Abstract
Alzheimer’s disease (AD) is a proteinopathy characterized by the accumulation of β-amyloid (Aβ) and tau. To date, clinical trials indicate that Aβ immunotherapy does not improve cognition. Consequently, it is critical to modulate other aspects of AD pathology. As such, tau represents an excellent target, as its accumulation better correlates with cognitive impairment. To determine the effectiveness of targeting pathological tau, with Aβ pathology present, we administered a single injection of AT8, or control antibody, into the hippocampus of aged 3xTg-AD mice. Extensive data indicates that phosphorylated Ser202 and Thr205 sites of tau (corresponding to the AT8 epitope) represent a pathologically relevant target for AD. We report that immunization with AT8 reduced somatodendritic tau load, p-tau immunoreactivity, and silver stained positive neurons, without affecting Aβ pathology. We also discovered that tau pathology soon reemerges post-injection, possibly due to persistent Aβ pathology. These studies provide evidence that targeting p-tau may represent an effective treatment strategy: potentially in conjunction with Aβ immunotherapy.
Keywords: Tau, Phosphorylated tau, Neurofibrillary tangles, Immunotherapy, Beta-amyloid, Alzheimer’s disease
1. Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder that is pathologically characterized by the presence of plaques, neurofibrillary tangles, and sever synaptic and neuronal loss [1]. β-amyloid (Aβ), generated from the amyloid precursor protein (APP), is the primary constituent of plaques, whereas hyperphosphorylation of tau promotes its aggregation into tangles. Overwhelming evidence, generated from over two decades of genetic and molecular research, supports the hypothesis that Aβ accumulation underlies the onset of AD; however, tau is needed to induce cognitive impairment in human APP mice [2]. Furthermore, the identification of mutations in the tau gene (MAPT), associated with frontotemporal dementia (FTD), provides a genetic link between tau, neurodegeneration, and cognitive impairment [3]. Tau’s physiological role is to stabilize microtubules, which is disrupted during the pathogenesis of AD, FTD, and other tauopathies. During the pathogenesis of AD, intraneuronal tau accumulation is first found in the transentorhinal cortex, and later in the hippocampus and neocortex [4]. The mechanisms underlining the spread of tau pathology remain unknown. It has been postulated that tau can diffuse from neuron to neuron in a prion-like manner. Recently, pathological forms of tau have been shown to spread, after direct injected into mice, or from human expressing MAPT neurons to neurons lacking human MAPT expression; leading to subsequent progression of tau pathology [5,6]. If tau is indeed spread through extracellular mechanisms, immunotherapy represents an attractive treatment strategy for AD patients.
The use of immunotherapy to target AD pathology is not a new concept. Over the past decade multiple studies have provided evidence that Aβ immunotherapy improves cognition in transgenic models of AD [7–10]. In contrast, Aβ immunotherapy in humans does not improve cognition, and may only stabilize cognitive decline [11–13]. While active immunization can clear established Aβ pathology in patients, no apparent effect on neurofibrillary tangles is observed [14]. Additionally, analyses of CSF tau levels in patients are inconclusive, as few patients show reduced levels after treatment [15,16]. In this context, the affect on tau pathology elicited by targeting Aβ alone, may not be sufficient to produced significant cognitive improvement in AD patients.
To date, there have been no clinical trials utilizing tau immunotherapy to treat AD. However, preclinical studies utilizing tau immunotherapy have been performed in transgenic models containing mutant human tau [17–19]. These models recapitulate critical neuropathological features of most tauopathies, but lack a critical feature of AD neuropathology; namely Aβ accumulation. We previously found that tau immunization, using an antibody that recognizes all isoforms of normal human tau, does not decrease tau or Aβ pathology [8]. However, it remains unknown whether tau immunization, targeting a pathological species, will effect either Aβ or tau pathology in a model possessing both. Herein, we investigate the impact of p-tau immunotherapy in aged 3xTg-AD mice, which have extensive plaque and tangle pathology. We report the novel findings that a single intrahippocampal injection of AT8, targeting phosphorylated Ser202, and Thr205 residues of tau [20], significantly reduces somatodendritic tau; without affecting Aβ. Our results reveal that targeting pathological tau can reduce tau pathology, in the presence of established Aβ pathology, and hence represents a potential therapeutic target for AD in combination with Aβ immunotherapy.
