Abstract
Introduction
Risk factors associated with erectile dysfunction (ED) that results from recurrent ischemic priapism (RIP) in sickle cell disease (SCD) are incompletely defined.
Aim
To determine and compare ED risk factors associated with SCD and non-SCD-related “minor” RIP, defined as having ≥2 episodes of ischemic priapism within the past 6 months, with the majority (>75%) of episodes lasting <5 hrs.
Methods
We performed a retrospective study of RIP in SCD and non-SCD patients presenting from June 2004 to March 2014 using the IIEF, IIEF-5, and priapism-specific questionnaires.
Main Outcome Measures
Prevalence rates and risk factor correlations for ED associated with RIP.
Results
The study was comprised of 59 patients {40 SCD (mean age 28.2 ± 8.9 yrs) and 19 non-SCD (15 idiopathic and 4 drug-related etiologies) (mean age 32.6 ± 11.7 yrs)}. Nineteen of 40 (47.5%) SCD patients vs 4 of 19 (21.1%) non-SCD patients (39% overall) had ED (IIEF<26 or IIEF-5<22) (p=0.052). SCD patients had a longer mean time-length with RIP than non-SCD patients (p=0.004). Thirty of 40 (75%) SCD patients vs 10 of 19 (52.6%) non-SCD patients (p=0.14) had “very minor” RIP (episodes regularly lasting ≤2 hrs). Twenty-eight of 40 (70%) SCD patients vs 14 of 19 (73.7%) non-SCD patients had weekly or more frequent episodes (p=1). Of all patients with very minor RIP, ED was found among 14 of 30 (46.7%) SCD patients vs none of 10 (0%) non-SCD patients (p=0.008). Using logistic regression analysis, the odds ratio for developing ED was 4.7 for SCD patients, when controlling for RIP variables (95% CI: 1.1-21.0).
Conclusions
ED is associated with RIP, occurring in nearly 40% of affected individuals overall. SCD patients are more likely to experience ED in the setting of “very minor” RIP episodes and are 5 times more likely to develop ED compared to non-SCD patients.
Keywords: Epidemiology, penis, anemia, erection
Introduction
Priapism is a pathologic condition involving prolonged erection unassociated with sexual interest [1, 2]. It is estimated to affect approximately 40% of patients with sickle cell disease (SCD) [3]. Ischemic priapism (distinct from non-ischemic priapism) comprises the vast majority of priapism presentations [2]. Major ischemic priapism episodes are characterized as typically lasting ∼6 hours or longer because of irreversible, time-dependent, histological changes to erectile tissue ultrastructure after this duration of time [4-7]. Beyond 24 hours, these episodes often render the devastating sequelae of erectile tissue necrosis and subsequent fibrosis as demonstrated by fibroblast proliferation [1, 2, 4]. ED is predictable once irreversible erectile tissue damage has occurred, with documented rates as high as 90% for priapism lasting longer than 24 hours [5]. However, reports of ED have also been related to minor ischemic priapism episodes, commonly referred to as “stuttering” or RIP, in which durations of priapism are observed to be a few hours or less [3, 8].
Major ischemic priapism episodes are commonly associated with ED [3] because this form displays sufficiently prolonged duration of episodes, which conceivably exert profound ischemic effects on the erectile tissue [9]. The basis of ED in RIP with briefer priapism durations is unexplained. Because ED occurs after RIP, prolonged duration of ischemic episodes alone insufficiently accounts for this outcome. Prior studies documenting the occurrence of ED among patients with RIP did not specify how these evaluations were made or employ standard contemporary ED assessment tools such as the International Index of Erectile Function (IIEF) [3, 8]. Although RIP was not clearly defined, Adeyoju et al. cautiously suggested that ED rates in patients with RIP were not significantly different compared to rates in patients with major episodes in a small sample of patients [8]. In a previous retrospective evaluation of clinical outcomes associated with the use of phosphodiesterase type 5 (PDE5) inhibitors in a very small number of RIP patients, the baseline prevalence of ED was observed to be ostensibly higher among patients with an SCD vs idiopathic etiology [10].
Further investigations into risk factors are needed to characterize ED in the setting of RIP. We particularly focused on “minor” RIP, differentiating this from RIP episodes of prolonged durations because of this clear association with irreversible erectile tissue damage. We conjectured that ED outcomes after RIP are different in SCD patients and thus elected to do a comparative analysis between patients with and without SCD.
