Abstract
Interferon regulatory factor 7 (IRF7) was originally identified in the context of Epstein–Barr virus (EBV) infection, and has since emerged as the crucial regulator of type I interferons (IFNs) against pathogenic infections, which activate IRF7 by triggering signaling cascades from pathogen recognition receptors (PRRs) that recognize pathogenic nucleic acids. Moreover, IRF7 is a multifunctional transcription factor, underscored by the fact that it is associated with EBV latency, in which IRF7 is induced as well as activated by the EBV principal oncoprotein latent membrane protein-1 (LMP1). Aberrant production of type I IFNs is associated with many types of diseases such as cancers and autoimmune disorders. Thus, tight regulation of IRF7 expression and activity is imperative in dictating appropriate type I IFN production for normal IFN-mediated physiological functions. Posttranslational modifications have important roles in regulation of IRF7 activity, exemplified by phosphorylation, which is indicative of its activation. Furthermore, mounting evidence has shed light on the importance of regulatory ubiquitination in activation of IRF7. Albeit these exciting findings have been made in the past decade since its discovery, many questions related to IRF7 remain to be addressed.
Keywords: IRF7, IFN, EBV, LMP1, PRR
Introduction
Interferon regulatory factors (IRFs) are a family of transcription factors comprised of nine members (IRF1-9) in mammalian cells. IRF10 has only been identified in avian species; its DNA homolog in humans is not expressed.1 The first member, IRF1, was identified in 1988 (ref. 2). Genes encoding IRFs exist in all principal metazoan groups although the family numbers are different.3 In addition, the herpesviruses Kaposi's sarcoma-associated herpesvirus and rhesus rhadinovirus encode 4 and 8 viral IRF-like proteins respectively, which antagonize the functions of cellular IRFs.4 Interestingly, the IRF family has coevolved with the nuclear factor (NF)κB family, both of which share some evolutionary characteristics:3 both families are activated by pathways signaling from the same pathogen recognition receptors (PRRs) and by the same kinase family IκB kinases (IKKs); both cooperate extensively in regulation of target cytokines such as interferon (IFN)β, and together they represent the major players in innate immune responses.3,5–8
The IRF family members share significant homology within the conserved N-terminal DNA-binding domain (DBD), which consists of a signature tryptophan pentad that is essential for DNA binding.9 Crystal structure studies have shown that DBD forms a helix-turn-helix structure, which recognizes the consensus DNA sequences that generally include at least two GAAA repeats,10–14 whereas other studies have suggested that IRFs may have broader flexible binding capacities to the consensus sequence 5′-AANNGAAA-3′ (refs 9, 13, 15, 16). The viral IRFs lack several of the tryptophan residues, thus they cannot bind to DNA and as a consequence function as dominant-negative mutants. The C-termini of IRFs are different and confer on each member distinct functions.5,17 In general, they contain an IRF-association domain, a nuclear export sequence, an autoinhibitory domain, and a signal-responding domain that has key serine residues subjected to phosphorylation upon infection with pathogens.9 The IRF family is the key player in multiple facets of host defense systems.5,18,19 In addition, IRFs have pivotal roles in immune cell development and regulation of oncogenesis.19,20
The Irf7 gene was originally cloned in 1997, in the context of latent Epstein–Barr virus (EBV) infection where the encoded protein binds to and regulates the EBNA1 Q promoter.21 The human Irf7 gene is located on chromosome 11p15.5, and encodes four isoforms, IRF7A, -B, -C and -D (-H).22 Human IRF7A protein consists of 503 amino acids with molecular size of 55kD, and mouse IRF7 consists of 457 amino acids with molecular size of 52kD. IRF3 is the closest family member to IRF7; together they are key regulators of the type I IFN (IFNα/β) responses, which are central to both innate and adaptive immunity.18
This review summarizes research on IRF7 since its discovery, with emphasis on recent findings of the potential roles of posttranslational modifications (PTMs) on activation as well as regulation of IRF7. Although most studies have focused on IRF7 phosphorylation leading to its activation and on its role in IFN antiviral innate immune responses, emerging evidence supports an important role for non-degradative ubiquitination in activation of IRFs including IRF7. Induction and activation of IRF7 in the EBV context indicate that IRF7 may have an important role in EBV latency and oncogenesis. Study of IRF7 in the context of EBV is thus important not only for understanding the interaction between EBV and host IRF7/IFN signaling in EBV oncogenesis, but also will provide valuable information on IRF7-mediated immune responses triggered by a wide range of pathogens.
Activation of IRF7
Activation of IRF7 is prerequisite for its functions as a transcription factor. Inactive IRF7 resides in the cytoplasm as a `latent' form. Pathogenic infection triggers IRF7 phosphorylation and translocation into the nucleus, where with other co-activators it forms a transcriptional complex that binds to the promoter regions of target genes to activate transcription.23,24 Crystal structure studies for IRF3 have supported a model for its activation: the IRF-association domain of IRF3 has a hydrophobic surface that is covered by an autoinhibitory domain in the `latent' form, and phosphorylation uncovers the hydrophobic surface for dimerization and functional interactions with other co-activators.12,25 Structural study of IRF5 has supported this model as a general mechanism for activation of IRFs.10 In addition, in line with this model, artificial deletion of an autoinhibitory domain of IRF7 (human IRF7A aa 247–467) can generate a constitutively active form of IRF7 (ref. 26). In contrast, another crystal structure study has indicated that phosphorylation is directly involved in dimerization and other functional interactions.11 In addition, analyses with truncated mutants have indicated that the C-terminus of IRF7 contains several other functional domains, which regulate IRF7 activity. Importantly, the virus-activation domain spanning aa 278–305 of human IRF7A is indispensable for IRF7 activation.26
Activation by PRRs
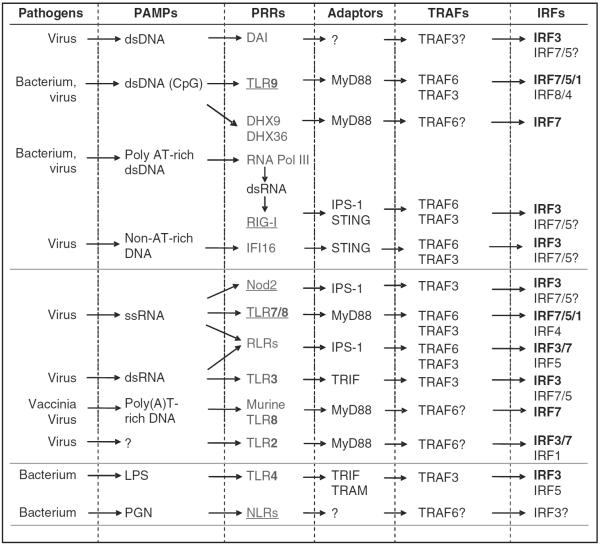
Pathogen-associated molecular patterns from invading pathogens launch innate immune responses by recognizing host PRRs, which include transmembrane Toll-like receptors (TLRs), and cytoplasmic nucleotide-binding oligomerization domain-like receptors, retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs), double-stranded RNA-dependent protein kinase,27 DNA-dependent activator of IRFs,28 absent in melanoma 2 (refs 29–32), and the recently identified DEAH box polypeptide 9 and −36 (ref. 33), IFN-inducible gene 16 (ref. 34) as well as RNA polymerase III;35–37 the last transcribes dsAT-rich microbial DNA into 5′-ppp dsRNA that is recognized by RIG-I.35–37
Among these identified PRRs, endolysosomal TLRs (endocytic TLR3 and TLR4, −7, −8 and −9) (ref. 38), and cytosolic RLRs,39 DNA-dependent activator of IRFs, RNA Pol III, nucleotide-binding oligomerization domain-2 (ref. 40), IFN-inducible gene 16, and DEAH box polypeptide 9 and −36, have been shown to activate IRFs for production of type I IFNs. Several recent reports have identified unexpected roles of TLR2 and −8 in activation of IRFs for production of type I IFNs in specific cell types.41–43 TLR2 can be internalized and activate IRF1, −3 and −7 in inflammatory monocytes41 and bone marrowderived macrophages,43 and murine TLR8, which was thought to be non-functional, mediates production of type I IFNs in murine plasmacytoid dendritic cells (pDCs) in response to poly(A)T-rich DNA derived from vaccinia virus.42 Core signaling components involved in signaling cascades leading to activation of IRFs include the adaptors TRIF (for TLR3 and −4) and MyD88 (for TLR2/7/8/9), IRAK1/4 (refs 44, 45), TRAF6 (for TLR7, −8 and −9 and RIG-I)46,47 and TRAF3 (for TLR3 and −4, RIG-I and nucleotide-binding oligomerization domain-2) (refs 48–50), and the IKKs, IKKε and TBK1. Core components unique to RLR signaling cascades leading to activation of IRFs include IFNβ promoter stimulator 1 (also called MAVS/VISA/CARDIF),51–53 stimulator of interferon genes (also called MITA/ERIS/MPYS)54–57 and Fas-associated death domain58 (Figure 1). Stimulator of interferon gene functions as an adaptor that links IFNβ promoter stimulator 1 to TBK1 for the activation of IRF3/7 following cytosolic non-CpG DNA-mediated signaling59 and other viral DNAs.34
Figure 1.
Activation of IRFs through signaling pathways triggered by different PRRs. Pathogen-associated molecular patterns (PAMPs) derived from invading pathogens are recognized by distinct PRRs located on the membranes of endosomal compartments and trigger signaling cascades, which lead to activation of IRFs and type I IFN production in the host cell.
IRF7 is activated by pathogenic nuclei acids through pathways mediated by TLR3, −7 and −9, RIG-I and likely DNA-dependent activator of IRF and IFI16, as well as by TLR2-mediated signaling pathway.41,43 IRF7 is predominantly activated by TLR7 in pDCs, in which both TLR7 and IRF7 are expressed at extra high levels that make these cells `professional' producers of type I IFNs. In contrast to TLR3, which requires both IRF3 and −7 for sufficient induction of type I IFNs, it is generally accepted that IRF7 is not activated by TLR4 (ref. 19). However, several reports have shown IRF7 can be activated by TLR4 under certain conditions, for example, in conventional DCs and monocytes after IFNβ priming.60,61
Increasing evidence supports the involvement of the phosphatidylinositol 3 kinase (PI3K) pathway in the regulation of activation of IRFs by TLRs.62 The PI3K pathway can be activated by various TLR ligands and can negatively or positively regulate TLR responses, depending on cell types and the ligands.63 Both TLR9 and -3 activate the PI3K/Akt/mTOR pathway leading to the activation of IRF7, -3 and -5 (refs 64–66). PI3K is critical for the nuclear translocation of IRF7 and type I IFN production by human pDCs in response to TLR7/9 activation,66 and mTOR Ser/Thr kinase activity is required for the interaction between MyD88 and IRF7 (ref. 64). In addition, induction of some IFN-stimulated genes such as Mx1, CXCL10, TNF-related apoptosis-inducing ligand by TLR7 in pDCs is dependent on the PI3K/p38MAPK pathway.67
Activation by EBV LMP1
Of special interest, IRF7, and likely IRF4, are induced as well as activated by the EBV oncoprotein latent membrane protein-1 (LMP1) (refs 68–71). LMP1 is a member of the tumor necrosis factor (TNF) receptor superfamily with constitutive activity. LMP1 exerts its functions by initiating both anti-apoptotic and proliferative growth factor-like signals, which are complex, have diverse outcomes and share signaling cascades with CD40, TLRs and a combination of TNF receptor-I and -II (refs 72–75). Our recent studies have shown that RIP1 and TRAF6, two key mediators participating in TNF signaling pathway, are also required for LMP1 activation of IRF7 (refs 70, 71). However, TRAF2 and -3, two other TRAFs involved in TNF signaling, are not required.76 Phosphorylation is believed to be the mechanism underlying IRF7 activation by LMP1 as shown by in vivo 32P-orthophosphate labeling68 and by the fact that the phosphorylation-deficient mutant, IRF7(S477A/479A), failed to be activated by LMP1 (ref. 71). Importantly, our recent studies has indicated that K63-linked non-degradative ubiquitination of IRF7 is indispensable for its activation by LMP1 as well as by IKKε,71 and probably the ubiquitination event is prerequisite for its phosphorylation.70
Regulation of IRF7
Regulation of IRF7 expression
IRF7 is a lymphoid-specific factor, which is constitutively expressed in the cytoplasm in B cells, pDCs and monocytes in the spleen, thymus, and peripheral blood lymphocytes, and is potently inducible by type I IFNs, virus infection and other stimuli such as 12-o-tetradecanoylphonol-13-acetate, TNFα and lipopolysaccharide in various cell types.21,77
The positive regulatory feedback between IRF7 and type I IFNs during antiviral immune responses is the major source for IRF7 expression in the cell.78–80 At the early `priming' stage of virus infection, the low level of endogenous IRF7 in the cell is phosphorylated and activated by signaling triggered from PRRs, and together with NFκB and IRF3, which are also activated by the same pathways, binds to the virus-responsive elements in the Ifna and Ifnb promoters and induces small amounts of type I IFNs, which secrete and bind to IFNA receptors on other cells. IRF7 has a pivotal role in the priming. Binding of IFNs to IFNA receptor results in the activation of the IFN Janus kinase-signal transducers and activator of transcription signaling cascade, leading to phosphorylation and activation of signal transducers and activator of transcription 1 and -2. The activated signal transducers and activator of transcription 1/2 then bind to IRF9 as a complex named `IFN-stimulated gene factor 3', which in turn binds to the IFN-stimulated response element on the IRF7 promoter and induces synthesis of more IRF7. Later, the newly synthesized IRF7 is activated and induces more IFNs so that more and more IRF7 and IFNs are produced, but IRF3 at late stages is degraded by virus infection.78–80
Induction of IRF7 by LMP1 in EBV latency is an important event as this occurs in a quite different biological context69 and this setting implies that IRF7 has another important role in oncogenesis, as also supported by a large body of other evidence (see later). Both LMP1 C-terminal functional domains, C-terminal activator regions 1 and -2, are involved in the induction of IRF7. NFκB, which is also activated by LMP1, was shown to be required for the induction.81 The IRF7 promoter contains at least 4 NFκB-binding sites in a 3 kb fragment starting from the translational start codon ATG.81,82 As it is induced by LMP1, IRF7 is associated with EBV latency programs; it is expressed at a low level in type I but at a high level in type III latency. IRF4, like IRF7, is also induced by LMP1 (ref. 83). However, another family member, IRF2, is not induced by LMP1 (ref. 69), although it is also highly expressed in type III latency.84
In addition, IRF7 is induced by TNFα and 12-o-tetradecanoylphonol-13-acetate through NFκB activation and chromosomal accessibility,85 and is silenced by promoter hypermethylation.82 Thus, reagents that can loosen chromatin structure such as topoisomerase II inhibitors can also induce IRF7 (ref. 85). We have shown that autoregulation of IRF7 also contributes to IRF7 induction in response to virus infection.86
IRF7 mRNA encodes four natural splicing variants, IRF7A, -B, -C and -D (-H).22 Moreover, IRF7 mRNA translation is repressed by the translational repressors, 4E-BP1/2. Production of type I IFNs is enhanced and virus infection is suppressed in 4E-BP1/2-deficient mouse embryo fibroblasts and knockout mice, correlated with upregulated IRF7 mRNA translation.87
Half-life of the IRF7 protein
Unlike its closest member IRF3 that is constitutively expressed and stable in most cells, IRF7 remains at low levels in most cell types, and has a very short half-life of 0.5~1 h in infected cells and approximately 5 h in uninfected cells but can be stabilized by the 26S proteasome inhibitor, MG132, or by a dominant-negative mutant of Cul1, a component of the SKP1-CUL1-F-box E3 ubiquitin ligase complex,88,89 suggesting that its stability is controlled by the ubiquitin–proteasome system. This claim is also supported by the fact that both murine and human IRF7, but not IRF3, contains a conserved potential prolineglutamic acid-serine-threonine sequence,88 which is essential for the target protein for destruction.
