Abstract
BACKGROUND
The role of Wnt/β-Catenin signaling in embryogenesis and carcinogenesis has been extensively studied in organs such as colon, lung and pancreas, but little is known about Wnt/β-Catenin signaling in the prostate. Although stabilizing mutations in APC and β-Catenin are rare in primary prostate tumors, recent studies suggest that cytoplasmic/nuclear β-Catenin is associated with advanced, metastatic, hormone-refractory prostate carcinoma.
METHODS
To better understand the role of β-Catenin in prostatic development and carcinogenesis, we studied Wnt expression during prostate development and activated Wnt/β-Catenin signaling in the developing and adult prostate.
RESULTS
Our results demonstrated that during prostate development Wnt ligands display a dynamic expression pattern. Activation of β-Catenin during prostate development caused epithelial hyperplasia followed by prostatic intraepithelial neoplasia (PIN) in prostate. In the adult prostate, activation of β-Catenin resulted in high grade PIN (HGPIN) and continuous prostatic growth after castration. As a result of activation of β-Catenin, AR was first up-regulated with the emergence of epithelial hyperplasia, but was later down-regulated when HGPIN developed. Furthermore, activation of β-Catenin induced Foxa2 re-expression in adult prostate which normally is only expressed in the embryonic budding stage during prostate development.
CONCLUSIONS
The results from this study strongly suggest that Wnt/β-Catenin signaling is involved in the regulation of prostate development and confirm that constitutive activation of this pathway enables the mouse prostate to grow after castration.
Keywords: castration, prostate, Wnt/β-Catenin, Foxa2
Introduction
Wnts are a family of secreted glycoproteins with 19 members found in mammals [1]. The Wnt ligands act in a short-range paracrine manner mediated through binding to a cell surface Frizzled (Fzd) receptor together with a co-receptor LDL-receptor-related protein (LRP). Wnt signaling is known to involve at least three main intracellular pathways: the canonical Wnt/β-Catenin pathway, the Wnt/Ca2+ pathway and the Wnt/polarity pathway. Accordingly, Wnts are often classified into three categories: canonical Wnts (including Wnt1, 2, 2b, 3, 3a, 6, 7b, 8a, and 8b), non-canonical Wnts (Wnt4, 5a, and 11) and non-classified Wnts (Wnt5b, 7a, 9a, 9b, 10a, 10b, and 16) [2].
The canonical Wnt/β-Catenin pathway has been extensively studied and is best understood. In this pathway, β-Catenin is the main mediator of Wnt signaling in the nucleus. β-Catenin has dual roles. First, β-Catenin is a junction molecule involved in cell-cell adhesion by forming a complex with E-Cadherin at the cell surface. β-Catenin is also present in the cytosol; however, without Wnt signaling cytoplasmic β-Catenin is rapidly degraded by a complex which contains adenomatous polyposis coli (APC), Axin and glycogen synthase kinase 3β (GSK3β). The constitutive proteosomal degradation of β-Catenin prevents its accumulation in the cytoplasm and maintains cellular β-Catenin at low levels. In the presence of Wnt signal, Wnt binds to its receptor frizzled (Fzd) and activates Disheveled. Disheveled blocks the degradation of β-Catenin, resulting in cytoplasmic accumulation of β-Catenin, followed by β-Catenin translocation into the nucleus through an unspecified mechanism(s) that, in the prostate, may involve interactions with AR and the Smad family of transcription factors [3, 4].
Once β-Catenin is transported to the nucleus, it acts as a transcription co-activator and activates TCF target genes such as c-Myc, cyclin D1 (CD1), and urokinase-type plasminogen activator (uPA) [5, 6]. Studies have shown that β-Catenin can activate AR target genes [7-9]. As a co-activator of AR, β-Catenin may have a significant influence on prostate development and more importantly on tumor initiation and progression [10]. Although stabilizing mutations in β-Catenin in primary prostate tumors have been shown to occur in approximately 5% of human cancer cases [11], they provide a selective advantage for the carcinoma cells during tumor progression [12, 13]. Furthermore, nuclear β-Catenin is strongly correlated with advanced stage and recurrence of prostate cancer [14, 15]. As a correlate, previous studies have shown that WIF1 (a Wnt inhibitor) is often down regulated in prostate cancer [14, 16]. In addition to Wnt signaling, β-Catenin also can be activated through cross-talk with other pathways. Studies have shown that β-Catenin is implicated in a network of several non-Wnt pathways that are important for prostate carcinogenesis including the PTEN/Akt [17], COX-2/PGE2 [18], TGF-β [19], NF-κB [20, 21], and PDGF pathways [22]. All these pathways are capable of activating β-Catenin and to some extent may thereby contribute to β-Catenin related prostate cancer progression in vivo.
To further clarify the role of Wnt dependent β-Catenin signaling in prostate development and carcinogenesis, we screened Wnt family mRNA to determine the pattern of expression during prostate development. We then used conditionally deleted β-Catenin exon 3 mouse models to activate Wnt/β-Catenin signaling during early prostate development using Nkx3.1-Cre or in late prostate development using Probasin-Cre (PBCre4). Our results demonstrated that activation of β-Catenin results in epithelial hyperplasia followed by PIN. Once HGPIN develops, the murine prostate continues to grow after androgen blockade. In addition, we found that activation of β-Catenin induced the re-expression of Foxa2, which is normally expressed only in the embryonic prostate. Our results suggest that β-Catenin is involved in prostate development and continuous prostatic growth in the absence of androgen.
Materials and Methods
Nkx3.1-Cre/Catnbfloxed (ex3) mouse breeding and UGS rescue
Catnbfloxed (ex3) mice (in C57BL/6 background) [23] were bred with Nkx3.1-Cre (in C57BL/ 6 background). Embryo age was determined by checking plug and the morning with plug was considered as E 0.5 day. UGS were dissected from E16 to E18 embryos and PCR was performed for genotyping. Rescued UGS were grafted into renal capsules of adult male nude mice, and rescued organs were recovered from host mice at 6-14 weeks.
PBCre4/Catnbfloxed (ex3) mouse breeding, castration, BrdU labeling and TUNEL assay
Catnbfloxed (ex3) mice (in C57BL/ 6 background) were bred with PBCre4 mice (crossed with C57BL/ 6 for more than ten generations) [24]. Male mice were castrated and sacrificed as indicated. BrdU was injected two hours before sacrifice according to manufacturer's instructions (Sigma, B5002 St Louis, MO.). TUNEL assay was performed using Apoptosis detection kit from Millipore (Billerica, MA)
Antibodies and Immunohistochemistry
Prostate lobes from PB-Cre/CatnbΔ (ex3) and control litter mate mice and tissues from rescued Nkx3.1-Cre/CatnbΔ(ex3) and litter mate UGS were fixed in 10% buffered formalin and embedded in paraffin as previously described [25-27]. Tissues were subjected to standard processing, and 5μm of sections were cut. H&E staining was performed following standard protocol. Immunostaining was performed using antibodies listed below and stained using Vectastain ABC kit (Vector Laboratories, Inc., Burlingame, CA). Sections were counterstained with Harris hematoxylin (Surgiport, Richmond, VA). AR (N20), Foxa2 (P19), and Probasin (M18) antibodies were purchased from Santa Cruz (Santa Cruz, CA). β-Catenin antibody was purchased from BD transduction laboratories (San Jose, CA). Secondary antibodies used for immunofluorescence staining were purchased from Molecular Probes (Eugene, Oregon). BrdU staining was performed in Vanderbilt Immunohistology Core Lab. Nkx3.1 antibody was kindly provided by Dr. Cory Abate-Shen at Herbert Irving Comprehensive Cancer Center, Columbia University Medical Center, New York, NY.
