Abstract
Muscle functional magnetic resonance imaging (MRI) refers to changes in the contrast properties of certain MR images that occur in exercising muscles. In part, these changes result indirectly from increased rates of cellular energy metabolism, which alter the image contrast properties by increasing the water content and by decreasing the intracellular pH. Also, increases in blood oxygen extraction cause a rapidly evolving, small, and negative contribution to signal. Together, these changes produce a complex time course of contrast changes during exercise. Analysis of this time course may provide insight into the physiology of exercising muscles. These contrast changes also provide a non-invasive method for determining the spatial pattern of muscle activation.
1. MRI – BASIC PHYSICAL PRINCIPLES
Magnetic resonance imaging (MRI) exploits the net magnetic moments of certain atomic nuclei, such as the protons in water. Exposing a system of magnetic nuclei ("spins") to a very strong magnetic field (B0) gives rise to an equilibrium condition in which a bulk magnetization vector (M0) lies parallel to the magnetic field and individual spins precess about B0 with a random phase distribution. Precessional motion is similar to the wobbling motion of a spinning top, and occurs at the Larmor frequency (ω0):
| (1) |
γ is a nucleus-specific constant called the gyromagnetic ratio. For protons, γ=42.57 MHz/Tesla. Subsequent application of a pulsed, radiofrequency (RF) magnetic field rotates the spins' magnetic moments such that M0 projects into the plane transverse to B0. The magnetic moments are initially phase-coherent, and their precession in the transverse plane creates a recordable signal. As the system returns to its equilibrium state, the signal decays.
The return of the system to equilibrium involves the loss of phase coherence and a realignment of the magnetic moments along B0. Realignment along B0 is caused by chemical and physical interactions of the spins with their surroundings and can be characterized by the longitudinal relaxation time constant, T1. This contributes to a net loss of transverse magnetization. In addition, interactions among the spins cause a loss of phase coherence. This process is characterized by the transverse relaxation time constant, T2. Finally, spatial inhomogeneity in the B0 field results in additional signal loss, which is characterized by the effective transverse relaxation time constant, T2*. While T1 and T2 represent a permanent loss of signal, the effects of B0 inhomogeneities can be reversed by applying a second RF pulse. T1 and T2 contribute to image contrast by determining the amount of MRI signal recorded from a particular tissue. Because the chemical, physical, and biological properties that contribute to these relaxation processes differ among tissues, T1 and T2 will differ among tissues as well. Localizing the MRI signal therefore gives rise to a spatially dependent contrast that allows the discrimination of different organs or parts of organs.
2. PHYSIOLOGICAL MRI OF MUSCLE
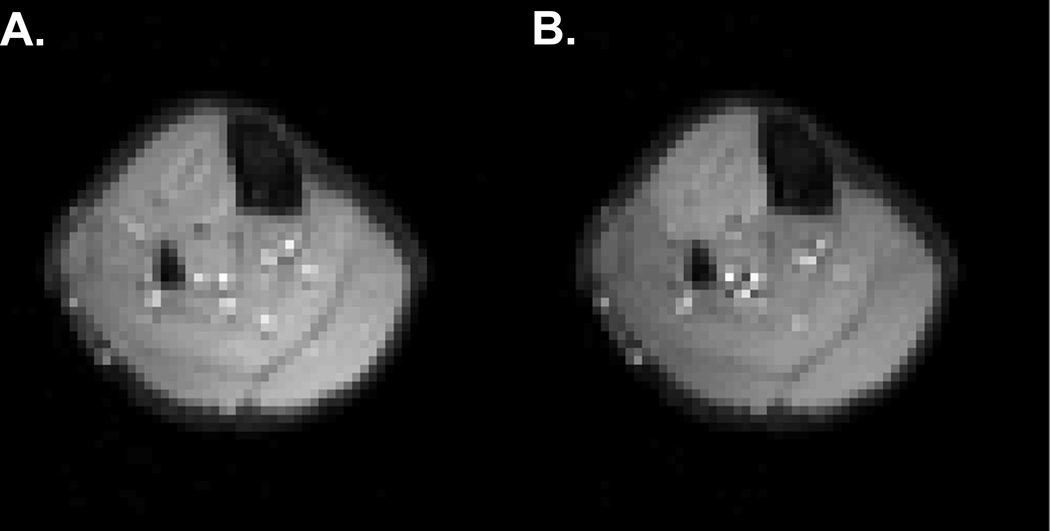
In contrast to the use of structural imaging to determine normal and pathologically altered anatomy, physiological MRI techniques are used to extract functional information during an experimental procedure. Two classes of physiological MRI techniques exist. One uses specialized sequences of RF pulses and/or magnetic field gradients to create an image that is sensitized to a specific physiological variable; for example, MRI methods exist for imaging perfusion, diffusion, and mechanical strain. The second class of physiological MRI techniques relies on localized signal intensity changes in images made using standard contrast generation schemes. The signal changes result from alterations in the chemical, physical, and/or biological properties of the tissue brought about by the experimental procedure. The best known example of this is brain functional MRI, in which transient elevations in blood volume, flow, and oxygenation associated with neuronal activity cause T2*-weighted signal increases in active brain areas. An analogous effect, which is the subject of this paper, is the increase in T2 that occurs in exercising muscles [1]. The lengthened T2 increases the signal from the active muscles (Fig. 1). The use of T2 and T2-weighted contrast changes to extract information about the functional properties of exercising muscles has become known as muscle functional MRI (mfMRI).