2. Materials and methods
2.1. Animal model
3xTg-AD mice containing the human mutations for APP-Swedish (KM670/671NL), tau (P301L), and presenilin 1 (M146V), were maintained on a C57BL6/129 background [21]. Male and female mice, from 15 to 18 months old, were used in the study. All procedures were performed in accordance to the guidelines of the University of California, Irvine, Institutional Animal Care and Use Committee.
2.2. Surgical procedure
Mice were anesthetized with isofluorane (Western Medical Supply, Arcadia, CA) and placed into a stereotaxic frame (KOPF Instruments, Tujunga, CA). Body temperature was maintained using an automated thermoregulation system. 2 µl of antibody, containing either 2 µg of AT8 (Thermo Scientific, Rockford, IL), or 4G8 (EMD Millipore, Billerica, MA), was injected into the CA1 subfield of the hippocampus. Equivalent control IgG (EMD Millipore) was administered into the contralateral hemisphere. The following coordinates relative to bregma were used; anteroposterior (A/P); −2.06 mm, dorsoventral (D/V), −1.95 mm; mediolateral (M/L); ±1.75 mm. After surgery, animals were placed into heated cages until the animal was fully recovered.
2.3. Tissue Isolation and Immunohistochemistry
2.3.1. Tissue isolation
Mice were anesthetized with euthasol (Virbac animal health, Fort Worth, TX), and perfused transcardially with phosphate-buffered saline (PBS). Brains were removed and drop-fixed in 4% paraformaldehyde for 48 h, then cryoprotected in 30% sucrose. Brains were then cut into 40 µm coronal sections on a freezing microtome (Leica SM 2010R, Leica, Buffalo, IL). Sections were stored in PBS with 0.02% sodium azide until use.
2.3.2. Histochemistry
Sections were dried on slides and subjected to antigen retrieval using citrate buffer. Sections were then oxidized with 3% H2O2 in PBS for 30 minutes followed by blocking in PBS containing 1% BSA, 0.2 g evaporated milk, 0.3% Triton X-100, then incubated overnight with primary antibodies; AT8, 4G8, 6E10 (Covance, Emeryville, CA), or HT7 (Fisher, Pittsburgh, PA). After washing with PBS + 0.3% Triton X-100, slides were treated with the appropriate secondary antibody (Vector Labs, Burlingame, CA). Immunoreactivity was detected with diaminobenzidine (DAB) substrate, in conjunction with the avidin-biotin horseradish peroxidase system (Vector labs). An average of 3 sections per animal were used. All analysis was done over a region representing 160 µm, obtained between 1.34 and 2.54 mm on the anterior/posterior axes. Images of stained CA1 hippocampal subfield were acquired using an Axiocam digital camera, and Axioskop 50 microscope (Carl Zeiss MicroImaging, Thornwood, NY).
2.4. Statistical analysis
Images were imported into the Scion Image system (NIH) for analysis. Pixel total for stained sections were calculated to determine the percent difference between the treated versus control injected sides. Comparisons between hemispheres utilized students paired t-test. Significance was set at 95% of confidence (p < 0.05) and all values are presented as mean ± SEM.
