Abstract
Objectives
In 2013, the Global Initiative for Chronic Obstructive Lung Disease (GOLD) updated the management strategy on COPD based on severity using a combined assessment of symptoms, degree of airflow limitation, and number of exacerbations. This study quantified prevalence and incidence of COPD in the United Kingdom and estimated disease severity by GOLD 2013 categories A/B (low risk) and C/D (high risk).
Methods
The Clinical Practice Research Datalink was used to identify COPD patients ≥40 years. Patient characteristics were described, and prevalence was calculated on December 31, 2013. Five-year incidence (2009–2013) was estimated, with rates standardized using 2011 UK population age and sex. To classify patients by GOLD categories, spirometry results, the modified British Medical Research Council grade, and history of exacerbations were used.
Results
The prevalent cohort comprised 49,286 patients with COPD with mean age 70 years; 51.0% were male. Overall prevalence was 33.3 per 1,000 persons (95% confidence interval [CI]: 33.1–33.6); 66.4% were classified as GOLD A/B and 33.6% as C/D. The standardized prevalence of GOLD A/B was 21.9 per 1,000 persons (95% CI: 21.7–22.1) and of C/D was 11.1 (95% CI: 10.9–11.2). A total of 27,224 newly diagnosed COPD patients were identified with mean age 67 years at diagnosis; 53.0% were male. Incidence was 2.2 per 1,000 person-years (95% CI: 2.2–2.3); 68.7% were classified in categories A/B and 31.3% in C/D, of which 17.2% did not receive COPD maintenance medication.
Conclusion
A third of COPD patients in the UK are considered high risk (GOLD 2013 categories C/D), and a third of patients are diagnosed for the first time at these severe stages. Given the progressive nature of the disease, results suggest that closer attention to respiratory symptoms for early detection, diagnosis, and appropriate treatment of COPD in the UK is warranted.
Keywords: COPD, prevalence, incidence, GOLD 2013, primary care management
Introduction
COPD is a progressive respiratory disease characterized by persistent airflow obstruction that is not reversible.1,2 Airflow obstruction in COPD is associated with increased morbidity and mortality, with cardiovascular diseases and lung cancer being the leading comorbidities and causes of death among patients with COPD.3–5 Acute exacerbations experienced by patients with COPD are defined by increased cough, dyspnea, or sputum purulence and are associated with the rapid decline of lung function.4
Existing epidemiological data on COPD incidence and prevalence show a wide variation in estimates due to differences in survey methods, diagnostic criteria, and analytic approaches.1,6,7 In the United Kingdom, it is estimated that more than 3 million people are diagnosed with COPD.2 In the context of UK’s aging population, these numbers are expected to increase as COPD becomes more commonly diagnosed with increasing age, posing significant economic burden to the UK National Health Service. Regardless of severity, the average annual cost of COPD management including the cost of exacerbations, COPD hospitalizations, and general practitioner (GP) visits and excluding nonexacerbation-related medications, is estimated at £2,108 per patient.8 Approximately 30,000 people in the UK die of COPD annually.2 Worldwide, it is estimated that COPD accounted for more than 3.1 million deaths in 2012, corresponding to 5.6% of all deaths, classifying COPD as the third leading cause of death globally.9
In the UK, the diagnosis of COPD in the primary care setting is made on the basis of symptoms and signs supported by spirometry according to the National Institute for Health and Care Excellence (NICE) guidelines.2 The new Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2013 classification aims to guide therapy by determining the severity of the disease, according to the combined evaluation of patients’ symptoms including dyspnea, severity of spirometric abnormality, risk of exacerbations based on prior history, and comorbidities.1
Given that guidance on treatment options is provided according to disease severity, it is important to assess patient characteristics, particularly in the primary care setting where the majority of care of COPD is delivered in the UK. In the present study, we used retrospective, observational primary care data to quantify the cross-sectional point prevalence of COPD at the end of 2013 and the 5-year incidence (2009–2012) in the UK according to the GOLD 2013 severity categories. In addition, the study investigated patient characteristics and medication use among patients with COPD in the UK.
Methods
Data
GPs are the primary point of contact for nonurgent patients within the UK National Health Service, often referred to as “gate-keepers” for patient specialist care. The majority of patients with chronic respiratory symptoms are diagnosed and managed by GPs in the primary care setting in the UK. The Quality and Outcomes Framework, an annual reward and incentive program detailing GP practice achievement results, includes eight indicators for COPD from initial diagnosis to ongoing management. Examples of such indicators are the percentage of patients with a first diagnosis confirmed by spirometry or the percentage of existing patients with recent records of spirometry and dyspnea assessment and are aimed to provide incentives for GPs to better record and subsequently assess patients with COPD.10,11
Primary care data were extracted from the Clinical Practice Research Datalink (CPRD). The CPRD contains longitudinal data from 630 real-life clinical practices and covers approximately 8% of the population in the UK. The patient population captured in the database is known to be broadly representative of the UK population. CPRD data have previously been used for research in the COPD area as well as epidemiological research.3–5,12–14 The study protocol was approved by the Independent Scientific Advisory Committee for Medicines and Healthcare products Regulatory Agency Database Research (protocol number 14_052).
Study population
Validated Read codes indicating COPD, chronic bronchitis, or emphysema were used to identify patients with a diagnosis of COPD at any time in their medical record until December 31, 2013, inclusive. Only patients aged 40 years and older at diagnosis were included in the analysis from practices whose data met “Acceptable Quality Standard” in the CPRD.
Patients with a diagnosis prior to January 1, 2009, and who were active on December 31, 2013, were entered into a prevalent cohort. Patients entered an incident cohort if they had a first-time COPD diagnosis between January 1, 2009, and December 31, 2013. In both cohorts, patients with less than 12 months of active data recoding history prior to cohort entry were excluded from the analysis.
Study measures
Clinical and demographic characteristics were assessed at the time of first diagnosis (index date) for the incident cohort and on December 31, 2013, for the prevalent cohort. These characteristics included age, sex, and smoking history. Commonly reported comorbid conditions in patients with COPD as previously identified15 were investigated: myocardial infarction, diabetes mellitus, hypertension, osteoporosis, anxiety, and depression. Chronic conditions were assessed from the whole patient record except for depression, for which the assessment period was 12 months prior to index date.
Prescribed maintenance therapies for COPD were identified in the 6 months prior to the point prevalence and index date for the prevalent and incident patients, respectively. The investigated treatments were those recommended by GOLD1 as monotherapy or combination therapy: short-acting beta agonists (SABA), short-acting anticholinergics, long-acting beta agonists (LABA), long-acting anticholinergics (LAMA), methylxanthines, inhaled corticosteroids (ICS), and oral corticosteroids (OCS). OCS prescriptions during exacerbation episodes were excluded and were assessed as a part of exacerbations.
