Abstract
Introduction
There is an unmet need for a new class of direct bronchodilators for the treatment of asthma and chronic obstructive lung disease. Unexpectedly, bitter taste receptors (TAS2Rs) have been localized on airway smooth muscle and when activated cause marked smooth muscle relaxation via a mechanism that is distinct from β2-adrenegic receptors. Thus TAS2R agonists have emerged as a novel class of bronchodilator.
Areas covered
A synopsis of the TAS2R family and its biology for bitter taste perception on the tongue is provided, followed by a review of the identification and molecular and physiological characterization of TAS2R subtypes on human and mouse airway smooth muscle. The proposed molecular mechanisms leading to the relaxation response are provided, along with gaps in our understanding at certain points in the signaling cascade. Unresolved issues that may need to be considered for drug development are discussed.
Expert opinion
TAS2R agnosts show promise as a new class of highly efficacious bronchodilators for treatment of obstructive lung disease. With tens of thousands of known natural and synthetic bitter compounds, there is substantial diversity within the known agonists, and, a ready source of agents for screening and further development of an inhaled TAS2R agonist for therapeutic purposes.
Keywords: asthma, COPD, smooth muscle, calcium, bronchospasm, β-agonist
1. Introduction
Asthma is characterized by chronic inflammation of the airways with obstruction due to mucous accumulation and actively contracted airway smooth muscle (termed bronchospasm) which decreases airway lumen diameter and thereby increases airflow resistance [1]. The increased work of breathing due to bronchospasm is the major source of morbidity and mortality in asthma. In persistent asthma (either moderate or severe), some degree of bronchospasm and thus symptoms are present virtually all the time, or can be readily evoked by activities such as exercise or allergen exposure. Treating the inflammatory component has led to moderate success in the treatment of asthma, but nevertheless bronchodilator therapy (either as-needed for “rescue” or on a continuous bases for “control”) is required for most asthmatics. Indeed, ~50% of asthmatics are not under adequate control [2–5]. Currently there is only one class of direct bronchodilators utilized for treating obstructive lung disease. These are the β-agonists, which act by binding to airway smooth muscle β2-adrenergic receptors (β2AR) [6]. The β2AR is a member of the large superfamily of cell surface, G-protein coupled receptors (GPCRs), whose effects within the cell come from activation of a receptor-G-protein-effector cascade. For airway β2AR, agonist binding stabilizes an active conformation of the receptor which evokes binding and dissociation of the heterotrimeric G-protein Gs, leading to activation of adenylyl cyclase, and enhancement of intracellular cAMP production from ATP. cAMP activates protein kinase A (PKA) with numerous PKA-dependent effects including phosphorylation of myosin light chain kinase leading to smooth muscle relaxation [6,7]. Interestingly, virtually all signals that cause smooth muscle contraction in asthma are due to locally produced factors that bind GPCRs which activate receptors coupled to the G-protein Gq, leading to an increase in intracellular Ca2+ ([Ca2+]i) [6,7]. Thus antagonists to receptors for histamine, acetylcholine, and leukotrienes are used for treating asthma, but are considered indirect bronchodilators. Thus the armamentarium for treating bronchospasm in asthma by direct bronchodilators is quite limited. β-agonist therapy has been associated with increased sensitivity to bronchospasm (bronchial hyperreactivity), decreased acute responsiveness (tachyphylaxis), worsening asthma, and death [8–13]. We have explored the potential for other airway receptors [14] that directly relax smooth muscle by novel mechanisms in order to develop bronchodilators for improving care of obstructive lung diseases. This review discusses the expression and function of bitter taste receptors (TAS2Rs) on airway smooth muscle [15,16] as targets for a new class of drugs for treating obstructive airways disease such as asthma and chronic obstructive pulmonary disease.
2. Bitter taste receptor (TAS2R) expression on airway smooth muscle
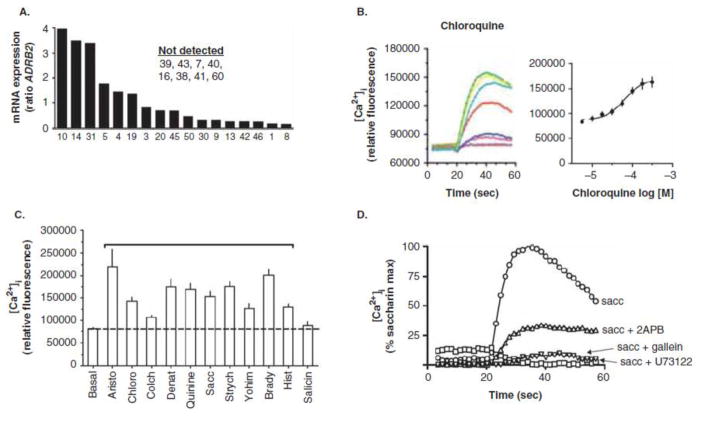
Using quantitative RT-PCR, we utilized primers for all 25 human TAS2R receptors with mRNA from a purified cell preparation of cultured, early passage, human airway smooth muscle cells (HASM) [15] to ascertain relative expression levels. These results are shown in Figure 1a, and are normalized to expression of the β2AR (ADRB2) to provide a reference. TAS2R subtypes 39, 43, 7, 40, 16, 38, 41, 60 were not detected, nor were the two sweet receptors TAS1R1 and R2. The expression of ADORA1 and LTB4R receptor transcripts served as high and low positive controls. Three TAS2Rs are clearly expressed at levels greater than ADRB2 (TAS2R 10, 14, 31), which we have termed “high expressors” and several additional receptors expressed at modest or low levels. Taken together we considered six TAS2Rs as potential drug targets: TAS2R10, 14, 31, 5, 4, and 19, with an emphasis on 10, 14 and 31 as the most attractive subtypes for the treatment of obstructive airways disease.
