Sensitivity is a critical issue in NMR spectroscopy, microscopy and imaging, and the factor that often limits the success of various applications. The origin of low sensitivity in NMR is well known to be due to the small magnetic moment of nuclear spins, which yields small Boltzmann polarizations and weak absorption signals. Historically, each advance in technology and methodology that has increased the signal-to-noise in NMR has shifted the boundary of what is achievable, often opening new areas of application and directions of research. The archetypal example of this phenomenon was the introduction of Fourier transform spectroscopy which led to increases of ∼ 102-fold in signal-to-noise, revolutionizing NMR and many other forms of spectroscopy.1 More recent technological developments of note include the continuing development of higher field superconducting magnets which increases polarization, and cryoprobes in which the excitation/detection coil is maintained at low temperatures increasing sensitivity through a higher probe Q and decreasing receiver noise.2 In addition, innovations in NMR methodology have improved sensitivity, classic examples being Hartmann–Hahn cross polarization,3,4 and J-coupling meditated5 transfer methods, and the introduction of 1H detection of 13C/15N resonances.6 Furthermore, techniques for non-inductive detection of resonance, such as the AFM-based technique of magnetic resonance force microscopy (MRFM), have recently allowed observation of a single electron spin,7 and ∼ 100 nuclear spins/√Hz.8
Another approach to enhancing the sensitivity in NMR experiments is to couple the nuclear spins to a reservoir with much higher polarization, such as unpaired electrons. This principle underlies such methods as laser-polarized noble gases, chemical induced dynamic nuclear polarization (CIDNP), parahydrogen induced polarization (PHIP), and microwave driven dynamic nuclear polarization (DNP). In the cases of CIDNP and PHIP, polarized states are generated by spin-sensitive chemical reactions, and, while they are very successful, they are generally system specific. In contrast, in essentially all experimental situations, unpaired electrons couple efficiently to the lattice and permit some degree of global sensitivity enhancement. For this reason, microwave-driven DNP experiments are evolving as a broadly applicable approach to enhancing signals in solid state and solution NMR and imaging. Currently, DNP improves the sensitivity in NMR spectra by ∼ 102 or reduces the acquisition time in multidimensional experiments by ∼ 104, thereby permitting studies of larger molecules, reaction dynamics, or high-throughput screening. In parallel, it can improve the information content by providing selectivity and contrast. For example, specific sections of a protein can be enhanced, metabolic cycles examined, and contrast in MRI spectra increased. In structural studies of proteins, additional distance and torsion angle constraints are available from electron-nuclear dipolar or scalar coupling and from paramagnetic shifts of sites in close proximity to spin labels or metal centers.
DNP is based on the transfer of the large electron spin polarization to nuclear spins (γe/γn > 657). This concept, originally proposed by Over-hauser in 1953,9 was first experimentally demonstrated in metals10 and subsequently in liquids,11,12 two distinct types of systems with mobile electrons. Thus, DNP is not a new area of scientific endeavor, but rather one undergoing a transition from low to high fields and frequencies; hence “renaissance” in the title. In the lead article, Charles Slichter (DOI: 10.1039/c003286g) describes the excitement of the early experiments performed in his group at the University of Illinois. Every scientist involved in DNP should read this paper, as many of the challenges that we confront today were also of concern to Charlie and his colleagues.