RT-PCR
Total RNA was extracted from urogenital sinus (UGS) of male embryos or dorsolateral prostate using RNeasy mini kit (Qiagen, Valencia CA) and treated with RNase free DNase (Qiagen, Valencia CA) to eliminate genomic DNA contamination. Primers specific for 19 Wnts were designed to span at least one intron (except when only a single exon exists) to ensure mRNA specific amplification. Primer sequences for 19 Wnts were listed in Table I. The PCR conditions were as follows: 94°C/4min, and 35 cycles of 94°C/30sec, 58°C/45sec, and 72°C/1min.
Table I.
Wnt PCR primer sequence
| Gene | PCR primer sequence | PCR product |
|---|---|---|
| Wnt1 | F: GTGCAAATGGCAATTCCGAAACCG R: AAATCGATGTTGTCACTGCAGCCC |
252bp |
| Wnt2 | F: AACTGCAACACCCTGGACAGAGAT R: CTACAAAGGCACGGGCAAACTTGA |
262bp |
| Wnt2b | F: TGAAGCTGCAGGGTGAGGATGAA R: TGTCTGGGTAGCGTTGACACAACT |
264bp |
| Wnt3 | F: TTCATGATCGCCGGCAAACTTCCT R: ACGCTGGGCATGATCTCGATGTAA |
247bp |
| Wnt3a | F: TCTGCAGGAACTACGTGGAGATCA R: TCCCGAGAGACCATTCCTCCAAAT |
340bp |
| Wnt4 | F: ATGCAGCAGTGGAGAACTGGAGAA R: GCCAGCCTCGTTGTTGTGAAGATT |
202bp |
| Wnt5a | F: TCGCCATGAAGAAGCCCATTGGAA R: TGTCCTTGAGAAAGTCCTGCCAGT |
241bp |
| Wnt5b | F: AGCTCCGCTTTGGAAGATGTTGGT R: AACTGACACAGCTTTCTCTGGCCT |
246bp |
| Wnt6 | F: TGTCAGTTCCAGTTCCGTTTCCGA R: GCTTGTGCTGCGCATCCATAAAGA |
352bp |
| Wnt7a | F: AGGCTGCCTTCACCTATGCGATTA R: ACACACCATGGCACTTACACTCCA |
309bp |
| Wnt7b | F: TACGTGAAGCTCGGAGCATTGTCA R: ACAGCCACAATTGCTCAGATTGCC |
342bp |
| Wnt8a | F: TGCCTGGTCAGTGAACAACTTCCT R: TCTGGCATCCTTCCCTTTCTCCAA |
409bp |
| Wnt8b | F: TCGGAGACTTTGACAACTGTGGCT R: GCACTTACACGTGCGTTTCATGGT |
230bp |
| Wnt9a | F: ACTGCTTTCCTCTACGCCATCTCT R: TTTGCAAGTGGTTTCCACTCCAGC |
294bp |
| Wnt9b | F: ATGTCAGTTCCAGTTCAGGCAGGA R: TTCAGATTGTCACCACACACACCC |
237bp |
| Wnt10a | F: CATCCATGAGTGCCAGCATCAGTT R: TTTGCACTTACGCCGCATGTTCTC |
514bp |
| Wnt10b | F: TCCGTGAGAGTGCTTTCTCCTTCT R: TCCCTGGAATCCAAGAAATCCCGA |
313bp |
| Wnt11 | F: GCCTGTGAAGGACTCAGAACTTGT R: CACCAGTGGTACTTGCAGTGACAT |
210bp |
| Wnt16 | F: ACTGTATGGTCGCCACTACCACTT R: AACTTTCTGCTGAACCACATGCCG |
271bp |
Western Blot
Fresh prostate tissue was obtained from PBCre4/CatnbΔ(ex3) or litter mate control mice. Western blot was performed as described before [25].
Results
1. Wnts display a dynamic expression pattern during prostate development
Wnt family member expression during prostate development was analyzed by RT-PCR. As shown in Fig. 1, of the 19 Wnts, Wnt 2, 3, 3a, 4, 5a, 5b, 6, 7a, 7b, 8b, 9a, 9b, 10a, 10b, 11, and 16 were expressed in prostate, but Wnt 1, Wnt 2b and Wnt 8a were not (data not shown: primers for these Wnts were tested using RNA from embryonic day 12 (E12) embryo or from mouse stem cells as positive controls). The expression of 16 Wnts displayed a dynamic pattern during prostate development: Wnt 3 and Wnt 7a were highly expressed in E16 urogenital sinus (UGS), decreased in E18 UGS, declined further after birth and were not detectable in the adult prostate; Wnt 3a was weakly expressed in E16 and E18 UGS, but not in postnatal day 1 or adult prostate; Wnt 2, 4, 5a, 5b, 7b, 8b, 9a, 10a, 10b and 11 were expressed throughout developing and adult prostate; Wnt6 was highly expressed in embryonic and postnatal day 1 prostate, but expression decreased in adult prostate; Wnt 9b was expressed only in embryo UGS at very low levels; Wnt 16 was highly expressed in embryonic and neonatal prostate, but totally disappeared in adult prostate (Fig. 1).
Fig. 1.
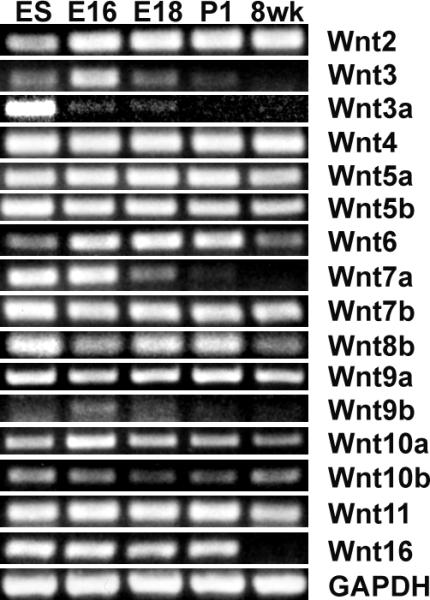
Expression of Wnts in prostate. Expression of Wnts during prostate development was analyzed by RT-PCR using total RNA extracted from embryo day 16 (E16) and day 18 (E18) UGS, postnatal day 1 (P1) and 8 week dorsolateral prostate (8 wk). RNA extracted from embryo stem cells (ES) served as positive control. 16 out of 19 Wnts were expressed in prostate.