GOLD classification parameters
In order to classify patients into the GOLD categories, parameters to assess the exacerbation risk and symptoms as outlined in the GOLD 2013 management strategy1 were investigated. Classification of airflow limitation severity was performed based on %predicted forced expiratory volume in 1 second (FEV1) test results recorded in CPRD, which were classified according to the GOLD 2013 stages: I (mild): FEV1 ≥80% predicted; II (moderate): FEV1 ≥50% and <80% predicted; III (severe): FEV1 ≥30% and <50% predicted; and IV (very severe): FEV1 <30% predicted. Dyspnea was assessed using the available Medical Research Council (MRC) score identified through Read codes and mapped to the modified MRC (mMRC) dyspnea grade used in GOLD 2013. The original MRC scale from 1 (least severe breathlessness) to 5 (more severe breathlessness) equals mMRC grades 0–4, with MRC grade 1 equal to mMRC grade 0.16 The most recent FEV1 and MRC measurements were assessed between December 31, 2012, and up to the end of data collection (February 2014) for the prevalent cohort, and within the 12 months prior to or after index date for the incident cohort.
In this study, exacerbation episodes were identified by the following:
A Read code in the clinical record indicating exacerbations or emergency admission to hospital due to COPD; or
An OCS and oral antibiotic prescription occurring on the same date with the OCS not being used as maintenance treatment; or
An OCS prescription or oral antibiotic prescription that is not part of a maintenance episode occurring on the same date as a specific list of Read codes indicating exacerbations.
To identify exacerbation episodes, a 21-day time period was used;17 that is, consecutive prescriptions of oral steroids, oral or injected antibiotics, or hospitalizations for the same reason of admission within 21 days of the first record were considered to be a single exacerbation. Episodes were identified in the 12 months prior to the prevalence point (prevalent cohort) and prior to index date (incident cohort).
Statistical analyses
Descriptive data on clinical and demographic characteristics, and the GOLD classification parameters for the prevalent and incident cohorts, were presented as mean (standard deviation [SD]), median (interquartile range), or percentages, as appropriate.
Cross-sectional point prevalence rate (per 1,000) were calculated by dividing the number of cases of COPD classified in each of the GOLD categories on December 31, 2013 by the number of subjects without COPD registered in CPRD at that point in time (total population). Total 5-year crude incidence rates (per 1,000 patient-years) were calculated by dividing the number of incident cases of COPD classified by GOLD category by the number of person-years at risk for subjects registered in CPRD over the 5-year study period. The analyses included stratification of prevalence and incidence rates by age and sex, using the 2012 and 2011 UK population estimates obtained from the UK Office of National Statistics.18 The 95% confidence intervals (CIs) were calculated based on the Poisson approximation.
All data programming and analyses were carried out using SAS (version 9.2) and Stata MP v12.
Results
Epidemiology of COPD by GOLD categories
The UK age- and sex-standardized prevalence of COPD was 21.9 per 1,000 persons (95% CI: 21.7–22.1) among patients in the low-risk GOLD categories A/B and 11.1 per 1,000 persons (95% CI: 10.9–11.2) in the high-risk GOLD categories C/D (Table 1).
Table 1.
Prevalence rates (per 1,000 persons) and incidence rates (per 1,000 person-years) by GOLD category
| GOLD category | Patients with COPD | Crude prevalence (95% CI) | Standardizeda prevalence (95% CI) | Total person-years at risk | Incident patients | Crude incidence (95% CI) | Standardizeda incidence (95% CI) |
|---|---|---|---|---|---|---|---|
| All categories | 49,286 | 33.3 (33.1–33.6) | 33.0 (32.7–33.3) | 12,207,060 | 27,224 | 2.2 (2.2–2.3) | 2.2 (2.2–2.2) |
| A/B | 32,723 | 22.1 (21.9–22.4) | 21.9 (21.7–22.1) | 12,187,799 | 18,709 | 1.5 (1.5–1.6) | 1.5 (1.5–1.5) |
| C/D | 16,563 | 11.2 (11.0–11.4) | 11.1 (10.9–11.2) | 12,163,295 | 8,515 | 0.7 (0.7–0.7) | 0.7 (0.7–0.7) |
Notes: Total CPRD population =1,478,325.
Standardized for age and sex.
Abbreviations: CI, confidence interval; CPRD, Clinical Practice Research Datalink; GOLD, Global Initiative for Chronic Obstructive Lung Disease; GOLD A, low symptoms, low risk; GOLD B, high symptoms, low risk; GOLD C, low symptoms, high risk; GOLD D, high symptoms, high risk.
Across high- and low-risk groups, prevalence was higher in males than in females. By age group, the prevalence of COPD was highest among patients aged 75 years and above for both high-risk and low-risk GOLD categories (Figure 1).
Figure 1.
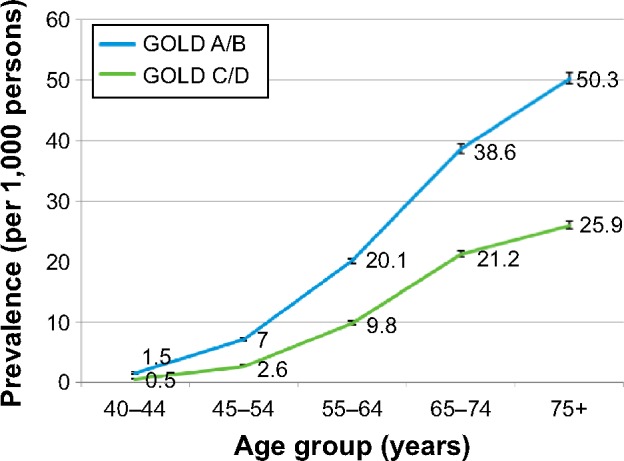
Age-stratified prevalence rates by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) category.
Abbreviations: GOLD A, low symptoms, low risk; GOLD B, high symptoms, low risk; GOLD C, low symptoms, high risk; GOLD D, high symptoms, high risk.
The overall age- and sex-standardized 5-year (2009–2013) incidence rate of COPD was 2.2 per 1,000 person-years (95% CI: 2.2, 2.2). The corresponding age- and sex-standardized prevalence of COPD in the low-risk GOLD categories A/B was 1.5 per 1,000 person-years (95% CI: 1.5, 1.5), which was higher than the prevalence of COPD in the high-risk GOLD categories C/D of 0.7 per 1,000 person-years (95% CI: 0.7, 0.7) (Table 1).
Incidence peaked in patients classified in high-risk categories C/D among patients aged 65–74 years old; this peak was also observed for the same age group in patients classified in low-risk categories A/B (Figure 2). Crude incidence was higher for males than females in both GOLD risk groups: 1.7 versus 1.4 per 1,000 in A/B; 0.8 versus 0.6 in C/D.
Figure 2.
