Abstract
Deciding how long to keep waiting for future rewards is a nontrivial problem, especially when the timing of rewards is uncertain. We report an experiment in which human decision makers waited for rewards in two environments, in which reward-timing statistics favored either a greater or lesser degree of behavioral persistence. We found that decision makers adaptively calibrated their level of persistence for each environment. Functional neuroimaging revealed signals that evolved differently during physically identical delays in the two environments, consistent with a dynamic and context-sensitive reappraisal of subjective value. This effect was observed in a region of ventromedial prefrontal cortex that is sensitive to subjective value in other contexts, demonstrating continuity between valuation mechanisms involved in discrete choice and in temporally extended decisions analogous to foraging. Our findings support a model in which voluntary persistence emerges from dynamic cost/benefit evaluation rather than from a control process that overrides valuation mechanisms.
Pursuing long-run rewards often requires persistence in the face of delay and short-run costs. The capacity to delay gratification is central to the notion of self-control in human decision making, and failures of persistence can appear to reflect impulsivity, inconsistency, or self-control failure1, 2. Here we used fMRI to examine brain activity associated with sustaining or curtailing persistence toward delayed rewards.
Much is known about neural systems involved in value-based decision making3–6, but it is unknown what role these mechanisms play in temporally extended persistence. Most intertemporal choice research focuses on discrete choices among outcomes that differ in delay7–9. Delay-of-gratification scenarios, in contrast, involve a prolonged delay period with a continuously available opportunity to give up1. These two types of future-oriented behavior are widely thought to involve different mental processes. Mischel and colleagues10 have argued that the initial selection of a delayed reward depends on a rational cost/benefit assessment, but that the subsequent ability to wait for it depends on self-regulatory dynamics (competition between hot and cool motivational systems11).
We previously hypothesized that both successes and apparent failures of persistence emerge from dynamic value maximization12, 13. Because the exact timing of future events is usually uncertain, there is no guarantee that a decision maker who was willing to begin waiting for a delayed reward should necessarily be willing to keep waiting indefinitely. In some situations, including many that seem to challenge self-control, a long delay so far is predictive of a longer-than-expected delay yet to come12–15. One way to navigate such situations would be to reassess the subjective value of the awaited reward as time passes, based on a continuously updated estimate of the remaining delay time12. Such a reassessment might be encoded in the same neural valuation system, comprised of ventromedial prefrontal cortex (VMPFC), ventral striatum (VS) and posterior cingulate cortex (PCC), that encodes subjective value in a highly general manner across many other kinds of decisions3–6. The subjective value representations encoded in VMPFC are known to be sensitive to both immediate and delayed outcomes7, 8, primary and secondary forms of reward3, 16, goal-related and temptation-related factors9, 17, and high-level task contingencies18, 19.
Other theoretical perspectives make different predictions. One alternative possibility is that successful persistence depends principally on cognitive control mechanisms external to the valuation system. Although some accounts hold that the VMPFC valuation system mediates cognitive control9, 17, 20, other accounts posit a form of control that overrides or competes with valuation2, 11, 20–23. If the latter control mechanism is paramount, successful persistence might be better understood as rule-adherence than as value-maximization, and curtailing persistence might reflect a lapse in control-related brain activity (e.g., in lateral PFC).
A second alternative possibility is based on the structural parallel between delay of gratification and certain kinds of foraging scenarios13, 15, 24–27. It has recently been hypothesized that single-alternative foraging decisions—e.g., whether to exploit one’s current food patch or depart to forage elsewhere—might depend, not on the VMPFC valuation system, but on a representation in dorsal anterior cingulate cortex (dACC) of the value of departing26, 28.
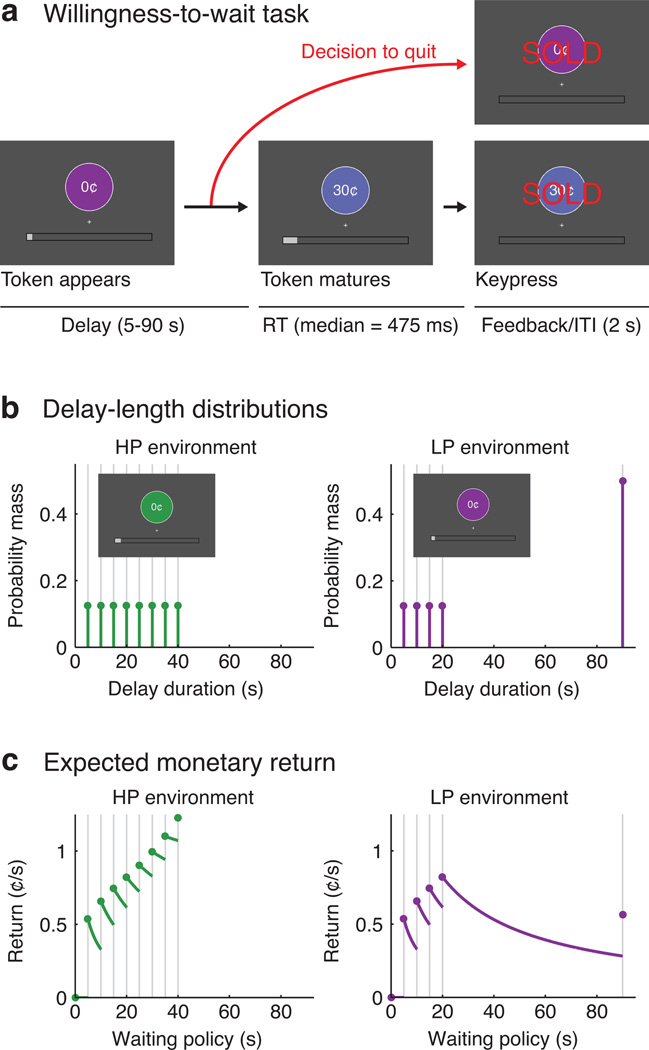
To examine valuation signals during temporally extended persistence we conducted an fMRI experiment in which participants repeatedly decided how long to keep waiting for future monetary rewards (Fig. 1a). On each trial the participant viewed a token, which had no initial value but matured to a value of 30¢ after a random delay. The participant could sell the token anytime and initiate a new trial, aiming to maximize total earnings in a fixed time period. Unlike some previous studies1, 13, no small reward was delivered if the participant quit early; instead, the main incentive to quit was the possibility that the next trial might mature with a shorter delay.
Figure 1.
Experimental task and timing conditions. A: Schematic of the willingness-to-wait task. B: Discrete probability distributions governing the scheduled delay times in each environment. C: Expected monetary rates of return under various waiting policies, where each policy is defined by a giving-up time. The reward-maximizing policy was to wait up to 40s in the HP environment (i.e., never to quit), but only up to 20s in the LP environment. These rates of return are contingent on the fixed 2s inter-trial interval (ITI).
The ideal strategy depended on the distribution of delay times, which differed between two environments (Fig. 1b,c). In a high-persistence (HP) environment the most productive strategy was to wait for every reward (up to 40s). In a limited-persistence (LP) environment the best strategy was to wait 20s and then quit if the reward had not arrived. Participants learned about the timing statistics through direct experience during preliminary training. The environments were presented in alternating 10-min runs, marked by different-colored tokens.
We predicted that participants would quit earlier in the LP environment than in the HP environment13. In addition, our theoretical model predicted that participants’ subjective valuation of the awaited token would evolve differently in the two environments, increasing more rapidly with elapsed time in the HP environment than the LP environment. Our neuroimaging experiment tested whether canonically value-responsive brain regions would reflect this dynamic reassessment. Our experiment could also detect alternative possibilities such as representations of subjective value elsewhere in the brain, a lapse in control-related activity associated with quitting, or a representation of the value of quitting in dACC.
Results
Behavioral results
Participants (n=20) quit before receiving the reward more often in the LP environment (median=50.0% of trials; IQR 46.6 to 57.6%) than the HP environment (median=3.1%; IQR 0 to 15.6%). In the LP environment the time waited before quitting (median of medians) was 29.3s (IQR 17.6 to 36.6). Within-subject (across-trial) variability in quit timing was comparatively small: the median size of the within-subject interquartile range was 9.1s.
