Abstract
Alzheimer disease (AD) is the leading cause of dementia among elderly. Currently, no effective treatment is available for AD. Analysis of transgenic mouse models of AD has facilitated our understanding of disease mechanisms and provided valuable tools for evaluating potential therapeutic strategies. In this review, we will discuss the strengths and weaknesses of current mouse models of AD and the contribution towards understanding the pathological mechanisms and developing effective therapies.
Keywords: Alzheimer disease, amyloid-beta, tau, transgenic mouse models
INTRODUCTION
Alzheimer disease (AD) is a progressive neurodegenerative disorder that causes nearly two-thirds of all dementia cases in the elderly population. Currently, over 35 million individuals worldwide and over 5 million in the U.S. suffer from this devastating disease, and the number of patients is expected to increase dramatically over the next 40 years particularly in developed countries where the average longevity and ratio of aged population are becoming more prominent [1-3]. The course of AD is prolonged and prognosis is poor. Over decades, neurodegeneration and dementia slowly and irreversibly proceed, and by the time patients receive medical attention for their symptoms, the neuropathology is well advanced, including structural alterations and atrophy to key brain regions. The chronic progression and latency, as in asymptomatic and/or mild cognitive impairment (MCI) cases, of the disease make it difficult to identify the pathogenic causes and underlying mechanisms of the disease.
The overwhelming majority of AD cases (~95%) occur sporadically, in which a definitive cause is not known, whereas less than 5% of the cases are hereditary/familial AD, caused by mutations or duplications genes encoding amyloid precursor protein (APP), presenilin-1 (PS1) and presenilin-2 (PS2). Epidemiological and twin studies have identified multiple genetic and environmental risk factors for sporadic AD, yet many of them have not been fully confirmed [4]. In contrast, it is well accepted that aging and the possession of an apolipoprotein E4 allele (apoE4) are strong risk factors for sporadic AD. Interestingly, regardless of whether the case is sporadic or familial, all AD patients develop common hallmark neuropathological lesions within the brain, providing us with clues to understand the pathogenic mechanisms that drive this disorder.
For the past decade, genetically engineered mouse models have been the mainstay of basic AD research and significantly contributed to the advancement of our knowledge in AD. In this review, we discuss the strengths and weaknesses of currently available transgenic mouse models of AD and challenges for the next generation of animal models. We will also analyze the application of these transgenic animals to the discovery and evaluation of molecular mechanisms and potential therapeutic approaches in AD.
NEUROPATHOLOGICAL HALLMARKS OF AD
Presently, a definitive diagnosis of AD is established when the presence of amyloid plaques and neurofibrillary tangles (NFTs) are confirmed in the post-mortem brain from the suspected patient. Amyloid plaques are largely composed of the β-amyloid (Aβ) peptide and are deposited in the extracellular spaces, whereas NFTs are found intracellularly and are made mostly of hyperphosphorylated tau protein [3, 5].
Aβ peptides may range from 38-43 amino acids, although the predominant species are considered to be 40 or 42 in length, and are generated from a ~100-kDa amyloid precursor protein (APP) by sequential proteolytic cleavage by β- and γ-secretases. The Aβ peptides found associated with plaques have mainly aggregated, having undergone conformational changes that lead to the formation of β-sheet fibrillar structures. The aggregated Aβ triggers innate immune responses, which are often observed in association with reactive astrocytes and activated microglia. Degenerative synapses and dystrophic neurites are also commonly detected around the plaques. These findings have long been suggestive of Aβ plaques as key pathological components for AD. However, recent advances in detection methods revealed that multiple forms of Aβ, including various sizes of soluble oligomers and proto-fibrils, also exist within the AD brain. Importantly, it has also been shown that these soluble species exhibit more potent neurotoxicity than plaques, suggesting that Aβ oligomers and proto-fibrils may be more important players in the pathogenesis of AD than fibrillar aggregates [6-8]. This hypothesis is further supported by identification of an APP mutation involving the deletion of glutamate residue at position 22 of Aβ (E22D) that results in higher oligomerization and resistance to degradation but not fibrillization [9], as well as a number of case reports describing cognitively intact individuals with large amyloid plaque burden [10, 11].
Neurofibrillary tangles (NFTs), a second hallmark lesion of AD are composed primarily of insoluble aggregates of the microtubule-associated protein tau (MAPT), which is normally unfolded and binds to microtubules to stabilize neuronal cytoskeleton [12]. However, when tau is hyperphosphorylated, it undergoes conformational changes to form abnormal paired helical filaments (PHFs), a primary component of NFTs, which become deposited in dystrophic neurites, dendrites and cell bodies. Interestingly, hyperphosphorylated tau accumulation or tau-containing NFT formation is also found in other neurodegenerative diseases, such as frontotemporal dementia and Parkinsonism linked to chromosome 17 (FTDP-17), Pick's disease, progressive supranuclear palsy, and corticobasal degeneration, which are collectively called tauopathies [13]. Although the Aβ plaques do not correlate well with the severity of dementia in AD, the presence of NFTs and tau in CSF have been reported to correlate with neuronal death and clinical phenotypes. Recent evidence suggests that soluble intermediates or oligomeric tau species, not aggregated tau or NFTs, are responsible in triggering neuronal death [14, 15]. C-terminal truncation of tau by caspase-3 cleavage has been detected in brains from early AD patients as well as in a transgenic mouse model [16, 17]. The cleaved tau exhibits toxicity and is susceptible to aggregation [18 19]. In addition, another important pathological function of tau involving excitotoxicity and synaptic dysfunction has recently been described in mouse models [20, 21]. Collectively, tau is implicated as a key molecule that triggers neuronal degeneration in AD.
Other neuropathological features of AD including loss of synapses and axonal pathology, and sustained inflammatory responses are associated with the two major hallmark lesions. Clinical pheno-types of AD measured as cognitive impairments are well correlated with synaptic loss, not amyloid burden in the brain [22-24]. Therefore, it is crucial to study AD in multiple aspects of the disease hallmarks.
GENETIC MUTATIONS IN AD
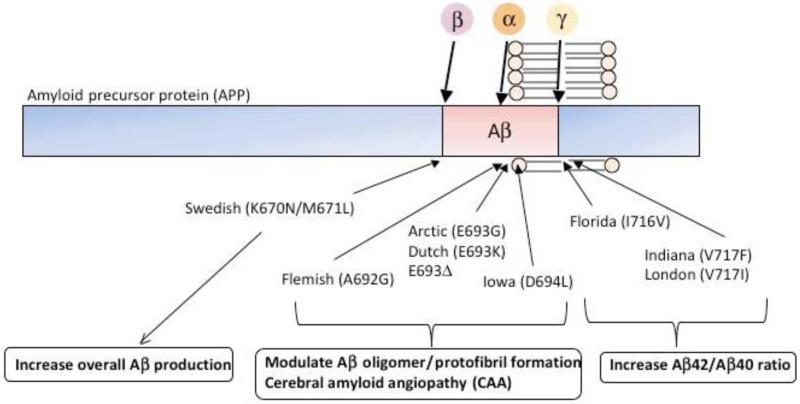
The understanding of AD was significantly advanced following the identification of genetic mutations linked to familial AD (FAD). At this moment, all autosomal dominant AD-associated mutations are found in three genes: APP, presenilin-1 (PS1) and presenilin-2 (PS2); the latter two are key components of the γ-secretase complex, whereas the former is the substrate from which Aβ is liberated. Each of these mutations result in mismetabolism of Aβ in varying ways Fig. (1). For example, mutations near the N-terminal of Aβ(Swedish double mutation) increase total Aβ production by modulating β-secretase cleavage [25]; mutations within the Aβ sequence appear to influence its conformation and promote oligomer or protofibril formations [9, 26-29] and; mutations at the C-terminal of Aβ as well as mutations in PS1 and PS2 cause a shift of γ-secretase cleavage, favoring the production of the more amyloidogenic Aβ42 [30-33]. These findings provided crucial support for the “amyloid cascade hypothesis”, which suggests that accumulation of Aß is the initiating event in AD pathogenesis and triggers the development of other downstream neuropathologies including NFTs [34]. Further support stems from the identification of patients harboring APP duplications, or APP triplication as in patients with Down syndrome, who develop Aβ plaques, AD-like pathologies and dementia [35, 36].
Fig. (1). Schematic diagram of APP mutations and its amyloidogenic effect.
Amyloid precursor protein (APP) is a type I transmembrane protein, and within its sequence, amyloid-β(Aβ) resides. APP is proteolytically cleaved by α-, β-, and γ-secretase activities, and α-secretase cleavage precludes toxic Aβ to be produced. FAD-linked genetic mutations on APP exhibit different effects on APP processing and amyloidogenic properties of Aβ peptides. Please refer main text for detailed explanation.
FAD-associated mutations in APP, PS1, and PS2 were quickly utilized to facilitate the development of transgenic mouse models of AD. These in vivo models have become invaluable tools to study the complex disease process and evaluate potential therapeutic strategies in a whole organism. Mice are commonly used as models for neurodegenerative diseases, because they have comparable, not identical, brain networks and neurobiological processes with humans. Mice are also relatively inexpensive to maintain and easy to manipulate genetically. Currently, numerous transgenic mouse models harboring single or combinations of FAD-associated mutations are readily available for research. Many of them overexpress human mutant APP in the brain, and these mice typically develop Aβ plaques and cognitive impairments in an age-dependent manner. However, it should be noted that none of the current transgenic mouse models of AD fully recapitulate the entire disease course, represented by plaque and tangle formation, cognitive decline, synaptic loss, and neurodegeneration in a progressive manner. Despite this, transgenic AD models have greatly contributed to the advancement of AD research, and based on the unique properties of each mutation, they remain highly useful to study specific aspect of the disease process. AD transgenic mouse models also allow us to evaluate potential therapeutic strategies and to examine the temporal changes in the disease progression, studies that are typically far more limited in human subjects or tissues.
APP TRANSGENIC MOUSE MODELS
Overexpression of FAD-associated mutant APP is the most common strategy to generate a transgenic mouse model of AD and is widely used in the field. Unlike humans, wildtype mice do not develop Aβ plaques during the course of normal aging, likely because of differences in three amino acids within the Aβ sequence between human and rodent [37]. Currently, over 50 different transgenic mouse models that overexpress human wildtype or FAD-associated mutant APP have been used in AD research (http://www.alzforum.org/res/com/tra/app/default.asp, also please see Table 1). The overexpression of wildtype human APP in the mouse brain was first generated, yet these mice only developed mild neuropathological changes without deposition of Aβ plaques, suggesting that wildtype APP overexpression may not be recapitulate the human disease efficiently in mice [38-40]. In order to robustly promote Aβ neuropathology in mice, FAD-associated mutant APP genes were utilized. In mice overexpressing mutant APP, an age-dependent development and maturation of Aβ plaques in the brain are commonly detected. The age of the onset of Aβ plaques formation is highly dependent on the types of mutations and promoters used for the transgene expression, as well as the resulting expression levels of transgene in the brain. Many, but not all, of these mutant APP transgenic mice also exhibit an age-dependent cognitive impairment that mimics the human disease [41-43]. Other neuropathological hallmarks similar to human AD are also observed, including dystrophic neurites, reactive astrocytes and activated microglia, elevated innate immune and inflammatory responses, synaptic loss, and deficits in electrophysiological and neurochemical signals. Almost all APP transgenic mice, however, do not exhibit robust neurodegeneration or NFT-like tau pathology in the brain, suggesting that even the expression of FAD-linked APP mutations is not sufficient to drive all pathological pathways in mice.
Table 1.
APP Transgenic Mouse Models
| Transgeni Mouse | Transgene | Promoter | Strain | Pathology |
|---|---|---|---|---|
| Lamb et al. (1993) | Wildtype human APP | Yeast artificial chromosome (YAC) APP promoter | C57BL/6J | No Aβ or AD-like pathology at 3 months |
| Buxbaum et al. (1993) | Wildtype human APP | YAC APP promoter | Increased APP expression No Aβ or AD-like pathology |
|
| Games et al. (1995) PDAPP | Human APP (Indiana) minigene | PDGFb | Swiss Webster × B6D2F1 | Aβ plaques at 6-9 months Gliosis, synaptic loss Neuronal loss in hippocampus and dentate gyrus at 3-4 months Behavior deficits |
| Hsiao et al. (1996) Tg2576 | Human APP695 (Swedish) | Hamster PrP | C57BL/6 | Aβ plaques at 10-12 months Oligomeric Aβ species Synaptic loss at 15-18 months Behavior deficits |
| Sturchler-Pierrat et al. (1997) APP23 | Human APP751 (Swedish) | Mouse Thy-1.2 | C57BL/6J | 7-fold APP expression over endogenous levels Aβ plaques by 6 months Gliosis Increased phospho-tau at 6 months Phospho-tau deposits around plaques at 12 months CA1 neuronal loss at 14-18 months Behavior deficits |
| Sturchler-Pierrat et al. (1997) APP22 | APP751 (Swedish/London) | Human Thy-1 | C57BL/6J | 2-fold APP expression over endogenous levels Aβ plaques at 18 months Tau phosphorylation and dystrophic neurites around plaques |
| Mucke et al. (2000) J20 | Human APP (Swedish/Indiana) minigene | PDGFb | C57BL/6 × DBA/2 F2 | Aβ plaques at 4-5 months Phospho-neurofilaments Synaptic loss behavior |
| Mucke et al. (2000) I5 | Human wildtype APP minigene | PDGFb | C57BL/6 × DBA/2 F2 | No plaques even at 24 months Mild synaptic loss |
| Chishti et al. (2001) TgCRND8 | Human APP695 (Swedish/Indiana) | Hamster PrP | C3H/He × C57BL/6 | Aβ plaques (Thioflavin S positive) at 3 months Dense core plaques at 5 months Dystrophic neurites around plaques 50% mortality at ~150 days Behavior deficits by 3 months |
| Borchelt et al. (1996) APP/PS1 | Mouse/human chimeric APP695 (Swedish) Human PS1 (A246E) |
Mouse PrP (APP and PS1) | C3H/HeJ × C57BL/6J | Increased Aβ42/Aβ40 ratio Accelerated Aβ plaques at 9 months Dystrophic neurites and gliosis at 12 months |
| Cheng et al. (2004) Arc6, Arc48 | Human APP (Arctic) minigene | PDGFb | C57BL/6N | Aβ plaques by 2 months (Arc48) or 4-6 months (Arc6) Increased Aβ fibrillar formation Decreased Aβ oligomers which correlate with attenuating behavior deficits |
| Wirths et al. (2009) TBA2 | mTRH-Aβ3Q-42 | Mouse Thy-1 | C57BL/6J | Mean survival around 70 days Massive Purkinje cell loss Diffuse plaques No phospho-tau |
| Tomiyama et al. (2010) | Human APP695 (E693D) | Mouse PrP | B6C3F1 (back-crossed to C57BL/6) | Increased Aβ oligomer intracellular deposits in hippocampus and cortex at 8 months No Aβ plaques PHF1-phospho-tau deposits in mossy fibers at 8 months Synaptic loss at 8 months Microglial activation at 12 months Astrocyte activation at 18 months Neuronal loss at 24 months Behavior deficits at 8 months |
Currently, more than 20 genetic mutations have been identified in the APP gene, and some of them are frequently used to generate widely used transgenic mouse models of AD. Almost half of APP transgenic mice overexpress the Swedish double mutation (K670N/M671L) of APP (APPSwe). One of the most widely used transgenic mouse models with this mutation is the Tg2576 mouse. In this model, APP695 with the Swedish mutation is driven under the control of hamster prion promoter, allowing the transgene expression in forebrain regions and spinal cord [44]. Tg2576 mice develop thioflavin-S-positive Aβ plaques at around 10-12 months of age along with other pathological changes. Tg2576 mice also produce oligomeric Aβ, which seems to be more toxic than fibrillar or aggregated Aβ in plaques [45]. Although Tg2576 mice failed to show any significant neuronal loss in affected brain regions [46], a recent study showed significant reduction of synaptic density in entorhinal cortex of Tg2576 mice at 15-18 months of age at electron microscopic level [47]. Similarly, the stability of dendritic spines in Tg2576 is significantly impaired compared to age-matched wildtype mice, resulting in accelerated loss of spines and impaired synaptic plasticity [48]. However, it still remains unclear whether Aβ oligomers, fibrils or plaques are responsible for the synaptic loss and contribute to subsequent cognitive impairments in the Tg2576 mouse model.