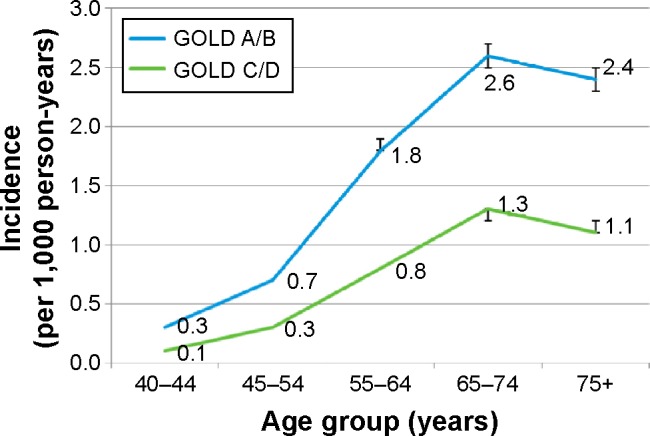
Age-stratified incidence rates by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) category.
Abbreviations: GOLD A, low symptoms, low risk; GOLD B, high symptoms, low risk; GOLD C, low symptoms, high risk; GOLD D, high symptoms, high risk.
Clinical and demographic patient characteristics
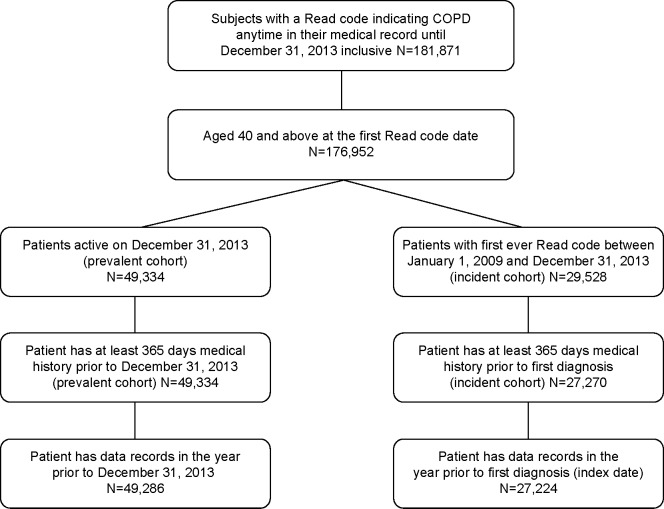
A total of 181,871 patients were identified with a Read code indicative of COPD anytime in their medical record prior to December 31, 2013. A total of 4,919 (2.7%) patients had their first COPD record at an age younger than 40 years and were excluded from the analysis. Of the 176,952 patients with COPD included in the study population, 49,286 patients met the criteria for inclusion in the prevalent cohort, and 27,224 newly diagnosed COPD cases were included in the incident cohort (Figure 3).
Figure 3.
Study patient identification.
Prevalent cohort
The average age at the time of diagnosis in the COPD prevalent cohort was 64.1 (SD 10.8) years, 51.0% were male. Of the patients, 78.2% were either current or former smokers (50.7% were ex-smokers and 27.5% were classified as current smokers) (Table 2). On December 31, 2013, the average time since diagnosis was 6.5 years. The most commonly recorded comorbidities were hypertension (34.5%), diabetes (19.4%), and osteoporosis (11.6%).
Table 2.
Characteristics of the prevalent and incident cohorts
| Prevalent cohort (N=49,286) |
Incident cohort (N=27,224) |
|
|---|---|---|
| Age at point prevalence, mean (SD) | 70.1 (10.9) | |
| Sex, n (%) | ||
| Male | 25,142 (51.0) | 14,550 (53.0) |
| Female | 24,144 (49.0) | 12,674 (47.0) |
| Age at diagnosis, mean (SD) | 64.1 (10.8) | 67.0 (11.4) |
| Time since diagnosis (months), mean (SD) | 77.4 (65.3) | |
| Comorbidities, n (%) | ||
| Myocardial infarction | 4,127 (8.4) | 2,068 (7.6) |
| Diabetes | 9,575 (19.4) | 3,731 (13.7) |
| Hypertension | 16,994 (34.5) | 8,417 (30.9) |
| Osteoporosis | 5,696 (11.6) | 1,740 (6.4) |
| Anxiety | 1,001 (2.0) | 623 (2.3) |
| Depression | 1,452 (3.0) | 970 (3.6) |
| Smoking history, n (%) | ||
| Unknown | 6,422 (13.0) | 3,103 (11.4) |
| Current smoker | 13,550 (27.5) | 11,122 (40.9) |
| Never smoked | 4,314 (8.8) | 1,853 (6.8) |
| Ex-smoker | 25,000 (50.7) | 11,146 (40.9) |
| Maintenance therapy, n (%) | ||
| Monotherapy | 12,710 (25.8) | 13,215 (48.5) |
| Combination therapy | 27,561 (55.9) | 7,167 (26.3) |
| Patients not on any treatment | 8,987 (18.2) | 6,835 (25.1) |
Abbreviations: n, number of patients; SD, standard deviation.
On December 31, 2013, the majority of prevalent patients with COPD (55.9%) were prescribed combination therapy (Table 3). The most frequent combination therapy was LABA + LAMA + ICS, prescribed for 28.6% of patients, followed by LABA + ICS (20.1%). Monotherapy was prescribed in 25.8% of the prevalent patients, with SABA and LAMA being the most frequently prescribed monotherapies (11.2% and 10.1%, respectively); 18.2% did not have a prescription of COPD medication in their medical record.
Table 3.
COPD therapy for stable COPD in the prevalent and incident cohorts
| Prevalent cohort (N=49,286), n (%) |
Incident cohort (N=27,224), n (%) |
|
|---|---|---|
| Total monotherapies | 12,710 (25.8) | 13,215 (48.5) |
| SABA | 5,499 (11.2) | 7,645 (28.1) |
| SAMA | 272 (0.6) | 403 (1.5) |
| LABA | 1,209 (2.5) | 1,323 (4.9) |
| LAMA | 4,977 (10.1) | 3,299 (12.1) |
| LAMA + SABA | 3,466 (69.6) | 2,462 (74.6) |
| LAMA + SAMA | 96 (1.9) | 90 (2.7) |
| Methylxanthines | 26 (0.1) | 2 (0.01) |
| ICS | 637 (1.3) | 493 (1.8) |
| OCS | 90 (0.2) | 50 (0.2) |
| Total combination therapies | 27,561 (55.9) | 7,167 (26.3) |
| LABA + ICS (as both FDC and combination) | 9,896 (20.1) | 3,686 (13.5) |
| LABA + ICS + SABA | 7,505 (75.8) | 2,980 (80.9) |
| LABA + ICS + SAMA | 1,219 (12.3) | 247 (6.7) |
| LABA + LAMA + ICS | 14,088 (28.6) | 2,044 (7.5) |
| LABA + LAMA + ICS + SABA | 12,137 (86.2) | 1,716 (84.0) |
| LABA + LAMA + ICS + SAMA | 382 (2.7) | 104 (5.1) |
| LAMA + ICS | 210 (0.4) | 106 (0.4) |
| LAMA + ICS + SABA | 155 (73.8) | 81 (76.4) |
| LAMA + ICS + SAMA | 5 (2.4) | 7 (6.6) |
| LABA + LAMA | 844 (1.7) | 480 (1.8) |
| SABA + SAMA only | 472 (1.0) | 647 (2.4) |
| Methylxanthines + ICS | 440 (0.9) | 55 (0.2) |
| Methylxanthines + LABA | 20 (0.0) | 24 (0.1) |
| Methylxanthines + LAMA | 67 (0.1) | 16 (0.1) |
| Methylxanthines + (LABA or LAMA or ICS) | 1,524 (3.1) | 109 (0.4) |
| Any other combinations | 28 (0.1) | 7 (0.03) |
| Patients not on any of the above treatments | 8,987 (18.2) | 6,835 (25.1) |
Abbreviations: ICS, inhaled corticosteroids; FDC, fixed-dose combination; LABA, long-acting beta agonists; LAMA, long-acting anticholinergics; n, number of patients; OCS, oral corticosteroids; SABA, short-acting beta agonists; SAMA, short-acting anticholinergics.