2. Activation of β-Catenin during embryogenesis results in PIN lesion and Foxa2 re-expression
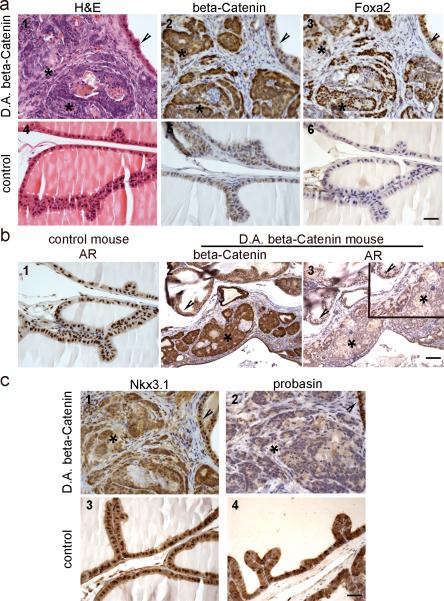
The RT-PCR results demonstrated that a subgroup of canonical Wnts (Wnt3, 3a, and 6) are expressed during early prostatic development and are decreased in the adult prostate. This prompted us to study how stabilized β-Catenin would affect prostate development. For this purpose, the Catnbfloxed(ex3) mouse model was utilized. In this model, exon 3 of β-Catenin was floxed. Breeding of Catnbfloxed(ex3) mice with tissue specific Cre mice will result in the deletion of exon 3 thus preventing degradation and causing accumulation of cytoplasmic/nuclear β-Catenin. In order to activate Wnt/β-Catenin signaling in early prostatic development, Catnbfloxed(ex3) mice were bred with Nkx3.1-Cre mice. Nkx3.1 is expressed in developing UGS and expression is maintained in the adult prostate epithelium [28]. Out of more than twenty litters from breeding of Catnbfloxed(ex3) mice with Nkx3.1-Cre mice, no offspring with the Nkx3.1-Cre/Catnbfloxed(ex3) genotype survived indicating that Nkx3.1-Cre/CatnbΔ(ex3) mice were embryonic lethal. This is consistent with a recent report that Nkx3.1-Cre is activated not only in the developing prostate, but also in several other organs during embryogenesis, including the lung, brain, heart, somites and spinal ridge [29]. In order to study prostate development in these mice, we used a renal grafting procedure that would rescue UGS and allow it to mature into a normal prostate [30]. Although the ratio is lower than Mendel's law, out of 130 embryos, we obtained 14 of Nkx3.1-Cre/Catnbfloxed(ex3) genotype. A total of 34 UGS from E16 to E18 embryos (14 of Nkx3.1-Cre/Catnbfloxed(ex3) genotype) were grafted into the renal capsules of adult male athymic nude mice. Rescued organs were recovered from host mice 6-14 weeks later. As shown in Fig. 2a, β-Catenin accumulated in prostate epithelial cells as a result of exon 3 deletion, and stabilization of β-Catenin in early developing prostate caused PIN lesions to develop. As β-Catenin accumulated in prostate epithelia, Foxa2 was re-expressed, which is normally expressed only in the early embryonic buds of the developing prostate but not in the adult gland [31]. Compared with the adjacent normal histology area, Foxa2 was only co-localized with the appearance of active β-Catenin (Fig. 2a). In addition, immunostaining for AR and the AR target gene-Nkx3.1 were decreased, and the adult prostatic differentiation marker-probasin, another AR target gene, was not expressed in PIN where β-Catenin was activated (Fig. 2b and 2c). These results indicate that activation of β-Catenin during embryogenesis results in PIN lesion formation and Foxa2 re-expression, as well as down-regulation of AR expression and signaling in the prostates of Nkx3.1-Cre/CatnbΔ (ex3) mice.
Fig. 2.
Constitutive activation of β-Catenin during prostate development caused prostatic intraepithelial neoplasia, induced Foxa2 re-expression, and reduced AR and AR signaling pathway. E16 to E18 UGS from Nkx3.1Cre/ CatnbΔ(ex3) and litter mate control male embryos were rescued and grafted under renal capsules of adult male nude mice. a, activation of β-Catenin caused PIN and Foxa2 re-expression. 1-3: serial sections of rescued prostate with Nkx3.1-Cre/ CatnbΔ(ex3) genotype; 4-6: serial sections of rescued prostate with Nkx3.1-Cre genotype. Asterisks in panel 1-3 indicate cytoplasmic/nuclear β-Catenin staining areas, and arrowheads indicate normal areas that show only membrane β-Catenin staining. At the areas where β-Catenin was activated (2, asterisks), PIN lesions developed (1, asterisks). While Foxa2 was not expressed in rescued control prostate (panel 6), Foxa2 expression was detected in β-Catenin accumulating prostate epithelia (panel 2-3). b, activation of β-Catenin reduced AR level. 1, AR staining performed on section from rescued control prostate. 2 and 3, β-Catenin or AR staining performed on serial sections from a rescued Nkx3.1Cre/ CatnbΔ(ex3) mouse prostate. At the area where β-Catenin was activated (2 and 3, asterisks), AR level was reduced compared with adjacent normal histology area (2 and 3, arrowheads) or with control prostate (1). inset: higher magnification. c, AR target genes (Nkx3.1 and probasin) were down-regulated in active β-Catenin mouse prostates. 1 and 2: immunostaining of Nkx3.1 or probasin performed on serial sections (same as those used in Fig2a, panel 1-3) from Nkx3.1-Cre/ CatnbΔ(ex3) prostate. 3 and 4: immunostaining performed on sections from rescued control prostate. Nkx3.1 antibody nicely stained the epithelial cell nuclei from control prostate (panel 3), or from the normal histology region of Nkx3.1-Cre/ CatnbΔ(ex3) prostate (panel 1, arrowhead), but at the areas that show activated β-Catenin (serial section from panel 2 in Fig.2a, asterisk), the Nkx3.1 staining was diffused (panel 1, asterisk) and the level was much lower when compared with adjacent normal histology area (panel 1, arrowhead) or with control prostate. Another AR target gene-probasin was lost at the area where β-Catenin was activated (panel 2, asterisk), while control prostate showed strong probasin staining. Scale bars represent 50 μm.
3. Activation of β-Catenin after birth results in epithelial hyperplasia with progression to HGPIN in the prostate
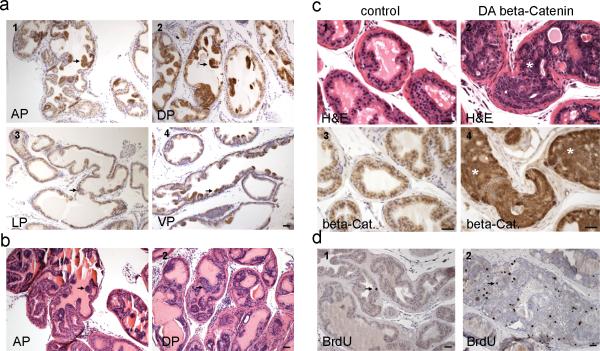
Breeding of PBCre4 mice with Catnbfloxed(ex3) mice resulted in the deletion of exon 3 of β-Catenin in prostate epithelial cells starting 2 weeks after birth, resulting in β-Catenin accumulation in the cytoplasm and nucleus in prostatic epithelial cells (Fig. 3a). This first resulted in prostatic epithelial hyperplasia at 12 weeks (Fig. 3b) followed by progression to HGPIN at 6 and up months (Fig. 3c, 9 months). With the stabilization of β-Catenin, epithelial cells proliferated actively as marked by the extensive BrdU incorporation (Fig. 3d). However, only HGPIN and no adenocarcinoma were observed even at 9 months and 1 year of age as epithelial cells were still confined by the basement membrane (Fig. 3c, panel 2 and table II). This indicates that β-Catenin activation after birth results in HGPIN in the prostate.
Fig. 3.
Activation of β-Catenin in adult prostate resulted in epithelial hyperplasia followed by progression to HGPIN. a, immunostaining for β-Catenin performed on a 12 week old PBCre4/ CatnbΔ(ex3) mouse prostate. With the deletion of exon 3, cytoplasmic/ nuclear β-Catenin accumulated focally in prostate epithelial cells at 12 weeks. AP, anterior prostate; DP, dorsal prostate; LP, lateral prostate; VP, ventral prostate. b, H&E staining performed on a 12 week old PBCre4/ CatnbΔ(ex3) mouse prostate. The accumulation of β-Catenin in cytoplasm/nucleus resulted in prostatic hyperplasia at 12 weeks. c, histology of PBCre4/ CatnbΔ(ex3) mouse prostate at 9 months. 1-2: H&E staining of a 9 month old control or PBCre4/ CatnbΔ(ex3) mouse prostate. 3-4: β-Catenin staining performed on serial sections from the above mouse prostates. At this age, prostatic epithelial cells displayed extensive cytoplasmic and nuclear β-Catenin (panel 4), which resulted in HGPIN to develop. Highly proliferating epithelial cells expanded and filled prostatic acini but were still restricted within stroma, and prostate basement membranes were still intact. d, BrdU staining. BrdU staining was performed on a 23 week old PBCre4/ CatnbΔ(ex3) (panel 2) or an age matched control mouse prostate (panel 1). BrdU incorporation in active β-Catenin prostate was significantly increased compared with control prostate (p<0.01). Scale bars represent 50 μm.