Another model that utilizes overexpression of APPSwe mutation is the APP23 transgenic line [43]. Unlike Tg2576 mice, APP23 transgenics utilize the longer 751 amino acids form of APP and expression is driven via the murine Thy-1.2 promoter. Approximately 7-fold overepxression of mutant APP in this model induces Congo-red-positive Aβ plaque formation at 6 months of age, which increases in number and size as mice age. A massive gliosis and microglial activation around plaques are apparent along with Aβ plaque formation, and tau phosphorylation is also detected around the plaques [43, 49, 50]. In addition, unlike Tg2576 mice, APP23 mice have increased neuronal loss in CA1 hippocampus at 14-18 months of age [51].
These two models as well as many of other models utilize cDNA of APP. The PDAPP transgenic mouse model, on the other hand, expresses the human Indiana mutation on APP minigene (V717F, APPInd) under the control of platelet-derived growth factor (PDGF) promoter [41, 52]. This APP minigene contains the introns 6-8, allowing alternative splicing of exons 7 and 8 to express splicing variants of APP; APP695, APP751 and APP770. Like other transgenic mice, PDAPP mice develop both diffuse and dense Aβ plaques in an age-dependent manner, starting from 6-9 months of age, and reactive astrocytes surround these plaques. Although aged PDAPP mice present significant synaptic loss, no clear evidence of neuronal loss in the entorhinal cortex, CA1 hippocampus or cingu-late gyrus is found in the brain of these animals with advanced Aβ pathology [41, 53]. Recent studies have demonstrated a reduction of 12-30% in the volume of hippocampus and dentate gyrus (DG), as well as a selective loss of granule cells in DG prior to Aβ deposits in 3-4 months old PDAPP mice, although it remains unclear whether this is caused by decreased adult neurogenesis as opposed to neuronal loss [54, 55]. An independent transgenic mouse (H6 line) generated using the same transgene as PDAPP mice also exhibits early synaptic and neuronal loss in hippocampus, which is independent from plaque burden in the brain, suggesting that perhaps other soluble forms of Aβ mediate neurodegeneration in these mice, a notion that is currently a central theme of Aβ neurotoxicity in AD [56].
Taken together, these studies clearly show that the development of Aβ pathology and other hallmark changes associated with AD in transgenic mice depend heavily on the expression of the transgene and the generation of particular Aβ species within the brain. Therefore, one approach to accelerate Aβ pathology is to combine multiple FAD-linked mutations. For example, although not found in humans, transgenic mouse models that overexpress multiple FAD-linked APP mutations in a single APP gene have been generated, such as J20, APP22 and TgCRND8 mice [40, 43, 57]. Most of these transgenic mice possess Swedish double mutation as it increases overall Aβ generation. In addition to the Swedish mutation, APP transgene contains mutation(s) that facilitates aggregation/fibrillization or increases generation of more toxic Aβ42 peptide. These mutations include E693G (Arctic), E693K (Dutch), V717F (Indiana), or V717I (London). Generally, these transgenic mice develop Aβ pathology much more aggressively than above described APP transgenic mice. For example, J20 mice overexpress APPSwe/Ind under the PDGF promoter developing Aβ deposits and synaptic loss as early as 5-7 months of age [40]. Interestingly, in this study, the synaptic loss does not correlate with Aβ plaque load or APP expression levels, rather it correlates well with brain Aβ levels. Similar results were found in the control transgenic mice overexpressing human wildtype APP (I5 mice) in comparable levels with J20 mice. I5 mice do not develop Aβ plaques throughout the lifespan, yet exhibit a significant reduction of synaptic protein with age, which also correlates with Aβ levels [40]. J20 mice also show significant impairment of cognitive function in different behavior tasks [58].
TgCRND8 transgenic mice were generated to induce an early development of Aβ pathology [57]. Like J20 mice, TgCRND8 mice overexpress APPSwe/Ind mutation, but it is driven by the prion promoter. TgCRND8 mice show significant cognitive declines within 3 months of age and develop Aβ plaque depositions [57], followed by activation of immune/inflammatory responses around the plaques [59] and loss of cholinergic input [60]. Clearly, the advantage of early and accelerated development of AD-like pathology in these mice is time-saving, however, critical components of the disease including aging may be difficult to study in such models.
Data from these APP transgenic mice as well as human AD cases suggest that Aβ oligomers, not plaques, may trigger clinical phenotypes. To further understand the pathogenic relationship between Aβ oligomers and plaques in AD, several transgenic mouse models overexpressing APP harboring the mutation in the gluta-mate residue at position 22 have been generated. Mutations at this amino acid residue affect conformational changes of Aβ and modify fibrogenesis. A transgenic mouse overexpressing Arctic APP (E693G) mutation significantly increases Aβ plaque formation and reduces Aβ oligomer levels [61]. The cognitive impairments, however, correlate well with the brain oligomer levels, but not plaque burden, in this mouse. In addition, a recently generated APP transgenic mouse model provides even more convincing evidence to a key role of oligomeric Aβ in the pathogenesis of AD and cognitive deficits. E693, mutation of APP (or E22, Aβ) overexpressed mouse exhibits increased accumulation of soluble Aβ oligomers in neurons without formation of extracellular Aβ plaques throughout its lifespan [62]. The synaptic loss and increased levels of phospho-tau in mossy fibers correlate with the accumulation of Aβ oligomers at 8 months of age in this mouse, yet interestingly, gliosis and neuronal loss at CA3 hippocampal region are detected at much later age (18-24 months). These findings raise a hypothesis that Aβ oligomers, not plaques, are major species to trigger neurotoxicity and cognitive deficits in AD, which has been supported from various studies in humans [63, 64]. However, additional studies are necessary to identify which oligomer species are more pathogenic in AD.
The localization of Aβ oligomers also plays an important aspect of the disease progression in AD. In vitro studies indicate that oligomer species may be generated and accumulate intracellularly in synapses [8, 65]. Several transgenic mouse models are found to accumulate Aβ species intracellularly, such as the APP-Swe/London/PS1M146L transgenic mice [66]. Moreover, a transgenic mouse model overexpressing the N-terminal truncated and pyroglutamate modified Aβ42 (Aβ3(pE)-42, TBA2 transgenic model) that results in robust intraneuronal accumulation of Aβ3(pE)-42 without extracellular Aβ plaques, exhibits a massive Purkinje cell loss suggesting a critical neurotoxic effect of intracellularly accumulated Aβ species [67]. Although other neurological changes commonly observed in AD are absent in this mouse model, these findings may reveal an important part of the neurodegenerative process in AD as it has been reported that AD brains contain high levels of Aβ3(pE)-42 [68]. Further studies, however, will be needed to examine the conformational states of Aβ3(pE)-42 in neurons in vivo as it has been shown to have a higher aggregation propensity and stability than full-length Aβ[69-71].
PRESENILIN TRANSGENIC MICE
Mutations in presenilins also cause FAD and nearly 200 mutations have been identified so far. Presenilins are components of -secretase complex along with nicastrin, presenilin enhancer 2 (PEN2) and anterior pharynx-defective 1 (APH1), and contain active proteolytic domains. Mutations lead to a shift of γ-cleavage in APP and produce more amyloidogenic Aβ42 peptides, which increase the ratio Aβ42/Aβ40 in the brain. Mutant PS1 or PS2 transgenic mice have been generated, and the pathological consequences studied. In contrast to APP transgenic mice, presenilin transgenic mice do not develop plaque pathology in the brain. This is likely a result of the Aβ sequence differences between mice and humans, that greatly diminish the tendency of Aβ to aggregate [37]. This issue was solved by introducing human APP into presenilin transgenic mice by crossing APP and presenilin transgenic lines, resulting in a double transgenic mouse with robust generation of Aβ42 and formation of Aβ plaques at much earlier ages than parental APP mice [72]. Interestingly, the ability of mutant presenilins to elevate Aβ42 levels is unrelated with their steady-state levels [73, 74]. These mice are now widely studied for amyloid pathology and for evaluation of anti-amyloid strategies. Single presenilin mutant mice, however, also exhibit some aspects of AD pathology without aberrant accumulation of Aβ in the brain. For example, several PS1 mutant mice exhibit a sign of age-dependent neurodegeneration in the CA1 subregion and synaptic loss in the stratum radiatum area of hippocampus [75-77]. Although no plaques are detected, a significant increase in intracellular accumulation of Aβ42, but not Aβ40, is also observed in PS1 (L286V) transgenic mice at 17-24 months of age [75]. In addition, mutant PS1 appears to facilitate NFT formation via activation of GSK-3β in neurons [78]. PS1 (I213T) knock-in mouse exhibits phospho-tau deposits in hippocampus as early as 7 months of age and later develops Congo red-, thioflavin T-, and various phospho- and conformational specific-tau antibodies-positive insoluble tau deposits in CA3 neurons at 14-16 months of age without apparent Aβ accumulation throughout [78]. This observation is in part supported by clinical evidence from individuals who possess this unique PS1 mutation and develop frontotemporal dementia (FTD)/Pick's disease with aberrant tau deposits but no Aβ pathology [79-84].
Knocking out PS1, but not PS2, is embryonic lethal in mice [85-87]. On the other hand, conditional knockout of PS1 in postnatal does not increase mortality, suggesting that PS1 plays a critical physiological role during the development [88]. This is further supported by the fact that expression of PS1 is higher during the development and that PS1 is critical for Notch signaling [89]. Conditional knockout of PS1 and/or PS2 lead to synaptic loss, deficits in long-term potentiation (LTP) and cognitive impairments [88, 90]. These pathophysiological changes are mediated by the reduction of CBP/CREB-mediated transcription and decreased levels of CREB target genes such as c-fos and brain-derived neurotrophic factor (BDNF). Interestingly, it has also been shown that presenilin mutations have a significant impact on calcium homeostasis mediated by ER, which may result in increased susceptibility to excitotoxicity in these mice [91].
TAU TRANSGENIC MICE
Increased phosphorylation of tau under pathological conditions results in dissociation of tau from microtubules and formation of tau inclusions or NFTs in dendrites, cell bodies and axons. As mentioned above, tau inclusions are not unique to AD but also found in other tauopathies. Subset of tauopathies are caused by mutations in the MAPT gene, which encodes tau, and nearly 40 mutations have been identified so far [92]. In AD, however, unlike APP, no genetic mutation has been found in the MAPT gene, suggesting that tau pathology in AD may be a downstream of APP/Aβ pathology. The lack of a genetic mutation in the MAPT gene in AD made it difficult to take a transgenic approach to generate a relevant mouse model Table 2. Transgenic mice overexpressing human wildtype tau have been generated by several laboratories and exhibited age-dependent changes [93-97]. For example, Gotz and colleagues generated a transgenic mouse overexpressing the longest form of human tau (tau40, 2N4R) under the control of Thy-1 promoter and observed accumulation of PHF-1-positive phospho-tau in somatodendritic compartments by 3 months of age [95]. However, no NFT-like pathology was detected. Similarly, transgenic mice over-expressing the shortest form of human tau (0N3R) developed phospho-tau deposits in somatodendritic compartments but no NFT-like structures were observed even at 19 months of age [93]. On the other hand, another transgenic mouse model overexpressing 0N3R tau developed straight filamentous structures in spinal cord and brain stem by 6 months of age [96]. Although axonal degeneration was also observed in one of these models, subsequent neurodegeneration or NFT formation in neurons was not clearly detected in wildtype tau overexpressing mice, suggesting a limitation of tau transgenic mice as a disease model.
Table 2.
Tau Transgenic Mouse Models
| Transgenic Mouse | Transgene | Promoter | Strain | Pathology |
|---|---|---|---|---|
| Gotz et al. (1995) ALZ17 | Human wildtype tau40 (2N4R) | Human Thy-1 | B6D2F1 × B6D2F1 (back-crossed to C57BL/6) | PHF1, AT8 positive phospho-tau at 3 months No Gallyas or Thioflavin-S positive deposits |
| Brion et al. (1999) | Human wildtype tau (0N3R) | HMGCR | C57BL/6J × CBA-F2 | AT180, PHF1, AT270 positive phospho-tau deposits in somatodendritic compartments at 6 months No NFTs |
| Duff et al. (2000) 5d, 8c lines | PAC human wildtype tau | Tau promoter | Swiss Webster × B6D2F1 | Express all 6 isoforms Tau distribution to neurites and synapses No pathology up to 8 months AT8, PHF1 positive tau |
| Lewis et al. (2000) JNPL3 | Human tau (P301L) (0N4R) | Mouse PrP | C57BL/DBA2/SW | AT8, AT180 positive tau and NFT-like pathology (Congo-red, Thioflavin-S, Gallyas, Bielschowsky, Bodian positive) in spinal cord Neuronal loss and gliosis in anterior horn Behavior deficits at 4.5 (homozygous) or 6.5 (hemizygous) months |
| Gotz et al. (2001) | Human tau40 (P301L) (2N4R) | Mouse Thy-1.2 | B6D2F1 × B6D2F1 (back-crossed to C57BL/6) | Abnormal filaments (straight or twisted structure, ~15 nm wide) at 8 months Phospho-tau (Thioflavin-S, Gallyas positive) in cortex, brain stem and spinal cord at 3 months Somatodendritic tau accumulation in CA1, amygdala, spinal cord and cortex by 3 months Activated astrocytes in cortex and amygdala, but not in hippocampus |
This difficulty was partially resolved by using the FTDP-17 tau mutations. These mutations in tau have been reported to cause similar neuropathology to AD – a formation of NFT-like phospho-tau accumulation in neurons. Transgenic mouse models harboring FTDP-17-associated tau mutations develop robust NFT-like tau pathology, and they have been used to investigate the tau-associated neuropathological changes in AD. The first model was generated by Lewis and colleagues using tau (P301L), the most common mutation in FTDP-17, under the control of mouse prion promoter [98]. This transgenic mouse, called JNPL3, shows tau-containing NFTs in an age-dependent manner and develops motor deficits and loss of motor neurons in spinal cord as early as 10 months of age. The NFTs are morphologically heterogeneous, ranging from flame- or globose-shaped NFTs to Pick bodies and irregular and dense cytoplasmic inclusions. Another transgenic mouse model with the same tau mutation (P301L) driven by mouse Thy1.2 promoter was also reported shortly after and develops NFT-like filamentous tau accumulations in neurons as early as 8 months of age [99]. The generation of the tau transgenic mice harboring various FTDP-17 mutations also exhibit NFT-like pathology, neuronal and synaptic loss and cognitive impairments in an age-dependent manner [100-104]. NFTs appear to be very resistant to degradation once formed inside neurons, yet they may not be a primary toxic mediator to trigger neuronal loss. For example, an inducible mutant tau model, rTg4510, was used to demonstrate that suppression of the mutant tau (P301L) transgene after the formation of NFTs effectively rescues cognitive function and halts neuronal loss, yet NFT formation is not attenuated [15, 105] Table 3. These findings suggest that other forms of tau, such as soluble tau oligomers or truncated species, may subsequently trigger neuronal loss and cognitive impairments [106].
