Abstract
Due to a blood supply shortage, articular cartilage has a limited capacity for self-healing once damaged. Articular chondrocytes, cartilage progenitor cells, embryonic stem cells, and mesenchymal stem cells are candidate cells for cartilage regeneration. Significant current attention is paid to improving chondrogenic differentiation capacity; unfortunately, the potential chondrogenic hypertrophy of differentiated cells is largely overlooked. Consequently, the engineered tissue is actually a transient cartilage rather than a permanent one. The development of hypertrophic cartilage ends with the onset of endochondral bone formation which has inferior mechanical properties. In this review, current strategies for inhibition of chondrogenic hypertrophy are comprehensively summarized; the impact of cell source options is discussed; and potential mechanisms underlying these strategies are also categorized. This paper aims to provide guidelines for the prevention of hypertrophy in the regeneration of cartilage tissue. This knowledge may also facilitate the retardation of osteophytes in the treatment of osteoarthritis.
Keywords: Adult stem cell, Cartilage regeneration, Cartilage repair, Cartilage tissue engineering, Chondrogenesis, Hypertrophy
Introduction
Articular cartilage is an avascular and aneural translucent tissue that functions by resisting compression, preventing adjacent bone contact, and maintaining a low-friction surface for joint articulation. Anatomically, normal articular cartilage is composed of four main zones: the superficial zone, the middle zone, the deep zone, and the zone with calcified cartilage; a tide-mark separates articular cartilage from subchondral bone.1 Articular cartilage is composed of chondrocytes and extracellular matrix (ECM). Cartilaginous ECM contains collagen type II (COLII), noncollagenous proteins, and proteoglycans, such as aggrecan (AGC), that have sulfated glycosaminoglycans (GAGs) that absorb water. Chondrocytes are responsible for the generation and maintenance of the ECM and are the sole, differentiated cellular resident of articular cartilage.
Articular cartilage has a relatively high incidence of damage and deterioration from common trauma such as sports injury and diseases such as osteoarthritis.2 Due to the avascular nature of articular cartilage, the progenitor cells in blood and bone marrow are inaccessible to the injured cartilage and the chondrocytes cannot move to the injured cartilage and generate ECM to repair the damaged cartilage; consequently, articular cartilage is weak in self-repairing and generally ends up with a fibrous repair tissue (fibrocartilage) which lacks the biomechanical characteristics necessary to withstand compressive stress during articulation. This fibrocartilage generally deteriorates over time, resulting in a return of the original symptoms and occasional progression to osteoarthritis.3
Taking the knee joint as an example, current methods for treatment of articular cartilage lesions4 consist of (1) Palliative Strategies, such as physiotherapy, weight loss, and systemic pain relief medications; (2) Non-reparative, Non-restorative Strategies, such as debridement, chondral shaving, and knee joint lavage; (3) Reparative Strategies, such as arthroscopic abrasion arthroplasty, microfracture, and subchondral drilling; (4) Restorative Strategies, such as high tibial osteotomy, unicompartmental knee arthroplasty, and total knee arthroplasty; and (5) Transplantation Strategies, such as osteochondral transplantation (osteochondral grafting), mosaicplasty, and autologous chondrocyte transplantation (ACT). Although these methods can relieve the pain to a certain extent and improve knee joint function, the effect is controversial.5
Of the above methods, ACT shows promise in clinical follow-up. This approach consists of harvesting chondrocytes from the non-weight bearing healthy area of articular cartilage followed by cell culturing for approximately 6 weeks and then transplantation of the cultured cells during open surgery. Despite the advantages of reducing the risk of immunological rejection and transmissible disease, ACT has many disadvantages including donor-site morbidity, the requirement for two surgeries, possible leakage of chondrocytes from the recipient site, uneven distribution of the cells in the defect, chondrocyte dedifferentiation in monolayer culture, long recovery time after operation, and finally, periosteal hypertrophy.6 The loss of chondrocyte phenotype prior to utilization in implantation is of grave concern for cartilage engineering and regeneration.
Unlike chondrocytes, mesenchymal stem cells (MSCs) are becoming a promising cell source for cartilage regeneration due to in vitro expansion without running the risk of losing their phenotype; however, MSCs tend to simultaneously acquire hypertrophic properties during chondrogenic induction, indicating the possibility of further differentiation toward endochondral bone formation.7, 8 It is becoming crucial to systematically assess current strategies for minimizing hypertrophy of chondrogenically differentiated cells to provide a high-quality cartilage tissue for clinical defect repair. A previous review covered molecular and biophysical mechanisms regulating hypertrophic differentiation in chondrocytes and MSCs9; this review will focus on strategies for preventing chondrogenic hypertrophy, including some new findings, such as the impact of different MSC sources and culture substrates. Potential mechanisms underlying the above strategies will also be delineated.
Definition and characterization of chondrogenic hypertrophy
Chondrogenic hypertrophy is marked by a more than 10-fold increase in cell volume and ECM structural remodeling.10 Cell volume expansion affects cell function.11 The explosive increase in the volume of hypertrophic chondrocytes involves changes in intracellular and extracellular osmolarity, ECM degradation around the cell, and an increase in the amount of organelles per cell.12 Osmotic swelling has been shown stereologically to be responsible for most of the cell volume increase. Swelling can be the result of either an increase in cytoplasmic concentration or a decrease in extracellular osmolarity followed by aquaporin-mediated movement of water to re-establish iso-osmotic conditions.13 Of all the ECM molecules, AGC is the prime contributor to the osmotic pressure generated in cartilage, both due to its abundance and its high negative fixed charge. It is not completely understood if expression of terminal markers results in increased cell volume or vice versa.
Chondrocyte hypertrophic differentiation is the gradual development process from chondrogenic differentiation to cartilage mineralization, which is characterized by a series of markers; each of these markers has its own function in the process of cartilage mineralization.14 For example, the transcription factors, runt-related transcription factor 2 (RUNX2) and myocyte enhancer factor-2C (MEF2C), drive the expression of terminal differentiation markers, including matrix metalloproteinase 13 (MMP13),9 collagen type X (COLX),15 Indian hedgehog (IHH),16 alkaline phosphatase (ALP), and vascular endothelial growth factor (VEGF),8, 17 which all functionally contribute to endochondral ossification. Secreted MMP13 degrades COLII and AGC, key ECM components of functional cartilage18; COLX serves as a framework for subsequent calcification through matrix vesicles (MV)19; ALP hydrolyses pyrophosphate (PPi) to inorganic phosphate (Pi) which, in the presence of calcium, forms hydroxyapatite20; and IHH induces the proliferation of non-hypertrophic chondrocytes.21
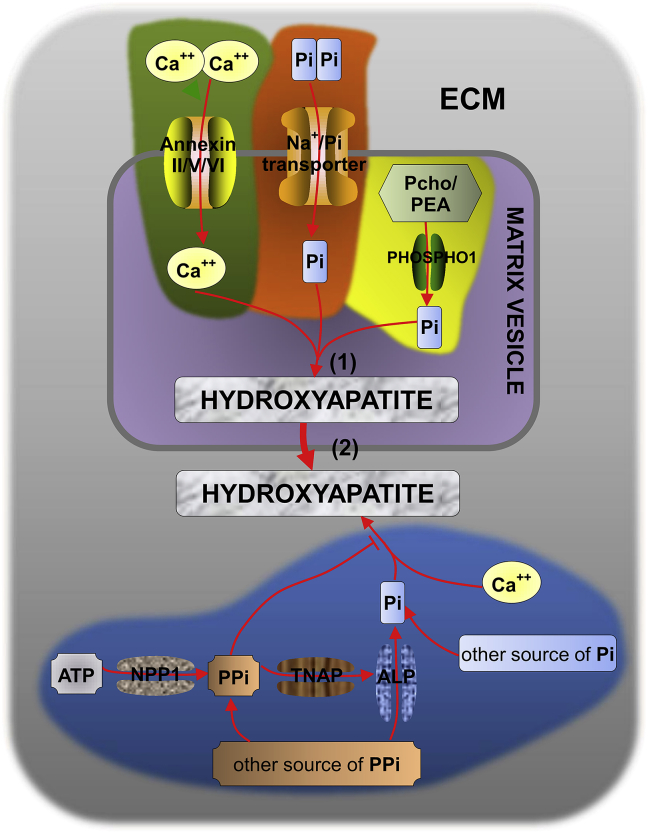
Calcification of cartilage ECM originates at MV.22 ECM mineralization to endochondral bone formation consists of three steps (Fig. 1): (1) Hydroxyapatite crystals are formed inside the MV; (2) Hydroxyapatite crystals penetrate MV into the ECM; and (3) Endochondral ossification. The final stages of endochondral ossification, including degradation of the calcified matrix, VEGF-mediated vascular invasion of the calcified zone, and deposition of osteoid on the calcified trabeculae by osteoblasts, are all under the control of MMPs.23 MMP is indispensable for the development of MV and it can calcify the growth plate; finally, calcification is substituted by endochondral bone. MMP13 binding to the MV membrane and cooperating with MMP9 could promote the release of VEGF in apoptotic chondrocytes, further accelerating the formation of vascularity in the growth plate.24
Figure 1.
ECM mineralization process: (1) Hydroxyapatite crystals are formed inside the MV (gray shading) when the concentration of calcium ion (influx through annexinII/V/VI calcium ion channels) and Pi [produced by the hydrolysis of Pcho and PEA via PHOSPHO1236, 237 and transferred into the MV by type-III Na+/Pi cotransporter238, 239 exceeds the solubility values.20, 238 (2) Hydroxyapatite crystals penetrate MV into the ECM (light grey shading). ATP, in the presence of nucleotide NPP1,240 can generate PPi which would in return inhibit the formation of hydroxyapatite.241 Pi could be produced through TNAP hydrolyzation of PPi238 and ALP dephosphorylation of PPi, promoting the formation of hydroxyapatite. PPi and Pi have antagonistic effects on the mineralization process.20, 242 Abbreviation: ALP: alkaline phosphatase; ATP: adenosine triphosphate; ECM: extracellular matrix; MV: matrix vesicles; NPP1: nucleotide pyrophosphatase phosphodiesterase 1; Pcho: phosphatidyl choline; PEA: phosphatidylethanolamine; PHOSPHO1: phosphoethanolamine/phosphocholine phosphatase; Pi: phosphatidylinositol; PPi: extracellular inorganic pyrophosphate; TNAP: tissue non-specific alkaline phosphatase.
Impact by cell sources chosen
Adult MSCs can differentiate into osteoblasts, adipocytes, muscle, and chondrocytes and are a promising cell source for tissue regeneration.25 Recent evidence indicates that great variability in differentiation capacity exists among tissue-specific stem cells,26 which might provide a theoretic foundation in regenerating a high-quality hyaline cartilage with minimum potential for hypertrophy.27
Articular chondrocytes
Articular chondrocytes are the earliest used cell sources for ACT.28 Due to limited availability, harvested chondrocytes need to be expanded in monolayer to obtain sufficient cells before implantation. However, monolayer expansion leads to a rapid chondrocyte dedifferentiation and causes loss of phenotype29 despite the limited restoration of chondrogenic properties of articular chondrocytes using growth factors and/or three dimensional (3D) culture.30, 31, 32 Dedifferentiated chondrocytes mostly become fibrous cartilage rather than hyaline cartilage33 with inferior biomechanical properties,34, 35 thus limiting the application of articular chondrocytes in cartilage repair.
Articular cartilage progenitor cells
Articular cartilage progenitor cells (ACPCs) usually exist in one third of the superficial zone of articular cartilage.36, 37, 38, 39 Recent findings show that ACPCs also exist in two thirds of the deep zone; the cell number in the deep zone is inferior to that in the superficial zone, but the chondrogenic and osteogenic differentiation capabilities are superior.40 McCarthy and colleagues found that both ACPCs and bone marrow stromal cells (BMSCs) could be induced for chondrogenic differentiation while COLX, RUNX2, and matrilin-1 existed only in the differentiated cells from BMSCs.41 ACPCs may therefore be considered superior to BMSCs in producing cartilage capable of functional repair despite a report showing ACPCs with inferior capability of producing cartilaginous matrix compared to articular chondrocytes.42 ACPCs isolated from the healthy area of articular cartilage could cause donor-site morbidity.43 When articular cartilage is injured severely, ACPCs begin to proliferate and migrate to the damaged site and act as alarmins promoting the development of inflammation44 while articular chondrocytes remain in a stationary state.45 The research on ACPCs is still in the early stage and needs further investigation to elucidate its role in cartilage regeneration.
Embryonic stem cells
Embryonic stem cells (ESCs) have the ability of infinite proliferation and self-renewal as well as differentiating into all kinds of human cells.46 The use of human ESCs to replace damaged cells and tissues is promising for cartilage tissue engineering in the future. However, in vitro chondrogenic induction of mouse ESCs also shows a tendency to hypertrophically differentiate.47 Complex ethical and legal questions as a result of the research needed to develop these cell-replacement therapies restrict the use of ESCs in research and clinical settings.
Mesenchymal stem cells
Mesenchymal stem cells are promising candidate cells for cartilage tissue engineering. High expression of cartilage hypertrophic markers such as COLX, MMP13, and phosphorylated ALP by BMSCs undergoing chondrogenic induction were observed in in vitro pellet culture.8 Like BMSCs, chondrogenesis of adipose-derived stem cells (ADSCs) was also associated with hypertrophy according to premature COLX expression and up-regulation of ALP activity as well as in vivo calcification of spheroids after ectopic transplantation in SCID mice.7
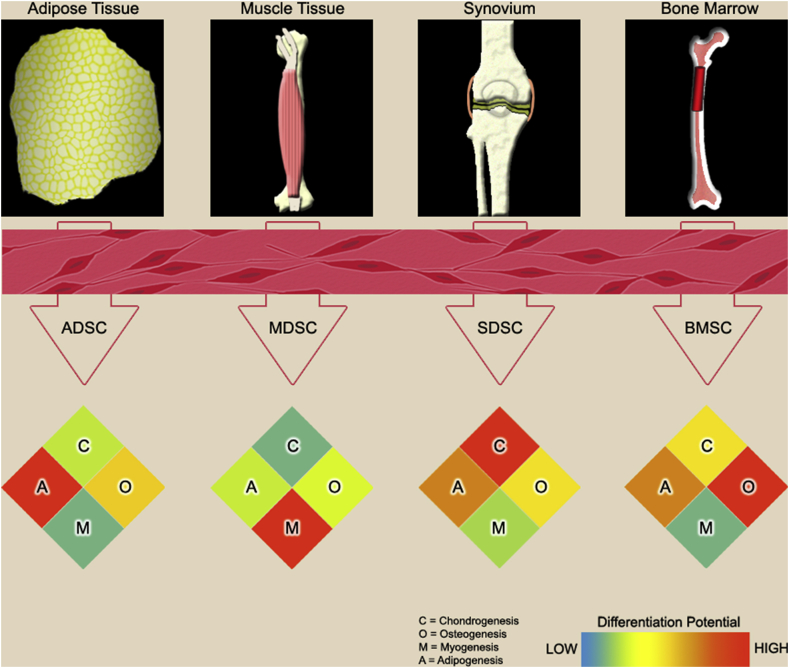
Recent evidence indicates that tissue-specific stem cells exhibit lineage-specific differentiation potential (Fig. 2).26 Compared to MSCs from adipose, bone marrow, and muscle, synovium-derived stem cells (SDSCs) display superior chondrogenic capacity with a limited potential toward hypertrophy.27 The lineage-specific differentiation ability of MSCs is also influenced by matrix microenvironment deposited by tissue-specific stem cells. For example, decellularized matrix deposited by SDSCs enhanced expanded SDSCs' chondrogenic potential rather than osteogenic capacity,48 while decellularized matrix deposited by BMSCs promoted expanded BMSCs' endochondral bone formation.49
Figure 2.
Adult stem cells can be derived from various tissues in the body. These viable and undifferentiated stem cell populations can be expanded in vitro and induced to undergo lineage-specific differentiation for chondrogenesis (C), osteogenesis (O), myogenesis (M), or adipogenesis (A). Although the cells may appear similar in morphology upon harvest, they are anything but identical. From the data presented in the paper entitled “Impact of tissue-specific stem cells on lineage-specific differentiation: a focus on the musculoskeletal system”,26 the efficacy of adult stem cells in lineage-specific differentiation is greatly affected by the type of resident tissue from which they are harvested. In the heatmap, the differentiation capacity is visualized by color ranging from low differentiation (blue) to high differentiation (red).