Figure 1.
Expression and pharmacology of TAS2Rs on isolated human airway smooth muscle cells.
3. The TAS2R family of receptors
TAS2Rs [17–19] are also GPCRs, which have been traditionally thought to be exclusively expressed on taste buds of the tongue [20]. They have been considered to have developed to avoid ingestion of toxic plants. These receptors couple to the G-protein gustducin, which activates via its βγ subunit phospholipase C (PLC). Activated PLC results in increases in inositol-3-phosphate (IP3), which binds to the sarcoendoplasmic reticulum IP3 receptor, releasing [Ca2+]i from intracellular stores. This activates a transient receptor potential channel (TRP channel) which depolarizes the taste cell membrane [21]. This depolarization results in release of neurotransmitter, which activates a neuronal connection to the brain. In humans, there are 25 TAS2R subtypes, differentially expressed on taste bud cells throughout the tongue [20]. They respond to a broad range of bitter tasting chemicals. These include naturally occurring compounds from plants (quinine, colchicine, yohimbine, strychnine) and synthetic agents currently utilized as dietary supplements or for treatment of a variety of diseases (saccharin, chloroquine, dapsone, flufenamic acid) [22]. Typically, the imputed affinity of these agents is relatively low (uM) for TAS2Rs [22], consistent with the fact that toxic plants would be in direct contact with the tongue. No core structural requirement for TAS2R agonists has been identified to date, suggesting that many agonists may be acting at allosteric as opposed to orthosteric sites. Thus these receptors appear to have evolved, at least on the tongue, as a broadly-tuned, low affinity, set of “aversion receptors”.
4. TAS2R agonists evoke bronchodilation
Functional studies were initially focused on agonist-promoted [Ca2+]i in HASM, since this is an early second messenger in TAS2R signaling in taste bud cells. As shown in Figure 1b, the benchmark bitter tastant chloroquine increase [Ca2+]i [15] in a dose dependent manner. Using a panel of components, we noted that strychnine (TAS2R10 agonist) evoked a greater response than the TAS2R4 agonist colchicine, which is a lower expressing TAS2R4 (Figure 1c). There was no [Ca2+]i response to salicin, which activates TAS2R16, which was not detected in these cells. Thus the pharmacological profile of [Ca2+]i stimulation (Figure 1c) was consistent with the differential expression levels ascertained by RT-PCR (Figure 1a). In these cells, TAS2R agonist-mediated [Ca2+]i was ablated by the βγ antagonist gallein and the PLC inhibitor antagonist U73112, and was significantly blunted by the IP3 receptor antagonist 2APB (Figure 1d). The increase in [Ca2+]i did not require extracellular Ca2+. Thus the signaling in HASM, up to the point of [Ca2+]i release, is consistent with TAS2R signaling in taste bud cells [15].
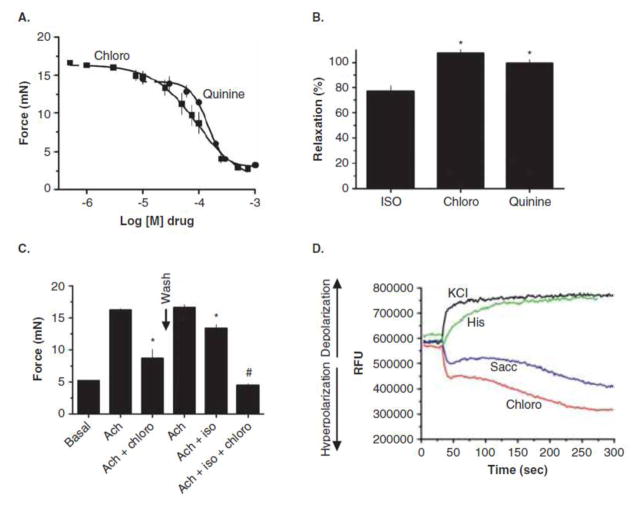
From these experiments, we expected that TAS2R agonists would cause contraction of HASM, since increased [Ca2+]i is the basis of known constrictive agents acting at their cognate receptors [6]. However, we found [15] in mouse airways studied ex vivo that bitter taste receptor agonists promote a profound relaxation (Figure 2a). Indeed, under most any condition that we tested (including contraction with serotonin and KCl), bitter tastants evoked relaxation back to baseline tension (i.e., 100% relaxation). Similarly, TAS2R agonists caused relaxation of excised human bronchi (Figure 2b) [15,16]. In both human and mouse airways, the relaxation response to isoproterenol (a full β-agonist) was less than that evoked by TAS2R agonists (Figure 2b). The most pronounced difference was in mouse, where β-agonists are known to have lower efficiencies than in human airways.
Figure 2.
Physiological consequences of TAS2R activation in mouse and human airways, and human airway smooth muscle cells.
In both mouse and human airways, the relaxation evoked by bitter tastants was rapid and fully reversible with wash-out [16]. One report suggests a lack of reversibility [23], but the methodologies differed from our original study, and in additional experiments we have now shown full reversibility with washout in both mouse and human airways with kinetics consistent with agonist-GPCR interactions [16]. Interestingly, the responses to β-agonist and submaximal concentrations of TAS2R agonist were found to be additive (Figure 2c) [15] suggesting that combined therapy with these two types of bronchodilators could provide clinical advantage over either agent alone. In additional studies no elevations in cAMP with any TAS2R agonist was observed in HASM cells [15]. Also, the concentration that evoked a 50% response (Ec50) was identical (~80uM) between HASM [Ca2+]i elevation and mouse airway relaxation, supporting a link between the two events. And indeed, depletion of intracellular Ca2+ with thapsigargin ablated TAS2R agonist-medicated airway relaxation [15]. Inhibitors of cyclooxygenase and nitric oxide synthase had no effect on TAS2R agonist mediated relaxation [15]. Finally, in contrast to the depolarization of taste cell membranes that occurs with TAS2R activation, in HASM these receptors evoke hyperpolarization [15] of the membrane (Figure 2d), which would be expected to cause relaxation.