Table II.
Histology of PBCre4/ CatnbΔ(ex3) mouse prostates at different age
| age | Mouse number | histology |
|---|---|---|
| 11-12 weeks | 5 | hyperplasia |
| 6 months | 7 | HGPIN |
| 9 months | 5 | HGPIN |
| 1 year | 2 | HGPIN |
4. Activation of β-Catenin causes continuous prostatic growth after castration
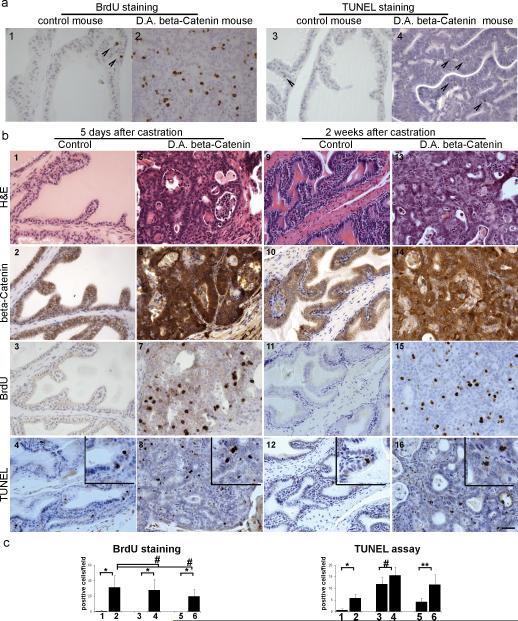
Accumulation of β-Catenin in the cytoplasm and nucleus is a late event in human prostate cancer and is associated with progression of prostate cancer to androgen-independence [14, 15]. To study whether Wnt/β-Catenin signaling is involved in controlling prostatic growth in the absence of androgen, PBCre4/CatnbΔ(ex3) and litter mate control mice were castrated and sacrificed 5 days or 2-4 weeks later (table III). Two hours before sacrifice, mice were injected with BrdU. TUNEL assay and BrdU staining were performed to study the influence of castration on PBCre4/CatnbΔ(ex3) prostatic cell apoptosis and proliferation, respectively. Apoptosis was detected in prostates from both control and PBCre4/CatnbΔ(ex3) mice after castration (TUNEL assay in Fig. 4b). However, while control mice showed no prostatic proliferation at any time point after castration (Fig. 4b panel 3 and 11), prostates derived from castrated PBCre4/CatnbΔ(ex3) mice continued to proliferate (BrdU staining Fig. 4b). Extensive BrdU incorporation was seen in the activated β-Catenin mouse prostates 5 days (panel 7) and 2 weeks (panel 15) post-castration. BrdU incorporation was significantly higher in the prostates of intact and castrated mice expressing nuclear β-catenin (PBCre4/CatnbΔ(ex3) ) when compared to wild type intact or castrated mice (Fig. 4c), indicating that activation of Wnt/β-Catenin induces prostatic cell proliferation in both the presence and absence of testicular androgens. TUNEL staining was significantly higher in the prostates of intact PBCre4/CatnbΔ(ex3) mice when compared to intact wild type mice (Fig. 4c). At 5-days post-castration, prostatic TUNEL staining was elevated but show no significant difference between wild type and PBCre4/CatnbΔ(ex3) mice. However, at 14-days post-castration, prostatic TUNEL staining was significantly higher in the PBCre4/CatnbΔ(ex3) prostate when compared to wild type prostate (Fig. 4c). Thus, after castration in the PBCre4/CatnbΔ(ex3) mice, the prostate continues to show both proliferation and cell death while the prostate of wild type mice only show cell death. These results suggest that activation of β-Catenin overrides androgen's control on prostate growth and causes continuous prostatic epithelial cell proliferation in the absence of testicular androgen.
Table III.
Mice used in castration study
| castration | Control mouse number | PBCre4/ CatnbΔ(ex3) mouse number |
|---|---|---|
| 5 days | 5 | 5 |
| ≥2 weeks | 7 | 7 |
Fig. 4.
Activation of β-Catenin caused continuous prostate growth after castration. PBCre4/ CatnbΔ(ex3) and litter mate control mice were castrated at 22-23 weeks and sacrificed 5 days or 2 weeks later. a, histology of control and PBCre4/ CatnbΔ(ex3) mouse prostates without castration. 1 and 2: BrdU staining; 3 and 4: TUNEL staining. Arrow heads indicate positive staining. b, histology of control and PBCre4/ CatnbΔ(ex3) mouse prostates 5 days or 2 weeks post castration. 1-4, serial sections from a control mouse prostate 5 days after castration; 5-8, serial sections from a PBCre4/ CatnbΔ(ex3) mouse prostate 5 days after castration; 9-12, serial sections from a control mouse prostate 2 weeks after castration; 13-16, serial sections from a PBCre4/ CatnbΔ(ex3) mouse prostate 2 weeks after castration. Panel 1, 5, 9, and 13: H&E staining. Activation of β-Catenin resulted in HGPIN in PBCre4/ CatnbΔ(ex3) mouse prostates. Panel 2, 6, 10, and 14: β-Catenin staining. While β-Catenin can only be detected on cell membranes of control prostates (panel 2 and 10), β-Catenin accumulated in cytoplasm/nucleus of PBCre4/ CatnbΔ(ex3) mouse prostates (panel 6 and 14). Panel 3, 7, 11, and 15: BrdU staining. While control prostate stopped grow after 5 days and 2 weeks castration, activation of β-Catenin enabled PBCre4/ CatnbΔ(ex3) mouse prostate to continuously proliferate after castration as marked by the extensive BrdU incorporation in panel 7 and 15. Panel 4, 8, 12, and 16: TUNEL assay by ApopTag staining (inset for higher magnification). Both control and PBCre4/ CatnbΔ(ex3) mouse prostates showed apoptosis after castration. c, quantification of BrdU and TUNEL staining. column 1: prostate from control mouse without castration; 2: prostate from PBCre4/ CatnbΔ(ex3) mouse without castration; 3: prostate from control mouse 5 days post castration; 4, prostate from PBCre4/ CatnbΔ(ex3) mouse 5 days post castration; 5, prostate from control mouse 2 weeks post castration; 6, prostate from PBCre4/ CatnbΔ(ex3) mouse 2 weeks post castration. * p< 0.01; ** p< 0.05, # p> 0.05. Scale bars represent 50 μm.
5. AR signaling pathway in PBCre4/CatnbΔ(ex3) mouse prostates
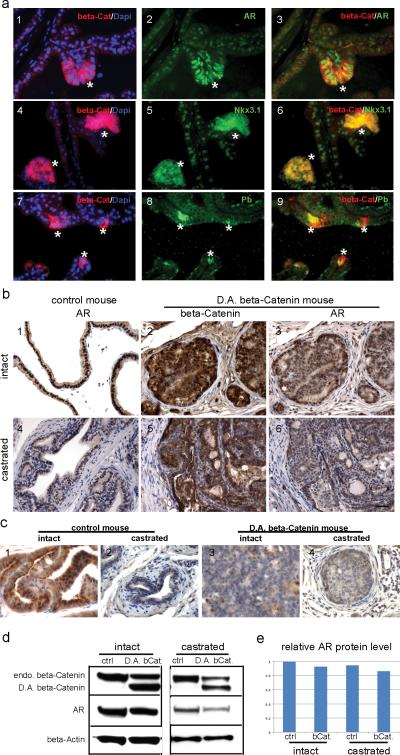
Up-regulation of AR expression has been suggested as one of the mechanisms to explain the continuous growth of the prostate following castration. Thus, the expression levels of AR, and AR associated target genes in both intact and castrated PBCre4/CatnbΔ(ex3) and control mouse prostates was examined. In prostates derived from intact PBCre4/CatnbΔ(ex3) mice, AR expression levels were at first up-regulated at 12 weeks, concomitant with the emergence of prostatic hyperplasia (Fig. 3b and Fig. 5a). In addition, AR expression was increased in prostate epithelial cells that have cytoplasmic/nuclear β-Catenin (Fig. 5a, asterisks) in 12 week-old PBCre4/CatnbΔ(ex3) mice, especially when compared with the adjacent areas that show only membrane β-Catenin staining. Furthermore, the AR target genes Nkx3.1 and probasin were up-regulated in the same cells at 12 weeks, when prostate epithelial hyperplasia developed (Fig. 5a). At this age, β-Catenin is only focally activated and the increased AR levels could not be detected by western blot.