Materials and Methods
Patient Selection
A retrospective analysis was performed using a prospectively managed database of patients with a history of priapism presenting to the urology and hematology clinics at this institution between June 2004 and March 2014. Ethical approval was granted by the Institutional Review Board at this institution. We defined RIP as having ≥2 episodes of priapism within the past 6 months, with the majority (>75%) of episodes lasting <5 hours. We termed episodes lasting 2-5 hours as “minor” RIP and those lasting ≤2 hours as “very minor” RIP. The presence of major episodes during this period was noted and defined as any episode lasting >5 hours. Intracavernosal aspiration, irrigation, and/or sympathomimetics were not exclusions; however, patients receiving surgical shunts, penile prostheses, or androgen ablative therapy were excluded on the basis that these interventions were considered to be likely ED risk confounding factors.
Patient demographic information, medical history, physical examination, and priapism history were obtained. The presence of ED was established based on a score of <26 on the IIEF EF Domain or <22 on the shorter form IIEF-5 [11, 12]. We also applied a secondary definition of ED that excluded those with mild ED [13-15]. Therefore the presence of ED in this alternative method was established based on a score of <22 on the IIEF EF Domain or <17 on the IIEF-5 [11, 12]. In the case of patient sexual inactivity over the 4 week or 6 month period specified by the IIEF or IIEF-5, respectively, a clinical evaluation based on patient report was used to assess for the presence of ED. Selected risk factors were race, marital status, etiology, age of priapism onset, associated circumstances, episode duration, episode frequency, disease duration, and perceived penile deformity.
Statistical Analysis
Priapism characteristics of SCD and non-SCD groups were evaluated. Means ± standard deviations were determined. Categorical data were compared using Chi Square or Fisher's Exact test where appropriate. Continuous data were compared using the Mann-Whitney U nonparametric test. Odds ratios were determined using logistic regression analysis and presented with corresponding 95% confidence intervals (CI). Data were analyzed using Stata 11 (StataCorp, College Station, Texas, USA) and GraphPad Prism 5 (GraphPad Software, Inc, La Jolla, CA, USA). A P value < 0.05 was considered statistically significant.
Results
Patient and Priapism Characteristics
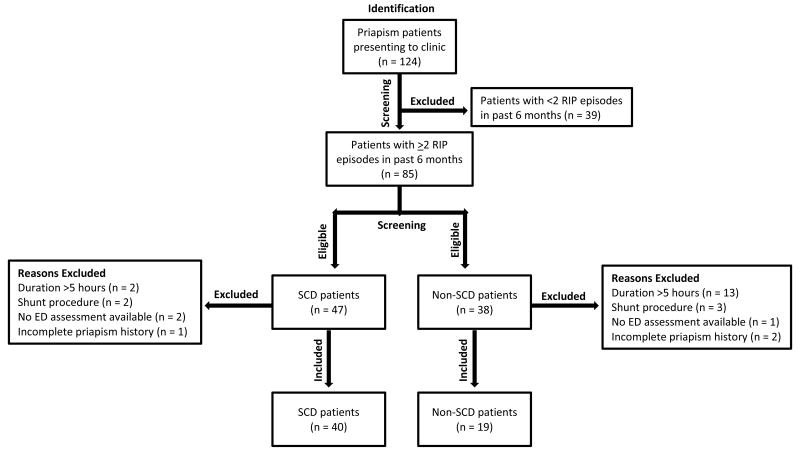
Of 124 patients reviewed, only 59 were enrolled, as a considerable number did not meet the eligibility criteria (Figure 1). Enrolled patients were comprised of 40 SCD (mean age 28.2 ± 8.9 years) and 19 non-SCD patients (mean age 32.6 ± 11.7 years), of whom 15 and 4 had idiopathic and drug-related etiologies, respectively. Additional demographic information is described in Table 1. On average, SCD patients were found to develop RIP in the late teenage years while non-SCD patients were found to develop RIP nearly a decade later. Consequently, SCD patients had a longer mean time-length with RIP than non-SCD patients (p = 0.0042). Three in 4 SCD patients reported episodes lasting ≤2 hours duration whereas only about half of non-SCD patients reported such a duration. Over two-thirds of patients in either group reported weekly or more frequent episodes. There was no significant difference in the overall occurrence of major episodes between groups (Table 2).