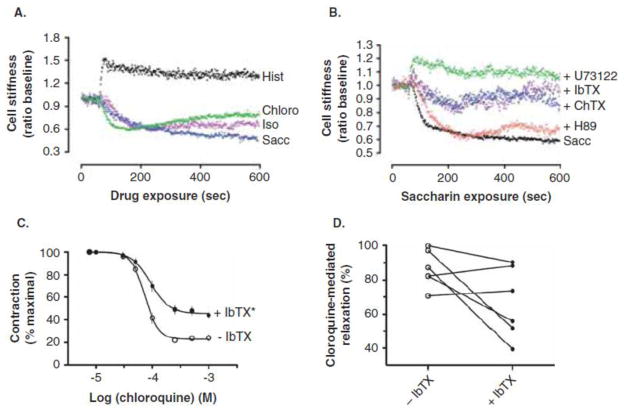
Using magnetic twisting cytometry (MTC) [24,25] the physiological consequences of agonist binding were assessed in cultured HASM [15]. Of note, these studies isolate the smooth muscle cell, thus effects that might be due to bitter tastants acting on airway epithelial cells which then communicate to the smooth muscle, can be excluded. As shown in Figure 3a, bitter tastants caused relaxation from baseline of HASM as determined by MTC. Histamine and isoproterenol had the expected contractile and relaxant effects, respectively. Additional studies showed that bitter tastants relax HASM as studied with MTC that had been pre-contracted with methacholine [26]. Consistent with the studies of [Ca2+]i release in HASM (Figure 1d), relaxation was blocked by the PLC inhibitor U73112 (Figure 3b) [15].
Figure 3.
Characteristics of airway smooth muscle responsiveness to TAS2R agonists.
5. Mechanism of TAS2R – mediated HASM relaxation
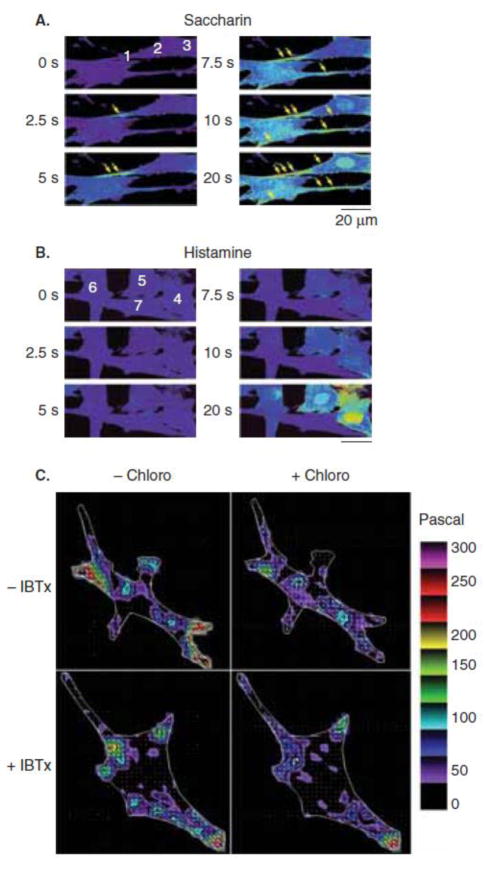
The unexpected finding that whole-cell readings of TAS2R-mediated [Ca2+]i increases were associated with HASM relaxation has raised the notion that the relaxation-based [Ca2+]i pool may exist in a distinct compartment (compared to contraction-associated [Ca2+]i), providing specialized signaling. To address this, high resolution real-time confocal microcopy was performed with Fluo-3 loaded HASM cells [15]. Spatiotemporal characteristics between saccharin and histamine-mediated [Ca2+]i were clearly evident when examined at the subcellular level (Figure 4a,b). For saccharin, the [Ca2+]i increase was seen as early as 2.5 sec and appeared to be mostly associated with the slender ends and sarcolemmal region of the cell. In contrast, no [Ca2+]i signal was seen with histamine until ~10 sec after agonist expression, and the response appeared to be more global in distribution. The TAS2R [Ca2+]i signal evoked by saccharin was very close to the cell membrane when examined using line scans [15].
Figure 4.
Specialized [Ca2+]i signaling by TAS2R agonists in human airway smooth muscle cells.
We considered that the specialized pool of [Ca2+]i evoked by TAS2R activation had characteristics (spatial, temporal, and concentration) consistent with activation of a channel such as the large capacitance Ca2+-dependent K+ channel (BKCa) that hyperpolarizes the membrane. To address this possibility, MTC was utilized with HASM in the absence or presence of the specific BKCa antagonist iberiotoxin (IbTx). As shown in Figure 3b, IbTx partially inhibited relaxation in HASM. In addition, partial inhibition was observed using intact mouse airways (Figure 3c). In human airways, the extent of the effect of IbTX varied between samples from different individuals [27], but the overall trend indicates that the BKCa toxin has an inhibitory effect on TAS2R-mediated relaxation (Figure 3d). This data suggests that BKCa activation may be necessary for the “full effect” of TAS2R-mediated relaxation. But clearly, other mechanisms are also involved, and depending on the physiological “readout”, the loss of a BKCa component may not be readily observed. In additional qualitative studies with isolated HASM, Fourier transform traction microscopy has been utilized to ascertain the effect of IbTx in the context of TAS2R activation [27]. As shown in Figure 4c, the forces within HASM are heterogeneous and asymmetric. The TAS2R agonist chloroquine caused a decrease in traction (consistent with relaxation) in several regions of the cell. This relaxation effect from TAS2R activation was attenuated or blocked in some, but not all, regions within the cell in the presence of IbTx. These data are all consistent with BKCa channel having some role in TAS2R-mediated relaxation, but also indicating that another mechanism is likely to be at play. Patch-clamp studies have also implicated other mechanisms, although these methodologies have been questioned as to their applicability [28].
