Abstract
Objective
Recent data suggest patients with epithelial ovarian cancers on statin therapy have improved survival. We have hypothesized that statins influence ovarian cancer outcome through alteration of lipoprotein profiles, and sought to determine correlations between lipoprotein levels and survival in women with advanced stage disease.
Methods
After IRB approval, we identified patients with stage IIIC/IV epithelial ovarian cancer with banked prediagnostic fasting serum. Serum was assayed for levels of total cholesterol (TC), high-density lipoprotein (HDL), and triglycerides (TG). LDL was calculated by subtraction of TG/5 and HDL from TC. Data were examined using Fisher's exact, Kaplan–Meier, and Cox regression analyses.
Results
One hundred thirty-two patients were studied. Twenty-six percent of patients had elevated LDL; 18% had elevated TC; 32% had elevated TG; and 48% had elevated HDL. No univariate associations were identified between elevated TC, HDL, TG, LDL and age, stage IV disease, high grade, or optimal cytoreduction. Median progression-free survival for patients with normal LDL levels was 27 months, compared to 12 months for patients with elevated LDL (p = 0.0004). Overall disease-specific survival was longer for patients with normal LDL levels (59 months) compared to those with elevated LDL (51 months, p = 0.04). Multivariate analysis indicated that LDL retained significance as an independent predictor of survival, after controlling for age, stage, grade, and suboptimal cytoreduction (p = 0.003).
Conclusions
These data suggest LDL is a significant predictor of clinical outcome, and warrant the further study of lipoproteins and statins on epithelial ovarian cancer biology.
Keywords: Ovarian cancer, Low-density lipoprotein, Survival
Introduction
Cardiovascular disease continues to be the leading cause of death in the United States, and the role of dyslipidemias in its pathogenesis is well documented. With the discovery of 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase inhibitors, or statins, application of these highly effective lipid-lowering therapeutics has grown in prevalence. While data examining statin use and risk of cancer remain controversial, other observational studies have suggested statins may significantly influence clinical outcome in hormone-driven solid malignancies. For example, in a large cohort of early stage breast cancer survivors, initiating statin therapy after diagnosis was associated with improved prognosis, and risk of recurrence decreased statistically with increasing duration of statin use [1]. In prostate cancer, the Health Professionals Follow-up Study identified a significantly lower risk of metastatic and fatal disease in statin users, and the risk of advanced disease was also statistically lower with longer statin use [2].We have previously examined an institutional cohort of women with advanced stage epithelial ovarian cancers, and observed a statistically longer time to progression and improved overall survival for those patients also on statin therapy. In this analysis, statin use retained significance as an independent positive prognostic factor [3].
The mechanisms by which statins modulate cancer biology and clinical outcome are not well established. Potential pro-apoptotic and antimetastatic properties of statins may result from altered cellular signaling pathways through inhibition of isoprenylation in the cholesterol cascade. Peptide dependent on this process include Ras, nuclear lamins, transducin g, rhodopsin kinase, Rho, and all of the heterotrimeric and small G proteins [reviewed in [4]. Alternatively, statins may modulate cancer growth and metastasis directly through lowering serum lipoprotein abundance. Cholesterol is a critical component in cell membranes, and in vitro studies, including in ovarian epithelial cells, suggest lipids promote tumor growth [5–7]. Furthermore, cholesterol is a known precursor in steroid hormone synthesis, which may underscore the potential mechanism by which lipid-lowering medications might impact breast, prostate, and ovarian malignancies.
In order to further explore the underlying relationship between statins and epithelial ovarian cancer biology, we have hypothesized that elevated lipoproteins correlate with clinical outcome in advanced stage disease. Our objectives in this study were to characterize lipid profiles in a cohort of women with stage III or IV epithelial ovarian or primary peritoneal cancer, and identify potential associations with clinico-pathologic prognostic factors and survival.