Figure 1. Flow chart of patient selection. RIP = Recurrent Ischemic Priapism, IIEF = International Index of Erectile Function, SCD = Sickle-Cell Disease.
Table 1. Patient demographics and medical comorbidities.
| Characteristic | SCD Patients (n=40) | Non-SCD Patients (n=19) | P-Value |
|---|---|---|---|
| Mean Age, yrs (SD) | 28.2 (8.9) | 32.6 (11.7) | 0.0943 |
| Race, n (%) | |||
| Caucasian | 0 (0) | 10 (52.6) | 0.0001 |
| African American | 40 (100) | 7 (36.8) | 0.0001 |
| Other | 0 (0) | 2 (10.5) | 0.0999 |
| Married, n (%) | 6 (15) | 7 (36.8) | 0.0916 |
| Hypertension, n (%) | 8 (20) | 5 (26.3) | 0.7381 |
| Dyslipidemia, n (%) | 1 (2.5) | 1 (5.3) | 0.5441 |
| Diabetes, n (%) | 0 (0) | 1 (5.3) | 0.3220 |
| Stroke, n (%) | 4 (10) | 0 (0) | 0.2945 |
| Kidney Disease, n (%) | 4 (10) | 0 (0) | 0.2945 |
| Tobacco Use, n (%) | 6 (15) | 8 (42.1) | 0.0460 |
SCD = sickle cell disease, n = number, yrs = years, SD = standard deviation
Table 2. Clinical characteristics.
| Characteristic | SCD Patients (n=40) | Non-SCD Patients (n=19) | P-Value |
|---|---|---|---|
| Mean Age of RIP Onset, yrs (SD) | 19.5 (8.2) | 29.1 (13.8) | 0.0024 |
| Mean Time-Length with RIP, yrs (SD) | 9.2 (6.9) | 4.6 (5.0) | 0.0042 |
| Episode Onset, n (%) | |||
| Sleep | 37 (92.5) | 15 (78.9) | 0.1030 |
| ≤2 Episode Duration, n (%) | 30 (75) | 10 (52.6) | 0.1351 |
| ≥ Weekly Episode Frequency, n (%) | 28 (70) | 14 (73.7) | 1.0000 |
| Major Episodes, n (%) | 14 (35) | 11 (57.9) | 0.0963 |
| Erectile Dysfunction (includes mild ED), n (%) | 19 (47.5) | 4 (21.1) | 0.0516 |
| Erectile Dysfunction (excludes mild ED), n (%) | 13 (32.5) | 3 (15.8) | 0.1773 |
| Perceived Penile Deformity or Scarring, n (%) |
n = 36 7 (19.4) |
n = 8 1 (12.5) |
1.0000 |
SCD = sickle cell disease, n = number, yrs = years, SD = standard deviation, RIP = recurrent ischemic priapism
Erectile Dysfunction
Overall, 23 of the 59 (39.0%) patients were identified as having ED; however patients with SCD had a 2-fold higher rate of ED than those without SCD (Table 2). SCD patients were also more likely to have ED than non-SCD patients among all patients with episodes lasting ≤2 hour or those occurring weekly or more frequently (p = 0.008 and 0.040, respectively) (Table 3).
Table 3. ED prevalence and association among RIP subgroups.