6. TAS2R function in a mouse model of asthma
Because signaling systems can undergo dynamic regulation by inflammation, TAS2R agonist function has been evaluated in the context of the ovalbumin-sensitized mouse model of asthma [15]. For these studies, BALB/c mice were sensitized to subcutaneous ovalbumin and subsequently challenged with inhaled albumin. This results in inflammation of the airways (Figure 5a,b) and enhanced bronchoconstriction to inhaled methacholine (bronchial hyperreactivity) compared to non-sensitized mice. Mice were then intubated, sedated, and ventilated and airway resistance measured at baseline, in response to inhaled methacholine, and during the methacholine induced constrictive phase, in response to inhaled TAS2R agonists quinine or denatonium. The positive control for bronchodilation was the β-agonist albuterol. These studies provide advantages over the ex vivo airway studies in that they are conducted during active inflammation and bronchial hyperreactivity in the intact mouse, and measurements of airway mechanics reflect net changes that may occur throughout the tracheobronchial tree. Also, drugs are administered by inhalation, which is what is envisioned for TAS2R agonists for the treatment of asthma. The main drawback is that multiple drugs/dosages and prolonged studies are often not tolerated, so the permutations available from these in vivo studies in a single mouse are not as broad as ex vivo ring experiments.
Figure 5.
Responsiveness of TAS2Rs in a mouse model of asthma and properties of desensitization.
As shown in Figure 5c, in control mice single doses of quinine or albuterol resulted in decreases in airway resistance to the same extent [15]. In the ovalbumin sensitized and challenged mice a lower amount of methacholine was required to increase airway resistance (consistent with the hyperresponsive phenotype). The decrease in airway resistance evoked by albuterol in these mice (Figure 5d) was markedly attenuated compared to the non-sensitized mice (~12% vs. ~60%, respectively). However, the response to quinine amounted to a >50% reduction in resistance in both non-sensitized and sensitized mice (Figure 5c,d). Similar results were also noted with the TAS2R agonist denatonium [15]. Taken together, these data show that TAS2R responses are maintained during the asthmatic milieu. This is in contrast to the β-agonist response, which becomes substantially decreased.
7. Desensitization of TAS2Rs
Desensitization is defined as a loss of receptor responsiveness during continuous or repetitive exposure to agonist [29]. This definition is strictly based on function, and does not specify mechanism or duration of exposure to the agonist. Function could be a physiological measurement (forced expiratory volume), a cell or tissue event (smooth muscle force, bronchial ring tension), or an intracellular signal (cAMP, IP3, Ca2+). Clinically, desensitization is observed as tolerance, or tachyphylaxis, to an administered agent. Many GPCRs undergo agonist-promoted desensitization, due to mechanisms such as receptor phosphorylation by various kinases, receptor internalization, and a loss of receptor expression (termed downregulation) [29–31]. Since agonist-promoted desensitization might limit therapeutic effectiveness of TAS2R agonists, initial studies have been performed to ascertain if desensitization of TAS2Rs occurs at the cellular or physiologic levels [32]. For the former studies, HASM cells were exposed to the TAS2R agonist quinine or carrier (control) for 15 minutes, rapidly washed, and the [Ca2+]i response to quinine rechallenge determined. A ~30% decrease in [Ca2+]i stimulation was observed with this pretreatment, indicating some degree of desensitization (Figure 5e) [32]. Of note, the [Ca2+]i response to endothelin was not effected by quinine pre-treatment, consistent with homologous desensitization of the TAS2R response (as opposed to an alteration in [Ca2+]i handling or other heterologous mechanisms). In addition, the function of the B2-bradykinin receptor (known to undergo agonist-promoted desensitization) showed profound (>90%) loss of function when HASM cells were pretreated with bradykinin, acting as a positive control for the experimental protocol [32]. This short-term desensitization was not blocked by protein kinase A or C inhibitors, pointing towards G-protein coupled receptor kinase (GRK)-mediated events as potential mechanisms for TAS2R desensitization. Of note, saccharin (TAS2R31 agonist) pretreatment evoked ~40% desensitization of the [Ca2+]i response to subsequent saccharin stimulation, suggesting that the quinine effects are acting through TAS2Rs, rather than some nonspecific effect. Similar studies using methacholine contracted rhesus macaque bronchial rings resulted in ~30% loss of relaxation to subsequent quinine exposure [32], showing that the desensitization of the [Ca2+]i response correlated with a relevant physiologic response in this non-human primate.
Given that β2AR and TAS2R relaxation appear to act additively (Figure 2d), it is likely that agonists for both receptors might be used concomitantly for treating asthma. However, GPCRs can “cross-regulate” each other, via early events in the signaling process or near the terminal physiologic endpoint [33–35]. Indeed dysregulation of a physiologic outcome might occur at a final common pathway for otherwise quite different signaling mechanisms. Thus β2AR and TAS2R might “interact” at the relaxation response of smooth muscle. Of particular interest has been whether TAS2Rs would still be functional under conditions of chronic β-agonist exposure, which results in β2AR desensitization. To address this, studies have been performed in isolated HASM measuring cell stiffness, the second messengers cAMP or [Ca2+]i, and relaxation of mouse and human airways using the ex vivo model [26]. With 18 hours of exposure to β-agonist, rechallenge with β-agonist showed >90% loss of cellular relaxation as assessed by MTC, indicative of significant physiological desensitization of the β2AR pathway. Consistent with this finding, β-agonist stimulated cAMP in HASM was also markedly desensitized. And, in mouse trachea, Figure 5d, and precision-cut human airway lung slices, a similar exposure to β-agonist resulted in a substantial loss of airway relaxation [26]. However, under these same conditions, the TAS2R agonist chloroquine stimulated [Ca2+]i to the same extent as unexposed cells, and chloroquine relaxed isolated HASM, mouse trachea, and human precision-cut lung slices without loss of efficacy [26]. Taken together, HASM TAS2R relaxation appears to be unaltered under extreme desensitization of the β2AR pathway.