During the 1960's and 70's, following the pioneering work of Overhauser, Carver and Slichter, DNP was used at low temperatures to produce highly polarized solid targets for nuclear scattering, and those experiments revealed multiple polarization transfer mechanisms. In particular, when the paramagnetic centers are localized, the so-called solid-state effect,13–15 cross-effect,16–19 and thermal mixing20 dominate the polarization transfer, and couple the nuclear spin to one, two or more electron spins, respectively. The theory for all three of these mechanisms predicts reduced transfer efficiencies at higher magnetic fields.20,21 This feature of the polarization transfer mechanisms, in combination with the paucity of high frequency microwave sources to excite electron spins at magnetic field strengths above 1 T, effectively relegated DNP to a position of an interesting scientific curiosity. Concurrently, during the 1970's and later, both solution and solid-state NMR moved briskly towards higher magnetic fields (∼5–20 T), yielding higher sensitivity and into multiple dimensions to achieve higher spectral resolution. Thus a dormant phase for DNP persisted until early 1993–95 when high field, solid state MAS DNP experiments, directed at structural biology and utilizing gyrotron microwave sources, were described by Griffin and coworkers.22,23 Subsequently, in 2003 the Nycomed/Amersham group reported the possibility of polarizing samples at very low temperatures followed by fast dissolution, heating, and observation of the liquid state spectrum.24 These two experimental approaches, and variations on these themes, received a good deal of attention in the magnetic resonance community and stimulated worldwide initiatives in the fields of solid and liquid state DNP and high-frequency microwave technology. Accordingly, a first international symposium on DNP was held in Nottingham in 2007 with 150 participants, resulting in a specialized DNP issue in Applied Magnetic Resonance.25 Two years later, the 2nd Symposium on DNP, held in Königstein and the EMAR Workshop on DNP, in Eberbach, highlighted the rapid pace of developments in this field. Thus, this themed issue on high field DNP, presenting the newest results and innovations, is timely.
A key barrier to the dissemination of high field DNP experiments to many laboratories remains the development of the required instrumentation. In particular, high frequency microwave technology is an area that generally remains outside the expertise of the primary consumers of the enhanced signal intensities available from DNP, namely the practitioners of solid state or solution NMR and MRI. This instrumentation includes high frequency microwave sources, efficient waveguides to transmit the microwaves from the source to the probe, and probes that must provide for irradiation of the polarizing electrons and NMR detection at multiple resonance frequencies—1H, 13C, 15N—often at cryogenic temperatures. Finally, there must be a suitable polarizing agent which requires expertise (or colleagues with expertise) in organic synthesis.
Currently, semiconductor diodes and vacuum electron devices are the microwave sources of choice in all DNP spectrometers. The effectiveness of semiconductor technology (Gunn and IMPATT diodes) plummets at frequencies of ∼100 GHz, corresponding to a magnetic field of 3.5 T (150 MHz 1H NMR). Higher frequencies can be attained most conveniently by generating higher harmonics and combining outputs from multiple sources, but with significant losses in power. Despite this limitation, several labs are successfully using diodes for high field DNP experiments, and some of their results appear in this issue. Alternatives are vacuum electron devices, where an accelerated electron beam is modulated by suitable slow wave structure or a magnetic field. Slow wave devices exist in number of different forms—backward wave oscillators (BWOs), orotrons, extended interaction oscillators and amplifiers (EIO and EIAs), etc.—and operate in continuous wave or pulsed mode, with variable or fixed frequencies. Because of the presence of a slow wave structure, which has a size comparable to the microwave wavelength, the electron beam power density close to this structure is limited, and leads to maximum deliverable CW microwave powers in the 0.1–1 W range. Gyrotrons, which are fast wave devices, circumvent this problem by replacing the slow wave structure with a cylindrical cavity immersed in a magnetic field. In this configuration, CW output powers in the range 10–100 watts have been achieved in devices designed specifically for DNP at MIT,26–28 more recently at Fukui University,29,30 and now in commercial instruments (DOI: 10.1039/c003685b). Gyrotrons are stable, spectrally pure, robust devices and can be operated continuously for weeks, which is essential for multidimensional NMR experiments. Examples of the use of all of these sources—diodes, slow wave devices, and gyrotons—appear in this issue (Rosay et al., DOI: 10.1039/c003685b; Armstrong et al., DOI: 10.1039/c002290j; Krahn et al., DOI: 10.1039/c003381b; Leggett et al., DOI: 10.1039/c002566f; Thurber and Tycko, DOI: 10.1039/c0cp00157k; Matsuki et al., DOI: 10.1039/c002268c; Hunter et al., DOI: 10.1039/c002251a; Barnes et al., DOI: 10.1039/c003763j; Denysenkov et al., DOI: 10.1039/c003697h).