3. Results
3.1. Intrahippocampal injection of AT8 reduces total tau levels without affecting Aβ load
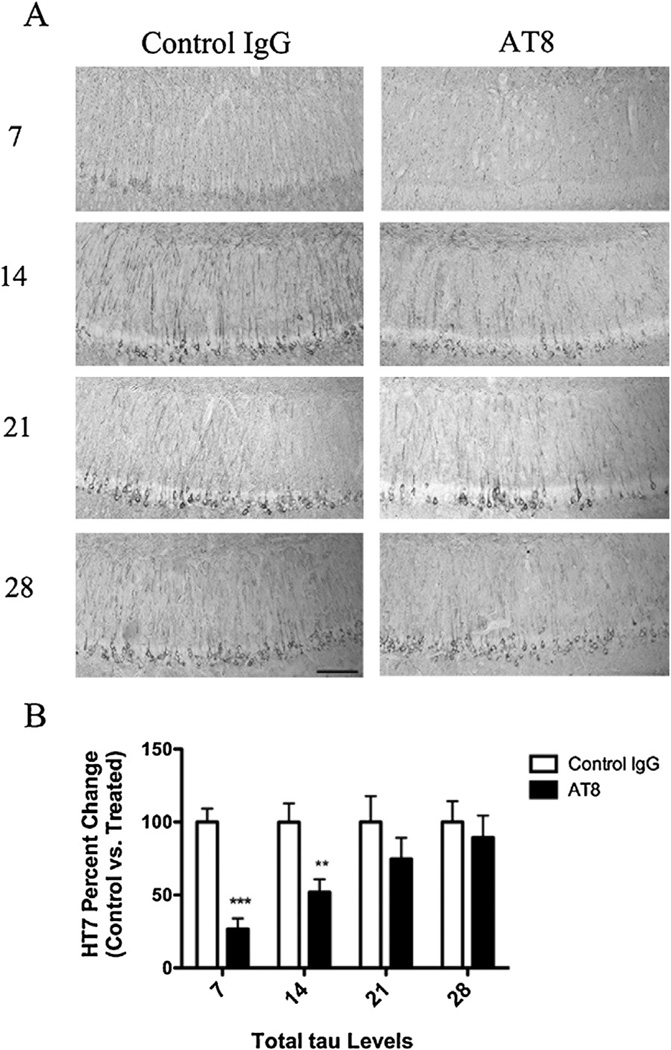
3xTg-AD mice harbor human mutant tau (P301L), and like other mutant tau transgenic mice, tau pathogenesis is age dependent. To better recapitulate the neuropathological state likely found at diagnosis, we treated aged 3xTg-AD mice with pre-existing pathology. To examine the affect of immunization against p-tau, a small cohort of mice was injected with either AT8, or control IgG (in the contralateral hemisphere), within the CA1 subfield of the hippocampus. To determine the temporal reoccurrence of pathology following a single injection of AT8, we collected tissue beginning 7 days post-injection, and each week thereafter, until day 28 postinjection. After one week, immunohistochemical analysis revealed a significant reduction in somatodendritic tau levels in AT8 treated vs. control hemispheres (Fig. 1). Levels did not return until week three. In contrast, AT8 immunotherapy had no effect on Aβ levels: at any time point (Fig. 2).
Fig. 1.
Immunization with the AT8-antibody reduces somatodendritic tau immunoreactivity. Total tau levels were quantified in 15–18-month-old 3xTg-AD mice following a single intrahippocampal injection with AT8 or a control IgG. Mice were sacrificed at post injection day 7, 14, 21, or 28. (A) Immunohistochemical analysis reveled sharp decreases in total tau (HT7) levels after immunization with AT8 at days 7 and 14, but not days 21 and 28. (B) Statistical analysis of the change in total tau between AT8 treated and control IgG treated hippocampal sides (t-test, ***P < 0.0001, **P < 0.008, N = 3–7). Scale bar equals 500 µm.
Fig. 2.
Tau immunization does not affect Aβ pathology. Total Aβ levels were also quantified following AT8 injection at post injection day 7, 14, 21, or 28. (A) In contrast to what was observed for tau, immunohistochemical analysis against Aβ (6E10) detected no differences between AT8 and control IgG treated hippocampi at either time point analyzed. (B) Statistical analysis of the change in total Aβ load between AT8 treated and control IgG treated hippocampal sides (N = 3–7). Scale bar equals 500 µm.