Fig. 5.
AR signaling in PBCre4/ CatnbΔ(ex3) mouse prostate. a, immunofluorescence staining performed on prostate sections from a 12 week old PBCre4/ CatnbΔ(ex3) mouse prostate. 1-3: dual staining of β-Catenin and AR. 4-6: dual staining of β-Catenin and Nkx3.1. 7-9: dual staining of β-Catenin and probasin (Pb). β-Catenin was in red; AR, Nkx3.1 and probasin were in green. At the areas where β-Catenin accumulated in cytoplasm/nucleus (indicated by asterisks), AR was up-regulated compared with other areas that showed only membrane β-Catenin staining. Concurrently, AR target genes-Nkx3.1 and probasin were up-regulated with the activation of β-Catenin. b, immunostaining performed on prostate sections from 22 week old intact (panel 1-3) or castrated (panel 4-6) control and PBCre4/ CatnbΔ(ex3) mice. At this age, AR level was slightly decreased in PBCre4/ CatnbΔ(ex3) mouse prostates compared with control under both intact and castration condition. c, probasin staining. d, western blot to analyze the AR level in intact or castrated control and PBCre4/ CatnbΔ(ex3) mouse prostates. e, quantification of western blot results. Scale bars represent 50 μm.
While AR expression in PBCre4/CatnbΔ(ex3) prostate epithelial cells was increased at 12 weeks, The development of HGPIN in PBCre4/CatnbΔ(ex3) prostates at 22 weeks was associated with decreased expression of AR (Fig. 5b, 5d, and 5e). AR was decreased by around 9% in PBCre4/CatnbΔ(ex3) mouse prostates compared to litter mate control (Fig. 5e). The actual decrease of AR in the PIN is likely greater then reflected by western analysis since the prostate contains areas of normal histology as well as PIN. Further, the Cre activation of the β-Catenin gene is directed by promoters that are only expressed in the epithelium likely leaving stromal AR unchanged.
At later ages (6 months), as seen in the Nkx3.1Cre/CatnbΔ(ex3) mouse, the PBCre4/CatnbΔ(ex3) mouse prostates failed to express probasin (Fig. 5c). Unlike the human Prostate Specific Antigen (PSA) that usually continues to be expressed by advanced human prostate cancer, probasin is consistently decreased in mouse models of PIN and prostate cancer.
Additionally, AR levels were also examined in castrated PBCre4/CatnbΔ(ex3) and castrated control mice. By immunohistochemistry staining (Fig 5b), AR levels appeared slightly decreased in castrated PBCre4/CatnbΔ(ex3) mouse prostates compared with castrated control. AR localization did not show obvious differences between the two groups. The slight down-regulation of AR in PBCre4/CatnbΔ(ex3) mouse prostates was confirmed by western blot (Fig. 5d). AR levels were decreased by around 8% in castrated PBCre4/CatnbΔ(ex3) mouse prostates compared with castrated litter mate controls (Fig. 5e). These results indicate that AR and AR signaling pathway were first up-regulated in epithelial cell hyperplasia as a result of activation of β-Catenin, but later AR levels were down-regulated when the prostate developed HGPIN.
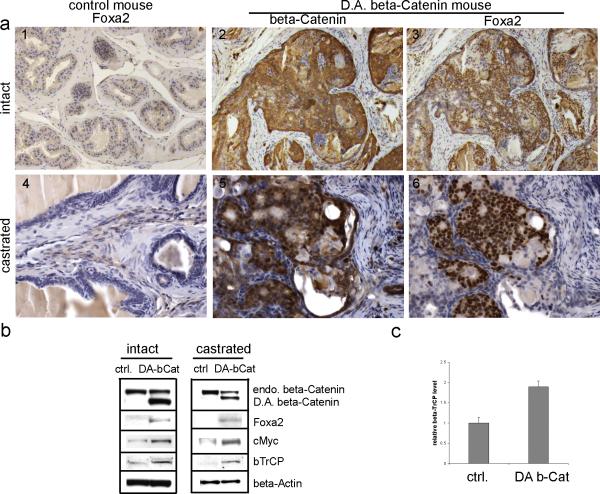
6. Gene expression is altered in PBCre4/CatnbΔ(ex3) mouse prostates
To elucidate the mechanisms that result in continuous prostate growth in the PBCre4/CatnbΔ(ex3) mouse after castration, we examined several nuclear β-Catenin regulated genes including Foxa2 (an embryonic prostate marker), cMyc (β-Catenin/TCF target gene, is involved in cell cycle and proliferation regulation), and β-TrCP (an ubiquitin ligase involved in regulation of several proteins’ degradation).
The Nkx3.1-Cre/CatnbΔ(ex3) mouse prostate showed Foxa2 expression coincided with the activation of nuclear β-Catenin (Fig 2). Since Nkx3.1-Cre is expressed in the embryonic prostate when Foxa2 is also expressed, it is possible that activation of β-Catenin allows Foxa2 expression to persist rather than to induce the expression of Foxa2. Our use of PBCre4/CatnbΔ(ex3) mice, in which the probasin promoter directs Cre expression to the prostatic epithelium starting around two weeks after birth, a time when Foxa2 is not detected in the prostate gland [31], enabled us to determine if Foxa2 was re-induced by the activation of β-Catenin. As shown in Fig 6, similar to the Nkx3.1-Cre/CatnbΔ(ex3) prostates, PBCre4/CatnbΔ(ex3) prostates showed Foxa2 expression. The expression of Foxa2 was detected at late time points such as 23 weeks (Fig. 6) but not at 12 weeks (data not shown). Thus, Foxa2 expression was detected in HGPIN lesions, but not in hyperplastic lesions, indicating that activation of Wnt/β-Catenin signaling induced Foxa2's re-expression in the prostate instead of sustaining Foxa2's expression from embryonic prostate. Furthermore, the re-expression of Foxa2 occurred when active nuclear β-Catenin appeared in the prostate of both intact and castrated mice (Fig. 6a). The induction of Foxa2 in PBCre4/CatnbΔ(ex3) prostates was further confirmed by western blot (Fig. 6b). In addition, western blot also showed increased cMyc levels in the prostates of intact and castrated mice once β-Catenin-signaling was activated as compared to controls (Fig. 6b).
Fig. 6.
genes altered in PBCre4/ CatnbΔ(ex3) mouse prostates. a, immunostaining performed on sections from intact (1-3) or castrated (4-6) mouse prostates. 1 and 4: Foxa2 staining performed on control prostates. Foxa2 was not detected in these prostates under either intact or castration condition. 2 and 3: serial sections from intact PBCre4/ CatnbΔ(ex3) mouse prostates. 5 and 6: serial section from castrated PBCre4/ CatnbΔ(ex3) mouse prostates. With the activation of β-Catenin, Foxa2 was induced under both intact and castration condition. b, western blot. Protein lysis was prepared from intact or castrated control and PBCre4/ CatnbΔ(ex3) mouse prostates. The deletion of exon 3 of β-Catenin resulted in truncated β-Catenin protein that was labeled as D.A. beta-Catenin. The endogenous β-Catenin band was labeled as endo. beta-Catenin. With the accumulation of D.A. β-Catenin, endogenous β-Catenin level was decreased. The induction of Foxa2 in PBCre4/ CatnbΔ(ex3) mouse prostates was confirmed in western blot. Protein levels of cMyc and βTrCP increased with the activation of β-Catenin. c, real-time RT-PCR to analyze βTrCP level.