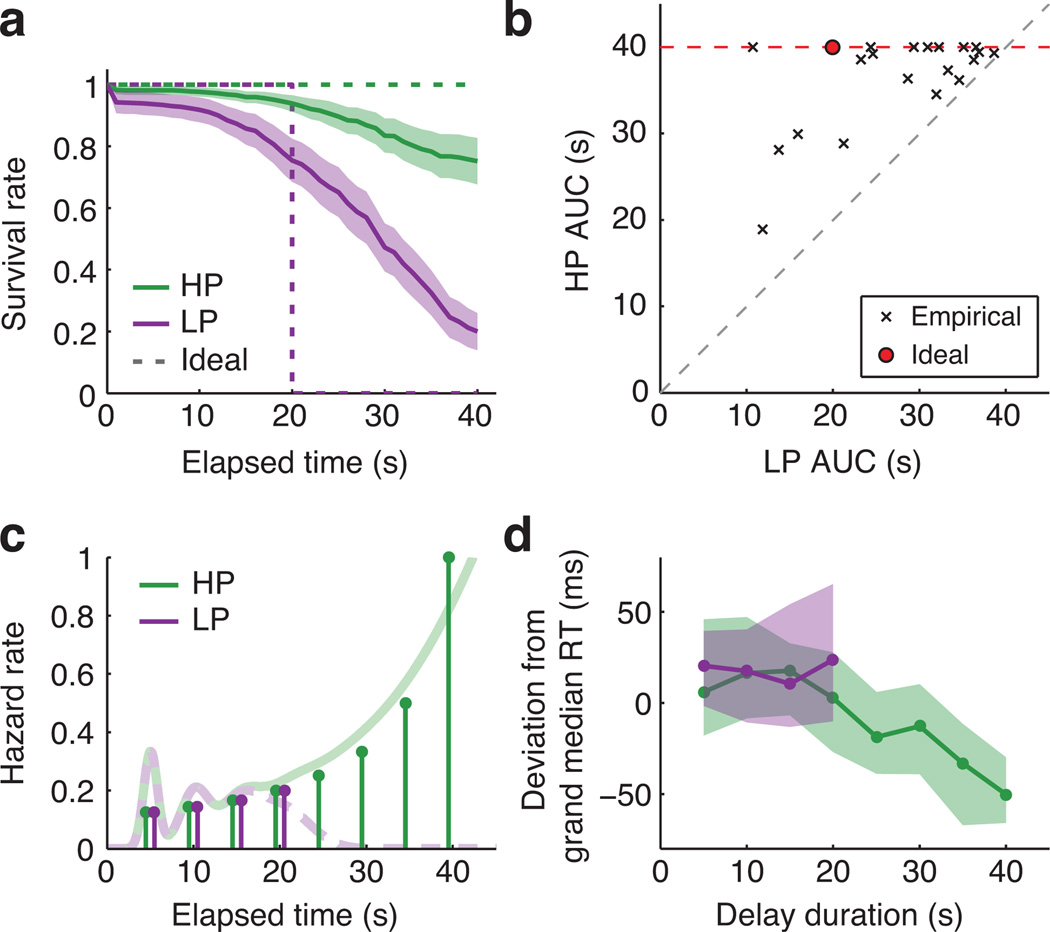
Participants were willing to wait longer in the HP environment than the LP environment. We used survival analysis to estimate each participant’s probability of “surviving” various lengths of time without quitting13. Fig. 2a shows averaged subject-wise empirical survival curves against ideal performance. The area under the curve (AUC) estimates how much of the first 40s a participant was willing to wait on average (Fig. 2b). Median AUC was 38.9s in the HP environment (IQR 35.4 to 40; ideal=40s) and 30.2s in the LP environment (IQR 22.3 to 34.9; ideal=20s). All 20 participants persisted longer in the HP environment (median difference=7.6s, IQR 3.0 to 14.2, signed-rank p<0.001). Persistence in the two environments was modestly correlated (Spearman ρn=20=0.37, p=0.11; Fig. 2b), and behavior was stable across the fMRI experiment (Supplementary Fig. 1).
Figure 2.
Behavioral results. A: Survival curves reflecting the probability that a participant was still waiting at each elapsed time, provided that the reward had not yet been delivered. Empirical survival curves were averaged across subjects at 1 s intervals (+/− SEM). Ideal performance is plotted for reference (dashed lines). B: Area under the curve (AUC) values calculated from individual participants’ survival curves. The maximum possible value was 40s. Red point marks ideal performance. All 20 participants persisted more in the HP environment. C: Stem plots show the ground-truth hazard rate for reward in each environment: i.e., the probability of the reward arriving at each time, conditional on not having arrived already. Faded lines illustrate hypothetical continuous hazard functions incorporating endogenous temporal uncertainty (see Methods). D: Reward RT at each delay (median and IQR of subject-wise medians). RTs are expressed as deviations from each subject’s grand-median RT (median=475ms, IQR 450 to 506ms) to display within-subject effects. RTs for 5–20s delays did not differ between the environments (HP median=472ms, IQR 454 to 538; LP median=494ms, IQR 443 to 522).
Reaction time (RT) to sell rewarded tokens tracked time-varying reward expectancy. When an event’s latency is uniformly distributed, expectancy theoretically increases with elapsed time28 (Fig. 2c). Accordingly, subject-wise Spearman correlations between delay and RT were reliably negative in the HP environment (median single-subject ρ=−0.27, IQR −0.36 to −0.16, signed-rank p<0.001), indicating faster responses to rewards that were preceded by longer delays (Fig. 2d) and implying that participants successfully encoded the task’s timing statistics.
Theoretical modeling
The passage of time can drive a dynamic reassessment of awaited rewards by furnishing information about the remaining delay12, 29. Intuitively, rewards in the HP environment grew nearer and more subjectively valuable as time passed, but rewards in the LP environment became progressively less likely to be delivered before the participant quit.
We formalized this intuition in a theoretical model of subjective valuation. The model estimated the awaited token’s subjective value at each point in the delay interval, accounting for the changing probability distribution over remaining delay durations. Our model extended a formalism from the optimal foraging literature known as the potential function25. The expected remaining delay was multiplied by the opportunity cost of time and subtracted from the expected reward. Subjective value at a given elapsed time equaled the expected net return in the remainder of the current trial, maximized over all possible giving-up times. Its minimum was zero since the agent could always quit immediately. If subjective value exceeded zero, this signified that the decision maker could do better by waiting than by quitting immediately. The level of subjective value at each time reflected the margin of preference for waiting over quitting (see Methods for details).
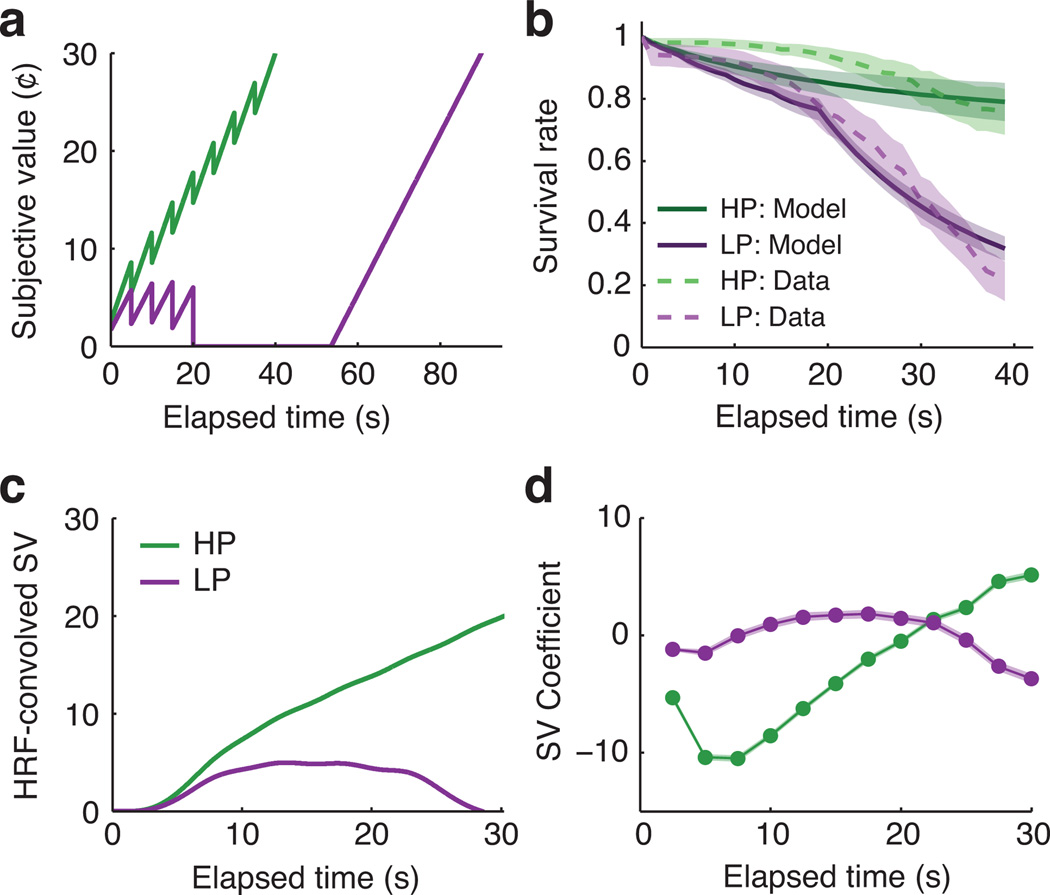
In the HP environment the token’s theoretical subjective value increased with elapsed time, reflecting the progressive shortening of the expected remaining delay (Fig. 3a). In the LP environment the token’s subjective value remained positive until 20s but then fell to zero, reflecting that the best strategy was to quit if the reward had not arrived by then. Differences between the subjective value trajectories in the two environments were primarily driven by the evolving probability that the reward would arrive before the optimal giving-up time (Supplementary Fig. 2).
Figure 3.
Theoretical subjective value of the awaited token as a function of elapsed time in each environment. A: A token's subjective value increased over time in the HP environment but not in the LP environment. These timecourses are based on the discrete ground-truth timing distributions and would be smoothed by subjective temporal uncertainty. B: Simulated behavior from a model in which subjective value linearly influenced the log-odds of continuing to wait (mean +/− SEM of subject-wise model fits). Data from Fig. 2a are overlaid for reference. C: Subjective value timecourses convolved with a canonical hemodynamic response function (HRF). D: Predicted BOLD timecourses obtained by applying our fMRI analysis to idealized synthetic data (mean +/− SEM of individual subject results). Visual differences from Panel C reflect that (1) the HP and LP environments had independent baselines, and (2) there was a small degree of carryover across trials. In spite of these differences the theoretical difference timecourses (HP minus LP) were highly correlated between Panels C and D (median r2=0.88, IQR 0.84 to 0.89).