Similar to wild-type IRF7, a phosphorylation-deficient mutant was also partially degraded in infected cells, but a phosphomimetic mutant is less stable than the wild type even in the absence of virus, suggesting that phosphorylation is not necessary for but may contribute to virus-stimulated IRF7 degradation.89 IRF7 stability is tissue specific. Compared with that in mouse embryo fibroblasts, IRF7 half-life is longer and virus infection stabilizes rather than degrades IRF7 in thymocytes and splenocytes. Further analyses indicated that virus-induced IRF7 stability in these cells is controlled by both IFN-dependent and virus-dependent/IFN-independent mechanisms.89 Another study has shown that, in human lymphocytes, endogenous IRF7 is very stable and the half-life is > 24 h even without virus infection, and even in EBV-transformed cells in which IRF7 is phosphorylated, which usually precedes protein degradation.81 Thus, mouse and human IRF7 may have significant differences in stability.
IRF7 transcriptional coregulators
The transcription factors NFκB, PU.1 and SMAD have important roles in immunity and are known to directly interact with IRFs.3 Upon activation, IRF7 forms a transcriptional complex enhanceosome together with IRF3, NFκB, c-Jun, activating transcription factor 2 and p300/CREB-binding protein on the promoter region of the Ifnb gene.16,23,90 IRF7 is also able to form homodimers or heterodimers with IRF5 in regulation of target genes.91,92 SMAD3, a key component in the transforming growth factor-β signaling, which modulates many aspects of immune functions, was identified as an IRF7-interacting protein by yeast two-hybrid screening, and cooperates with IRF7 in the induction of IFNβ.93 In fact, the C-termini of IRFs share structural similarity with the SMAD/Forkhead-associated domain.94 These lines of evidence strongly suggest that IRF7 does not bind to the target promoters as a sole molecule.
To our knowledge, no transcriptional corepressor has been identified for IRF7 so far. Recently, the transcription factor, MafB, which impairs the interaction of co-activators with IRF3, is reported also to inhibit IRF7-dependent transcription, probably through interfering with the binding of IRF7 to target promoters, as efficient interaction between MafB and IRF7 requires IRF7 DBD. Thus, MafB may contribute to the limitation of type I IFN production spontaneously induced by constitutive IRF7 activity under physiological conditions such as in pDCs and macrophages in which high constitutive levels of IRF7 is detected.95
Regulation of IRF7 activity and protein stability by viral proteins
Owing to their pivotal role in host immunity, IRF7 and -3 are counteracted by numerous viruses through different strategies.96,97 For example, the Thogoto virus non-essential accessory protein ML strongly inhibits both IRF3 and -7 (refs 98, 99). Rotavirus nonstructural protein 1 mediates the degradation of IRF3, -5 and -7 (ref. 100). Human papillomavirus E6 oncoprotein binds to IRF3 and inhibits its transcriptional activity through a proteasome-independent mechanism.101 Ebola Zaire virus VP35 promotes sumoylation of both IRF3 and -7 and therefore blocks type I IFN production.102 Hepatitis C virus NS3/4A protease cleaves IFNβ promoter stimulator 1 and TRIF and therefore inhibits activation of IRF3 and -7 during hepatitis C virus infection.103
As to the herpesvirus family, to ensure successful infection and establishment of latency in the host, many virus-encoded immediate-early (IE) proteins can antagonize the effect of IRFs. For Kaposi's sarcoma-associated herpesvirus, IRF7 is negatively regulated by the IE lytic transactivator RTA/ORF50, which possesses ubiquitin E3 ligase activity, through ubiquitination-mediated degradation,104 and by another IE protein ORF45 that inhibits IRF7 phosphorylation and nuclear translocation.105,106 In addition, the four viral IRFs function as dominant-negative mutants of cellular IRFs.4,107–109 For example, vIRF3 inhibits cellular IRF5 (ref. 107) and −7 (refs 109, 110). For herpes simplex virus, the IE transactivator ICP0, which possesses ubiquitin E3 ligase activity, inhibits IRF3/7 activation in different ways, including induction of proteasome-dependent degradation of DNA-PK that targets IRF3 N-terminal Thr135 phosphorylation,111 and likely recruitment of cellular USP7/HAUSP, the herpesvirus-associated ubiquitinspecific protease.112–114
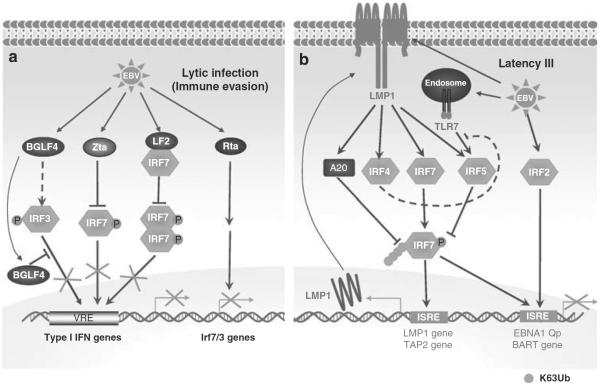
As to EBV, the IE proteins BZLF1 (also called Zta)115 and LF2 (also called BILF4),116 have recently been found to bind to IRF7 and repress its activity, and another IE transactivator, BRLF1 (also called RTA), downregulates expression of multiple IRFs including IRF7 and −3 and therefore impairs IFNβ production during EBV reactivation.117 LF2 interacts with the IRF-association domain of IRF7 and prevents its dimerization.116 Another EBV IE protein BGLF4, the only herpesvirus protein kinase conserved in EBV,118 has been identified as an IRF3-interacting protein by yeast two-hybrid screening.119 BGLF4 phosphorylates IRF3 in vitro, does not prevent IRF3 dimerization and nuclear translocation, but prevents its DNA-binding activity in response to poly(I:C) treatment119 (Figure 2a).
Figure 2.
Regulation of IRFs in EBV lytic and latent infections. (a) Lytic infection. BGLF4 phosphorylates IRF3, but blocks its DNA-binding activity. Zta inhibits IRF7 transcriptional activity and RTA downregulates expression of IRF7 and -3 through unclear mechanisms. LF2 interacts with IRF7 and therefore blocks its dimerization and transcriptional activity. (b) Latency 3. EBV LMP1 induces expression of IRF7, -4, -5 and A20. LMP1 also activates IRF7 through ubiquitination, but A20 inhibits LMP1-stimulated IRF7 ubiquitination and activity. IRF7 induces expression of LMP1 and TAP2, but inhibits BamH I-A rightward transcript (BART) transcript. EBV also induces expression of TLR7 and its downstream target IRF5, and IRF5 inhibits IRF7 through dimerization with IRF7. EBV also induces IRF2 through unknown mechanism.
Regulation of IRFs in EBV latency
EBV latency has an intimate association with the IRF family, as manifested by several lines of evidence: (i) Several members including IRF7 (refs 69, 120), −2 (ref. 84) and −4 (refs 83, 121, 122), which have oncogenic properties, as well as IRF5 (refs 92, 121, 123), are all expressed in much higher abundance in EBV latency III; (ii) both IRF4 (refs 83, 121) and −7 (ref. 69) are induced by LMP1, and IRF5 is induced by both TLR7 and LMP1 (refs 121, 124), whereas IRF2 is induced through an unclear mechanism;69 (iii) IRF7, and likely IRF4, are activated by LMP1 (refs 70, 71, 83) (Figure 2b).
In addition to IRF5 that negatively regulates IRF7 activity through heterodimerization with it in EBV latency,92 we have recently identified the dual function ubiquitinediting enzyme A20, which is also induced by LMP1 (refs 125, 126), as another negative regulator, which regulates IRF7 activity through regulation of its ubiquitination,127 indicating that IRF7 is complexly modulated in the EBV context. In addition, as IRF7 in EBV latency is constitutively phosphorylated and ubiquitinated, we believe that some other cellular proteins contribute to regulation of these important events in EBV latency through distinct signaling pathways, and we are conducting proteomic screening coupled with mass spectrometry to profile IRF7-interacting proteins from EBV-transformed cells. Further functional analyses of these proteins in the EBV/IRF7 interface are important not only for understanding how regulation of IRF7 contributes to the delicate balance of EBV latency as well as the interaction between EBV and IFN signaling pathways, but also will expand our knowledge in regulation of IRF7-mediated innate antiviral immune responses.
Modifications of IRF7
Posttranslational modifications of IRF7
Phosphorylation
Like other transcription factors, IRF7 undergoes various PTMs, the most important of which is phosphorylation. For human IRF7A, the most convincing major phosphorylation sites, serines 477 and 479, have been identified by serine–alanine substitution, which resulted in abrogation of IRF7 ability to transactivate Ifn gene promoters,26 and a phosphorylation-specific antibody has been developed which recognizes these two phosphorylation sites.128 Other substitution studies have shown that phosphorylation of Ser471, 472, 483, 484 and 487 also contributes to its activation.129–131 For mouse IRF7, Ser425 and 426 are considered as the critical phosphorylation/activation sites.78
The kinases identified for IRF7 phosphorylation include IKKε, TBK1 (refs 132–135), IRAK1 (ref. 136) and IKKα,137 which may phosphorylate IRF7 in cell typespecific manner. Study with knockout mice has indicated that the two IκB kinases, IKKε and TBK1, are the major kinases for IRF3/7 phosphorylation and activation.134 IRAK1 is involved in MyD88-dependent TLR7/9 signaling leading to IRF7 activation in pDCs.136 IRAK1 phosphorylates IRF7 in vitro, and kinase-dead IRAK1 can inhibit MyD88-dependent production of type I IFNs. IRAK1 is autophosphorylated, dissociates from the receptor complex, and associates with and activates TRAF6, which subsequently activates TAK1. At the same time, IRAK1 autophosphorylation may lead to its inactivation and therefore inhibits IRF7 in TLR and RLR responses. So IRAK1 may not be a kinase for IRF7 in vivo.138 IKKα is critical for IRF7 activation in TLR7/9 signaling cascades in pDCs.137 A later study has shown that the NFκB-inducing kinase differentially regulates IKKα in IRF7/3 activation.139 NFκB-inducing kinase can phosphorylate IKKα at Ser176 and Ser180. Substitution of these two residues with glutamate mimics IKKα phosphorylation. Although IKKα(S176E) activates IRF3/7, IKKα(S180E) does not.139
LMP1 also promotes IRF7 phosphorylation and activation,68 and likely targets the same two major sites, Ser477/479, as the phosphorylation-deficient mutant, IRF7(S477A/479A), could not be activated by LMP1 (ref. 71). Further investigation is necessary to identify the kinases involved in LMP1 activation of IRF7.