As shown in Fig. 6b, the high level of the non-degradable exon 3 deleted β-Catenin (D.A. beta-Catenin) was in sharp contrast to the lower level of the endogenous wild type β-Catenin. To explain this difference, we examined the levels of F-box β-TrCP ubiquitin ligase, an enzyme that causes the degradation of β-Catenin and functions as a feedback inhibitor to Wnt signaling [32-34]. Levels of β-TrCP mRNA were increased 1.9-fold in the CatnbΔ(ex3) expressing mouse prostate compared with control prostate (p<0.01) (Fig. 6c). Similarly, the levels of β-TrCP protein were increased from control levels when Wnt/β-Catenin signaling was active (Fig. 6b). The up-regulated β-TrCP caused the wild type β-Catenin to undergo increased degradation while the non-degradable CatnbΔ(ex3) protein continued to accumulate. The altered gene expressions of Foxa2, cMyc, and β-TrCP in PBCre4/CatnbΔ(ex3) mouse prostates suggests that these genes contribute to prostatic growth after castration.
Discussion
To study Wnt/β-Catenin signaling in prostate development, we initially screened the expression profiles of 19 Wnt family members by RT-PCR. We found that the Wnt family members displayed a dynamic expression pattern during prostate development (Fig. 1). Several of the canonical Wnts were expressed in developing prostate but decreased or were expressed below the level of detection in the adult prostate (Wnt 3, 3a, and 6). The canonical Wnts are capable of activating β-Catenin signaling, suggesting that Wnt dependent β-Catenin signaling is active during prostate development. Wnt 16, which is highly expressed only in developing prostate, is a non-classified Wnt that has been implicated in bone morphogenesis. Non-canonical Wnts including Wnt 4, 5a and 11 are expressed throughout the developing and adult prostate. These results suggest that non-canonical Wnts are required to maintain normal prostatic homeostasis.
In order to study how continuous activation of Wnt/β-Catenin affects prostate development, a mouse model with constitutively activated β-Catenin was utilized where exon 3 of β-Catenin was deleted thus preventing its degradation and resulting in the accumulation of cytoplasmic and nuclear β-Catenin. Two types of prostate-specific Cre mice were used to activate β-Catenin: Nkx3.1-Cre which starts to express Cre around E14.5 and the expression continues into adulthood; and PBCre4 which specifically expresses Cre in the mouse prostate starting about 2 weeks after birth. Both lines activated the Catnbfloxed(ex3) in the prostate resulting in the re-expression of Foxa2. Foxa2 is forkhead transcription factor that is normally expressed in the developing prostate only at E18 through postnatal day 1 when the prostate is undergoing budding [31]. The re-expression of Foxa2 in the Nkx3.1-Cre/CatnbΔ(ex3) and PB-Cre/CatnbΔ(ex3) mouse prostates suggests that either β-Catenin activation endows prostate an embryonic feature and/or that Wnt signaling directly controls Foxa2 levels. β-Catenin and Sox17 have been reported to regulate Foxa2 expression [35]. Sox17 is expressed in the adult prostate (unpublished data), which, with the activation of Wnt/β-Catenin, may explain the expression of Foxa2 in adult prostate.
Androgen ablation therapy is commonly used to treat patients with clinically advanced prostate cancer. However, prostate cancer almost uniformly escapes androgen blockade and becomes androgen depletion independent [36] or “androgen refractory.” The mechanism(s) that controls nonmalignant prostate growth in the absence of androgen may also explain how prostate cancer progresses to the castrate resistant stage during androgen ablation therapy. Our results demonstrate that activation of Wnt/β-Catenin signaling did result in continuous prostatic cell proliferation in the absence of testicular androgen as marked by the extensive BrdU incorporation (Fig. 4). We have demonstrated that either activation of the NF-κB [37] or β-Catenin activation can result in nonmalignant prostatic tissue to continue to grow after castration.
In an effort to explain our observation that constitutively active Wnt/β-Catenin signaling promotes continue prostate growth after castration, we examined several pathways. First, we examined AR pathway. Cross-talk between β-Catenin and AR pathways have been reported in vitro [10], and the activation of AR pathway by β-Catenin is androgen dependent since β-Catenin only activates AR signaling in the presence of androgen. In addition, over-expression of the AR can activate β-Catenin signaling in the presence of low level of androgen (castrate levels of androgen), but not in the presence of physiological level of androgen [38]. Simultaneous activation of β-Catenin and over-expression of the AR enabled prostate cancer cells to grow at castrate levels of androgen, suggesting the pivotal role of the interaction of these two pathways in the progression to castrate resistant prostate cancer. We used Nkx3.1 and PB directed Cre to activate β-Catenin in developing and adult murine prostates, respectively. Rescued Nkx3.1-Cre/CatnbΔ(ex3) mouse prostates developed HGPIN lesions following the activation of β-Catenin (Fig. 2) and displayed decreased levels of AR expression. PBCre4/CatnbΔ(ex3) mouse prostates develop hyperplasia (12 weeks) followed by HGPIN (6 months). Interestingly, while AR levels are up-regulated in early hyperplastic lesions, AR expression levels were subsequently down-regulated in HGPIN (6 months) (Fig. 5).
In hyperplastic lesions (12 weeks), concurrent with the increase of AR level, expression of Nkx3.1 and probasin was increased in epithelial cells where β-Catenin is activated. Our findings provide in vivo evidence for the up-regulation of AR action by nuclear β-Catenin on two androgen target genes, probasin and Nkx3.1. In HGPIN mice, under both intact and castrate conditions, the PBCre4/CatnbΔ(ex3) mouse prostates showed slightly decreased AR protein levels compared with control mouse prostates by westerns. Nuclear AR staining was diminished in both PBCre4/CatnbΔ(ex3) and control mouse prostates after castration. These results indicate that activation of Wnt/β-Catenin signaling promotes prostatic cell proliferation in the absence of testicular androgens. This can be due to castrate levels of androgens activating the AR, nuclear β-Catenin enhancing AR activity, and/or by pathway(s) other than AR signaling that would controls prostatic growth.
Our results showed that Foxa2 was re-expressed in the PBCre4/CatnbΔ(ex3) mice in both intact and castrated conditions. The Foxa2 is an embryonic prostate marker. The re-expression of Foxa2 in PBCre4/CatnbΔ(ex3) mouse prostates suggests the gaining of progenitor cell feature, which may enable these prostatic cells to grow after castration.
In the PBCre4/CatnbΔ(ex3) mouse prostates, we also found that cMyc was up-regulated (Fig. 6b). cMyc, a well-known oncogenic gene homologue to the viral v-myc, is a transcription factor that can activate a variety of target genes involved in the regulation of cell growth, proliferation, and apoptosis. Over-expression of cMyc in benign human prostatic epithelium causes a cancer phenotype [39], and over-expression of cMyc converts prostate cancer cell line LNCaP from androgen dependent into androgen independent [40]. These studies suggest that wnt/β-Catenin control of the cMyc pathway may play a pivotal role in promoting continuous prostate growth after castration.