Materials and methods
The Gynecologic Oncology service at Cedars-Sinai Medical Center maintains an Institutional Review Board (IRB)-approved prospective database of patients with banked tissue and serum. Patients undergoing surgery by a gynecologic oncologist are routinely approached to participate in donation of tissue and/or serum prior to diagnostic surgery. We queried this database under a separate IRB-approved protocol for patients with epithelial ovarian or primary peritoneal carcinoma with available pre-diagnostic fasting serum. We specified selection criteria to include patients who had undergone primary exploratory laparotomy with the intent of complete surgical resection of metastatic disease, followed by at least six cycles of platinum- and taxane-based adjuvant chemotherapy. Only patients with stage III or IV disease were included for study, and no patients underwent intraperitoneal chemotherapy. Patients on concurrent statin therapy were specifically excluded from this cohort. Finally, patients with other malignancies, non-epithelial tumor histologies, borderline tumors, and those who underwent neoadjuvant chemotherapy were also excluded.
We defined optimal surgical resection as residual disease less than 1 cm. Patients with subsequent recurrent disease were treated with surgery and/or chemotherapy at the discretion of the treating physician.
We assayed frozen serum for levels of total cholesterol (TC), high-density lipoprotein (HDL), and triglycerides (TG) using the ATAC 8000 Random Access Chemistry System (Elan Diagnostics, Brea, CA). By convention, LDL was calculated by subtraction of TG/5 and HDL from TC. Medical records for all eligible patients were reviewed and abstracted data included clinico-pathologic factors and time to disease recurrence and death. Patients at our institution do not undergo routine lipid evaluations prior to diagnostic exploratory laparotomy, and so those with hyperlipidemias and/or hypertriglyceridemias were presumed to be undiagnosed. All lipid assays were performed after recruitment of the retrospective cohort, and thus no data regarding elevated levels were available to clinicians prior to surgery.
For statistical considerations, we followed guidelines published by the American Heart Association, and defined elevated lipid levels as TC>201, LDL>101, TG>151, and HDL>51 mg/dl [8]. Data were analyzed using Fisher's exact test, Chi square, Kaplan–Meier survival, and Cox regression analyses. A p value of less than 0.05 was considered to be statistically significant.
Results
One hundred thirty-two patients were included in this analysis. The mean age of the entire cohort was 60 years (range, 30–89). The majority of patients had stage III (115, or 87%) and grade 3 disease (122, or 92%) with papillary serous histology (122, or 92%). One hundred nineteen patients (92%) underwent optimal cytoreductive surgery at initial exploration to residual disease less than 1 cm. No patients in this cohort were taking statins at time of diagnosis.
Lipoprotein assays revealed mean levels as follows: TC, 155.68 mg/dl (range, 42.15–302.20); HDL, 42.12 mg/dl (range, 14.55–85.50); TG, 155.80 mg/dl (range, 49.00–1717.50); and LDL, 84.91 mg/dl (range, 30.82–188.18). Twenty-four (18%) patients were considered as having elevated TC levels; 63 (48%) with elevated HDL; 42 (32%) with elevated TG; and 35 (27%) patients with elevated LDL.
To determine whether specific lipoproteins correlated with established clinical and pathologic prognostic factors in this disease, we performed univariate analyses for TC, HDL, TG, and LDL. There were no statistical associations between TC, HDL, or TG and age, stage IV disease, high grade tumors, non-serous histology, or incidence of suboptimal surgical cytoreduction (data not shown). For LDL, distribution of age, stage and grade of disease, incidence of non-serous histologies, and incidence of suboptimal resection were also evenly matched (Table 1).
Table 1.
Distribution of clinico-pathologic prognosticators between patients with and without elevated serum LDL.