Besides various site-dependent MSCs, cell composition also plays a critical role in MSC-based cartilage engineering and regeneration. BMSCs are multi-lineage potential non-hematopoietic cells, accounting for only a small percentage of nucleated cells in bone marrow.50 Hematopoietic cells could yield short-term selected (STS) cells; passaging enriches more primitive, uniformly Sca-1 expressing, long-term selected (LTS) cells. In STS cells, chondrogenesis progressed rapidly to terminal differentiation while LTS cells differentiated at a slower rate with no hypertrophy. These data indicate the impact of stromal cell composition on the chondrogenic differentiation profile, which is an important aspect to be considered when developing MSC-based cartilage repair technologies.51
Strategies for inhibition of chondrogenic hypertrophy
Specific intervention
Current evidence indicates that chondrogenic hypertrophy can be prevented with interventions (Table 1) at a protein level, such as parathyroid hormone-related peptide (PTHrP),52 transforming growth factor beta1 (TGF-β1),53 MMP13 inhibitor,54 and extracellular signal-regulated protein kinases 1 and 2 (Erk1/2) inhibitor,55 and at a gene level, such as NK3 homeobox 2 (Nkx3.2),56 histone deacetylase 4 (HDAC4),57 and Chondromodulin 1 (ChM1).58, 59
Table 1.
Specific intervention to prevent hypertrophy during chondrogenic differentiation.
| Hypertrophy inhibitors | Target molecules | |
|---|---|---|
| Protein level | PTHrP | Nkx3.2/PKC/cAMP/CaMKII |
| TGF-β | Smad 2/3 or Smad1/5/8 | |
| BMP-4/7/13 | Bapx1/Nkx3.2 | |
| GG86/2 | MMP13 | |
| Dorsomorphin | BMPIR | |
| PD98059 | Erk1/2 | |
| NSC23766 | Rac1 | |
| FK506 | Calcineurin | |
| Gene level | Nkx3.2 | RUNX2 |
| SOX9 | β-catenin/PTHrP/RUNX2 | |
| Smad6 | Smad1/5/8 | |
| HDAC4 | Runx2/MEF2C | |
| ChM1 | p21 | |
| sFlt-1 | VEGF | |
| C-1-1 | RUNX2/COLX/ALP |
Abbreviation: ALP: alkaline phosphatase; Bapx1: bagpipe homeobox homolog 1; BMP: bone morphogenetic protein; BMPIR: bone morphogenetic protein receptor I; cAMP: cyclic adenosine monophosphate; CaMKII: Ca2+/calmodulin-dependent protein kinase II; ChM1: Chondromodulin 1; COLX: type X collagen; Erk1/2: extracellular regulated protein kinases 1/2; HDAC4: histone deacetylase-4; MEF2C: myocyte-specific enhancer factor 2C; MMP13: matrix metalloproteinase 13; Nkx3.2: NK3 homeobox 2; PKC: protein kinase C; PTHrP: parathyroid hormone-related peptide; Rac1: Ras-related C3 botulinum toxin substrate 1; RUNX2: runt-related transcription factor 2; sFlt-1: soluble Flt-1; Smad: mothers against decapentaplegic homolog (Drosophila); SOX9: sex determining region Y-type high mobility group box 9; TGF-β1: transforming growth factor beta 1; VEGF: vascular endothelial growth factor.
Protein-level intervention
PTHrP
During the development of cartilage, PTHrP, belonging to the family of parathyroid hormones (PTHs), maintained chondrocytes in a proliferative state and inhibited their terminal differentiation into hypertrophic chondrocytes.60 The addition of PTHrP in a dose-dependent fashion increased DNA and GAG contents in the pellets of both human BMSCs and ADSCs with down-regulation of COL10A1 and RUNX2 and up-regulation of SRY (sex determining region Y)-box 9 (SOX9) and COL2A1, suggesting PTHrP could promote chondrogenesis and suppress hypertrophy during in vitro chondrogenesis.61 Mueller and coworkers found that PTHrP(1–40) treatment reduced ALP expression in human BMSC pellets cultured under standard chondrogenic conditions in a dose-dependent manner; however, when cultured under hypertrophy-enhancing conditions, PTHrP(1–40) could not diminish the induced enhancement of hypertrophy in the MSC pellets.52 Kafienah and colleagues also found that the inclusion of PTHrP at a dose of 1 μM or 10 μM in chondrogenic induction medium resulted in significant suppression of COL10A1 and ALP activity in cartilage constructs engineered from BMSCs of patients with osteoarthritis.62 Of four PTHrP isoforms (1–34, 1–86, 7–34, and 107–139), Lee and Im found that PTHrP(1–34) most significantly enhanced chondrogenesis and suppressed hypertrophy in human BMSCs, supporting its use for cartilage tissue engineering.63
TGF-β
Shintani and coworkers reported that TGF-β1 enhanced the bone morphogenetic protein 2 (BMP-2)-induced chondrogenesis of bovine synovial explants, improved the hyaline-like properties of the neocartilage, and arrested the downstream differentiation of cells at an early stage of hypertrophy.53 Mello and Tuan found that TGF-β1 stimulated limb mesenchymal cell proliferation and suppressed hypertrophy from chick embryos, while the thyroid hormone triiodothyronine stimulated hypertrophy and apoptosis.64 Intriguingly, Cals and coworkers found that human BMSC pellets cultured with TGF-β1 had significantly less mineralization than pellets cultured with TGF-β3.65 Consistent with this finding, Pei and colleagues found that, despite a comparable chondrogenic capacity during in vitro induction, the supplementation of TGF-β3 up-regulated COL10A1 and ALP in porcine SDSC pellets compared to TGF-β1.66 However, Mueller and coworkers did not observe significant TGF-β subtype-dependent differences in suppressing hypertrophy in chondrogenic induction of human BMSCs.67 Interestingly, TGF-β-depletion during expansion improved the re-differentiation capacity of chondrocytes and inhibited hypertrophy68 while TGF-β1 administration during expansion of human articular chondrocytes in a serum-free medium redirected cell phenotype toward hypertrophy.69
BMP-4/7/13 and Dorsomorphin
Belonging to the superfamily of TGF-β, BMP enhanced the TGF-β-induced chondrogenesis of MSCs.70 BMP-4 promoted MSC chondrogenic differentiation while suppressing hypertrophy.71 Caron and coworkers found that BMP-2 acted as a specific inducer of chondrocyte hypertrophy while BMP-7 appeared to increase or maintain chondrogenic potential and prevent chondrocyte hypertrophy; bagpipe homeobox homolog 1 (Bapx1)/Nkx3.2 was involved in the BMP-7 mediated suppression of chondrocyte hypertrophy in ATDC5 cells (clonal mouse embryonal carcinoma cells).72 BMP-13 inhibited osteogenic differentiation of human BMSCs in vitro.73 Those studies indicated that BMP-4/7/13 can increase or maintain chondrogenic potential and prevent chondrocyte hypertrophy. Terminal differentiation and mineralization of chondrocytes can be regulated by the Smad1/5/8 pathway.74, 75, 76 Yu and colleagues found that Dorsomorphin inhibited the BMP type I receptors activin receptor-like kinase 2 (ALK-2), ALK-3, and ALK-6 and thus blocked BMP-mediated Smad1/5/8 phosphorylation.77
MMP13 inhibitor
As one of the hypertrophic markers, proteolysis involving MMP13 was required for chondrocyte differentiation that occurs as part of growth plate development and was associated with matrix mineralization.78 Bertram and coworkers found that broad spectrum pan-MMP inhibitors suppressed proteoglycan deposition, COLII and COLX staining, ALP activity, and reduced SOX9 and COL2A1 expression in a dose-dependent fashion; a selective MMP13 inhibitor GG86/2 allowed chondrogenesis and showed only weak effects on ALP activity, indicating that, in future therapeutic applications of diseased joints, the tested MMP13-specific inhibitor GG86/2 promises suppression of COLII degradation without imposing a risk of impairment of MSC-driven regeneration processes.54
Erk1/2 inhibitor
Erk1/2 signaling, one of the mitogen-activated protein kinase (MAPK) pathways, increased chondrogenic hypertrophy.79 Kim and Im found that the addition of PD98059, an Erk1/2 inhibitor, in chondrogenic medium suppressed hypertrophy and promoted chondrogenesis of human MSCs in a pellet culture system.80 Furthermore, the in vitro culture results showed that the PD98059-impregated poly(lactic-co-glycolic acid) (PLGA) scaffold is more effective in suppressing hypertrophy than the TGF-β2-immobilized scaffold while both scaffolds enhance chondrogenesis from human BMSCs. After 10 weeks of in vivo implantation in rabbits, osteochondral defects were successfully repaired in both PD98059-impregnated and TGF-β2-immobilized scaffolds seeded with rabbit BMSCs when evaluated grossly and microscopically. However, COLX was not observed from regenerated cartilage in PD98059-impregnated scaffold, whereas it was detected around chondrocytes in the TGF-β2-impregnated scaffolds. These results suggested that the use of the PD98059-impregnated scaffold led to articular cartilage regeneration of better quality and prevented hypertrophy when implanted in osteochondral defects.55
Rac1 inhibitor
Rac1 belongs to the Rho family of small GTPases and can promote chondrocyte hypertrophy within the growth plate.81 Rac1 inhibitor was also reported to decrease the expression of COL10A1 in human ADSCs.82 Activated Rac1 promoted expression of MMP13, a disintegrin and metalloproteinase with thrombospondin motifs 5 (ADAMTS5), and COLX in chondrocytes, and accelerated osteoarthritis progression while inhibition of Rac1 activity by NSC23766 delayed osteoarthritis development.83 Furthermore, TGF-β3 promoted ADSC chondrogenic differentiation and NSC23766 prevented differentiated cells from hypertrophy in vitro. The combination of ADSCs, TGF-β3, and NSC23766 promoted the quality of osteochondral defect repair in rats with much less chondrocyte hypertrophy and significantly higher International Cartilage Repair Society macroscopic and microscopic scores.84
FK506
FK506 (Tacrolimus) exerts its immunosuppressive effect via a common mechanism, calcineurin inhibition, after binding to intracellular proteins FK506-binding protein (FKBP). FK506 was found to induce chondrogenic differentiation of ATDC5 cells in a concentration-dependent manner (0.1–1000 ng/ml).85 van der Windt and colleagues found that inhibition of calcineurin activity by FK506 increased the expression of chondrogenic markers via endogenous TGF-β1 production in human articular chondrocytes.86 Furthermore, FK506 at physiologic tonicity (380 mOsm) exerted a superior effect compared to the physiologic tonicity or FK-506 alone, increasing anabolic markers while suppressing hypertrophic and catabolic markers.87
Gene-level intervention
Nkx3.2
Nkx3.2 is a transcription factor inhibitor promoting chondrogenesis88 which acts as a negative regulator of chondrocyte maturation in vivo.89 Lengner and colleagues found that transfection of Nkx3.2 into pluripotent C3H10T1/2 cells showed dose-responsive repression of the RUNX2 promoter. Bypassing RUNX2 repression by adenoviral-mediated introduction of RUNX2 into C3H10T1/2 cells prevented the induction of chondrogenesis, but could not reverse the chondrogenic phenotype once it was initiated, as evidenced by SOX9 and COL2A1 expression and ECM deposition. These results suggest that RUNX2 is a direct transcriptional target of Nkx3.2 and that repression of RUNX2 at the onset of chondrogenesis is a prerequisite for the activation of a chondrocyte-specific program of gene expression.56 RUNX2 is a critical link in BMP-2-mediated initiation of mesenchymal chondrogenesis that results in activation of SOX9 at least in part through the Nkx3.2-dependent repression of RUNX2.56
SOX9 and/or SOX5/SOX6
Sox proteins are necessary in the process of cartilage formation. SOX9 mutation can cause serious cartilage dysplasia, in terms of campomelic dysplasia.90 SOX5 and SOX6 knockout also can cause serious cartilage dysplasia, leading to death at birth in a mouse model.91 Overexpression of SOX9 promoted the ability for chondrogenesis in mouse BMSCs.92 The SOX5, SOX6, and SOX9 combination (the SOX trio) successfully induced chondrocyte differentiation of mouse ESCs.93 Moreover, Ikeda and coauthors found that, among various combinations examined, only the SOX trio successfully induced chondrocyte differentiation in all cell types tested, including nonchondrogenic types, and the induction occurred regardless of the culture system used (monolayer, spheroid, and 3D). Contrary to the conventional chondrogenic techniques, the SOX trio suppressed hypertrophic and osteogenic differentiation at the same time.94 Venkatesan and coworkers found that recombinant adeno-associated virus (rAAV)-FLAG-hSOX9 delivery in human BMSCs strongly enhanced chondrogenic differentiation and reduced the levels of markers of hypertrophy and terminal and osteogenic/adipogenic differentiation. These effects were accompanied with decreased levels of β-catenin (an indicator of osteoblast lineage differentiation) and enhanced PTHrP expression via SOX9 treatment.95
SMAD6
Smad6 and Smad ubiquitin regulatory factor 1 (Smurf1) blocked the signal transduction of the BMP-Smad1/5/8 pathway.96, 97 SMAD6 transgenic mice showed postnatal dwarfism with osteopenia and inhibition of Smad1/5/8 phosphorylation in chondrocytes.96 In cultured human articular chondrocytes, stimulation with interleukin 1β (IL-1β) showed up-regulation of SMAD7, whereas SMAD6, aggrecan (ACAN), and COL2A1 were down-regulated,98 suggesting a potentially important role of IL-1β signaling in chondrocytes via indirect influencing of the BMP/TGF-β signaling cascade. Histological analysis of appendicular bones revealed delayed onset of hypertrophic differentiation and mineralization at midgestation in SMAD6−/− mice.99 By late gestation, however, an expanded hypertrophic zone associated with an increased pool of proliferating cells undergoing hypertrophy was evident in SMAD6 mutant growth plates. Loss of SMAD6 in mice led to defects in both axial and appendicular skeletal development.99 Overall, those results show that Smad6 is required to limit BMP signaling during endochondral bone formation.
HDAC4
HDACs modulate cell growth and differentiation by governing chromatin structure and repressing the activity of specific transcription factors.100, 101 HDAC4-null mice display premature ossification of developing bones due to ectopic and early onset chondrocyte hypertrophy, mimicking the phenotype that results from constitutive RUNX2 expression in chondrocytes. Conversely, overexpression of HDAC4 in proliferating chondrocytes in vivo inhibited chondrocyte hypertrophy and differentiation, mimicking a RUNX2 loss-of-function phenotype.102 These results establish HDAC4 as a central regulator of chondrocyte hypertrophy and skeletogenesis and suggest general roles for class II HDACs in the control of cellular hypertrophy. Similarly, Pei and coauthors found that, in the presence of TGF-β1, adenovirus-mediated HDAC4 transduction in porcine SDSCs sped up and maintained a high level of chondrogenesis while down-regulating COL10A1 expression.57 Shimizu and coworkers found that HDAC4 interacted with RUNX2, bound the MMP13 promoter, and suppressed MMP13 gene transcription in the rat osteoblastic cell line, UMR 106-01. PTH induces the rapid cyclic adenosine monophosphate (cAMP)-dependent protein kinase-dependent release of HDAC4 from the MMP13 promoter and subsequent transcription of MMP13. Knock-out of HDAC4 either by small interfering RNA (siRNA) in vitro or by gene deletion in vivo led to an increase in MMP13 expression; overexpression of HDAC4 decreased the PTH induction of MMP13, indicating that HDAC4 represses MMP13 gene transcription in bone.103
Chondromodulin 1/soluble Flt-1
ChM1 is significant in articular cartilage and could induce the chondrocyte phenotype and inhibit the invasion of vessel structures.104 Klinger and colleagues found that transplantation of porcine osteochondral progenitor cells infected with AAV-ChM1 to cartilage lesions in the knee joints of miniature pigs that were treated by the microfracture technique stabilized the chondrocyte phenotype by supporting chondrogenesis but inhibiting chondrocyte hypertrophy and endochondral ossification.105 The underlying mechanism is unclear; some studies indicated that ChM1 promotes expression of the cell cycle inhibitor p21WAF1/Cip1,106 preventing expression of ALP and COL10A1.58, 59
VEGF increased the BMP-4-induced endochondral bone formation of muscle-derived stem cells (MDSCs) in vitro.107 The soluble Flt-1 (sFlt1) gene, a VEGF antagonist, delayed the development of mouse osteoarthritis.108 Kubo and coworkers found that sFlt-1 gene therapy improved BMP-4- and TGF-β3-induced in vitro chondrogenesis of MDSCs and promoted the persistence of articular cartilage repair by preventing vascularization and bone invasion into the repaired articular cartilage.109 Matsumoto and colleagues also reported that, after intraarticular injection of stem cells for cartilage repair in an immunodeficient rat osteoarthritis model, a combination of sFlt-1- and BMP-4-transduced MDSCs demonstrated better repair without osteophyte formation macroscopically and histologically when compared with a combination of VEGF- and BMP-4-transduced MDSCs or with BMP-4-transduced MDSCs alone.110 However, some reports also indicated that VEGF is a necessary survival factor for stem cells and chondrocytes in the process of cartilage development. In VEGF knockout mice, a lot of chondrocytes died in the epiphyseal region during the process of cartilage development.111, 112
ERG/C-1-1
The ETS-related gene (ERG) belongs to the family of erythroblast transformation-specific (ETS) transcription factors.113 Iwamoto and coworkers found that, during limb development, C-1-1 and ch-ERG had diverse biological properties and distinct expression patterns. Virally driven expression of C-1-1 maintained chondrocytes in a stable and immature phenotype, blocked their maturation into hypertrophic cells, and prevented the replacement of cartilage with bone. In contrast, virally driven expression of ch-ERG significantly stimulated chondrocyte maturation in culture, as indicated by increases in ALP activity and deposition of a mineralized matrix; however, it had modest effects in vivo. Growth of articular chondrocytes in culture was accompanied by decreasing C-1-1 expression after several passages, while expression of hypertrophic markers increased. Expression of C-1-1 in cultured chondrocytes inhibited cell hypertrophy, ALP activity, and cartilage matrix mineralization. In contrast, over-expression of ch-ERG promoted chondrocyte maturation and mineralization.114 Those findings suggest that C-1-1 plays a crucial role in the development process of cartilage formation and is important for steering the cells toward alternative developmental paths in the epiphyseal region; it also makes chondrocytes in the epiphyseal region acquire a permanent articular chondrocyte phenotype.