Ubiquitination
The immune system is under stringent regulation in order to orchestrate an appropriate immune response.140 As with its role in other biological processes, ubiquitination is one of the most important regulatory mechanisms in the immune system.140 The well studied type of ubiquitination is K48-linked polyubiquitination, which is known as the principal process whereby proteins are targeted for proteasomal degradation through the 26S proteosome. In recent years, proteasome-independent functions for non-degradative ubiquitination, especially K63-linked polyubiquitination and monoubiquitination, have been identified. The importance of these ubiquitination and other ubiquitination-like events such as sumoylation and acetylation in a variety of cellular processes, including receptor internalization (endocytosis), vesicle trafficking, DNA repair, stress responses and protein kinase activation, is becoming increasingly recognized.141–143
IRFs are also regulated by ubiquitination.144 IRF3 (ref. 97) and IRF7 (refs 89, 104, 145) have been reported to be degraded by virus infection through the ubiquitination pathway. Ubiquitination of IRF7 and −3 by the HECT E3 ligase RAUL results in their degradation and consequently restricts type I IFN responses.145 Ubiquitination of IRF3 by the E3 ubiquitin ligase RNF54, which is also called RBCC protein interacting with PKC1 (RBCK1) (ref. 146), or by the TRIM E3 ligase TRIM21/Ro52 (ref. 147) results in IRF3 degradation through proteosome-dependent pathway. A recent study has also shown that Ro52 interacts with IRF7 and promotes IRF7 ubiquitination and degradation.148 IRF6 has recently been linked to degradation through the ubiquitination pathway induced by cellular proliferation signaling.149 IRF8 is degraded by Cbl-mediated ubiquitination.150 The cellular levels of IRF1 (ref. 151) and IRF7 (ref. 89) under normal physiological conditions are also controlled by ubiquitination.
More interesting and importantly, accumulating evidence has supported an innovative concept that ubiquitination is required for activation of IRF7 (refs 70, 71), IRF3 (refs 152, 153), IRF5 (ref. 154), IRF2 (ref. 155) and IRF8 (ref. 156). Our recent study has shown that the TRAF6 E3 ligase promotes K63-linked polyubiquitination of IRF7 (ref. 70), and this modification is required for IRF7 activation by EBV LMP1 and IKKε.70 Similarly, TRAF6 also promotes K63-linked polyubiquitination of IRF5, and this polyubiquitination is required for IRF5 transactivation of the Ifna promoter and nuclear translocation.154 Biochemical evidence from a cell-free system has shown that Ubc5-mediated K63-linked polyubiquitination has a key role in IRF3 activation.153 Thus, K63-linked polyubiquitination may be a common mechanism for activation of IRFs, and is likely a prerequisite for their phosphorylation. We are now investigating this hypothesis using IRF7 as a model; this study may uncover the mechanism of regulatory ubiquitination as a key missing link in the activation of IRFs.
In addition to its role in activation of IRFs and regulation of their stability, ubiquitination also positively and negatively regulates the transcriptional activity of IRFs.97 For example, ubiquitination of IRF3 by TRIM21 was recently shown to be required for sustaining IRF3 activity after viral infection.157 The murine hepatitis virus-encoded deubiquitinase PLP2 deubiquitinates IRF3 and prevents its nuclear translocation, and therefore impairs IRF3-mediated type I IFN production.152 In contrast to RNF54 that specifically interacts with IRF3, TRIM21 also interacts with IRF7 and −8. However, TRIM21-mediated ubiquitination of IRF8 enhances its cytokine production ability in macrophages.156 MDM2, a ubiquitin E3 ligase targeting P53 for degradation, has been identified as E3 ligase for IRF2, and ubiquitination of IRF2 by MDM2 may attenuate the function of IRF2 as a transcriptional repressor.155
Sumoylation
Small ubiquitin-related modifiers (SUMOs) are a ubiquitin-like protein family, which includes SUMO1, −2 and −3 in higher eukaryotes. Protein modification mediated by SUMOs (sumoylation) generally occurs on lysines located in the motif ψKxE where ψ is a large hydrophobic residue. Like non-degradative ubiquitination, the functions of sumoylation include but are not limited to: regulation of subcellular localization, protein–protein interaction, DNA-binding, transcription, DNA repair, chromosomal functions and signal transduction.158,159
Recently, mouse IRF7 was reported to be sumoylated in response to viral infection, and the authors also identified K406 as the sumoylation site,160 a site corresponding to human IRF7 K452, which we also have shown to be likely a site for TRAF6-mediated ubiquitination.70 IRF7 sumoylation is mediated by PIAS1 (ref. 102). However, mutation of K406 to R406 did not abolish IRF7 sumoylation promoted by Ebola Zaire virus VP35 protein, indicating that there are other potential sumoylation site(s), one of which is K43 (corresponding to human IRF7A K45),102 and human IRF7A(K45R) can barely activate IFN promoter constructs.161 In contrast to K63-linked ubiquitination that promotes IRF7 activation, sumoylation negatively regulates the transcriptional activity of IRF7 and -3 (ref. 160). Similarly, IRF1 (refs 162–164), IRF2 (ref. 165) and IRF3 (refs 102, 160) have been shown to be sumoylated. Sumoylated IRF1 was elevated in tumor cells,164 and displays oncogenic potential by interfering with IRF1-mediated apoptosis,163 as well as enhanced resistance to degradation.163 PIAS3 has been identified as a SUMO1 ligase for IRF1, and the PIAS3-mediated sumoylation represses IRF1 transcriptional activity in reporter assays,162 but the physiological significance has not been investigated. Sumoylation of IRF2 by PIAS4 has no significant effects on its nuclear localization and DNA-binding activity, but increases its ability to inhibit IRF1 transcriptional activity and decreases its ability to activate the IFN-stimulated response element and H4 promoters.165
Acetylation
In addition to phosphorylation, ubiquitination and sumoylation, IRF7 also undergoes lysine acetylation in response to Newcastle disease virus infection. IRF7 is acetylated by the histone acetyltransferases p300/CREB-binding protein-associated factor and GCN5 on the position K92, and the acetylation impairs IRF7 DNA-binding activity.166 The mutant, IRF7(K92R) lost DNA-binding activity whereas another mutant, IRF7(G90T/T93R), which retains K92 but cannot be acetylated, showed increased DNA-binding ability compared with wild-type IRF7 (ref. 166), suggesting other modification(s) on K92 may be critical for IRF7 DNA binding. IRF1 and -2 were also shown to be acetylated by p300 and p300/CREB-binding protein-associated factor following 12-o-tetradecanoylphonol-13-acetate treatment, which induces expression of p300 and p300/CREB-binding protein-associated factor.167,168 Under physiological conditions, acetylation of IRF2 is likely required for its transactivation of the H4 promoter, which is dependent on cell growth.167 Besides K92, K45 and K120 of human IRF7 were also acetylated when overexpressed, as identified by mass spectrometry.169 Other IRF family members also undergo lysine acetylation.169 In contrast to IRF7, acetylation of IRF3 by CREB-binding protein unmasks its DBD and thus facilitates its activation.170 Acetylation of IRF9 on K81 may be required for its DNA binding, as the mutant, IRF9(K81R) lost DNA-binding activity.169 However, this mutant may mask other modifications on this site such as ubiquitination, which may indeed be required for IRF9 DNA binding.
Promoter methylation
The IRF7 promoter contains a putative CpG island flanking the TATA box. The CpG island is endogenously methylated in the human fibroblasts, 2fTGH. Methylation of the IRF7 promoter results in Irf7 gene silencing in these cells, even in the presence of IFN treatment. 5-AzaC, an inhibitor of methyltransferase, can restore IRF7 expression in 2fTGH cells.82 Methylation of the IRF7 promoter is also found in immortalized fibroblasts from Li-Fraumeni syndrome171 and in lung cancer cell lines.172
Functions of IRF7
IRFs have been involved in regulation of a variety of cellular functions. Besides their roles in IFN-mediated immune responses, IRFs also have a role in leukemia and other malignancies in many cell types,173 and in regulation of cell growth and apoptosis, and they therefore affect susceptibility to and the progression of cancer. Another important function conferred by IRFs is the regulation of immune cell differentiation and activation. In addition, IRFs, especially IRF5, has been shown to be involved in several autoimmune diseases such as systemic lupus erythematosus (SLE).174
IRF7 as the `master' regulator of type I IFN production
Type I IFNs, encoded by a single Ifnb and 13 closely related Ifna genes that are all clustered on human chromosome 9p22, constitute a highly pleiotropic cytokine family that participates not only in immune modulation, including pathogen-elicited innate immune and subsequent adaptive immune responses, as well as autoimmune responses, but also in oncogenesis, cellular development and homeostasis. Aberrant production of IFNs is associated with many types of diseases, such as cancers, immune disorders and multiple sclerosis.175–179 Thus, fine regulation of type I IFN production is critical to maintain homeostasis. IFNs exert their functions through induction of >300 IFN-inducible genes such as dsRNA-dependent protein kinase, inducible nitric oxide synthase, STAT1, 2′,5′-OAS, major histocompatibility complex class I, Mx GTPase, IKKε, TRIM5α, caspase-1, melanoma differentiation associated gene 5, P53 and IRF7 (refs 175, 180).
Five IRFs, IRF1, -3, -5, -7 and -8 (ref. 181) are positive regulators of type I IFNs in different cell types,182 but IRF1, -5 and -8 are not necessary for induction of type I IFNs. Although both IRF3 and -7 are required for efficient IFN production in most immune cells, their functions are not redundant and have distinct roles. A recent study has shown that IRF3, which is constitutively expressed in most cells, is crucial for initial induction of IFNβ and IFNα1 (ref. 183). However, the constitutive low levels of IRF7 present in most immune cells are indispensable for this priming, and more importantly, IRF7 has a major role in subsequent feedback amplification of both IFNαs and IFNβ when new IRF7 is synthesized but IRF3 is degraded, in spite of the dependence on MyD88 and in both innate and adaptive immunity.18 In IRF7 knockout mice, MyD88-independent production of type I IFNs was severely impaired. No type I IFNs were produced in MyD88-dependent pathway in pDCs.174 The late-phase production of IFNs greatly amplifies protective responses and is important for initiation of adaptive immunity. In addition, at specific stages, IRF3 and -7 physically bind and together with other transcription factors such as NFκB, cooperatively regulate the Ifnb promoter.18,80
pDCs are `professional' producers for type I IFNs, in which both TLR7 and IRF7 are expressed at extremely high levels even in the absence of virus infection. TLR7 recognizes single-stranded RNA released from many RNA viruses such as human immunodeficiency virus,184 hepatitis C virus,185 vescular stomatitis virus and influenza virus, and produce type I IFNs mainly through activation of IRF7. TLR9, which recognizes CpG DNA from DNA viruses such as herpes simplex virus 1, is also expressed at a high level in these cells and dominantly activates IRF7 for production of type I IFNs.186
IRF7 in regulation of oncogenesis and apoptosis
In addition to regulation of immune responses, another important function of IRFs is their role in regulation of oncogenesis and apoptosis.5,19,182,187
IRF1 can induce cell cycle arrest and apoptosis, has obvious tumor-suppressor function, and is a new member of the tumor susceptibility genes.188 IRF2 and -4 have oncogenic potential.83,189 IRF2 may execute its oncogenic function via antagonizing IRF1. IRF4 targets c-Myc and vice versa.189 Knockdown of IRF4 by short hairpin RNA in EBV-positive lymphoblastoid cells decreases cell division and increases apoptosis,83 and induces a rapid and profound non-apoptotic cell death in multiple myeloma cell lines.189 Single-nucleotide polymorphisms of Irf4 3′-UTR are strongly associated with chronic lymphocytic leukemia susceptibility.190 IRF4 may need additional factors to exert its oncogenic function. IRF3 can mediate virus-induced apoptosis191–193 through activation of the Bcl2 family member Bax.194 Aberrant expression of IRF3 and its variants have also been detected in human lung cancer.195 IRF5 mediates stress-induced cell cycle arrest and p53-independent, TNF-related apoptosis-inducing ligand/Fas-dependent apoptosis in a cell type-dependent manner, and inhibits the growth of tumor cells both in vitro and in vivo.196–198 TNF-related apoptosis-inducing ligand triggers a signal leading to IRF5 phosphorylation and nuclear translocation,198 whereas activation of Fas receptor did not result in IRF5 nuclear translocation.196 Moreover, IRF5 is a direct target of the tumor suppressor p53, and like p53, is a candidate tumor suppressor.199 In addition, IRF4 and -8 regulate telomerase activity in immune cells.200 IRF8 also has tumor-suppressor function and downregulates Bcl-2 expression,201 and its mRNA expression is reduced in chronic myeloid leukemia and acute myelogenous leukemia.202 IRF6 expression is reduced or absent in breast carcinomas, and may be a tumor suppressor.149
Like IRF2 and -4, IRF7 has oncogenic properties, as indicated by several lines of evidence from our studies and others: (i) IRF7 is induced and activated by EBV LMP1 in EBV latency programs, which are associated with a variety of malignancies.203 (ii) IRF7 is over-expressed in EBV-positive central nervous system lymphomas,204 as well as in EBV-negative acute lymphocytic leukemia, chronic lymphocytic leukemia, acute myelogenous leukemia, and adult T-cell leukemia/lymphoma.205,206 (iii) IRF7 induces expression of the EBV principal oncoprotein LMP1 so that they form a regulatory circuit that may potentiate oncogenic effects of both factors.120 (iv) Moreover, IRF7-expressing NIH 3T3 cells induce tumor formation in nude mice.