| Mean age (years) |
Stage IV | Grade 3 | Non-serous histology |
Suboptimal cytoreduction |
|
|---|---|---|---|---|---|
| Normal LDL (n = 98) | 60 | 14 (14%) | 91 (93%) | 7 (7%) | 9 (9%) |
| Elevated LDL (n = 34) | 61 | 3 (9%) | 31 (91%) | 3 (9%) | 4 (12%) |
| P | 0.46 | 0.56 | 0.72 | 0.72 | 0.74 |
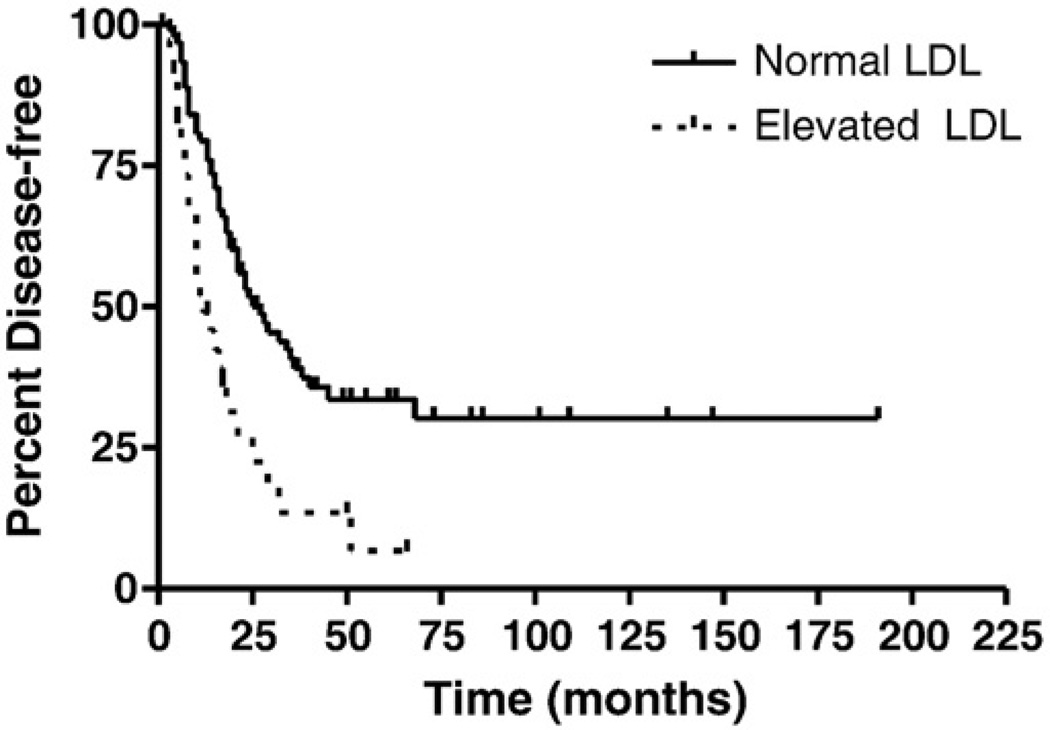
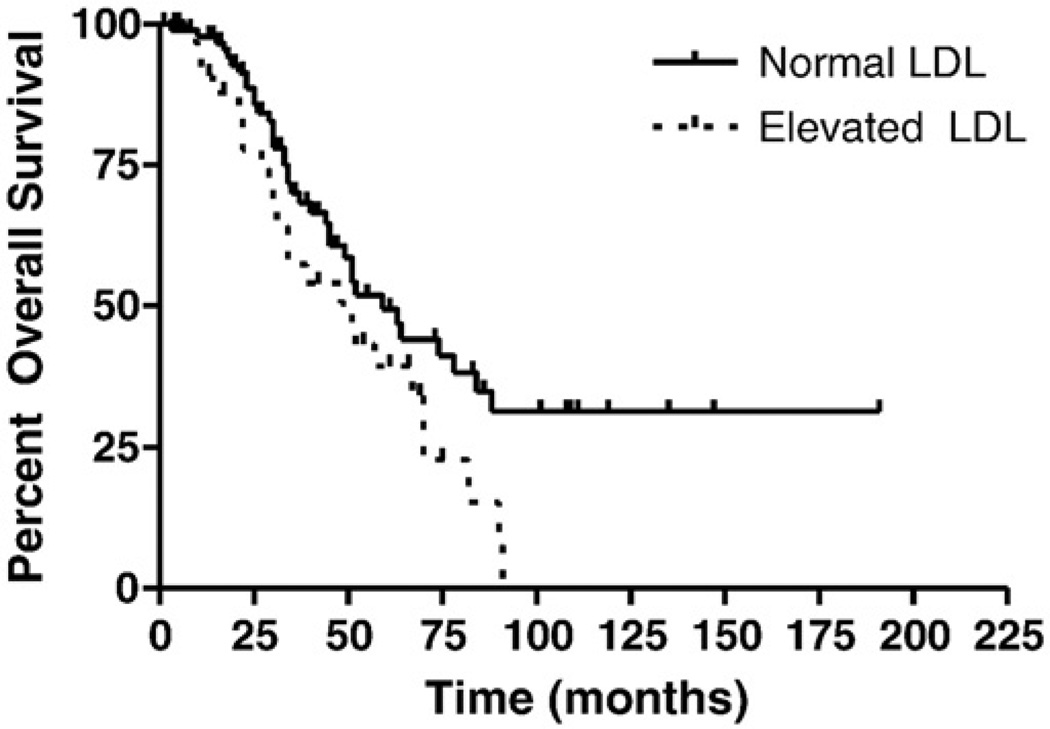
To examine correlations between elevated lipoprotein levels and disease progression and overall survival, we performed Kaplan-Meier survival analyses. In this cohort, no patients died of non-disease related events. Using a 2-sided log-rank test, a statistical difference in time to recurrence was observed for patients with normal LDL levels (median progression-free survival of 27 months) compared to those with elevated LDL (12 months, p = 0.0004; Fig. 1). A statistically significant difference was also identified for overall disease-specific survival, with a median survival of 59 months for patients with normal LDL levels, compared to 51 months for those with elevated LDL (p = 0.04; Fig. 2). Analyses for both progression-free and overall disease-specific survival did not identify statistical differences for patients with elevated TC, TG, or HDL.
Fig. 1.
Effect of LDL levels on progression-free survival. Median progression-free survival was statistically longer in patients with normal LDL (27 months), compared to those with elevated LDL (12 months, p = 0.0004).
Fig. 2.
Effect of LDL levels on overall disease-specific survival. Overall disease-specific survival was statistically longer in patients with normal LDL (59 months) compared to those with elevated LDL (51 months, p = 0.04).
While outcome data in this analysis was abstracted for disease-specific survival, dyslipidemias may be associated with patient co-morbidities that could influence treatment plans. Review of admission data from the gynecologic oncologist, the referring internist, and from nursing records could not