Co-culture
The use of a co-culture system of articular chondrocytes and MSCs could enhance chondrogenesis and suppress hypertrophy during chondrogenesis of MSCs.115, 116 Aung and colleagues found that human BMSCs co-cultured with primary osteoarthritic chondrocytes underwent chondrogenic differentiation even in the absence of growth factors; however, the same effect could not be replicated using osteoarthritic chondrocyte-conditioned medium or expanded cells. Additionally, the co-culture environment down-regulated hypertrophic differentiation of human BMSCs.117 Bian and coworkers found that mixed cell populations (human BMSCs and human articular chondrocytes) encapsulated in hydrogels exhibited significantly higher Young's moduli, dynamic moduli, GAG levels, and collagen content than did constructs seeded with only BMSCs or chondrocytes. In addition, the deposition of COLX was significantly lower in the co-culture constructs than in the constructs seeded with BMSCs alone.118
Articular chondrocyte-derived soluble factors and direct co-culture are potent means of improving chondrogenesis and suppressing the hypertrophic development of MSCs. PTHrP secreted by chondrocytes is an important candidate soluble factor involved in this effect.119, 120 Two distinct cell types (human BMSCs and rabbit articular chondrocytes) were encapsulated in alginate hydrogels singly or in various ratios and cultured under chondrogenic conditions; Mo and coworkers found that newly synthesized cartilaginous ECM and COL2A1 were up-regulated with greater human BMSC ratios and longer culture periods. However, a specific COL2A1 human gene probe was found only in single human BMSC groups and was absent in all co-culture groups, which indicated that the enhanced cartilaginous phenotype originated from the co-cultured rabbit chondrocytes.121 Bovine articular chondrocytes co-cultured with BMSCs also resulted in redifferentiation of passaged chondrocytes and the trophic effect of MSCs may significantly increase the chondrogenic potential of articular chondrocytes.122 Those studies indicated that both MSCs and articular chondrocytes stabilized the chondrocyte phenotype. MSCs drive chondrocytes to synthesize cartilaginous ECM; chondrocytes also inhibit or minimize the hypertrophy of chondrogenic MSCs.
Culture substrates
Culture substrates can be synthetic material, purified single matrix component, or comprehensive decellularized matrix from mature tissue or grown cells. Cell phenotype, adhesion, migration, proliferation, and differentiation can be affected by culture substrates, such as ECM components and structure.123
Nitrogen-rich plasma polymer
One of the most important properties of MSCs is adherent growth. The capability for adhesion of stem cells can be enhanced by nitrogen (N)-rich plasma polymer layers (PPE:N).124 Petit and colleagues found that PPE:N suppressed COL10A1 in fetal bovine growth plate chondrocytes but had no significant effect on COL2A1, ACAN, MMP13, and cyclin B2 (CCNB2) levels,125 indicating that PPE:N is beneficial for MSC chondrogenesis. Similarly, Mwale and coworkers found that PPE:N almost completely suppressed the expression not only of COL10A1, but also of osteogenic marker genes such as ALP, bone sialoprotein (BSP), and osteocalcin (BGLAP) in human BMSCs. In contrast, neither ACAN nor COL1A2 expression was significantly affected.126 Rampersad and coworkers further confirmed the potential of two different types of PPE:N surfaces (low-pressure-PPE:N [L-PPE:N] and high-pressure-PPE:N [H-PPE:N]) in suppressing COL10A1 expression, more so on the latter. Interestingly, when human BMSCs were transferred to pellet cultures, the expression level of COL10A1 was further decreased by preincubation on H-PPE:N, suggesting that these kinds of coatings show promise for tissue engineering of cartilage and disc tissues.127
Chondroitin sulfate
Chondroitin sulfate (CS), a chemical that is normally found in articular cartilage, has been widely used in cartilage tissue engineering.128, 129, 130 Varghese and colleagues found that the aggregation of goat BMSCs in poly(ethylene glycol) (PEG)/CS hydrogels resulted in an enhancement of chondrogenic genes and matrix production and a significant down-regulation of COLX expression compared to control PEG hydrogels containing no CS-moieties.131 Similarly, a 3D alginate microbead platform was coated with cartilaginous ECM components hyaluronic acid, CS, and COLII to emulate an in vivo chondrogenic microenvironment for the differentiation of BMSCs; Wu and coworkers found that CS- and COLII-coated microbeads enhanced the chondrogenic differentiation of human BMSCs. In addition, COLII-coated microbeads resulted in hypertrophic maturation of the differentiated chondrocytes, similar to conventional pellet culture, while CS-coated microbeads were able to retain the pre-hypertrophy state of the differentiated cells.132 Those findings demonstrated that CS coatings are beneficial to induce MSCs to differentiate into chondrocytes and prevent hypertrophy.
Decellularized matrix
ECM, an indispensable niche for stem cells in vivo, not only provides support scaffold, but also participates in the regulation of self-renewal, proliferation, and differentiation of stem cells.133 Decellularization of natural ECM, eliminating the cells and antigen composition, can avoid disease transmission, reduce inflammation and immune response, and maintain the integrity of ECM.134 The advantages of using ECM in cartilage engineering and regeneration include not only the formation of functional specific tissue dependent on several conditions, such as cytokines inside ECM and unique surface anatomical features,135, 136 but also the in vitro microenvironment for stem cell rejuvenation during in vitro expansion.48, 49, 137, 138
Cartilage matrix
A porous scaffold derived from adult porcine articular cartilage has the ability to induce chondrogenic differentiation of human ADSCs without exogenous growth factors, with significant synthesis and accumulation of ECM macromolecules, and with the development of mechanical properties approaching those of native cartilage.139 In a further study, acellular cartilage matrix (ACM) powders and human SDSCs were mixed into collagen gel for in vitro culture. The data showed that ACM powders had the potential of promoting COL2A1 expression in an environment with no growth factors; a synergistic effect between ACM powders and chondrogenic growth factors was observed in the formation of engineered cartilage and reduction of hypertrophy in chondrogenesis of SDSCs.140
Stem cell matrix
He and coworkers found that decellularized matrix deposited by stem cells (DSCM) could provide a cell expansion system for the rejuvenation of porcine SDSCs, in terms of “tiny and spindle shaped typical stem cell morphology”, “higher cell yields (about 44 times more than those expanded on plastic flasks for two continuing passages)”, and “high-quality cells (in subsequent 14-day chondrogenic induction, ECM expanded SDSCs exhibited a higher ratio of GAG to DNA than plastic expansion, 39.37 ± 3.05 versus 5.19 ± 1.11)”, despite the pellets from the DSCM expanded SDSC group having half the level of COL10A1.48 From the same laboratory, Li and Pei found that both basic fibroblast growth factor (FGF2) and DSCM could promote porcine SDSCs' proliferation and chondrogenic potential; however, in the subsequent chondrogenic induction, DSCM expanded SDSCs yielded pellets with significantly lower levels of COL10A1, ALP, and MMP13 compared to FGF2 pretreated SDSCs.141
Chondrocyte matrix
A cell-derived ECM scaffold was constructed using cultured porcine chondrocytes via a freeze-drying method; Jin and colleagues demonstrated its ability to promote cartilage formation both in vitro and in vivo.142, 143 From the same group, Choi and colleagues evaluated the chondrogenic capacity of rabbit BMSCs after growth on ECM scaffold deposited by porcine chondrocytes and found that the ECM scaffold evoked chondrogenic differentiation of BMSCs earlier and produced more cartilaginous tissues than the polyglycolic acid (PGA) scaffold. Next, BMSCs in each scaffold were preconditioned with chondrogenic media in vitro for 1 week and implanted in the backs of nude mice for 6 weeks. The initially formed cartilaginous tissues turned into bone matrix. This phenomenon progressed much more rapidly in the PGA group than in the ECM group. In the ECM group, the chondrogenic phenotypes of BMSCs were also maintained longer than in the PGA group. The loss of chondrogenic phenotypes was accompanied by the calcification of matrix and hypertrophic changes by immunohistochemistry for osteocalcin and COLI and COLX. Blood vessel invasion took place more deeply and intensively in the PGA group. These results suggested that the ECM scaffold not only strongly supports chondrogenic differentiation of rabbit MSCs, but also helps maintain its phenotype in vivo.137
Low oxygen
Cartilage is an avascular tissue and thus resides in a microenvironment with reduced oxygen tension, indicating that low oxygen might benefit in vitro chondrogenic differentiation of MSCs. Expansion under hypoxia was reported to enhance the preservation of “stemness” properties and inhibit osteogenic potential of marrow-isolated adult multilineage inducible (MIAMI) cells144 as well as to increase chondrogenic differentiation of ADSCs while suppressing hypertrophy.145, 146 Similarly, chondrogenic induction in a low oxygen environment would increase chondrogenic differentiation and suppress hypertrophy of MSCs cultured in both pellets and hydrogels used in tissue engineering strategies.147 Hirao and coworkers found that hypoxia promoted BMP2-induced GAG production and suppressed ALP activity and mineralization of the pluripotent mesenchymal cell line C3H10T1/2. Thus, hypoxia promoted chondrocytic commitment rather than osteoblastic differentiation. In the mouse embryo forelimb organ culture, hypoxia increased cartilaginous matrix synthesis. These effects were primarily mediated by p38 MAPK activation, independent of SOX9. Hypoxia inhibited COL10A1 expression via down-regulation of RUNX2 activity by Smad suppression and HDAC4 activation. These findings indicated that hypoxia promotes chondrocytic differentiation and cartilage matrix synthesis and suppresses terminal chondrocyte differentiation.148 Furthermore, Kawato and colleagues demonstrated that Nkx3.2-dependent suppression of RUNX2 was a crucial factor in hypoxia-dependent maintenance of chondrocyte identity.149
Biomechanical stimulation
Cartilage cells live in an environment heavily influenced by mechanical forces. Wong and coworkers found that cyclic tension (shear stress) could up-regulate the expression of RUNX2, MMP13, connective tissue growth factor (CTGF), COL10A1, and VEGF and down-regulate the expression of the tissue inhibitor of metalloproteinase-1 (TIMP1) in bovine chondrocyte-seeded alginate constructs. Cyclic tension also up-regulated the expression of COL2A1, cartilage oligomeric matrix protein (COMP) and Proteoglycan 4 (PRG4), but did not change the expression of SOX9 and ACAN. Cyclic hydrostatic pressure down-regulated the expression of MMP13 and COL1A1 and up-regulated expression of TIMP1 compared to the unloaded controls. Hydrostatic pressure may slow chondrocyte differentiation and have a chondroprotective, anti-angiogenic influence on cartilage tissue. These findings suggested that cyclic tension activates the RUNX2/MMP13 pathway and increases the expression of terminal differentiation hypertrophic markers.150 Bian and colleagues found that dynamic compressive loading increased the mechanical properties, as well as the glycosaminoglycan (GAG) and collagen contents of human BMSC-seeded hyaluronic acid hydrogel constructs in a seeding density dependent manner and was also shown to significantly reduce the expression of hypertrophic markers and to suppress the degree of calcification in MSC-seeded hyaluronic acid hydrogels.151
Low-intensity ultrasound (LUS) was shown to enhance TGF-β-mediated chondrogenic differentiation of human BMSCs in a pellet culture system.152 Similarly, Cui and coworkers found that LUS pretreatment could significantly increase chondrogenic genes while decreasing hypertrophic genes in rabbit BMSCs seeded on PGA scaffold; four weeks after subcutaneous transplantation into nude mice, the LUS and LUS/TGF groups were significantly better at forming hyaline cartilage-like tissue than the non-LUS groups. The development of osteogenic phenotypes shown by von Kossa staining was highly suppressed until four weeks in the LUS groups, along with compressive strength comparable to the positive control.153
Potential mechanisms underlying chondrogenic hypertrophy
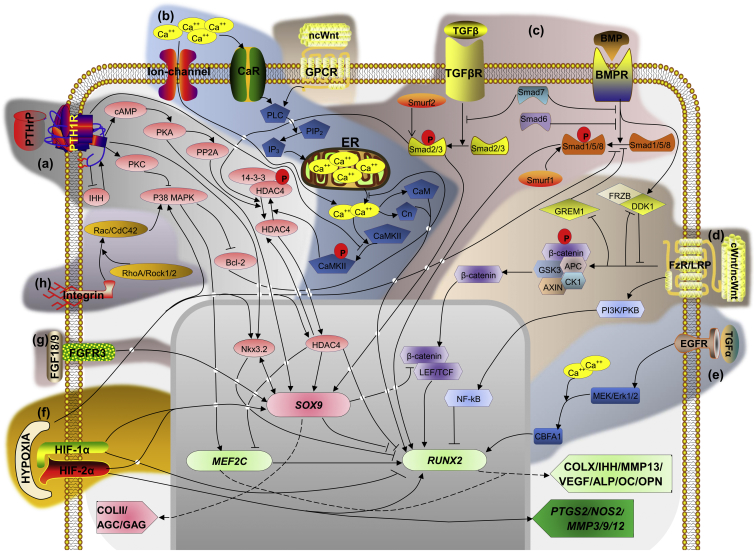
Many signaling pathways and biophysical factors have been involved in the process of chondrogenic hypertrophy, so understanding these pathways (Fig. 3) will benefit the control of hypertrophy in cartilage regeneration.
Figure 3.
Potential signaling pathways of chondrocyte hypertrophy, including but not limited to (a): PTHrP/IHH signaling, (b): calcium ion channel signaling, (c): TGF-β/BMP signaling, (d): Wnt signaling, (e): MAPK (TGF-a) signaling, (f): HIF signaling, (g) FGF signaling, and (h): integrin signaling. The main transcription factors regulating hypertrophy are SOX9, which is responsible for the expression of COLII and AGC, and RUNX2 which regulates transcription of COL10A1, IHH, MMP13, VEGF, ALP, OC, and OPN genes. SOX9 was shown to repress RUNX2162 through Nkx3.289 and LEF/TCF/β-catenin complex.184, 185 Nkx3.2 and SOX9 mutually induce each other's expression.162 Nkx3.2 is induced by PTHrP89 and acts synergistically with SOX9243 to inhibit RUNX2.56 MEF2C is proposed to be the main regulator of RUNX2,15 and drives the expression of the terminal differentiation markers.15 ‘P’ depicts phosphorylation/dephosphorylation. ‘→’ means ‘increase’, ‘T’ means ‘inhibit’.