We modeled the empirical behavioral data as a stochastic function of theoretical subjective value using logistic regression (Fig. 3b; see Methods for details). Greater subjective value was associated with higher odds of waiting (median coefficient=0.26, IQR 0.05 to 0.78, signed-rank p<0.001). The subjective value model significantly outperformed an intercept-only model (subject-wise likelihood-ratio tests: median z=4.26, IQR 1.79 to 7.97, signed-rank p<0.001) and an alternative model that directly fit different overall rates of quitting in the HP and LP conditions (subject-wise difference of model deviances: median=6.45, IQR −1.85 to 32.02, signed-rank p=0.033).
Neuroimaging results
Our fMRI analyses tested for brain signals that evolved differently during physically identical delay intervals in the two environments. Trial-onset-locked BOLD timecourses were flexibly estimated in each environment using a finite impulse response (FIR) model; i.e., a series of single-timepoint basis functions in a general linear model (GLM). Each trial was modeled from onset up to 1s before the outcome (reward cue or quit response). Because trials had different durations, earlier timepoints were observed on more trials than later timepoints (Supplementary Fig. 3). Group analyses focused on the interval from 2.5–30s, for which 19 of 20 participants contributed complete data. Because the HP and LP conditions were presented in separate scanning runs with independent baselines our analyses focused on differential change over time, not the overall offset between the two conditions. Significance was assessed using whole-brain permutation tests to control for multiple comparisons (see Methods).
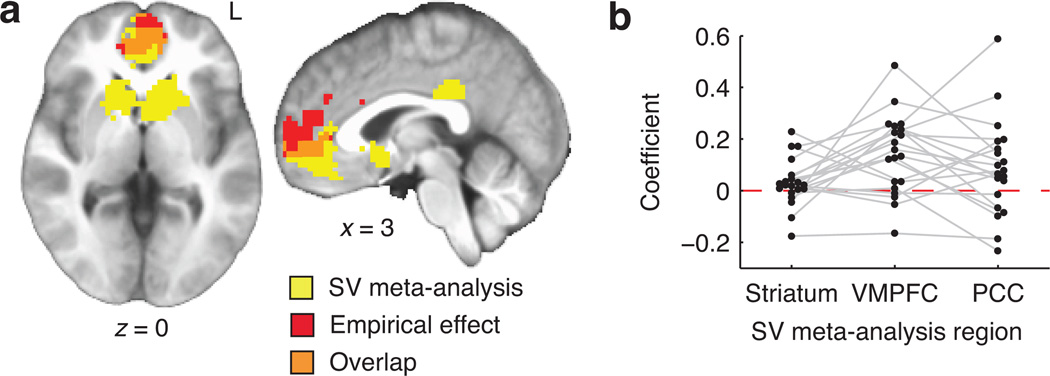
A model-based fMRI contrast tested directly for effects of theoretical subjective value on BOLD activity. For each subject and voxel, the empirical difference timecourse (HP minus LP) was regressed on the predicted difference (Fig. 3c,d; see Methods) and a constant intercept. The resulting contrast coefficient reflected the degree to which BOLD signal increased more steeply with elapsed time in the HP environment than the LP environment. Coefficients were submitted to a whole-brain, two-tailed, group-level test (n=20). This identified a single significant cluster, located in VMPFC (Fig. 4a and Table 1a), in which BOLD signal was positively related to theoretical subjective value. No negative effects of subjective value on BOLD were identified, even in follow-up analyses tailored to detect signals reflecting the difficulty of persistence (Supplementary Fig. 4).
Figure 4.
Model-based contrast results. A: Whole-brain analysis. Displayed in red is the VMPFC cluster that showed a significant relationship with the theoretical subjective value timecourses in Fig. 3d. In yellow, for reference, are regions identified in a previous meta-analysis of valuation effects (the regions reported in Fig. 3D of Bartra et al.3). Overlap was observed in VMPFC, though not in PCC or striatum. B: Model-based contrast values for each participant, spatially averaged within meta-analytic ROIs. Subjective value effects were significantly positive in VMPFC, and significantly greater in VMPFC than striatum.
Table 1.
| Region | x | y | z | Cluster extent |
Peak value |
Cluster p value |
|---|---|---|---|---|---|---|
| A: Trial-onset locked timecourses: Model-based contrast (t statistic) | ||||||
| VMPFC | 0 | 60 | 3 | 311 | 5.38 | 0.014 |
| B: Trial-onset locked timecourses: Condition-by-time interaction (F statistic) | ||||||
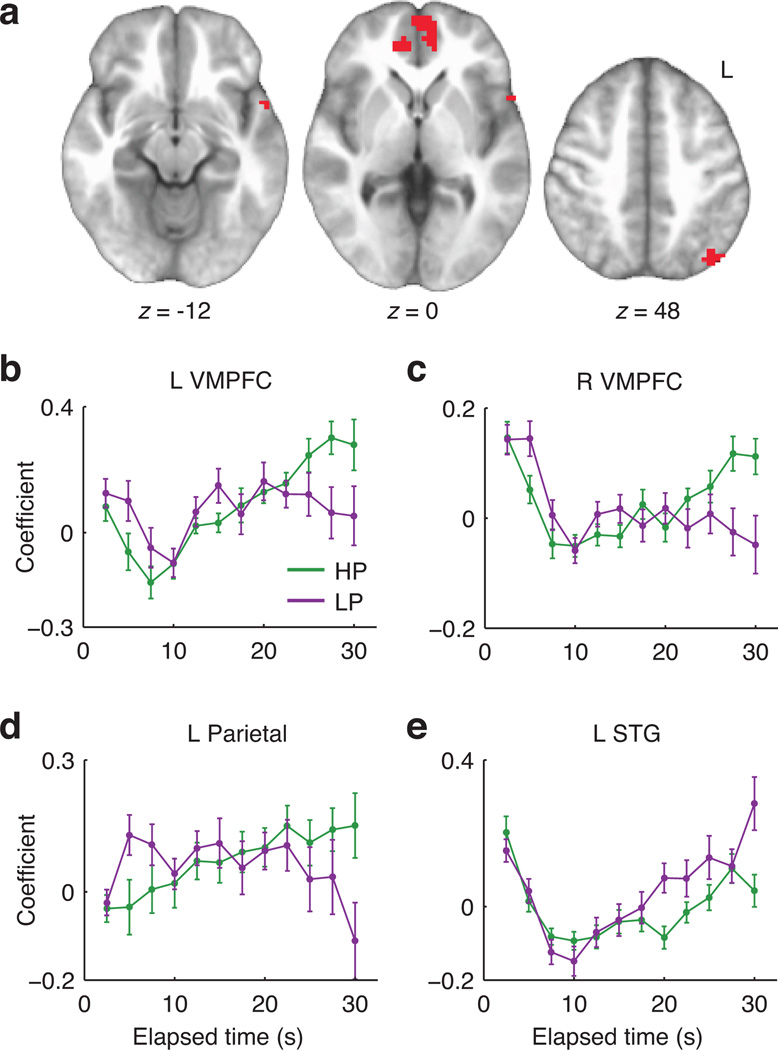
| L VMPFC | −3 | 60 | 3 | 84 | 5.54 | 0.001 |
| R VMPFC | 12 | 42 | 3 | 38 | 4.23 | 0.005 |
| L posterior parietal | −42 | −72 | 45 | 42 | 4.79 | 0.003 |
| L superior temporal gyrus | −54 | 6 | −15 | 16 | 4.09 | 0.034 |
|
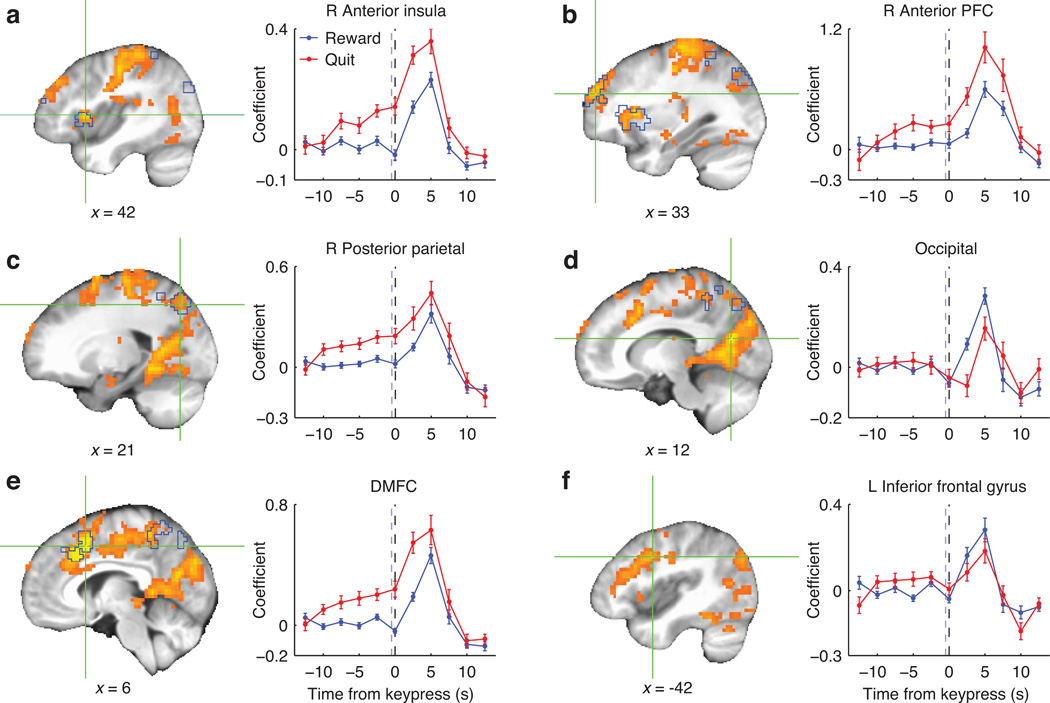
C: Anticipatory activity in quit-related timecourses: Main effect of time (F statistic), −12.5s to −2.5s | ||||||
| R posterior parietal | 21 | −72 | 51 | 393 | 9.32 | 0.001 |
| L posterior parietal | −21 | −72 | 48 | 59 | 5.20 | 0.042 |
| R anterior insula | 33 | 27 | 6 | 140 | 10.32 | 0.005 |
| DMFC | 3 | 12 | 45 | 127 | 7.45 | 0.007 |
| R anterior PFC | 33 | 54 | 27 | 70 | 7.65 | 0.024 |
The observed effect in VMPFC echoes effects of subjective value that are seen in a broad range of other contexts3–6. We formally juxtaposed our results with previous findings by quantifying the spatial overlap between our empirical results and canonically valuation-related brain regions derived from a 206-study meta-analysis3 (Fig. 4a). The meta-analysis had identified clusters showing preferentially positive effects of value in VMPFC (9.67cm3), striatum (21.41cm3), and PCC (2.62cm3). There was a 100-voxel (2.70cm3) region of overlap in VMPFC (27.9% of the canonical region and 32.2% of the empirical cluster).