Importantly, our data show that IRF4 upregulates expression of miR-155 in EBV latency. However, in this setting, IRF7 has little if any effect (to be published). In addition to its role in innate immunity,207,208 miR-155 is the first oncogenic microRNA shown to have increased expression in cancers,209 and is now believed to be critical for various types of cancers.210,211 Like IRF7 and -4, miR-155 is also associated with EBV latency as it is induced by LMP1 (refs 212–214).
On the other hand, IRF7 has antitumor effects in macrophages,192 partially through induction of TNF-related apoptosis-inducing ligand,215 a TNF superfamily member that induces caspase-8-dependent apoptosis selectively in tumor cells but not in normal cells. Other experimental data supporting IRF7's antitumor effects include that IRF7 is induced by the tumor-suppressor BRCA1 in response to IFN-γ signaling in MDA-derived breast cancer cell lines and overexpressed IRF7 inhibits growth of MCF-7 breast cancer cells,216 and that IRF7 may be potentially activated by DNA-damaging reagents as exogenously expressed IRF7 is translocated to the nucleus in response to these reagents in HeLa,217 a cervical carcinoma cell line. In addition, IRF7 is not expressed in fibrosarcoma cells,82 Li-Fraumeni Syndrome fibroblasts171 and lung cancer cell lines,172 because of promoter methylation. Thus, IRF7 regulation of oncogenesis may be cell type dependent.
Role of IRF7 in the EBV context
As stated above, the IRF family is tightly associated with EBV latency. Although IRF2, −4 and −7 are believed to be positive regulators of EBV oncogenesis, IRF5 may be a negative regulator of it. IRF5 expressed in EBV-infected cells has at least two natural variants, a truncation mutant V12 (ref. 121) and a point mutant IRF5(A68P),123 both of which function as dominant-negative mutants. In fact, we have shown that IRF5 in EBV-infected B cells negatively regulates LMP1-promoted IRF7 activity.92 The point mutant IRF5(A68P) also exists in adult T cell leukemia and chronic lymphocytic leukemia.123 Both IRF5(V12) and IRF4 in the EBV context are able to neutralize TLR7 ligand-induced cell growth inhibition. Like IRF7, IRF2 is constitutively expressed at a low level in type I latency,218,219 but at a higher level and represses Qp in type III latency.84 In addition, as with its role in antiviral responses,220 IRF4 may negatively regulate TLR7 activation of IRF5 in EBV latency (Figure 2).
Although along with others, we have presented overwhelming evidence that IRF7 is associated with EBV latency and has oncogenic properties, the role of IRF7 in oncogenesis remains unclear. IRF7 itself may be an oncoprotein targeted by LMP1. More likely, as a transcription factor, IRF7 may exert this function through regulation of a set of genes involved in regulation of cell growth and proliferation in EBV-transformed cells. However, until now, no such targets have been identified except the gene encoding the viral oncoprotein LMP1 (ref. 120), and BamH I-A rightward transcript P1 promoter221 (Figure 2). BamH I-A rightward transcript-derived microRNAs downregulate LMP1 expression and regulate LMP1 signaling in nasopharyngeal carcinoma cells and therefore have a role in EBV oncogenesis.222 By microarray analysis from shIRF7-expressing EBV-transformed cells, we have identified many cellular genes targeted by IRF7 such as apoptosis inhibitor 5 and c-IAP1/2 (data not shown). Some of these genes overlap with those targeted by the LMP1/NFκB pathway. Thus, the LMP1/IRF7 pathway may cooperate with the LMP1/NFκB pathway in EBV oncogenesis.
Although IRF7 is the key regulator of type I IFNs, EBV does not induce substantial type I IFNs in latency where it activates IRF7 (ref. 22). Understanding the underlying mechanism of this paradox is of vital importance as the outcomes of the interaction between EBV and IFN signaling shape the immune response to viral infection as well as affect aspects of host cell proliferation and survival. In addition, LMP1-activated IRF7 induces TAP2, type I IFNs and other IFN-stimulated genes upon superinfection and therefore helps to establish cellular antiviral states in EBV latency68,223,224 (Figure 2).
Besides these roles, the LMP1/IRF7 pathway also contributes to maintenance of EBV long-term latency as IRF7 inhibits activity of the EBNA1 Q promoter in type III latency.21
IRF7 in autoimmune diseases
Although PRR recognition of pathogenic nucleic acids orchestrates immune responses for defense, recognition of cellular `self' nucleic acids derived from tissue damage produces aberrant type I IFNs, which have received particular attention because of their role in autoimmune diseases such as SLE and rheumatoid arthritis.225 SLE is characterized by `IFN signature' in the serum.226 Nucleic acid-recognizing TLRs (TLR3, −7, −8 and −9) and RLRs, have been implicated in autoimmune diseases orchestrated by autoantibodies, which recognize DNA or RNA-containing immune complexes such as snRNPs and debris of apoptotic cells.18,177,226 RNAs in these immune complexes have 5′-triphosphates, which are required for RIG-I recognition.39 In addition, whether the recently identified PRR, DNA-dependent activator of IRF, is also able to recognize `self' DNA and contributes to autoimmune diseases merits study.
It is evident that single-nucleotide polymorphisms of Irf5 are linked to lupus,227 although the functional interaction is unclear. Recent work has shown that high levels of IRF1 are expressed in myelodysplastic syndrome patients with autoimmune disease.228 Given that IRF7 is also activated by TLR3/7/9 and RIG-I in response to nucleic acids, and that pDCs, in which the TLR7/IRF7 pathway governs type I IFN production, secrete a large amount of IFNα in response to immune complexes and has a role in autoimmune diseases, IRF7 may also be involved in these diseases.18,225,229 In fact, IRF7/3 recognize undegraded chromosomal DNAs released from apoptotic cells in macrophages,230 and furthermore, a genome-wide association study has identified rs4963128, a KIAA1542 single-nucleotide polymorphism 23 kb telomeric to IRF7, in strong association with SLE.231 A recent study of single-nucleotide polymorphisms in the Irf7/Phrf1 locus also has indicated that genetic variants of Irf7 act as risk factors for SLE, but the role needs to be further clarified.232 Another recent study has shown that the functional IRF7 variant rs1131665 (Q412R) is associated with SLE.233 In addition, IRF7 is implicated in the pathogenesis of type I diabetes.234
IRF7 in cell differentiation
The role of IRFs in regulation of the development and activation of hematopoietic cells accounts for their involvement in both innate and adaptive immune responses. IRF4, −8, −1 and −2 have been shown to have diverse roles in controlling the development, maturation and function of hematopoietic cells, including lymphoid and myeloid lineages, as well as DC subsets, which can be derived from both lymphoid and myeloid progenitors. Both IRF4 and −8 interact with PU.1, an immune cell-specific protein of the Ets family.20,182,235 IRF5 has recently been shown to have a critical role in regulation of B-cell differentiation.236
Involvement of IRF7 in cell differentiation is poorly understood, and only one study has shown that IRF7 is required for monocyte differentiation to macrophages, but the mechanism remains unclear.237 Given that virus-induced type I IFNs can drive pDC maturation from immature circulating cells to differentiated cells that contribute to adaptive immunity, IRF7 may have an indispensable role in DC differentiation. IRF7 is also involved in expression of CCL19, which has a central role in DC biology.238 In addition, we have results showing that IRF7 regulates expression of MyD88 and macrophage colony-stimulating factor, two critical factors involved in cell differentiation (to be published).
Other functions
Compared with the closest family member IRF3 that has a strict DNA-binding specificity to GAAANNGAAANN, IRF7 binds to a wider DNA consensus sequence GAAWNYGAAANY.239 This means IRF7 has the ability to regulate a larger range of target genes, which may be involved in diverse cellular processes. For example, IRF7, but not IRF3, is associated with EBV latency. IRF7 directly induces expression of IRF1 and LMP2, two key players in adaptive immunity.240 IRF3 has only been found to function in antiviral innate immunity and virus-induced apoptosis.17,192 Moreover, the consensus DNA-binding motif of IRF7 has been found in promoters of many oncogenes,18 and microarray results from the stable cell line BJAB–IRF7 have shown that IRF7 induces a subset of pro-apoptotic proteins, as well as a group of mitochondrial genes and genes affecting the DNA structure.241
Questions
The importance of IRF7 in the immune system has been quickly recognized since its discovery in the EBV context. However, IRF7 has been less studied in immunity compared with IRF3, in apoptosis compared with IRF1 and −5, in oncogenesis compared with IRF2 and −4, in cell differentiation compared with IRF4 and −8, and in autoimmune diseases compared with IRF5 and −1. There are many questions to be addressed.
-
(1)
Structural and functional domains. Crystal structure has been reported for other family members but not for IRF7. The functional domains of IRF7 have only been characterized by deletion analysis, and the underlying mechanisms are not understood. For example, why is the virus-activation domain important for its activation? Moreover, functions of isoforms other than IRF7A are not clear. Recently, IRF7C has been shown to inhibit IRF7A transcriptional activation of Ifn genes, and like IRF7A, is associated with EBV latency and has oncogenic potential, as it produces anchorage-independent growth of NIH 3T3 cells.242
-
(2)
Physiological targets. Transcriptional target genes of IRF7 in the EBV context and in the immune system have not been profiled. As a transcription factor, comprehensive profiling of IRF7 target genes is not only important for better understanding of its known roles in immune responses and EBV latency, but also important for revealing its novel functions in other biological processes. Although one report has identified genes targeted by IRF7 using BJAB cells stably expressing IRF7 (ref. 241), these identified genes may not represent those targets under physiological settings.
-
(3)
Regulation of expression. Study of IRF7 regulation is important as its expression is lymphoid specific. Although it has been shown that pDCs express a very high level of the translational inhibitors 4E-BP1 and -2, which may account for the extremely high level of IRF7 in pDCs,87 the molecular mechanism requires further clarification. In addition to the binding sites responsible for NFκB and IFN induction, which have been identified, other potentially functional cis-regulatory elements recognized by transcription factors such as c-myc, Sp1 and SREBP exist in the IRF7 promoter.82 Regulation of IRF7 expression by microRNA has not been reported.
-
(4)
Functional regulation. Deregulation of type I IFNs is associated with many types of diseases including cancers. As the `master' regulator of type I IFNs, fine regulation of IRF7 activity is thus necessary for its functions for normal immune responses as well as many other fundamental cellular processes involving IFNs. However, most studies have focused on its activation although accumulating evidence has identified viral proteins, which counteract its functions. Cellular proteins such as transcriptional co-regulators involved in regulation of IRF7 have been rarely identified.