| Subgroups | SCD Patients | Non-SCD Patients | P-Value |
|---|---|---|---|
| Including Mild ED | n = 19 | n = 4 | |
| Mean Age of RIP among ED patients, yrs (SD) | 18.5 (6.3) | 22.8 (5.0) | 0.0944 |
| Mean Time-Length with RIP among ED patients, yrs (SD) | 9.5 (6.7) | 3.8 (4.2) | 0.0807 |
| ≤2 hours Duration, n (%) | n = 30 14 (46.7) |
n = 10 0 (0) |
0.0075 |
| 2-5 hours Duration, n (%) | n = 10 5 (50) |
n = 9 4 (44.4) |
1.0000 |
| ≥Weekly Frequency, n (%) | n = 28 13 (46.4) |
n = 14 2 (14.3) |
0.0404 |
| ≤Monthly Frequency, n (%) | n = 12 6 (50) |
n = 5 2 (40) |
1.0000 |
| Major Episodes among ED patients, n (%) | n = 19 7 (36.8) |
n = 4 2 (50) |
1.0000 |
| Excluding Mild ED | n = 13 | n = 3 | |
| Mean Age of RIP among ED patients, yrs (SD) | 16.1 (2.5) | 25.0 (2.6) | 0.0120 |
| Mean Time-Length with RIP among ED patients, yrs (SD) | 11.1 (7.1) | 2.1 (3.0) | 0.0221 |
| ≤2 hours Duration, n (%) | n = 30 10 (33.3) |
n = 10 0 (0) |
0.0428 |
| 2-5 hours Duration, n (%) | n = 10 3 (30) |
n = 9 3 (33.3) |
1.0000 |
| ≥Weekly Frequency, n (%) | n = 28 9 (32.1) |
n = 14 1 (7.1) |
0.1249 |
| ≤Monthly Frequency, n (%) | n = 12 4 (33.3) |
n = 5 2 (40) |
1.0000 |
| Major Episodes among ED patients, n (%) | n = 13 5 (38.5) |
n = 3 2 (66.7) |
0.5500 |
SCD = sickle cell disease, n = number, yrs = years, SD = standard deviation RIP = recurrent ischemic priapism, ED = erectile dysfunction
When controlling for RIP duration, presence of major episodes, episode duration, and episode frequency using logistic regression analysis, patients with SCD were found to be 4.7 times as likely to develop ED compared to those without SCD (95% CI: 1.06-20.96, p = 0.041). When using our alternative definition of ED, the odds ratio was 3.6; however, this relationship was no longer statistically significant (95% CI: 0.69-18.35, p = 0.131).
Discussion
ED is associated with major priapism, but little is known regarding its relationship to RIP, specifically in the setting of SCD. In this study, we confirmed RIP to be a risk factor for ED with a higher prevalence rate of ED among SCD compared to non-SCD patients. Additionally, we showed that a number of risk factors are associated with this condition including duration of RIP (longer), episode duration (≤2 hours), and episode frequency (≥weekly).
The prevalence of ED found here (47.5%) among SCD patients was slightly greater than in previous reports (29-36%); however, these studies did not clearly define how ED was assessed and did not utilize the IIEF [3, 8]. In this study, we also employed an alternative definition of ED excluding mild ED and found a closer agreement between the rate found using this approach (32.5%) and previous studies. Teloken et al. demonstrated that nearly 70% of patients classified with mild ED on the IIEF indicated that they were capable of having sexual intercourse whenever they wished [14]. As such, cutoffs representing normal function may overestimate functional erection loss and mild ED may represent a functionally acceptable threshold [13].
As expected, patients with SCD had a younger mean age of RIP onset and thus a longer mean time-length with RIP. Therefore, it is no surprise that the prevalence of ED was found to be higher among SCD patients compared to non-SCD patients. Interestingly, when adjusting for the duration of RIP and presence of major episodes in logistic regression analysis, this relationship was maintained. This may indicate a risk of ED that is inherent to SCD unlike other etiologies. In evaluating differentiating factors among patient subgroups, having SCD was found to be an associated risk factor amongst patients with very minor or frequent (≥weekly) RIP episodes in the development of ED. When adjusting for several of these factors, having SCD was shown to be predictive of ED. In our secondary analysis excluding mild ED (as it likely represents sufficient erectile functionality), the association between ED and very minor episode duration, but not frequency, continued to be statistically significant. Additionally, the prevalence rate of this modified definition of ED was not significantly different between the two groups; however the increasing trend among these features in SCD patients persisted.
The occurrence of major priapism episodes is a well-established determinant of ED consequent to irreversible cavernosal damage [16]. We found that 36.8% and 50% of non-SCD patients and SCD patients with ED, respectively, reported a major episode within our evaluation period, similar to previous findings [8]. When controlling for the presence of major episodes within this evaluation period, SCD patients continued to demonstrate a higher likelihood of ED than non-SCD patients.
