Abstract
Pleural photodynamic therapy (PDT) has been used as an adjuvant treatment with lung-sparing surgical treatment for mesothelioma with remarkable results. In the current intrapleural PDT protocol, a moving fiber-based point source is used to deliver the light and the light dose are monitored by 7 detectors placed in the pleural cavity. To improve the delivery of light dose uniformity, an infrared (IR) camera system is used to track the motion of the light sources. A treatment planning system uses feedback from the detectors as well as the IR camera to update light fluence distribution in real-time, which is used to guide the light source motion for uniform light dose distribution. We have improved the GUI of the light dose calculation engine to provide real-time light fluence distribution suitable for guiding the surgery to delivery light more uniformly. A dual-correction method is used in the feedback system, so that fluence calculation can match detector readings using both direct and scatter light models. An improved measurement device is developed to automatically acquire laser position for the point source. Comparison of the effects of the guidance is presented in phantom study.
Keywords: Photodynamic therapy, light fluence, intracavitory treatment planning, light dosimetry, navigation system, Pleural PDT
1. INTRODUCTION
PDT is a local treatment, suitable to treat malignant, localized tumors such as those observed in malignant pleural mesothelioma (MPM).[1, 2] MPM has no standard treatment and the median survival for diagnosed patients is 6 to 17 months, depending on the disease stage. To treat MPM, PDT is coupled with surgical debulking of the tumorous tissue, part of a trend in multi-modal regimes to increase survival rates. The photosensitizer is administered to the patient, followed by a latent period referred to as the incubation time. After incubation time, debulking surgery is performed, followed by light delivery. The Phase I pleural treatment program at Penn treats patients with MPM or pleural effusion. The photosensitizing drug, HPPH®, is administered 24–48 hours before light irradiation. The irradiation is applied using a laser of wavelength 665 nm at 15- 60 J/cm2. Within the thoracic cavity, the light delivery is continuously administered by a moving point source applied by the surgeon (Fig. 1). In the current protocol, the light uniformity is monitored using 7 detectors distributed in various locations inside the cavity.
Figure 1.
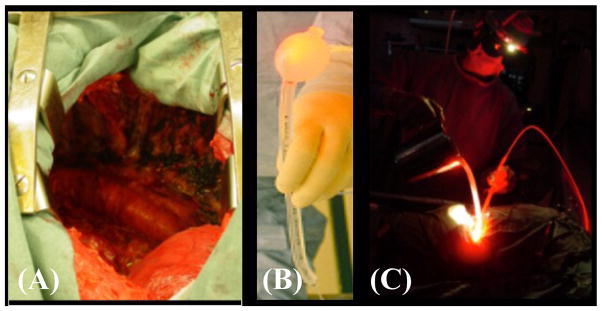
illustrations of pleural PDT treatment of the pleural cavity (left picture, A) using a moving light source made of a modified endotracheal tube filled with 0.1% intralipid (middle picture, B) and light treatment (right picture, C).
Light, photosensitizers, and oxygen are the three most important factors for photodynamic therapy (PDT).[3] Light distribution over the treatment area is of great importance in terms of treatment efficacy. To improve the light dose uniformity, we have proposed to use an IR navigation system to track the movement of the light source during PDT in order to calculate the light fluence distribution on the treatment area.[4] In this paper, we describe the results in a clinical study of 12 patients. To improve the agreement between calculation and measurements at the 7 isotropic detector locations, we have developed a dual-correction method to correct the calculated value to match that at the detector locations.
2. MATERIALS AND METHODS
2.1 Light fluence calculation algorithm
The light from the point source is the sum of the direct and the scatter lights:
| (1) |
where S is the power of the point source and r(t) is the distance from the point source to point of interest at time t. The first term is the direct light, where the correction factor CF(t) is applied based on a dual fluence correction methodology described previously to match the detector readings at the 7 sites.[5] Due to the integrating sphere effect, the scattered light is a constant b due to multiple scattering, independent of the shape of the lung cavity:[6, 7]
| (2) |
where As is the surface area of the treatment area, ρ is the diffuse reflectance, and μa is the absorption coefficient of the water medium inside the lung cavity. Several studies have been performed to characterize the patient dependence of the scatter light fluence, particularly the quantification of μa and ρ.[8, 9] Figure 2 shows a comparison of the direct and scatter light to the total measured light fluence in a cavity phantom with known optical properties.[7] In this study, b is treated as a constant as part of a dual-correction scheme [5] without using Eq. 2.