Transmitting the microwaves to the sample with minimal loss, and monitoring the microwave power output, is important experimentally. Corrugated wave guides are more suitable than fundamental mode wave guides because they are far less lossy (<1–2 dB) and are more efficient for free-space propagation of the Gaussian beams typically used for quasi-optical transmission from the microwave source to the probe.31,32 In addition, detection of the EPR signal requires quasioptical duplexing devices to prohibit the strong excitation power from reaching the microwave detector. Different designs of such microwave transmission and detection systems are described in this issue.
Fig. 1 illustrates some typical spectrometer configurations for DNP/NMR experiments at high magnetic fields. The upper two instruments are configured to polarize liquid samples, whereas the polarization step in the lower two is performed in the solid, often frozen state. Several applications of HF-liquid DNP (high frequency liquid DNP, upper left), with in situ microwave excitation at the NMR detection field, are reported in this issue, with very promising enhancements at high magnetic fields (up to 10 T) (Denysenkov et al., 10.1039/c003697h; Kryukov et al., DOI: 10.1039/c003189e; Türke et al., DOI: 10.1039/c002814m; Bennati et al., DOI: 10.1039/c002304n; Villanueva-Garibay et al., DOI: 10.1039/c002554m). Theoretical and experimental investigations of the success of the electron-nuclear polarization transfer will be important to understand the underlying physical principles of these results. This understanding is also important for optimizing the polarizing field for Shuttle DNP (upper right) apparatus (Krahn et al., DOI: 10.1039/c003381b), where the liquid sample is rapidly moved from the low field, where the polarization is performed, to a high field region for NMR detection. A new two-center magnet for such a DNP system is also described in this issue (Leggett et al., DOI: 10.1039/c002566f). High field MAS DNP (lower left) was developed at MIT17,33–36 and enhancements of up to ∼300 have been observed with biradical polarizing agents.37 In most experiments the enhanced 1H polarization is transferred to 13C with cross polarization and used for 1D and 2D MAS NMR applications in proteins.27,35,38,39 An oft misunderstood part of this process is the fact that 1H spin-diffusion distributes this polarization uniformly throughout the sample, even if it is heterogeneous, e.g., in the case of a membrane protein in a bilayer or a protein in an amyloid fibril. This process, and resolution in low temperature MAS experiments, is addressed here by Barnes et al. (DOI: 10.1039/c003763j). Recently a commercial MAS DNP spectrometer became available that is described in this issue together with some recent results obtained with the instrument (Rosay et al., DOI: 10.1039/c003685b; Debelouchina et al., DOI: 10.1039/c003661g). Also in this issue, direct transfers to low-γ nuclei (2H, 13C, etc.) are discussed, and the enhancements, field profiles, and preferred polarizing agents are shown to be system dependent (Maly et al., DOI: 10.1039/c003705b).40 In Dissolution DNP (lower right) the sample is polarized in the solid state at very low temperatures (typically 1–4 K) and magnetic fields of 3–7 T, rapidly dissolved, and finally transferred to either a high resolution NMR spectrometer or a MR imager (Leggett et al., DOI: 10.1039/c002566f; Bowen and Hilty, DOI: 10.1039/c002316g). Very high enhancements (relative to room temperature) for 13C can be retained during the dissolution and transfer process, arising from the product of DNP enhancement (∼250) and Boltzmann polarization (∼250). This issue introduces several new approaches and improvements to the experiment. Applications of this method range from MR imaging of metabolites to studies of chemical reaction mechanisms (Bowen and Hilty, DOI: 10.1039/c002316g; Ludwig et al., DOI: 10.1039/c002700f; Panek et al., DOI: 10.1039/c002710n; Cudalbu et al., DOI: 10.1039/c002309b).