As an alternative test of the same question, the three canonical valuation areas were tested as regions of interest (ROIs). Model-based contrast coefficients were spatially averaged in each ROI for each participant. The effect of subjective value was significant in VMPFC (signed-rank p=0.002) but non-significant, albeit with a positive trend, in striatum (p=0.079) and PCC (p=0.062; Fig. 4b). Paired-samples comparisons identified a greater effect in VMPFC than striatum (signed-rank p=0.012) and no significant differences between the other two pairs of ROIs (ps>0.11).
In summary, results suggested that the region of VMPFC previously found to encode subjective value during discrete choices and outcomes also reflected a dynamic reassessment of subjective value during voluntary persistence. This was true to a greater degree for VMPFC than striatum.
We additionally conducted a less-constrained analysis that could detect BOLD timecourse differences predicted either by our model or alternative frameworks. Trial-onset-locked timecourses were analyzed at the group level in a whole-brain voxelwise repeated-measures ANOVA (n=19), with factors for condition (HP vs. LP) and timepoint. We focused on the condition-by-timepoint interaction, seeking to identify signals that exhibited different patterns of change over time in the two environments. This analysis avoids a priori assumptions about either the form of the difference or the location of effects in the brain. A significant interaction was observed in left and right VMPFC, left posterior parietal cortex, and a small region of left superior temporal gyrus (Table 1b and Fig. 5a). Timecourse plots (Fig. 5b – e) suggested that in VMPFC and parietal regions the effect took the form of a greater signal increase with elapsed time in the HP environment, consistent with theoretical subjective value.
Figure 5.
Model-free analysis of trial-onset-locked BOLD timecourses. A: Clusters showing a significant timepoint-by-environment interaction (Table 1b). B–E: Spatially averaged signal timecourses for significant clusters (mean +/− SEM), illustrating the form of the observed interactions. Although voxel selection effects would distort follow-up inferential tests of these timecourses, we descriptively summarized their resemblance to our theoretical predictions in terms of the correlation between the average theoretical (Fig. 3d) and observed HP-minus-LP difference timecourses. The resulting Pearson r values were 0.91, 0.89, 0.90, and −0.68 for the results in Panels B–E, respectively.
Further analyses tested for evidence of reward prediction error (RPE) signals30. When a reward occurs, RPE is the difference between the obtained and expected outcome. Because reward expectancy theoretically rose over time in the HP environment (Fig. 2c; see also RT data above and heart rate data below), rewards at short delays should have been more surprising and evoked larger RPEs than rewards at longer delays. We tested whether the amplitude of the phasic BOLD response to reward was modulated by the delay duration that preceded it. A negative effect would reflect an RPE-like pattern (smaller reward responses after longer delays, a pattern seen previously in the firing rates of dopaminergic midbrain neurons31). To focus on phasic reward responses while controlling for nonspecific effects of elapsed time, we compared the modulatory effect in the post-reward epoch against the same effect in a pre-reward epoch.
We observed no significant negative modulatory effect of elapsed time on the reward-related BOLD response in any location. We did, however, identify an occipitoparietal cluster with an effect in the opposite direction: a higher-amplitude BOLD response to rewards at longer delays, which theoretically were more strongly anticipated (Supplementary Fig. 5). Expectancy-driven amplification of brain responses has been seen before32, including in visual cortex33; these effects bear a family resemblance to the facilitatory effects of spatial attention34.
Numerous brain areas responded differentially to reward and quit keypresses, including some that exhibited a ramp-up in activity prior to quit responses. We used a GLM to estimate subject-wise perievent timecourses for the two event types separately (using all keypresses across all four runs), and submitted the difference between reward-related and quit-related timecourses to a group-level ANOVA. Significant effects occurred diffusely across DMFC, lateral PFC, anterior insula, precentral sulcus, and occipital and posterior parietal cortex (Fig. 6a – f). In DMFC, anterior insula, posterior parietal cortex, and anterior PFC the difference consisted of an earlier elevated response for quit responses than reward responses. Other regions, including occipital cortex and left inferior frontal gyrus (IFG), responded more strongly to rewards. Broadly, these effects reflect that rewards involved a visual cue whereas quitting was freely timed and volitional.
Figure 6.
Regions in which BOLD signal differentiated reward-related and quit-related keypresses, assessed on the basis of the event type (reward vs. quit) by timepoint interaction. Warm colors represent F statistics for the analysis of full timecourses, and crosshairs mark local peaks. Blue outlines mark regions significant in the analysis of pre-quit timepoints only. Timecourses (mean +/− SEM) are plotted for a 6mm-radius (33-voxel) sphere centered at each depicted focus point. Black dashed lines mark the keypress time; blue dashed lines mark the median reward cue time (for reward-related keypresses).
To test directly for signal changes that preceded decisions to quit, we performed a group-level ANOVA on only the first 5 points in the quit-related timecourse (−12.5 to −2.5s). A significant effect of timepoint within this anticipatory interval was observed in posterior parietal cortex, DMFC, anterior insula, and anterior PFC (Fig. 6a – f and Table 1c). VMPFC showed no effects in either of the above analyses; that is, there was no evidence that subjective value effects in VMPFC could be alternatively explained in terms of a role in response preparation.
Somatic arousal
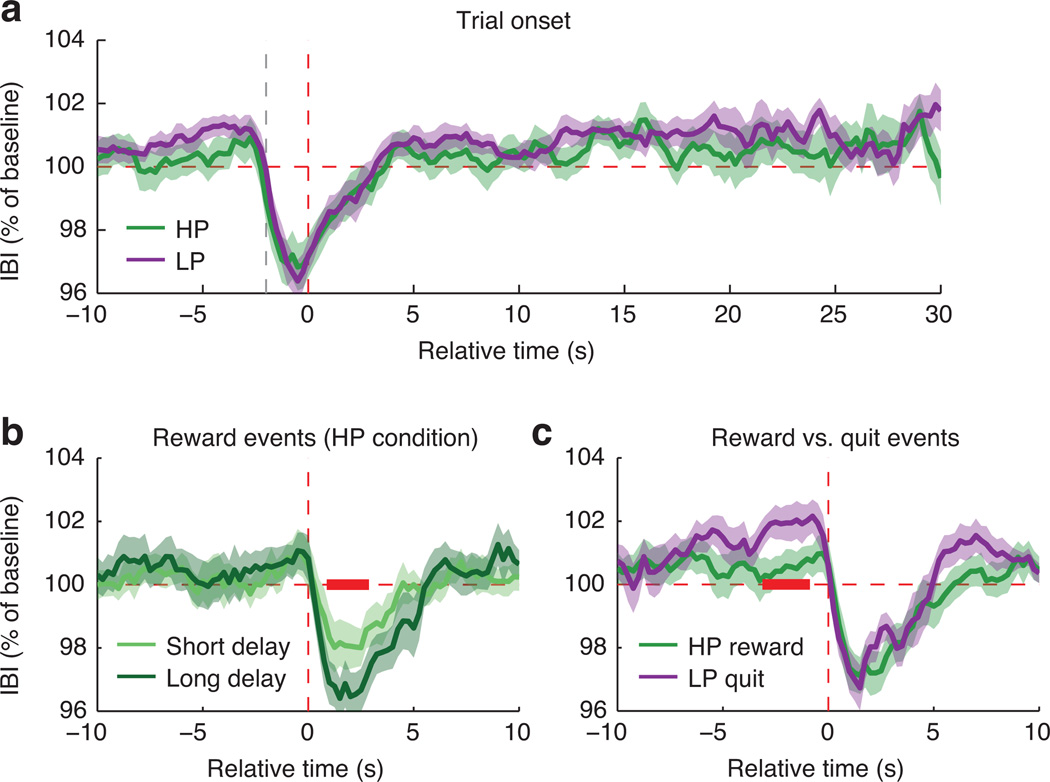
To test whether subjective value effects in BOLD activity were accompanied by changes in general physiological arousal, we performed exploratory analyses of heart rate (inter-beat interval measured via pulse oximetry; n=17) as a function of task events. Heart rate transiently accelerated after keypresses, but did not differ between the two conditions as a function of delay time (Fig. 7a). In the HP condition there was greater transient cardiac acceleration for rewards preceded by longer delays (Fig. 7b), bolstering our conclusion—also supported by RTs and occipitoparietal BOLD effects—that subjective reward expectancy increased with elapsed time in the HP condition. Comparing heart-rate timecourses for reward and quit events revealed cardiac deceleration, a well-known correlate of motor preparation35, prior to quit responses (Fig. 7c). In summary, pre- and post-keypress brain responses (Fig. 6a – f) co-occurred with changes in general somatic arousal, but there was no evidence that arousal effects (as indexed by heart rate) accompanied the trial-onset-locked BOLD effects of theoretical subjective value (Figs. 4 and 5).