Recent discoveries have uncovered critical components of the pathophysiologic mechanism of SCD-associated, ischemic priapism relating primarily to aberrations in nitric oxide (NO) regulatory signaling, as well as adenosine and RhoA/ROCK pathways [17-20]. It is believed that derangements in these regulatory mechanisms lower the set point of smooth muscle tone control, allowing for recurrent episodes [21]. Patients with SCD may have an increased susceptibility to the reversible ischemic tissue injury associated with RIP episodes, or perhaps over time, these episodes have a cumulative effect in producing cavernosal damage independent of major episodes. Chronically decreased NO bioavailability, an environment influenced from multiple sources in SCD [22, 23], may offer an explanation for the differing ED development rates between SCD and non-SCD populations. Additionally, several studies have demonstrated oxidative stress as a contributing factor to ED development [24] with greater oxidative/nitrosative stress found in cavernosal tissues of priapism associated with SCD compared to non-SCD [25, 26]. This increased oxidative stress found in SCD patients may be a result of a compounding effect of both the cavernosal tissue reperfusion injury that follows episodic resolution of ischemia [27] and the elevated oxidative/nitrosative stress resulting from reactive species generated chronically in SCD [28]. This in turn, may also contribute to the difference in ED occurrence rates as reactive oxygen species have been shown to inactivate NO and are attributed to the subsequent impairment of endothelium-dependent vasodilation [28]. This impairment along with changes in hematocyte properties associated with oxidative stress [28] may be the basis for the histologic changes involving occasional endothelial defects and lymphocytic infiltration previously described in a population of patients with priapism suggestive of RIP [9]. Identification of these pathophysiologic mechanisms may suggest potential new strategies to treat SCD-associated ED [10, 19, 26, 29-31].
We acknowledge potential limitations of this study. The accuracy of the associations regarding analyzed risk factors may be decreased due to the study's small sample size and therefore underpowered. This likely contributes to the decreased statistical significance of the difference in ED rates and risk factors between groups using our secondary ED definition. The differences in ED rates attributed to RIP as shown may be greater because the significantly greater history of tobacco use, a cardiovascular risk factor for ED, among non-SCD patients may have contributed to their ED rates. Although the small sample size is owed in part to the rarity of this condition, this study represents one of the larger retrospectively identified cohorts of SCD-associated priapism patients studied in a comparable time period [16, 32]. While our extensive eligibility criteria resulted in less than a 50% enrollment rate, it advantaged us to establish a rather homogenous subset of the population from which likely valid conclusions were drawn. Rather, if too inclusive in our selection, the assignment and qualification of the variables identified in our analysis would be weakened. We attempted to control for the comparable prevalence of major episodes between groups during the evaluation period in the development of ED; however, we concede that we were unable to fully account for the number and severity of major episodes that may have occurred before or even during our evaluation period due to limitations associated with medical record review. Additionally, pre-existing baseline ED may have also confounded our measures of erectile function. We also acknowledge the potential for patient recall bias regarding responses to priapism-history questionnaire items. Finally, we recognize that we were unable to account for SCD management status as a potential risk factor in our assessment of ED outcomes.
Conclusion
ED is associated with RIP, occurring in nearly 40% of affected individuals overall. Frequency and very minor episode durations (≤2 hours) appear to be related to the development of ED in SCD-associated RIP. SCD patients with RIP are nearly 5 times more likely to develop ED compared with those having RIP associated with non-SCD etiologies.
Acknowledgments
The authors thank Drs. Linda Smith-Resar and Sophie Lanzkron for providing patients in the development of our database.
Footnotes
Conflict of Interest: The authors declare that they have no conflicts of interest.