8. Unresolved Issues for development of TAS2R agnostic for asthma therapy
8.1 Which TAS2R subtype?
As introduced earlier, three TAS2R subtypes, 10, 14 and 31, represent a cluster of the highest expressing bitter taste receptors on human airway smooth muscle [15]. Current studies do not indicate if maximal bronchodilation requires activation of more than one receptor, or, if a single receptor is sufficient. Ascertaining this information using highly specific agonists [22] has important indications for drug development. For example, an agonist may have relatively low affinity for any one receptor, but if it activates all three TAS2Rs the efficacy may be adequate. Similarly, desensitization may not be clinically observed if the “net TAS2R reserve” is high under conditions of these receptors being activated. On the other hand, these agents will likely need to be administered via nebulized inhalation, thereby passing the tongue. Activation of a single TAS2R subtype might be less offensive than activation of several. In addition, there are structural differences between these three receptors [32], particularly between the third intracellular loops and cytoplasmic tails (typical regions where desensitization and accessory protein binding occurs). Thus the selection of a TAS2R subtype to target may be dependent on functions or characteristics of the signaling other than acute smooth muscle relaxation per se.
8.2 What are the mechanisms of desensitization?
Current understanding of TAS2R desensitization is limited in terms of mechanism, and dose-and time-dependency. In addition, it is recognized that certain agonist-stabilized conformations of GPCRs can be biased away from desensitization, and this aspect is dictated by the structure of the agonist [36–38]. These issues can be resolved by first using “benchmark” agonists to gain some initial insight, and then as new agonists are developed they can be integrated into any paradigms that have been established. The notion that TAS2R desensitization mechanisms are similar to any other GPCR (or any specific TAS2R subtype) cannot be assumed, but rather should be investigated in an impartial manner.
8.3 Is it necessary to increase potency?
Most TAS2R agonists discovered so far has assumed affinities (based on potency values) for their receptors in the uM range. Like the free fatty acid receptors, high throughput screening and molecular modeling could result in the synthesis of compounds with increased potencies by several orders of magnitude [39]. To our knowledge such studies have not been undertaken, but would be a natural consequence for achieving an optimal set of agonists to move forward in clinical studies. Of note, in studies of the intact mouse, we found maximal bronchodilation with 150 μg quinine compared to 3 μg albuterol. In the ovalbumin sensitized mouse, the equieffective dose of quinine compared to albuterol was 50 μg for bronchodilation. Extrapolation to humans yields ~175 mg of inhaled quinine per dose. This is within the limits of modern clinical nebulizers. For example, tobramycin (similar molecular weight to quinine) is administered at 300 mg per inhalation. So it may not be absolutely necessary to obtain subnanomolar potencies, but such efforts should be undertaken to attain a readily formulated, deliverable, and convenient agonist for inhalation. Of interest is whether full or partial agonists would be preferred for treatment of obstructive lung disease. One might consider that a full agonist is preferred, however it is not uncommon that desensitization and alternative signaling outcomes are more likely with full agonists, as might be off-target effects. Understanding the “receptor reserve” for a TAS2R in relaxing smooth muscle may be helpful in understanding whether full or partial agonism is preferred.
8.4 Off-target effects
It is now clear that taste receptors, including the bitter taste receptors, are expressed in organs outside of the oral cavity [40]. They may represent previously unrecognized and physiologically relevant signaling pathways, or some may be “vestigial proteins” without significant consequences when activated. As with inhaled β-agonists and cholinergic antagonists targeted to airway smooth muscle, and inhaled corticosteroids targeted to multiple cell types in the airway, the inhaled route has allowed for clinically effective dosing with little if any systemic, off-target, effects. And, we note that purposeful ingestion of bitter compounds in foods or pharmaceuticals has not been specifically linked to adverse systemic effects. Nevertheless, this issue must be considered as new agents are developed.
Interestingly, lung airway epithelial cells express TAS2Rs which act to increase ciliary beat frequency [41], as do solitary chemosensory cells in the trachea that may regulate respiratory rate [42]. In addition, circulating lymphocytes express TAS2Rs, which may regulate inflammatory mediator release [43]. While none of these non-smooth muscle sites would be expected to cause direct airway relaxation, the outcome of their activation may nevertheless be towards a beneficial effect for obstructive lung disease. It is intriguing to consider the evolutionary basis of TAS2Rs expression on airway smooth muscle. One scenario that we have considered is the protective effect of bronchodilation during bronchitis. Gram negative bacteria secrete acyl-homoserine lactones [44], and these are highly efficacious TAS2R agonists [45]. Thus during active bronchitis the secreted agonists may act to open the airway, providing an advantage for clearance of bacteria and cellular debris.
8.5 Method of administration
Finally, one commentary was puzzled as to how TAS2R agonists reach the smooth muscle of the airway, since they would need to pass the epithelium [46]. The in vivo studies of inhaled TAS2R agonists clearly demonstrate direct relaxation of the airway. Whatever the mechanism of movement to smooth muscle, it does not appear to be due to the hydrophobicity of the compound. Albuterol, terbutaline, metaproterenol and the benchmark β-agonist isoproterenol are hydrophilic and do not cross the cell membrane, yet are rapid and effective bronchodilators via their activation of airway smooth muscle β2AR. Tight junctions between epithelial cells and other mechanisms are likely in play to provide these agents access to smooth muscle receptors. β-agonists such as salmeterol and formoterol are indeed lipophilic. So, there is no a priori rule that predicts drug access to smooth muscle receptors based on hydrophobicity. This issue does stress, however, the need for studying potential new compounds in the in vivo setting early in the screening process.
