Droz S, Mühlemann K. Rapid screening for carriage of methicillin-resistant Staphylococcus aureus by PCR and associated costs. J Clin Microbiol. 2008;46(7):2151–2154. doi: 10.1128/JCM.01957-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taguchi H, Matsumoto T, Ishikawa H, Ohta S, Yukioka T. Prevalence of methicillin-resistant Staphylococcus aureus based on culture and PCR in inpatients at a tertiary care center in Tokyo, Japan. J Infect Chemother. 2012;18(5):630–636. doi: 10.1007/s10156-012-0385-8. [DOI] [PubMed] [Google Scholar]
- 7.Yanagihara K, Kitagawa Y, Tomonaga M, et al. Evaluation of pathogen detection from clinical samples by real-time polymerase chain reaction using a sepsis pathogen DNA detection kit. Crit Care. 2010;14(4):R159. doi: 10.1186/cc9234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seki M, Gotoh K, Nakamura S, et al. Fatal sepsis caused by an unusual Klebsiella species that was misidentified by an automated identification system. J Med Microbiol. 2013;62(Pt 5):801–803. doi: 10.1099/jmm.0.051334-0. [DOI] [PubMed] [Google Scholar]
- 9.National Nosocomial Infections Surveillance System National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32(8):470–485. doi: 10.1016/S0196655304005425. [DOI] [PubMed] [Google Scholar]
- 10.Girou E, Pujade G, Legrand P, Cizeau F, Brun-Buisson C. Selective screening of carriers for control of methicillin-resistant Staphylococcus aureus (MRSA) in high-risk hospital areas with a high level of endemic MRSA. Clin Infect Dis. 1998;27(3):543–550. doi: 10.1086/514695. [DOI] [PubMed] [Google Scholar]
- 11.Talon D, Rouget C, Cailleaux V, et al. Nasal carriage of Staphylococcus aureus and cross-contamination in a surgical intensive care unit: efficacy of mupirocin ointment. J Hosp Infect. 1995;30(1):39–49. doi: 10.1016/0195-6701(95)90247-3. [DOI] [PubMed] [Google Scholar]
- 12.Lucet JC, Chevret S, Durand-Zaleski I, Chastang C, Régnier B, Multicenter Study Group Prevalence and risk factors for carriage of methicillin-resistant Staphylococcus aureus at admission to the intensive care unit: results of a multicenter study. Arch Intern Med. 2003;163(2):181–188. doi: 10.1001/archinte.163.2.181. [DOI] [PubMed] [Google Scholar]
- 13.Warren DK, Guth RM, Coopersmith CM, Merz LR, Zack JE, Fraser VJ. Epidemiology of methicillin-resistant Staphylococcus aureus colonization in a surgical intensive care unit. Infect Control Hosp Epidemiol. 2006;27(10):1032–1040. doi: 10.1086/507919. [DOI] [PubMed] [Google Scholar]
- 14.Davis KA, Stewart JJ, Crouch HK, Florez CE, Hospenthal DR. Methicillin-resistant Staphylococcus aureus (MRSA) nares colonization at hospital admission and its effect on subsequent MRSA infection. Clin Infect Dis. 2004;39(6):776–782. doi: 10.1086/422997. [DOI] [PubMed] [Google Scholar]
- 15.Ghazal SS, Hakawi AM, Demeter CV, Joseph MV, Mukahal MA. Intervention to reduce the incidence of healthcare-associated methicillin-resistant Staphylococcus aureus infection in a Tertiary Care Hospital in Saudi Arabia. Infect Control Hosp Epidemiol. 2011;32(4):411–413. doi: 10.1086/659256. [DOI] [PubMed] [Google Scholar]
- 16.Farley JE, Stamper PD, Ross T, Cai M, Speser S, Carroll KC. Comparison of the BD GeneOhm methicillin-resistant Staphylococcus aureus (MRSA) PCR assay to culture by use of BBL CHROMagar MRSA for detection of MRSA in nasal surveillance cultures from an at-risk community population. J Clin Microbiol. 2008;46(2):743–746. doi: 10.1128/JCM.02071-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sumitani Y, Kobayashi Y. Comparative evaluation of a rapid MRSA detection assay based on multiplex real-time PCR versus MRSA screening cultures containing egg yolk. J Infect Chemother. 2009;15(4):262–265. doi: 10.1007/s10156-009-0685-9. [DOI] [PubMed] [Google Scholar]
- 18.Senn L BP, Nahimana I, Zanetti G, Blanc DS. Which anatomical sites should be sampled for screening of methicillin-resistant Staphylococcus aureus carriage by culture or by rapid PCR test? Clin Microbiol Infect. 2012;18(2):E31–E33. doi: 10.1111/j.1469-0691.2011.03724.x. [DOI] [PubMed] [Google Scholar]