PTHrP/IHH signaling
As one of the key anti-hypertrophy factors, PTHrP functions by activating protein kinase A (PKA) and, to a lesser extent, protein kinase C (PKC).154 cAMP-dependent PKA phosphorylates SOX9 and activates protein phosphatase II (PP2A)155 leading to the dephosphorylation of HDAC4 and the inactivation of MEF2C.156 PKC inhibits the activity of p38 MAPK157 reducing MEF2C phosphorylation158 and, ultimately, hypertrophic gene expression.159 PTHrP also interferes with the calcium pathway by dephosphorylation of calcium/calmodulin (CaM)-dependent protein kinase II (CaMKII) because endogenous CaMKII activity is up-regulated prior to hypertrophy; the loss of CaMKII function substantially blocks the transition from proliferation to hypertrophy.160
In addition, PTHrP blocks hypertrophy by stimulating Nkx3.289 and preventing RUNX2 expression.89, 161 Sonic Hedgehog (SHH) and BMP signals work in sequence to establish a positive regulatory loop between SOX9 and Nkx3.2.162 Interestingly, IHH and PTHrP signaling play crucial roles in regulating the onset of chondrocyte hypertrophy by forming a negative feedback loop, in which IHH signaling regulates chondrocyte hypertrophy by controlling PTHrP expression. IHH signaling is also found to promote chondrocyte hypertrophy of PTHrP independently.163, 164
Ion channels
Accumulation of cytosolic Ca2+ concentration is indispensable to proper chondrogenesis.165 This cytoplasmic Ca2+ accumulation occurs through at least two distinct pathways. Extracellular Ca2+ can directly pass through ion channels in the cell membrane or, alternatively, it can activate G-protein coupled receptors (GPCRs), such as the calcium-sensing receptor, CaR, which is expressed in elevated levels in hypertrophic chondrocytes166 that stimulate intracellular Ca2+ release from the endoplasmic reticulum (ER).167 Activation of CaR could increase the expression of COLX. The Ca2+ release from the ER is regulated by phospholipase C (PLC) activation, prompting hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) to inositol 1,4,5-triphosphate (IP3), which triggers the opening of ER ion-channels (IP3R).168 A series of changes of signaling pathways are triggered by cytoplasmic calcium via CaM, CaMKII, and calcineurin.169
Phosphorylation of HDAC4 by CaMKII promotes nuclear export and prevents nuclear import of HDAC4 with consequent derepression of chondrocyte hypertrophy.170 PTHrP inhibited the activity of p38 MAPK, which led to an increase in Bcl-2 activity which was further shown to inhibit IP3R channel opening.171 Administration of PTHrP or the overexpression of its receptor abolished the effect of elevated calcium to increase terminal differentiation markers via dephosphorylation of CaMKII activity.160 A synergistic relationship exists between the stimulatory effect of active CaMKII and RUNX2 on IHH expression.160 Increasing Ca2+ concentration results in significant up-regulation of Erk1/2 phosphorylation promoting chondrocyte hypertrophy and mineralization.172
TGF-β/BMP signaling
Hellingman and colleagues found that blocking Smad2/3 after the onset of chondrogenesis resulted in a halt in COLII production; blocking Smad1/5/8 resulted in decreased expression of MMP13, COLX, and ALP while allowing COLII production. Moreover, blocking Smad1/5/8 prevented mineralization. This finding indicated that, while Smad2/3 is important for continuation of COLII deposition, Smad1/5/8 phosphorylation is associated with terminal differentiation and mineralization.173 TGF-β-dependent Smad3 signaling pathways present a key role for the SOX9-dependent transcriptional activation in primary chondrogenesis174 and also leads to RUNX2 inhibition through dephosphorylation of HDAC4.175 The Smad2/3 and Smad1/5/8 pathways can be blocked by Smad7, while only the Smad1/5/8 pathway can be inhibited by Smad6. Therefore, hypertrophy can be suppressed by up-regulating the expression of Smad6.176, 177 Smurf1 can suppress terminal differentiation via blocking the Smad1/5/8 pathway178 and Smurf2 can decrease chondrogenic differentiation by blocking the Smad2/3 pathway.179 Thus, endochondral ossification can be stimulated by overexpression of Smurf2.180
Wnt signaling
The Wingless/Int (Wnt) signaling, including canonical Wnt (cWnt) and non-canonical Wnt (ncWnt), is another pathway involved in regulation of chondrocyte hypertrophy. cWnt signaling controls the fate of β-catenin via the Frizzled receptor (FzR).181 In the absence of cWnt, β-catenin is bound by a degradation complex consisting of glycogen synthase kinase 3 (GSK3), adenomatous polyposis coli (APC), AXIN, and casein kinase 1α (CKI) which phosphorylate β-catenin initiating its ubiquitination and proteosomal degradation.182 cWnt activation of the FzR and coreceptor low density lipoprotein receptor-related protein 5 and 6 (LRP5/6) interferes with the degradation complex; β-catenin can translocate to the nucleus where it binds to the lymphoid enhancer factor (LEF) and T cell factor (TCF) proteins. The LEF/TCF/β-catenin complex promotes RUNX2-expression inducing hypertrophy.183 SOX9 inhibits this signaling through phosphorylation/degradation of β-catenin.184, 185 The inhibition of cWnt leads to an increase in COL2A1 and ACAN expression but does not affect COL10A1 expression in MSC pellet culture.186 The ncWnts (such as Wnt5a) exhibit dual functions during chondrogenesis of MSCs. At early stages, Wnt5a induces chondrogenesis and hypertrophy through intracellular Ca2+ release via GPCR activation. Later, it acts as an inhibitor of hypertrophy by activating the phosphoinositide 3-kinase (PI3K)/protein kinase B (PKB or Akt)-dependent pathway which, in turn, activates nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), an inhibitor of RUNX2.187
Gremlin 1 (GREM1), frizzled related protein (FRZB), and dickkopf 1 homolog (Xenopus laevis) (DKK1) are enriched in articular cartilage compared with other hyaline cartilage types and these BMP and cWnt antagonists are important regulators of articular cartilage homeostasis by preventing hypertrophic differentiation of chondrocytes.188 GREM1, FRZB, and DKK1 mRNA expression levels are down-regulated in osteoarthritis.189, 190 FRZB and DKK1 are cWnt antagonists and GREM1 is a BMP antagonist. GREM1 is also able to inhibit Wnt signaling via unknown indirect mechanisms and BMP signaling is able to repress Wnt signaling.191 The crosstalk between BMP and cWnt signaling might act as a feedback loop that balances the activity of both pathways.190
MAPK signaling
MAPKs play a key role in a variety of cellular responses including proliferation, differentiation, and apoptosis of cells.192 The Erk1/2 MAPK pathway is activated by growth factors and the JNK/p38 MAPK pathways are activated by cellular stress, cytokines, and hypoxia.193, 194 Inhibition of p38 MAPK in hypertrophic chondrocytes by either PTH, SB203580, or both together leads to a decrease of COL10A1 mRNA and an increase of the expression of prehypertrophic marker cartilage matrix protein. Therefore, inhibition of p38 converts a hypertrophic cell phenotype to a prehypertrophic one, thereby preventing precocious chondrocyte hypertrophy.157, 158
Activation of the Erk1/2 pathway promoted chondrocyte hypertrophy.79, 195 TGF-a, an activator of epidermal growth factor receptor (EGFR) signaling, was up-regulated in articular chondrocytes in experimentally induced and human osteoarthritis; inhibition of MAPK kinase (MEK)/Erk1/2 could prevent TGF-a-induced deterioration of COLII and AGC.196 Stimulation of EGFR signaling in articular chondrocytes by TGF-a resulted in the activation of RhoA/Rho-associated protein kinase (ROCK), MEK/ERK, PI3K, and p38 MAPK pathways and cartilage degradation. Therefore, inhibition of p38 MAPK and Erk1/2 is an effective strategy to minimize hypertrophy in cartilage regeneration.
HIF signaling
Hypoxia inducible factor 1 alpha (HIF-1α), an oxygen-sensitive transcription factor, was reported to promote chondrogenesis of MSCs197 in part by activating SOX9 via a HIF-1α-dependent mechanism.198 Strobel and coauthors demonstrated that the chondrogenic and anti-catabolic effect (MMP13) of hypoxia in pellet culture of human articular chondrocytes disappeared with the addition of a HIF-1α inhibitor.199 The exact role of HIF-2α in chondrogenesis is currently undetermined. Hypoxic conditions enhanced robust chondrogenesis with an up-regulation of HIF-2α but suppressed COL10A1 in BMSC pellet cultures regardless of the oxygen tension during BMSC isolation and propagation.200 Lafont and coworkers found that hypoxia promoted cartilage matrix synthesis specifically through HIF-2α-mediated SOX9 induction of key cartilage genes.201 Interestingly, HIF-2α was also reported to enhance promoter activities of COL10A1, MMP13, and VEGFA through specific binding to the respective hypoxia-responsive elements and was essential for endochondral ossification of cultured chondrocytes and embryonic skeletal growth in mice.202 In addition, HIF-2α caused cartilage destruction by regulating crucial catabolic genes, such as MMPs, ADAMTS4, nitric oxide synthase-2 (NOS2), and prostaglandin-endoperoxide synthase-2 (PTGS2).203
FGF signaling
FGF receptor 3 (FGFR3) is a tyrosine kinase receptor expressed in proliferating chondrocytes and early hypertrophic chondrocytes in the growth plate; its deficiency in mice resulted in the expansion of both the proliferative zone (PZ) and hypertrophic zone (HZ), indicating FGFR3 suppresses both proliferation and differentiation.204, 205 The major ligands of FGFR3 in the growth plate include FGF18206, 207 and FGF9.208 Mice lacking FGF18 display expanded PZ and HZ, similar to FGFR3-null mice. Recently, Shung and colleagues found that FGFR3 expression increased SOX9 and decreased β-catenin levels and transcriptional activity in cultured mesenchymal cells. Since both SOX9 overexpression and β-catenin deletion independently block hypertrophic differentiation of chondrocytes, activation of FGF signaling may inhibit the hypertrophic differentiation of chondrocytes.
Integrin signaling
The mutual recognition and adhesion between chondrocytes and ECM can translate extracellular stimulation into intracellular cascades via integrin activation.209 The Rho family of GTPases is a family of small signaling G proteins and a subfamily of the Ras superfamily. Three members of the Rho GTPase family, Cdc42, Rac1, and RhoA, are implicated in the formation of actin cytoskeletal organization in fibroblasts. Activating the Integrin-Rho signal pathway led to the formation of stress fibers and focal adhesions and regulated the signal transduction process between cell membrane receptor and cytoskeleton.210
Xu and coworkers found that TGF-β treated SDSCs illustrated the activation of the RhoA/ROCK pathway and concomitantly induced cytoskeletal reorganization and gene expression of SOX9, COL2A1, and ACAN.211 RhoA overexpression in chondrogenic ATDC5 cells resulted in increased proliferation and a marked delay of hypertrophic differentiation.212 Overexpression of Rac1/Cdc42 in ATDC5 cells activated the p38 MAPK pathway, which plays a crucial role in hypertrophy,158 resulting in decreased proliferation and a marked acceleration of hypertrophic differentiation.81 Integrin α1β1213 and integrin α5β1214 were demonstrated to induce GTP-bound transglutaminase 2 (TG2) mobilization to the cell surface, phosphorylation of p38 MAPK, and expression of MEF2C, indicating that blocking integrin β1, a COLII and COLX binding integrin, is a successful strategy to suppress COLX expression in chondrocytes.
Epigenetic regulation
Many differences in gene expression arise during development and are subsequently retained through mitosis. Stable alterations of this kind are said to be ‘epigenetic’215 because they are heritable in the short term but do not involve mutations of the DNA itself. Histone (de)acetylation, DNA methylation, and microRNAs (miRs) are epigenetic events strongly involved in chondro-specific differentiation.216, 217
HDAC4
Up-regulation of HDAC4 increased chondrogenesis as well as suppressing hypertrophy by inhibiting RUNX2 and MEF2C.57, 102, 103, 218 CaMKIV induced chondrocyte differentiation through regulation of HDAC4 subcellular relocation, from the nucleus to the cytoplasm, which resulted in increased activity of RUNX2 and the transition of chondrocytes from the proliferative to the prehypertrophic stage.219 Nuclear HDAC4 is not tethered in the nucleus, but instead shuttles between the nucleus and the cytoplasm. Phosphorylation-induced 14-3-3 binding biases the balance of nucleo-cytoplasmic shuttling toward the cytoplasm by inhibiting nuclear import.220 HDAC4 nuclear localization was enhanced by TGF-β and PTHrP,102, 156, 218 whereas increased calcium concentration led to its phosphorylation and nuclear export.170 In addition to HDAC4, HDAC5, and HDAC7 suppressed MEF2C and RUNX2, respectively.221, 222
MicroRNA 140
MicroRNAs are a class of noncoding regulatory small RNA about 22 nucleotides (∼22 nt) in length that probably function as antisense regulators of other RNAs and profoundly affect cell proliferation, differentiation, apoptosis, and individual growth and development.223, 224 Previous studies found reduced miR-140 expression in human osteoarthritic cartilage,225, 226 which may contribute to the abnormal gene expression pattern characteristic of osteoarthritis. The expression of miR-140 shadows SOX9 expression,227 and the deletion of SOX9 diminishes miR-140 expression during embryogenesis, indicating that miR-140 is subject to SOX9 regulation in chondrocytes. Transfection of human chondrocytes with double-stranded-miR-140 down-regulated IL-1β-induced ADAMTS5 expression.226, 228 Buechli and coworkers demonstrated that miR-140 was highly expressed in normal equine articular cartilage and chondrogenically induced equine cord blood-derived MSCs; miR-140 expression closely paralleled that of the cartilage-specific transcription factor SOX9 while up-regulation of miR-140 repressed chemokine (CXC motif) ligand 12 (CXCL12) and ADAMTS5.229
DNA methylation
DNA methylation of mammals occurs in the cytosine of the CpG dinucleotide via a reaction catalyzed by proteins called DNA methyltransferases.230 Ezura and coworkers found that the DNA methylation levels of CpG-rich promoters of genes related to chondrocyte phenotypes were largely kept low during chondrogenesis in human SDSCs.231 The findings from Zimmermann and colleagues indicated that methylation-based COL10A1 gene silencing is established in cartilage tissue and human articular chondrocytes. Altered methylation levels at 2 CpG sites of COL10A1 in MSCs and their demethylation during chondrogenesis may facilitate induction of COL10A1 as observed during in vitro chondrogenesis of MSCs.232 These studies show that chondrogenic differentiation can be regulated by the level of DNA methylation.217 With osteoarthritis, the methylation of MMPs present in healthy cartilage was lost and the de-methylation led to the expression of MMP-3/9/13 and ADAMTS4.233, 234 The application of the nonspecific de-methylating agent 5-aza-deoxycytidine on chondrogenic differentiation yielded mixed results.235 The role of gene silencing through DNA methylation during chondrogenesis and hypertrophy is still in its infancy and further study is needed.
Conclusion and future perspective
Seed cells, growth factors, and scaffolds are three main elements of tissue engineering; mechanical stimulation also has a great impact on differentiation, so strategies to minimize hypertrophy for cartilage regeneration could be explored from those aspects.
Great variability in the differential capacity certainly exists between tissue-specific stem cells, which may vary within the same cell type.26, 88 For researchers investigating stem cell-based tissue engineering, it is necessary to choose the most appropriate type of MSC naturally suited to the research goals and objectives. SDSCs have a great capacity for proliferation and chondrogenic differentiation, a small potential for hypertrophy, and are tissue specific for cartilage rehabilitation; to our knowledge, SDSCs are the most appropriate stem cell source for cartilage engineering and regeneration.27 Chondrogenic differentiation of MSCs is also influenced by specific intervention, such as growth factors and transduction signals, and the comprehensive environment, such as co-culture with articular chondrocytes, hypoxia, and biomechanical stimulation. Recently, culture substrates, such as decellularized ECM, have received intense attention in lowering hypertrophic potential during chondrogenic induction.
In summary, to get adequate, high quality chondrocytes in vitro, the first challenge that needs to be overcome is hypertrophy of chondrogenic MSCs in cartilage tissue engineering. Understanding a series of signaling pathways and biophysical factors which have been involved in the process of chondrocyte hypertrophy will be beneficial to controlling hypertrophy during cartilage regeneration.
Conflicts of interest
All authors have none to declare.