Table 3.
Inducible Transgenic Mouse Models
| Transgenic Mouse | Transgene | Promoter | Strain | Pathology |
|---|---|---|---|---|
| SantaCruz et al. (2005) rTg4510 | Human tau (P301L) (0N4R) | CaMKIIa (tTA) TRE (tau) | 129S6 × FVB/N | Massive neuronal loss and forebrain atrophy by 5-6 months Phospho-tau deposits by 2.5 months NFT-like pathology at 4 months (cortex) to 5.5 months (hippocampus) Cognitive and motor impairments by 4 months Cognitive impairments but not NFT formation is rescued following transgene suppression |
| Jankowsky et al. (2005) | Mouse/human chimeric APP695 (Swedish/Indiana) | CaMKIIa (tTA) TRE (APP) | C57BL/6J × C3HeJF1 | Aβ plaques in hippocampus Neuritic and glial pathology These pathologies are unchanged following transgene suppression |
Together, mutant tau transgenic mice provide important evidence that NFT-like neuropathology can form in absence of amyloid pathology. However, the formation of NFT-like neuropathology in mutant tau transgenic mice is significantly accelerated following the addition of Aβ fibrils [107]. Therefore, it is critical to investigate the molecular interaction between Aβ and tau in order to fully understand the pathogenesis of AD.
TRANSGENIC MOUSE MODELS DEVELOPING BOTH AB AND TAU NEUROPATHOLOGIES
There have been numerous attempts to create a mouse model that develops more comprehensive phenotypic changes of AD - Aβ plaques and tau tangles. As described above, single expression of mutant APP or tau gene in mice does not robustly trigger the other pathology, making it an incomplete animal model of AD. To overcome this issue, the first mouse model that successfully exhibits both hallmark pathologies in AD was generated by crossing the two independent transgenic lines, Tg2576 and JNPL3 mice [108] Table 4. Interestingly, while Aβ plaque pathology was developed in the same manner as the parental Tg2576 mice, the development of NFTs was significantly accelerated and increased in AD-related brain regions (olfactory cortex, entorhinal cortex and amygdala) versus the parental JNPL3 mice [108]. The double transgenic mice also develop NFTs in areas, such as subiculum and hippocampus, where the tangle pathology was rarely detected in JNPL3 mice, suggesting that Aβ potentiates tau pathology. The same phenomena was observed by crossing JNPL3 mice with another APP transgenic mouse model, APP23, showing that the resulting double transgenic mice develop exacerbated tau pathology in areas with high Aβ plaques [109]. Similarly, Boutajangout and colleagues generated a transgenic mouse harboring only FAD-associated mutations, APP751 (Swedish/London) and PS1 (M146L), together with over-expression of human wildtype tau by crossing the three independent lines [110]. Although Aβ plaque pathology develops as early as 3-4 months of age, and phospho-tau deposits are associated with these Aβ plaques, these mice do not develop NFTs even at 18 months of age. Collectively, these findings indicate that mice, in general, may be more resistant to the development of these pathologies and/or additional factors may be required.
Table 4.
Transgenic Mouse Models with Plaques and Tangles
| Transgenic Mouse | Transgene | Promoter | Strain | Pathology |
|---|---|---|---|---|
| Lewis et al. (2001) | Tg2576 × JNPL3 | Hamster PrP (APP) Mouse PrP (tau) |
AP pathology is comparable with Tg2576 Accelerated tau pathology |
|
| Oddo et al. (2003) 3xTg-AD | Human APP695 (Swedish) Human tau (P301L, 0N4R) PS1 (M146V) |
Mouse Thy-1.2 (APP and tau) Knock-in (PS1) |
C57BL/6 × 129SvJ | Intracellular amyloid deposits at 4-6 months Aβ plaque pathology at 9-12 months Thioflavin-S, Gallyas positive NFT-like pathology at 12 months Gliosis and activated microglia around plaques Behavior deficits at 6 months |
| Boutajungout et al. (2004) | Human wildtype tau (0N3R) Human APP751 (Swedish/London) PS1 (M146V) |
HMGCR (tau and PS1) Thy-1 (APP) |
Aβ plaques at 3-4 months Dystrophic neurites associated with plaques Phospho-tau deposits associated with plaques No NFT pathology up to 18 months |
|
| Bolmont et al. (2007) | APP23 × JNPL3 | Human Thy-1 (APP) Mouse PrP (tau) |
Accelated tau pathology around plaques |
These mouse models clearly showed that Aβ and tau interplay during the development and progression of the AD. Another model that develop both Aβ and tau pathologies was generated using a more aggressive approach. The 3xTg-AD mouse model possesses three disease mutant genes, two of which are FAD mutations, Swedish mutations (K670N/M671L) on APP695 and PS1M146V, and one mutation is associated with FTDP-17, tau (P301L) [111]. Both APP and tau transgenes are overexpressed in forebrain region under the control of Thy1.2 promoter, and these transgenes were comicroinjected in a single cell embryo from the PS1M146V knock-in mouse. The 3xTg-AD mouse develops an age-dependent Aβ and tau pathologies, with intracellular Aβ accumulation at 6 months of age and Aβ plaques at 9-12 months of age, while the accumulation of phospho-tau and NFT-like formation at later ages [111]. Other hallmark pathologies including astrogliosis, activated microglia, loss of synapses and neurodegeneration are also detected [112-115]. The 3xTg-AD mice also show age- and pathology-dependent cognitive decline that makes the model suitable to assess the relationship between neuropathology and cognition [116].
The 3xTg-AD mouse model helps to elucidate molecular interplay between Aβ and tau as modulating Aβ42 levels directly influences tau pathology [117, 118]. Mechanistically, the ubiquitination of tau by carboxyl terminus Hsc70-interacting protein (CHIP) and subsequent degradation by proteasome are impaired by accumulation of Aβ [118-120]. Furthermore, Aβ oligomers, but not monomers, target the proteasome, thereby facilitating tau accumulation [121]. These findings supports the amyloid cascade hypothesis and implies the impairment of ubiquitin proteasome mechanisms in pathogenesis of AD. In AD brain, Uch-L1, an enzyme responsible for ubiquitin recycling, is downregulated [122], and increasing expression of Uch-L1 rescues synaptic and cognitive deficits in the APP/PS1 mouse model [123, 124].
APOE X APP MICE
In addition to transgenic mouse models with FAD mutations, numbers of other mouse models have been generated to elucidate the pathogenic mechanisms of AD. Mouse models with human ApoE are among these models. ApoE gene is a strong risk factor for a late-onset AD. In humans, 3 isoforms exist; ε2, ε3, and ε4, and they are different by two amino acids at positions 112 and 158 [125]. It is well documented that the possession of ε4 allele (apoE4) dose-dependently increases the risk of developing AD [126]. PDAPP transgenic mice with apoE knockout show a significant and apoE gene dosage-dependent reduction of Aβ deposits and other pathological changes including astrogliosis and microglial activation, while behavioral tasks are either unaffected or even impaired in these mice [127-129]. The generation of transgenic mice possessing human apoE isoforms provided a better understanding in the pathogenic role of apoE in AD. The introduction of apoE3 or apoE4 in APP transgenic mice show that the presence of apoE4 results in significant increase of Aβ deposits particularly in the molecular layer of dentate gyrus compared to apoE3 [130]. These findings are further confirmed by different transgenic mouse models [131-136], whose revealed underlying mechanisms may in part explain the genetic risk of apoE4 and AD in humans. In addition, recent studies using these mouse models have further identified a differential function of apoE isoforms on inflammatory responses, which modulate AD pathology [137, 138].
The importance of the lipidation state of apoE and its modula-tory role in Aβ pathology was also discovered using these transgenic mouse models. ABCA1 (ATP-binding cassette A1) transfers cellular cholesterol and phospholipids to lipid-poor apolipoproteins, including apoE, for distribution of cholesterols and lipids in the body. APP transgenic mice knockout for ABCA1 accumulate more insoluble, lipid-poor apoE, though the overall apoE levels are reduced, and more Aβ plaques than the parental APP transgenic mice [139-141]. These lipid-poor apoE are also found to be colocalized with plaques, suggesting that they may facilitate the formation and maturation of Aβ plaques. From these findings, it is speculated that increase in lipidation state of apoE or increased ABCA1 activity may facilitate Aβ clearance (or reduce Aβ deposits), and this approach could be a potential therapeutic strategy for AD [137, 140, 142-144].
NEUROINFLAMMATION AND AD MOUSE MODELS
Neuroinflammation is another, yet not less, important pathological hallmark in AD. Epidemiologically, the use of anti-inflammatory drugs (NSAIDs) has been suggested to lower the risk for AD [4, 145, 146]. Genetic studies suggest that certain polymorphisms in inflammatory-related genes (IL-1α, IL-1β, IL-6, etc) are found more frequently among AD patients though it is still under debate [147]. In addition, pro-inflammatory cytokines (e.g. IL-6, IL-1β) are elevated in earlier stages and may even precede by years the formation of plaques and other neuropathological lesions in AD and Down syndrome [148, 149]. Therefore, the role of inflammation in AD has recently received more attention, and various transgenic mouse models have helped to understand the mechanistic basis of inflammation in AD. Here we will review some of the recent advancements in this area.
APP transgenic mice exhibit robust astrogliosis and microglial activation particularly around Aβ plaques. Acute activation of microglia in these mice has been reported to clear Aβ plaques, yet chronic inflammatory responses exacerbate Aβ pathology in APP-Swe transgenic mice [150]. Soon after, we and others have reported that tau pathology is directly modulated by chronic inflammatory responses in the brain using both the 3xTg-AD mice and mutant tau transgenic mice [104, 114]. To further understand underlying mechanisms and identify key molecules, APP or tau transgenic mice were crossed with other transgenic mice.
APP transgenic mice crossed with CD11-thimidine kinase (TK) mice reveals that resident microglia is not critically involved in plaque formation or clearance [151]. On the other hand, the administration of macrophagy colony stimulating factor (M-CSF) to APP-Swe/PS1 transgenic mice significantly increased bone marrow-derived macrophages and cleared Aβ plaques [152]. Together, these studies suggest a differential role of subpopulation of micro-glia/macrophages in AD pathogenesis.
Not ablating the entire population of microglia, but just eliminating one receptor significantly modulates AD pathology in mice. CX3CR1, a fractalkine receptor only found in microglia in CNS appears to be important to AD pathology, as crossing CX3CR1 knockout mice with 3xTg-AD mice results in reduced neuronal loss [113, 153]. On the other hand, a significant activation of p38 MAPK and increased tau phosphorylation and aggregation in neurons is observed after crossing CX3CR1 knockout mice with tau transgenic mice [154]. This microglia-neuron interaction is found to be mediated by IL-1, confirming previous findings [104, 114]. Interestingly, the effect of CX3CR1 knockout on Aβ pathology is opposite as APP transgenic mice crossed with the CX3CR1 knockout mice ameliorate Aβ plaque pathology [155]. It is implicated that CX3CR1 deficiency and alteration of cytokine profiles promote alternative activation of microglia and reduce Aβ plaques in the brain. The similar effects have been reported in several APP transgenic mouse models with overproduction of pro-inflammatory cytokines, such as IL-1β, IL-6 or IFNγ[156-158].
MOUSE MODEL WITH ACCELERATED SENESCENCE AND AD
These transgenic mouse models of AD described above are still incomplete models of the disease in several aspects. First, none of these mouse models develops progressive cognitive deficits, pathological hallmarks of AD and neuronal degeneration to the same extent with human cases. Second, all mouse models harbor FAD-associated mutant genes, which would be suitable models for FAD, but not sporadic AD, which accounts for over 95% of all cases in humans. There is a growing attention to the investigation of risk factors for AD and the understanding of the pathogenic mechanisms that underlie these factors. Mouse models with human APOE gene in part elucidate this key aspect of the disease. However, the strongest risk factors for AD is still aging, and this is a challenging component to study in animal models. Many of transgenic mouse models of AD, particularly those with mutant APP overexpression, develop Aβ pathology in much earlier ages, making it difficult to study the aging component in the disease progression. Interestingly, overexpression of human wildtype APP in mice does not lead to development of Aβ plaques even after 2 years of life [40]. However, in APP/PS1 transgenic mouse model, aging significantly influences the activation status of microglia, suggesting that it may exacerbate the progression of AD-related neuropathology [159]. To better study aging and AD using mouse models, it has been suggested that the use of a mouse model with accelerated senescence may be helpful.
The senescence-accelerated prone mouse (SAMP) model was generated by phenotypic selection of AKR/J strain [160-162]. Among 14 SAMP strains, SAMP8 mice exhibit progressive synaptic loss and develop deficits in learning and memory as early as 4 months of age [163, 164]. Several studies demonstrated that the expression of APP and the production of Aβ peptides in the brain of SAMP8 mice are markedly increased with age and in comparison with its control strain, SAMR1 (senescence-accelerated resistant mouse) [163, 165]. In particular, a recent study demonstrates that SAMP8 mice develop an age-dependent accumulation of Aβ deposits in the hippocampus as early as 6 months of age [166]. Injection of anti-Aβ antibodies intraventricularly to SAMP8 mice rescued impaired cognitions [167, 168]. SAMP8 mice also accumulate phospho-tau, and aberrant cdk5 activation may be responsible for the tau phosphorylation in these mice [169].
Despite the limitations, the examples above highlight the importance of the combination of various transgenic mouse models for the study of the pathological mechanisms involved in AD.
LIMITATIONS ON TRANSGENIC MOUSE MODELS OF AD
Although mouse models have provided invaluable data toward understanding the pathogenesis of AD and evaluating numerous potential therapeutic strategies, there are some fundamental discrepancies between human AD and mouse models. Therefore, the use of transgenic mouse models and interpretation of data obtained from them should be carefully analyzed. Neuropathologically, on top of discrepancies described above, Aβ plaques observed in mouse model may be physically different from senile plaques in AD as evidenced by the lack of binding to Pittsburg Compound B (PIB) and much less dense core formation [170]. Mouse models, in addition, require high levels of APP expression (at least 8-fold over the endogenous levels) in order to develop Aβ pathology in the brain as opposed to human cases in which an increase of only 50% in APP levels is sufficient to develop AD-like neuropathology in Down syndrome [171]. These differences may be due to the time required to develop the pathology. In humans, it takes at least from a few decades (in the case of Down's syndrome) to 50 years or more (in the case of AD) to form and mature Aβ plaques, while transgenic mice are designed to develop plaques within a year or less because (i) the average lifespan for mice is 2-3 years, and (ii) the cost to house and age mice is high. Exclusion of the aging component may also impact other neuropathological discrepancies observed between humans and mice including tau pathology and neuronal loss.