References
- 1.Berger R, Billups K, Brock G, Broderick GA, Dhabuwala CB, Goldstein I, Hakim LS, Hellstrom W, Honig S, Levine LA, Lue T, Munarriz R, Montague DK, Mulcahy JJ, Nehra A, Rogers ZR, Rosen R, Seftel AD, Shabsigh R, Steers W. Evaluation ATLPo, Treatment of P. Report of the American Foundation for Urologic Disease (AFUD) Thought Leader Panel for evaluation and treatment of priapism. Int J Impot Res. 2001;13(Suppl 5):S39–43. doi: 10.1038/sj.ijir.3900777. [DOI] [PubMed] [Google Scholar]
- 2.Montague DK, Jarow J, Broderick GA, Dmochowski RR, Heaton JP, Lue TF, Nehra A, Sharlip ID. American Urological Association guideline on the management of priapism. J Urol. 2003;170:1318–24. doi: 10.1097/01.ju.0000087608.07371.ca. [DOI] [PubMed] [Google Scholar]
- 3.Emond AM, Holman R, Hayes RJ, Serjeant GR. Priapism and impotence in homozygous sickle cell disease. Arch Intern Med. 1980;140:1434–7. [PubMed] [Google Scholar]
- 4.Kovac JR, Mak SK, Garcia MM, Lue TF. A pathophysiology-based approach to the management of early priapism. Asian J Androl. 2013;15:20–6. doi: 10.1038/aja.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broderick GA. Priapism and sickle-cell anemia: diagnosis and nonsurgical therapy. J Sex Med. 2012;9:88–103. doi: 10.1111/j.1743-6109.2011.02317.x. [DOI] [PubMed] [Google Scholar]
- 6.Ralph DJ, Garaffa G, Muneer A, Freeman A, Rees R, Christopher AN, Minhas S. The immediate insertion of a penile prosthesis for acute ischaemic priapism. Eur Urol. 2009;56:1033–8. doi: 10.1016/j.eururo.2008.09.044. [DOI] [PubMed] [Google Scholar]
- 7.Rees RW, Kalsi J, Minhas S, Peters J, Kell P, Ralph DJ. The management of low-flow priapism with the immediate insertion of a penile prosthesis. BJU Int. 2002;90:893–7. doi: 10.1046/j.1464-410x.2002.03058.x. [DOI] [PubMed] [Google Scholar]
- 8.Adeyoju AB, Olujohungbe AB, Morris J, Yardumian A, Bareford D, Akenova A, Akinyanju O, Cinkotai K, O'Reilly PH. Priapism in sickle-cell disease; incidence, risk factors and complications - an international multicentre study. BJU Int. 2002;90:898–902. doi: 10.1046/j.1464-410x.2002.03022.x. [DOI] [PubMed] [Google Scholar]
- 9.Spycher MA, Hauri D. The ultrastructure of the erectile tissue in priapism. J Urol. 1986;135:142–7. doi: 10.1016/s0022-5347(17)45549-2. [DOI] [PubMed] [Google Scholar]
- 10.Burnett AL, Bivalacqua TJ, Champion HC, Musicki B. Feasibility of the use of phosphodiesterase type 5 inhibitors in a pharmacologic prevention program for recurrent priapism. J Sex Med. 2006;3:1077–84. doi: 10.1111/j.1743-6109.2006.00333.x. [DOI] [PubMed] [Google Scholar]
- 11.Cappelleri JC, Rosen RC, Smith MD, Mishra A, Osterloh IH. Diagnostic evaluation of the erectile function domain of the International Index of Erectile Function. Urology. 1999;54:346–51. doi: 10.1016/s0090-4295(99)00099-0. [DOI] [PubMed] [Google Scholar]
- 12.Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Pena BM. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11:319–26. doi: 10.1038/sj.ijir.3900472. [DOI] [PubMed] [Google Scholar]
- 13.Mulhall JP, Bivalacqua TJ, Becher EF. Standard operating procedure for the preservation of erectile function outcomes after radical prostatectomy. J Sex Med. 2013;10:195–203. doi: 10.1111/j.1743-6109.2012.02885.x. [DOI] [PubMed] [Google Scholar]
- 14.Teloken P, Valenzuela R, Parker M, Mulhall J. The correlation between erectile function and patient satisfaction. J Sex Med. 2007;4:472–6. doi: 10.1111/j.1743-6109.2005.00164.x. [DOI] [PubMed] [Google Scholar]
- 15.Garcia FJ, Violette PD, Brock GB, Pautler SE. Predictive factors for return of erectile function in robotic radical prostatectomy: case series from a single centre. Int J Impot Res. 2014 doi: 10.1038/ijir.2014.20. [DOI] [PubMed] [Google Scholar]
- 16.El-Bahnasawy MS, Dawood A, Farouk A. Low-flow priapism: risk factors for erectile dysfunction. BJU Int. 2002;89:285–90. doi: 10.1046/j.1464-4096.2001.01510.x. [DOI] [PubMed] [Google Scholar]