Incident cohort
The mean (SD) age of the incident cohort was 67 (11.4) years, 53.0% were males, 81.8% of the patients were either current or former smokers (40.9% were ex-smokers and 40.9% of patients were classified as current smokers). The most commonly recorded comorbidities were hypertension (30.9%), diabetes (13.7%), and myocardial infarction (7.6%).
Incident patients were most frequently prescribed monotherapies (48.5%), with 28.1% being prescribed SABA as monotherapy. At the time of first diagnosis, 25.1% of patients did not have a prescription of COPD medication in their medical record.
GOLD assessment
The proportion of prevalent patients with a FEV1% predicted measurement recorded was 55.2% and 66.0% for patients with an MRC score around point prevalence date (Table 4). Almost half the patients with available FEV1 records (53.5%) had moderate airflow limitation, followed by 25.6% with severe to very severe airflow limitation, and 20.8% with mild airflow limitation. Of patients with mMRC scores, the largest proportion had grade 1 (38.9%) followed by grade 2 (26.6%) dyspnea. Exacerbations were defined for all patients. Evidence of at least one exacerbation episode in the 12 months prior to December 31, 2013, was present in 51.7% of patients. Two or more exacerbations were identified among 25.5% of prevalent patients.
Table 4.
GOLD classification parameters for the prevalent and incident cohorts
| Prevalent cohort (N=49,286), n (%) |
Incident cohort (N=27,224), n (%) |
|
|---|---|---|
| FEV1 (at least one test recorded) | 27,184 (55.2) | 22,214 (81.6) |
| I. Mild: FEV1 >80% | 5,072 (20.8) | 3,669 (18.3) |
| II. Moderate FEV1 50%–80% | 13,027 (53.5) | 12,230 (61.0) |
| III. Severe FEV1 30%–50% | 5,218 (21.4) | 3,653 (18.2) |
| IV. Very severe FEV1 <30% | 1,016 (4.2) | 508 (2.5) |
| mMRC (at least one score recorded) | 32,515 (66.0) | 22,144 (81.3) |
| Grade 0 | 5,069 (15.6) | 4,885 (22.1) |
| Grade 1 | 12,644 (38.9) | 9,785 (44.2) |
| Grade 2 | 8,655 (26.6) | 5,071 (22.9) |
| Grade 3 | 5,066 (15.6) | 2,057 (9.3) |
| Grade 4 | 1,081 (3.3) | 346 (1.6) |
| Number of patients with exacerbations | ||
| 0 | 23,865 (48.4) | 14,301 (52.5) |
| 1 | 12,886 (26.2) | 7,786 (28.6) |
| 2+ | 12,535 (25.5) | 5,137 (18.9) |
Abbreviations: FEV1, forced expiratory volume in 1 second; GOLD, Global Initiative for Chronic Obstructive Lung Disease; mMRC, modified Medical Research Council Dyspnea Scale.
In the incident cohort, 81.6% of patients had a least one valid FEV1% predicted record in the year prior to diagnosis. The distribution of patients by airflow limitation was similar to that in the prevalent cohort, with 61.0% of patients with moderate airflow limitation, followed by 20.7% with severe to very severe, and 18.2% with mild airflow limitation. MRC scores were available for 81.3% of patients in the 12 months around diagnosis (Table 4) and were mapped to the mMRC dyspnea scale. Most newly diagnosed patients had grade 1 (44.2%), while 22.9% had grade 2 dyspnea. Records for both FEV1% predicted and the mapped mMRC were present in 65.5% of the incident cohort. A total of 47.5% of the incident cohort had evidence of at least one exacerbation in their record, in the year prior to diagnosis. Two or more exacerbations were identified among 18.9% of newly diagnosed patients.
As data were not available for mMRC scores required for symptom assessment, 16,771 (34.0%) patients in the prevalent cohort and 5,080 (18.7%) patients in the incident cohort could not be classified into one of the A, B, C, or D GOLD categories. On the basis of history of exacerbations, total patients in both cohorts were classified into combined GOLD categories A/B or C/D, representing low and high risk, respectively. Of the 49,286 prevalent patients, 66.4% were classified in GOLD categories A/B and 33.6% were classified in categories C/D. Of the 27,224 patients identified in the incident cohort, 68.7% were classified in GOLD categories A/B and 31.3% in GOLD categories C/D.
Clinical and demographic patient characteristics by GOLD categories
Prevalent cohort
Prevalent patients in GOLD A/B categories had a mean age of 64 (SD 11.0) years at diagnosis, 51.1% were male, 49.1% were ex-smokers, and 27.1% were classified as current smokers (Table 5). Patients classified in GOLD categories C/D were slightly younger with a mean age of 63.7 (10.4), 50.8% were males, 54.0% were ex-smokers, and 28.4% were classified as current smokers. The prevalence of comorbidities was similar in both incident and prevalent groups, with the most common conditions being hypertension (GOLD A/B: 34.7%; GOLD C/D: 34.0%), diabetes (GOLD A/B: 19.0%; GOLD C/D: 20.2%), and osteoporosis (GOLD A/B: 10.5%; GOLD C/D: 13.7%).
Table 5.
Characteristics of the prevalent cohort: GOLD A/B and GOLD C/D
| GOLD category A/B | GOLD category C/D | |
|---|---|---|
| Sample size, n (%) | 32,723 (66.4) | 16,563 (33.6) |
| Age at point prevalence, mean (SD) | 69.9 (11.2) | 70.5 (10.3) |
| Sex, n (%) | ||
| Male | 16,725 (51.1) | 8,417 (50.8) |
| Female | 15,998 (48.9) | 8,146 (49.2) |
| Age at diagnosis, mean (SD) | 64.3 (11.0) | 63.7 (10.4) |
| Time since diagnosis (months), mean (SD) | 72.5 (62.0) | 87.1 (70.5) |
| Comorbidities, n (%) | ||
| Myocardial infarction | 2,620 (8.0) | 1,507 (9.1) |
| Diabetes | 6,227 (19.0) | 3,348 (20.2) |
| Hypertension | 11,361 (34.7) | 5,633 (34.0) |
| Osteoporosis | 3,430 (10.5) | 2,266 (13.7) |
| Anxiety | 564 (1.7) | 437 (2.6) |
| Depression | 902 (2.8) | 550 (3.3) |
| Smoking history, n (%) | ||
| Unknown | 4,643 (14.2) | 1,779 (10.7) |
| Current smoker | 8,852 (27.1) | 4,698 (28.4) |
| Never smoked | 3,176 (9.7) | 1,138 (6.9) |
| Ex-smoker | 16,052 (49.1) | 8,948 (54.0) |
Abbreviations: GOLD, Global Initiative for Chronic Obstructive Lung Disease; GOLD A, low symptoms, low risk; GOLD B, high symptoms, low risk; GOLD C, low symptoms, high risk; GOLD D, high symptoms, high risk; n, number of patients; SD, standard deviation.