Figure 7.
Effects of task events on mean cardiac inter-beat interval (IBI; lower values correspond to faster heart rate). Error bands show SEM; red bands mark significant differences. A: Mean trial-onset-locked IBI timecourse in each condition. Vertical red dashed line marks trial onset; gray dashed line marks the preceding keypress. Each trial contributed data until 1 s before the trial ended (later timepoints therefore have fewer observations than earlier timepoints). No significant differences were observed. B: Comparison between rewards arriving at shorter (5–20s) vs. longer (25–40s) delays in the HP condition. The amplitude of post-keypress heart-rate acceleration was greater for rewards that followed longer delays (lag +1s to +2.75s; permutation-based p=0.018). C: Comparison between reward events in the HP condition and quit events in the LP condition, each restricted to trials with duration >10s. Vertical red dashed line marks the time of the reward cue or quit keypress. Results suggested transient cardiac deceleration prior to quit responses (lag −1s to −3s; permutation-based p=0.045).
Discussion
Decision makers faced with uncertain delay should reappraise awaited rewards as time passes. Depending on the statistics of the environment, the passage of time may either decrease or increase one’s estimate of how long a delay remains. This type of dynamic reassessment offers a rationale for sustaining or curtailing persistence.
We elicited either greater or lesser willingness to persist in laboratory environments by manipulating the timing statistics that governed reward delivery. Decision makers calibrated their level of persistence adaptively; this extends previous demonstrations of environment-specific calibration of intertemporal choice behavior13, 36. Convergent RT, BOLD and heart-rate data suggested participants encoded the relevant timing statistics, responding more vigorously to more strongly expected rewards28. Behavior still fell short of optimality, and an important goal for future work is to determine whether this was due to inexact statistical learning, strong prior expectations, stochastic noise, unmodeled sources of value (e.g., anticipation37) or other causes. Future work should also test whether performance would differ if immediate or viscerally tempting rewards were at stake (e.g., appetizing foods instead of money)1.
The success of the behavioral manipulation enabled us to examine time-dependent brain signals associated with either high or limited behavioral persistence. We observed signals in VMPFC consistent with a dynamic and context-sensitive reassessment of the awaited outcome’s subjective value. This effect was identified using both model-guided and exploratory fMRI timecourse analyses, both at the whole-brain level and in ROIs previously implicated in subjective evaluation.
VMPFC and persistence
Persistence toward future rewards has been classically understood to depend on self-regulatory psychological processes that compete with and override more impulsive, reward-sensitive processes1, 2, 11. Dual-system psychological models have given rise to the neuroscientific hypothesis that competitive dynamics exist between brain regions subserving cognitive control and reward processing21–23.
In contrast to this standard view, we have proposed that delay-of-gratification decisions depend on a dynamic reappraisal of the awaited future reward12, 13. This account attributes differences in waiting behavior across individuals and situations to factors such as temporal beliefs, perceived outcome values and the perceived cost of time, not merely to differences in the capacity to exert self-control12. Here we elicited differences in waiting by manipulating temporal beliefs and obtained evidence for a time-varying representation of subjective value. The hypothesized signal is context dependent, evolving over time in a manner that depends on the timing statistics of the current environment. A corresponding BOLD trajectory was identified in VMPFC, a cortical region regarded as part of a final common pathway in the prospective evaluation of choice alternatives6. These results are consistent with the view that persistence depends on the same neural and cognitive processes that guide other forms of reward evaluation and economic choice. This view implies that adaptive persistence depends on accurately representing the value of waiting, and need not depend on the engagement of effortful inhibitory control processes38. Our results add to the large body of evidence that VMPFC valuation processes utilize a detailed representation of higher-order task structure18, 19. Our findings also extend current conceptions of VMPFC function; while VMPFC activity is known to encode phasic subjective value during discrete choices3–8, we found that it also tracked subjective value in a temporally extended manner (see Jimura et al.39 for a related finding).
Our neuroimaging results suggest there is no need to posit antagonistic dynamics between neural reward systems and control systems to explain voluntary persistence (though we cannot, of course, rule out such dynamics in other situations). Our analyses could have detected patterns suggestive of dual-system competition. For example, the analyses in Figs. 4 and 5 and Supplementary Fig. 4 could have detected activity scaling with the difficulty of persistence, but no such effects were found. The analyses in Fig. 6 could have detected a lapse in control-related activity before decisions to quit, but instead the opposite occurred: an ensemble of regions previously implicated in cognitive control—lateral PFC, DMFC, insula, and parietal cortex—increased activity prior to quits, consistent with brain responses found to precede shifts of strategy in other task paradigms26, 40.
Our findings are more compatible with the hypothesis that cognitive control operates via value modulation9, 17, 20. The value modulation hypothesis stipulates that control mechanisms in lateral PFC operate by modulating subjective value representations in VMPFC. The hypothesis therefore posits a VMPFC signal that incorporates all relevant information and suffices as a final common pathway to guide decisions, consistent with the present findings. It additionally posits that this signal depends on lateral PFC inputs. On this point our data are mostly silent. We found no evidence for condition-dependent activation trajectories in lateral PFC; nevertheless we assume value computation involves interactions among multiple brain regions, and we cannot exclude the possibility that lateral PFC plays a role.
Value representation during foraging
The problem of calibrating persistence in our willingness-to-wait task is closely analogous to the patch departure problem in foraging24–26. It has recently been hypothesized that foraging, which typically involves a succession of accept/reject decisions, imposes fundamentally different information-processing demands from standard multi-alternative economic choice41. Recent work has implicated dACC in signaling the value of exiting foraging patches26 or of shifting away from default alternatives41, although other findings have questioned this idea42.
We did not find evidence for continuous, prospective encoding of the value of quitting (analogous to patch departure) in dACC. Such a signal would theoretically have followed an inverted version of the value of waiting (Fig. 3c,d), and could have been detected in either our model-based analysis (as a negative effect) or our exploratory timecourse analyses. We did, however, observe a response in dACC and other frontal and parietal regions in anticipation of quit decisions. This pattern is consistent with general motor preparation as well as with the possibility that decision-related signals in dACC manifest predominantly during overt choice execution26, 43.
The present results point to a role for VMPFC valuation signals even in a foraging-like situation where decision makers encountered one opportunity at a time and sought to maximize their overall rate of return. VMPFC activity correlated with the value of the current opportunity (waiting for the current token). This finding agrees with the idea that VMPFC encodes a “best minus next-best” comparative value signal41 even when the “next-best” is the constant background option of moving on to a new opportunity. This parallels previous demonstrations that VMPFC reflects the subjective value of individual options that are evaluated in turn against a fixed reference alternative7. Our findings suggest continuity between the valuation mechanisms involved in temporally extended foraging scenarios and multi-option economic choice.
Reward prediction error
The willingness-to-wait task theoretically involves both positive and negative RPE. Positive RPE should accompany reward delivery since, given temporal uncertainty, rewards are not fully predicted at the specific time they are delivered31. Conversely, the pre-reward interval (when the reward could have occurred but does not) presumably involves negative RPE44, 45. Long delays in the HP environment highlight the dissociability of value and RPE signals. Reward expectancy ramps up over time (Fig. 2c), so nonreward should be associated with progressively larger negative RPE even as the awaited reward’s subjective value steadily increases (Fig. 3a). Even though decision makers may be increasingly surprised that the reward did not come now, they are also increasingly confident that it will arrive soon. One potential explanation for the lack of clear RPE signals in our neuroimaging data might be that, at least at the resolution of fMRI, RPE and subjective value signals were superimposed.
Subjective value is canonically associated with BOLD activity in VMPFC, PCC, and striatum3–5, and a broad standing question is how these regions might differ in their computational contributions to decision behavior. One possibility is that striatum preferentially encodes RPE46, 47 whereas VMPFC preferentially encodes prospective decision values6, 47. The present findings appear compatible with such a distinction: a dynamic signal of prospective subjective value was observed in VMPFC, but was significantly less evident in striatum. However, these results will need to be integrated with insights gained using other neuroscientific techniques; recent evidence from direct dopamine recordings suggests striatum may indeed exhibit a ramping pre-reward signal48, and other work points to an important role for serotonergic neuromodulation in behavioral persistence49. It will also be important for future research to assess the fidelity with which VMPFC encodes the individual components of subjective value (Supplementary Fig. 2), to isolate valuation from related factors such as moment-by-moment reward probability33, 50, and to test the generality of these effects across different magnitudes and types of rewards. Research on these topics will yield an enriched picture of how the brain's valuation mechanisms contend with the complexity of real-world decision environments.
Methods
Participants
The participants were 20 members of the University of Pennsylvania community (age 18–30, mean=22, 11 female). Two additional participants were excluded for head movement (shifts of at least 0.5mm between >5% of adjacent timepoints). Participants were paid a show-up fee ($15/hr) plus rewards earned in the task (median=$19.80). All participants provided informed consent. The procedures were approved by the University of Pennsylvania Internal Review Board. No statistical methods were used to predetermine sample size but our sample size was similar to those reported in previous publications16, 18, 19, 32, 42, 47.