Acknowledgements
We thank Suzanne Danley for editing the manuscript. This project was partially supported by Research Grants from the AO Foundation (S-12-19P) and the National Institutes of Health (R03 AR062763-01A1) to M.P. and the National Science Foundation for Distinguished Young Scholars of China (81000798) and the Science and Technology Commission of Shanghai Municipality, China (15ZR14140) to P.L.F.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Johnstone B., Alini M., Cucchiarini M. Tissue engineering for articular cartilage repair–the state of the art. Eur Cell Mater. 2013;25:248–267. doi: 10.22203/ecm.v025a18. [DOI] [PubMed] [Google Scholar]
- 2.Mahmoudifar N., Doran P.M. Chondrogenesis and cartilage tissue engineering: the longer road to technology development. Trends Biotechnol. 2012;30:166–176. doi: 10.1016/j.tibtech.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Falah M., Nierenberg G., Soudry M., Hayden M., Volpin G. Treatment of articular cartilage lesions of the knee. Int Orthop. 2010;34:621–630. doi: 10.1007/s00264-010-0959-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed T.A., Hincke M.T. Strategies for articular cartilage lesion repair and functional restoration. Tissue Eng Part B Rev. 2010;16:305–329. doi: 10.1089/ten.TEB.2009.0590. [DOI] [PubMed] [Google Scholar]
- 5.Karnes J., Zhang Y., Pei M. Cell therapy for the creation of cartilage and related clinical trials. In: Templeton N.S., editor. Gene and Cell Therapy: Therapeutic Mechanisms and Strategies. 4th ed. Taylor & Francis/CRC Press; 2015. pp. 1123–1135. [Google Scholar]
- 6.Hubka K.M., Dahlin R.L., Meretoja V.V., Kasper F.K., Mikos A.G. Enhancing chondrogenic phenotype for cartilage tissue engineering: monoculture and coculture of articular chondrocytes and mesenchymal stem cells. Tissue Eng Part B Rev. 2014;20:641–654. doi: 10.1089/ten.teb.2014.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hennig T., Lorenz H., Thiel A. Reduced chondrogenic potential of adipose tissue derived stromal cells correlates with an altered TGFbeta receptor and BMP profile and is overcome by BMP-6. J Cell Physiol. 2007;211:682–691. doi: 10.1002/jcp.20977. [DOI] [PubMed] [Google Scholar]
- 8.Mueller M.B., Tuan R.S. Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arthritis Rheum. 2008;58:1377–1388. doi: 10.1002/art.23370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Studer D., Millan C., Ozturk E., Maniura-Weber K., Zenobi-Wong M. Molecular and biophysical mechanisms regulating hypertrophic differentiation in chondrocytes and mesenchymal stem cells. Eur Cell Mater. 2012;24:118–135. doi: 10.22203/ecm.v024a09. discussion 135. [DOI] [PubMed] [Google Scholar]
- 10.Bush P.G., Parisinos C.A., Hall A.C. The osmotic sensitivity of rat growth plate chondrocytes in situ; clarifying the mechanisms of hypertrophy. J Cell Physiol. 2008;214:621–629. doi: 10.1002/jcp.21249. [DOI] [PubMed] [Google Scholar]
- 11.Chao P.H., West A.C., Hung C.T. Chondrocyte intracellular calcium, cytoskeletal organization, and gene expression responses to dynamic osmotic loading. Am J Physiol Cell Physiol. 2006;291:C718–C725. doi: 10.1152/ajpcell.00127.2005. [DOI] [PubMed] [Google Scholar]
- 12.Mackie E.J., Tatarczuch L., Mirams M. The skeleton: a multi-functional complex organ: the growth plate chondrocyte and endochondral ossification. J Endocrinol. 2011;211:109–121. doi: 10.1530/JOE-11-0048. [DOI] [PubMed] [Google Scholar]
- 13.Wang F., Zhu Y. Aquaporin-1: a potential membrane channel for facilitating the adaptability of rabbit nucleus pulposus cells to an extracellular matrix environment. J Orthop Sci. 2011;16:304–312. doi: 10.1007/s00776-011-0055-1. [DOI] [PubMed] [Google Scholar]
- 14.Hellingman C.A., Koevoet W., van Osch G.J. Can one generate stable hyaline cartilage from adult mesenchymal stem cells? A developmental approach. J Tissue Eng Reg Med. 2012;6:e1–e11. doi: 10.1002/term.502. [DOI] [PubMed] [Google Scholar]
- 15.Arnold M.A., Kim Y., Czubryt M.P. MEF2C transcription factor controls chondrocyte hypertrophy and bone development. Dev Cell. 2007;12:377–389. doi: 10.1016/j.devcel.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Yoshida C.A., Yamamoto H., Fujita T. Runx2 and Runx3 are essential for chondrocyte maturation, and Runx2 regulates limb growth through induction of Indian hedgehog. Genes Dev. 2004;18:952–963. doi: 10.1101/gad.1174704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwon T.G., Zhao X., Yang Q. Physical and functional interactions between Runx2 and HIF-1alpha induce vascular endothelial growth factor gene expression. J Cell Biochem. 2011;112:3582–3593. doi: 10.1002/jcb.23289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fosang A.J., Last K., Knauper V., Murphy G., Neame P.J. Degradation of cartilage aggrecan by collagenase-3 (MMP-13) FEBS Lett. 1996;380:17–20. doi: 10.1016/0014-5793(95)01539-6. [DOI] [PubMed] [Google Scholar]
- 19.Shen G. The role of type X collagen in facilitating and regulating endochondral ossification of articular cartilage. Orthod Craniofac Res. 2005;8:11–17. doi: 10.1111/j.1601-6343.2004.00308.x. [DOI] [PubMed] [Google Scholar]
- 20.Anderson H.C. Matrix vesicles and calcification. Curr Rheumatol Rep. 2003;5:222–226. doi: 10.1007/s11926-003-0071-z. [DOI] [PubMed] [Google Scholar]
- 21.van Donkelaar C.C., Huiskes R. The PTHrP-Ihh feedback loop in the embryonic growth plate allows PTHrP to control hypertrophy and Ihh to regulate proliferation. Biomech Model Mechanobiol. 2007;6:55–62. doi: 10.1007/s10237-006-0035-0. [DOI] [PubMed] [Google Scholar]
- 22.Iannotti J.P., Naidu S., Noguchi Y., Hunt R.M., Brighton C.T. Growth plate matrix vesicle biogenesis. The role of intracellular calcium. Clin Orthop Relat Res. 1994:222–229. [PubMed] [Google Scholar]
- 23.Ortega N., Behonick D.J., Werb Z. Matrix remodeling during endochondral ossification. Trends Cell Biol. 2004;14:86–93. doi: 10.1016/j.tcb.2003.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ortega N., Behonick D., Stickens D., Werb Z. How proteases regulate bone morphogenesis. Ann N Y Acad Sci. 2003;995:109–116. doi: 10.1111/j.1749-6632.2003.tb03214.x. [DOI] [PubMed] [Google Scholar]
- 25.Caplan A.I. Review: mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005;11:1198–1211. doi: 10.1089/ten.2005.11.1198. [DOI] [PubMed] [Google Scholar]
- 26.Pizzute T., Lynch K., Pei M. Impact of tissue-specific stem cells on lineage-specific differentiation: a focus on the musculoskeletal system. Stem Cell Rev. 2014 doi: 10.1007/s12015-014-9546-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones B.A., Pei M. Synovium-derived stem cells: a tissue-specific stem cell for cartilage engineering and regeneration. Tissue Eng Part B Rev. 2012;18:301–311. doi: 10.1089/ten.TEB.2012.0002. [DOI] [PubMed] [Google Scholar]
- 28.Brittberg M., Lindahl A., Nilsson A., Ohlsson C., Isaksson O., Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. New Engl J Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 29.Cournil-Henrionnet C., Huselstein C., Wang Y. Phenotypic analysis of cell surface markers and gene expression of human mesenchymal stem cells and chondrocytes during monolayer expansion. Biorheology. 2008;45:513–526. [PubMed] [Google Scholar]
- 30.Benya P.D., Shaffer J.D. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- 31.Jakob M., Demarteau O., Schafer D. Specific growth factors during the expansion and redifferentiation of adult human articular chondrocytes enhance chondrogenesis and cartilaginous tissue formation in vitro. J Cell Biochem. 2001;81:368–377. doi: 10.1002/1097-4644(20010501)81:2<368::aid-jcb1051>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 32.Wolf F., Candrian C., Wendt D. Cartilage tissue engineering using pre-aggregated human articular chondrocytes. Eur Cell Mater. 2008;16:92–99. doi: 10.22203/ecm.v016a10. [DOI] [PubMed] [Google Scholar]
- 33.Tew S.R., Clegg P.D. Analysis of post transcriptional regulation of SOX9 mRNA during in vitro chondrogenesis. Tissue Eng Part A. 2011;17:1801–1807. doi: 10.1089/ten.tea.2010.0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peterson L., Minas T., Brittberg M., Nilsson A., Sjogren-Jansson E., Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;374:212–234. doi: 10.1097/00003086-200005000-00020. [DOI] [PubMed] [Google Scholar]
- 35.Roberts S., McCall I.W., Darby A.J. Autologous chondrocyte implantation for cartilage repair: monitoring its success by magnetic resonance imaging and histology. Arthritis Res Ther. 2003;5:R60–R73. doi: 10.1186/ar613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dowthwaite G.P., Bishop J.C., Redman S.N. The surface of articular cartilage contains a progenitor cell population. J Cell Sci. 2004;117:889–897. doi: 10.1242/jcs.00912. [DOI] [PubMed] [Google Scholar]
- 37.Hattori S., Oxford C., Reddi A.H. Identification of superficial zone articular chondrocyte stem/progenitor cells. Biochem Biophys Res Commun. 2007;358:99–103. doi: 10.1016/j.bbrc.2007.04.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khan I.M., Bishop J.C., Gilbert S., Archer C.W. Clonal chondroprogenitors maintain telomerase activity and Sox9 expression during extended monolayer culture and retain chondrogenic potential. Osteoarthr Cartil. 2009;17:518–528. doi: 10.1016/j.joca.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Williams R., Khan I.M., Richardson K. Identification and clonal characterisation of a progenitor cell sub-population in normal human articular cartilage. PLoS One. 2010;5:e13246. doi: 10.1371/journal.pone.0013246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu Y., Zheng H., Buckwalter J.A., Martin J.A. Single cell sorting identifies progenitor cell population from full thickness bovine articular cartilage. Osteoarthr Cartil. 2014;22:1318–1326. doi: 10.1016/j.joca.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCarthy H.E., Bara J.J., Brakspear K., Singhrao S.K., Archer C.W. The comparison of equine articular cartilage progenitor cells and bone marrow-derived stromal cells as potential cell sources for cartilage repair in the horse. Vet J. 2012;192:345–351. doi: 10.1016/j.tvjl.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 42.Zhou C., Zheng H., Seol D., Yu Y., Martin J.A. Gene expression profiles reveal that chondrogenic progenitor cells and synovial cells are closely related. J Orthop Res. 2014;32:981–988. doi: 10.1002/jor.22641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mathur D., Pereira W.C., Anand A. Emergence of chondrogenic progenitor stem cells in transplantation biology-prospects and drawbacks. J Cell Biochem. 2012;113:397–403. doi: 10.1002/jcb.23367. [DOI] [PubMed] [Google Scholar]
- 44.Sokolove J., Lepus C.M. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis. 2013;5:77–94. doi: 10.1177/1759720X12467868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garcia-Arnandis I., Guillen M.I., Gomar F., Pelletier J.P., Martel-Pelletier J., Alcaraz M.J. High mobility group box 1 potentiates the pro-inflammatory effects of interleukin-1beta in osteoarthritic synoviocytes. Arthritis Res Ther. 2010;12:R165. doi: 10.1186/ar3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karlsson C., Emanuelsson K., Wessberg F. Human embryonic stem cell-derived mesenchymal progenitors–potential in regenerative medicine. Stem Cell Res. 2009;3:39–50. doi: 10.1016/j.scr.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 47.Jukes J.M., Both S.K., Leusink A., Sterk L.M., van Blitterswijk C.A., de Boer J. Endochondral bone tissue engineering using embryonic stem cells. Proc Natl Acad Sci USA. 2008;105:6840–6845. doi: 10.1073/pnas.0711662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He F., Chen X., Pei M. Reconstruction of an in vitro tissue-specific microenvironment to rejuvenate synovium-derived stem cells for cartilage tissue engineering. Tissue Eng Part A. 2009;15:3809–3821. doi: 10.1089/ten.TEA.2009.0188. [DOI] [PubMed] [Google Scholar]
- 49.Pei M., He F., Kish V.L. Expansion on extracellular matrix deposited by human bone marrow stromal cells facilitates stem cell proliferation and tissue-specific lineage potential. Tissue Eng Part A. 2011;17:3067–3076. doi: 10.1089/ten.tea.2011.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pittenger M.F., Mackay A.M., Beck S.C. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 51.Taipaleenmaki H., Suomi S., Hentunen T., Laitala-Leinonen T., Saamanen A.M. Impact of stromal cell composition on BMP-induced chondrogenic differentiation of mouse bone marrow derived mesenchymal cells. Exp Cell Res. 2008;314:2400–2410. doi: 10.1016/j.yexcr.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 52.Mueller M.B., Fischer M., Zellner J. Effect of parathyroid hormone-related protein in an in vitro hypertrophy model for mesenchymal stem cell chondrogenesis. Int Orthop. 2013;37:945–951. doi: 10.1007/s00264-013-1800-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shintani N., Siebenrock K.A., Hunziker E.B. TGF-ss1 enhances the BMP-2-induced chondrogenesis of bovine synovial explants and arrests downstream differentiation at an early stage of hypertrophy. PLoS One. 2013;8:e53086. doi: 10.1371/journal.pone.0053086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bertram H., Boeuf S., Wachters J. Matrix metalloprotease inhibitors suppress initiation and progression of chondrogenic differentiation of mesenchymal stromal cells in vitro. Stem Cells Dev. 2009;18:881–892. doi: 10.1089/scd.2008.0306. [DOI] [PubMed] [Google Scholar]
- 55.Lee J.M., Kim J.D., Oh E.J., Oh S.H., Lee J.H., Im G.I. PD98059-impregnated functional PLGA scaffold for direct tissue engineering promotes chondrogenesis and prevents hypertrophy from mesenchymal stem cells. Tissue Eng Part A. 2014;20:982–991. doi: 10.1089/ten.tea.2013.0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lengner C.J., Hassan M.Q., Serra R.W. Nkx3.2-mediated repression of Runx2 promotes chondrogenic differentiation. J Biol Chem. 2005;280:15872–15879. doi: 10.1074/jbc.M411144200. [DOI] [PubMed] [Google Scholar]
- 57.Pei M., Chen D., Li J., Wei L. Histone deacetylase 4 promotes TGF-beta1-induced synovium-derived stem cell chondrogenesis but inhibits chondrogenically differentiated stem cell hypertrophy. Differentiation. 2009;78:260–268. doi: 10.1016/j.diff.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 58.Ben-Eliezer M., Phillip M., Gat-Yablonski G. Leptin regulates chondrogenic differentiation in ATDC5 cell-line through JAK/STAT and MAPK pathways. Endocrine. 2007;32:235–244. doi: 10.1007/s12020-007-9025-y. [DOI] [PubMed] [Google Scholar]
- 59.Mikami Y., Asano M., Honda M.J., Takagi M. Bone morphogenetic protein 2 and dexamethasone synergistically increase alkaline phosphatase levels through JAK/STAT signaling in C3H10T1/2 cells. J Cell Physiol. 2010;223:123–133. doi: 10.1002/jcp.22017. [DOI] [PubMed] [Google Scholar]
- 60.Kronenberg H.M. PTHrP and skeletal development. Ann N Y Acad Sci. 2006;1068:1–13. doi: 10.1196/annals.1346.002. [DOI] [PubMed] [Google Scholar]
- 61.Kim Y.J., Kim H.J., Im G.I. PTHrP promotes chondrogenesis and suppresses hypertrophy from both bone marrow-derived and adipose tissue-derived MSCs. Biochem Biophys Res Commun. 2008;373:104–108. doi: 10.1016/j.bbrc.2008.05.183. [DOI] [PubMed] [Google Scholar]
- 62.Kafienah W., Mistry S., Dickinson S.C., Sims T.J., Learmonth I., Hollander A.P. Three-dimensional cartilage tissue engineering using adult stem cells from osteoarthritis patients. Arthritis Rheum. 2007;56:177–187. doi: 10.1002/art.22285. [DOI] [PubMed] [Google Scholar]