9. Expert Opinion
Bitter taste receptor agonists appear to be efficacious relaxants of airway smooth muscle and a target for a novel class of direct bronchodilators. The strength of the research thus far centers on identification of the most abundant TAS2Rs subtype, on airway smooth muscle, the initial signal transduction pathway, verification of a direct effect of bitter tastants on airway smooth muscle function, and efficacy of inhaled agonists in a mouse model of asthma. The link between activation of TAS2Rs, [Ca2+]i release, and membrane hyperpolarization needs additional study. The characteristics of the specialized [Ca2+]i pool to which TAS2Rs apparently communicate with, and the nature of the channels involved, remain underdeveloped. In addition, the presence and extent of agonist-promoted desensitization and the determination of its mechanism would aid in understanding which airway smooth muscle TAS2R subtype to target with a specific agonist. The choice of the “ideal” subtype may also depend on which TAS2R provides for the most robust relaxation as a single subtype. Potential regulation of TAS2R subtype expression, or function, by the human asthmatic milieu may also direct drug development to a specific subtype.
With the availability of thousands [22] of TAS2R agonists, the goal of identifying agonists acting at one or more of the higher expressing TAS2Rs on airway smooth muscle through high throughput screening should be readily achievable. Structural modifications of these compounds to achieve greater potency and selectivity would be desirable. Secondary screening techniques using intact cell mechanics, as well as human bronchi, will be necessary throughout the process of candidate development to assure that the physiological response is maintained, and, to compare with agents such as β-agonists. Moderate throughput of these physiological endpoints can be realized using MCT (isolated cells) and precision cut lung slices (bronchi) derived from normal and diseased human lung.
These efforts are likely to provide for TAS2R agonists that can be utilized for the treatment of asthma and chronic obstructive pulmonary disease. This will fill an unmet need in the treatment of these diseases, where many patients experience inadequate therapeutic responses to the only other class of direct bronchodilators, the β-agonists.
Article highlights.
Bitter taste receptors (TAS2Rs) are expressed on human airway smooth muscle cells.
When activated they cause direct relaxation of airway smooth muscle, as demonstrated by in vitro, ex vivo, and in vivo techniques.
TAS2R activation in human airway smooth muscle cells causes hyperpolarization of the membrane, consistent with the observed relaxation response.
TAS2R promotion of membrane hyperpolarization may be linked to receptor coupling to a specialized [Ca2+]i pool within the airway smooth muscle cell.
TAS2R-mediated airway relaxation is mechanistically different than that of the β-agonists, which act via β2-adreneigic receptors in a cAMP-dependent manner.
If developed for therapeutic purposes, TAS2R agonists will represent a distinct class of direct bronchodilator which will fill an unmet need in the treatment of obstructive lung diseases.
Acknowledgments
The author thanks his collaborators for generation of primary data and Charmaine Disimile for manuscript preparation. Supported by National Institute of Health grant HL045967 and HL071609.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.McFadden ER. Diseases of the Respiratory System: Asthma. In: Braunwald E, Fauci AS, Kasper DL, Hauser SL, Longo DL, Jameson JL, editors. Harrison’s 15th Edition Principles of Internal Mediicne. 15. McGraw-Hill; 2001. pp. 1456–1463. [Google Scholar]
- 2.Drazen JM, Silverman EK, Lee TH. Heterogeneity of therapeutic responses in asthma. Br Med Bull. 2000;56(4):1054–1070. doi: 10.1258/0007142001903535. [DOI] [PubMed] [Google Scholar]
- 3.Malmstrom K, Rodriguez-Gomez G, Guerra J, Villaran C, Pineiro A, Wei LX, et al. Oral montelukast, inhaled beclomethasone, and placebo for chronic asthma. A randomized, controlled trial. Montelukast/Beclomethasone Study Group. Ann Intern Med. 1999;130(6):487–495. doi: 10.7326/0003-4819-130-6-199903160-00005. [DOI] [PubMed] [Google Scholar]
- 4.National Health Interview Survey Public Use Data File. 2007 http://wwwcdcgov/NCHS/nhis/nhis_2007_data_releasehtm.