Task
The task was programmed using Matlab (The MathWorks, Natick, MA) with Psychophysics Toolbox extensions51, 52. A circular token, colored green or purple, appeared in the center of the screen, labeled “0¢.” After a random delay the token turned blue and its value changed to 30¢. Participants could sell the token anytime by pressing a key with their right hand. The word “SOLD” appeared in red over the token for 1 s. After a 1 s blank screen, a new token appeared. The previous token’s value was added to the participant’s total earnings, which were displayed only at the end of each scanning run. Setting the token’s initial value to 0¢ meant that, unlike earlier work using this paradigm13, participants received no immediate reward upon quitting. This served to simplify the task without significantly altering either its incentive structure or the resulting pattern of behavior.
A white progress bar marked the amount of time the current token had been on the screen. The bar’s full length corresponded to 100 s. It grew continuously from the left and reset when a new token appeared. The progress bar was included to reduce interval-timing demands and discourage a strategy of covertly counting time.
The scanning session was divided into four 10-min runs. New tokens were presented until time was up. Each run presented one timing environment (i.e., token color). The two environments alternated in successive runs. The order of environments and the mapping of token color to environment were counterbalanced across participants.
Each participant completed a preliminary behavioral training session consisting of 4 10-min runs alternating between the HP and LP environments. Participants were explicitly instructed that the green and purple tokens might differ in their timing, but that they had to learn the nature of the differences from direct experience and were free to adopt any behavioral strategy they preferred. During behavioral training (but not during scanning) the screen displayed the time left in the 10-min run and the amount earned so far, to help ensure that participants understood the structure of the task. Each token during behavioral training was worth 10¢. Participants explored the task environments during training, waiting through full 90s delays in the LP condition on a median of 3.5 trials (IQR 1 to 5.5; >0 for 18/20 subjects). Participants completed two additional 5-min runs (one per condition) outside the scanner just before the fMRI session. Waiting behavior over time across training, practice, and fMRI sessions is plotted in Supplementary Fig. 1.
Participants would have faced fundamentally the same trial-by-trial decision problem if they had received explicit information about the probabilistic contingencies in lieu of experience-based training (cf. Luhmann et al.53). However, there is evidence that probabilistic information is encoded differently when learned from description vs. direct experience54–56; our training procedure was designed to involve the type of experience-based, implicit statistical learning that is thought to guide beliefs and expectations in real-world domains29, including ecological foraging environments. Future work might introduce explicit information to help assess whether deviations from optimal behavior were due to inexact encoding of the relevant probabilities.
The delay duration on each trial was randomly drawn from a discrete probability distribution (Fig 1b). In the HP environment delays were drawn uniformly from the values 5, 10, 15, 20, 25, 30, 35, and 40s. In the LP environment delays were set to 90s with probability 0.5, and otherwise drawn uniformly from the values 5, 10, 15, and 20s. By design, reward probabilities were identical between the two environments for the first 20s of the delay, the period of greatest interest in our neuroimaging analyses. We imposed longer delays here than in our previous work13 in order to obtain fluctuations in subjective value across a time period on the order of 30s, which is well suited for detecting BOLD effects (this corresponds to the time scale of a blocked design with ~15 s blocks; see further discussion and simulation results below).
Delays were sampled in a pseudorandom manner that approximately balanced the first-order transition statistics between delays in successive trials. This helped ensure that the scheduled delays were representative of the ground-truth distribution, while avoiding the negative autocorrelation that would result from strictly balanced frequencies.
The HP environment was richer by design, with all participants receiving more rewards in the HP environment (median=44, IQR 41 to 46.5) than in the LP environment (median=25, IQR 21 to 26). The difference in overall richness was not the factor that determined the ideal behavioral strategy (one could design richer LP environments and poorer HP environments13), but emerged here as a side-effect of our decision to match the sizes of individual rewards and the reward probabilities over the first 20s. These design choices maximized the comparability of the two conditions for purposes of our neuroimaging contrasts.
We quantified behavioral persistence using Kaplan-Meier survival curves57, which estimated the probability of “surviving” various lengths of time without quitting. This technique accommodated the fact that reward delivery censored observed waiting times13.
Modeling ideal performance
The rate-maximizing strategy was to wait through all delays in the HP environment (up to 40 s), but to give up after 20 s in the LP environment. We determined this by calculating the expected rate of return for various giving-up times (Fig. 1c). This calculation follows previous work13, and has precedent in stochastic foraging models25.
The reward’s arrival time treward is a random variable. For a policy of quitting at time T, let pT equal the probability of receiving the reward, pT = Pr(treward ≤ T), and let τT equal the expected delay if the reward is received, τT = E(treward | treward ≤ T). Each trial’s expected rate of return, in ¢/s, is:
| (1) |
The numerator is a trial’s expected gain in cents and the denominator is a trial’s expected cost in seconds, assuming a 30¢ reward and a 2 s inter-trial interval. The goal is to find the value of T that maximizes RT. We use R* to denote the best available rate of return. Fig. 1c plots RT as a function of T. The best policy in the HP environment was to wait 40s (R* = 1.22¢/s), whereas the best policy in the LP environment was to wait 20s (R* = 0.82¢/s).
Modeling subjective value as a function of elapsed time
At each point in a trial, the token's subjective value depended on three factors: (1) the expected earnings from that token, (2) the expected additional time to be spent on that token, and (3) the monetary value of time, which corresponds to R* from above. We denote the expected earnings as aT(t) and the expected time as bT(t). Each of these depends jointly on the current elapsed time t and the intended future quitting time T. For given values of t and T, the expected return is:
| (2) |
The current trial’s subjective value (denoted “potential” in the model’s original formulation25) equals the maximum value of gT across all possible quitting times:
| (3) |
Put differently, g(t) is the expected net return in the remainder of the current trial, accounting for the cost of time, under the best available waiting policy. Its minimum is zero because there is always an option to quit immediately (we treat the ITI as part of the subsequent trial). The decision maker should continue waiting if g(t) > 0.
Fig. 3a shows g(t) as a function of t in each environment (see Supplementary Fig. 2 for decomposition of g(t) into its components). The function approaches 30¢ at the last possible reward time, when a 30¢ reward is expected with no further delay. The best strategy is to wait up to 40 s in the HP environment but quit at 20 s in the LP environment. If a decision maker in the LP environment were to have waited 53.5 s already it would be better at that point to continue waiting for the reward that was sure to arrive at 90 s. We obtained very similar results if we used each participant’s actual environment-specific reward rate in place of the theoretical maximum, R* (Supplementary Fig. 6).
Behavior could be well characterized as a stochastic function of theoretical subjective value. To evaluate this we represented each subject’s behavior as a series of pseudo-choices between waiting and quitting, placed every 1s throughout all delay intervals in the experiment. We then modeled pseudo-choice outcomes (1=wait, 0=quit) as a function of subjective value and a constant intercept in subject-wise logistic regressions. Subject-wise maximum-likelihood coefficients were tested at the group level using a Wilcoxon signed-rank test. We additionally used likelihood-ratio tests at the single-subject level to compare the full model to the (nested) intercept-only model, and tested the resulting z statistics at the group level. Finally, we tested an alternative model that, in place of subjective value, coded the HP and LP conditions categorically. This model had the same number of parameters as the subjective value model and could represent the possibility that participants merely quit more often in the LP than HP environment. Subject-wise differences in model deviance were tested against zero using a group-level Wilcoxon signed-ranks test.
Allowing for endogenous temporal uncertainty did not substantially alter the theoretical results described above. Fig. 2c displays hypothetical continuous hazard functions allowing for subjective uncertainty in time-interval perception58. For an interval of true duration t, subjective uncertainty is typically well characterized by a Gaussian distribution with mean µ = t and standard deviation σ = t × CV, where CV is a fixed coefficient of variation. We modeled temporal uncertainty by converting each discrete distribution in Fig. 1b to a Gaussian mixture distribution. A Gaussian component was placed at each possible reward time t, with µ = t, σ = t × CV, and weight equal to Pr(treward=t). We set CV=0.16 on the basis of human behavioral findings (the median CV from Table 2 of Rakitin et al.59 after converting the unit of variability from full-width-at-half-maximum to SD). The continuous functions in Fig. 2c are scaled by a factor of 5 for comparability with the corresponding discrete functions.
Blurring the ground-truth timing distributions to allow for subjective uncertainty did not change any of our model-based theoretical predictions. If rates of return (Fig. 1c) were calculated using the Gaussian mixture distribution, the best policy was to wait 40 s in the HP environment and 22.1 s in the LP environment. Endogenous uncertainty smoothed the theoretical subjective value functions (Fig. 3a) without altering their general shape.
MRI data acquisition and preprocessing
MRI data were acquired on a 3T Siemens Trio with a 32-channel head coil. Functional data were acquired using a gradient-echo echoplanar imaging (EPI) sequence (3mm isotropic voxels, 64×64 matrix, 44 axial slices tilted 30° from the AC-PC plane, TR=2500 ms, TE=25 ms, flip angle=75°). There were 4 runs, each with 246 images (10 min, 15 s). At the end of the session we acquired matched fieldmap images (TR=1000 ms, TE=2.69 and 5.27 ms, flip angle=60°) and a T1-weighted MPRAGE structural image (0.9375×0.9375×1 mm voxels, 192×256 matrix, 160 axial slices, TI=1100 ms, TR=1630 ms, TE=3.11 ms, flip angle=15°).