The mouse strain background may also affect neuropathological and behavioral outcomes. Hsiao et al. first reported that the APP overexpression (wildtype or mutant) in FVB/N background significantly shortened lifespan [44], whereas other backgrounds did not seem to be affected [41]. We have recently tested whether the strain background determines some of the neuropathological features. FVB/N strain is known to be more susceptible to excitotoxicity and exhibit extensive neuronal death compared to C57BL/6 strain [172, 173]. In order to test whether this difference accounts for a resistance against neuronal loss in transgenic mice, the background strain of the 3xTg-AD mice (C57BL/6 × 129SvJ) was changed to FVB/N by backcrossing for 10 generations. Interestingly, both Aβ and tau pathologies were significantly reduced compared to the original background, suggesting that background strain influences the pathology (unpublished observations).
The selection of transgene promoters also needs to be carefully considered in each study. Most transgenic mouse models of AD use heterologous, brain specific promoters, such as prion, calmodulin kinase II (CaMKII), Thy1.2, and PDGF, to drive the transgene expression predominantly in areas relevant to AD. Therefore, the temporal and spatial patterns of the transgene expression are likely different from those in human AD cases. In this regard, many of these mice may not be suitable tools for researches investigating risk factors whose mechanisms are related to the modulation of expression of AD-associated genes.
The advantages of using mouse models over other species include better cost performance, easiness to generate transgenic mice, easiness to handle, the development of pathology in relatively short time (within 1-2 years), and similarity to human CNS anatomy. Recently, the advancement of transgenic technology has allowed the generation of transgenic rat models more readily [174]. These new models will be of high benefit as rats have larger tissue volume and are a better model for testing cognition. Higher vertebrate species, such as dogs [175], and non-human primates [176, 177], have also been used to study AD.
TRANSGENIC MOUSE MODELS AND DRUG DISCOVERY
Transgenic mouse models of AD have been extensively utilized in studies to evaluate potential therapeutic strategies and candidate drugs. Any pre-clinical data from animal models would be useful for improving these therapies, however the data obtained need to be used cautiously. Although AD is characterized as plaques and tangles, and all mouse models are predominantly focused in these two pathological hallmarks, patients with clinically diagnosed dementia or even AD often have mixed pathological signatures [178-181]. In other words, patients with pure AD, as represented in most mouse models, are relatively rare cases. In this regard, potential therapies tested in mouse models of AD may not be as effective as expected from pre-clinical studies.
Only a few drugs are currently available for the management of AD, namely acetylcholine esterase (AChE) inhibitors and the NMDA receptor antagonist. These drugs, however, are mainly symptomatic and ameliorate memory impairments by modulating neurotransmitter signals. The mechanism-based and disease-modifying drugs and therapeutic strategies are highly needed. Here, we will review several mechanism-based therapeutic approaches in AD, and how transgenic mouse models contribute to the advancement of drug discovery.
TARGETING Aβ PATHOLOGY
Immunotherapy against Aβ, either by active or passive immunization, has been well established as an effective method to prevent Aβ aggregation. Early studies where PDAPP mice were actively immunized against Aβ found a significant reduction in Aβ aggregation in both young, pre-pathological mice, as well as in aged mice with established pathology [182-184]. Further studies in PDAPP mice found that passive delivery of Aβ antibodies to the periphery is also effective in reducing soluble and insoluble Aβ, and in Tg2576 mice this strategy led to a reversal in cognitive deficits [185, 186].
Studies from our lab provided insight into the effect of immunotherapy on both Aβ and tau pathology in the 3xTg-AD model. Administration of Aβ antibodies into the hippocampus lead to reductions in soluble and insoluble Aβ, as well as phosphorylated tau [117]. This finding supports the amyloid cascade hypothesis and suggests that targeting Aβ alone may be sufficient to affect multiple aspects of AD pathology. Interestingly, hyperphosphorylated tau was unaffected by immunotherapy, indicating that pathology may become more resistant to antibody-mediated clearance as the disease progresses. In a subsequent study, we demonstrated that the clearance of soluble Aβ alone was insufficient to improve cognition in 3xTg-AD mice [187]. Concomitant reduction in soluble Aβ and tau was necessary to rescue memory impairments, thus highlighting the utility of a mouse that models both Aβ and tau pathology.
These findings in transgenic mice have resulted in numerous clinical trials of Aβ immunotherapy. One of the earlier and more well-known trials was of a synthetic Aβ42 peptide called AN1792, developed by Elan Pharmaceuticals and Wyeth. Although the phase 2 clinical trial was halted early due to development of meningoencephalitis in about 6% of patients that received the vaccine, a follow-up study found that some participants with high antibody titers had a significantly reduced rate of cognitive decline [188]. Neuro-pathological examination of many participants indicated a reduction of Aβ and tau load relative to controls [189-191]. However, some of patients responded well with the vaccine treatment and had virtually complete plaque removal still exhibited severe end-stage dementia at a time of death [189]. These findings suggest certain beneficial and disease-modifying effects of Aβ vaccination, yet more detail studies will be required to confirm its therapeutic effects and safety. Currently, safer Aβ vaccination approaches, such as DNA vaccination and oral vaccination are being tested in animal models [192].
These vaccination strategies clear Aβ possibly via an activation of opsonization and phagocytosis by immune cells. Although at enodogenous levels, resident microglia may not significantly contribute to the plaque formation and clearance [151], once they are properly activated, microglia cells may effectively remove Aβ plaques in the brain. Studies from acute LPS treatment in APP transgenic mice and recent evidence that chronically upregulated pro-inflammatory cytokines in the brain markedly reduce Aβ plaques possibly through mechanisms involved in microglial activation support an ability of biological clearance of Aβ [155-158 193-195]. These results promise an alternative and safer strategy to target Aβ pathology.
SECRETASE INHIBITORS
BACE1 (β-secretase) and γ-secretase are obvious therapeutic targets for AD as they are responsible for generating Aβ peptides from APP. BACE1 is considered a rate-limiting enzyme for Aβ generation [196]. In AD, the steady-state levels and activity of BACE1 have been reported to be upregulated [197]. The genetic deletion of BACE1 in mice is a model that offers valuable information on the physiological importance of this enzyme. The BACE1 knockout mouse has been reported to be viable and does not develop any significant adverse signs and phenotypes [198-201], although recent studies reveal that it exhibits a mild schizophrenia-like behavior and seizures [202 203]. Despite these mild neuropsychiatric phenotypes, crossing APP transgenic mouse with BACE1 knockout mouse still rescues AD-associated cognitive deficits and attenuates Aβ pathology and neuronal loss [199 204 205], suggesting that the inhibition of BACE1 activity would still be an attractive therapeutic strategy for AD. Unfortunately, not many in vivo studies evaluating BACE1 inhibitors have been published. The efficacy of peptide-based BACE1 inhibitors has been tested in Tg2576 mice with promising results, but the bioavailability, stability and blood-brain barrier (BBB) penetration need to be further improved [206]. To overcome these issues, non-peptide-based drugs have been designed and screened in vitro and in vivo using several APP transgenic mouse models [207 208]. In particular, a recent study by Fukumoto and colleagues reported more comprehensive effects of a promising non-peptidic BACE1 inhibitor on Tg2576 mouse model. Chronic oral administration of TAK-070 to Tg2576 mice resulted in approximately 15-30% reduction of both soluble and insoluble Aβ and 22% increase in α-secretase cleaved N-terminal APP fragment (sAPPα) levels in the brain as well as rescued cognitive impairments [207]. These data from transgenic mouse models would serve as important pre-clinical data to support future clinical trials of candidate drugs.
The γ-secretase complex is another potential target to block the production of Aβ. However, unlike BACE1, gene deletion mouse models reveal that the γ-secretase activity plays critical roles during the development as well as in adults as described above. Therefore, inhibition of γ-secretase activity may cause significant adverse effects. It is critical to develop candidate drugs that selectively inhibit γ-secretase while minimizing any toxic effects in the body. To evaluate the safety and therapeutic potential of γ-secretase inhibitors, mouse models of AD have been serving as valuable tools. For example, Tg2576 or PDAPP mice have been used to test various drugs including N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester (DAPT), LY-411575 (Pfizer), MRK-560 (Merck), GSI-953 (Begacestat, Wyeth), and these drugs lower Aβ levels in plasma, CSF and/or brain as well as rescue synaptic function and cognition in mice [209-214]. In addition, some of the compounds exhibit selective inhibition of γ-secretase cleavage on APP over Notch in mice, a feature that may result in decreased adverse or toxic side-effects [213]. Interestingly, recent studies identified novel proteins associated with γ-secretase complex that modulate selective cleavage of APP over Notch [215, 216]. Targeting these proteins to selectively ablate γ-secretase cleavage of APP while sparing Notch will reduce adverse effects. The knock-down of one protein called γ-secretase activating protein (GSAP) in the double transgenic mouse model of AD (APPswe/PS1DE9) significantly reduced Aβ generation and Aβ plaque burden without causing typical adverse signs of the inhibition of Notch signaling, such as intestinal mucosal cell metaplasia or marginal-zone lymphoid depletion in spleen [216]. These findings point out GSAP as a potential drug target for γ-secretase modulation.
A recent study identified a new class of γ-secretase modulator (GSM) that selectively reduces Aβ generation without affecting Notch signaling, γ-cleavage of APP and other γ-secretase substrates [217]. In this study, a candidate GSM, a derivative of 2-amino-thiazole, was screened from over 80,000 compounds, and Tg2576 mice chronically treated with this compound for 7 months exhibited a significant reduction of Aβ40 and Aβ42 production as well as plaque formation while no sign of adverse effects typically observed following treatment with γ-secretase inhibitors. These promising data from transgenic mouse models will certainly help to move these drugs to clinical trials and possibly establish new therapies for AD.
Most of the studies to evaluate β- or γ-secretase inhibitors as drug targets for AD were conducted using relatively young or prepathologic transgenic mice and showed promising reduction of plaque load and improvement in cognition. Nevertheless, additional studies evaluating whether these drugs reverse AD neuropathology and restore cognition in mice with advanced pathology or even in AD patients are still needed. An inducible transgenic mouse of APP reveals that even after 6 months of mutant APP suppression, Aβ plaques that were deposited prior to the APP suppression were not cleared or degraded, implicating that once Aβ plaques are deposited, they are hardly removed from the brain [218]. This study suggests that therapeutic approaches based on reducing Aβ production may only be effective to slow the progression of the disease process, but not reverse the disease.
STEM CELL THERAPY
Stem cell-related research has made remarkable advances in many fields in the last few years. The versatility of embryonic stem cells and inducible pluripotent stem (iPS) offers a vast number of potential applications, and one of them is regenerative or replacement therapy. In neuroscience, this idea is particularly attractive in spinal cord injury, where regeneration or replacement of damaged/dead motor neurons by stem cells could be an ultimate cure. The same approach may be applied in other neurodegenerative disorders, such as AD, Parkinson disease and amyotrophic lateral sclerosis (ALS), although its safety, ethical issues and number of other hurdles need to be clarified prior to the initiation of human trials. Pre-clinically, the therapeutic potential of stem cells has been tested using several transgenic mouse models of AD. In the aged 3xTg-AD mice with fully developed plaques and tangles, intrahippocampal injection of haplotype-matched murine neuronal stem cells restores cognition without attenuating either plaques or tangle pathology [112]. Transplanted neuronal stem cells secrete brain-derived neurotrophic factor (BDNF) and promote synaptogenesis, hence restoring cognition. On the other hand, bone marrow-derived mesenchymal stem cells (BM-MSCs) transplant reduces APP expression and Aβ plaques in the APPswe/PS1(M146V) double transgenic mice [219]. In transplanted mice, increased number of phagocytic microglia is associated with plaques, suggesting that BM-MSCs promote alternative activation of microglia, which then degrade Aβ plaques in the brain.
These studies elegantly demonstrated that transplanted stem cells support existing neurons or monocytes by secreting trophic factors, however it was not clear whether neuronal replacement contributed any significant improvement of brain function. At least, our study revealed that transplanted neuronal stem cells differentiated mostly into astrocytes (approximately 40%), while only ~6% of stem cells differentiated into neurons [112]. These differentiated neurons exhibit pyramidal neuronal morphology and dendritic spines, suggesting that they may be capable of forming connections with existing neurons, although the low percentage of neuronal differentiation suggests that it is unlikely that neuronal replacement is involved in the behavioral recovery. One approach to increase neuronal differentiation has been proposed by Marutle and colleagues [220] using APP23 transgenic mice. Combined with the APP-lowering drug (+)-phenserine, transplantation of human neuronal stem cells into young APP23 mice resulted in a marked reduction of glial differentiation (up to 40% from saline-treated mice), while the neuronal differentiation was significantly increased (as much as 2-fold increase) particularly in CA1 and CA2 regions of hippocampus and in motor and sensory cortices, with no changes in dentate gyrus [220]. Whether those newly replaced neurons successfully integrate existing neuronal network and exhibit functional activity remain to be further investigated.
TAU THERAPY
Although Aβ immunotherapy dramatically reduced Aβ plaque burden and rescued cognitive function in various transgenic mouse models of AD, the clinical trials failed not only to show its safety, but also to rescue or improve cognitive function despite clearance of Aβ plaques in the brain [189, 221]. These findings suggest that targeting Aβ plaques may provide limited therapeutic outcomes. NFTs, on the other hand, are not unique to AD but also observed in other tauopathies. In AD, cognitive impairments correlate with NFT/CSF tau, and in transgenic mouse models, neuronal death has also been positively associated with NFT formation. Therefore, targeting tau pathology may be an alternative approach for AD as well as other tauopathies.
Tau immunotherapy has been tested in several transgenic mouse models [222, 223]. Injection of phospho-tau immunogens in tau mice successfully generated antibodies against phospho-tau and significantly reduced phospho-tau-bearing neurons in the brain without any obvious adverse side effects. P301L tau transgenic mice are known to develop extensive tau pathology in spinal cord and subsequent motor impairment. Importantly, the administration of phospho-tau immunogen to these mice significantly improved motor function, whereas cognition was unaffected [222].
The search for small chemical compounds targeting tau pathology has been extensive, and several candidates have been identified, yet in vivo studies evaluating the therapeutic efficacy of these compounds have not been documented so far [224, 225]. The therapeutic potential of minocycline has been tested in several transgenic mouse models [226-228]. Minocycline suppresses caspase-3 activation via inhibiting cytochrome c release from mitochondria and subsequently reduces generation of truncated tau and tau aggregation in human tau transgenic mice [226]. However, in the 3xTg-AD mouse model, four months of minocycline treatment did not attenuate tau pathology [227]. Its safety and mechanisms of action should be further investigated and clarified in different mouse models prior to moving forward to clinical trials as it shows some adverse effects in ALS patients [229].
Overall, none of the potential therapeutic strategies tested in mouse models with highly promising outcomes have as of yet been successfully translated to AD patients.