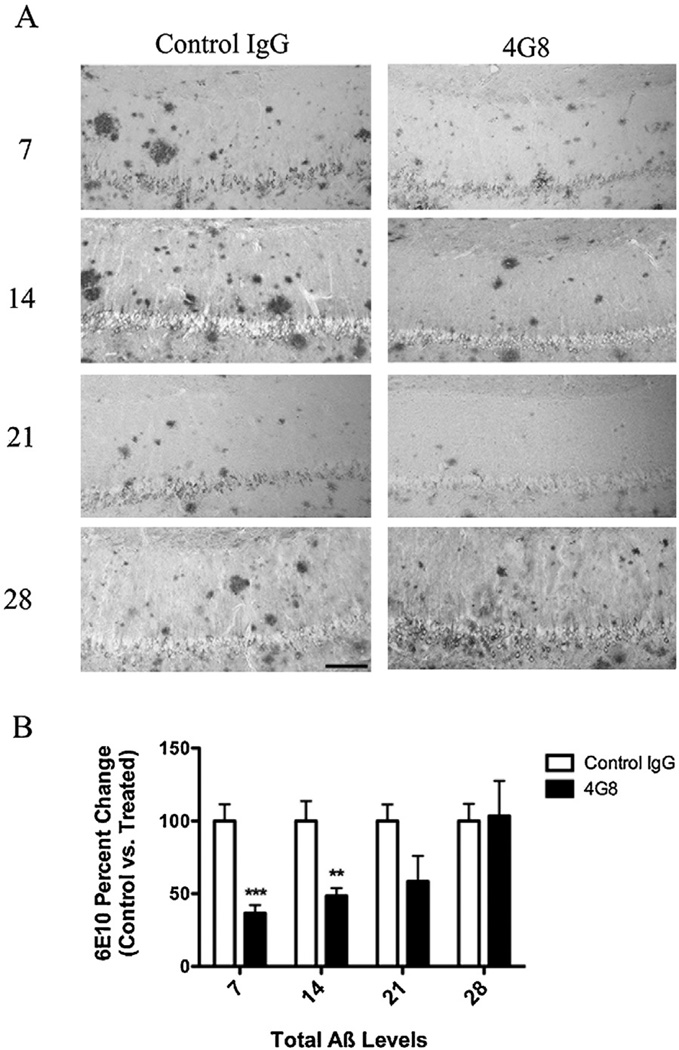
Additionally, we administered a single injection of 4G8 (targeting residues 17–24 of Aβ) in a second cohort of animals. 4G8 treatment resulted in a reduction of intra- and extracellular Aβ, corroborating our previous results (Fig. 3) [8]. This suggests that targeting p-tau, in AD patients with advance Aβ pathology, can reduce early pathological species of tau without an immediate counteracting effect from Aβ.
Fig. 3.
Immunization with the 4G8-antibody reduces intra- and extracellular Aβ. In addition to treatment with AT8, a small cohort of 15–18-month-old 3xTg-AD mice was treated with either the anti-Aβ antibody 4G8 or a control IgG. (A) Staining for total Aβ load (6E10) revealed that 4G8 injection drastically reduced intra- and extracellular Aβ at days 7 and 14. (B) Statistical analysis of Aβ immunization in 4G8 vs. control IgG hippocampal sections (t-test, ***P < 0.0003, **P < 0.0057, N = 3–7). Scale bar equals 500 µm.
3.2. Single injection with AT8 reduces both early and late pathological tau
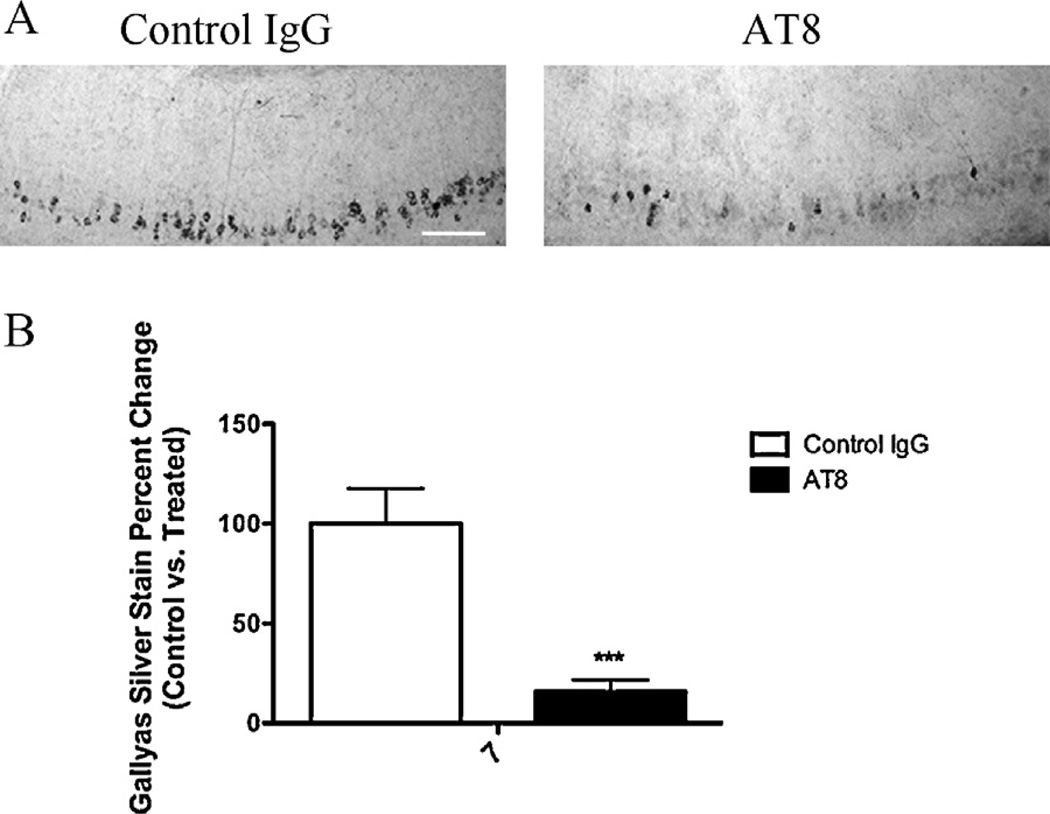
To investigate whether AT8 injection would diminish pathological tau, we conducted an assessment of early and late tau pathology. We found that treated hemispheres had significantly less AT8 reactivity in the CA1 subfield. This observation was only significant at one week post-injection (Fig. 4). To determine if there was a similar effect on more advance stages of tau pathology, we used the Gallyas silver stain method to stain for NFTs. The number of Gallyas positive neurons, within CA1, was reduced one week after AT8 injection compared to control hemispheres (Fig. 5). These findings show proof of concept that immunotherapy targeting p-tau can reduce different pools of pathological tau.