identify statistical differences in the incidence of hypertension (21% for those with elevated LDL compared to 29% with normal LDL levels, p = 0.63), diabetes mellitus (3% compared to 7%, p = 0.68), or coronary artery disease (3% compared to 9%, p = 0.44). The mean body mass index was 25.1 mg/m2 for women with and without elevated LDL.
Finally, in order to determine the independent prognostic impact of LDL levels in this cohort, we performed multivariate Cox regression analyses. Established prognostic factors as well as LDL levels (examined as a continuous variable) were included in the analysis. We observed that LDL levels retained statistical significance after controlling for age, stage, grade and optimal cytoreduction (p = 0.003; Table 2).
Table 2.
Multivariable Cox proportional hazard analysis of potential prognostic factors on survival.
| Variable | Hazard ratio | 95% Confidence interval | p value |
|---|---|---|---|
| Agea | 1.0471 | 0.6138–1.7861 | 0.86 |
| Stageb | 0.9795 | 0.6247–1.5371 | 0.93 |
| Gradea | 0.9363 | 0.5302–1.6537 | 0.82 |
| Optimal cytoreduction | 0.9988 | 0.9938–1.0038 | 0.64 |
| LDLa | 1.0087 | 1.0029–1.0146 | 0.003 |
Examined as a continuous variable.
Stage III versus stage IV.
Discussion
We have hypothesized that statin use may influence epithelial ovarian cancer biology through alteration of lipoprotein profiles. In this study, we sought to determine potential correlations between lipoprotein levels and survival in women with advanced stage disease. While the high prevalence of grade 3 and stage III disease may have limited identifying differences in these factors between patients with and without elevated LDL levels, we did identify a statistically significant association between elevated LDL levels and progression-free and overall survival. We also observed that LDL levels retained significance as an independent prognostic factor. We did not identify correlations in survival in patients with abnormal TC, HDL, or TG levels.