-
(5)
Posttranslational modifications. Accumulating evidence shows that IRF7 undergoes many types of PTMs including phosphorylation, ubiquitination, sumoylation and acetylation, which are critical for regulation of its functions. Thus, understanding these PTMs in regulation of IRF7 may have an important impact on the field of innate immunity. However, except for phosphorylation, other PTMs of IRF7 and their interplay are poorly understood; even its phosphorylation sites have not been fully profiled. Moreover, the role of ubiquitination in activating and regulating IRF7 activity is not established. In addition, all the sites undergoing modifications have been identified by amino acid substitution, which may mask other PTMs or its function. Thus, employment of contemporary approaches such as mass spectrometry, which is still under development in this field, will be important for identification of these sites.
-
(6)
Can any signaling pathways other than those triggered by LMP1 and TLR/RLR innate immunity activate IRF7? For example, LMP1 mimics CD40, BCR, TNF receptor and TLR in many facets of signaling transduction.73,75 Since LMP1 and TLR activate IRF7, does any other similar signaling pathway activate IRF7? Activation of IRF7 by other signaling pathways may endow IRF7 with important novel functions in the corresponding biological contexts. In fact, IRF7 is potentially activated by DNA-damaging reagents through MKK4-JNK pathway,217 suggesting that IRF7 may have a role in DNA damage response.
-
(7)
Key signaling intermediaries for IRF7 activation. In past years, extensive efforts have been focused on the immune signaling pathways leading to IRF3/7 activation, and an increasing list of the signaling intermediaries have been identified, including STING, TRAF3, IKKs and others. However, an entire and clear picture of the signaling cascade is still a distant prospect.
-
(8)
All known functions for IRF7 so far are as a transcription factor in the nucleus. Does IRF7 have other functions in the cytoplasm? P53 is believed to promote apoptotic events directly in the cytoplasm, independent of its role as a transcription factor.243
Acknowledgements
This work is supported by the State of Florida Biomedical Research Programs (1BN-07) and NCI (1P30CA147890-01).
Footnotes
Conflict of interest The authors declare no conflict interest.
References
- 1.Nehyba J, Hrdlickova R, Burnside J, Bose HR., Jr A novel interferon regulatory factor (IRF), IRF10, has a unique role in immune defense and is induced by the v-Rel oncoprotein. Mol Cell Biol. 2002;22:3942–3957. doi: 10.1128/MCB.22.11.3942-3957.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miyamoto M, Fujita T, Kimura Y, Maruyama M, Harada H, Sudo Y, et al. Regulated expression of a gene encoding a nuclear factor, IRF1, that specifically binds to IFN-β gene regulatory elements. Cell. 1988;54:903–913. doi: 10.1016/s0092-8674(88)91307-4. [DOI] [PubMed] [Google Scholar]
- 3.Nehyba J, Hrdlickova R, Bose HR., Jr Dynamic evolution of immune system regulators: the history of the interferon regulatory factor (IRF) family. Mol Biol Evol. 2009;26:2539–2550. doi: 10.1093/molbev/msp167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee HR, Kim MH, Lee JS, Liang C, Jung JU. Viral interferon regulatory factors. J Interferon Cytokine Res. 2009;29:621–628. doi: 10.1089/jir.2009.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takaoka A, Tamura T, Taniguchi T. Interferon regulatory factor family of transcription factors and regulation of oncogenesis. Cancer Sci. 2008;99:467–478. doi: 10.1111/j.1349-7006.2007.00720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghosh S, May MJ, Kopp EB. NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh S, Hayden MS. New regulators of NF-κB in inflammation. Nat Rev Immunol. 2008;8:837–848. doi: 10.1038/nri2423. [DOI] [PubMed] [Google Scholar]
- 8.Hiscott J. Convergence of the NF-κB and IRF pathways in the regulation of the innate antiviral response. Cytokine Growth Factor Rev. 2007;18:483–490. doi: 10.1016/j.cytogfr.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Chen W, Royer W. Structural insights into interferon regulatory factor activation. Cell Signal. 2010;22:883–887. doi: 10.1016/j.cellsig.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen W, Lam SS, Srinath H, Jiang Z, Correia JJ, Schiffer CA, et al. Insights into interferon regulatory factor activation from the crystal structure of dimeric IRF5. Nat Struct Mol Biol. 2008;15:1213–1220. doi: 10.1038/nsmb.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahasi K, Suzuki NN, Horiuchi M, Mori M, Suhara W, Okabe Y, et al. X-ray crystal structure of IRF3 and its functional implications. Nat Struct Mol Biol. 2003;10:922–927. doi: 10.1038/nsb1001. [DOI] [PubMed] [Google Scholar]
- 12.Qin BY, Liu C, Lam SS, Srinath H, Delston R, Correia JJ, et al. Crystal structure of IRF3 reveals mechanism of autoinhibition and virus-induced phosphoactivation. Nat Struct Mol Biol. 2003;10:913–921. doi: 10.1038/nsb1002. [DOI] [PubMed] [Google Scholar]
- 13.Fujii Y, Shimizu T, Kusumoto M, Kyogoku Y, Taniguchi T, Hakoshima T. Crystal structure of an IRF-DNA complex reveals novel DNA recognition and cooperative binding to a tandem repeat of core sequences. EMBO J. 1999;18:5028–5041. doi: 10.1093/emboj/18.18.5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kusumoto M, Fujii Y, Tsukuda Y, Ohira T, Kyougoku Y, Taniguchi T, et al. Crystallographic characterization of the DNA-binding domain of interferon regulatory factor-2 complexed with DNA. J Stract Biol. 1998;121:363–366. doi: 10.1006/jsbi.1998.3970. [DOI] [PubMed] [Google Scholar]
- 15.Escalante CR, Nistal-Villβn E, Shen L, Garcia-Sastre A, Aggarwal AK. Structure of IRF-3 bound to the PRDIII-I regulatory element of the human interferon-β enhancer. Mol Cell. 2007;26:703–716. doi: 10.1016/j.molcel.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 16.Panne D, Maniatis T, Harrison SC. An atomic model of the interferon-β enhanceosome. Cell. 2007;129:1111–1123. doi: 10.1016/j.cell.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiscott J. Triggering the innate antiviral response through IRF3 activation. J Biol Chem. 2007;282:15325–15329. doi: 10.1074/jbc.R700002200. [DOI] [PubMed] [Google Scholar]
- 18.Honda K, Taniguchi T. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat Rev Immunol. 2006;6:644–658. doi: 10.1038/nri1900. [DOI] [PubMed] [Google Scholar]
- 19.Savitsky D, Tamura T, Yanai H, Taniguchi T. Regulation of immunity and oncogenesis by the IRF transcription factor family. Cancer Immunol Immunother. 2010;59:489–510. doi: 10.1007/s00262-009-0804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gabriele L, Ozato K. The role of the interferon regulatory factor (IRF) family in dendritic cell development and function. Cytokine Growth Factor Rev. 2010;18:503–510. doi: 10.1016/j.cytogfr.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L, Pagano JS. IRF7, a new interferon regulatory factor associated with Epstein-Barr virus latency. Mol Cell Biol. 1997;17:5748–5757. doi: 10.1128/mcb.17.10.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L, Pagano JS. Structure and function of IRF7. J Interferon Cytokine Res. 2002;22:95–102. doi: 10.1089/107999002753452700. [DOI] [PubMed] [Google Scholar]
- 23.Wathelet MG, Lin CH, Parekh BS, Ronco LV, Howley PM, Maniatis T. Virus infection induces the assembly of coordinately activated transcription factors on the IFN-β enhancer in vivo. Mol Cell. 1998;1:507–518. doi: 10.1016/s1097-2765(00)80051-9. [DOI] [PubMed] [Google Scholar]
- 24.Yang H, Lin CH, Ma G, Baffi MO, Wathelet MG. Interferon regulatory factor (IRF)-7 synergizes with other transcription factors through multiple interactions with p300/CBP coactivators. J Biol Chem. 2003;278:15495–15504. doi: 10.1074/jbc.M212940200. [DOI] [PubMed] [Google Scholar]
- 25.Qin BY, Liu C, Srinath H, Lam SS, Correia JJ, Derynck R, et al. Crystal structure of IRF-3 in complex with CBP. Structure. 2005;13:1269–1277. doi: 10.1016/j.str.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 26.Lin R, Mamane Y, Hiscott J. Multiple regulatory domains control IRF7 activity in response to virus infection. J Biol Chem. 2000;275:34320–34327. doi: 10.1074/jbc.M002814200. [DOI] [PubMed] [Google Scholar]
- 27.Balachandran S, Roberts PC, Brown LE, Truong H, Pattnaik AK, Archer DR, et al. Essential role for the dsRNA-dependent protein kinase PKR in innate immunity to viral infection. Immunity. 2000;13:129–141. doi: 10.1016/s1074-7613(00)00014-5. [DOI] [PubMed] [Google Scholar]
- 28.Takaoka A, Wang Z, Choi MK, Yanai H, Negishi H, Ban T, et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–505. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 29.Burckstummer T, Baumann C, Bluml S, Dixit E, Durnberger G, Jahn H, et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol. 2009;10:266–272. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- 30.Fernandes-Alnemri T, Yu JW, Datta P, Wu J. Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey D, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts TL, Idris A, Dunn JA, Kelly GM, Burnton CM, Hodgson S, et al. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science. 2009;323:1057–1060. doi: 10.1126/science.1169841. [DOI] [PubMed] [Google Scholar]
- 33.Kim T, Pazhoor S, Bao M, Zhang Z, Hanabuchi S, Facchinetti V, et al. Aspartate-glutamate-alanine-histidine box motif (DEAH)/RNA helicase A helicases sense microbial DNA in human plasmacytoid dendritic cells. Proc Natl Acad Sci USA. 2010;107:15181–15186. doi: 10.1073/pnas.1006539107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Unterholzner L, Keating SE, Baran M, Horan KA, Jensen SB, Sharma S, et al. IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol. 2010;11:997–1004. doi: 10.1038/ni.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ablasser A, Bauernfeind F, Hartmann G, Latz E, Fitzgerald KA, Hornung V. RIG-I-dependent sensing of poly(dA:dT) through the induction of an RNA polymerase III-transcribed RNA intermediate. Nat Immunol. 2009;10:1065–1072. doi: 10.1038/ni.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiu YH, MacMillan JB, Chen ZJ. RNA polymerase III detects cytosolic DNA and induces Type I interferons through the RIG-I pathway. Cell. 2009;138:576–591. doi: 10.1016/j.cell.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi MK, Wang Z, Ban T, Yanai H, Lu Y, Koshiba R, et al. A selective contribution of the RIG-I-like receptor pathway to type I interferon responses activated by cytosolic DNA. Proc Natl Acad Sci USA. 2009;106:17870–17875. doi: 10.1073/pnas.0909545106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barton GM, Kagan JC. A cell biological view of Toll-like receptor function: regulation through compartmentalization. Nat Rev Immunol. 2009;9:535–542. doi: 10.1038/nri2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakhaei P, Genin P, Civas A, Hiscott J. RIG-I-like receptors: sensing and responding to RNA virus infection. Semin Immunol. 2009;21:215–222. doi: 10.1016/j.smim.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 40.Sabbah A, Chang TH, Harnack R, Frohlich V, Tominaga K, Dube PH, et al. Activation of innate immune antiviral responses by Nod2. Nat Immunol. 2009;10:1073–1080. doi: 10.1038/ni.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barbalat R, Lau L, Locksley RM, Barton GM. Toll-like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands. Nat Immunol. 2009;10:1200–1207. doi: 10.1038/ni.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martinez J, Huang X, Yang Y. Toll-like receptor 8-mediated activation of murine plasmacytoid dendritic cells by vaccinia viral DNA. Proc Natl Acad Sci USA. 2010;107:6442–6447. doi: 10.1073/pnas.0913291107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dietrich N, Lienenklaus S, Weiss S, Gekara NO. Murine toll-like receptor 2 activation induces type I interferon responses from endolysosomal compartments. PLoS ONE. 2010;5:e10250. doi: 10.1371/journal.pone.0010250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim TW, Staschke K, Bulek K, Yao J, Peters K, Oh KH, et al. A critical role for IRAK4 kinase activity in Toll-like receptor-mediated innate immunity. J Exp Med. 2007;204:1025–1036. doi: 10.1084/jem.20061825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koziczak-Holbro M, Joyce C, Gluck A, Kinzel B, Muller M, Tschopp C, et al. IRAK-4 kinase activity is required for Interleukin-1 (IL-1) receptor- and Toll-like Receptor 7-mediated signaling and gene expression. J Biol Chem. 2007;282:13552–13560. doi: 10.1074/jbc.M700548200. [DOI] [PubMed] [Google Scholar]