Fig. 4.
Targeting p-tau via immunization significantly reduces early AT8 immunoreactivity. The phosphorylated residues of tau that make up the AT8 epitope also represent some of the earliest modifications observed in tau pathogenesis. (A) Staining for phosphorylated residues Ser199 and Thr202 (AT8) show a large decreased in reactive at 7 days post-injection. (B) Statistical analysis of AT8 immunoreactivity, at post injection day 7, reveals a significant decrease in AT8 treated vs. control IgG treated sections (t-test, ***P < 0.0001, N = 7). Scale bar equals 250 µm.
Fig. 5.
3xTg-AD mice immunized with AT8 have reduced Gallyas positive neurons one week post-injection. (A) Using the Gallyas silver stain method, we observed fewer Gallyas positive neurons in hemispheres treated with AT8 at 7 days. (B) Statistical analysis of Gallyas staining after AT8 treatment (t-test, ***P < 0.0001, N = 7). Scale bar equals 250 µm.
4. Discussion
Here, we demonstrate that a single intrahippocampal injection of AT8 significantly reduces early and late tau pathology in 3xTg-AD mice: within 7 days post-injection. In contrast, there were no changes in Aβ pathology. The effect of AT8 administration was no longer evident by three weeks post-injection. The excessive intra- and extracellular Aβ that persists following AT8 treatment may contribute to the reemergence of tau pathology. Our results provide proof of concept that targeting pathological tau, via passive immunization, can reduce tau pathology, even when advanced Aβ pathology is present.