There remains a relative paucity of data examining the influence of lipid parameters on survival in women with gynecologic malignancies. Several older studies, representing large population-based health screening trials, have not consistently demonstrated a significant relationship between elevated lipoprotein levels and cancer mortality. For example, two reports from Scotland and the Netherlands could not identify any consistent significant trends between total cholesterol and deaths due to gastrointestinal, breast, lung, or ovary in their female cohorts [9,10]. Similarly, in a subcohort of Greek men from an international prospective study conducted from 1960 to 2000 (the Seven Countries study), an analysis of total serum cholesterol did not reveal any associations between higher values and overall cancer mortality [11]. In contrast, a Swedish health screening trial of 46,570 women, initiated in the 1960s, found that the relative risk of cancer mortality decreased with increases in serum cholesterol [12]. The inconsistencies in these findings may have resulted from a potentially confounding factor common to all of these studies: plasma cholesterol levels were routinely assayed from a casual, non-fasting blood draw.
More recently, studies have examined lipid levels and cancer in more focused cohorts of patients with specific disease sites. Bahl and colleagues [13] examined fasting lipid panels from 520 women with early staged breast cancer, and observed that both elevations in TC and LDL correlated weakly with disease recurrence. Elevated total cholesterol was also associated with poorer overall survival in 99 patients with colorectal cancer [14]. In patients with epithelial ovarian cancers, however, studies have not demonstrated consistent trends in lipid profiles in patients with malignancies compared to benign controls [15–17]. Furthermore, examination of serum lipoproteins in the context of clinical outcome has not been reported.
Molecular studies support a role for elevated LDL in ovarian cancer biology. Chemoresistant ovarian carcinoma cells may overexpress ABCA2, an ATP-binding cassette transporter, which functions in the trafficking of LDL-derived free cholesterol [reviewed in [18]. More recently, Scoles and colleagues [7] identified a dose-dependent increase in cellular proliferation of ovarian carcinoma cell lines CaOV3, OVCAR3, and SKOV3 with increasing oxidized LDL concentrations. They also observed that oxidized LDL reduced cisplatin sensitivity of both platinum-sensitive CaOV3 and platinum-resistant SKOV3 cells. While these data remain limited, they suggest abnormal lipoprotein profiles may promote aggressive tumor biology.
Our study is limited by its retrospective design, the correlative nature of the analyses, and the relatively small cohort; in addition, examination of a single pretreatment LDL level may not adequately reflect the variances in lipoprotein profile over the clinical course of this disease. Furthermore, patients with undiagnosed hyperlipidemias may have additional co-morbidities influencing survival. However, these data are the first to examine fasting lipid panels as a predictor of clinical outcome in this disease, and suggest one mechanism by which statins may favorably influence epithelial ovarian cancer biology could potentially be through a LDL-lowering mechanism (in addition to potential direct effects on tumor biology). Further molecular studies examining statins and lipoproteins are underway to support the potential use of statins in the management of women with this disease.
Footnotes
Conflict of interest statement
None of the authors have actual, apparent, or potential conflicts of interest to report.