CONCLUDING REMARKS
It has been less than two decades since the mutations of AD were identified and the first transgenic mouse model of AD was generated. Many mouse models of AD have been generated since then in order to examine specific disease processes, and many models have also been used to evaluate potential therapeutic targets and strategies. Although mouse models do not fully recapitulate the human disease course of AD, they have significantly contributed to understanding the disease mechanisms and the development of potential therapies. Currently, over 50 compounds are being tested in clinical trials for AD, and many of them have been screened and identified based on their safety and efficacy in transgenic mouse models. Many more compounds and strategies are being evaluated pre-clinically, and it is hoped that such studies will help to identify approaches that can effectively treat AD in the near future.
ACKNOWLEDGEMENT
We thank Dr. Mathew Blurton-Jones for his professional comments on this review.
REFERENCES
- 1.Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–7. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol 2003. 60:1119–22. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 3.Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362:329–44. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 4.Patterson C, Feightner JW, Garcia A, Hsiung GY, MacKnight C, Sadovnick AD. Diagnosis and treatment of dementia: 1. Risk assessment and primary prevention of Alzheimer disease. Cmaj. 2008;178:548–56. doi: 10.1503/cmaj.070796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selkoe DJ, Schenk D. Alzheimer's disease: molecular understanding predicts amyloid-based therapeutics. Annu Rev Pharmacol Toxicol. 2003;43:545–84. doi: 10.1146/annurev.pharmtox.43.100901.140248. [DOI] [PubMed] [Google Scholar]
- 6.Glabe CG. Structural classification of toxic amyloid oligomers. J Biol Chem. 2008;283:29639–43. doi: 10.1074/jbc.R800016200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lublin AL, Gandy S. Amyloid-beta oligomers: possible roles as key neurotoxins in Alzheimer's Disease. Mt Sinai J Med. 2010;77:43–9. doi: 10.1002/msj.20160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walsh DM, Tseng BP, Rydel RE, Podlisny MB, Selkoe DJ. The oligomerization of amyloid beta-protein begins intracellularly in cells derived from human brain. Biochemistry. 2000;39:10831–9. doi: 10.1021/bi001048s. [DOI] [PubMed] [Google Scholar]
- 9.Tomiyama T, Nagata T, Shimada H, et al. A new amyloid beta variant favoring oligomerization in Alzheimer's-type dementia. Ann Neurol. 2008;63:377–87. doi: 10.1002/ana.21321. [DOI] [PubMed] [Google Scholar]
- 10.Aizenstein HJ, Nebes RD, Saxton JA, et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol. 2008;65:1509–17. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mintun MA, Larossa GN, Sheline YI, et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–52. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- 12.Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW. A protein factor essential for microtubule assembly. Proc Natl Acad Sci USA. 1975;72:1858–62. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nat Rev Neurosci. 2007;8:663–72. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- 14.Le Corre S, Klafki HW, Plesnila N, et al. An inhibitor of tau hyperphosphorylation prevents severe motor impairments in tau transgenic mice. Proc Natl Acad Sci USA. 2006;103:9673–8. doi: 10.1073/pnas.0602913103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santacruz K, Lewis J, Spires T, et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309:476–81. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guillozet-Bongaarts AL, Garcia-Sierra F, Reynolds MR, et al. Tau truncation during neurofibrillary tangle evolution in Alzheimer's disease. Neurobiol Aging. 2005;26:1015–22. doi: 10.1016/j.neurobiolaging.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Rissman RA, Poon WW, Blurton-Jones M, et al. Caspase-cleavage of tau is an early event in Alzheimer disease tangle pathology. J Clin Invest. 2004;114:121–30. doi: 10.1172/JCI20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung CW, Song YH, Kim IK, et al. Proapoptotic effects of tau cleavage product generated by caspase-3. Neurobiol Dis. 2001;8:162–72. doi: 10.1006/nbdi.2000.0335. [DOI] [PubMed] [Google Scholar]
- 19.Cribbs DH, Poon WW, Rissman RA, Blurton-Jones M. Caspase-mediated degeneration in Alzheimer's disease. Am J Pathol. 2004;165:353–5. doi: 10.1016/S0002-9440(10)63302-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberson ED, Scearce-Levie K, Palop JJ, et al. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer's disease mouse model. Science. 2007;316:750–4. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- 21.Ittner LM, Ke YD, Delerue F, et al. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer's disease mouse models. Cell. 2010;142:387–97. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 22.DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer's disease: correlation with cognitive severity. Ann Neurol. 1990;27:457–64. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- 23.Masliah E, Mallory M, Alford M, et al. Altered expression of synaptic proteins occurs early during progression of Alzheimer's disease. Neurology. 2001;56:127–9. doi: 10.1212/wnl.56.1.127. [DOI] [PubMed] [Google Scholar]
- 24.Scheff SW, Price DA, Sparks DL. Quantitative assessment of possible age-related change in synaptic numbers in the human frontal cortex. Neurobiol Aging. 2001;22:355–65. doi: 10.1016/s0197-4580(01)00222-6. [DOI] [PubMed] [Google Scholar]
- 25.Mullan M, Crawford F, Axelman K, et al. A pathogenic mutation for probable Alzheimer's disease in the APP gene at the N-terminus of beta-amyloid. Nat Genet. 1992;1:345–7. doi: 10.1038/ng0892-345. [DOI] [PubMed] [Google Scholar]
- 26.Grabowski TJ, Cho HS, Vonsattel JP, Rebeck GW, Greenberg SM. Novel amyloid precursor protein mutation in an Iowa family with dementia and severe cerebral amyloid angiopathy. Ann Neurol. 2001;49:697–705. doi: 10.1002/ana.1009. [DOI] [PubMed] [Google Scholar]
- 27.Hendriks L, van Duijn CM, Cras P, et al. Presenile dementia and cerebral haemorrhage linked to a mutation at codon 692 of the beta-amyloid precursor protein gene. Nat Genet. 1992;1:218–21. doi: 10.1038/ng0692-218. [DOI] [PubMed] [Google Scholar]
- 28.Levy E, Carman MD, Fernandez-Madrid IJ, et al. Mutation of the Alzheimer's disease amyloid gene in hereditary cerebral hemorrhage, Dutch type. Science. 1990;248:1124–6. doi: 10.1126/science.2111584. [DOI] [PubMed] [Google Scholar]
- 29.Nilsberth C, Westlind-Danielsson A, Eckman CB, et al. The ‘Arctic’ APP mutation (E693G) causes Alzheimer's disease by enhanced Abeta protofibril formation. Nat Neurosci. 2001;4:887–93. doi: 10.1038/nn0901-887. [DOI] [PubMed] [Google Scholar]
- 30.Chartier-Harlin MC, Crawford F, Houlden H, et al. Early-onset alzheimer's disease caused by mutations at codon 717 of the beta-amyloid precursor protein gene. Nature. 1991;353:844–6. doi: 10.1038/353844a0. [DOI] [PubMed] [Google Scholar]
- 31.Goate A, Chartier-Harlin MC, Mullan M, et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature. 1991;349:704–6. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- 32.Murrell J, Farlow M, Ghetti B, Benson MD. A mutation in the amyloid precursor protein associated with hereditary Alzheimer's disease. Science. 1991;254:97–9. doi: 10.1126/science.1925564. [DOI] [PubMed] [Google Scholar]
- 33.Murrell JR, Hake AM, Quaid KA, Farlow MR, Ghetti B. Early-onset alzheimer disease caused by a new mutation (V717L) in the amyloid precursor protein gene. Arch Neurol. 2000;57:885–7. doi: 10.1001/archneur.57.6.885. [DOI] [PubMed] [Google Scholar]
- 34.Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer's disease. Trends Pharmacol Sci. 1991;12:383–8. doi: 10.1016/0165-6147(91)90609-v. [DOI] [PubMed] [Google Scholar]
- 35.Glenner GG, Wong CW. Alzheimer's disease and Down's syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984;122:1131–5. doi: 10.1016/0006-291x(84)91209-9. [DOI] [PubMed] [Google Scholar]
- 36.Rovelet-Lecrux A, Hannequin D, Raux G, et al. APP locus duplication causes autosomal dominant early-onset alzheimer disease with cerebral amyloid angiopathy. Nat Genet. 2006;38:24–6. doi: 10.1038/ng1718. [DOI] [PubMed] [Google Scholar]
- 37.Dyrks T, Dyrks E, Masters CL, Beyreuther K. Amyloidogenicity of rodent and human beta A4 sequences. FEBS Lett. 1993;324:231–6. doi: 10.1016/0014-5793(93)81399-k. [DOI] [PubMed] [Google Scholar]
- 38.Buxbaum JD, Christensen JL, Ruefli AA, Greengard P, Loring JF. Expression of APP in brains of transgenic mice containing the entire human APP gene. Biochem Biophys Res Commun. 1993;197:639–45. doi: 10.1006/bbrc.1993.2527. [DOI] [PubMed] [Google Scholar]
- 39.Lamb BT, Sisodia SS, Lawler AM, et al. Introduction and expression of the 400 kilobase amyloid precursor protein gene in transgenic mice [corrected]. Nat Genet. 1993;5:22–30. doi: 10.1038/ng0993-22. [DOI] [PubMed] [Google Scholar]
- 40.Mucke L, Masliah E, Yu GQ, et al. High-level neuronal expression of abeta 1-42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20:4050–8. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Games D, Adams D, Alessandrini R, et al. Alzheimer-type neuro-pathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature. 1995;373:523–7. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- 42.Hsiao K, Chapman P, Nilsen S, et al. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 43.Sturchler-Pierrat C, Abramowski D, Duke M, et al. Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc Natl Acad Sci USA. 1997;94:13287–92. doi: 10.1073/pnas.94.24.13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hsiao KK, Borchelt DR, Olson K, et al. Age-related CNS disorder and early death in transgenic FVB/N mice overexpressing Alzheimer amyloid precursor proteins. Neuron. 1995;15:1203–18. doi: 10.1016/0896-6273(95)90107-8. [DOI] [PubMed] [Google Scholar]
- 45.Lesne S, Koh MT, Kotilinek L, et al. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–7. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- 46.Irizarry MC, McNamara M, Fedorchak K, Hsiao K, Hyman BT. APPSw transgenic mice develop age-related A beta deposits and neuropil abnormalities, but no neuronal loss in CA1. J Neuropathol Exp Neurol. 1997;56:965–73. doi: 10.1097/00005072-199709000-00002. [DOI] [PubMed] [Google Scholar]
- 47.Dong H, Martin MV, Chambers S, Csernansky JG. Spatial relationship between synapse loss and beta-amyloid deposition in Tg2576 mice. J Comp Neurol. 2007;500:311–21. doi: 10.1002/cne.21176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spires-Jones TL, Meyer-Luehmann M, Osetek JD, et al. Impaired spine stability underlies plaque-related spine loss in an Alzheimer's disease mouse model. Am J Pathol. 2007;171:1304–11. doi: 10.2353/ajpath.2007.070055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bornemann KD, Wiederhold KH, Pauli C, et al. Abeta-induced inflammatory processes in microglia cells of APP23 transgenic mice. Am J Pathol. 2001;158:63–73. doi: 10.1016/s0002-9440(10)63945-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stalder M, Phinney A, Probst A, Sommer B, Staufenbiel M, Jucker M. Association of microglia with amyloid plaques in brains of APP23 transgenic mice. Am J Pathol. 1999;154:1673–84. doi: 10.1016/S0002-9440(10)65423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Calhoun ME, Wiederhold KH, Abramowski D, et al. Neuron loss in APP transgenic mice. Nature. 1998;395:755–6. doi: 10.1038/27351. [DOI] [PubMed] [Google Scholar]
- 52.Rockenstein EM, McConlogue L, Tan H, Power M, Masliah E, Mucke L. Levels and alternative splicing of amyloid beta protein precursor (APP) transcripts in brains of APP transgenic mice and humans with Alzheimer's disease. J Biol Chem. 1995;270:28257–67. doi: 10.1074/jbc.270.47.28257. [DOI] [PubMed] [Google Scholar]
- 53.Irizarry MC, Soriano F, McNamara M, et al. Abeta deposition is associated with neuropil changes, but not with overt neuronal loss in the human amyloid precursor protein V717F (PDAPP) transgenic mouse. J Neurosci. 1997;17:7053–9. doi: 10.1523/JNEUROSCI.17-18-07053.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Redwine JM, Kosofsky B, Jacobs RE, et al. Dentate gyrus volume is reduced before onset of plaque formation in PDAPP mice: a magnetic resonance microscopy and stereologic analysis. Proc Natl Acad Sci USA. 2003;100:1381–6. doi: 10.1073/pnas.242746599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu CC, Chawla F, Games D, et al. Selective vulnerability of den-tate granule cells prior to amyloid deposition in PDAPP mice: digital morphometric analyses. Proc Natl Acad Sci USA. 2004;101:7141–6. doi: 10.1073/pnas.0402147101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hsia AY, Masliah E, McConlogue L, et al. Plaque-independent disruption of neural circuits in Alzheimer's disease mouse models. Proc Natl Acad Sci USA. 1999;96:3228–33. doi: 10.1073/pnas.96.6.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chishti MA, Yang DS, Janus C, et al. Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J Biol Chem. 2001;276:21562–70. doi: 10.1074/jbc.M100710200. [DOI] [PubMed] [Google Scholar]
- 58.Harris JA, Devidze N, Halabisky B, et al. Many neuronal and behavioral impairments in transgenic mouse models of Alzheimer's disease are independent of caspase cleavage of the amyloid precursor protein. J Neurosci. 2010;30:372–81. doi: 10.1523/JNEUROSCI.5341-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dudal S, Krzywkowski P, Paquette J, et al. Inflammation occurs early during the Abeta deposition process in TgCRND8 mice. Neurobiol Aging. 2004;25:861–71. doi: 10.1016/j.neurobiolaging.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 60.Bellucci A, Luccarini I, Scali C, et al. Cholinergic dysfunction, neuronal damage and axonal loss in TgCRND8 mice. Neurobiol Dis. 2006;23:260–72. doi: 10.1016/j.nbd.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 61.Cheng IH, Scearce-Levie K, Legleiter J, et al. Accelerating amyloid-beta fibrillization reduces oligomer levels and functional deficits in Alzheimer disease mouse models. J Biol Chem. 2007;282:23818–28. doi: 10.1074/jbc.M701078200. [DOI] [PubMed] [Google Scholar]