- 46.Konno H, Yamamoto T, Yamazaki K, Gohda J, Akiyama T, Semba K, et al. TRAF6 establishes innate immune responses by activating NF-κB and IRF7 upon sensing cytosolic viral RNA and DNA. PLoS ONE. 2009;4:e5674. doi: 10.1371/journal.pone.0005674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kawai T, Sato S, Ishii KJ, Coban C, Hemmi H, Yamamoto M, et al. Interferon α induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat Immunol. 2004;5:1061–1068. doi: 10.1038/ni1118. [DOI] [PubMed] [Google Scholar]
- 48.Saha SK, Pietras EM, He JQ, Kang JR, Liu SY, Oganesyan G, et al. Regulation of antiviral responses by a direct and specific interaction between TRAF3 and Cardif. EMBO J. 2006;25:3257–3263. doi: 10.1038/sj.emboj.7601220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oganesyan G, Saha SK, Guo B, He JQ, Shahangian A, Zarnegar B, et al. Critical role of TRAF3 in the Toll-like receptor-dependent and -independent antiviral response. Nature. 2006;439:208–211. doi: 10.1038/nature04374. [DOI] [PubMed] [Google Scholar]
- 50.Hacker H, Redecke V, Blagoev B, Kratchmarova I, Hsu LC, Wang GG, et al. Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. Nature. 2006;439:204–207. doi: 10.1038/nature04369. [DOI] [PubMed] [Google Scholar]
- 51.Kawai T, Takahashi K, Sato S, Coban C, Kumar H, Kato H, et al. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol. 2005;6:981–988. doi: 10.1038/ni1243. [DOI] [PubMed] [Google Scholar]
- 52.Kumar H, Kawai T, Kato H, Sato S, Takahashi K, Coban C, et al. Essential role of IPS-1 in innate immune responses against RNA viruses. J Exp Med. 2006;203:1795–1803. doi: 10.1084/jem.20060792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu LG, Wang YY, Han KJ, Li LY, Zhai ZH, Shu HB. VISA is an adapter protein required for virus-triggered IFN-beta signaling. Mol Cell. 2005;19:727–740. doi: 10.1016/j.molcel.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 54.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jin L, Waterman PM, Jonscher KR, Short CM, Reisdorph NA, Cambier JC. MPYS, a novel membrane tetraspanner, is associated with major histocompatibility complex class II and mediates transduction of apoptotic signals. Mol Cell Biol. 2008;28:5014–5026. doi: 10.1128/MCB.00640-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhong B, Yang Y, Li S, Wang YY, Li Y, Diao F, et al. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity. 2008;29:538–550. doi: 10.1016/j.immuni.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 57.Sun W, Li Y, Chen L, Chen H, You F, Zhou X, et al. ERIS, an endoplasmic reticulum IFN stimulator, activates innate immune signaling through dimerization. Proc Natl Acad Sci USA. 2009;106:8653–8658. doi: 10.1073/pnas.0900850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Balachandran S, Thomas E, Barber GN. A FADD-dependent innate immune mechanism in mammalian cells. Nature. 2004;432:401–405. doi: 10.1038/nature03124. [DOI] [PubMed] [Google Scholar]
- 59.Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461:788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Richez C, Yasuda K, Watkins AA, Akira S, Lafyatis R, van Seventer JM, et al. TLR4 ligands induce IFN-α production by mouse conventional dendritic cells and human monocytes after IFN-β priming. J Immunol. 2009;182:820–828. doi: 10.4049/jimmunol.182.2.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sakaguchi S, Negishi H, Asagiri M, Nakajima C, Mizutani T, Takaoka A, et al. Essential role of IRF-3 in lipopolysaccharide-induced interferon-[beta] gene expression and endotoxin shock. Biochem Biophys Res Commun. 2003;306:860–866. doi: 10.1016/s0006-291x(03)01049-0. [DOI] [PubMed] [Google Scholar]
- 62.Hiscott J. Another detour on the Toll road to the interferon antiviral response. Nat Struct Mol Biol. 2004;11:1028–1030. doi: 10.1038/nsmb1104-1028. [DOI] [PubMed] [Google Scholar]
- 63.Fukao T, Koyasu S. PI3K and negative regulation of TLR signaling. Trends Immunol. 2003;24:358–363. doi: 10.1016/s1471-4906(03)00139-x. [DOI] [PubMed] [Google Scholar]
- 64.Schmitz F, Heit A, Dreher S, Eisenacher K, Mages J, Haas T, et al. Mammalian target of rapamycin (mTOR) orchestrates the defense program of innate immune cells. Eur J Immunol. 2008;38:2981–2992. doi: 10.1002/eji.200838761. [DOI] [PubMed] [Google Scholar]
- 65.Cao W, Manicassamy S, Tang H, Kasturi SP, Pirani A, Murthy N, et al. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI3K-mTOR-p70S6K pathway. Nat Immunol. 2008;9:1157–1164. doi: 10.1038/ni.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guiducci C, Ghirelli C, Marloie-Provost MA, Matray T, Coffman RL, Liu YJ, et al. PI3K is critical for the nuclear translocation of IRF7 and type I IFN production by human plasmacytoid predendritic cells in response to TLR activation. J Exp Med. 2008;205:315–322. doi: 10.1084/jem.20070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Di Domizio J, Blum A, Gallagher-Gambarelli M, Molens JP, Chaperot L, Plumas J. TLR7 stimulation in human plasma-cytoid dendritic cells leads to the induction of early IFN-inducible genes in the absence of type I IFN. Blood. 2009;114:1794–1802. doi: 10.1182/blood-2009-04-216770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang L, Pagano JS. Interferon regulatory factor 7 mediates activation of Tap-2 by Epstein-Barr virus latent membrane protein 1. J Virol. 2001;75:341–350. doi: 10.1128/JVI.75.1.341-350.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang L, Pagano JS. Interferon regulatory factor 7 is induced by Epstein-Barr virus latent membrane protein 1. J Virol. 2000;74:1061–1068. doi: 10.1128/jvi.74.3.1061-1068.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ning S, Campos AD, Darnay B, Bentz G, Pagano JS. TRAF6 and the three C-terminal lysine sites on IRF7 are required for its ubiquitination-mediated activation by the tumor necrosis factor receptor family member latent membrane protein 1. Mol Cell Biol. 2008;28:6536–6546. doi: 10.1128/MCB.00785-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huye LE, Ning S, Kelliher M, Pagano JS. IRF7 is activated by a viral oncoprotein through RIP-dependent ubiquitination. Mol Cell Biol. 2007;27:2910–2918. doi: 10.1128/MCB.02256-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dempsey PW, Doyle SE, He JQ, Cheng G. The signaling adaptors and pathways activated by TNF superfamily. Cytokine Growth Factor Rev. 2003;14:193–209. doi: 10.1016/s1359-6101(03)00021-2. [DOI] [PubMed] [Google Scholar]
- 73.Eliopoulos AG, Young LS. LMP1 structure and signal transduction. Semin Cancer Biol. 2001;11:435–444. doi: 10.1006/scbi.2001.0410. [DOI] [PubMed] [Google Scholar]
- 74.Hayden MS, Ghosh S. Signaling to NF-κB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 75.Li HP, Chang YS. Epstein-Barr virus latent membrane protein 1: structure and functions. J Biomed Sci. 2003;10:490–504. doi: 10.1007/BF02256110. [DOI] [PubMed] [Google Scholar]
- 76.Song YJ, Izumi KM, Shinners NP, Gewurz BE, Kieff E. IRF7 activation by Epstein-Barr virus latent membrane protein 1 requires localization at activation sites and TRAF6, but not TRAF2 or TRAF3. Proc Natl Acad Sci USA. 2008;105:18448–18453. doi: 10.1073/pnas.0809933105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Au W-C, Moore PA, LaFleur DW, Tombal B, Pitha PM. Characterization of the interferon regulatory factor-7 and its potential role in the transcription activation of interferon A genes. J Biol Chem. 1998;273:29210–29217. doi: 10.1074/jbc.273.44.29210. [DOI] [PubMed] [Google Scholar]
- 78.Marie I, Durbin JE, Levy DE. Differential viral induction of distinct interferon-α genes by positive feedback through interferon regulatory factor-7. EMBO J. 1998;17:6660–6669. doi: 10.1093/emboj/17.22.6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sato M, Hata N, Asagiri M, Nakaya T, Taniguchi T, Tanaka N. Positive feedback regulation of type I IFN genes by the IFN-inducible transcription factor IRF7. FEBS Lett. 1998;441:106–110. doi: 10.1016/s0014-5793(98)01514-2. [DOI] [PubMed] [Google Scholar]
- 80.Honda K, Takaoka A, Taniguchi T. Type I interferon gene induction by the interferon regulatory factor family of transcription factors. Immunity. 2006;25:349–360. doi: 10.1016/j.immuni.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 81.Zhang L, Wu L, Hong K, Pagano JS. Intracellular signaling molecules activated by Epstein-Barr virus for induction of interferon regulatory factor 7. J Virol. 2001;75:12393–12401. doi: 10.1128/JVI.75.24.12393-12401.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lu R, Au W-C, Yeow W-S, Hageman N, Pitha PM. Regulation of the promoter activity of interferon regulatory factor-7 gene-activation by interferon and silencing by hypermethylation. J Biol Chem. 2000;275:31805–31812. doi: 10.1074/jbc.M005288200. [DOI] [PubMed] [Google Scholar]
- 83.Xu D, Zhao L, Del Valle L, Miklossy J, Zhang L. Interferon regulatory factors 4 is involved in Epstein-Barr virus-mediated transformation of human B lymphocytes. J Virol. 2008;82:6251–6258. doi: 10.1128/JVI.00163-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang L, Pagano JS. Interferon regulatory factor 2 represses the Epstein-Barr virus BamH I Q latency promoter in type III latency. Mol Cell Biol. 1999;19:3216–3223. doi: 10.1128/mcb.19.4.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lu R, Moore PA, Pitha PM. Stimulation of IRF7 gene expression by tumor necrosis factor α: requirement for NF-κB transcription factor and gene accessibility. J Biol Chem. 2002;277:16592–16598. doi: 10.1074/jbc.M111440200. [DOI] [PubMed] [Google Scholar]
- 86.Ning S, Huye LE, Pagano JS. Regulation of the transcriptional activity of the IRF7 promoter by a pathway independent of interferon signaling. J Biol Chem. 2005;285:12262–12270. doi: 10.1074/jbc.M404260200. [DOI] [PubMed] [Google Scholar]
- 87.Colina R, Costa-Mattioli M, Dowling RJO, Jaramillo M, Tai LH, Breitbach CJ, et al. Translational control of the innate immune response through IRF7. Nature. 2008;452:323–328. doi: 10.1038/nature06730. [DOI] [PubMed] [Google Scholar]
- 88.Sato M, Suemori H, Hata N, Asagiri M, Ogasawara K, Nakao K, et al. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-α/β gene induction. Immunity. 2000;13:539–548. doi: 10.1016/s1074-7613(00)00053-4. [DOI] [PubMed] [Google Scholar]
- 89.Prakash A, Levy DE. Regulation of IRF7 through cell type-specific protein stability. Biochem Biophys Res Commun. 2006;342:50–56. doi: 10.1016/j.bbrc.2006.01.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Agalioti T, Lomvardas S, Parekh B, Yie J, Maniatis T, Thanos D. Ordered recruitment of chromatin modifying and general transcription factors to the IFN-β promoter. Cell. 2000;103:667–678. doi: 10.1016/s0092-8674(00)00169-0. [DOI] [PubMed] [Google Scholar]
- 91.Barnes BJ, Field AE, Pitha PM. Virus-induced heterodimer formation between IRF-5 and IRF-7 modulates assembly of the IFNA enhanceosome in vivo and transcriptional activity of IFNA genes. J Biol Chem. 2003;278:16630–16641. doi: 10.1074/jbc.M212609200. [DOI] [PubMed] [Google Scholar]