- 63.Lee J.M., Im G.I. PTHrP isoforms have differing effect on chondrogenic differentiation and hypertrophy of mesenchymal stem cells. Biochem Biophys Res Commun. 2012;421:819–824. doi: 10.1016/j.bbrc.2012.04.096. [DOI] [PubMed] [Google Scholar]
- 64.Mello M.A., Tuan R.S. Effects of TGF-beta1 and triiodothyronine on cartilage maturation: in vitro analysis using long-term high-density micromass cultures of chick embryonic limb mesenchymal cells. J Orthop Res. 2006;24:2095–2105. doi: 10.1002/jor.20233. [DOI] [PubMed] [Google Scholar]
- 65.Cals F.L., Hellingman C.A., Koevoet W., Baatenburg de Jong R.J., van Osch G.J. Effects of transforming growth factor-beta subtypes on in vitro cartilage production and mineralization of human bone marrow stromal-derived mesenchymal stem cells. J Tissue Eng Reg Med. 2012;6:68–76. doi: 10.1002/term.399. [DOI] [PubMed] [Google Scholar]
- 66.Pei M., He F., Li J., Tidwell J.E., Jones A.C., McDonough E.B. Repair of large animal partial-thickness cartilage defects through intraarticular injection of matrix-rejuvenated synovium-derived stem cells. Tissue Eng Part A. 2013;19:1144–1154. doi: 10.1089/ten.TEA.2012.0351. [DOI] [PubMed] [Google Scholar]
- 67.Mueller M.B., Fischer M., Zellner J. Hypertrophy in mesenchymal stem cell chondrogenesis: effect of TGF-beta isoforms and chondrogenic conditioning. Cells Tissues Organs. 2010;192:158–166. doi: 10.1159/000313399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Narcisi R., Signorile L., Verhaar J.A., Giannoni P., van Osch G.J. TGFbeta inhibition during expansion phase increases the chondrogenic re-differentiation capacity of human articular chondrocytes. Osteoarthr Cartil. 2012;20:1152–1160. doi: 10.1016/j.joca.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 69.Narcisi R., Quarto R., Ulivi V., Muraglia A., Molfetta L., Giannoni P. TGF beta-1 administration during ex vivo expansion of human articular chondrocytes in a serum-free medium redirects the cell phenotype toward hypertrophy. J Cell Physiol. 2012;227:3282–3290. doi: 10.1002/jcp.24024. [DOI] [PubMed] [Google Scholar]
- 70.Shen B., Wei A., Tao H., Diwan A.D., Ma D.D. BMP-2 enhances TGF-beta3-mediated chondrogenic differentiation of human bone marrow multipotent mesenchymal stromal cells in alginate bead culture. Tissue Eng Part A. 2009;15:1311–1320. doi: 10.1089/ten.tea.2008.0132. [DOI] [PubMed] [Google Scholar]
- 71.Miljkovic N.D., Cooper G.M., Marra K.G. Chondrogenesis, bone morphogenetic protein-4 and mesenchymal stem cells. Osteoarthr Cartil. 2008;16:1121–1130. doi: 10.1016/j.joca.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 72.Caron M.M., Emans P.J., Cremers A. Hypertrophic differentiation during chondrogenic differentiation of progenitor cells is stimulated by BMP-2 but suppressed by BMP-7. Osteoarthr Cartil. 2013;21:604–613. doi: 10.1016/j.joca.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 73.Shen B., Bhargav D., Wei A. BMP-13 emerges as a potential inhibitor of bone formation. Int J Biol Sci. 2009;5:192–200. doi: 10.7150/ijbs.5.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Retting K.N., Song B., Yoon B.S., Lyons K.M. BMP canonical Smad signaling through Smad1 and Smad5 is required for endochondral bone formation. Development. 2009;136:1093–1104. doi: 10.1242/dev.029926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shi Y., Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 76.van der Kraan P.M., Blaney Davidson E.N., Blom A., van den Berg W.B. TGF-beta signaling in chondrocyte terminal differentiation and osteoarthritis: modulation and integration of signaling pathways through receptor-Smads. Osteoarthr Cartil. 2009;17:1539–1545. doi: 10.1016/j.joca.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 77.Yu P.B., Hong C.C., Sachidanandan C. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4:33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu C.W., Tchetina E.V., Mwale F. Proteolysis involving matrix metalloproteinase 13 (collagenase-3) is required for chondrocyte differentiation that is associated with matrix mineralization. J Bone Miner Res. 2002;17:639–651. doi: 10.1359/jbmr.2002.17.4.639. [DOI] [PubMed] [Google Scholar]
- 79.Bobick B.E., Kulyk W.M. Regulation of cartilage formation and maturation by mitogen-activated protein kinase signaling. Birth Defects Res C Embryo Today. 2008;84:131–154. doi: 10.1002/bdrc.20126. [DOI] [PubMed] [Google Scholar]
- 80.Kim H.J., Im G.I. The effects of ERK1/2 inhibitor on the chondrogenesis of bone marrow- and adipose tissue-derived multipotent mesenchymal stromal cells. Tissue Eng Part A. 2010;16:851–860. doi: 10.1089/ten.TEA.2009.0070. [DOI] [PubMed] [Google Scholar]
- 81.Wang G., Beier F. Rac1/Cdc42 and RhoA GTPases antagonistically regulate chondrocyte proliferation, hypertrophy, and apoptosis. J Bone Miner Res. 2005;20:1022–1031. doi: 10.1359/JBMR.050113. [DOI] [PubMed] [Google Scholar]
- 82.Jungmann P.M., Mehlhorn A.T., Schmal H., Schillers H., Oberleithner H., Sudkamp N.P. Nanomechanics of human adipose-derived stem cells: small GTPases impact chondrogenic differentiation. Tissue Eng Part A. 2012;18:1035–1044. doi: 10.1089/ten.TEA.2011.0507. [DOI] [PubMed] [Google Scholar]
- 83.Zhu S., Lu P., Liu H. Inhibition of Rac1 activity by controlled release of NSC23766 from chitosan microspheres effectively ameliorates osteoarthritis development in vivo. Ann Rheum Dis. 2015;74:285–293. doi: 10.1136/annrheumdis-2013-203901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhu S., Chen P., Wu Y. Programmed application of transforming growth factor beta3 and Rac1 inhibitor NSC23766 committed hyaline cartilage differentiation of adipose-derived stem cells for osteochondral defect repair. Stem Cells Transl Med. 2014;3:1242–1251. doi: 10.5966/sctm.2014-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nishigaki F., Sakuma S., Ogawa T., Miyata S., Ohkubo T., Goto T. FK506 induces chondrogenic differentiation of clonal mouse embryonic carcinoma cells, ATDC5. Eur J Pharmacol. 2002;437:123–128. doi: 10.1016/s0014-2999(02)01269-4. [DOI] [PubMed] [Google Scholar]
- 86.van der Windt A.E., Jahr H., Farrell E., Verhaar J.A., Weinans H., van Osch G.J. Calcineurin inhibitors promote chondrogenic marker expression of dedifferentiated human adult chondrocytes via stimulation of endogenous TGFbeta1 production. Tissue Eng Part A. 2010;16:1–10. doi: 10.1089/ten.TEA.2009.0082. [DOI] [PubMed] [Google Scholar]
- 87.van der Windt A.E., Haak E., Kops N., Verhaar J.A., Weinans H., Jahr H. Inhibiting calcineurin activity under physiologic tonicity elevates anabolic but suppresses catabolic chondrocyte markers. Arthritis Rheum. 2012;64:1929–1939. doi: 10.1002/art.34369. [DOI] [PubMed] [Google Scholar]
- 88.Demoor M., Ollitrault D., Gomez-Leduc T. Cartilage tissue engineering: molecular control of chondrocyte differentiation for proper cartilage matrix reconstruction. Biochim Biophys Acta. 2014;1840:2414–2440. doi: 10.1016/j.bbagen.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 89.Provot S. Nkx3.2/Bapx1 acts as a negative regulator of chondrocyte maturation. Development. 2006;133:651–662. doi: 10.1242/dev.02258. [DOI] [PubMed] [Google Scholar]
- 90.Wagner T., Wirth J., Meyer J. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell. 1994;79:1111–1120. doi: 10.1016/0092-8674(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 91.Smits P., Li P., Mandel J. The transcription factors L-Sox5 and Sox6 are essential for cartilage formation. Dev Cell. 2001;1:277–290. doi: 10.1016/s1534-5807(01)00003-x. [DOI] [PubMed] [Google Scholar]
- 92.Tsuchiya H., Kitoh H., Sugiura F., Ishiguro N. Chondrogenesis enhanced by overexpression of sox9 gene in mouse bone marrow-derived mesenchymal stem cells. Biochem Biophys Res Commun. 2003;301:338–343. doi: 10.1016/s0006-291x(02)03026-7. [DOI] [PubMed] [Google Scholar]
- 93.Lefebvre V., Li P., de Crombrugghe B. A new long form of Sox5 (L-Sox5), Sox6 and Sox9 are coexpressed in chondrogenesis and cooperatively activate the type II collagen gene. EMBO J. 1998;17:5718–5733. doi: 10.1093/emboj/17.19.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ikeda T., Kamekura S., Mabuchi A. The combination of SOX5, SOX6, and SOX9 (the SOX trio) provides signals sufficient for induction of permanent cartilage. Arthritis Rheum. 2004;50:3561–3573. doi: 10.1002/art.20611. [DOI] [PubMed] [Google Scholar]
- 95.Venkatesan J.K., Ekici M., Madry H., Schmitt G., Kohn D., Cucchiarini M. SOX9 gene transfer via safe, stable, replication-defective recombinant adeno-associated virus vectors as a novel, powerful tool to enhance the chondrogenic potential of human mesenchymal stem cells. Stem Cell Res Ther. 2012;3:22. doi: 10.1186/scrt113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Horiki M., Imamura T., Okamoto M. Smad6/Smurf1 overexpression in cartilage delays chondrocyte hypertrophy and causes dwarfism with osteopenia. J Cell Biol. 2004;165:433–445. doi: 10.1083/jcb.200311015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nishihara A., Fujii M., Sampath T.K., Miyazono K., Reddi A.H. Bone morphogenetic protein signaling in articular chondrocyte differentiation. Biochem Biophys Res Commun. 2003;301:617–622. doi: 10.1016/s0006-291x(02)03068-1. [DOI] [PubMed] [Google Scholar]
- 98.Kaiser M., Haag J., Soder S., Bau B., Aigner T. Bone morphogenetic protein and transforming growth factor beta inhibitory Smads 6 and 7 are expressed in human adult normal and osteoarthritic cartilage in vivo and are differentially regulated in vitro by interleukin-1beta. Arthritis Rheum. 2004;50:3535–3540. doi: 10.1002/art.20750. [DOI] [PubMed] [Google Scholar]
- 99.Estrada K.D., Retting K.N., Chin A.M., Lyons K.M. Smad6 is essential to limit BMP signaling during cartilage development. J Bone Miner Res. 2011;26:2498–2510. doi: 10.1002/jbmr.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McKinsey T.A., Zhang C.L., Olson E.N. Signaling chromatin to make muscle. Curr Opin Cell Biol. 2002;14:763–772. doi: 10.1016/s0955-0674(02)00389-7. [DOI] [PubMed] [Google Scholar]
- 101.Thiagalingam S., Cheng K.H., Lee H.J., Mineva N., Thiagalingam A., Ponte J.F. Histone deacetylases: unique players in shaping the epigenetic histone code. Ann N Y Acad Sci. 2003;983:84–100. doi: 10.1111/j.1749-6632.2003.tb05964.x. [DOI] [PubMed] [Google Scholar]
- 102.Vega R.B., Matsuda K., Oh J. Histone deacetylase 4 controls chondrocyte hypertrophy during skeletogenesis. Cell. 2004;119:555–566. doi: 10.1016/j.cell.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 103.Shimizu E., Selvamurugan N., Westendorf J.J., Olson E.N., Partridge N.C. HDAC4 represses matrix metalloproteinase-13 transcription in osteoblastic cells, and parathyroid hormone controls this repression. J Biol Chem. 2010;285:9616–9626. doi: 10.1074/jbc.M109.094862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shukunami C., Iyama K., Inoue H., Hiraki Y. Spatiotemporal pattern of the mouse chondromodulin-I gene expression and its regulatory role in vascular invasion into cartilage during endochondral bone formation. Int J Dev Biol. 1999;43:39–49. [PubMed] [Google Scholar]
- 105.Klinger P., Surmann-Schmitt C., Brem M. Chondromodulin 1 stabilizes the chondrocyte phenotype and inhibits endochondral ossification of porcine cartilage repair tissue. Arthritis Rheum. 2011;63:2721–2731. doi: 10.1002/art.30335. [DOI] [PubMed] [Google Scholar]
- 106.Mera H., Kawashima H., Yoshizawa T. Chondromodulin-1 directly suppresses growth of human cancer cells. BMC Cancer. 2009;9:166. doi: 10.1186/1471-2407-9-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Peng H., Wright V., Usas A. Synergistic enhancement of bone formation and healing by stem cell-expressed VEGF and bone morphogenetic protein-4. J Clin Invest. 2002;110:751–759. doi: 10.1172/JCI15153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Afuwape A.O., Feldmann M., Paleolog E.M. Adenoviral delivery of soluble VEGF receptor 1 (sFlt-1) abrogates disease activity in murine collagen-induced arthritis. Gene Ther. 2003;10:1950–1960. doi: 10.1038/sj.gt.3302104. [DOI] [PubMed] [Google Scholar]
- 109.Kubo S., Cooper G.M., Matsumoto T. Blocking vascular endothelial growth factor with soluble Flt-1 improves the chondrogenic potential of mouse skeletal muscle-derived stem cells. Arthritis Rheum. 2009;60:155–165. doi: 10.1002/art.24153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Matsumoto T., Cooper G.M., Gharaibeh B. Cartilage repair in a rat model of osteoarthritis through intraarticular transplantation of muscle-derived stem cells expressing bone morphogenetic protein 4 and soluble Flt-1. Arthritis Rheum. 2009;60:1390–1405. doi: 10.1002/art.24443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Haigh J.J., Gerber H.P., Ferrara N., Wagner E.F. Conditional inactivation of VEGF-A in areas of collagen2a1 expression results in embryonic lethality in the heterozygous state. Development. 2000;127:1445–1453. doi: 10.1242/dev.127.7.1445. [DOI] [PubMed] [Google Scholar]
- 112.Zelzer E., Mamluk R., Ferrara N., Johnson R.S., Schipani E., Olsen B.R. VEGFA is necessary for chondrocyte survival during bone development. Development. 2004;131:2161–2171. doi: 10.1242/dev.01053. [DOI] [PubMed] [Google Scholar]
- 113.Wasylyk B., Hagman J., Gutierrez-Hartmann A. Ets transcription factors: nuclear effectors of the Ras-MAP-kinase signaling pathway. Trends Biochem Sci. 1998;23:213–216. doi: 10.1016/s0968-0004(98)01211-0. [DOI] [PubMed] [Google Scholar]
- 114.Iwamoto M., Higuchi Y., Koyama E. Transcription factor ERG variants and functional diversification of chondrocytes during limb long bone development. J Cell Biol. 2000;150:27–40. doi: 10.1083/jcb.150.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Acharya C., Adesida A., Zajac P. Enhanced chondrocyte proliferation and mesenchymal stromal cells chondrogenesis in coculture pellets mediate improved cartilage formation. J Cell Physiol. 2012;227:88–97. doi: 10.1002/jcp.22706. [DOI] [PubMed] [Google Scholar]
- 116.Lettry V., Hosoya K., Takagi S., Okumura M. Coculture of equine mesenchymal stem cells and mature equine articular chondrocytes results in improved chondrogenic differentiation of the stem cells. Jpn J Vet Res. 2010;58:5–15. [PubMed] [Google Scholar]
- 117.Aung A., Gupta G., Majid G., Varghese S. Osteoarthritic chondrocyte-secreted morphogens induce chondrogenic differentiation of human mesenchymal stem cells. Arthritis Rheum. 2011;63:148–158. doi: 10.1002/art.30086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bian L., Zhai D.Y., Mauck R.L., Burdick J.A. Coculture of human mesenchymal stem cells and articular chondrocytes reduces hypertrophy and enhances functional properties of engineered cartilage. Tissue Eng Part A. 2011;17:1137–1145. doi: 10.1089/ten.tea.2010.0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fischer J., Dickhut A., Rickert M., Richter W. Human articular chondrocytes secrete parathyroid hormone-related protein and inhibit hypertrophy of mesenchymal stem cells in coculture during chondrogenesis. Arthritis Rheum. 2010;62:2696–2706. doi: 10.1002/art.27565. [DOI] [PubMed] [Google Scholar]
- 120.Weiss S., Hennig T., Bock R., Steck E., Richter W. Impact of growth factors and PTHrP on early and late chondrogenic differentiation of human mesenchymal stem cells. J Cell Physiol. 2010;223:84–93. doi: 10.1002/jcp.22013. [DOI] [PubMed] [Google Scholar]
- 121.Mo X.T., Guo S.C., Xie H.Q. Variations in the ratios of co-cultured mesenchymal stem cells and chondrocytes regulate the expression of cartilaginous and osseous phenotype in alginate constructs. Bone. 2009;45:42–51. doi: 10.1016/j.bone.2008.07.240. [DOI] [PubMed] [Google Scholar]