References
- 1.Kwan ML, Habel LA, Flick ED, Quesenberry CP, Caan B. Post-diagnosis statin use and breast cancer recurrence in a prospective cohort study of early stage breast cancer survivors. Breast Cancer Res Treat. 2008;109:573–579. doi: 10.1007/s10549-007-9683-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Platz EA, Leitzmann MF, Visvanathan K, et al. Statin drugs and risk of advanced prostate cancer. J Natl Cancer Inst. 2006;98:1819–1825. doi: 10.1093/jnci/djj499. [DOI] [PubMed] [Google Scholar]
- 3.Elmore RG, Ioffe YI, Scoles DR, Karlan BY, Li AJ. Impact of statin therapy on survival in epithelial ovarian cancer. Gynecol Oncol. 2008;111:102–105. doi: 10.1016/j.ygyno.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Mo H, Elson CE. Studies of the isoprenoid-mediated inhibition of mevalonate synthesis applied to cancer chemotherapy and chemoprevention. Exp Biol Med. 2004;229:567–585. doi: 10.1177/153537020422900701. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein JL, Brown MS. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- 6.Ifere GO, Barr E, Equan A, et al. Differential effects of cholesterol and phytosterols on cell proliferation, apoptosis and expression of a prostate specific gene in prostate cancer cell lines. Cancer Detect Prev. 2009;32:319–328. doi: 10.1016/j.cdp.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Scoles DR, Karahashi H, Karlan B. Ovarian carcinoma cell proliferation mediated by oxidized low-density lipoprotien is inhibited by liver × receptor activation (abstract #93) Gynecol Oncol. 2008;108:S43. [Google Scholar]
- 8.American Heart Association. Updated 12/19/2008. http://www.americanheart.org/presenter.jhtml?identifier=183. [Google Scholar]
- 9.Schuit A, Van Dijk CE, Dekker JM, Schouten EG, Kok FJ. Inverse association between serum total cholesterol and cancer mortality in Dutch civil servants. Am J Epidemiol. 1993;137:966–976. doi: 10.1093/oxfordjournals.aje.a116769. [DOI] [PubMed] [Google Scholar]
- 10.Isles CG, Hole DJ, Gillis CR, Hawthorne VM, Lever AF. Plasma cholesterol, coronary heart disease, and cancer in the Renfrew and Paisley survey. British Med J. 1989;298:920–924. doi: 10.1136/bmj.298.6678.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panagiotakos DB, Pitsavos C, Polychronopoulos E, et al. Total cholesterol and body mass index in relation to 40-year cancer mortality (the Corfu cohort of the Seven Countries Study) Cancer Epidemiol Biomarkers Prev. 2005;14:1797–1801. doi: 10.1158/1055-9965.EPI-04-0907. [DOI] [PubMed] [Google Scholar]
- 12.Tornberg SA, Holm LE, Carstensen JM, Eklund GA. Cancer incidence and cancer mortality in relation to serum cholesterol. J Natl Cancer Inst. 1989;81:1917–1921. doi: 10.1093/jnci/81.24.1917. [DOI] [PubMed] [Google Scholar]
- 13.Bahl M, ennis M, Tannock IF, et al. Serum lipids and outcome of early-stage breast cancer: results of a prospective cohort study. Breast Cancer Res Treat. 2005;94:135–144. doi: 10.1007/s10549-005-6654-9. [DOI] [PubMed] [Google Scholar]
- 14.Cengiz O, Kocer B, Surmeli S, Santicky MJ, Soran A. Are pretreatment serum albumin and cholesterol levels prognostic tools in patients with colorectal carcinoma? Med Sci Monit. 2006;12:CR240–CR247. [PubMed] [Google Scholar]
- 15.Delimaris I, Faviou E, Antonakos G, Stathopoulou E, Zachari A, Dionyssiou-Asteriou A. Oxidized LDL, serum oxidizability and serum lipid levels in patients with breast or ovarian cancer. Clin Biochem. 2007;40:1129–1134. doi: 10.1016/j.clinbiochem.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Qadir MI, Malik SA. Plasma lipid profile in gynecologic cancers. Eur J Gynaecol Oncol. 2008;29:158–161. [PubMed] [Google Scholar]
- 17.Genkinger JM, Hunter DJ, Spiegelman D, et al. A pooled analysis of 123 cohort studies of dietary fat, cholesterol and egg intake and ovarian cancer. Cancer Causes Control. 2006;17:273–285. doi: 10.1007/s10552-005-0455-7. [DOI] [PubMed] [Google Scholar]
- 18.Mack JT, Townsend DM, Beljanski V, Tew KD. The ABCA2 transporter: intracellular roles in trafficking and metabolism of LDL-derived cholesterol and sterol-related compounds. Curr Drug Metab. 2007;8:47–57. doi: 10.2174/138920007779315044. [DOI] [PMC free article] [PubMed] [Google Scholar]