- 62.Tomiyama T, Matsuyama S, Iso H, et al. A mouse model of amyloid beta oligomers: their contribution to synaptic alteration, abnormal tau phosphorylation, glial activation, and neuronal loss in vivo. J Neurosci. 2010;30:4845–56. doi: 10.1523/JNEUROSCI.5825-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McLean CA, Cherny RA, Fraser FW, et al. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer's disease. Ann Neurol. 1999;46:860–6. doi: 10.1002/1531-8249(199912)46:6<860::aid-ana8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 64.Naslund J, Haroutunian V, Mohs R, et al. Correlation between elevated levels of amyloid beta-peptide in the brain and cognitive decline. Jama. 2000;283:1571–7. doi: 10.1001/jama.283.12.1571. [DOI] [PubMed] [Google Scholar]
- 65.Takahashi RH, Almeida CG, Kearney PF, et al. Oligomerization of Alzheimer's beta-amyloid within processes and synapses of cultured neurons and brain. J Neurosci. 2004;24:3592–9. doi: 10.1523/JNEUROSCI.5167-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blanchard V, Moussaoui S, Czech C, et al. Time sequence of maturation of dystrophic neurites associated with Abeta deposits in APP/PS1 transgenic mice. Exp Neurol. 2003;184:247–63. doi: 10.1016/s0014-4886(03)00252-8. [DOI] [PubMed] [Google Scholar]
- 67.Wirths O, Breyhan H, Cynis H, Schilling S, Demuth HU, Bayer TA. Intraneuronal pyroglutamate-Abeta 3-42 triggers neurodegeneration and lethal neurological deficits in a transgenic mouse model. Acta Neuropathol. 2009;118:487–96. doi: 10.1007/s00401-009-0557-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harigaya Y, Saido TC, Eckman CB, Prada CM, Shoji M, Younkin SG. Amyloid beta protein starting pyroglutamate at position 3 is a major component of the amyloid deposits in the Alzheimer's disease brain. Biochem Biophys Res Commun. 2000;276:422–7. doi: 10.1006/bbrc.2000.3490. [DOI] [PubMed] [Google Scholar]
- 69.He W, Barrow CJ. The A beta 3-pyroglutamyl and 11-pyroglutamyl peptides found in senile plaque have greater beta-sheet forming and aggregation propensities in vitro than full-length A beta. Biochemistry. 1999;38:10871–7. doi: 10.1021/bi990563r. [DOI] [PubMed] [Google Scholar]
- 70.Kuo YM, Webster S, Emmerling MR, De Lima N, Roher AE. Irreversible dimerization/tetramerization and post-translational modifications inhibit proteolytic degradation of A beta peptides of Alzheimer's disease. Biochim Biophys Acta. 1998;1406:291–8. doi: 10.1016/s0925-4439(98)00014-3. [DOI] [PubMed] [Google Scholar]
- 71.Schilling S, Lauber T, Schaupp M, et al. On the seeding and oligomerization of pGlu-amyloid peptides (in vitro). Biochemistry. 2006;45:12393–9. doi: 10.1021/bi0612667. [DOI] [PubMed] [Google Scholar]
- 72.Borchelt DR, Ratovitski T, van Lare J, et al. Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Neuron. 1997;19:939–45. doi: 10.1016/s0896-6273(00)80974-5. [DOI] [PubMed] [Google Scholar]
- 73.Duff K, Eckman C, Zehr C, et al. Increased amyloid-beta42(43) in brains of mice expressing mutant presenilin 1. Nature. 1996;383:710–3. doi: 10.1038/383710a0. [DOI] [PubMed] [Google Scholar]
- 74.Wen PH, Hof PR, Chen X, et al. The presenilin-1 familial Alzheimer disease mutant P117L impairs neurogenesis in the hippocampus of adult mice. Exp Neurol. 2004;188:224–37. doi: 10.1016/j.expneurol.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 75.Chui DH, Tanahashi H, Ozawa K, et al. Transgenic mice with Alzheimer presenilin 1 mutations show accelerated neurodegeneration without amyloid plaque formation. Nat Med. 1999;5:560–4. doi: 10.1038/8438. [DOI] [PubMed] [Google Scholar]
- 76.Rutten BP, Van der Kolk NM, Schafer S, et al. Age-related loss of synaptophysin immunoreactive presynaptic boutons within the hippocampus of APP751SL, PS1M146L, and APP751SL/PS1M146L transgenic mice. Am J Pathol. 2005;167:161–73. doi: 10.1016/S0002-9440(10)62963-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sadowski M, Pankiewicz J, Scholtzova H, et al. Amyloid-beta deposition is associated with decreased hippocampal glucose metabolism and spatial memory impairment in APP/PS1 mice. J Neuropathol Exp Neurol. 2004;63:418–28. doi: 10.1093/jnen/63.5.418. [DOI] [PubMed] [Google Scholar]
- 78.Tanemura K, Chui DH, Fukuda T, et al. Formation of tau inclusions in knock-in mice with familial Alzheimer disease (FAD) mutation of presenilin 1 (PS1). J Biol Chem. 2006;281:5037–41. doi: 10.1074/jbc.M509145200. [DOI] [PubMed] [Google Scholar]
- 79.Amtul Z, Lewis PA, Piper S, et al. A presenilin 1 mutation associated with familial frontotemporal dementia inhibits gamma-secretase cleavage of APP and notch. Neurobiol Dis. 2002;9:269–73. doi: 10.1006/nbdi.2001.0473. [DOI] [PubMed] [Google Scholar]
- 80.Dermaut B, Kumar-Singh S, Engelborghs S, et al. A novel presenilin 1 mutation associated with Pick's disease but not beta-amyloid plaques. Ann Neurol. 2004;55:617–26. doi: 10.1002/ana.20083. [DOI] [PubMed] [Google Scholar]
- 81.Evin G, Smith MJ, Tziotis A, et al. Alternative transcripts of presenilin-1 associated with frontotemporal dementia. Neuroreport. 2002;13:917–21. doi: 10.1097/00001756-200205070-00036. [DOI] [PubMed] [Google Scholar]
- 82.Gomez-Isla T, Growdon WB, McNamara MJ, et al. The impact of different presenilin 1 andpresenilin 2 mutations on amyloid deposition, neurofibrillary changes and neuronal loss in the familial Alzheimer's disease brain: evidence for other phenotype-modifying factors. Brain. 1999;122(Pt 9):1709–19. doi: 10.1093/brain/122.9.1709. [DOI] [PubMed] [Google Scholar]
- 83.Tang-Wai D, Lewis P, Boeve B, et al. Familial frontotemporal dementia associated with a novel presenilin-1 mutation. Dement Geriatr Cogn Disord. 2002;14:13–21. doi: 10.1159/000058328. [DOI] [PubMed] [Google Scholar]
- 84.Yoshiyama Y, Lee VM, Trojanowski JQ. Frontotemporal dementia and tauopathy. Curr Neurol Neurosci Rep. 2001;1:413–21. doi: 10.1007/s11910-001-0100-0. [DOI] [PubMed] [Google Scholar]
- 85.Herreman A, Hartmann D, Annaert W, et al. Presenilin 2 deficiency causes a mild pulmonary phenotype and no changes in amyloid precursor protein processing but enhances the embryonic lethal phenotype of presenilin 1 deficiency. Proc Natl Acad Sci USA. 1999;96:11872–7. doi: 10.1073/pnas.96.21.11872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shen J, Bronson RT, Chen DF, Xia W, Selkoe DJ, Tonegawa S. Skeletal and CNS defects in Presenilin-1-deficient mice. Cell. 1997;89:629–39. doi: 10.1016/s0092-8674(00)80244-5. [DOI] [PubMed] [Google Scholar]
- 87.Wong PC, Zheng H, Chen H, et al. Presenilin 1 is required for Notch1 and DII1 expression in the paraxial mesoderm. Nature. 1997;387:288–92. doi: 10.1038/387288a0. [DOI] [PubMed] [Google Scholar]
- 88.Yu H, Saura CA, Choi SY, et al. APP processing and synaptic plasticity in presenilin-1 conditional knockout mice. Neuron. 2001;31:713–26. doi: 10.1016/s0896-6273(01)00417-2. [DOI] [PubMed] [Google Scholar]
- 89.Lee MK, Slunt HH, Martin LJ, et al. Expression of presenilin 1 and 2 (PS1 and PS2) in human and murine tissues. J Neurosci. 1996;16:7513–25. doi: 10.1523/JNEUROSCI.16-23-07513.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Saura CA, Choi SY, Beglopoulos V, et al. Loss of presenilin function causes impairments of memory and synaptic plasticity followed by age-dependent neurodegeneration. Neuron. 2004;42:23–36. doi: 10.1016/s0896-6273(04)00182-5. [DOI] [PubMed] [Google Scholar]
- 91.Leissring MA, Akbari Y, Fanger CM, Cahalan MD, Mattson MP, LaFerla FM. Capacitative calcium entry deficits and elevated luminal calcium content in mutant presenilin-1 knockin mice. J Cell Biol. 2000;149:793–8. doi: 10.1083/jcb.149.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gotz J, Deters N, Doldissen A, et al. A decade of tau transgenic animal models and beyond. Brain Pathol. 2007;17:91–103. doi: 10.1111/j.1750-3639.2007.00051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brion JP, Tremp G, Octave JN. Transgenic expression of the shortest human tau affects its compartmentalization and its phosphorylation as in the pretangle stage of Alzheimer's disease. Am J Pathol. 1999;154:255–70. doi: 10.1016/S0002-9440(10)65272-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Duff K, Knight H, Refolo LM, et al. Characterization of pathology in transgenic mice over-expressing human genomic and cDNA tau transgenes. Neurobiol Dis. 2000;7:87–98. doi: 10.1006/nbdi.1999.0279. [DOI] [PubMed] [Google Scholar]
- 95.Gotz J, Probst A, Spillantini MG, et al. Somatodendritic localization and hyperphosphorylation of tau protein in transgenic mice expressing the longest human brain tau isoform. Embo J. 1995;14:1304–13. doi: 10.1002/j.1460-2075.1995.tb07116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ishihara T, Hong M, Zhang B, et al. Age-dependent emergence and progression of a tauopathy in transgenic mice overexpressing the shortest human tau isoform. Neuron. 1999;24:751–62. doi: 10.1016/s0896-6273(00)81127-7. [DOI] [PubMed] [Google Scholar]
- 97.Spittaels K, Van den Haute C, Van Dorpe J, et al. Prominent axonopathy in the brain and spinal cord of transgenic mice overexpressing four-repeat human tau protein. Am J Pathol. 1999;155:2153–65. doi: 10.1016/S0002-9440(10)65533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lewis J, McGowan E, Rockwood J, et al. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet. 2000;25:402–5. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- 99.Gotz J, Chen F, Barmettler R, Nitsch RM. Tau filament formation in transgenic mice expressing P301L tau. J Biol Chem. 2001;276:529–34. doi: 10.1074/jbc.M006531200. [DOI] [PubMed] [Google Scholar]
- 100.Sahara N, Lewis J, DeTure M, et al. Assembly of tau in transgenic animals expressing P301L tau: alteration of phosphorylation and solubility. J Neurochem. 2002;83:1498–508. doi: 10.1046/j.1471-4159.2002.01241.x. [DOI] [PubMed] [Google Scholar]
- 101.Tanemura K, Akagi T, Murayama M, et al. Formation of filamentous tau aggregations in transgenic mice expressing V337M human tau. Neurobiol Dis. 2001;8:1036–45. doi: 10.1006/nbdi.2001.0439. [DOI] [PubMed] [Google Scholar]
- 102.Tanemura K, Murayama M, Akagi T, et al. Neurodegeneration with tau accumulation in a transgenic mouse expressing V337M human tau. J Neurosci. 2002;22:133–41. doi: 10.1523/JNEUROSCI.22-01-00133.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tatebayashi Y, Miyasaka T, Chui DH, et al. Tau filament formation and associative memory deficit in aged mice expressing mutant (R406W) human tau. Proc Natl Acad Sci USA. 2002;99:13896–901. doi: 10.1073/pnas.202205599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yoshiyama Y, Higuchi M, Zhang B, et al. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53:337–51. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 105.Ramsden M, Kotilinek L, Forster C, et al. Age-dependent neurofibrillary tangle formation, neuron loss, and memory impairment in a mouse model of human tauopathy (P301L). J Neurosci. 2005;25:10637–47. doi: 10.1523/JNEUROSCI.3279-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.de Calignon A, Fox LM, Pitstick R, et al. Caspase activation precedes and leads to tangles. Nature. 2010;464:1201–4. doi: 10.1038/nature08890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gotz J, Chen F, van Dorpe J, Nitsch RM. Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Abeta 42 fibrils. Science. 2001;293:1491–5. doi: 10.1126/science.1062097. [DOI] [PubMed] [Google Scholar]
- 108.Lewis J, Dickson DW, Lin WL, et al. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293:1487–91. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- 109.Bolmont T, Clavaguera F, Meyer-Luehmann M, et al. Induction of tau pathology by intracerebral infusion of amyloid-beta -containing brain extract and by amyloid-beta deposition in APP × Tau transgenic mice. Am J Pathol. 2007;171:2012–20. doi: 10.2353/ajpath.2007.070403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Boutajangout A, Authelet M, Blanchard V, et al. Characterisation of cytoskeletal abnormalities in mice transgenic for wild-type human tau and familial Alzheimer's disease mutants of APP and presenilin-1. Neurobiol Dis. 2004;15:47–60. doi: 10.1016/j.nbd.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 111.Oddo S, Caccamo A, Shepherd JD, et al. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–21. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 112.Blurton-Jones M, Kitazawa M, Martinez-Coria H, et al. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci USA. 2009;106:13594–9. doi: 10.1073/pnas.0901402106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fuhrmann M, Bittner T, Jung CK, et al. Microglial Cx3cr1 knockout prevents neuron loss in a mouse model of Alzheimer's disease. Nat Neurosci. 2010;13:411–3. doi: 10.1038/nn.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kitazawa M, Oddo S, Yamasaki TR, Green KN, LaFerla FM. Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer's disease. J Neurosci. 2005;25:8843–53. doi: 10.1523/JNEUROSCI.2868-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Oddo S, Caccamo A, Kitazawa M, Tseng BP, LaFerla FM. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer's disease. Neurobiol Aging. 2003;24:1063–70. doi: 10.1016/j.neurobiolaging.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 116.Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM. Intraneuronal Abeta causes the onset of early Alzheimer's disease-related cognitive deficits in transgenic mice. Neuron. 2005;45:675–88. doi: 10.1016/j.neuron.2005.01.040. [DOI] [PubMed] [Google Scholar]