Fig. 1.
Axial mfMRI images obtained before (A) and after (B) 2 min. of sustained isometric dorsiflexion exercise, illustrating contrast changes in the anterior compartment.
T2 changes during and following exercise have been extensively characterized with respect to traditional indices of exercise performance, such as intensity, duration, and work. T2 can increase by as much as 30% during exercise, causing the signal intensity in active muscles to increase by 15% or more. T2 increases vary directly with the relative exercise intensity [2]. Moreover, T2 and T2-weighted signal intensity changes evolve as a complex function of exercise duration [3–5]. As we discuss below, this complexity results from the superposition of the several physiological factors that contribute to the T2 change. Generally, these factors contribute to a slowly evolving, large magnitude, positive T2 change indirectly related to cellular energy metabolism or to a rapidly evolving, low magnitude, negative T2 change resulting from blood oxygenation changes.
3. METABOLICALLY DRIVEN T2 CHANGES
A major determinant of tissue relaxation times is the water content (or rather, the protein concentration). Proteins affect the T2 of the surrounding water because chemical exchange occurs between the water and side groups on the proteins. In muscle, the intracellular space contains a large amount of highly oriented proteins (~20% of wet weight). As a result, the intracellular T2 of muscle fibers (~35 ms; [6–8]) is shorter than the T2 of the corresponding interstitial space (~150 ms; [6–8]), in which the protein concentration is lower. As we show below, modulation of protein-water chemical exchange pathways by physiological variables accounts for much of the T2 changes observed during exercise.
The importance of protein content on the intracellular T2 of muscle was first demonstrated by Belton and Packer [9], who observed that dessicated frog gastrocnemius muscle has a lower intracellular T2 than fully hydrated muscle. Damon et al. [10] exposed frog sartorius muscles to Ringer’s solutions modified to be hypotonic or hypertonic, causing water to enter or exit cells; there were increases and decreases, respectively, in T2. The finding in this study that T2 changes follow alterations in intracellular water content is similar to the findings of Belton and Packer, but was reached by altering cell volume within its physiological range. The in vivo importance of intracellular volume changes to the T2 was demonstrated by Meyer et al. [11], who electrically stimulated the tail muscles of lobsters (an osmoconformer with an intracellular osmolarity of ~1 Osm/l) and freshwater crayfish (an osmoregulator with an intracellular osmolarity of ~300mOsm/l). Contractions induced a greater T2 change in the osmoregulators, which can be explained as follows. There were similar absolute levels of osmolyte accumulation in the two species, but different relative levels. The greater relative osmolyte accumulation in the osmoregulators would cause a greater relative change in intracellular volume. Collectively, these data indicate that decreases in the intracellular protein concentration, such as those brought about by osmotic water shifts, increase the intracellular T2.
In addition, the intracellular pH influences T2. Fung and Puon [12] placed permeabilized muscle strips in low (5), physiological (7) and high (9) pH solutions revealed that T2 is inversely related to intracellular pH. Later, Damon et al. [10] manipulated intracellular pH throughout the physiological range (6.5–7.4) by transient exposure to the ammonium ion (NH4+). They too observed that T2 is inversely related to intracellular pH. Both authors suggested the T2 and pH relationship was due to a pH modulated chemical exchange effect. Recent results from our lab have demonstrated explicitly that this is the case, with amide groups proposed to play a particularly important role in the exchange process [13].