Activation of β-Catenin signaling resulted in prostatic hyperplasia, low grade PIN, and progression to HGPIN. A recently published paper demonstrates that inactivation of APC in the mouse prostate results in foci of prostate cancer at 7 months and continued proliferation of the prostate cancer after castration [41]. Varied genetic backgrounds may contribute to the slightly different phenotype observed in APC deficient mouse compared to the PBCr4/CatnbΔ(ex3) mouse. Both floxed β-Catenin and PBCre4 mouse lines have been crossed into C57BL/6 for more than ten generations while the APC deficient mice are on a mixed background of C57Bl/6J and 129Sv/J. However, in PBCr4/CatnbΔ(ex3) mouse prostate, we found that with the accumulation of the exon 3 deleted non-degradable β-Catenin in the cytoplasm, endogenous β-Catenin levels decreased. The decreased endogenous β-Catenin is attributed to the increased expression of the F-box β-TrCP ubiquitin ligase that is a feedback inhibitor to block Wnt signaling by the degradation of β-Catenin [32-34]. Although the levels of β-TrCP mRNA measured by qRT-PCR are only 1.9-fold higher than controls (p < 0.01), this value must be an underestimate since the RNA was isolated from the complete prostate that contained areas of normal histology as well as PIN. Increased levels of F-box proteins are particular important in cancer [42] since they control the degradation of multiple signaling pathways that are required for proliferation. Although the endogenous β-Catenin decreased, the exon 3 deleted β-Catenin can still function normally in the E-Cadherin complex. We detect no decrease in E-Cadherin levels with the loss of the endogenous β-Catenin (data not shown). Thus, the exon 3 deleted non-degradable β-Catenin may serve to stabilize E-Cadherin, which may account for the lack of prostate carcinoma in PBCr4/CatnbΔ(ex3) mouse .
Conclusions
The present report focused on the study of Wnt/β-Catenin signaling in prostate development and carcinogenesis. In this study, we have now found that Wnt/β-Catenin signaling is active in early prostate development and constitutive activation of β-Catenin results in HGPIN in the prostate. Further, we have shown that accumulation of β-Catenin enables the prostate to grow after androgen blockade. In addition, we have found that with the activation of Wnt/β-Catenin signaling, Foxa2 was induced in adult prostates. Foxa2 is normally expressed in embryonic prostate buds during development but not in adult prostate. These findings suggest that Wnt/β-Catenin signaling plays an important role in prostate development and in the progression of prostate cancer.
Acknowledgments
We would like to thank Dr. Marie-Claire Orgebin-Crist for critical reading of this manuscript, Dr. Michael R. Dohn and Dr. David Degraff for manuscript revision, Tom Case and Manik Paul for technical support. This research was supported by NIH grant R01-AG023490, R01-CA76142, and R01-DK55748 and the Frances Preston Laboratories of the T.J. Martel Foundation to RJM, by NIH grants R01-CA115985 and R01-DK076602 to MMS, and by NIH grants R01-CA59705 and R01-CA113392 to PRB. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- AR
androgen receptor
- HGPIN
high grade prostatic intraepithelial neoplasia
- UGS
urogenital sinus
References
- 1.Miller JR. The Wnts. Genome Biol. 2002;3 doi: 10.1186/gb-2001-3-1-reviews3001. REVIEWS3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mulholland DJ, Dedhar S, Coetzee GA, Nelson CC. Interaction of nuclear receptors with the Wnt/beta-catenin/Tcf signaling axis: Wnt you like to know? Endocr Rev. 2005;26:898–915. doi: 10.1210/er.2003-0034. [DOI] [PubMed] [Google Scholar]
- 3.Mulholland DJ, Cheng H, Reid K, Rennie PS, Nelson CC. The androgen receptor can promote Beta -catenin nuclear translocation independently of adenomatous polyposis coli. J Biol Chem. 2002;277:17933–43. doi: 10.1074/jbc.M200135200. [DOI] [PubMed] [Google Scholar]
- 4.Edlund S, Lee SY, Grimsby S, Zhang S, Aspenström P, Heldin CH, Landström M. Interaction between Smad7 and beta-catenin: importance for transforming growth factor beta-induced apoptosis. Mol Cell Biol. 2005;25:1475–88. doi: 10.1128/MCB.25.4.1475-1488.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–12. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 6.Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R, Ben Ze'ev A. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci U S A. 1999;96:5522–7. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Truica CI, Byers S, Gelmann EP. Beta-catenin affects androgen receptor transcriptional activity and ligand specificity. Cancer Res. 2000;60:4709–13. [PubMed] [Google Scholar]
- 8.Yang F, Li X, Sharma M, Sasaki CY, Longo DL, Lim B, Sun Z. Linking beta-catenin to androgen-signaling pathway. J Biol Chem. 2002;277:11336–44. doi: 10.1074/jbc.M111962200. [DOI] [PubMed] [Google Scholar]
- 9.Verras M, Sun Z. Roles and regulation of Wnt signaling and beta-catenin in prostate cancer. Cancer Lett. 2006;237:22–32. doi: 10.1016/j.canlet.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Terry S, Yang X, Chen MW, Vacherot F, Buttyan R. Multifaceted interaction between the androgen and Wnt signaling pathways and the implication for prostate cancer. J Cell Biochem. 2006;99:402–10. doi: 10.1002/jcb.20983. [DOI] [PubMed] [Google Scholar]
- 11.Voeller HJ, Truica CI, Gelmann EP. Beta-catenin mutations in human prostate cancer. Cancer Res. 1998;58:2520–3. [PubMed] [Google Scholar]
- 12.Yardy GW, Brewster SF. Wnt signalling and prostate cancer. Prostate Cancer Prostatic Dis. 2005;8:119–26. doi: 10.1038/sj.pcan.4500794. [DOI] [PubMed] [Google Scholar]
- 13.Chesire DR, Ewing CM, Gage WR, Isaacs WB. In vitro evidence for complex modes of nuclear beta-catenin signaling during prostate growth and tumorigenesis. Oncogene. 2002;21:2679–94. doi: 10.1038/sj.onc.1205352. [DOI] [PubMed] [Google Scholar]
- 14.Chen G, Shukeir N, Potti A, Sircar K, Aprikian A, Goltzman D, Rabbani SA. Up-regulation of Wnt-1 and beta-catenin production in patients with advanced metastatic prostate carcinoma: potential pathogenetic and prognostic implications. Cancer. 2004;101:1345–56. doi: 10.1002/cncr.20518. [DOI] [PubMed] [Google Scholar]
- 15.de la TA, Rubin MA, Chen MW, Vacherot F, de Medina SG, Burchardt M, Buttyan R, Chopin D. Beta-catenin-related anomalies in apoptosis-resistant and hormone-refractory prostate cancer cells. Clin Cancer Res. 2003;9:1801–7. [PubMed] [Google Scholar]
- 16.Wissmann C, Wild PJ, Kaiser S, Roepcke S, Stoehr R, Woenckhaus M, Kristiansen G, Hsieh JC, Hofstaedter F, Hartmann A, Knuechel R, Rosenthal A, Pilarsky C. WIF1, a component of the Wnt pathway, is down-regulated in prostate, breast, lung, and bladder cancer. J Pathol. 2003;201:204–12. doi: 10.1002/path.1449. [DOI] [PubMed] [Google Scholar]