- 117.Oddo S, Billings L, Kesslak JP, Cribbs DH, LaFerla FM. Abeta immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron. 2004;43:321–32. doi: 10.1016/j.neuron.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 118.Oddo S, Caccamo A, Tseng B, et al. Blocking Abeta42 accumulation delays the onset and progression of tau pathology via the C terminus of heat shock protein70-interacting protein: a mechanistic link between Abeta and tau pathology. J Neurosci. 2008;28:12163–75. doi: 10.1523/JNEUROSCI.2464-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dickey CA, Kamal A, Lundgren K, et al. The high-affinity HSP90-CHIP complex recognizes and selectively degrades phosphorylated tau client proteins. J Clin Invest. 2007;117:648–58. doi: 10.1172/JCI29715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dickey CA, Yue M, Lin WL, et al. Deletion of the ubiquitin ligase CHIP leads to the accumulation, but not the aggregation, of both endogenous phospho- and caspase-3-cleaved tau species. J Neurosci. 2006;26:6985–96. doi: 10.1523/JNEUROSCI.0746-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tseng BP, Green KN, Chan JL, Blurton-Jones M, LaFerla FM. Abeta inhibits the proteasome and enhances amyloid and tau accumulation. Neurobiol Aging. 2008;29:1607–18. doi: 10.1016/j.neurobiolaging.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.de Vrij FM, Fischer DF, van Leeuwen FW, Hol EM. Protein quality control in Alzheimer's disease by the ubiquitin proteasome system. Prog Neurobiol. 2004;74:249–70. doi: 10.1016/j.pneurobio.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 123.Gong B, Cao Z, Zheng P, et al. Ubiquitin hydrolase Uch-L1 rescues beta-amyloid-induced decreases in synaptic function and contextual memory. Cell. 2006;126:775–88. doi: 10.1016/j.cell.2006.06.046. [DOI] [PubMed] [Google Scholar]
- 124.Smith DL, Pozueta J, Gong B, Arancio O, Shelanski M. Reversal of long-term dendritic spine alterations in Alzheimer disease models. Proc Natl Acad Sci USA. 2009;106:16877–82. doi: 10.1073/pnas.0908706106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fan J, Donkin J, Wellington C. Greasing the wheels of Abeta clearance in Alzheimer's disease: the role of lipids and apolipoprotein E. Biofactors. 2009;35:239–48. doi: 10.1002/biof.37. [DOI] [PubMed] [Google Scholar]
- 126.Bales KR, Dodart JC, DeMattos RB, Holtzman DM, Paul SM. Apolipoprotein E, amyloid, and Alzheimer disease. Mol Interv. 2002;2363-75:339. doi: 10.1124/mi.2.6.363. [DOI] [PubMed] [Google Scholar]
- 127.Bales KR, Verina T, Cummins DJ, et al. Apolipoprotein E is essential for amyloid deposition in the APP(V717F) transgenic mouse model of Alzheimer's disease. Proc Natl Acad Sci USA. 1999;96:15233–8. doi: 10.1073/pnas.96.26.15233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bales KR, Verina T, Dodel RC, et al. Lack of apolipoprotein E dramatically reduces amyloid beta-peptide deposition. Nat Genet. 1997;17:263–4. doi: 10.1038/ng1197-263. [DOI] [PubMed] [Google Scholar]
- 129.Dodart JC, Mathis C, Bales KR, Paul SM, Ungerer A. Behavioral deficits in APP(V717F) transgenic mice deficient for the apolipoprotein E gene. Neuroreport. 2000;11:603–7. doi: 10.1097/00001756-200002280-00034. [DOI] [PubMed] [Google Scholar]
- 130.Holtzman DM, Bales KR, Tenkova T, et al. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer's disease. Proc Natl Acad Sci USA. 2000;97:2892–7. doi: 10.1073/pnas.050004797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Buttini M, Yu GQ, Shockley K, et al. Modulation of Alzheimer-like synaptic and cholinergic deficits in transgenic mice by human apolipoprotein E depends on isoform, aging, and overexpression of amyloid beta peptides but not on plaque formation. J Neurosci. 2002;22:10539–48. doi: 10.1523/JNEUROSCI.22-24-10539.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Carter DB, Dunn E, McKinley DD, et al. Human apolipoprotein E4 accelerates beta-amyloid deposition in APPsw transgenic mouse brain. Ann Neurol. 2001;50:468–75. doi: 10.1002/ana.1134. [DOI] [PubMed] [Google Scholar]
- 133.Dodart JC, Bales KR, Johnstone EM, Little SP, Paul SM. Apolipoprotein E alters the processing of the beta-amyloid precursor protein in APP(V717F) transgenic mice. Brain Res. 2002;955:191–9. doi: 10.1016/s0006-8993(02)03437-6. [DOI] [PubMed] [Google Scholar]
- 134.Fagan AM, Watson M, Parsadanian M, Bales KR, Paul SM, Holtzman DM. Human and murine ApoE markedly alters A beta metabolism before and after plaque formation in a mouse model of Alzheimer's disease. Neurobiol Dis. 2002;9:305–18. doi: 10.1006/nbdi.2002.0483. [DOI] [PubMed] [Google Scholar]
- 135.Fryer JD, Taylor JW, DeMattos RB, et al. Apolipoprotein E markedly facilitates age-dependent cerebral amyloid angiopathy and spontaneous hemorrhage in amyloid precursor protein transgenic mice. J Neurosci. 2003;23:7889–96. doi: 10.1523/JNEUROSCI.23-21-07889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Oddo S, Caccamo A, Cheng D, LaFerla FM. Genetically altering Abeta distribution from the brain to the vasculature ameliorates tau pathology. Brain Pathol. 2009;19:421–30. doi: 10.1111/j.1750-3639.2008.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jiang Q, Lee CY, Mandrekar S, et al. ApoE promotes the proteolytic degradation of Abeta. Neuron. 2008;58:681–93. doi: 10.1016/j.neuron.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhao L, Lin S, Bales KR, et al. Macrophage-mediated degradation of beta-amyloid via an apolipoprotein E isoform-dependent mechanism. J Neurosci. 2009;29:3603–12. doi: 10.1523/JNEUROSCI.5302-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hirsch-Reinshagen V, Maia LF, Burgess BL, et al. The absence of ABCA1 decreases soluble ApoE levels but does not diminish amyloid deposition in two murine models of Alzheimer disease. J Biol Chem. 2005;280:43243–56. doi: 10.1074/jbc.M508781200. [DOI] [PubMed] [Google Scholar]
- 140.Koldamova R, Staufenbiel M, Lefterov I. Lack of ABCA1 considerably decreases brain ApoE level and increases amyloid deposition in APP23 mice. J Biol Chem. 2005;280:43224–35. doi: 10.1074/jbc.M504513200. [DOI] [PubMed] [Google Scholar]
- 141.Wahrle SE, Jiang H, Parsadanian M, et al. Deletion of Abca1 increases Abeta deposition in the PDAPP transgenic mouse model of Alzheimer disease. J Biol Chem. 2005;280:43236–42. doi: 10.1074/jbc.M508780200. [DOI] [PubMed] [Google Scholar]
- 142.Fitz NF, Cronican A, Pham T, et al. Liver X receptor agonist treatment ameliorates amyloid pathology and memory deficits caused by high-fat diet in APP23 mice. J Neurosci. 2010;30:6862–72. doi: 10.1523/JNEUROSCI.1051-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wahrle SE, Jiang H, Parsadanian M, et al. Overexpression of ABCA1 reduces amyloid deposition in the PDAPP mouse model of Alzheimer disease. J Clin Invest. 2008;118:671–82. doi: 10.1172/JCI33622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Donkin JJ, Stukas S, Hirsch-Reinshagen V, et al. ATP-binding cassette transporter A1 mediates the beneficial effects of the liver-X-receptor agonist GW3965 on object recognition memory and amyloid burden in APP/PS1 mice. J Biol Chem. 2010 doi: 10.1074/jbc.M110.108100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Breitner JC, Gau BA, Welsh KA, et al. Inverse association of anti-inflammatory treatments and Alzheimer's disease: initial results of a co-twin control study. Neurology. 1994;44:227–32. doi: 10.1212/wnl.44.2.227. [DOI] [PubMed] [Google Scholar]
- 146.McGeer PL, Schulzer M, McGeer EG. Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer's disease: a review of 17 epidemiologic studies. Neurology. 1996;47:425–32. doi: 10.1212/wnl.47.2.425. [DOI] [PubMed] [Google Scholar]
- 147.Licastro F, Veglia F, Chiappelli M, Grimaldi LM, Masliah E. A polymorphism of the interleukin-1 beta gene at position +3953 influences progression and neuro-pathological hallmarks of Alzheimer's disease. Neurobiol Aging. 2004;25:1017–22. doi: 10.1016/j.neurobiolaging.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 148.Forlenza OV, Diniz BS, Talib LL, et al. Increased serum IL-1beta level in Alzheimer's disease and mild cognitive impairment. Dement Geriatr Cogn Disord. 2009;28:507–12. doi: 10.1159/000255051. [DOI] [PubMed] [Google Scholar]
- 149.Mrak RE, Griffin WS. Trisomy 21 and the brain. J Neuropathol Exp Neurol. 2004;63:679–85. doi: 10.1093/jnen/63.7.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sheng JG, Bora SH, Xu G, Borchelt DR, Price DL, Koliatsos VE. Lipopolysaccharide-induced-neuroinflammation increases intracellular accumulation of amyloid precursor protein and amyloid beta peptide in APPswe transgenic mice. Neurobiol Dis. 2003;14:133–45. doi: 10.1016/s0969-9961(03)00069-x. [DOI] [PubMed] [Google Scholar]
- 151.Grathwohl SA, Kalin RE, Bolmont T, et al. Formation and maintenance of Alzheimer's disease beta-amyloid plaques in the absence of microglia. Nat Neurosci. 2009;12:1361–3. doi: 10.1038/nn.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Boissonneault V, Filali M, Lessard M, Relton J, Wong G, Rivest S. Powerful beneficial effects of macrophage colony-stimulating factor on beta-amyloid deposition and cognitive impairment in Alzheimer's disease. Brain. 2009;132:1078–92. doi: 10.1093/brain/awn331. [DOI] [PubMed] [Google Scholar]
- 153.Cardona AE, Pioro EP, Sasse ME, et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–24. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- 154.Bhaskar K, Konerth M, Kokiko-Cochran ON, Cardona A, Ranso-hoff RM, Lamb BT. Regulation of tau pathology by the microglial fractalkine receptor. Neuron. 2010;68:19–31. doi: 10.1016/j.neuron.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lee S, Varvel NH, Konerth ME, et al. CX3CR1 Deficiency Alters Microglial Activation and Reduces Beta-Amyloid Deposition in Two Alzheimer's Disease Mouse Models. Am J Pathol. 2010 doi: 10.2353/ajpath.2010.100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chakrabarty P, Ceballos-Diaz C, Beccard A, et al. IFN-gamma promotes complement expression and attenuates amyloid plaque deposition in amyloid beta precursor protein transgenic mice. J Immunol. 2010;184:5333–43. doi: 10.4049/jimmunol.0903382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chakrabarty P, Jansen-West K, Beccard A, et al. Massive gliosis induced by interleukin-6 suppresses Abeta deposition in vivo: evidence against inflammation as a driving force for amyloid deposition. Faseb J. 2010;24:548–59. doi: 10.1096/fj.09-141754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shaftel SS, Kyrkanides S, Olschowka JA, Miller JN, Johnson RE, O'Banion MK. Sustained hippocampal IL-1 beta overexpression mediates chronic neuroinflammation and ameliorates Alzheimer plaque pathology. J Clin Invest. 2007;117:1595–604. doi: 10.1172/JCI31450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jimenez S, Baglietto-Vargas D, Caballero C, et al. Inflammatory response in the hippocampus of PS1M146L/APP751SL mouse model of Alzheimer's disease: age-dependent switch in the microglial phenotype from alternative to classic. J Neurosci. 2008;28:11650–61. doi: 10.1523/JNEUROSCI.3024-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hosokawa M, Abe T, Higuchi K, et al. Management and design of the maintenance of SAM mouse strains: an animal model for accelerated senescence and age-associated disorders. Exp Gerontol. 1997;32:111–6. doi: 10.1016/s0531-5565(96)00078-2. [DOI] [PubMed] [Google Scholar]
- 161.Miyamoto M. Characteristics of age-related behavioral changes in senescence-accelerated mouse SAMP8 and SAMP10. Exp Gerontol. 1997;32:139–48. doi: 10.1016/s0531-5565(96)00061-7. [DOI] [PubMed] [Google Scholar]
- 162.Takeda T, Hosokawa M, Takeshita S, et al. A new murine model of accelerated senescence. Mech Ageing Dev. 1981;17:183–94. doi: 10.1016/0047-6374(81)90084-1. [DOI] [PubMed] [Google Scholar]
- 163.Flood JF, Morley JE. Learning and memory in the SAMP8 mouse. Neurosci Biobehav Rev. 1998;22:1–20. doi: 10.1016/s0149-7634(96)00063-2. [DOI] [PubMed] [Google Scholar]
- 164.Sugiyama H, Akiyama H, Akiguchi I, Kameyama M, Takeda T. [Loss of dendritic spines in hippocampal CA1 pyramidal cells in senescence accelerated mouse (SAM)--a quantitative Golgi study]. Rinsho Shinkeigaku. 1987;27:841–5. [PubMed] [Google Scholar]
- 165.Takemura M, Nakamura S, Akiguchi I, et al. Beta/A4 proteinlike immunoreactive granular structures in the brain of senescence-accelerated mouse. Am J Pathol. 1993;142:1887–97. [PMC free article] [PubMed] [Google Scholar]
- 166.Del Valle J, Duran-Vilaregut J, Manich G, et al. Early amyloid accumulation in the hippocampus of SAMP8 mice. J Alzheimers Dis. 2010;19:1303–15. doi: 10.3233/JAD-2010-1321. [DOI] [PubMed] [Google Scholar]
- 167.Morley JE, Farr SA, Flood JF. Antibody to amyloid beta protein alleviates impaired acquisition, retention, and memory processing in SAMP8 mice. Neurobiol Learn Mem. 2002;78:125–38. doi: 10.1006/nlme.2001.4047. [DOI] [PubMed] [Google Scholar]
- 168.Morley JE, Kumar VB, Bernardo AE, et al. Beta-amyloid precursor polypeptide in SAMP8 mice affects learning and memory. Peptides. 2000;21:1761–7. doi: 10.1016/s0196-9781(00)00342-9. [DOI] [PubMed] [Google Scholar]
- 169.Canudas AM, Gutierrez-Cuesta J, Rodriguez MI, et al. Hyperphosphorylation of microtubule-associated protein tau in senescence-accelerated mouse (SAM). Mech Ageing Dev. 2005;126:1300–4. doi: 10.1016/j.mad.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 170.Maeda J, Ji B, Irie T, et al. Longitudinal, quantitative assessment of amyloid, neuroinflammation, and anti-amyloid treatment in a living mouse model of Alzheimer's disease enabled by positron emission tomography. J Neurosci. 2007;27:10957–68. doi: 10.1523/JNEUROSCI.0673-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Duff K, Suleman F. Transgenic mouse models of Alzheimer's disease: how useful have they been for therapeutic development? Brief Funct Genomic Proteomic. 2004;3:47–59. doi: 10.1093/bfgp/3.1.47. [DOI] [PubMed] [Google Scholar]
- 172.Schauwecker PE. Modulation of cell death by mouse genotype: differential vulnerability to excitatory amino acid-induced lesions. Exp Neurol. 2002;178:219–35. doi: 10.1006/exnr.2002.8038. [DOI] [PubMed] [Google Scholar]
- 173.Schauwecker PE. Genetic basis of kainate-induced excitotoxicity in mice: phenotypic modulation of seizure-induced cell death. Epilepsy Res. 2003;55:201–10. doi: 10.1016/s0920-1211(03)00115-3. [DOI] [PubMed] [Google Scholar]