T2 changes during exercise occur mainly the intracellular space [10, 14, 15], and osmotically induced water shifts and changes in intracellular pH are the key mediators of these changes. Fleckenstein et el. [16] demonstrated a central role for metabolism in the T2 change of exercise in their study of patients with myophosphorylase deficiency (MPD). In MPD, glycogenolysis is blocked and lactate does not accumulate; therefore, the change in muscle volume during exercise is smaller than in controls, there is a slight alkalosis, and the T2 does not change at all. Prior et al. [17] also showed a metabolic dependence for the T2 change by examining the relationships between fiber type and T2 increases in stimulated rat muscles. After muscle stimulation, the T2 increase was greater in the white portion of the gastrocnemius muscle than in the red portion. In a separate experiment, these authors ligated the feed artery during exercise. They observed that the muscle volume and T2 changes did not become maximal until the ligature was released. This shows that osmotic water shifts and maximal T2 changes require that the intracellular space has access to an essentially infinite reservoir of extracellular water, a condition that is met only when the muscle is perfused. Finally, in an isolated muscle model of exercise, Damon et al. [10] demonstrated that changes in pH and intracellular water content explain the majority (70%) of the T2 change of exercise. The general conclusion from these studies is that osmolyte accumulation during exercise causes water entry, diluting the myofibrillar proteins and increasing T2. The T2 changes are further enhanced by the decrease in intracellular pH that results from anaerobic glycolysis.
4. T2 SENSITIVITY TO BLOOD OXYGENATION
T2 changes can also result from changes in blood oxygenation (i.e., the blood oxygenation level-dependent, or BOLD, effect). The physical basis for the BOLD effect is that the magnetic properties of hemoglobin (Hb) depend on its oxygenation state. Oxygenated Hb (HbO2) is diamagnetic, meaning that it has a small, negative magnetic susceptibility. Deoxygenated Hb (HHb) is paramagnetic, meaning that it has a small, positive magnetic susceptibility. The magnetic field around the Hb molecule therefore depends on its oxygenation state. It transpires that water is diamagnetic. Therefore, protons in the near vicinity of an HHb molecule will experience a different magnetic field than those that are more distant from it. By examining Eq. 1, we see that the precessional frequency depends directly on B0. Thus the slightly inhomogeneous magnetic field caused by the presence of HHb will lead to a loss of phase coherence among the spins, and decrease the T2 and T2*.
In tissues, the paramagnetic effect of HHb plays out in two ways. First, it affects the T2 and T2* values for blood itself (the intravascular BOLD effect). Water exchanges very rapidly across the red blood cell membrane. Because of this, the overall T2 of the blood represents the T2 of both the plasma (with a long T2 value) and the red blood cells (with a short T2). The T2 of whole blood decreases when the blood oxygenation level decreases and increases when the hematocrit (Hct) increases.
The second way in which blood oxygenation affects the MRI signal is by affecting the relaxation of extravascular spins (the extravascular BOLD effect). The presence of HHb causes the magnetic susceptibility of the blood to differ from that of the tissue parenchyma. As a result, magnetic susceptibility gradients form around blood vessels. As water diffuses in and out of these gradients, its Larmor frequency will change. The strength of this effect will depend first on the size of the gradient (determined by %HbO2, Hct, B0, the capillary orientation, and the relative blood volume) [18]. Also, the magnitude of the extravascular BOLD effect will depend on how rapidly water samples each magnetic environment, which is determined by the diffusion coefficient for water and the vessel radius [18]. In general, extravascular BOLD effects in muscle are predicted to be small [19]. We have confirmed this experimentally by comparing the T2 and T2* values made with the leg oriented at 5 and 15 degrees to the B0 field, which do not differ significantly [20].
BOLD effects can be seen in several types of exercise studies. Following brief isometric contractions, a hyperemic response provides increased oxygen to meet the metabolic demands of recovery. However, the oxygen supply initially exceeds the demand, and so a temporary overshoot of %HbO2 occurs. This transiently elevates the MRI signal in T2- or T2*-weighted images [21–23]. Endurance-trained subjects exhibit a greater hyperemic response than untrained subjects, and so their post-contraction, BOLD-sensitive signal change is greater [23]. Also, BOLD effects are important contributors to mfMRI signal intensity changes during prolonged exercise because the rate of muscle oxygen consumption is tightly linked and linearly proportional to the exercise intensity. Increases in oxygen supply cannot keep up with the increased oxygen demand, and so oxygen extraction increases; HbO2 saturation levels can reach 20–30% with prolonged, intense exercise. These large changes in blood oxygenation produce an up-to ~1% loss of signal in T2-weighted images [19]. While this absolute signal change seems small, at some exercise durations its relative importance can be large, contributing to as much as 35% of the contrast change in mfMRI [19].