Data were preprocessed using FSL60–63 and AFNI64, 65 software. Functional data were temporally aligned to midpoint of each acquisition (AFNI's 3dTshift), motion corrected (FSL's MCFLIRT), undistorted and warped to MNI space (see below), outlier-attenuated (AFNI's 3dDespike), smoothed with a 6 mm FWHM Gaussian kernel (FSL's fslmaths), and intensity-scaled by a single grand-mean value per run. To warp the data to MNI space, functional data were aligned to the structural image (FSL's FLIRT) using boundary-based registration66, simultaneously incorporating fieldmap-based geometric undistortion. Separately, the structural image was nonlinearly coregistered to the MNI template (FSL's FLIRT and FNIRT). The two transformations were concatenated and applied to the functional data.
fMRI analysis
Voxelwise general linear models (GLMs) were fit using ordinary least squares (AFNI's 3dDeconvolve). GLMs were estimated for each subject individually using data concatenated across the 4 runs. There were 12 baseline terms per run: a constant, 5 low-frequency drift terms (first-through-fifth-order Legendre polynomials), and 6 motion parameters.
Event-related BOLD signal timecourses were flexibly estimated by fitting piecewise linear splines (“tent” basis functions). For trial-onset-locked timecourses, basis functions were centered every 2.5 s beginning at 2.5 s and ending 1 s before the end of each trial (for example, the basis function regressor corresponding to “10 s” had a peak 10 s after trial onset for every trial that lasted at least 11 s). For reward-related and quit-related timecourses, basis functions were centered every 2.5 s from 12.5 s before to 12.5 s after the event.
We conducted simulations to confirm the validity of our analysis procedures. We calculated theoretical subjective value over the course of each subject’s entire experimental session using the actual timing of task events together with the ideal model in Fig. 3a. These full-session timecourses were convolved with a hemodynamic response function (HRF) to generate subject-specific synthetic BOLD timecourses representing idealized theoretical predictions. In order to verify that the theoretical signal had a suitable time scale and could be distinguished from baseline drift, we fit these synthetic BOLD timecourses in a GLM that contained only the constant and drift terms. For each subject, the residuals were highly correlated with a merely de-meaned version of the original synthetic BOLD timecourses (median r2=0.90, IQR 0.88 to 0.93), indicating that the theoretical signal could indeed be clearly distinguished from baseline fluctuations. Next we used the synthetic BOLD timecourses as inputs to the analysis procedure described above for estimating trial-onset-locked timecourses. The resulting timecourses, shown in averaged form in Fig. 3d, constituted our subject-by-subject theoretical predictions.
The model-based analysis was performed voxelwise on all 20 subjects across 2.5–30 s from trial onset. Each subject’s empirically estimated difference timecourse (HP minus LP) was regressed on the theoretical difference timecourse (Fig. 3d) together with a constant intercept. Using the simplified HRF-convolved theoretical timecourses in Fig. 3c yielded equivalent results. Timepoints lacking data in either environment for a given subject were omitted (this resulted in the omission of 3 timepoints for one subject; see Supplementary Fig. 3). We adopted a two-step approach (first estimating the timecourses and then submitting them to the model-based contrast) so that included timepoints were weighted uniformly. Otherwise, early timepoints, which were sampled more frequently (Supplementary Fig. 3), would have received greater weight, and the pattern of timepoint weighting could have differed between environments for individual subjects. Contrast coefficients were tested against zero at the group level in 2-tailed voxelwise t-tests.
An additional open-ended analysis tested for condition-by-timepoint interactions in the trial-onset-locked BOLD timecourses (using n=19 participants with complete data; Supplementary Fig. 3). The main effect of timepoint is of limited interest because it captures nonspecific effects of time-from-keypress; similarly, the main effect of condition is uninformative because the two conditions were presented in separate runs with independent baselines. The condition-by-timepoint interaction tests for a difference in BOLD trajectories between the two environments without constraining the form of the difference. In a repeated-measures framework this is equivalent to testing the main effect of timepoint on the difference in signal between the two environments. Accordingly, we performed a voxelwise one-way repeated-measures ANOVA on the difference timecourses (HP minus LP) at the group level. An equivalent procedure was used to compare BOLD timecourses aligned to reward-related and quit-related keypresses.
The RPE analysis was limited to the HP environment, in which the sustained rise in reward expectancy supported clear predictions. Within a GLM we estimated FIR coefficients for the peri-reward timecourse (from 7.5 s before to 10 s after each reward). Eight terms modeled the mean timecourse, and another 8 terms modeled amplitude modulation at each timepoint as a function of the preceding delay duration. We then computed a contrast of the modulatory effect for 3 post-reward timepoints (2.5 to 7.5 s) minus 3 earlier timepoints (–5 to 0 s). The value of this contrast reflected modulation of the phasic reward response as a function of preceding delay time, over and above any nonspecific effect of elapsed time on the pre-reward baseline.
All whole-brain, group-level analyses assessed statistical significance on the basis of cluster mass, with the cluster-defining threshold set to the nominal p<0.01 level. Corrected p-values were determined using permutation testing67 (FSL's randomise; 5000 iterations), and results were thresholded at corrected p<0.05. For F tests, each random iteration shuffled timepoints within subject. For one-sample t-tests, each iteration randomly sign-flipped individual subjects’ coefficient maps.
Heart rate data acquisition and analysis
Pulse oximetry data were recorded at 50 Hz using the MRI system’s built-in oximeter, which also performed automatic heartbeat detection. Timestamped data were successfully recorded for 17 of the 20 participants. Heartbeat times were converted to inter-beat interval (IBI). IBI values farther than 30% above or below the grand median were treated as missing (median = 1.6% of points removed; IQR 0.5 to 3.6%). Since IBI varied across individuals (median=820 ms; IQR 760 to 950 ms), IBI values were converted to a percentage of the individual’s grand median. Mean perievent timecourses were calculated on a 0.25 s grid for each subject and event type. For comparisons, timecourses for two event types were subtracted to yield single-subject difference timecourses, which were then tested at the group level for significant excursions from zero. Entire timecourses were tested using cluster-based control for multiple comparisons. Cluster size was defined as the number of adjacent timepoints with nominal p<0.05 in single-timepoint Wilcoxon signed-rank tests. A cluster was assigned a corrected p-value based on its percentile in the empirical null distribution for cluster size, which was obtained via permutation testing (10,000 iterations with randomized sign-flipping of individual subjects’ difference timecourses).
A supplementary methods checklist is available.
Supplementary Material
Acknowledgements
This research was supported by NIH grants DA030870 to JTM and DA029149 to JWK.
Footnotes
Author contributions
J.T.M and J.W.K. designed the experiment, developed the analysis procedures, and wrote the paper. J.T.M. collected and analyzed the data and developed the theoretical model.
Contributor Information
Joseph T. McGuire, Email: mcguirej@psych.upenn.edu.
Joseph W. Kable, Email: kable@psych.upenn.edu.