Immunotherapy targeting tau has been previously examined in transgenic mouse models containing human mutant tau only [18,19,22,23]. While many successes have been reported, there is not yet consciences on the optimal approach; i.e. active vs. passive immunization, or, which tau species to target. A recent study by d’Abramo et al., using a passive immunization approach, compared the effectiveness of the DA31 and MC1 monoclonal antibodies in P301L mutant tau mice. The authors found that the antibody MC1, which targets PHF-tau, was more efficacious, vs. the DA31 antibody, which targets normal tau, and suggest that the antibody target, and not its affinity, is most critical in determining its in vivo effectiveness [24]. In a separate study by Boutajangout et al., also utilizing a passive immunization approach, administration of the PHF1 antibody, which targets pathological tau, lead to better performance on the traverse beam task, and 58% less tau pathology in the hippocampus [25]. While Boutajangout et al., and others using immunotherapy against pathological tau, have shown positive results, the use of tau only transgenic models makes extrapolating the results to potential efficacy in human AD patients difficult. Therefore, there remains a great need to test tau immunotherapy in more complete models of AD. Between 15 and 18 months of age, 3xTg-AD mice develop extensive thioflavin-S positive plaques and Gallyas positive tangles, thus better recapitulating the human AD brain. Our lab has previously demonstrated that Aβ immunotherapy decreases some soluble tau pools, but not Sarkosyl-insoluble tau, in aged 3xTg-AD mice [8,21]. In the same study, we found that immunotherapy against normal human tau was ineffective in reducing tau or Aβ pathology: supporting the idea that antibody specificity is critical in determining immunization effectiveness. In the current study, we chose to administer the p-tau antibody AT8, based on extensive evidence implicating the p-tau residues Ser202, and Thr205 as markers of intra- and extracellular pathological tau. It is our belief that a passive immunization approach is more adventitious at this time, based on several disadvantages associated with active immunization, specifically autoimmune side effects, and inconsistent antibody titers amount patients.
One of the most critical remaining questions regarding tau immunization involves identifying the mechanism behind its effects. Once generated, it is possible for a small percentage of circulating IgG to enter the brain under normal circumstances [26], despite the presence of an intact blood brain barrier (BBB). However, in disorders such as AD, the BBB is likely compromised, which would allow for greater antibody numbers to reach their target within the brain. Indeed, several studies have reported that peripherally administered antibodies can reach their targets within the diseased brain. In the study by Asuni et al., the authors were able to detect FITC-labeled IgG in the brains of tau transgenic mice after intracarotid injection [23]. While it is possible that peripherally delivered tau antibodies can reach the brain in efficacious concentrations, it remains to be elucidated as to how pathological tau might be targeted by this approach. One possible method, arising form studies conducted using Aβ, suggest that the removal of extracellular tau could create a shift in the equilibrium between intracellular and extracellular pools of tau; and therefore, promote the release of intracellular tau. Extracellular antibodytau complexes could subsequently be phagocytized by microglia, astrocytes, or neurons, and degraded. Another possibility, also barrowing from observations from studies targeting Aβ, is that unbound antibody can enter neurons: targeting tau directly within the cells. Neurons have been shown to possess receptors that can bind and internalize IgG [27], and thus, could actively internalized antibodies to tau, which would then subsequently target intraneuronal pools of aggregated tau. Once the antibody-tau complex is formed, it has been hypothesized that a final destination is likely the endosomal/lysosomal system for degradation [22]. Indeed, evidence has been reported that antibody-tau complexes can be found co-localized with markers of the endosomal/lysosomal system ex vivo, supporting the endosomal/lysosomal hypothesis [28]. This is likely the case in our model, as we saw evidence for increased lysosomal activation after AT8 administration in treated hemispheres (data not shown). However, the increase did not reach significance after a single administration.
Overall, we found that a single intrahippocampal injection of AT8 significantly reduces total tau levels, and early and late pathological tau, in just one-weeks time. Unfortunately, a single injection of the AT8 did not affect pre-established Aβ pathology. This supports the hypothesis that Aβ pathology presides tau pathology, or that once initiated Aβ pathology cannot be attenuated by the removal of tau alone. In addition, our study shows that tau pathology begins to reemerge at 21 days post-injection after single treatment, which was surprising based on evidence that tau pathology can occur over a very short time period [29]. Nevertheless, a single administration of AT8 was able to reduce total tau, and early pathological tau; and attenuate the generation of any new tau pathology for several days. Though AT8 reactivity is recognized as occurring late in the pathogenesis of tau, it is possible that the mechanism underlying our observations involves a shift in the pathological pools of tau. The removal of pre-established NFTs may drive new NFT formation, thereby decreasing the amounts of smaller aggregates of tau. Indeed, our results support this hypothesis; however, we acknowledge more studies are needed. To conclude, our studies provide valuable evidence that targeting pathological tau alone may not be sufficient for AD therapy, and that future work investigating the efficacy of tau immunotherapy for AD should be conducted in more complete transgenic models.