The majority of patients for both risk groups were treated with a combination therapy (74.6% in most severe patients), with the most frequent combination LAMA + LABA + ICS, having been prescribed for 21.1% of low-risk A/B patients and for 43.4% among high-risk C/D patients (Table 6). Almost one-quarter of the patients in GOLD A/B categories and 5.9% of patients in GOLD C/D categories did not receive any of the treatments.
Table 6.
COPD therapy for stable COPD in the prevalent cohort: GOLD A/B and GOLD C/D
| GOLD category A/B (N=32,723), n (%) |
GOLD category C/D (N=16,563), n (%) |
|
|---|---|---|
| Total monotherapies | 9,493 (29.0) | 3,217 (19.4) |
| SABA | 4,262 (13.0) | 1,237 (7.5) |
| SAMA | 214 (0.7) | 58 (0.4) |
| LABA | 879 (2.7) | 330 (2.0) |
| LAMA | 3,520 (10.8) | 1,457 (8.8) |
| LAMA + SABA | 2,342 (66.5) | 1,124 (77.1) |
| LAMA + SAMA | 56 (1.6) | 40 (2.8) |
| Methylxanthines | 20 (0.1) | 6 (0.0) |
| ICS | 514 (1.6) | 123 (0.7) |
| OCS | 84 (0.3) | 6 (0.04) |
| Total combination therapies | 15,208 (46.5) | 12,353 (74.6) |
| LABA + ICS (as both FDC and combination) | 6,522 (19.9) | 3,374 (20.4) |
| LABA + ICS + SABA | 4,677 (71.7) | 2,828 (83.8) |
| LABA + ICS + SAMA | 666 (10.2) | 553 (16.4) |
| LABA + LAMA + ICS | 6,894 (21.1) | 7,194 (43.4) |
| LABA + LAMA + ICS + SABA | 5,654 (82.0) | 6,483 (90.1) |
| LABA + LAMA + ICS + SAMA | 133 (1.9) | 249 (3.5) |
| LAMA + ICS | 143 (0.4) | 67 (0.4) |
| LAMA + ICS + SABA | 100 (69.9) | 55 (82.1) |
| LAMA + ICS + SAMA | 2 (1.4) | 3 (4.5) |
| LABA + LAMA | 519 (1.6) | 325 (2.0) |
| SABA + SAMA only | 330 (1.0) | 142 (0.9) |
| Methylxanthines + ICS | 222 (0.7) | 218 (1.3) |
| Methylxanthines + LABA | 13 (0.04) | 7 (0.04) |
| Methylxanthines + LAMA | 36 (0.1) | 31 (0.2) |
| Methylxanthines + (LABA or LAMA or ICS) | 529 (1.6) | 995 (6.0) |
| Any other combinations | 14 (0.04) | 14 (0.1) |
| Patients not on any of the above treatments | 8,008 (24.5) | 979 (5.9) |
Abbreviations: FDC, fixed-dose combination; GOLD, Global Initiative for Chronic Obstructive Lung Disease; GOLD A, low symptoms, low risk; GOLD B, high symptoms, low risk; GOLD C, low symptoms, high risk; GOLD D, high symptoms, high risk; ICS, inhaled corticosteroids; LABA, long-acting beta agonists; LAMA, long-acting anticholinergics; OCS, oral corticosteroids; SABA, short-acting beta agonists; SAMA, short-acting anticholinergics.
Incident cohort
Newly diagnosed patients in GOLD A/B categories had a mean (SD) age of 67 (11.5) years at first diagnosis, 53.5% were male, 39.9% were ex-smokers, and 40.4% were classified as current smokers (Table 7). Incident patients in GOLD C/D categories had a mean (SD) age of 67 (11.1) years and a high proportion (41.9%) of patients were classified as current smokers. Similar to the prevalent cohort, the most common comorbidities among patients, regardless of severity, were hypertension (GOLD A/B: 30.8%; GOLD C/D: 31.2%), diabetes (GOLD A/B: 13%; GOLD C/D: 15.3%), and myocardial infarction (GOLD A/B: 7.3%; GOLD C/D: 8.3%).
Table 7.
Characteristics of the incident cohort: GOLD A/B and GOLD C/D
| GOLD category A/B | GOLD category C/D | |
|---|---|---|
| Sample size, n (%) | 18,709 (68.7) | 8,515 (31.3) |
| Age at diagnosis, mean (SD) | 66.8 (11.5) | 67.4 (11.1) |
| Sex, n (%) | ||
| Male | 10,008 (53.5) | 4,542 (53.3) |
| Female | 8,701 (46.5) | 3,973 (46.7) |
| Comorbidities, n (%) | ||
| Myocardial infarction | 1,358 (7.3) | 710 (8.3) |
| Diabetes | 2,429 (13.0) | 1,302 (15.3) |
| Hypertension | 5,763 (30.8) | 2,654 (31.2) |
| Osteoporosis | 1,153 (6.2) | 587 (6.9) |
| Anxiety | 405 (2.2) | 218 (2.6) |
| Depression | 675 (3.6) | 295 (3.5) |
| Smoking history, n (%) | ||
| Unknown | 2,411 (12.9) | 692 (8.1) |
| Current smoker | 7,554 (40.4) | 3,568 (41.9) |
| Never smoked | 1,272 (6.8) | 581 (6.8) |
| Ex-smoker | 7,472 (39.9) | 3,674 (43.2) |
| Maintenance therapy, n (%) | ||
| Total monotherapies | 9,163 (49.0) | 4,052 (47.6) |
| Total combination therapies | 4,169 (22.3) | 2,998 (35.2) |
| Patients not on any treatment | 5,373 (28.7) | 1,462 (17.2) |
Abbreviations: GOLD, Global Initiative for Chronic Obstructive Lung Disease; GOLD A, low symptoms, low risk; GOLD B, high symptoms, low risk; GOLD C, low symptoms, high risk; GOLD D, high symptoms, high risk; n, number of patients; SD, standard deviation.