Fig. 1.
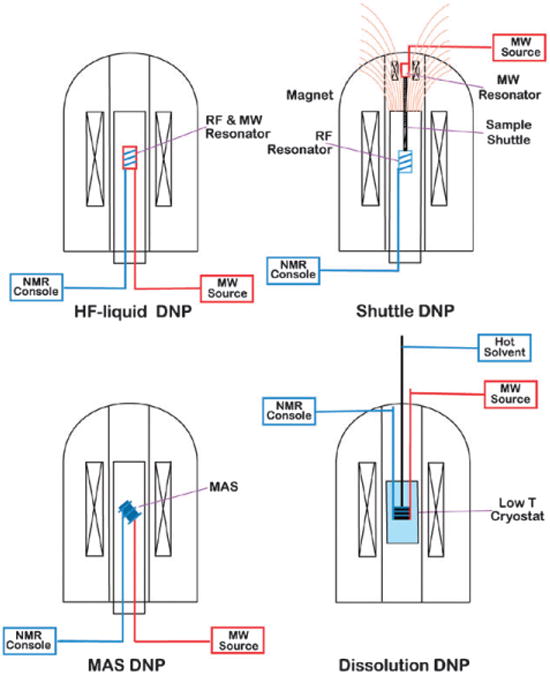
Typical experimental approaches for dynamic nuclear polarization spectrometers.
An essential ingredient of every DNP experiment is a stable polarizing agent, and for the first 50 years of DNP these consisted of readily available monomeric paramagnetic centers such as a metal, or organic radicals like BDPA or TEMPO. More recently, several new polarizing agents have been introduced that are more efficient in that they produce larger enhancements at lower concentrations.41–44 Four articles describe these new agents: narrow line trityl radicals, biradicals and spin labeled polymers that separate at higher temperatures and therefore preserve resolution (Paniagua et al., DOI: 10.1039/c003291n; Dollmann et al., DOI: 10.1039/c003349a; Ysacco et al., DOI: 10.1039/c002591g; Macholl et al., DOI: 10.1039/c002699a).
Finally, two other important and exciting topics are discussed in contributions to this volume: Thurber and Tycko (DOI: 10.1039/c0cp00157k) consider the possibility of using DNP enhancements in solid state imaging to improve the resolution of images of cells and other biological systems; Pomplun and Glaser (DOI: 10.1039/c003751f) discuss theoretical methods for optimizing time domain DNP experiments, an area that has thus far received little attention.
All of these approaches are potentially applicable to a wide range of important NMR experiments in biology, chemistry, physics and medicine, and their successful development will have an enormous impact on the field. Accordingly, a number of academic and industrial research groups have recently initiated efforts to overcome the current limitations of the techniques. Technical advances in the area of high-frequency microwave sources and components, and of various DNP approaches (Fig. 1), will be of vital importance for the further development of the DNP method, especially at the highest magnetic fields available for NMR (<20 T). In addition, implementation of microwave time domain experiments should open many new areas of application, just as rf time domain experiments did for high resolution solid state and solution NMR. Other avenues, such as the optimization of polarizing agents, the development of new types of polarization transfer methods, and the design of new experiments focusing on selectivity, contrast and additional structural restraints, are ripe for investigation. Thus, collaborative efforts among researchers from chemistry, physics, biology, medicine, and the engineering disciplines will be required to optimize DNP for applications in high-field NMR and MRI. We foresee a very bright and expansive future for this field, well into the 21st century.