- 17.Champion HC, Bivalacqua TJ, Takimoto E, Kass DA, Burnett AL. Phosphodiesterase-5A dysregulation in penile erectile tissue is a mechanism of priapism. Proc Natl Acad Sci U S A. 2005;102:1661–6. doi: 10.1073/pnas.0407183102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bivalacqua TJ, Musicki B, Kutlu O, Burnett AL. New insights into the pathophysiology of sickle cell disease-associated priapism. J Sex Med. 2012;9:79–87. doi: 10.1111/j.1743-6109.2011.02288.x. [DOI] [PubMed] [Google Scholar]
- 19.Bivalacqua TJ, Burnett AL. Priapism: new concepts in the pathophysiology and new treatment strategies. Curr Urol Rep. 2006;7:497–502. doi: 10.1007/s11934-006-0061-6. [DOI] [PubMed] [Google Scholar]
- 20.Bivalacqua TJ, Ross AE, Strong TD, Gebska MA, Musicki B, Champion HC, Burnett AL. Attenuated RhoA/Rho-kinase signaling in penis of transgenic sickle cell mice. Urology. 2010;76:510, e7–12. doi: 10.1016/j.urology.2010.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan J, Desouza R, Westney OL, Wang R. Insights of priapism mechanism and rationale treatment for recurrent priapism. Asian J Androl. 2008;10:88–101. doi: 10.1111/j.1745-7262.2008.00314.x. [DOI] [PubMed] [Google Scholar]
- 22.Akinsheye I, Klings ES. Sickle cell anemia and vascular dysfunction: the nitric oxide connection. J Cell Physiol. 2010;224:620–5. doi: 10.1002/jcp.22195. [DOI] [PubMed] [Google Scholar]
- 23.Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO, 3rd, Schechter AN, Gladwin MT. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8:1383–9. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 24.Kanika ND, Melman A, Davies KP. Experimental priapism is associated with increased oxidative stress and activation of protein degradation pathways in corporal tissue. Int J Impot Res. 2010;22:363–73. doi: 10.1038/ijir.2010.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lagoda G, Sezen SF, Cabrini MR, Musicki B, Burnett AL. Molecular analysis of erection regulatory factors in sickle cell disease associated priapism in the human penis. J Urol. 2013;189:762–8. doi: 10.1016/j.juro.2012.08.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lagoda G, Sezen SF, Hurt KJ, Cabrini MR, Mohanty DK, Burnett AL. Sustained nitric oxide (NO)-releasing compound reverses dysregulated NO signal transduction in priapism. FASEB J. 2014;28:76–84. doi: 10.1096/fj.13-228817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munarriz R, Park K, Huang YH, Saenz de Tejada I, Moreland RB, Goldstein I, Traish AM. Reperfusion of ischemic corporal tissue: physiologic and biochemical changes in an animal model of ischemic priapism. Urology. 2003;62:760–4. doi: 10.1016/s0090-4295(03)00484-9. [DOI] [PubMed] [Google Scholar]
- 28.Wood KC, Granger DN. Sickle cell disease: role of reactive oxygen and nitrogen metabolites. Clin Exp Pharmacol Physiol. 2007;34:926–32. doi: 10.1111/j.1440-1681.2007.04639.x. [DOI] [PubMed] [Google Scholar]
- 29.Bivalacqua TJ, Musicki B, Hsu LL, Berkowitz DE, Champion HC, Burnett AL. Sildenafil citrate-restored eNOS and PDE5 regulation in sickle cell mouse penis prevents priapism via control of oxidative/nitrosative stress. PLoS One. 2013;8:e68028. doi: 10.1371/journal.pone.0068028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burnett AL, Bivalacqua TJ, Champion HC, Musicki B. Long-term oral phosphodiesterase 5 inhibitor therapy alleviates recurrent priapism. Urology. 2006;67:1043–8. doi: 10.1016/j.urology.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 31.Burnett AL, Anele UA, Trueheart IN, Strouse JJ, Casella JF. Randomized controlled trial of sildenafil for preventing recurrent ischemic priapism in sickle cell disease. Am J Med. 2014;127:664–8. doi: 10.1016/j.amjmed.2014.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bennett N, Mulhall J. Sickle cell disease status and outcomes of African-American men presenting with priapism. J Sex Med. 2008;5:1244–50. doi: 10.1111/j.1743-6109.2008.00770.x. [DOI] [PubMed] [Google Scholar]