HIGHLIGHTS.
We investigated the impact of targeting phosphorylated tau in 3xTg-AD mice.
3xTg-AD mice contain both amyloid-β and tau pathology at time of injection.
We examined for both changes in tau and amyloid-β pathology.
Tau immunization does not affect established amyloid-β pathology.
Targeting phosphorylated tau decreases both soluble and insoluble tau levels.
Acknowledgement
This work was supported by the National Institutes of Health-National Institute of Aging Grant AG027544 (F.M.L).
References
- 1.Querfurth HW, LaFerla FM. Alzheimer’s disease. N. Engl. J. Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 2.Roberson ED2, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, Gerstein H, Yu GQ, Mucke L. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer’s disease mouse model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- 3.Dumanchin C, Camuzat A, Campion D, Verpillat P, Hannequin D, Dubois B, Saugier-Veber P, Martin C, Penet C, Charbonnier F, Agid Y, Frebourg T, Brice A. Segregation of a missense mutation in the microtubule-associated protein tau gene with familial frontotemporal dementia and parkinsonism. Hum. Mol. Genet. 1998;7:1825–1829. doi: 10.1093/hmg/7.11.1825. [DOI] [PubMed] [Google Scholar]
- 4.Goedert M, Klug A, Crowther RA. Tau protein, the paired helical filament and Alzheimer’s disease. J. Alzheimers Dis. 2006;9:195–207. doi: 10.3233/jad-2006-9s323. [DOI] [PubMed] [Google Scholar]
- 5.deCalignon A, Polydoro M, Suarez-Calvet M, William C, Adamowicz DH, Kopeikina KJ, Pitstick R, Sahara N, Ashe KH, Carlson GA, Spires-Jones TL, Hyman BT. Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron. 2012;73:685–697. doi: 10.1016/j.neuron.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, Probst A, Fraser G, Stalder AK, Beibel M, Staufenbiel M, Jucker M, Goedert M, Tolnay M. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009;11:909–913. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee EB, Leng LZ, Zhang B, Kwong L, Trojanowski JQ, Abel T, Lee VM. Targeting amyloid-beta peptide (Abeta) oligomers by passive immunization with a conformation-selective monoclonal antibody improves learning and memory in Abeta precursor protein (APP) transgenic mice. J. Biol. Chem. 2006;281:4292–4299. doi: 10.1074/jbc.M511018200. [DOI] [PubMed] [Google Scholar]
- 8.Oddo S, Billings L, Kesslak JP, Cribbs DH, LaFerla FM. Abeta immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron. 2004;43:321–332. doi: 10.1016/j.neuron.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Oddo S, Vasilevko V, Caccamo A, Kitazawa M, Cribbs DH, LaFerla FM. Reduction of soluble Abeta and tau, but not soluble Abeta alone, ameliorates cognitive decline in transgenic mice with plaques and tangles. J. Biol. Chem. 2006;281:39413–39423. doi: 10.1074/jbc.M608485200. [DOI] [PubMed] [Google Scholar]
- 10.Hartman RE, Izumi Y, Bales KR, Paul SM, Wozniak DF, Holtzman DM. Treatment with an amyloid-beta antibody ameliorates plaque load, learning deficits, and hippocampal long-term potentiation in a mouse model of Alzheimer’s disease. J. Neurosci. 2005;25:6213–6220. doi: 10.1523/JNEUROSCI.0664-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boche D, Denham N, Holmes C, Nicoll JA. Neuropathology after active Abeta42 immunotherapy: implications for Alzheimer’s disease pathogenesis. Acta Neuropathol. 2010;120:369–384. doi: 10.1007/s00401-010-0719-5. [DOI] [PubMed] [Google Scholar]
- 12.Serrano-Pozo A, William CM, Ferrer I, Uro-Coste E, Delisle MB, Maurage CA, Hock C, Nitsch RM, Masliah E, Growdon JH, Frosch MP, Hyman BT. Beneficial effect of human anti-amyloid-beta active immunization on neurite morphology and tau pathology. Brain. 2010;133:1312–1327. doi: 10.1093/brain/awq056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, Jones RW, Bullock R, Love S, Neal JW, Zotova E, Nicoll JA. Long-term effects of Abeta42 immunisation in Alzheimer’s disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372:216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- 14.Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nat. Med. 2003;9:448–452. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- 15.Fox NC, Black RS, Gilman S, Rossor MN, Griffith SG, Jenkins L, Koller M. Effects of Abeta immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology. 2005;64:1563–1572. doi: 10.1212/01.WNL.0000159743.08996.99. [DOI] [PubMed] [Google Scholar]