References
- 1.Ernst RR, Anderson WA. Rev Sci Instrum. 1966;37:93–102. [Google Scholar]
- 2.Styles P, Soffe N, Scott C, Cragg D, Row F, White D, White P. J Magn Reson. 1984;60:397–404. doi: 10.1016/j.jmr.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Hartmann SR, Hahn EL. Phys Rev. 1962;128:2042–2053. [Google Scholar]
- 4.Pines A, Gibby MG, Waugh JS. J Chem Phys. 1972;56:1776. [Google Scholar]
- 5.Morris G, Freeman R. J Am Chem Soc. 1979;101:760–762. [Google Scholar]
- 6.Bodenhausen G, Ruben DJ. Chem Phys Lett. 1980;69:185–189. [Google Scholar]
- 7.Rugar D, Budakian R, Mamin HJ, Chui BW. Nature. 2004;329:430–432. doi: 10.1038/nature02658. [DOI] [PubMed] [Google Scholar]
- 8.Mamin SHJ, Oosterkamp TH, Poggio M, Degen CL, Rettner CT, Rugar D. Nano Lett. 2009;9:3020–3024. doi: 10.1021/nl901466p. [DOI] [PubMed] [Google Scholar]
- 9.Overhauser AW. Phys Rev. 1953;92:411–415. [Google Scholar]
- 10.Carver TR, Slichter CP. Phys Rev. 1953;92:212–213. [Google Scholar]
- 11.Carver TR, Slichter CP. Phys Rev. 1956;102:975–980. [Google Scholar]
- 12.Hausser KH, Stehlik D. Adv Magn Reson. 1968;3:79. [Google Scholar]
- 13.Jefferies CD. Phys Rev. 1957;106:164–165. [Google Scholar]
- 14.Abragam A, Proctor WG. Comptes Rendus Hebdomadaries des Seances de L'Academia des Sciences. 1958;246:2253–2256. [Google Scholar]
- 15.Jefferies CD. Phys Rev. 1960;117:1056–1069. [Google Scholar]
- 16.Kessenikh AV, Manenkov AA. Soviet Physics-Solid State. 1963;5:835–837. [Google Scholar]
- 17.Hwang CF, Hill DA. Phys Rev Lett. 1967;19:1011–1013. [Google Scholar]
- 18.Hwang CF, Hill DA. Phys Rev Lett. 1967;18:110–112. [Google Scholar]
- 19.Wollan DS. Phys Rev B: Solid State. 1976;13:3671–3685. [Google Scholar]
- 20.Goldman M. Spin Temperature and Nuclear Magnetic Resonance in Solids. Oxford University Press; London: 1970. [Google Scholar]
- 21.Wind RA, Duijvestijn MJ, Vanderlugt C, Manenschijn A, Vriend J. Prog Nucl Magn Reson Spectrosc. 1985;17:33–67. [Google Scholar]
- 22.Becerra LR, Gerfen GJ, Temkin RJ, Singel DJ, Griffin RG. Phys Rev Lett. 1993;71:3561–3564. doi: 10.1103/PhysRevLett.71.3561. [DOI] [PubMed] [Google Scholar]
- 23.Gerfen GJ, Becerra LR, Hall DA, Griffin RG, Temkin RJ, Singel DJ. J Chem Phys. 1995;102:9494–9497. [Google Scholar]
- 24.Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K. Proc Natl Acad Sci U S A. 2003;100:10158–10163. doi: 10.1073/pnas.1733835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prisner TF, Koeckenberger W. Appl Magn Reson. 2008;34:213–218. and other papers therein. [Google Scholar]
- 26.Becerra LR, Gerfen GJ, Bellew BF, Bryant JA, Hall DA, Inati SJ, Weber RT, Un S, Prisner TF, McDermott AE, Fishbein KW, Kreischer KE, Temkin RJ, Singel DJ, Griffin RG. J Magn Reson, Ser A. 1995;117:28–40. [Google Scholar]