Figure 2.
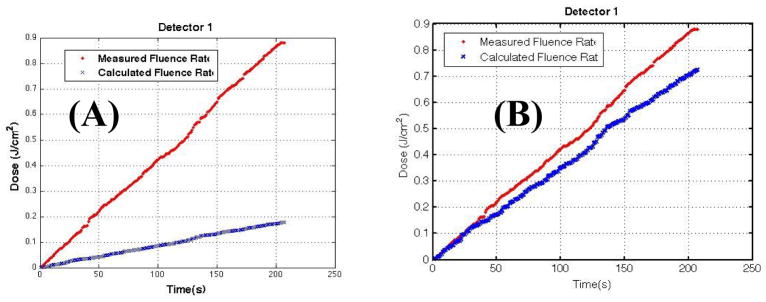
Comparison of measurement (red) and calculation (blue) assuming (a) direct light (first term of Eq. 1, CF=1) and (b) scatter light (Eq. 2) in a cavity phantom with known optical properties. [Taken from [7]].
2.2 Real-time determination of laser positions and patient surface contours
To perform real-time light fluence calculation, the first critical task is to determine the laser source position. We have developed a method to perform the task using a single detector. [4] However, this procedure requires 8 individual measurements at different locations on the operational room (OR) table and is proven to be difficult to accomplish easily. To speedup the process to obtain the laser source position, we have developed a modified laser source calibration jig incorporating 8 detectors positioned in known locations in the laser jig (Fig. 3b). A reference wand (arrow in Fig. 3b) is used to determine the relative positions of the detectors in the OR room. We were able to determine the laser source position using the fitting algorithm [4] about 2 minutes after the measurements were done.
Figure 3.
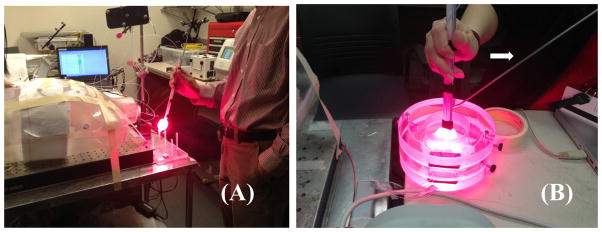
Laser source position determination using (a) a single isotropic detector and (b) modified laser source calibration jig that incorporates 8 isotropic detectors so that all 8 measurements can be performed at once. Arrow points to the positioning wand for the jig.
2.3 A new GUI for determination of patient surface contour and isotropic detector positions
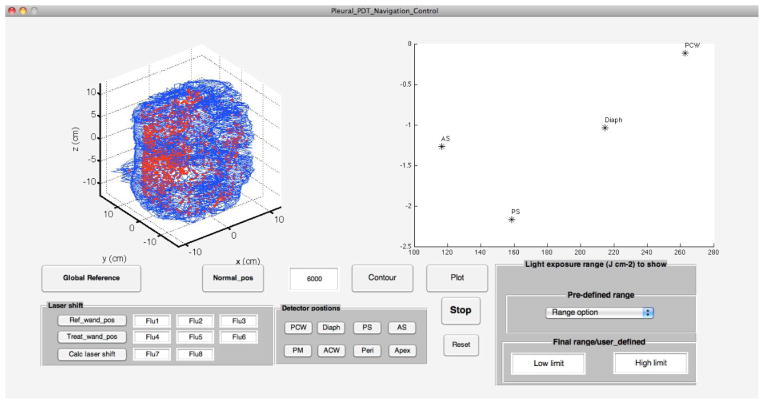
In order to calculate the light fluence, it is important to determine the surface contour where the calculation will be made. This is currently done with a fake treatment (moving the wand in all locations as if one is doing a PDT but the laser light is not on) for 3 minutes before PDT. The result of our current measurement has been programmed into a new GUI interface build using Matlab software (Fig. 4).