- 122.Meretoja V.V., Dahlin R.L., Kasper F.K., Mikos A.G. Enhanced chondrogenesis in co-cultures with articular chondrocytes and mesenchymal stem cells. Biomaterials. 2012;33:6362–6369. doi: 10.1016/j.biomaterials.2012.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Streuli C. Extracellular matrix remodelling and cellular differentiation. Curr Opin Cell Biol. 1999;11:634–640. doi: 10.1016/s0955-0674(99)00026-5. [DOI] [PubMed] [Google Scholar]
- 124.Girard-Lauriault P.-L., Mwale F., Iordanova M., Demers C., Desjardins P., Wertheimer M.R. Atmospheric pressure deposition of micropatterned nitrogen-rich plasma-polymer films for tissue engineering. Plasma Process Polym. 2005;2:263–270. [Google Scholar]
- 125.Petit A., Demers C.N., Girard-Lauriault P.L. Effect of nitrogen-rich cell culture surfaces on type X collagen expression by bovine growth plate chondrocytes. Biomed Eng Online. 2011;10:4. doi: 10.1186/1475-925X-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mwale F., Girard-Lauriault P.L., Wang H.T., Lerouge S., Antoniou J., Wertheimer M.R. Suppression of genes related to hypertrophy and osteogenesis in committed human mesenchymal stem cells cultured on novel nitrogen-rich plasma polymer coatings. Tissue Eng. 2006;12:2639–2647. doi: 10.1089/ten.2006.12.2639. [DOI] [PubMed] [Google Scholar]
- 127.Rampersad S., Ruiz J.C., Petit A. Stem cells, nitrogen-rich plasma-polymerized culture surfaces, and type X collagen suppression. Tissue Eng Part A. 2011;17:2551–2560. doi: 10.1089/ten.TEA.2010.0723. [DOI] [PubMed] [Google Scholar]
- 128.Li Q., Williams C.G., Sun D.D., Wang J., Leong K., Elisseeff J.H. Photocrosslinkable polysaccharides based on chondroitin sulfate. J Biomed Mater Res A. 2004;68:28–33. doi: 10.1002/jbm.a.20007. [DOI] [PubMed] [Google Scholar]
- 129.Muzzarelli R.A., Greco F., Busilacchi A., Sollazzo V., Gigante A. Chitosan, hyaluronan and chondroitin sulfate in tissue engineering for cartilage regeneration: a review. Carbohydr Polym. 2012;89:723–739. doi: 10.1016/j.carbpol.2012.04.057. [DOI] [PubMed] [Google Scholar]
- 130.Roughley P., Martens D., Rantakokko J., Alini M., Mwale F., Antoniou J. The involvement of aggrecan polymorphism in degeneration of human intervertebral disc and articular cartilage. Eur Cell Mater. 2006;11:1–7. discussion 7. [PubMed] [Google Scholar]
- 131.Varghese S., Hwang N.S., Canver A.C., Theprungsirikul P., Lin D.W., Elisseeff J. Chondroitin sulfate based niches for chondrogenic differentiation of mesenchymal stem cells. Matrix Biol. 2008;27:12–21. doi: 10.1016/j.matbio.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 132.Wu Y.N., Yang Z., Hui J.H., Ouyang H.W., Lee E.H. Cartilaginous ECM component-modification of the micro-bead culture system for chondrogenic differentiation of mesenchymal stem cells. Biomaterials. 2007;28:4056–4067. doi: 10.1016/j.biomaterials.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 133.Toh W.S., Foldager C.B., Pei M., Hui J.H. Advances in mesenchymal stem cell-based strategies for cartilage repair and regeneration. Stem Cell Rev. 2014;10:686–696. doi: 10.1007/s12015-014-9526-z. [DOI] [PubMed] [Google Scholar]
- 134.Badylak S.F., Taylor D., Uygun K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Ann Rev Biomed Eng. 2011;13:27–53. doi: 10.1146/annurev-bioeng-071910-124743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Benders K.E., van Weeren P.R., Badylak S.F., Saris D.B., Dhert W.J., Malda J. Extracellular matrix scaffolds for cartilage and bone regeneration. Trends Biotechnol. 2013;31:169–176. doi: 10.1016/j.tibtech.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 136.Tottey S., Johnson S.A., Crapo P.M. The effect of source animal age upon extracellular matrix scaffold properties. Biomaterials. 2011;32:128–136. doi: 10.1016/j.biomaterials.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Choi K.H., Choi B.H., Park S.R., Kim B.J., Min B.H. The chondrogenic differentiation of mesenchymal stem cells on an extracellular matrix scaffold derived from porcine chondrocytes. Biomaterials. 2010;31:5355–5365. doi: 10.1016/j.biomaterials.2010.03.053. [DOI] [PubMed] [Google Scholar]
- 138.Pei M., Li J.T., Shoukry M., Zhang Y. A review of decellularized stem cell matrix: a novel cell expansion system for cartilage tissue engineering. Eur Cell Mater. 2011;22:333–343. doi: 10.22203/ecm.v022a25. discussion 343. [DOI] [PubMed] [Google Scholar]
- 139.Cheng N.C., Estes B.T., Awad H.A., Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells by a porous scaffold derived from native articular cartilage extracellular matrix. Tissue Eng Part A. 2009;15:231–241. doi: 10.1089/ten.tea.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chang C.H., Chen C.C., Liao C.H., Lin F.H., Hsu Y.M., Fang H.W. Human acellular cartilage matrix powders as a biological scaffold for cartilage tissue engineering with synovium-derived mesenchymal stem cells. J Biomed Mater Res A. 2014;102:2248–2257. doi: 10.1002/jbm.a.34897. [DOI] [PubMed] [Google Scholar]
- 141.Li J., Pei M. Optimization of an in vitro three-dimensional microenvironment to reprogram synovium-derived stem cells for cartilage tissue engineering. Tissue Eng Part A. 2011;17:703–712. doi: 10.1089/ten.TEA.2010.0339. [DOI] [PubMed] [Google Scholar]
- 142.Jin C.Z., Choi B.H., Park S.R., Min B.H. Cartilage engineering using cell-derived extracellular matrix scaffold in vitro. J Biomed Mater Res A. 2010;92:1567–1577. doi: 10.1002/jbm.a.32419. [DOI] [PubMed] [Google Scholar]
- 143.Jin C.Z., Park S.R., Choi B.H., Park K., Min B.H. In vivo cartilage tissue engineering using a cell-derived extracellular matrix scaffold. Artif Organs. 2007;31:183–192. doi: 10.1111/j.1525-1594.2007.00363.x. [DOI] [PubMed] [Google Scholar]
- 144.D'Ippolito G., Diabira S., Howard G.A., Roos B.A., Schiller P.C. Low oxygen tension inhibits osteogenic differentiation and enhances stemness of human MIAMI cells. Bone. 2006;39:513–522. doi: 10.1016/j.bone.2006.02.061. [DOI] [PubMed] [Google Scholar]
- 145.Ronziere M.C., Perrier E., Mallein-Gerin F., Freyria A.M. Chondrogenic potential of bone marrow- and adipose tissue-derived adult human mesenchymal stem cells. Biomed Mater Eng. 2010;20:145–158. doi: 10.3233/BME-2010-0626. [DOI] [PubMed] [Google Scholar]
- 146.Xu Y., Malladi P., Chiou M., Bekerman E., Giaccia A.J., Longaker M.T. In vitro expansion of adipose-derived adult stromal cells in hypoxia enhances early chondrogenesis. Tissue Eng. 2007;13:2981–2993. doi: 10.1089/ten.2007.0050. [DOI] [PubMed] [Google Scholar]
- 147.Sheehy E.J., Buckley C.T., Kelly D.J. Oxygen tension regulates the osteogenic, chondrogenic and endochondral phenotype of bone marrow derived mesenchymal stem cells. Biochem Biophys Res Commun. 2012;417:305–310. doi: 10.1016/j.bbrc.2011.11.105. [DOI] [PubMed] [Google Scholar]
- 148.Hirao M., Tamai N., Tsumaki N., Yoshikawa H., Myoui A. Oxygen tension regulates chondrocyte differentiation and function during endochondral ossification. J Biol Chem. 2006;281:31079–31092. doi: 10.1074/jbc.M602296200. [DOI] [PubMed] [Google Scholar]
- 149.Kawato Y., Hirao M., Ebina K. Nkx3.2-induced suppression of Runx2 is a crucial mediator of hypoxia-dependent maintenance of chondrocyte phenotypes. Biochem Biophys Res Commun. 2011;416:205–210. doi: 10.1016/j.bbrc.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 150.Wong M., Siegrist M., Goodwin K. Cyclic tensile strain and cyclic hydrostatic pressure differentially regulate expression of hypertrophic markers in primary chondrocytes. Bone. 2003;33:685–693. doi: 10.1016/s8756-3282(03)00242-4. [DOI] [PubMed] [Google Scholar]
- 151.Bian L., Zhai D.Y., Zhang E.C., Mauck R.L., Burdick J.A. Dynamic compressive loading enhances cartilage matrix synthesis and distribution and suppresses hypertrophy in hMSC-laden hyaluronic acid hydrogels. Tissue Eng Part A. 2012;18:715–724. doi: 10.1089/ten.tea.2011.0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ebisawa K., Hata K., Okada K. Ultrasound enhances transforming growth factor beta-mediated chondrocyte differentiation of human mesenchymal stem cells. Tissue Eng. 2004;10:921–929. doi: 10.1089/1076327041348437. [DOI] [PubMed] [Google Scholar]
- 153.Cui J.H., Park S.R., Park K., Choi B.H., Min B.H. Preconditioning of mesenchymal stem cells with low-intensity ultrasound for cartilage formation in vivo. Tissue Eng. 2007;13:351–360. doi: 10.1089/ten.2006.0080. [DOI] [PubMed] [Google Scholar]
- 154.Li T.F., Dong Y., Ionescu A.M. Parathyroid hormone-related peptide (PTHrP) inhibits Runx2 expression through the PKA signaling pathway. Exp Cell Res. 2004;299:128–136. doi: 10.1016/j.yexcr.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 155.Huang W., Zhou X., Lefebvre V., de Crombrugghe B. Phosphorylation of SOX9 by cyclic AMP-dependent protein kinase A enhances SOX9's ability to transactivate a Col2a1 chondrocyte-specific enhancer. Mol Cell Biol. 2000;20:4149–4158. doi: 10.1128/mcb.20.11.4149-4158.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kozhemyakina E., Cohen T., Yao T.P., Lassar A.B. Parathyroid hormone-related peptide represses chondrocyte hypertrophy through a protein phosphatase 2A/histone deacetylase 4/MEF2 pathway. Mol Cell Biol. 2009;29:5751–5762. doi: 10.1128/MCB.00415-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhen X., Wei L., Wu Q., Zhang Y., Chen Q. Mitogen-activated protein kinase p38 mediates regulation of chondrocyte differentiation by parathyroid hormone. J Biol Chem. 2001;276:4879–4885. doi: 10.1074/jbc.M004990200. [DOI] [PubMed] [Google Scholar]
- 158.Stanton L.A., Sabari S., Sampaio A.V., Underhill T.M., Beier F. p38 MAP kinase signalling is required for hypertrophic chondrocyte differentiation. Biochem J. 2004;378:53–62. doi: 10.1042/BJ20030874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Han Y.S., Bang O.S., Jin E.J., Park J.H., Sonn J.K., Kang S.S. High dose of glucose promotes chondrogenesis via PKCalpha and MAPK signaling pathways in chick mesenchymal cells. Cell Tissue Res. 2004;318:571–578. doi: 10.1007/s00441-004-0993-4. [DOI] [PubMed] [Google Scholar]
- 160.Li Y., Ahrens M.J., Wu A., Liu J., Dudley A.T. Calcium/calmodulin-dependent protein kinase II activity regulates the proliferative potential of growth plate chondrocytes. Development. 2011;138:359–370. doi: 10.1242/dev.052324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhang M., Xie R., Hou W. PTHrP prevents chondrocyte premature hypertrophy by inducing cyclin-D1-dependent Runx2 and Runx3 phosphorylation, ubiquitylation and proteasomal degradation. J Cell Sci. 2009;122:1382–1389. doi: 10.1242/jcs.040709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zeng L., Kempf H., Murtaugh L.C., Sato M.E., Lassar A.B. Shh establishes an Nkx3.2/Sox9 autoregulatory loop that is maintained by BMP signals to induce somitic chondrogenesis. Genes Dev. 2002;16:1990–2005. doi: 10.1101/gad.1008002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kobayashi T., Soegiarto D.W., Yang Y. Indian hedgehog stimulates periarticular chondrocyte differentiation to regulate growth plate length independently of PTHrP. J Clin Invest. 2005;115:1734–1742. doi: 10.1172/JCI24397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mak K.K., Kronenberg H.M., Chuang P.T., Mackem S., Yang Y. Indian hedgehog signals independently of PTHrP to promote chondrocyte hypertrophy. Development. 2008;135:1947–1956. doi: 10.1242/dev.018044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Matta C., Fodor J., Szijgyarto Z. Cytosolic free Ca2+ concentration exhibits a characteristic temporal pattern during in vitro cartilage differentiation: a possible regulatory role of calcineurin in Ca-signalling of chondrogenic cells. Cell Calcium. 2008;44:310–323. doi: 10.1016/j.ceca.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 166.Chang W., Tu C., Chen T.H. Expression and signal transduction of calcium-sensing receptors in cartilage and bone. Endocrinology. 1999;140:5883–5893. doi: 10.1210/endo.140.12.7190. [DOI] [PubMed] [Google Scholar]
- 167.Wu S., Palese T., Mishra O.P., Delivoria-Papadopoulos M., De Luca F. Effects of Ca2+ sensing receptor activation in the growth plate. FASEB J. 2004;18:143–145. doi: 10.1096/fj.03-0294fje. [DOI] [PubMed] [Google Scholar]
- 168.D'Andrea P., Calabrese A., Capozzi I., Grandolfo M., Tonon R., Vittur F. Intercellular Ca2+ waves in mechanically stimulated articular chondrocytes. Biorheology. 2000;37:75–83. [PubMed] [Google Scholar]
- 169.Steward A.J., Kelly D.J., Wagner D.R. The role of calcium signalling in the chondrogenic response of mesenchymal stem cells to hydrostatic pressure. Eur Cell Mater. 2014;28:358–371. doi: 10.22203/ecm.v028a25. [DOI] [PubMed] [Google Scholar]
- 170.Backs J., Song K., Bezprozvannaya S., Chang S., Olson E.N. CaM kinase II selectively signals to histone deacetylase 4 during cardiomyocyte hypertrophy. J Clin Invest. 2006;116:1853–1864. doi: 10.1172/JCI27438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bassik M.C., Scorrano L., Oakes S.A., Pozzan T., Korsmeyer S.J. Phosphorylation of BCL-2 regulates ER Ca2+ homeostasis and apoptosis. EMBO J. 2004;23:1207–1216. doi: 10.1038/sj.emboj.7600104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Orfanidou T., Malizos K.N., Varitimidis S., Tsezou A. 1,25-Dihydroxyvitamin D(3) and extracellular inorganic phosphate activate mitogen-activated protein kinase pathway through fibroblast growth factor 23 contributing to hypertrophy and mineralization in osteoarthritic chondrocytes. Exp Biol Med. 2012;237:241–253. doi: 10.1258/ebm.2011.011301. [DOI] [PubMed] [Google Scholar]
- 173.Hellingman C.A., Davidson E.N., Koevoet W. Smad signaling determines chondrogenic differentiation of bone-marrow-derived mesenchymal stem cells: inhibition of Smad1/5/8P prevents terminal differentiation and calcification. Tissue Eng Part A. 2011;17:1157–1167. doi: 10.1089/ten.TEA.2010.0043. [DOI] [PubMed] [Google Scholar]
- 174.Furumatsu T., Tsuda M., Taniguchi N., Tajima Y., Asahara H. Smad3 induces chondrogenesis through the activation of SOX9 via CREB-binding protein/p300 recruitment. J Biol Chem. 2005;280:8343–8350. doi: 10.1074/jbc.M413913200. [DOI] [PubMed] [Google Scholar]
- 175.Kang J.S., Alliston T., Delston R., Derynck R. Repression of Runx2 function by TGF-beta through recruitment of class II histone deacetylases by Smad3. EMBO J. 2005;24:2543–2555. doi: 10.1038/sj.emboj.7600729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Konrad L., Scheiber J.A., Bergmann M., Eickelberg O., Hofmann R. Identification of a new human Smad6 splice variant. Andrologia. 2008;40:358–363. doi: 10.1111/j.1439-0272.2008.00869.x. [DOI] [PubMed] [Google Scholar]
- 177.Scharstuhl A., Diepens R., Lensen J. Adenoviral overexpression of Smad-7 and Smad-6 differentially regulates TGF-beta-mediated chondrocyte proliferation and proteoglycan synthesis. Osteoarthr Cartil. 2003;11:773–782. doi: 10.1016/s1063-4584(03)00165-1. [DOI] [PubMed] [Google Scholar]
- 178.Ying S.X., Hussain Z.J., Zhang Y.E. Smurf1 facilitates myogenic differentiation and antagonizes the bone morphogenetic protein-2-induced osteoblast conversion by targeting Smad5 for degradation. J Biol Chem. 2003;278:39029–39036. doi: 10.1074/jbc.M301193200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lin X., Liang M., Feng X.H. Smurf2 is a ubiquitin E3 ligase mediating proteasome-dependent degradation of Smad2 in transforming growth factor-beta signaling. J Biol Chem. 2000;275:36818–36822. doi: 10.1074/jbc.C000580200. [DOI] [PubMed] [Google Scholar]