The majority of low-risk patients were prescribed a monotherapy (GOLD A/B: 49.0%), with SABA being the most commonly used monotherapy (29.3%), whereas 22.4% received combination treatment (Table 8). For high-risk patients in GOLD C/D categories, 47.6% were on monotherapy and 35.2% received combination treatment, with LABA + ICS being the most frequently prescribed treatment (17.0% of GOLD C/D patients). However, around 17.2% of the most severe cases did not receive any of the specified treatments.
Table 8.
Chronic obstructive pulmonary disease maintenance therapy in the incident cohort: GOLD A/B and GOLD C/D
| GOLD category A/B (N=18,709), n (%) |
GOLD category C/D (N=8,515), n (%) |
|
|---|---|---|
| Total monotherapies | 9,163 (49.0) | 4,052 (47.6) |
| SABA | 5,481 (29.3) | 2,164 (25.4) |
| SAMA | 283 (1.5) | 120 (1.4) |
| LABA | 868 (4.6) | 455 (5.3) |
| LAMA | 2,127 (11.4) | 1,172 (13.8) |
| LAMA + SABA | 1,505 (70.8) | 957 (81.7) |
| LAMA + SAMA | 53 (2.5) | 37 (3.2) |
| Methylxanthines | 2 (0.01) | 0 (0.0) |
| ICS | 363 (1.9) | 130 (1.5) |
| OCS | 39 (0.2) | 11 (0.1) |
| Total combination therapies | 4,169 (22.3) | 2,998 (35.2) |
| LABA + ICS (as both FDC and combination) | 2,237 (12.0) | 1,449 (17.0) |
| LABA + ICS + SABA | 1,756 (78.5) | 1,224 (84.5) |
| LABA + ICS + SAMA | 122 (5.5) | 125 (8.6) |
| LABA + LAMA + ICS | 1,096 (5.9) | 948 (11.1) |
| LABA + LAMA + ICS + SABA | 876 (79.9) | 840 (88.6) |
| LABA + LAMA + ICS + SAMA | 39 (3.6) | 65 (6.9) |
| LAMA + ICS | 65 (0.4) | 41 (0.5) |
| LAMA + ICS + SABA | 46 (70.8) | 35 (85.4) |
| LAMA + ICS + SAMA | 4 (6.2) | 3 (7.3) |
| LABA + LAMA | 261 (1.4) | 219 (2.6) |
| SABA + SAMA only | 422 (2.3) | 225 (2.6) |
| Methylxanthines + ICS | 25 (0.1) | 30 (0.4) |
| Methylxanthines + LABA | 9 (0.1) | 15 (0.2) |
| Methylxanthines + LAMA | 7 (0.04) | 9 (0.1) |
| Methylxanthines + (LABA or LAMA or ICS) | 47 (0.3) | 62 (0.7) |
| Any other combinations | 4 (0.02) | 3 (0.04) |
| Patients not on any of the above treatments | 5,373 (28.7) | 1,462 (17.2) |
Abbreviations: FDC, fixed-dose combination; GOLD, Global Initiative for Chronic Obstructive Lung Disease; GOLD A, low symptoms, low risk; GOLD B, high symptoms, low risk; GOLD C, low symptoms, high risk; GOLD D, high symptoms, high risk; ICS, inhaled corticosteroids; LABA, long-acting beta agonists; LAMA, long-acting anticholinergics; n, number of patients; OCS, oral corticosteroids; SABA, short-acting beta agonists; SAMA, short-acting anticholinergics.
Discussion
A third of patients with COPD in the UK are classified as high risk based on lung function and exacerbation history. In line with previously published studies,6,19 the overall age- and sex-standardized prevalence of COPD in the UK was found to be 33.0 per 1,000 persons at the end of 2013; this rate was higher in the low-risk COPD categories A/B compared with the high-risk COPD categories C/D. The age-and sex-standardized 5-year incidence of COPD was 2.2 per 1,000 person-years between 2009 and 2013, equivalent to 5,000 newly diagnosed patients per year. The incidence and prevalence rates were calculated among patients who sought medical attention for their symptoms, as indicated in their medical records. These rates are lower than those expected in the general population as 60%–85% of people with COPD, mainly with mild-to-moderate disease, are thought to remain undiagnosed.20 In our study, a third of the patients were diagnosed for the first time at a high-risk stage, in line with previously reported results that COPD is often not detected until the airflow obstruction is severe.21 It has been argued that subjects may not seek medical attention until the disease becomes advanced because they gradually adapt to the symptoms or their doctor may not notice them.22,23
The incidence of high-risk COPD (C/D categories) was 0.7 per 1,000 person-years, with a significant proportion (31.3%) of newly diagnosed patients with COPD being diagnosed at high risk. Previous epidemiological studies using smaller patient samples have reported the prevalence and incidence of COPD in the UK;12,24 however, to our knowledge this is the first study to report prevalence and incidence COPD rates in the UK using a large population-based database by disease severity, as defined by the GOLD 2013 management strategy document.1 Prior to 2011, GOLD strategies for the management of COPD were solely based on spirometry evaluation. However, FEV1 was found to be a poor indicator of disease status leading to the update of the GOLD management strategy first published in 2011 and most recently updated in 2013, which incorporates important aspects of the disease such as symptoms and future risk of disease progression, especially exacerbations. In the present study, by using FEV1 measurements only, as opposed to the combined assessment of symptoms and risk of future exacerbations, we would have classified 25.6% of the prevalent patients in GOLD III and IV (severe/very severe COPD categories) versus 33.6% patients classified as severe/very severe using the new GOLD 2013 management strategy recommendations.
Comparison with other study populations, based on the same severity assessment framework as in the present study, reveals a similar distribution of patients across severity groups. Using data collected by the National Service for Health Improvement, Haughney et al described the distribution of 6,283 patients with COPD in the UK by the GOLD 2011 categories, which classified 44.9% of patients with COPD in the high-risk C/D categories.24 Jones et al using international cross-sectional survey data, investigated the distribution of a COPD population across the GOLD 2011 severity groups. Using the mMRC scale for symptom assessment, 42.2% of patients were allocated in the high-risk categories C/D.25 In addition, a previous study revealed that people with COPD are more likely to be older, male, and socioeconomically deprived, than those without the disease.7 Our findings confirm that higher prevalence rates were associated with older age, with the highest rate for patients ≥75 years old.
In our study we found that a significant proportion of newly diagnosed patients with COPD are diagnosed when their condition is considered high risk. This may be explained by findings from a recent retrospective cohort study that investigated pharmacotherapy use in the UK primary care setting using the CPRD.13 Prior to the first COPD diagnosis recorded, many patients were treated with COPD pharmacotherapy to reduce symptoms, indicating a potential delay of a definite COPD diagnosis in primary care, until later stages of the disease.