- 5.World Health Report 2002-reducing risks, promoting healthy life. 2002 doi: 10.1080/1357628031000116808. http://wwwwhoint/whr/2002/en/ [DOI] [PubMed]
- 6.Green SA, Liggett SB. G-protein-coupled receptor signaling in the lung. In: Liggett SB, Meyers DA, editors. The Genetics of Asthma. New York: Marcel Dekker, Inc; 1996. pp. 67–90. [Google Scholar]
- 7.Paul RJ, de Lanerolle P. Regulation of Smooth Muscle Contractility. In: Liggett SB, Meyers DA, editors. The Genetics of Asthma. New York: Marcel Dekker, Inc; 1996. pp. 91–117. [Google Scholar]
- 8.Lipworth BJ. Airway subsensitivity with long-acting beta 2-agonists. Is there cause for concern? Drug Saf. 1997;16:295–308. doi: 10.2165/00002018-199716050-00002. [DOI] [PubMed] [Google Scholar]
- 9.Kraan J, Koeter GH, van der Mark TW, Sluiter HJ, De Vries K. Changes in bronchial hyperreactivity induced by 4 weeks of treatment with antiasthmatic drugs in patients with allergic asthma: a comparison between budesonide and terbutaline. J Allergy Clin Immunol. 1985;76:636–636. doi: 10.1016/0091-6749(85)90786-9. [DOI] [PubMed] [Google Scholar]
- 10.Cheung D, Timmers MC, Zwinderman AH, Bel EH, Dijkman JH, Sterk PJ. Long-term effects of a long acting β2-adrenoceptor agonist, salmeterol, on airway hyperresponsiveness in patients with mild asthma. N Engl J Med. 1992;327:1198–1203. doi: 10.1056/NEJM199210223271703. [DOI] [PubMed] [Google Scholar]
- 11.Beasley R, Pearce N, Crane J, Burgess C. Beta-agonists: what is the evidence that their use increases the risk of asthma morbidity and mortality? J Allergy Clin Immunol. 1999;103:S18–S30. doi: 10.1016/s0091-6749(99)70270-8. [DOI] [PubMed] [Google Scholar]
- 12.Sears MR, Taylor DR. The β2-agonist controversy: Observations, explanations and relationship to asthma epidemiology. Drug Saf. 1994;11(4):259–283. doi: 10.2165/00002018-199411040-00005. [DOI] [PubMed] [Google Scholar]
- 13.Salpeter SR, Wall AJ, Buckley NS. Long-acting beta-agonists with and without inhaled corticosteroids and catastrophic asthma events. Am J Med. 2010;123(4):322–328. doi: 10.1016/j.amjmed.2009.07.035. [DOI] [PubMed] [Google Scholar]
- 14•.Einstein R, Jordan H, Zhou W, Brenner M, Moses EG, Liggett SB. Alternative splicing of the G protein-coupled receptor superfamily in human airway smooth muscle diversifies the complement of receptors. Proc Natl Acad Sci U S A. 2008;105(13):5230–5235. doi: 10.1073/pnas.0801319105. Defines the complement of GPCRs on human airway smooth muscle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15••.Deshpande DA, Wang WCH, McIlmoyle EL, Robinett KS, Schillinger RM, An SS, et al. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat Med. 2010;16(11):1299–1304. doi: 10.1038/nm.2237. Initial identification and characterization of TAS2Rs on airway smooth muscle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deshpande DA, Robinett KS, Wang WC, Sham JS, An SS, Liggett SB. Bronchodilator activity of bitter tastants in human tissue. Nat Med. 2011;17(7):776–778. doi: 10.1038/nm0711-776b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJ, Zuker CS. A novel family of mammalian taste receptors. Cell. 2000;100(6):693–702. doi: 10.1016/s0092-8674(00)80705-9. [DOI] [PubMed] [Google Scholar]
- 18•.Chandrashekar J, Mueller KL, Hoon MA, Adler E, Feng L, Guo W, et al. T2Rs function as bitter taste receptors. Cell. 2000;100(6):703–711. doi: 10.1016/s0092-8674(00)80706-0. Initial discovery of bitter taste receptors. [DOI] [PubMed] [Google Scholar]
- 19.Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS. The receptors and cells for mammalian taste. Nature. 2006;444(7117):288–294. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
- 20•.Meyerhof W. Elucidation of mammalian bitter taste. Rev Physiol Biochem Pharmacol. 2005;154:37–72. doi: 10.1007/s10254-005-0041-0. Excellent review of TAS2R organization and function. [DOI] [PubMed] [Google Scholar]
- 21.Margolskee RF. Molecular mechanisms of bitter and sweet taste transduction. J Biol Chem. 2002;277(1):1–4. doi: 10.1074/jbc.R100054200. [DOI] [PubMed] [Google Scholar]
- 22••.Meyerhof W, Batram C, Kuhn C, Brockhoff A, Chudoba E, Bufe B, et al. The molecular receptive ranges of human TAS2R bitter taste receptors. ChemSenses. 2010;35(2):157–170. doi: 10.1093/chemse/bjp092. Excellent data derived from recombinantly expressed TAS2Rs in HEK-293 cells with many bitter compounds. [DOI] [PubMed] [Google Scholar]
- 23.Morice AH, Bennett RT, Chaudhry MA, Cowen ME, Griffin SC, Loubani M. Effect of bitter tastants on human bronchi. Nat Med. 2011;17(7):775. doi: 10.1038/nm0711-775. [DOI] [PubMed] [Google Scholar]
- 24.An SS, Fabry B, Trepat X, Wang N, Fredberg JJ. Do biophysical properties of the airway smooth muscle in culture predict airway hyperresponsiveness? Am J Respir Cell Mol Biol. 2006;35(1):55–64. doi: 10.1165/rcmb.2005-0453OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.An SS, Laudadio RE, Lai J, Rogers RA, Fredberg JJ. Stiffness changes in cultured airway smooth muscle cells. Am J Physiol Cell Physiol. 2002;283(3):C792–C801. doi: 10.1152/ajpcell.00425.2001. [DOI] [PubMed] [Google Scholar]