- 16.Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, Eisner L, Kirby L, Rovira MB, Forette F, Orgogozo JM. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64:1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- 17.Bi M, Ittner A, Ke YD, Gotz J, Ittner LM. Tau-targeted immunization impedes progression of neurofibrillary histopathology in aged P301L tau transgenic mice. PLoS One. 2011;6:e26860. doi: 10.1371/journal.pone.0026860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chai X, Wu S, Murray TK, Kinley R, Cella CV, Sims H, Buckburner H, Hanmer J, Daves P, O’Neil MJ, Mutton ML, Citron M. Passive immunization with anti-Tau antibodies in two transgenic models: reduction of Tau pathology and delay of disease progression. J. Biol. Chem. 2011;286:34457–34467. doi: 10.1074/jbc.M111.229633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boutajangout A, Quartermain D, Sigurdsson EM. Immunotherapy targeting pathological tau prevents cognitive decline in a new tangle mouse model. J. Neurosci. 2010;30:16559–16566. doi: 10.1523/JNEUROSCI.4363-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goedert M, Jakes R, Vanmechelen E. Monoclonal antibody AT8 recognises tau protein phosphorylated at both serine 202 and threonine 205. Neurosci. Lett. 1995;189:167–169. doi: 10.1016/0304-3940(95)11484-e. [DOI] [PubMed] [Google Scholar]
- 21.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 22.Boimel M, Grigoriadis N, Lourbopoulos A, Haber E, Abramsky O, Rosenmann H. Efficacy and safety of immunization with phosphorylated tau against neurofibrillary tangles in mice. Exp. Neurol. 2010;224:472–485. doi: 10.1016/j.expneurol.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Asuni AA, Boutajangout A, Quartermain D, Sigurdsson EM. Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. J. Neurosci. 2007;27:9115–9129. doi: 10.1523/JNEUROSCI.2361-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.d’Abramo C, Acker CM, Jimenez HT, Davies P. Tau passive immunotherapy in mutant P301L mice: antibody affinity versus specificity. PLoS One. 2013;8:e62402. doi: 10.1371/journal.pone.0062402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boutajangout A, Ingadottir J, Davies P, Sigurdsson EM. Passive immunization targeting pathological phospho-tau protein in a mouse model reduces functional decline and clears tau aggregates from the brain. J. Neurochem. 2011;118:658–667. doi: 10.1111/j.1471-4159.2011.07337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nerenberg ST, Prasad R. Radioimmunoassays for Ig classes G, A, M, D, and E in spinal fluids: normal values of different age groups. J. Lab. Clin. Med. 1975;86:887–898. [PubMed] [Google Scholar]
- 27.van der Kleij H, Charles N, Karimi K, Mao YK, Foster J, Janssen L, Chang Yang P, Kunze W, Rivera J, Bienenstock J. Evidence for neuronal expression of functional Fc (epsilon and gamma) receptors. J. Allergy Clin. Immunol. 2010;125:757–760. doi: 10.1016/j.jaci.2009.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krishnamurthy PK, Deng Y, Sigurdsson EM. Mechanistic Studies of Antibody-Mediated Clearance of Tau Aggregates Using an ex vivo Brain Slice Model. Front. Psychiatry. 2011;2:59. doi: 10.3389/fpsyt.2011.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Calignon A, Fox LM, Pitstick R, Carlson GA, Bacskai BJ, Spires-Jones TL, Hyman BT. Caspase activation precedes and leads to tangles. Nature. 2010;464:1201–1204. doi: 10.1038/nature08890. [DOI] [PMC free article] [PubMed] [Google Scholar]