Figure 4.
A GUI is developed to determine the pleural contour positions along with the detector positions just before PDT. Blue contours are the generated surface contour and red points are the raw data corresponding to the laser source position.
Figure 4 shows the 3D surface contour obtained using an algorithm [4] described previously. The entire calculation takes about 80 s after the fake treatment. So the entire surface contour can be generated in less than 5 minutes if we include 3 minutes fake treatment time. Once the surface contour is defined, the locations of the isotropic detectors can be obtained and displayed on the stretched out treatment area (right side of Fig. 4) by clicking the corresponding button when the tip of the IR positioning wand (arrow in Fig. 3b) is placed on the detector location.
2.4 Real-time feedback of light fluence rate calculation
In order to provide real-time feedback to the measured values, we have developed another Matlab-based GUI to display the calculated light fluence on a 2D unwrapped surface. OpenIGTLink was used to provide a platform communicating between the NDI Polaris camera and a PC. The light source position data were acquired at a frequency of 20 Hz from the NDI data acquisition driver. When the PC receives the light source position data, Matlab was used to calculate the cumulative light fluence on every point of the pleural cavity contour, and to display/update the unwrapped light fluence distribution map at the frequency of 20 Hz. In the light fluence distribution map, 3D points on the pleural cavity contour were unwrapped into 2D φ-z maps, given the condition that on each x–y plane of the cavity contour, there is only one point for each angle φ, where φ=atan(y/x). Calculation engine based on the direct light term of Eq. 1 (first term with CF = 1) was used so that the cumulative light fluence was displayed in real-time during treatment.
2.5 Treatment results with and without IR navigation in phantom
Figure 5 shows the comparison of light fluence distribution for a treatment on a phantom (a) without and (b) with IR navigation assistance. Four evenly distributed isotropic detectors were used for the treatment. The goal of the phantom treatment is to deliver 1 J/cm2 light fluence uniformly on the entire phantom surface (Fig. 4). The blue dark line is the calculated mean light fluence (along the patient superior and posterior direction, see Fig. 4). The dark shaded area designates the standard deviation of all calculated light fluence at each z position along all angles φ. Each line is the light fluence vs. z distribution for a different φ of the unwrapped plane of the pleural surface.
Figure 5.
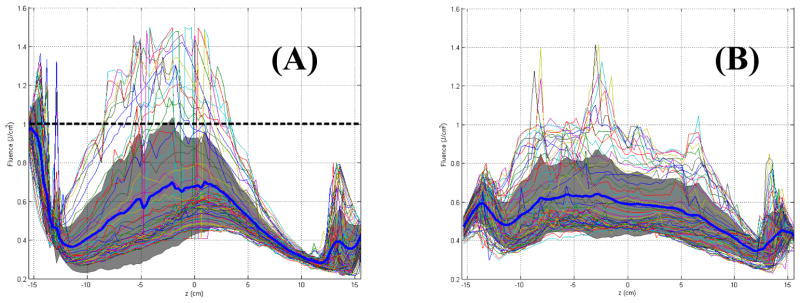
Calculated spatial distribution of light fluence using direct light only (first term of Eq. 1) in a phantom: (a) without and (b) with IR navigation guidance.
This resulting light fluence distribution is much more uniform with IR navigation than that without IR navigation as indicated by the mean blue line and the narrower shades of the light fluence spread in Fig. 5. One must notice the calculated light fluence is still lower than the target light fluence of 1 J/cm2. This is due to the fact that light fluence calculated using direct light alone is smaller than the actual light fluence. Thus the actual light fluence should be closer to the detector reading (1 J/cm2) than the calculation. Since the IR navigation system ensure the operator spend the same amount of time in each surface location to achieve uniform light fluence, this method, even without matching the measured light fluence, will result in better light fluence distribution overall.
2.6 Summary of IR navigation system clinical application
Table 1 summarizes the results of the current application of the IR navigation system to 20 patients. In the column for “effective data”, “×”/“√” means sufficient data about laser position during treatment is not/is obtained for calculation. In the column for “Reference”, “×” ”(“√”) means the laser position is not (is) relative to an independent reference mount that is fixed to patient table. Even if the data is not obtained relative to the reference mount, one can still analyze the calculated data. Among the 20 patients, we obtained good data in 70% (14/20) patients. Among patients with good data, we can apply correction to 86% (12/14) patients since we didn’t get the detector positions in the other 2 patients.