- 92.Ning S, Huye LE, Pagano JS. Interferon regulatory factor 5 represses expression of the Epstein-Barr virus oncoprotein LMP1: braking of the IRF7/LMP1 regulatory circuit. J Virol. 2005;79:11671–11676. doi: 10.1128/JVI.79.18.11671-11676.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Qing J, Liu C, Choy L, Wu RY, Pagano JS, Derynck R. Transforming growth factor β/Smad3 signaling regulates IRF7 function and transcriptional activation of the beta interferon promoter. Mol Cell Biol. 2004;24:1411–1425. doi: 10.1128/MCB.24.3.1411-1425.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Eroshkin A, Mushegian A. Conserved transactivation domain shared by interferon regulatory factors and Smad morphogens. J Mol Med. 1999;77:403–405. doi: 10.1007/s001090050369. [DOI] [PubMed] [Google Scholar]
- 95.Kim H, Seed B. The transcription factor MafB antagonizes antiviral responses by blocking recruitment of coactivators to the transcription factor IRF3. Nat Immunol. 2010;11:743–750. doi: 10.1038/ni.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Haller O, Kochs G, Weber F. The interferon response circuit: induction and suppression by pathogenic viruses. Virology. 2006;344:119–130. doi: 10.1016/j.virol.2005.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tsuchida T, Kawai T, Akira S. Inhibition of IRF3-dependent antiviral responses by cellular and viral proteins. Cell Res. 2009;19:3–4. doi: 10.1038/cr.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Buettner N, Vogt C, Martinez-Sobrido L, Weber F, Waibler Z, Kochs G. Thogoto virus ML protein is a potent inhibitor of the IRF7 transcription factor. J Gen Virol. 2010;91:220–227. doi: 10.1099/vir.0.015172-0. [DOI] [PubMed] [Google Scholar]
- 99.Jennings S, MartØnez-Sobrido L, GarcØa-Sastre A, Weber F, Kochs G. Thogoto virus ML protein suppresses IRF3 function. Virology. 2005;331:63–72. doi: 10.1016/j.virol.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 100.Barro M, Patton JT. Rotavirus NSP1 inhibits expression of type I interferon by antagonizing the function of interferon regulatory factors IRF3, IRF5, and IRF7. J Virol. 2007;81:4473–4481. doi: 10.1128/JVI.02498-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ronco LV, Karpova AY, Vidal M, Howley PM. Human papillomavirus 16 E6 oncoprotein binds to interferon regulatory factor-3 and inhibits its transcriptional activity. Genes Dev. 1998;12:2061–2072. doi: 10.1101/gad.12.13.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chang TH, Kubota T, Matsuoka M, Jones S, Bradfute SB, Bray M, et al. Ebola Zaire virus blocks type I interferon production by exploiting the host SUMO modification machinery. PLoS Pathog. 2009;5:e1000493. doi: 10.1371/journal.ppat.1000493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li K, Foy E, Ferreon JC, Nakamura M, Ferreon ACM, Ikeda M, et al. Immune evasion by hepatitis C virus NS3/4A protease-mediated cleavage of the Toll-like receptor 3 adaptor protein TRIF. Proc Natl Acad Sci USA. 2005;102:2992–2997. doi: 10.1073/pnas.0408824102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yu Y, Wang SE, Hayward GS. The KSHV immediate-early transcription factor RTA encodes ubiquitin E3 ligase activity that targets IRF7 for proteosome-mediated degradation. Immunity. 2005;22:59–70. doi: 10.1016/j.immuni.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 105.Zhu FX, King SM, Smith EJ, Levy DE, Yuan Y. A Kaposi's sarcoma-associated herpesviral protein inhibits virus-mediated induction of type I interferon by blocking IRF7 phosphorylation and nuclear accumulation. Proc Natl Acad Sci USA. 2002;99:5573–5578. doi: 10.1073/pnas.082420599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhu FX, Sathish N, Yuan Y. Antagonism of host antiviral responses by Kaposi's sarcoma-associated herpesvirus tegument protein ORF45. PLoS ONE. 2010;5:e10573. doi: 10.1371/journal.pone.0010573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wies E, Hahn AS, Schmidt K, Viebahn C, Rohland N, Lux A, et al. The Kaposi's sarcoma-associated herpesvirus encoded vIRF-3 inhibits cellular IRF-5. J Biol Chem. 2009;284:8525–8538. doi: 10.1074/jbc.M809252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liang C, Lee JS, Jung JU. Immune evasion in Kaposi's sarcoma-associated herpes virus associated oncogenesis. Semin Cancer Biol. 2008;18:423–436. doi: 10.1016/j.semcancer.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Joo CH, Shin YC, Gack M, Wu L, Levy D, Jung JU. Inhibition of IRF7-mediated interferon signal transduction by Kaposi's sarcoma-associated herpesvirus vIRF3. J Virol. 2007;81:8282–8292. doi: 10.1128/JVI.00235-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lubyova B, Pitha PM. Characterization of a novel human herpesvirus 8-encoded protein, vIRF3, that shows homology to viral and cellular interferon regulatory factors. J Virol. 2000;74:8194–8201. doi: 10.1128/jvi.74.17.8194-8201.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Karpova AY, Trost M, Murray JM, Cantley LC, Howley PM. Interferon regulatory factor-3 is an in vivo target of DNA-PK. Proc Natl Acad Sci USA. 2002;99:2818–2823. doi: 10.1073/pnas.052713899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lin R, Noyce RS, Collins SE, Everett RD, Mossman KL. The herpes simplex virus ICP0 RING finger domain inhibits IRF3- and IRF7-mediated activation of interferon-stimulated genes. J Virol. 2004;78:1675–1684. doi: 10.1128/JVI.78.4.1675-1684.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Eidson KM, Hobbs WE, Manning BJ, Carlson P, DeLuca NA. Expression of herpes simplex virus ICP0 inhibits the induction of interferon-stimulated genes by viral infection. J Virol. 2002;76:2180–2191. doi: 10.1128/jvi.76.5.2180-2191.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Daubeuf S, Singh D, Tan Y, Liu H, Federoff HJ, Bowers WJ, et al. HSV ICP0 recruits USP7 to modulate TLR-mediated innate response. Blood. 2009;113:3264–3275. doi: 10.1182/blood-2008-07-168203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hahn AM, Huye LE, Ning S, Webster-Cyriaque J, Pagano JS. Interferon regulatory factor 7 is negatively regulated by the Epstein-Barr virus immediate-early gene, BZLF1. J Virol. 2005;79:10040–10052. doi: 10.1128/JVI.79.15.10040-10052.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wu L, Fossum E, Joo CH, Lee K, Shin YC, Johannsen E, et al. Epstein-Barr virus LF2: an antagonist to type I interferon. J Virol. 2009;83:1140–1146. doi: 10.1128/JVI.00602-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bentz G, Liu R, Hahn A, Shackelford J, Pagano JS. Epstein-Barr virus immediate-early protein RTA negatively regulates interferon regulatory factors. Virology. 2010;402:121–128. doi: 10.1016/j.virol.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gershburg E, Pagano JS. Conserved herpesvirus protein kinases. Biochimica et Biophysica Acta (BBA)—Proteins Proteomics. 2008;1784:203–212. doi: 10.1016/j.bbapap.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang JT, Doong SL, Teng SC, Lee CP, Tsai CH, Chen MR. Epstein-Barr virus BGLF4 kinase suppresses the interferon regulatory factor 3 signaling pathway. J Virol. 2009;83:1856–1869. doi: 10.1128/JVI.01099-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ning S, Hahn AM, Huye LE, Joseph PS. Interferon regulatory factor 7 regulates expression of Epstein-Barr virus latent membrane protein 1: a regulatory circuit. J Virol. 2003;77:9359–9368. doi: 10.1128/JVI.77.17.9359-9368.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Martin HJ, Lee JM, Walls D, Hayward SD. Manipulation of the TLR7 signaling pathway by Epstein-Barr virus. J Virol. 2007;81:9748–9758. doi: 10.1128/JVI.01122-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cahir-McFarland ED, Carter K, Rosenwald A, Giltnane JM, Henrickson SE, Staudt LM, et al. Role of NF-κB in cell survival and transcription of latent membrane protein 1-expressing or Epstein-Barr virus latency III-infected cells. J Virol. 2004;78:4108–4119. doi: 10.1128/JVI.78.8.4108-4119.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yang L, Zhao T, Shi X, Nakhaei P, Wang Y, Sun Q, et al. Functional analysis of a dominant negative mutation of interferon regulatory factor 5. PLoS ONE. 2009;4:e5500. doi: 10.1371/journal.pone.0005500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dirmeier U, Hoffmann R, Kilger E, Schultheiss U, Briseno C, Gires O, et al. Latent membrane protein 1 of Epstein-Barr virus coordinately regulates proliferation with control of apoptosis. Oncogene. 2005;24:1711–1717. doi: 10.1038/sj.onc.1208367. [DOI] [PubMed] [Google Scholar]
- 125.Laherty CD, Hu HM, Opipari AW, Wang F, Dixit VM. The Epstein-Barr virus LMP1 gene product induces A20 zinc finger protein expression by activating nuclear factor kappa B. J Biol Chem. 1992;267:24157–24160. [PubMed] [Google Scholar]
- 126.Fries KL, Miller WE, Raab-Traub N. Epstein-Barr virus latent membrane protein 1 blocks p53-mediated apoptosis through the induction of the A20 gene. J Virol. 1996;70:8653–8659. doi: 10.1128/jvi.70.12.8653-8659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ning S, Pagano J. The A20 deubiquitinase activity negatively regulates LMP1 activation of IRF7. J Virol. 2010;84:6130–6138. doi: 10.1128/JVI.00364-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhao T, Yang L, Sun Q, Arguello M, Ballard DW, Hiscott J, et al. The NEMO adaptor bridges the nuclear factor-κB and interferon regulatory factor signaling pathways. Nat Immunol. 2007;8:592–600. doi: 10.1038/ni1465. [DOI] [PubMed] [Google Scholar]
- 129.Marie I, Smith E, Prakash A, Levy DE. Phosphorylation-induced dimerization of interferon regulatory factor 7 unmasks DNA binding and a bipartite transactivation domain. Mol Cell Biol. 2000;20:8803–8814. doi: 10.1128/mcb.20.23.8803-8814.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Au W-C, Yeow W-S, Pitha PM. Analysis of functional domains of interferon regulatory factor 7 and its association with IRF3. Virology. 2001;280:273–282. doi: 10.1006/viro.2000.0782. [DOI] [PubMed] [Google Scholar]
- 131.tenOever BR, Sharma S, Zou W, Sun Q, Grandvaux N, Julkunen I, et al. Activation of TBK1 and IKKε kinases by vesicular stomatitis virus infection and the role of viral ribonucleoprotein in the development of interferon antiviral immunity. J Virol. 2004;78:10636–10649. doi: 10.1128/JVI.78.19.10636-10649.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sharma S, tenOever BR, Grandvaux N, Zhou GP, Lin R, Hiscott J. Triggering the interferon antiviral response through an IKK-related pathway. Science. 2003;300:1148–1151. doi: 10.1126/science.1081315. [DOI] [PubMed] [Google Scholar]
- 133.Iwamura T, Yoneyama M, Yamaguchi K, Suhara W, Mori W, Shiota K, et al. Induction of IRF-3/-7 kinase and NF-kappaB in response to double-stranded RNA and virus infection: common and unique pathways. Genes Cells. 2001;6:375–388. doi: 10.1046/j.1365-2443.2001.00426.x. [DOI] [PubMed] [Google Scholar]
- 134.Hemmi H, Takeuchi O, Sato S, Yamamoto M, Kaisho T, Sanjo H, et al. The roles of two IκB kinase-related kinases in lipopolysaccharide and double stranded RNA signaling and viral infection. J Exp Med. 2004;199:1641–1650. doi: 10.1084/jem.20040520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fitzgerald KA, McWhirter SM, Faia KL, Rowe DC, Latz E, Golenbock DT, et al. IKKε and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol. 2003;4:491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- 136.Uematsu S, Sato S, Yamamoto M, Hirotani T, Kato H, Takeshita F, et al. Interleukin-1 receptor-associated kinase-1 plays an essential role for Toll-like receptor (TLR)7- and TLR9-mediated interferon-α induction. J Exp Med. 2005;201:915–923. doi: 10.1084/jem.20042372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hoshino K, Sugiyama T, Matsumoto M, Tanaka T, Saito M, Hemmi H, et al. IκB kinase-α is critical for interferon-α production induced by Toll-like receptors 7 and 9. Nature. 2006;440:949–953. doi: 10.1038/nature04641. [DOI] [PubMed] [Google Scholar]
- 138.An H, Hou J, Zhou J, Zhao W, Xu H, Zheng Y, et al. Phosphatase SHP-1 promotes TLR- and RIG-I-activated production of type I interferon by inhibiting the kinase IRAK1. Nat Immunol. 2008;9:542–550. doi: 10.1038/ni.1604. [DOI] [PubMed] [Google Scholar]