- 174.Leon WC, Canneva F, Partridge V, et al. A novel transgenic rat model with a full Alzheimer's-like amyloid pathology displays pre-plaque intracellular amyloid-beta-associated cognitive impairment. J Alzheimers Dis. 2010;20:113–26. doi: 10.3233/JAD-2010-1349. [DOI] [PubMed] [Google Scholar]
- 175.Ruehl WW, Bruyette DS, DePaoli A, et al. Canine cognitive dys-function as a model for human age-related cognitive decline, dementia and Alzheimer's disease: clinical presentation, cognitive testing, pathology and response to 1-deprenyl therapy. Prog Brain Res. 1995;106:217–25. doi: 10.1016/s0079-6123(08)61218-2. [DOI] [PubMed] [Google Scholar]
- 176.Price DL, Sisodia SS. Cellular and molecular biology of Alzheimer's disease and animal models. Annu Rev Med. 1994;45:435–46. doi: 10.1146/annurev.med.45.1.435. [DOI] [PubMed] [Google Scholar]
- 177.Lemere CA, Beierschmitt A, Iglesias M, et al. Alzheimer's disease abeta vaccine reduces central nervous system abeta levels in a non-human primate, the Caribbean vervet. Am J Pathol. 2004;165:283–97. doi: 10.1016/s0002-9440(10)63296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Langa KM, Foster NL, Larson EB. Mixed dementia: emerging concepts and therapeutic implications. Jama. 2004;292:2901–8. doi: 10.1001/jama.292.23.2901. [DOI] [PubMed] [Google Scholar]
- 179.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 180.Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol. 2009;66:200–8. doi: 10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Snowdon DA. Aging and Alzheimer's disease: lessons from the Nun Study. Gerontologist. 1997;37:150–6. doi: 10.1093/geront/37.2.150. [DOI] [PubMed] [Google Scholar]
- 182.Lemere CA, Maron R, Spooner ET, et al. Nasal A beta treatment induces anti-A beta antibody production and decreases cerebral amyloid burden in PD-APP mice. Ann N Y Acad Sci. 2000;920:328–31. doi: 10.1111/j.1749-6632.2000.tb06943.x. [DOI] [PubMed] [Google Scholar]
- 183.Schenk D, Barbour R, Dunn W, et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–7. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 184.Weiner HL, Lemere CA, Maron R, et al. Nasal administration of amyloid-beta peptide decreases cerebral amyloid burden in a mouse model of Alzheimer's disease. Ann Neurol. 2000;48:567–79. [PubMed] [Google Scholar]
- 185.Bard F, Cannon C, Barbour R, et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–9. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 186.Kotilinek LA, Bacskai B, Westerman M, et al. Reversible memory loss in a mouse transgenic model of Alzheimer's disease. J Neurosci. 2002;22:6331–5. doi: 10.1523/JNEUROSCI.22-15-06331.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Oddo S, Vasilevko V, Caccamo A, Kitazawa M, Cribbs DH, LaFerla FM. Reduction of soluble Abeta and tau, but not soluble Abeta alone, ameliorates cognitive decline in transgenic mice with plaques and tangles. J Biol Chem. 2006;281:39413–23. doi: 10.1074/jbc.M608485200. [DOI] [PubMed] [Google Scholar]
- 188.Vellas B, Black R, Thal LJ, et al. Long-term follow-up of patients immunized with AN1792: reduced functional decline in antibody responders. Curr Alzheimer Res. 2009;6:144–51. doi: 10.2174/156720509787602852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Holmes C, Boche D, Wilkinson D, et al. Long-term effects of Abeta42 immunisation in Alzheimer's disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372:216–23. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- 190.Masliah E, Hansen L, Adame A, et al. Abeta vaccination effects on plaque pathology in the absence of encephalitis in Alzheimer disease. Neurology. 2005;64:129–31. doi: 10.1212/01.WNL.0000148590.39911.DF. [DOI] [PubMed] [Google Scholar]
- 191.Nicoll JA, Barton E, Boche D, et al. Abeta species removal after abeta42 immunization. J Neuropathol Exp Neurol. 2006;65:1040–8. doi: 10.1097/01.jnen.0000240466.10758.ce. [DOI] [PubMed] [Google Scholar]
- 192.Ishii-Katsuno R, Nakajima A, Katsuno T, et al. Reduction of amyloid beta-peptide accumulation in Tg2576 transgenic mice by oral vaccination. Biochem Biophys Res Commun. 2010;399:593–9. doi: 10.1016/j.bbrc.2010.07.120. [DOI] [PubMed] [Google Scholar]
- 193.DiCarlo G, Wilcock D, Henderson D, Gordon M, Morgan D. Intrahippocampal LPS injections reduce Abeta load in APP+PS1 transgenic mice. Neurobiol Aging. 2001;22:1007–12. doi: 10.1016/s0197-4580(01)00292-5. [DOI] [PubMed] [Google Scholar]
- 194.Herber DL, Mercer M, Roth LM, et al. Microglial activation is required for Abeta clearance after intracranial injection of lipopolysaccharide in APP transgenic mice. J Neuroimmune Pharmacol. 2007;2:222–31. doi: 10.1007/s11481-007-9069-z. [DOI] [PubMed] [Google Scholar]
- 195.Herber DL, Roth LM, Wilson D, et al. Time-dependent reduction in Abeta levels after intracranial LPS administration in APP transgenic mice. Exp Neurol. 2004;190:245–53. doi: 10.1016/j.expneurol.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 196.Cole SL, Vassar R. The role of amyloid precursor protein processing by BACE1, the beta-secretase, in Alzheimer disease pathophysiology. J Biol Chem. 2008;283:29621–5. doi: 10.1074/jbc.R800015200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Stockley JH, O'Neill C. The proteins BACE1 and BACE2 and beta-secretase activity in normal and Alzheimer's disease brain. Biochem Soc Trans. 2007;35:574–6. doi: 10.1042/BST0350574. [DOI] [PubMed] [Google Scholar]
- 198.Hu X, Hicks CW, He W, et al. Bace1 modulates myelination in the central and peripheral nervous system. Nat Neurosci. 2006;9:1520–5. doi: 10.1038/nn1797. [DOI] [PubMed] [Google Scholar]
- 199.Luo Y, Bolon B, Kahn S, et al. Mice deficient in BACE1, the Alzheimer's beta-secretase, have normal phenotype and abolished beta-amyloid generation. Nat Neurosci. 2001;4:231–2. doi: 10.1038/85059. [DOI] [PubMed] [Google Scholar]
- 200.Roberds SL, Anderson J, Basi G, et al. BACE knockout mice are healthy despite lacking the primary beta-secretase activity in brain: implications for Alzheimer's disease therapeutics. Hum Mol Genet. 2001;10:1317–24. doi: 10.1093/hmg/10.12.1317. [DOI] [PubMed] [Google Scholar]
- 201.Sankaranarayanan S, Price EA, Wu G, et al. In vivo beta-secretase 1 inhibition leads to brain Abeta lowering and increased alpha-secretase processing of amyloid precursor protein without effect on neuregulin-1. J Pharmacol Exp Ther. 2008;324:957–69. doi: 10.1124/jpet.107.130039. [DOI] [PubMed] [Google Scholar]
- 202.Hu X, Zhou X, He W, et al. BACE1 deficiency causes altered neuronal activity and neurodegeneration. J Neurosci. 2010;30:8819–29. doi: 10.1523/JNEUROSCI.1334-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Savonenko AV, Melnikova T, Laird FM, Stewart KA, Price DL, Wong PC. Alteration of BACE1-dependent NRG1/ErbB4 signaling and schizophrenia-like phenotypes in BACE1-null mice. Proc Natl Acad Sci USA. 2008;105:5585–90. doi: 10.1073/pnas.0710373105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ohno M, Cole SL, Yasvoina M, et al. BACE1 gene deletion prevents neuron loss and memory deficits in 5XFAD APP/PS1 transgenic mice. Neurobiol Dis. 2007;26:134–45. doi: 10.1016/j.nbd.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ohno M, Sametsky EA, Younkin LH, et al. BACE1 deficiency rescues memory deficits and cholinergic dysfunction in a mouse model of Alzheimer's disease. Neuron. 2004;41:27–33. doi: 10.1016/s0896-6273(03)00810-9. [DOI] [PubMed] [Google Scholar]
- 206.Ghosh AK, Gemma S, Tang J. beta-Secretase as a therapeutic target for Alzheimer's disease. Neurotherapeutics. 2008;5:399–408. doi: 10.1016/j.nurt.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Fukumoto H, Takahashi H, Tarui N, et al. A noncompetitive BACE1 inhibitor TAK-070 ameliorates Abeta pathology and behavioral deficits in a mouse model of Alzheimer's disease. J Neurosci. 2010;30:11157–66. doi: 10.1523/JNEUROSCI.2884-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hussain I, Hawkins J, Harrison D, et al. Oral administration of a potent and selective non-peptidic BACE-1 inhibitor decreases beta-cleavage of amyloid precursor protein and amyloid-beta production in vivo. J Neurochem. 2007;100:802–9. doi: 10.1111/j.1471-4159.2006.04260.x. [DOI] [PubMed] [Google Scholar]
- 209.Best JD, Jay MT, Otu F, et al. Quantitative measurement of changes in amyloid-beta(40) in the rat brain and cerebrospinal fluid following treatment with the gamma-secretase inhibitor LY-411575 [N2-[(2S)-2-(3,5-difluorophenyl)-2-hydroxyethanoyl]-N1-[(7S)-5-methyl-6-oxo-6,7-dihydro-5H-dibenzo[b,d]azepin-7-yl]-L-alaninamide]. J Pharmacol Exp Ther. 2005;313:902–8. doi: 10.1124/jpet.104.081174. [DOI] [PubMed] [Google Scholar]
- 210.Dovey HF, John V, Anderson JP, et al. Functional gamma-secretase inhibitors reduce beta-amyloid peptide levels in brain. J Neurochem. 2001;76:173–81. doi: 10.1046/j.1471-4159.2001.00012.x. [DOI] [PubMed] [Google Scholar]
- 211.Lanz TA, Himes CS, Pallante G, et al. The gamma-secretase inhibitor N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester reduces A beta levels in vivo in plasma and cerebrospinal fluid in young (plaque-free) and aged (plaque-bearing) Tg2576 mice. J Pharmacol Exp Ther. 2003;305:864–71. doi: 10.1124/jpet.102.048280. [DOI] [PubMed] [Google Scholar]
- 212.Lanz TA, Hosley JD, Adams WJ, Merchant KM. Studies of Abeta pharmacodynamics in the brain, cerebrospinal fluid, and plasma in young (plaque-free) Tg2576 mice using the gamma-secretase inhibitor N2-[(2S)-2-(3,5-difluorophenyl)-2-hydroxyethanoyl]-N1-[(7S)-5-methyl-6-oxo-6,7-dihydro-5H-dibenzo[b,d]azepin-7-yl]-L-alaninamide (LY-411575). J Pharmacol Exp Ther. 2004;309:49–55. doi: 10.1124/jpet.103.060715. [DOI] [PubMed] [Google Scholar]
- 213.Martone RL, Zhou H, Atchison K, et al. Begacestat (GSI-953): a novel, selective thiophene sulfonamide inhibitor of amyloid precursor protein gamma-secretase for the treatment of Alzheimer's disease. J Pharmacol Exp Ther. 2009;331:598–608. doi: 10.1124/jpet.109.152975. [DOI] [PubMed] [Google Scholar]
- 214.Townsend M, Qu Y, Gray A, et al. Oral treatment with a gamma-secretase inhibitor improves long-term potentiation in a mouse model of Alzheimer's disease. J Pharmacol Exp Ther. 2010;333:110–9. doi: 10.1124/jpet.109.163691. [DOI] [PubMed] [Google Scholar]
- 215.Chen F, Hasegawa H, Schmitt-Ulms G, et al. TMP21 is a presenilin complex component that modulates gamma-secretase but not epsilon-secretase activity. Nature. 2006;440:1208–12. doi: 10.1038/nature04667. [DOI] [PubMed] [Google Scholar]
- 216.He G, Luo W, Li P, et al. Gamma-secretase activating protein is a therapeutic target for Alzheimer's disease. Nature. 2010;467:95–8. doi: 10.1038/nature09325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kounnas MZ, Danks AM, Cheng S, et al. Modulation of gamma-secretase reduces beta-amyloid deposition in a transgenic mouse model of Alzheimer's disease. Neuron. 2010;67:769–80. doi: 10.1016/j.neuron.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Jankowsky JL, Slunt HH, Gonzales V, et al. Persistent amyloidosis following suppression of Abeta production in a transgenic model of Alzheimer disease. PLoS Med. 2005;2:e355. doi: 10.1371/journal.pmed.0020355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Lee JK, Jin HK, Endo S, Schuchman EH, Carter JE, Bae JS. Intracerebral transplantation of bone marrow-derived mesenchymal stem cells reduces amyloid-beta deposition and rescues memory deficits in Alzheimer's disease mice by modulation of immune responses. Stem Cells. 2010;28:329–43. doi: 10.1002/stem.277. [DOI] [PubMed] [Google Scholar]
- 220.Marutle A, Ohmitsu M, Nilbratt M, Greig NH, Nordberg A, Sugaya K. Modulation of human neural stem cell differentiation in Alzheimer (APP23) transgenic mice by phenserine. Proc Natl Acad Sci USA. 2007;104:12506–11. doi: 10.1073/pnas.0705346104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Orgogozo JM, Gilman S, Dartigues JF, et al. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology. 2003;61:46–54. doi: 10.1212/01.wnl.0000073623.84147.a8. [DOI] [PubMed] [Google Scholar]
- 222.Asuni AA, Boutajangout A, Quartermain D, Sigurdsson EM. Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. J Neurosci. 2007;27:9115–29. doi: 10.1523/JNEUROSCI.2361-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Boimel M, Grigoriadis N, Lourbopoulos A, Haber E, Abramsky O, Rosenmann H. Efficacy and safety of immunization with phosphorylated tau against neurofibrillary tangles in mice. Exp Neurol. 2010;224:472–85. doi: 10.1016/j.expneurol.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 224.Bulic B, Pickhardt M, Mandelkow EM, Mandelkow E. Tau protein and tau aggregation inhibitors. Neuropharmacology. 2010;59:276–89. doi: 10.1016/j.neuropharm.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 225.Noble W, Hanger DP, Gallo JM. Transgenic mouse models of tauopathy in drug discovery. CNS Neurol Disord Drug Targets. 2010;9:403–28. doi: 10.2174/187152710791556131. [DOI] [PubMed] [Google Scholar]
- 226.Noble W, Garwood C, Stephenson J, Kinsey AM, Hanger DP, Anderton BH. Minocycline reduces the development of abnormal tau species in models of Alzheimer's disease. Faseb J. 2009;23:739–50. doi: 10.1096/fj.08-113795. [DOI] [PubMed] [Google Scholar]
- 227.Parachikova A, Vasilevko V, Cribbs DH, LaFerla FM, Green KN. Reductions in amyloid-beta-derived neuroinflammation, with minocycline, restore cognition but do not significantly affect tau hyperphosphorylation. J Alzheimers Dis. 2010;21:527–42. doi: 10.3233/JAD-2010-100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Seabrook TJ, Jiang L, Maier M, Lemere CA. Minocycline affects microglia activation, Abeta deposition, and behavior in APP-tg mice. Glia. 2006;53:776–82. doi: 10.1002/glia.20338. [DOI] [PubMed] [Google Scholar]
- 229.Gordon PH, Moore DH, Miller RG, et al. Efficacy of minocycline in patients with amyotrophic lateral sclerosis: a phase III randomised trial. Lancet Neurol. 2007;6:1045–53. doi: 10.1016/S1474-4422(07)70270-3. [DOI] [PubMed] [Google Scholar]