- 26.An SS, Wang WC, Koziol-White CJ, Ahn K, Lee DY, Kurten RC, et al. TAS2R activation promotes airway smooth muscle relaxation despite beta(2)-adrenergic receptor tachyphylaxis. Am J Physiol Lung Cell Mol Physiol. 2012;303(4):L304–11. doi: 10.1152/ajplung.00126.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.An SS, Robinett KS, Deshpande DA, Wang WC, Liggett SB. Reply to: Activation of BK channels may not be required for bitter tastant-induced bronchodilation. Nat Med. 2012;18(5):650–1. doi: 10.1038/nm.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28•.Janssen LJ. Airway smooth muscle electrophysiology in a state of flux? Am J Physiol Lung Cell Mol Physiol. 2012;302(8):L730–2. doi: 10.1152/ajplung.00032.2012. Highlights potential methodologic problems with conventional electrophysiological methods when applied to airway smooth muscle. [DOI] [PubMed] [Google Scholar]
- 29•.Liggett SB, Lefkowitz RJ. Adrenergic receptor-coupled adenylyl cyclase systems: regulation of receptor function by phosphorylation, sequestration and downregulation. In: Sibley D, Houslay M, editors. Regulation of cellular signal transduction pathways by desensitization and amplification. London: John Wiley & Sons; 1993. pp. 71–97. Review of common mechanism of GPCR desensitization. [Google Scholar]
- 30.Liggett SB, Raymond JR. Pharmacology and molecular biology of adrenergic receptors. In: Bouloux PM, editor. Catecholamines. London: W.B. Saunders Co; 1993. pp. 279–306. [DOI] [PubMed] [Google Scholar]
- 31.McGraw DW, Liggett SB. Molecular mechanisms of beta2-adrenergic receptor function and regulation. Proc Am Thorac Soc. 2005;2(4):292–296. doi: 10.1513/pats.200504-027SR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32•.Robinett KS, Deshpande DA, Malone MM, Liggett SB. Agonist-promoted homologous desensitization of human airway smooth muscle bitter taste receptors. Am J Respir Cell Mol Biol. 2011;45:1069–1074. doi: 10.1165/rcmb.2011-0061OC. Initial studies of the potential for tachyphylaxis to TAS2R agonists in the airway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGraw DW, Mihlbachler KA, Schwarb MR, Rahman FF, Small KM, Almoosa KF, et al. Airway smooth muscle prostaglandin-EP1 receptors directly modulate beta2-adrenergic receptors within a unique heterodimeric complex. J Clin Invest. 2006;116(5):1400–1409. doi: 10.1172/JCI25840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGraw DW, Fogel KM, Kong S, Litonjua AA, Kranias EG, Aronow BJ, et al. Transcriptional response to persistent β2-adrenergic receptor signaling reveals regulation of phospholamban which alters airway contractility. Physiol Genomics. 2006;27:171–177. doi: 10.1152/physiolgenomics.00044.2006. [DOI] [PubMed] [Google Scholar]
- 35.McGraw DW, Almoosa KF, Paul RJ, Kobilka BK, Liggett SB. Antithetic regulation by β-adrenergic receptors of Gq-receptor signaling via phospholipase-C underlies the airway β-agonist paradox. J Clin Invest. 2003;112(4):619–626. doi: 10.1172/JCI18193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eason MG, Jacinto MT, Liggett SB. Contribution of ligand structure to activation of α2AR subtype coupling to Gs. Mol Pharmacol. 1994;45:696–702. [PubMed] [Google Scholar]
- 37.Jewell-Motz EA, Small KM, Liggett SB. α2A/α2C-adrenergic receptor third loop chimera show that agonist interaction with receptor-subtype backbone establishes G protein-coupled receptor kinase phosphorylation. JBC. 2000;275:28989–28993. doi: 10.1074/jbc.M005381200. [DOI] [PubMed] [Google Scholar]
- 38.Stallaert W, Christopoulos A, Bouvier M. Ligand functional selectivity and quantitative pharmacology at G protein-coupled receptors. Expert opinion on drug discovery. 2011;6(8):811–25. doi: 10.1517/17460441.2011.586691. [DOI] [PubMed] [Google Scholar]
- 39.Hudson BD, Smith NJ, Milligan G. Experimental challenges to targeting poorly characterized GPCRs: uncovering the therapeutic potential for free fatty acid receptors. Advances in pharmacology (San Diego, Calif) 2011;62:175–218. doi: 10.1016/B978-0-12-385952-5.00006-3. [DOI] [PubMed] [Google Scholar]
- 40•.Clark AA, Liggett SB, Munger SD. Extraoral bitter taste receptors as mediators of off-target drug effects. FASEB J. 2012;26(12):4827–31. doi: 10.1096/fj.12-215087. Discussion of TAS2Rs outside of the oral cavity and their potential functions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shah AS, Ben Shahar Y, Moninger TO, Kline JN, Welsh MJ. Motile cilia of human airway epithelia are chemosensory. Science (New York, NY) 2009;325(5944):1131–1134. doi: 10.1126/science.1173869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krasteva G, Canning BJ, Hartmann P, Veres TZ, Papadakis T, Muhlfeld C, et al. Cholinergic chemosensory cells in the trachea regulate breathing. Proc Natl Acad Sci U S A. 2011;108(23):9478–83. doi: 10.1073/pnas.1019418108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pietras CO, James A, Konradsen JR, Nordlund B, Soderhall C, Pulkkinen V, et al. Transcriptome analysis reveals upregulation of bitter taste receptors in severe asthmatics. Eur Respir J. 2012 doi: 10.1183/09031936.00077712. [DOI] [PubMed] [Google Scholar]
- 44.Sbarbati A, Tizzano M, Merigo F, Benati D, Nicolato E, Boschi F, et al. Acyl homoserine lactones induce early response in the airway. Anat Rec (Hoboken) 2009;292:439–448. doi: 10.1002/ar.20866. [DOI] [PubMed] [Google Scholar]
- 45.Brockhoff A, Behrens M, Massarotti A, Appendino G, Meyerhof W. Broad tuning of the human bitter taste receptor hTAS2R46 to various sesquiterpene lactones, clerodane and labdane diterpenoids, strychnine, and denatonium. J Agric Food Chem. 2007;55(15):6236–6243. doi: 10.1021/jf070503p. [DOI] [PubMed] [Google Scholar]
- 46.Tizzano M, Cristofoletti M, Sbarbati A, Finger TE. Expression of taste receptors in solitary chemosensory cells of rodent airways. BMC Pulm Med. 2011;11(3) doi: 10.1186/1471-2466-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]