Combination therapy with LABA + ICS or LAMA + LABA (if ICS is not tolerated) and LAMA monotherapy is recommended by NICE guidelines2 for the management of severe stable COPD, while a combination of LAMA + LABA + ICS is recommended to be considered in cases of persistent exacerbations or breathlessness. Among newly diagnosed patients with COPD classified in the high-risk GOLD categories C/D, 44.5% received medications recommended by NICE at the time of diagnosis. However, 38.3% of patients received monotherapies (other than LAMA) or other combination medications that were not recommended for severe stable COPD, indicating that they were in early stages of disease management. Interestingly, for 17.2% of severe cases no prescriptions for COPD medication were found, whereas in the prevalent cohort this percentage was only 5.2%. These results suggest possible undertreatment of the high-risk patient population in real-world practice in the UK, particularly when newly diagnosed.
The importance of recognizing and treating comorbidities in COPD has been discussed in previous research.26 In the absence of a systematic screening protocol for prevalent comorbidities among COPD patients, it is likely that these are underreported. The current study assessed the prevalence of most common comorbidities among COPD patients as reported in previous studies.15 The prevalence of comorbidities was found to be high for both cohorts, among all categories, with the highest proportions in the high-risk categories C/D. These results are in agreement with findings from recent cross-sectional survey for an international COPD population suggesting a link between disease severity assessed by GOLD and prevalence of metabolic and cardiovascular comorbidities.24
A major strength of the current study is the use of CPRD data that offer a large sample size, validated in prior studies,27 and well known to be representative of the demographic breakdown and primary care for the whole UK population.
One limitation of the study was the lack of complete mMRC records for symptom assessment, which did not allow us to report the study results in each of the GOLD categories A, B, C, and D, separately. Using available records, we were able to classify 66.0% of prevalent patients and 81.3% of incident patients into the GOLD categories A, B, C, and D. Patients with incomplete records were, on average, older, had a higher prevalence of comorbidities, and a high proportion (31.0% of prevalent patients and 38.6% of newly diagnosed patients) had not been prescribed any medication for the management of stable COPD when disease severity was being assessed (data not shown). Underperformance of the adequate diagnostic tests during follow-up on behalf of the GPs could possibly imply that these patients presented with milder COPD; however, the evidence from our study is not sufficient to infer any conclusions regarding the severity of COPD among these patients. For both cohorts, to mitigate potential misclassification bias, results were reported by aggregate groups A/B and C/D, given that the exacerbation history was complete for all patients.
Nevertheless, in this study, better recording of MRC and spirometry measurements among the incident cohort with patients diagnosed from 2009 onward revealed an improvement in data quality in more recent years, with about 81.3% of newly diagnosed patients with COPD having at least one record of MRC grade and about 81.6% of patients having at least one spirometry test recorded. This observation is in agreement with previous studies, confirming the association of improved data quality in recent years and the inclusion of MRC dyspnea scale, and spirometry indicators within the Quality and Outcomes Framework in April 2009.10,12 Futures studies could collect information on parameters for all COPD patients that would allow a more detailed classification and the consideration of important outcomes such as mortality, as seen in previous research.28
A total of 18.2% and 25.1% of patients in the prevalent and incident cohorts, respectively, were found to have not received any medication for the management of stable COPD. We were only able to capture medications prescribed in the primary care setting; hence, any medications that may have been prescribed by a specialist or during a hospital visit were not recorded. The percentage of these prescriptions is expected to be low due to the COPD management guidelines in the UK.2
The difficulty of recording exacerbation episodes accurately in the clinical practice has been described previously.29,30 Following a similar approach to Donaldson et al31 we identified possible exacerbation history through Read codes, indicating exacerbations or COPD-related hospitalization and prescriptions of oral antibiotics and oral steroids. In this study, we improved the exacerbation definition by linking prescriptions to the events identified by Read codes. However, a limitation of this study was that the exacerbation definition relied upon hospitalizations recorded in primary care. It is possible that GPs fail to record milder exacerbations or that some exacerbations may not be captured in primary care13 (those that occur at home or at the hospital setting); thus, our results may underestimate the frequency of exacerbations. Nonetheless, in the UK, follow-up care is managed by primary care physicians, and most exacerbation episodes are likely to be captured by prescriptions of antibiotics or OCS; therefore, the underestimation of the frequency of exacerbations is expected to be low. Future research could look to complement primary care data with English Hospital Episodes Statistics database in order to validate the reporting of COPD-related hospitalizations in CPRD.
Latest updated GOLD management guidelines in 2015 were revised to classify patients as high risk with two or more exacerbations or at least one exacerbation-related hospital admission,32 compared to the GOLD 2013 release, where high risk was based on at least two exacerbations alone. It is estimated that the inclusion of hospitalization data in future research could lead to a shift of the patient distribution to higher severity categories and would be an interesting analyses in future research.
Conclusion
On December 31, 2013, 33.3 patients in 1,000 were living with COPD in the UK, and a third (33.6%) was considered high risk and most severe (GOLD 2013 classification severity levels C/D). This was an elderly subgroup of the population (average age 70 years old) with other chronic and severe comorbidities, and even though more than half of the patients were treated with combination therapy, results showed a potential undertreatment for 38.3% of patients who were not on any of the medications recommended by NICE for the management of severe stable COPD. Every year, 5,000 patients in the UK are expected to be newly diagnosed with COPD. In our study, a third of newly diagnosed patients (31.3%) were diagnosed at an older age (68 years old), when presenting symptoms and airflow limitation is more severe (GOLD 2013 categories C/D). However, the absence of any combination treatment for almost half of these patients indicated they were in the early stages of their COPD management. Earlier diagnosis may allow identification of milder cases that could be better controlled and appropriately treated. Further research into COPD management and management of COPD exacerbations by GOLD stage would allow better understanding of the impact of the management of the disease on patients’ health and overall health care resource utilization.
Acknowledgments
The authors are grateful to Evie Merinopoulou, Research Associate, Evidera, for her excellent research assistance in the development of this article.
Footnotes
Disclosure
This analysis was sponsored by Takeda Pharmaceuticals International, Inc. Mireia Raluy-Callado, Dimitra Lambrelli, and Sharon MacLachlan are full-time employees of Evidera and served as paid consultants to Takeda Pharmaceuticals International Inc. for conducting this study. Javaria Mona Khalid is a full-time employee of Takeda Development Center Europe Ltd. The authors have indicated that they have no other conflicts of interest.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global Strategy for the Diagnosis, Management and Prevention of COPD. 2013. [Accessed January 29, 2014]. Available from: http://www.goldcopd.org/
- 2.National Institute for Health and Care Excellence (NICE) National Clinical Guideline Centre for Acute and Chronic Conditions. Chronic Obstructive Pulmonary Disease. Management of Chronic Obstructive Pulmonary Disease in Adults in Primary and Secondary Care. 2010. [Accessed January 29, 2014]. Available from: http://guidance.nice.org.uk/cg101/guidance/pdf/english.