- 17.Persad S, Troussard AA, McPhee TR, Mulholland DJ, Dedhar S. Tumor suppressor PTEN inhibits nuclear accumulation of beta-catenin and T cell/lymphoid enhancer factor 1-mediated transcriptional activation. J Cell Biol. 2001;153:1161–74. doi: 10.1083/jcb.153.6.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castellone MD, Teramoto H, Williams BO, Druey KM, Gutkind JS. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science. 2005;310:1504–10. doi: 10.1126/science.1116221. [DOI] [PubMed] [Google Scholar]
- 19.Lei S, Dubeykovskiy A, Chakladar A, Wojtukiewicz L, Wang TC. The murine gastrin promoter is synergistically activated by transforming growth factor-beta/Smad and Wnt signaling pathways. J Biol Chem. 2004;279:42492–502. doi: 10.1074/jbc.M404025200. [DOI] [PubMed] [Google Scholar]
- 20.Lamberti C, Lin KM, Yamamoto Y, Verma U, Verma IM, Byers S, Gaynor RB. Regulation of beta-catenin function by the IkappaB kinases. J Biol Chem. 2001;276:42276–86. doi: 10.1074/jbc.M104227200. [DOI] [PubMed] [Google Scholar]
- 21.Carayol N, Wang CY. IKKalpha stabilizes cytosolic beta-catenin by inhibiting both canonical and non-canonical degradation pathways. Cell Signal. 2006;18:1941–6. doi: 10.1016/j.cellsig.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 22.Yang L, Lin C, Liu ZR. P68 RNA helicase mediates PDGF-induced epithelial mesenchymal transition by displacing Axin from beta-catenin. Cell. 2006;127:139–55. doi: 10.1016/j.cell.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 23.Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, Taketo MM. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 1999;18:5931–42. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu X, Wu J, Huang J, Powell WC, Zhang J, Matusik RJ, Sangiorgi FO, Maxson RE, Sucov HM, Roy-Burman P. Generation of a prostate epithelial cell-specific Cre transgenic mouse model for tissue-specific gene ablation. Mech Dev. 2001;101:61–9. doi: 10.1016/s0925-4773(00)00551-7. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y-Q, Kasper S, Yuan J, Jin RJ, Zhang ZF, Ishii K, Wills ML, Hayward SW, Matusik RJ. Androgen dependent prostatic epithelial cell selection by targeting ARR2PBNeo to the LPB-Tag transgenic model of prostate cancer. Lab Invest. 2006;86:1074–88. doi: 10.1038/labinvest.3700463. [DOI] [PubMed] [Google Scholar]
- 26.Yu X, Gupta A, Wang Y-Q, Suzuki K, Mirosevich J, Orgebin-Crist MC, Matusik RJ. Foxa1 and Foxa2 interact with the androgen receptor to regulate prostate and epididymal genes differentially. Annals New York Academy of Sciences. 2005;1061:77–93. doi: 10.1196/annals.1336.009. [DOI] [PubMed] [Google Scholar]
- 27.Yu X, Suzuki K, Wang Y, Gupta A, Jin R, Orgebin-Crist MC, Matusik R. The role of forkhead box A2 to restrict androgen-regulated gene expression of lipocalin 5 in the mouse epididymis. Mol Endocrinol. 2006;20:2418–31. doi: 10.1210/me.2006-0008. [DOI] [PubMed] [Google Scholar]
- 28.Sciavolino PJ, Abrams EW, Yang L, Austenberg LP, Shen MM, Abate-Shen C. Tissue-specific expression of murine Nkx3.1 in the male urogenital system. Dev Dyn. 1997;209:127–38. doi: 10.1002/(SICI)1097-0177(199705)209:1<127::AID-AJA12>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 29.Stanfel MN, Moses KA, Carson JA, Zimmer DB, DeMayo F, Schwartz RJ, Zimmer WE. Expression of an Nkx3.1-CRE gene using ROSA26 reporter mice. Genesis. 2006;44:550–5. doi: 10.1002/dvg.20250. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Hayward SW, Donjacour AA, Young P, Jacks T, Sage J, Dahiya R, Cardiff RD, Day ML, Cunha GR. Sex hormone-induced carcinogenesis in Rb-deficient prostate tissue. Cancer Res. 2000;60:6008–17. [PubMed] [Google Scholar]
- 31.Mirosevich J, Gao N, Matusik RJ. Expression of Foxa transcription factors in the developing and adult murine prostate. Prostate. 2005;62:339–52. doi: 10.1002/pros.20131. [DOI] [PubMed] [Google Scholar]
- 32.Noubissi FK, Elcheva I, Bhatia N, Shakoori A, Ougolkov A, Liu J, Minamoto T, Ross J, Fuchs SY, Spiegelman VS. CRD-BP mediates stabilization of betaTrCP1 and c-myc mRNA in response to beta-catenin signalling. Nature. 2006;441:898–901. doi: 10.1038/nature04839. [DOI] [PubMed] [Google Scholar]
- 33.Fuchs SY, Spiegelman VS, Kumar KG. The many faces of beta-TrCP E3 ubiquitin ligases: reflections in the magic mirror of cancer. Oncogene. 2004;23:2028–36. doi: 10.1038/sj.onc.1207389. [DOI] [PubMed] [Google Scholar]
- 34.Fuchs SY. The role of ubiquitin-proteasome pathway in oncogenic signaling. Cancer Biol Ther. 2002;1:337–41. [PubMed] [Google Scholar]
- 35.Sinner D, Rankin S, Lee M, Zorn AM. Sox17 and beta-catenin cooperate to regulate the transcription of endodermal genes. Development. 2004;131:3069–80. doi: 10.1242/dev.01176. [DOI] [PubMed] [Google Scholar]
- 36.Roy-Burman P, Tindall DJ, Robins DM, Greenberg NM, Hendrix MJ, Mohla S, Getzenberg RH, Isaacs JT, Pienta KJ. Androgens and prostate cancer: are the descriptors valid? Cancer Biol Ther. 2005;4:4–5. doi: 10.4161/cbt.4.1.1563. [DOI] [PubMed] [Google Scholar]
- 37.Jin RJ, Lho Y, Connelly L, Wang Y-Q, Yu X, Saint Jean L, Case T, Ellwood-Yen K, Sawyers CL, Bhowmick NA, Blackwell TS, Yull FE, Matusik RJ. The Nuclear Factor kappa B Pathway Controls Progression of Prostate Cancer to Androgen Independent Growth. Cancer Res. 2008;68:6762–9. doi: 10.1158/0008-5472.CAN-08-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schweizer L, Rizzo CA, Spires TE, Platero JS, Wu Q, Lin TA, Gottardis MM, Attar RM. The androgen receptor can signal through Wnt/beta-Catenin in prostate cancer cells as an adaptation mechanism to castration levels of androgens. BMC Cell Biol. 2008;9:4. doi: 10.1186/1471-2121-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams K, Fernandez S, Stien X, Ishii K, Love HD, Lau YF, Roberts RL, Hayward SW. Unopposed c-MYC expression in benign prostatic epithelium causes a cancer phenotype. Prostate. 2005;63:369–84. doi: 10.1002/pros.20200. [DOI] [PubMed] [Google Scholar]
- 40.Bernard D, Pourtier-Manzanedo A, Gil J, Beach DH. Myc confers androgen-independent prostate cancer cell growth. J Clin Invest. 2003;112:1724–31. doi: 10.1172/JCI19035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bruxvoort KJ, Charbonneau HM, Giambernardi TA, Goolsby JC, Qian CN, Zylstra CR, Robinson DR, Roy-Burman P, Shaw AK, Buckner-Berghuis BD, Sigler RE, Resau JH, Sullivan R, Bushman W, Williams BO. Inactivation of apc in the mouse prostate causes prostate carcinoma. Cancer Res. 2007;67:2490–6. doi: 10.1158/0008-5472.CAN-06-3028. [DOI] [PubMed] [Google Scholar]
- 42.Maser RS, Choudhury B, Campbell PJ, Feng B, Wong KK, Protopopov A, O'Neil J, Gutierrez A, Ivanova E, Perna I, Lin E, Mani V, Jiang S, McNamara K, Zaghlul S, Edkins S, Stevens C, Brennan C, Martin ES, Wiedemeyer R, Kabbarah O, Nogueira C, Histen G, Aster J, Mansour M, Duke V, Foroni L, Fielding AK, Goldstone AH, Rowe JM, Wang YA, Look AT, Stratton MR, Chin L, Futreal PA, DePinho RA. Chromosomally unstable mouse tumours have genomic alterations similar to diverse human cancers. Nature. 2007;447:966–71. doi: 10.1038/nature05886. [DOI] [PMC free article] [PubMed] [Google Scholar]