- 180.Wu Q., Chen D., Zuscik M.J., O'Keefe R.J., Rosier R.N. Overexpression of Smurf2 stimulates endochondral ossification through upregulation of beta-catenin. J Bone Miner Res. 2008;23:552–563. doi: 10.1359/JBMR.071115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Angers S., Moon R.T. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol. 2009;10:468–477. doi: 10.1038/nrm2717. [DOI] [PubMed] [Google Scholar]
- 182.Ma B., Landman E.B., Miclea R.L. WNT signaling and cartilage: of mice and men. Calcif Tissue Int. 2013;92:399–411. doi: 10.1007/s00223-012-9675-5. [DOI] [PubMed] [Google Scholar]
- 183.Dong Y.F., Soung do Y., Schwarz E.M., O'Keefe R.J., Drissi H. Wnt induction of chondrocyte hypertrophy through the Runx2 transcription factor. J Cell Physiol. 2006;208:77–86. doi: 10.1002/jcp.20656. [DOI] [PubMed] [Google Scholar]
- 184.Topol L., Chen W., Song H., Day T.F., Yang Y. Sox9 inhibits Wnt signaling by promoting beta-catenin phosphorylation in the nucleus. J Biol Chem. 2009;284:3323–3333. doi: 10.1074/jbc.M808048200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yano F., Kugimiya F., Ohba S. The canonical Wnt signaling pathway promotes chondrocyte differentiation in a Sox9-dependent manner. Biochem Biophys Res Commun. 2005;333:1300–1308. doi: 10.1016/j.bbrc.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 186.Im G.I., Lee J.M., Kim H.J. Wnt inhibitors enhance chondrogenesis of human mesenchymal stem cells in a long-term pellet culture. Biotechnol Lett. 2011;33:1061–1068. doi: 10.1007/s10529-010-0514-3. [DOI] [PubMed] [Google Scholar]
- 187.Bradley E.W., Drissi M.H. WNT5A regulates chondrocyte differentiation through differential use of the CaN/NFAT and IKK/NF-kappaB pathways. Mol Endocrinol. 2010;24:1581–1593. doi: 10.1210/me.2010-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Leijten J.C., Emons J., Sticht C. Gremlin 1, frizzled-related protein, and Dkk-1 are key regulators of human articular cartilage homeostasis. Arthritis Rheum. 2012;64:3302–3312. doi: 10.1002/art.34535. [DOI] [PubMed] [Google Scholar]
- 189.Gelse K., Ekici A.B., Cipa F. Molecular differentiation between osteophytic and articular cartilage–clues for a transient and permanent chondrocyte phenotype. Osteoarthr Cartil. 2012;20:162–171. doi: 10.1016/j.joca.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 190.Leijten J.C., Bos S.D., Landman E.B. GREM1, FRZB and DKK1 mRNA levels correlate with osteoarthritis and are regulated by osteoarthritis-associated factors. Arthritis Res Ther. 2013;15:R126. doi: 10.1186/ar4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gazzerro E., Smerdel-Ramoya A., Zanotti S. Conditional deletion of gremlin causes a transient increase in bone formation and bone mass. J Biol Chem. 2007;282:31549–31557. doi: 10.1074/jbc.M701317200. [DOI] [PubMed] [Google Scholar]
- 192.Stanton L.A., Underhill T.M., Beier F. MAP kinases in chondrocyte differentiation. Dev Biol. 2003;263:165–175. doi: 10.1016/s0012-1606(03)00321-x. [DOI] [PubMed] [Google Scholar]
- 193.Pearson G., Robinson F., Beers Gibson T. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 194.Xiao G., Gopalakrishnan R., Jiang D., Reith E., Benson M.D., Franceschi R.T. Bone morphogenetic proteins, extracellular matrix, and mitogen-activated protein kinase signaling pathways are required for osteoblast-specific gene expression and differentiation in MC3T3-E1 cells. J Bone Miner Res. 2002;17:101–110. doi: 10.1359/jbmr.2002.17.1.101. [DOI] [PubMed] [Google Scholar]
- 195.Provot S., Nachtrab G., Paruch J., Chen A.P., Silva A., Kronenberg H.M. A-raf and B-raf are dispensable for normal endochondral bone development, and parathyroid hormone-related peptide suppresses extracellular signal-regulated kinase activation in hypertrophic chondrocytes. Mol Cell Biol. 2008;28:344–357. doi: 10.1128/MCB.00617-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Appleton C.T., Usmani S.E., Mort J.S., Beier F. Rho/ROCK and MEK/ERK activation by transforming growth factor-alpha induces articular cartilage degradation. Lab Invest. 2010;90:20–30. doi: 10.1038/labinvest.2009.111. [DOI] [PubMed] [Google Scholar]
- 197.Kanichai M., Ferguson D., Prendergast P.J., Campbell V.A. Hypoxia promotes chondrogenesis in rat mesenchymal stem cells: a role for AKT and hypoxia-inducible factor (HIF)-1alpha. J Cell Physiol. 2008;216:708–715. doi: 10.1002/jcp.21446. [DOI] [PubMed] [Google Scholar]
- 198.Robins J.C., Akeno N., Mukherjee A. Hypoxia induces chondrocyte-specific gene expression in mesenchymal cells in association with transcriptional activation of Sox9. Bone. 2005;37:313–322. doi: 10.1016/j.bone.2005.04.040. [DOI] [PubMed] [Google Scholar]
- 199.Strobel S., Loparic M., Wendt D. Anabolic and catabolic responses of human articular chondrocytes to varying oxygen percentages. Arthritis Res Ther. 2010;12:R34. doi: 10.1186/ar2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Adesida A.B., Mulet-Sierra A., Jomha N.M. Hypoxia mediated isolation and expansion enhances the chondrogenic capacity of bone marrow mesenchymal stromal cells. Stem Cell Res Ther. 2012;3:9. doi: 10.1186/scrt100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lafont J.E., Talma S., Murphy C.L. Hypoxia-inducible factor 2alpha is essential for hypoxic induction of the human articular chondrocyte phenotype. Arthritis Rheum. 2007;56:3297–3306. doi: 10.1002/art.22878. [DOI] [PubMed] [Google Scholar]
- 202.Saito T., Fukai A., Mabuchi A. Transcriptional regulation of endochondral ossification by HIF-2alpha during skeletal growth and osteoarthritis development. Nat Med. 2010;16:678–686. doi: 10.1038/nm.2146. [DOI] [PubMed] [Google Scholar]
- 203.Yang S., Kim J., Ryu J.H. Hypoxia-inducible factor-2alpha is a catabolic regulator of osteoarthritic cartilage destruction. Nat Med. 2010;16:687–693. doi: 10.1038/nm.2153. [DOI] [PubMed] [Google Scholar]
- 204.Colvin J.S., Bohne B.A., Harding G.W., McEwen D.G., Ornitz D.M. Skeletal overgrowth and deafness in mice lacking fibroblast growth factor receptor 3. Nat Genet. 1996;12:390–397. doi: 10.1038/ng0496-390. [DOI] [PubMed] [Google Scholar]
- 205.Deng C., Wynshaw-Boris A., Zhou F., Kuo A., Leder P. Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell. 1996;84:911–921. doi: 10.1016/s0092-8674(00)81069-7. [DOI] [PubMed] [Google Scholar]
- 206.Liu Z., Xu J., Colvin J.S., Ornitz D.M. Coordination of chondrogenesis and osteogenesis by fibroblast growth factor 18. Genes Dev. 2002;16:859–869. doi: 10.1101/gad.965602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ohbayashi N., Shibayama M., Kurotaki Y. FGF18 is required for normal cell proliferation and differentiation during osteogenesis and chondrogenesis. Genes Dev. 2002;16:870–879. doi: 10.1101/gad.965702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hung I.H., Yu K., Lavine K.J., Ornitz D.M. FGF9 regulates early hypertrophic chondrocyte differentiation and skeletal vascularization in the developing stylopod. Dev Biol. 2007;307:300–313. doi: 10.1016/j.ydbio.2007.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Millward-Sadler S.J., Salter D.M. Integrin-dependent signal cascades in chondrocyte mechanotransduction. Ann Biomed Eng. 2004;32:435–446. doi: 10.1023/b:abme.0000017538.72511.48. [DOI] [PubMed] [Google Scholar]
- 210.Amano M., Chihara K., Kimura K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–1311. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- 211.Xu T., Wu M., Feng J., Lin X., Gu Z. RhoA/Rho kinase signaling regulates transforming growth factor-beta1-induced chondrogenesis and actin organization of synovium-derived mesenchymal stem cells through interaction with the Smad pathway. Int J Mol Med. 2012;30:1119–1125. doi: 10.3892/ijmm.2012.1107. [DOI] [PubMed] [Google Scholar]
- 212.Wang G., Woods A., Sabari S., Pagnotta L., Stanton L.A., Beier F. RhoA/ROCK signaling suppresses hypertrophic chondrocyte differentiation. J Biol Chem. 2004;279:13205–13214. doi: 10.1074/jbc.M311427200. [DOI] [PubMed] [Google Scholar]
- 213.Johnson K.A., Rose D.M., Terkeltaub R.A. Factor XIIIA mobilizes transglutaminase 2 to induce chondrocyte hypertrophic differentiation. J Cell Sci. 2008;121:2256–2264. doi: 10.1242/jcs.011262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Tanaka K., Yokosaki Y., Higashikawa F., Saito Y., Eboshida A., Ochi M. The integrin alpha5beta1 regulates chondrocyte hypertrophic differentiation induced by GTP-bound transglutaminase 2. Matrix Biol. 2007;26:409–418. doi: 10.1016/j.matbio.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 215.Jaenisch R., Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33(suppl):245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 216.Barter M.J., Bui C., Young D.A. Epigenetic mechanisms in cartilage and osteoarthritis: DNA methylation, histone modifications and microRNAs. Osteoarthr Cartil. 2012;20:339–349. doi: 10.1016/j.joca.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 217.Furumatsu T., Ozaki T. Epigenetic regulation in chondrogenesis. Acta Med Okayama. 2010;64:155–161. doi: 10.18926/AMO/40007. [DOI] [PubMed] [Google Scholar]
- 218.Wang A.H., Bertos N.R., Vezmar M. HDAC4, a human histone deacetylase related to yeast HDA1, is a transcriptional corepressor. Mol Cell Biol. 1999;19:7816–7827. doi: 10.1128/mcb.19.11.7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Guan Y., Chen Q., Yang X. Subcellular relocation of histone deacetylase 4 regulates growth plate chondrocyte differentiation through Ca2+/calmodulin-dependent kinase IV. Am J Physiol Cell Physiol. 2012;303:C33–C40. doi: 10.1152/ajpcell.00348.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Nishino T.G., Miyazaki M., Hoshino H., Miwa Y., Horinouchi S., Yoshida M. 14-3-3 regulates the nuclear import of class IIa histone deacetylases. Biochem Biophys Res Commun. 2008;377:852–856. doi: 10.1016/j.bbrc.2008.10.079. [DOI] [PubMed] [Google Scholar]
- 221.Backs J., Backs T., Bezprozvannaya S., McKinsey T.A., Olson E.N. Histone deacetylase 5 acquires calcium/calmodulin-dependent kinase II responsiveness by oligomerization with histone deacetylase 4. Mol Cell Biol. 2008;28:3437–3445. doi: 10.1128/MCB.01611-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Jensen E.D., Gopalakrishnan R., Westendorf J.J. Bone morphogenic protein 2 activates protein kinase D to regulate histone deacetylase 7 localization and repression of Runx2. J Biol Chem. 2009;284:2225–2234. doi: 10.1074/jbc.M800586200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ambros V. microRNAs: tiny regulators with great potential. Cell. 2001;107:823–826. doi: 10.1016/s0092-8674(01)00616-x. [DOI] [PubMed] [Google Scholar]
- 224.Stefani G., Slack F.J. Small non-coding RNAs in animal development. Nat Rev Mol Cell Biol. 2008;9:219–230. doi: 10.1038/nrm2347. [DOI] [PubMed] [Google Scholar]
- 225.Iliopoulos D., Malizos K.N., Oikonomou P., Tsezou A. Integrative microRNA and proteomic approaches identify novel osteoarthritis genes and their collaborative metabolic and inflammatory networks. PLoS One. 2008;3:e3740. doi: 10.1371/journal.pone.0003740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Miyaki S., Nakasa T., Otsuki S. MicroRNA-140 is expressed in differentiated human articular chondrocytes and modulates interleukin-1 responses. Arthritis Rheum. 2009;60:2723–2730. doi: 10.1002/art.24745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Nakamura Y., He X., Kato H. Sox9 is upstream of microRNA-140 in cartilage. Appl Biochem Biotechnol. 2012;166:64–71. doi: 10.1007/s12010-011-9404-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Miyaki S., Sato T., Inoue A. MicroRNA-140 plays dual roles in both cartilage development and homeostasis. Genes Dev. 2010;24:1173–1185. doi: 10.1101/gad.1915510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Buechli M.E., Lamarre J., Koch T.G. MicroRNA-140 expression during chondrogenic differentiation of equine cord blood-derived mesenchymal stromal cells. Stem Cells Dev. 2013;22:1288–1296. doi: 10.1089/scd.2012.0411. [DOI] [PubMed] [Google Scholar]
- 230.Quina A.S., Buschbeck M., Di Croce L. Chromatin structure and epigenetics. Biochem Pharmacol. 2006;72:1563–1569. doi: 10.1016/j.bcp.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 231.Ezura Y., Sekiya I., Koga H., Muneta T., Noda M. Methylation status of CpG islands in the promoter regions of signature genes during chondrogenesis of human synovium-derived mesenchymal stem cells. Arthritis Rheum. 2009;60:1416–1426. doi: 10.1002/art.24472. [DOI] [PubMed] [Google Scholar]
- 232.Zimmermann P., Boeuf S., Dickhut A., Boehmer S., Olek S., Richter W. Correlation of COL10A1 induction during chondrogenesis of mesenchymal stem cells with demethylation of two CpG sites in the COL10A1 promoter. Arthritis Rheum. 2008;58:2743–2753. doi: 10.1002/art.23736. [DOI] [PubMed] [Google Scholar]
- 233.Cheung K.S., Hashimoto K., Yamada N., Roach H.I. Expression of ADAMTS-4 by chondrocytes in the surface zone of human osteoarthritic cartilage is regulated by epigenetic DNA de-methylation. Rheumatol Int. 2009;29:525–534. doi: 10.1007/s00296-008-0744-z. [DOI] [PubMed] [Google Scholar]
- 234.Roach H.I., Yamada N., Cheung K.S. Association between the abnormal expression of matrix-degrading enzymes by human osteoarthritic chondrocytes and demethylation of specific CpG sites in the promoter regions. Arthritis Rheum. 2005;52:3110–3124. doi: 10.1002/art.21300. [DOI] [PubMed] [Google Scholar]
- 235.El-Serafi A.T., Oreffo R.O., Roach H.I. Epigenetic modifiers influence lineage commitment of human bone marrow stromal cells: differential effects of 5-aza-deoxycytidine and trichostatin A. Differentiation. 2011;81:35–41. doi: 10.1016/j.diff.2010.09.183. [DOI] [PubMed] [Google Scholar]
- 236.Houston B., Stewart A.J., Farquharson C. PHOSPHO1-A novel phosphatase specifically expressed at sites of mineralisation in bone and cartilage. Bone. 2004;34:629–637. doi: 10.1016/j.bone.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 237.Roberts S., Narisawa S., Harmey D., Millan J.L., Farquharson C. Functional involvement of PHOSPHO1 in matrix vesicle-mediated skeletal mineralization. J Bone Miner Res. 2007;22:617–627. doi: 10.1359/jbmr.070108. [DOI] [PubMed] [Google Scholar]
- 238.Balcerzak M., Hamade E., Zhang L. The roles of annexins and alkaline phosphatase in mineralization process. Acta Biochim Pol. 2003;50:1019–1038. [PubMed] [Google Scholar]
- 239.Kirsch T., Harrison G., Golub E.E., Nah H.D. The roles of annexins and types II and X collagen in matrix vesicle-mediated mineralization of growth plate cartilage. J Biol Chem. 2000;275:35577–35583. doi: 10.1074/jbc.M005648200. [DOI] [PubMed] [Google Scholar]
- 240.Terkeltaub R.A. Inorganic pyrophosphate generation and disposition in pathophysiology. Am J Physiol Cell Physiol. 2001;281:C1–C11. doi: 10.1152/ajpcell.2001.281.1.C1. [DOI] [PubMed] [Google Scholar]
- 241.Addison W.N., Azari F., Sorensen E.S., Kaartinen M.T., McKee M.D. Pyrophosphate inhibits mineralization of osteoblast cultures by binding to mineral, up-regulating osteopontin, and inhibiting alkaline phosphatase activity. J Biol Chem. 2007;282:15872–15883. doi: 10.1074/jbc.M701116200. [DOI] [PubMed] [Google Scholar]
- 242.Xiao Z., Blonder J., Zhou M., Veenstra T.D. Proteomic analysis of extracellular matrix and vesicles. J Proteomics. 2009;72:34–45. doi: 10.1016/j.jprot.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 243.Yamashita S., Andoh M., Ueno-Kudoh H., Sato T., Miyaki S., Asahara H. Sox9 directly promotes Bapx1 gene expression to repress Runx2 in chondrocytes. Exp Cell Res. 2009;315:2231–2240. doi: 10.1016/j.yexcr.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]