- 3.Kiri VA, Soriano J, Visick G, Fabbri L. Recent trends in lung cancer and its association with COPD: an analysis using the UK GP Research Database. Prim Care Respir J. 2010;19(1):57–61. doi: 10.4104/pcrj.2009.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez LA, Wallander MA, Martin-Merino E, Johansson S. Heart failure, myocardial infarction, lung cancer and death in COPD patients: a UK primary care study. Respir Med. 2010;104(11):1691–1699. doi: 10.1016/j.rmed.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 5.Schneider C, Bothner U, Jick SS, Meier CR. Chronic obstructive pulmonary disease and the risk of cardiovascular diseases. Eur J Epidemiol. 2010;25(4):253–260. doi: 10.1007/s10654-010-9435-7. [DOI] [PubMed] [Google Scholar]
- 6.Simpson CR, Hippisley-Cox J, Sheikh A. Trends in the epidemiology of chronic obstructive pulmonary disease in England: a national study of 51 804 patients. Br J Gen Pract. 2010;60(576):277–284. doi: 10.3399/bjgp10X514729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shahab L, Jarvis MJ, Britton J, West R. Prevalence, diagnosis and relation to tobacco dependence of chronic obstructive pulmonary disease in a nationally representative population sample. Thorax. 2006;61(12):1043–1047. doi: 10.1136/thx.2006.064410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Punekar YS, Shukla A, Mullerova H. COPD management costs according to the frequency of COPD exacerbations in UK primary care. Int J Chron Obstruct Pulmon Dis. 2014;9:65–73. doi: 10.2147/COPD.S54417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization (WHO) The 10 Leading Causes of Death in the World 2000 and 2012. Fact sheet 310. 2014. [Accessed June 11, 2014]. Available from: http://www.who.int/mediacentre/factsheets/fs310/en/
- 10.Health and Social Care Information Centre Quality and Outcomes Framework. 2014. [Accessed July 29, 2014]. Available from: http://www.hscic.gov.uk/gpes/qof.
- 11.hscic (Health & Social Care Information Centre) Indicators for Quality Improvement. 2014. [Accessed July 29, 2014]. Available from: http://www.hscic.gov.uk/iqi.
- 12.Mullerova H, Lu C, Li H, Tabberer M. Prevalence and burden of breathlessness in patients with chronic obstructive pulmonary disease managed in primary care. PLoS One. 2014;9(1):e85540. doi: 10.1371/journal.pone.0085540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wurst KE, Shukla A, Muellerova H, Davis KJ. Respiratory pharmacotherapy use in patients newly diagnosed with chronic obstructive pulmonary disease in a primary care setting in the UK: a retrospective cohort study. COPD. 2014;11(5):521–530. doi: 10.3109/15412555.2014.922064. [DOI] [PubMed] [Google Scholar]
- 14.Thomas M, Radwan A, Stonham C, Marshall S. COPD exacerbation frequency, pharmacotherapy and resource use: an observational study in UK primary care. COPD. 2014;11(3):300–309. doi: 10.3109/15412555.2013.841671. [DOI] [PubMed] [Google Scholar]
- 15.Chatila WM, Thomashow BM, Minai OA, Criner GJ, Make BJ. Comorbidities in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5(4):549–555. doi: 10.1513/pats.200709-148ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanders DB, Bittner RC, Rosenfeld M, Redding GJ, Goss CH. Pulmonary exacerbations are associated with subsequent FEV1 decline in both adults and children with cystic fibrosis. Pediatr Pulmonol. 2011;46(4):393–400. doi: 10.1002/ppul.21374. [DOI] [PubMed] [Google Scholar]
- 18.Office of National Statistics (ONS) Population Estimates for UK, England and Wales, Scotland and Northern Ireland, Mid-2011 and Mid-2012. 2013. [Accessed January 29, 2014]. Available from: http://www.ons.gov.uk/ons/rel/pop-estimate/population-estimates-for-uk--england-and-wales--scotland-and-northern-ireland/mid-2011-and-mid-2012/index.html.
- 19.James GPI, Donaldson G, Wedzicha W. P213 Longitudinal changes in the rate and mean age of incidence and prevalence of COPD in the UK, 2000–2009. Thorax. 2011;66(4):A154. [Google Scholar]
- 20.Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet. 2012;379:1341–1351. doi: 10.1016/S0140-6736(11)60968-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johns DP, Walters JA, Walters EH. Diagnosis and early detection of COPD using spirometry. J Thorac Dis. 2014;6(11):1557–1569. doi: 10.3978/j.issn.2072-1439.2014.08.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albers M, Schermer T, Molema J, et al. Do family physicians’ records fit guideline diagnosed COPD? Fam Pract. 2009;26(2):81–87. doi: 10.1093/fampra/cmp005. [DOI] [PubMed] [Google Scholar]
- 23.van Schayck CP, Loozen JMC, Wagena E, Akkermans RP, Wesseling GJ. Detecting patients at a high risk of developing chronic obstructive pulmonary disease in general practice: cross sectional case finding study. BMJ. 2002;324:1370. doi: 10.1136/bmj.324.7350.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haughney J, Gruffydd-Jones K, Roberts J, Lee AJ, Hardwell A, McGarvey L. The distribution of COPD in UK general practice using the new GOLD classification. Eur Respir J. 2014;43(4):993–1002. doi: 10.1183/09031936.00065013. [DOI] [PubMed] [Google Scholar]
- 25.Jones PW, Nadeau G, Small M, Adamek L. Characteristics of a COPD population categorised using the GOLD framework by health status and exacerbations. Respir Med. 2014;108(1):129–135. doi: 10.1016/j.rmed.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 26.Smith MC, Wrobel JP. Epidemiology and clinical impact of major comorbidities in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2014;9:871–888. doi: 10.2147/COPD.S49621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jick SS, Kaye JA, Vasilakis-Scaramozza C, et al. Validity of the general practice research database. Pharmacotherapy. 2003;23(5):686–689. doi: 10.1592/phco.23.5.686.32205. [DOI] [PubMed] [Google Scholar]
- 28.Leivseth L, Brumpton BM, Nilsen TI, Mai XM, Johnsen R, Langhammer A. GOLD classifications and mortality in chronic obstructive pulmonary disease: the HUNT Study, Norway. Thorax. 2013;68(10):914–921. doi: 10.1136/thoraxjnl-2013-203270. [DOI] [PubMed] [Google Scholar]
- 29.Donaldson GC, Wedzicha JA. COPD exacerbations. 1: epidemiology. Thorax. 2006;61(2):164–168. doi: 10.1136/thx.2005.041806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gruffydd-Jones K. GOLD guidelines 2011: what are the implications for primary care? Prim Care Respir J. 2012;21(4):437–441. doi: 10.4104/pcrj.2012.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donaldson GC, Hurst JR, Smith CJ, Hubbard RB, Wedzicha JA. Increased risk of myocardial infarction and stroke following exacerbation of COPD. Chest. 2010;137(5):1091–1097. doi: 10.1378/chest.09-2029. [DOI] [PubMed] [Google Scholar]
- 32.Global Initiative for Chronic Obstructive Lung Disease (GOLD) At-a-glance Outpatient Management Reference for Chronic Obstructive Pulmonary Disease (COPD) 2015. Available from: http://www.gold-copd.org/uploads/users/files/GOLD_AtAGlance_2015_Feb18.pdf.