5. POTENTIAL APPLICATIONS OF MFMRI
The elevated signal intensity in the anterior compartment shown in Figure 1 suggests an application for mfMRI: detecting the spatial pattern of muscle activation. mfMRI-determined muscle activation patterns correspond to those determined by traditional electromyography methods [24]. mfMRI analyses have also revealed that kinetically and kinematically similar cycling movements can be brought about by very different patterns of muscle activity among synergistic muscles [25]. The ability to detect these patterns would be of great use in modelling the mechanical and metabolic aspects of an exercise, because it allows the non-invasive detection of muscle activity in both superficial and deep muscles. Also, it could be used to guide the placement of surface RF coils and regions of interest in MR spectroscopy studies.
Because the metabolic and hemodynamic determinants of T2-weighted signal changes evolve with different time courses, a complex time course of signal intensity changes exists. Examining this time course reveals that metabolic and/or hemodynamic heterogeneity exists within muscles during exercise. For example, during sustained isometric dorsiflexion exercise performed at 30% of maximum voluntary contraction (MVC) force, T2-weighted signal changes are similar in the extensor digitorum longus (EDL) and the superficial and deep compartments of the tibialis anterior (S-TA and D-TA, respectively) muscles. However, at 60% of MVC, the signal change is almost two-fold greater in the S-TA than in the other regions [19]. This might indicate a higher level of neural activation of the S-TA (causing an elevated T2 response in this compartment) or a relatively greater perfusion restriction in the D-TA (attenuating the T2 response in analagous manner to the arterial ligation study cited above). Either interpretation supports the hypothesis that spatially heterogeneous neuromuscular function exists within the TA muscle during exercise.
In conclusion, the T2 of skeletal muscle is sensitive to many of the physiological variables altered during exercise, including water content, pH, and blood volume and oxygenation. These changes alter the contrast properties of T2-weighted MR images. Careful analysis of these changes may provide new insight into individually varying spatial patterns of muscle activity and the metabolic and hemodynamic responses of muscles to exercise.
ACKNOWLEDGMENTS
Grant Support: NIH/NIAMS R01 AR050101
REFERENCES
- 1.Bratton CB, et al. Nuclear Magnetic Resonance Studies of Living Muscle. Science. 1965;147:738–739. doi: 10.1126/science.147.3659.738. [DOI] [PubMed] [Google Scholar]
- 2.Reid RW, et al. Effect of Aerobic Capacity on the T2 Increase in Exercised Skeletal Muscle. J Appl Physiol. 2001;90:897–902. doi: 10.1152/jappl.2001.90.3.897. [DOI] [PubMed] [Google Scholar]
- 3.Damon BM, Gore JC. Physiological Basis of Muscle Functional MRI: Predictions Using a Computer Model. J Appl Physiol. 2005;98:264–273. doi: 10.1152/japplphysiol.00369.2004. [DOI] [PubMed] [Google Scholar]
- 4.Damon BM, et al. Cluster Analysis of Muscle Functional MRI Data. J Appl Physiol. 2003;95:1287–1296. doi: 10.1152/japplphysiol.00178.2003. [DOI] [PubMed] [Google Scholar]
- 5.Jenner G, et al. Changes in Magnetic Resonance Images of Muscle Depend on Exercise Intensity and Duration, Not Work. J Appl Physiol. 1994;76:2119–2124. doi: 10.1152/jappl.1994.76.5.2119. [DOI] [PubMed] [Google Scholar]
- 6.Belton PS, et al. Pulsed NMR Studies of Water in Striated Muscle. I. Transverse Nuclear Spin Relaxation Times and Freezing Effects. Biochim Biophys Acta. 1972;286:16–25. doi: 10.1016/0304-4165(72)90084-0. [DOI] [PubMed] [Google Scholar]