- 27.Bajaj VS, Farrar CT, Hornstein MK, Mastovsky I, Vieregg J, Bryant J, Elena B, Kreischer KE, Temkinand RJ, Griffin RG. J Magn Reson. 2003;160:85–90. doi: 10.1016/s1090-7807(02)00192-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bajaj VS, Hornstein MK, Kreischer KE, Sirigiri JR, Woskov PP, Mak-Jurkauskas ML, Herzfeld J, Temkin RJ, Griffin RG. J Magn Reson. 2007;189:251–279. doi: 10.1016/j.jmr.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Idehara T, Ogawa I, Mitsudo S, Pereyaslavets M, Nishida N, Yoshida K. IEEE Trans Plasma Sci. 1999;27:340–354. [Google Scholar]
- 30.Idehara T, Saito T, Ogawa I, Mitsudo S, Tatematsu Y, Agusu L, Mori H, Kobayashi S. Appl Magn Reson. 2008;34:265. [Google Scholar]
- 31.Woskov PW, Bajaj VS, Hornstein MK, Temkin RJ, Griffin RG. IEEE Trans Microwave Theory Tech. 2005;53:1863–1869. doi: 10.1109/TMTT.2005.848097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Denysenkov VP, Prandolini MJ, Krahn A, Gafurov M, Endeward B, P TF. Appl Magn Reson. 2008;34:289. [Google Scholar]
- 33.Hall DA, Maus DC, Gerfen GJ, Inati SJ, Becerra LR, Dahlquist FW, Griffin RG. Science. 1997;276:930–932. doi: 10.1126/science.276.5314.930. [DOI] [PubMed] [Google Scholar]
- 34.Rosay M, Weis V, Kreischer KE, Temkin RJ, Griffin RG. J Am Chem Soc. 2002;124:3214–3215. doi: 10.1021/ja0176752. [DOI] [PubMed] [Google Scholar]
- 35.Rosay M, Lansing JC, Haddad KC, Bachovchin WW, Herzfeld J, Temkin RJ, Griffin RG. J Am Chem Soc. 2003;125:13626–13627. doi: 10.1021/ja036898k. [DOI] [PubMed] [Google Scholar]
- 36.van der Wel PCA, Hu KN, Lewandowski J, Griffin RG. J Am Chem Soc. 2006;128:10840–10846. doi: 10.1021/ja0626685. [DOI] [PubMed] [Google Scholar]
- 37.Song C, Hu KN, Joo CG, Swager TM, Griffin RG. J Am Chem Soc. 2006;128:11385–11390. doi: 10.1021/ja061284b. [DOI] [PubMed] [Google Scholar]
- 38.Hu KN, Yu HH, Swager TM, Griffin RG. J Am Chem Soc. 2004;126:10844–10845. doi: 10.1021/ja039749a. [DOI] [PubMed] [Google Scholar]
- 39.Bajaj VS, Mak-Jurkauskas ML, Belenky M, Herzfeld J, Griffin RG. Proc Natl Acad Sci U S A. 2009;106:9244–9249. doi: 10.1073/pnas.0900908106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maly T, Miller AF, Griffin RG. Chem Phys Chem. 2010;11:999–1001. doi: 10.1002/cphc.200900908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu KN, Song C, Yu Hh, Swager TM, Griffin RG. J Chem Phys. 2008;128:052321. doi: 10.1063/1.2816783. [DOI] [PubMed] [Google Scholar]
- 42.Hu KN, Bajaj VS, Rosay MM, Griffin RG. J Chem Phys. 2007;126:044512. doi: 10.1063/1.2429658. [DOI] [PubMed] [Google Scholar]
- 43.Hu KN., PhD MIT. 2008 [Google Scholar]
- 44.Matsuki Y, Maly T, Ouari O, Lyubenova S, Herzfeld J, Prisner T, Tordo P, Griffin RG. Angew Chem, Int Ed. 2009;48:4996–5000. doi: 10.1002/anie.200805940. [DOI] [PMC free article] [PubMed] [Google Scholar]