References
- 1.Mischel W, Ebbesen EB. Attention in delay of gratification. Journal of Personality and Social Psychology. 1970;16:329–337. doi: 10.1037/h0032198. [DOI] [PubMed] [Google Scholar]
- 2.Baumeister RF, Vohs KD, Tice DM. The strength model of self-control. Current Directions in Psychological Science. 2007;16:351–355. [Google Scholar]
- 3.Bartra O, McGuire JT, Kable JW. The valuation system: A coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. NeuroImage. 2013;76:412–427. doi: 10.1016/j.neuroimage.2013.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clithero JA, Rangel A. Informatic parcellation of the network involved in the computation of subjective value. Social Cognitive and Affective Neuroscience. 2013 doi: 10.1093/scan/nst106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu X, Hairston J, Schrier M, Fan J. Common and distinct networks underlying reward valence and processing stages: A meta-analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews. 2011;35:1219–1236. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levy DJ, Glimcher PW. The root of all value: A neural common currency for choice. Current Opinion in Neurobiology. 2012;22:1027–1038. doi: 10.1016/j.conb.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nature Neuroscience. 2007;10:1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kable JW, Glimcher PW. An "as soon as possible" effect in human intertemporal decision making: Behavioral evidence and neural mechanisms. Journal of Neurophysiology. 2010;103:2513–2531. doi: 10.1152/jn.00177.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- 10.Mischel W, Ayduk O, Mendoza-Denton R. Sustaining delay of gratification over time: A hot-cool systems perspective. In: Loewenstein G, Read D, Baumeister RF, editors. Time and decision: Economic and psychological perspectives on intertemporal choice. New York: Russell Sage Foundation; 2003. pp. 175–200. [Google Scholar]
- 11.Metcalfe J, Mischel W. A hot/cool-system analysis of delay of gratification: dynamics of willpower. Psychological Review. 1999;106:3–19. doi: 10.1037/0033-295x.106.1.3. [DOI] [PubMed] [Google Scholar]
- 12.McGuire JT, Kable JW. Rational temporal predictions can underlie apparent failures to delay gratification. Psychological Review. 2013;120:395–410. doi: 10.1037/a0031910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGuire JT, Kable JW. Decision makers calibrate behavioral persistence on the basis of time-interval experience. Cognition. 2012;124:216–226. doi: 10.1016/j.cognition.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rachlin H. The science of self-control. Cambridge, MA: Harvard University Press; 2000. [Google Scholar]
- 15.Dasgupta P, Maskin E. Uncertainty and hyperbolic discounting. American Economic Review. 2005;95:1290–1299. [Google Scholar]
- 16.Kim H, Shimojo S, O'Doherty JP. Overlapping responses for the expectation of juice and money rewards in human ventromedial prefrontal cortex. Cerebral Cortex. 2011;21:769–776. doi: 10.1093/cercor/bhq145. [DOI] [PubMed] [Google Scholar]
- 17.Hare TA, Malmaud J, Rangel A. Focusing attention on the health aspects of foods changes value signals in vmPFC and improves dietary choice. Journal of Neuroscience. 2011;31:11077–11087. doi: 10.1523/JNEUROSCI.6383-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hampton AN, Bossaerts P, O'Doherty JP. The role of the ventromedial prefrontal cortex in abstract state-based inference during decision making in humans. Journal of Neuroscience. 2006;26:8360–8367. doi: 10.1523/JNEUROSCI.1010-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daw ND, Gershman SJ, Seymour B, Dayan P, Dolan RJ. Model-based influences on humans' choices and striatal prediction errors. Neuron. 2011;69:1204–1215. doi: 10.1016/j.neuron.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hutcherson CA, Plassmann H, Gross JJ, Rangel A. Cognitive regulation during decision making shifts behavioral control between ventromedial and dorsolateral prefrontal value systems. Journal of Neuroscience. 2012;32:13543–13554. doi: 10.1523/JNEUROSCI.6387-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casey BJ, et al. Behavioral and neural correlates of delay of gratification 40 years later. Proceedings of the National Academy of Sciences. 2011;36:14998–15003. doi: 10.1073/pnas.1108561108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Figner B, et al. Lateral prefrontal cortex and self-control in intertemporal choice. Nature Neuroscience. 2010;13:538–539. doi: 10.1038/nn.2516. [DOI] [PubMed] [Google Scholar]
- 23.Heatherton TF, Wagner DD. Cognitive neuroscience of self-regulation failure. Trends in Cognitive Sciences. 2011;15:132–139. doi: 10.1016/j.tics.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charnov EL. Optimal foraging, the marginal value theorem. Theoretical Population Biology. 1976;9:129–136. doi: 10.1016/0040-5809(76)90040-x. [DOI] [PubMed] [Google Scholar]
- 25.McNamara J. Optimal patch use in a stochastic environment. Theoretical Population Biology. 1982;21:269–288. [Google Scholar]
- 26.Hayden BY, Pearson JM, Platt ML. Neuronal basis of sequential foraging decisions in a patchy environment. Nature Neuroscience. 2011;14:933–939. doi: 10.1038/nn.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fawcett TW, McNamara JM, Houston AI. When is it adaptive to be patient? A general framework for evaluating delayed rewards. Behavioural Processes. 2012;89:128–136. doi: 10.1016/j.beproc.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 28.Nickerson RS. Response time to the second of two successive signals as a function of absolute and relative duration of intersignal interval. Perceptual and Motor Skills. 1965;21:3–10. doi: 10.2466/pms.1965.21.1.3. [DOI] [PubMed] [Google Scholar]
- 29.Griffiths TL, Tenenbaum JB. Optimal predictions in everyday cognition. Psychological Science. 2006;17:767–773. doi: 10.1111/j.1467-9280.2006.01780.x. [DOI] [PubMed] [Google Scholar]
- 30.Montague PR, Dayan P, Sejnowski TJ. A framework for mesencephalic dopamine systems based on predictive Hebbian learning. Journal of Neuroscience. 1996;16:1936–1947. doi: 10.1523/JNEUROSCI.16-05-01936.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fiorillo CD, Newsome WT, Schultz W. The temporal precision of reward prediction in dopamine neurons. Nature Neuroscience. 2008;11:966–973. doi: 10.1038/nn.2159. [DOI] [PubMed] [Google Scholar]
- 32.Cui X, Stetson C, Montague PR, Eagleman DM. Ready…go: Amplitude of the FMRI signal encodes expectation of cue arrival time. PLoS biology. 2009;7:e1000167. doi: 10.1371/journal.pbio.1000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bueti D, Bahrami B, Walsh V, Rees G. Encoding of temporal probabilities in the human brain. Journal of Neuroscience. 2010;30:4343–4352. doi: 10.1523/JNEUROSCI.2254-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tootell RBH, et al. The retinotopy of visual spatial attention. Neuron. 1998;21:1409–1422. doi: 10.1016/s0896-6273(00)80659-5. [DOI] [PubMed] [Google Scholar]
- 35.Lacey JI, Lacey BC. Some autonomic-central nervous system interrelationships. In: Black P, editor. Physiological correlates of emotion. New York: Academic Press; 1970. pp. 205–227. [Google Scholar]
- 36.Schweighofer N, et al. Humans can adopt optimal discounting strategy under real-time constraints. PLoS Computational Biology. 2006;2:e152. doi: 10.1371/journal.pcbi.0020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loewenstein G. Anticipation and the valuation of delayed consumption. The Economic Journal. 1987;97:666–684. [Google Scholar]
- 38.Duckworth AL, Gendler TS, Gross JJ. Self-control in school-age children. Educational Psychologist. 2014;49:199–217. [Google Scholar]
- 39.Jimura K, Chushak MS, Braver TS. Impulsivity and self-control during intertemporal decision making linked to the neural dynamics of reward value representation. Journal of Neuroscience. 2013;33:344–357. doi: 10.1523/JNEUROSCI.0919-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Helfinstein SM, et al. Predicting risky choices from brain activity patterns. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:2470–2475. doi: 10.1073/pnas.1321728111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rushworth MFS, Kolling N, Sallet J, Mars RB. Valuation and decision-making in frontal cortex: One or many serial or parallel systems? Current Opinion in Neurobiology. 2012;22:946–955. doi: 10.1016/j.conb.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 42.Shenhav A, Straccia MA, Cohen JD, Botvinick MM. Anterior cingulate engagement in a foraging context reflects choice difficulty, not foraging value. Nature Neuroscience. 2014;17:1249–1254. doi: 10.1038/nn.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blanchard TC, Hayden BY. Neurons in dorsal anterior cingulate cortex signal postdecisional variables in a foraging task. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:646–655. doi: 10.1523/JNEUROSCI.3151-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McClure SM, Berns GS, Montague PR. Temporal prediction errors in a passive learning task activate human striatum. Neuron. 2003;38:339–346. doi: 10.1016/s0896-6273(03)00154-5. [DOI] [PubMed] [Google Scholar]
- 45.Hollerman JR, Schultz W. Dopamine neurons report an error in the temporal prediction of reward during learning. Nature Neuroscience. 1998;1:304–309. doi: 10.1038/1124. [DOI] [PubMed] [Google Scholar]
- 46.Berns GS, McClure SM, Pagnoni G, Montague PR. Predictability modulates human brain response to reward. Journal of Neuroscience. 2001;21:2793–2798. doi: 10.1523/JNEUROSCI.21-08-02793.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hare TA, O'Doherty J, Camerer CF, Schultz W, Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. Journal of Neuroscience. 2008;28:5623–5630. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Howe MW, Tierney PL, Sandberg SG, Phillips PEM, Graybiel AM. Prolonged dopamine signalling in striatum signals proximity and value of distant rewards. Nature. 2013;500:575–579. doi: 10.1038/nature12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miyazaki KW, et al. Optogenetic activation of dorsal raphe serotonin neurons enhances patience for future rewards. Current Biology. 2014;24:2033–2040. doi: 10.1016/j.cub.2014.07.041. [DOI] [PubMed] [Google Scholar]
- 50.Janssen P, Shadlen MN. A representation of the hazard rate of elapsed time in macaque area LIP. Nature Neuroscience. 2005;8:234–241. doi: 10.1038/nn1386. [DOI] [PubMed] [Google Scholar]
- 51.Brainard DH. The psychophysics toolbox. Spatial Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- 52.Pelli DG. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision. 1997;10:437–442. [PubMed] [Google Scholar]
- 53.Luhmann CC, Chun MM, Yi D-J, Lee D, Wang X-J. Neural dissociation of delay and uncertainty in intertemporal choice. Journal of Neuroscience. 2008;28:14459–14466. doi: 10.1523/JNEUROSCI.5058-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ungemach C, Chater N, Stewart N. Are probabilities overweighted or underweighted when rare outcomes are experienced (rarely)? Psychological Science. 2009;20:473–479. doi: 10.1111/j.1467-9280.2009.02319.x. [DOI] [PubMed] [Google Scholar]
- 55.FitzGerald THB, Seymour B, Bach DR, Dolan RJ. Differentiable neural substrates for learned and described value and risk. Current Biology. 2010;20:1823–1829. doi: 10.1016/j.cub.2010.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hertwig R, Barron G, Weber EU, Erev I. Decisions from experience and the effect of rare events in risky choice. Psychological Science. 2004;15:534–539. doi: 10.1111/j.0956-7976.2004.00715.x. [DOI] [PubMed] [Google Scholar]
- 57.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- 58.Gibbon J. Scalar expectancy theory and Weber's law in animal timing. Psychological Review. 1977;84:279–325. [Google Scholar]
- 59.Rakitin BC, et al. Scalar expectancy theory and peak-interval timing in humans. Journal of Experimental Psychology: Animal Behavior Processes. 1998;24:15–33. doi: 10.1037//0097-7403.24.1.15. [DOI] [PubMed] [Google Scholar]
- 60.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 61.Smith SM, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 62.Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. NeuroImage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 63.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 64.Cox RW. AFNI: What a long strange trip it's been. NeuroImage. 2012;62:743–747. doi: 10.1016/j.neuroimage.2011.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 66.Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. NeuroImage. 2009;48:63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: A primer with examples. Human Brain Mapping. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.