Table 1.
Summary of clinical application among 20 patients treated with IR navigation system
| ID | Effective data | Reference | Detector |
|---|---|---|---|
| 10 | × | × | × |
| 12 | × | × | × |
| 13 | √ | × | √ |
| 15 | × | √ | × |
| 16 | √ | × | √ |
| 18 | √ | √ | × |
| 20 | √ | √ | √ |
| 21 | √ | × | × |
| 22 | √ | √ | √ |
| 23 | × | × | × |
| 24 | √ | √ | × |
| 25 | √ | √ | √ |
| 26 | √ | √ | √ |
| 28 | √ | √ | √ |
| 27 | √ | √ | √ |
| 30 | √ | √ | √ |
| 31 | √ | √ | √ |
| 32 | × | × | × |
| 34 | √ | √ | √ |
| 33 | √ | √ | √ |
The dual correction method with the scattering term b (Eq. 1) allows us to correct data for 86% of the patient to be in agreement.
3. CONCLUSION
We have presented evidence that improved methods for obtaining the laser source position and patient contour allows us to perform real-time feedback guidance in phantom studies. The light distribution in phantom is improved with IR guidance. This result, coupled with a dual correction method to match the calculation with measurements in the 7 detector locations will allow us to apply the technique for clinical use to improve treatment for pleural PDT patients. This will be the goal of our next phase of study.
Acknowledgments
This work is supported by grant from National Institute of Health (NIH) P01 CA87971 and R01 CA 154562.
References
- 1.Pass HI, DeLaney TF, Tochner Z, Smith PE, Temeck BK, Pogrebniak HW, Kranda KC, Russo A, Friauf WS, Cole JW, et al. Intrapleural photodynamic therapy: results of a phase I trial. Ann Surg Oncol. 1994;1(1):28–37. doi: 10.1007/BF02303538. [DOI] [PubMed] [Google Scholar]
- 2.Pass HI, Tochner Z, DeLaney T, Smith P, Friauf W, Glatstein E, Travis W. Intraoperative photodynamic therapy for malignant mesothelioma. Ann Thorac Surg. 1990;50(4):687–8. doi: 10.1016/0003-4975(90)90230-4. [DOI] [PubMed] [Google Scholar]
- 3.Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. Photodynamic therapy. J Natl Cancer Inst. 1998;90 (12):889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu TC, Liang X, Chang C, Sandell J, Finlay JC, Dimofte A, Rodriguez C, Cengel K, Friedberg J, Glatstein E, Hahn SM. An IR navigation system for real-time treatment guidance of pleural PDT. Proc SPIE. 2011;7886:28860L. doi: 10.1117/12.875635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu TC, Liang X, Sandell J, Finlay JC, Dimofte A, Rodriguez C, Cengel K, Friedberg J, Hahn SM, Glatstein E. A real-time treatment guidance system for pleural PDT. Proc SPIE. 2012;8210:821009. doi: 10.1117/12.908032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandell J, Chang C, Finlay JC, Zhu TC. A treatment planning system for pleural PDT. Proc SPIE. 2010;7551:75510C. doi: 10.1117/12.843044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sandell J, Liang X, Zhu TC. Light dose verification for pleural PDT. Proc SPIE. 2012;8210:821010. doi: 10.1117/12.908223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dimofte A, Zhu TC, Finlay JC, Cullighan M, Edmonds CE, Friedberg JS, Cengel K, Hahn SM. In-vivo light dosimetry for pleural PDT. Proc SPIE. 2009;7164:71640A. doi: 10.1117/12.809548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dimofte A, Zhu TC, Finlay JC, Cullighan M, Edmonds CE, Friedberg JS, Cengel K, Hahn SM. In-vivo light dosimetry for HPPH-mediaed pleural PDT. Proc SPIE. 2010;7551:755115. doi: 10.1117/12.809548. [DOI] [PMC free article] [PubMed] [Google Scholar]