- 7.Hazlewood CF, et al. Nuclear Magnetic Resonance Transverse Relaxation Times of Water Protons in Skeletal Muscle. Biophys J. 1974;14:583–606. doi: 10.1016/S0006-3495(74)85937-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cole WC, et al. The Origin of Biexponential T2 Relaxation in Muscle Water. Magn Reson Med. 1993;29:19–24. doi: 10.1002/mrm.1910290106. [DOI] [PubMed] [Google Scholar]
- 9.Belton PS, Packer KJ. Pulsed NMR Studies of Water in Striated Muscle. III. The Effects of Water Content. Biochim Biophys Acta. 1974;354:305–314. doi: 10.1016/0304-4165(74)90015-4. [DOI] [PubMed] [Google Scholar]
- 10.Damon BM, et al. Intracellular Acidification and Volume Increases Explain R2 Decreases in Exercising Muscle. Magn Reson Med. 2002;47:14–23. doi: 10.1002/mrm.10043. [DOI] [PubMed] [Google Scholar]
- 11.Meyer RA, et al. Contraction Increases the T2 of Muscle in Fresh Water but Not in Marine Invertebrates. NMR Biomed. 2001;14:199–203. doi: 10.1002/nbm.702. [DOI] [PubMed] [Google Scholar]
- 12.Fung BM, Puon PS. Nuclear Magnetic Resonance Transverse Relaxation in Muscle Water. Biophys J. 1981;33:27–37. doi: 10.1016/S0006-3495(81)84870-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Louie EA, et al. Joint Annual Meeting ISMRM-ESMRMB. Berlin, Germany: 2007. Magnetization Transfer and T2 Measurements of Isolated Muscle: Effect of pH. [Google Scholar]
- 14.Ploutz-Snyder LL, et al. Different Effects of Exercise and Edema on T2 Relaxation in Skeletal Muscle. Magn Reson Med. 1997;37:676–682. doi: 10.1002/mrm.1910370509. [DOI] [PubMed] [Google Scholar]
- 15.Saab G, et al. Effects of Exercise on Muscle Transverse Relaxation Determined by MR Imaging and in Vivo Relaxometry. J Appl Physiol. 2000;88:226–233. doi: 10.1152/jappl.2000.88.1.226. [DOI] [PubMed] [Google Scholar]
- 16.Fleckenstein JL, et al. Absence of Exercise-Induced MRI Enhancement of Skeletal Muscle in McArdle's Disease. J Appl Physiol. 1991;71:961–969. doi: 10.1152/jappl.1991.71.3.961. [DOI] [PubMed] [Google Scholar]
- 17.Prior BM, et al. Fiber Type and Metabolic Dependence of T2 Increases in Stimulated Rat Muscles. J Appl Physiol. 2001;90:615–623. doi: 10.1152/jappl.2001.90.2.615. [DOI] [PubMed] [Google Scholar]
- 18.Stables LA, et al. Asymmetric Spin-Echo Imaging of Magnetically Inhomogeneous Systems: Theory, Experiment, and Numerical Studies. Magn Reson Med. 1998;40:432–442. doi: 10.1002/mrm.1910400314. [DOI] [PubMed] [Google Scholar]
- 19.Damon BM, et al. Exercise Intensity Effects on the Muscle Functional MRI Signal Intensity Time Course: I. Absolute and Relative Contributions of Bold Effects. Magn Reson Med. 2007 doi: 10.1002/mrm.21319. in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanchez OA, et al. Joint Annual Meeting ISMRM-ESMRMB. Berlin, Germany: 2007. Effects of Capillary Orientation on Muscle T2/T2*: Comparison of Numerical Simulations with Empirical Data. [Google Scholar]
- 21.Meyer RA, et al. Bold MRI Mapping of Transient Hyperemia in Skeletal Muscle after Single Contractions. NMR Biomed. 2004;17:392–398. doi: 10.1002/nbm.893. [DOI] [PubMed] [Google Scholar]
- 22.Damon BM, et al. Dual Gradient-Echo MRI of Post-Contraction Changes in Skeletal Muscle Blood Volume and Oxygenation. Magn Reson Med. 2007;47:670–679. doi: 10.1002/mrm.21191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Towse TF, et al. Effect of Physical Activity on MRI-Measured Blood Oxygen Level-Dependent Transients in Skeletal Muscle after Brief Contractions. J Appl Physiol. 2005;99:715–722. doi: 10.1152/japplphysiol.00272.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Price TB, et al. Comparison of MRI with EMG to Study Muscle Activity Associated with Dynamic Plantar Flexion. Magn Reson Imaging. 2003;21:853–861. doi: 10.1016/s0730-725x(03)00183-8. [DOI] [PubMed] [Google Scholar]
- 25.Hug F, et al. Heterogeneity of Muscle Recruitment Pattern During Pedaling in Professional Road Cyclists: A Magnetic Resonance Imaging and Electromyography Study. Eur J Appl Physiol. 2004;92:334–342. doi: 10.1007/s00421-004-1096-3. [DOI] [PubMed] [Google Scholar]