- 139.Wang RP, Zhang M, Li Y, Diao FC, Chen D, Zhai Z, et al. Differential regulation of IKKα-mediated activation of IRF3/7 by NIK. Mol Immunol. 2008;45:1926–1934. doi: 10.1016/j.molimm.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 140.Bhoj VG, Chen ZJ. Ubiquitylation in innate and adaptive immunity. Nature. 2009;458:430–437. doi: 10.1038/nature07959. [DOI] [PubMed] [Google Scholar]
- 141.Chen ZJ, Sun LJ. Nonproteolytic functions of ubiquitin in cell signaling. Mol Cell. 2009;33:275–286. doi: 10.1016/j.molcel.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 142.Mukhopadhyay D, Riezman H. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science. 2007;315:201–205. doi: 10.1126/science.1127085. [DOI] [PubMed] [Google Scholar]
- 143.Welchman RL, Gordon C, Mayer RJ. Ubiquitin and ubiquitin-like proteins as multifunctional signals. Nat Rev Mol Cell Biol. 2005;6:599–609. doi: 10.1038/nrm1700. [DOI] [PubMed] [Google Scholar]
- 144.Higgs R, Jefferies CA. Targeting IRFs by ubiquitination: regulating antiviral responses. Biochem Soc Trans. 2008;36:453–458. doi: 10.1042/BST0360453. [DOI] [PubMed] [Google Scholar]
- 145.Yu Y, Hayward GS. The ubiquitin E3 ligase RAUL negatively regulates type I interferon through ubiquitination of the transcription factors IRF7 and IRF3. Immunity. 2010;33:863–877. doi: 10.1016/j.immuni.2010.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhang M, Tian Y, Wang RP, Gao D, Zhang Y, Diao FC, et al. Negative feedback regulation of cellular antiviral signaling by RBCK1-mediated degradation of IRF3. Cell Res. 2008;18:1096–1104. doi: 10.1038/cr.2008.277. [DOI] [PubMed] [Google Scholar]
- 147.Higgs R, Gabhann JN, Larbi NB, Breen EP, Fitzgerald KA, Jefferies CA. The E3 ubiquitin ligase Ro52 negatively regulates IFN-β production post-pathogen recognition by polyubiquitin-mediated degradation of IRF3. J Immunol. 2008;181:1780–1786. doi: 10.4049/jimmunol.181.3.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Higgs R, Lazzari E, Wynne C, NÃ-Gabhann J, Espinosa A, Wahren-Herlenius M, et al. Self protection from anti-viral responses—Ro52 promotes degradation of the transcription factor IRF7 downstream of the viral toll-like receptors. PLoS ONE. 2010;5:e11776. doi: 10.1371/journal.pone.0011776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bailey CM, Abbott DE, Margaryan NV, Khalkhali-Ellis Z, Hendrix MJC. IRF6 promotes cell cycle arrest and is regulated by the proteasome in a cell cycle-dependent manner. Mol Cell Biol. 2008;28:2235–2243. doi: 10.1128/MCB.01866-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Xiong H, Li H, Kong HJ, Chen Y, Zhao J, Xiong S, et al. Ubiquitin-dependent degradation of interferon regulatory factor-8 mediated by Cbl down-regulates interleukin-12 expression. J Biol Chem. 2005;280:23531–23539. doi: 10.1074/jbc.M414296200. [DOI] [PubMed] [Google Scholar]
- 151.Nakagawa K, Yokosawa H. Degradation of transcription factor IRF1 by the ubiquitin-proteasome pathway. The C-terminal region governs the protein stability. Eur J Biochem. 2000;267:1680–1686. doi: 10.1046/j.1432-1327.2000.01163.x. [DOI] [PubMed] [Google Scholar]
- 152.Zheng D, Chen G, Guo B, Cheng G, Tang H. PLP2, a potent deubiquitinase from murine hepatitis virus, strongly inhibits cellular type I interferon production. Cell Res. 2008;18:1105–1113. doi: 10.1038/cr.2008.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zeng W, Xu M, Liu S, Sun L, Chen ZJ. Key role of Ubc5 and lysine-63 polyubiquitination in viral activation of IRF3. Mol Cell. 2009;36:315–325. doi: 10.1016/j.molcel.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Balkhi MY, Fitzgerald KA, Pitha PM. Functional regulation of MyD88-activated interferon regulatory factor 5 by K63-linked polyubiquitination. Mol Cell Biol. 2008;28:7296–7308. doi: 10.1128/MCB.00662-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pettersson S, Kelleher M, Pion E, Wallace M, Ball KL. Role of MDM2 acid domain interactions in recognition and ubiquitination of the transcription factor IRF2. Biochem J. 2009;418:575–585. doi: 10.1042/BJ20082087. [DOI] [PubMed] [Google Scholar]
- 156.Kong HJ, Anderson DE, Lee CH, Jang MK, Tamura T, Tailor P, et al. Cutting edge: autoantigen Ro52 is an interferon inducible E3 ligase that ubiquitinates IRF8 and enhances cytokine expression in macrophages. J Immunol. 2007;179:26–30. doi: 10.4049/jimmunol.179.1.26. [DOI] [PubMed] [Google Scholar]
- 157.Yang K, Shi HX, Liu XY, Shan YF, Wei B, Chen S, et al. TRIM21 is essential to sustain IFN regulatory factor 3 activation during antiviral response. J Immunol. 2009;182:3782–3792. doi: 10.4049/jimmunol.0803126. [DOI] [PubMed] [Google Scholar]
- 158.Meulmeester E, Melchior F. Cell biology: SUMO. Nature. 2008;452:709–711. doi: 10.1038/452709a. [DOI] [PubMed] [Google Scholar]
- 159.Bergink S, Jentsch S. Principles of ubiquitin and SUMO modifications in DNA repair. Nature. 2009;458:461–467. doi: 10.1038/nature07963. [DOI] [PubMed] [Google Scholar]
- 160.Kubota T, Matsuoka M, Chang TH, Tailor P, Sasaki T, Tashiro M, et al. Virus infection triggers SUMOylation of IRF3 and IRF7, leading to the negative regulation of type I interferon gene expression. J Biol Chem. 2008;283:25660–25670. doi: 10.1074/jbc.M804479200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhang T, Wang Y, Zhang L, Liu B, Xie J, Wood C, et al. Lysine residues of interferon regulatory factor 7 affect the replication and transcription activator-mediated lytic replication of Kaposi′s sarcoma-associated herpesvirus/human herpesvirus 8. J Gen Virol. 2011;92:181–187. doi: 10.1099/vir.0.021816-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nakagawa K, Yokosawa H. PIAS3 induces SUMO-1 modification and transcriptional repression of IRF1. FEBS Lett. 2002;530:204–208. doi: 10.1016/s0014-5793(02)03486-5. [DOI] [PubMed] [Google Scholar]
- 163.Park SM, Chae M, Kim BK, Seo T, Jang IS, Choi JS, et al. SUMOylated IRF-1 shows oncogenic potential by mimicking IRF-2. Biochem Biophys Res Commun. 2010;391:926–930. doi: 10.1016/j.bbrc.2009.11.166. [DOI] [PubMed] [Google Scholar]
- 164.Park J, Kim K, Lee EJ, Seo YJ, Lim SN, Park K, et al. Elevated level of SUMOylated IRF1 in tumor cells interferes with IRF1-mediated apoptosis. Proc Natl Acad Sci USA. 2007;104:17028–17033. doi: 10.1073/pnas.0609852104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Han KJ, Jiang L, Shu HB. Regulation of IRF2 transcriptional activity by its sumoylation. Biochem Biophys Res Commun. 2008;372:772–778. doi: 10.1016/j.bbrc.2008.05.103. [DOI] [PubMed] [Google Scholar]
- 166.Caillaud A, Prakash A, Smith E, Masumi A, Hovanessian AG, Levy DE, et al. Acetylation of interferon regulatory factor-7 by p300/CREB-binding protein (CBP)-associated factor (PCAF) impairs its DNA binding. J Biol Chem. 2002;277:49417–49421. doi: 10.1074/jbc.M207484200. [DOI] [PubMed] [Google Scholar]
- 167.Masumi A, Yamakawa Y, Fukazawa H, Ozato K, Komuro K. Interferon regulatory factor-2 regulates cell growth through its acetylation. J Biol Chem. 2003;278:25401–25407. doi: 10.1074/jbc.M213037200. [DOI] [PubMed] [Google Scholar]
- 168.Masumi A, Ozato K. Coactivator p300 acetylates the interferon regulatory factor-2 in U937 cells following phorbol ester treatment. J Biol Chem. 2001;276:20973–20980. doi: 10.1074/jbc.M101707200. [DOI] [PubMed] [Google Scholar]
- 169.Tang X, Gao JS, Guan Yj, McLane KE, Yuan ZL, Ramratnam B, et al. Acetylation-dependent signal transduction for type I interferon receptor. Cell. 2007;131:93–105. doi: 10.1016/j.cell.2007.07.034. [DOI] [PubMed] [Google Scholar]
- 170.Suhara W, Yoneyama M, Iwamura T, Yoshimura S, Tamura K, Namiki H, et al. Analyses of virus-induced homomeric and heteromeric protein associations between IRF3 and coactivator CBP/p300. J Biochem (Tokyo) 2000;128:301–307. doi: 10.1093/oxfordjournals.jbchem.a022753. [DOI] [PubMed] [Google Scholar]
- 171.Li Q, Tang L, Roberts PC, Kraniak JM, Fridman AL, Kulaeva OI, et al. Interferon regulatory factors IRF5 and IRF7 inhibit growth and induce senescence in immortal li-fraumeni fibroblasts. Mol Cancer Res. 2008;6:770–784. doi: 10.1158/1541-7786.MCR-07-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Fukasawa M, Kimura M, Morita S, Matsubara K, Yamanaka S, Endo C, et al. Microarray analysis of promoter methylation in lung cancers. J Hum Genet. 2006;51:368–374. doi: 10.1007/s10038-005-0355-4. [DOI] [PubMed] [Google Scholar]
- 173.Ozato K, Tailor P, Kubota T. The interferon regulatory factor family in host defense: mechanism of action. J Biol Chem. 2007;282:20065–20069. doi: 10.1074/jbc.R700003200. [DOI] [PubMed] [Google Scholar]
- 174.Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, et al. IRF7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434:772–777. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- 175.Bonjardim CA, Ferreira PCP, Kroon EG. Interferons: signaling, antiviral and viral evasion. Immunol Lett. 2009;122:1–11. doi: 10.1016/j.imlet.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Borden EC, Sen GC, Uze G, Silverman RH, Ransohoff RM, Foster GR, et al. Interferons at age 50: past, current and future impact on biomedicine. Nat Rev Drug Discov. 2007;6:975–990. doi: 10.1038/nrd2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol. 2006;6:823–835. doi: 10.1038/nri1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Randall RE, Goodbourn S. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J Gen Virol. 2008;89:1–47. doi: 10.1099/vir.0.83391-0. [DOI] [PubMed] [Google Scholar]
- 179.Stetson DB, Medzhitov R. Type I interferons in host defense. Immunity. 2006;25:373–381. doi: 10.1016/j.immuni.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 180.Sadler AJ, Williams BRG. Interferon-inducible antiviral effectors. Nat Rev Immunol. 2008;8:559–568. doi: 10.1038/nri2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Tailor P, Tamura T, Kong HJ, Kubota T, Kubota M, Borghi P, et al. The feedback phase of type I interferon induction in dendritic cells requires Interferon Regulatory Factor 8. Immunity. 2007;27:228–239. doi: 10.1016/j.immuni.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tamura T, Yanai H, Savitsky D, Taniguchi T. The IRF family transcription factors in immunity and oncogenesis. Annu Rev Immunol. 2008;26:535–584. doi: 10.1146/annurev.immunol.26.021607.090400. [DOI] [PubMed] [Google Scholar]
- 183.Genin P, Lin R, Hiscott J, Civas A. Differential regulation of human interferon-A genes expression by Interferon Regulatory Factor 3 and 7. Mol Cell Biol. 2009;29:3435–3450. doi: 10.1128/MCB.01805-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Meier A, Alter G, Frahm N, Sidhu H, Li B, Bagchi A, et al. MyD88-dependent immune activation mediated by human immunodeficiency virus type 1-encoded Toll-like receptor ligands. J Virol. 2007;81:8180–8191. doi: 10.1128/JVI.00421-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Takahashi K, Asabe S, Wieland S, Garaigorta U, Gastaminza P, Isogawa M, et al. Plasmacytoid dendritic cells sense hepatitis C virus-infected cells, produce interferon, and inhibit infection. Proc Natl Acad Sci USA. 2011;107:7431–7436. doi: 10.1073/pnas.1002301107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kawai T, Akira S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int Immunol. 2009;21:317–337. doi: 10.1093/intimm/dxp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Tanaka N, Taniguchi T. The interferon regulatory factors and oncogenesis. Semin Cancer Biol. 2000;10:73–81. doi: 10.1006/scbi.2000.0310. [DOI] [PubMed] [Google Scholar]
- 188.Liebermann DA, Hoffman B. Good and bad IRF-1: role in tumor suppression versus autoimmune disease. Leukemia Res. 2009;33:1301–1302. doi: 10.1016/j.leukres.2009.03.038. [DOI] [PubMed] [Google Scholar]
- 189.Shaffer AL, Emre NCT, Lamy L, Ngo VN, Wright G, Xiao W, et al. IRF4 addiction in multiple myeloma. Nature. 2008;454:226–231. doi: 10.1038/nature07064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Shaffer AL, Emre NC, Romesser PB, Staudt LM. IRF4: immunity. Malignancy! Therapy? Clin Cancer Res. 2009;15:2954–2961. doi: 10.1158/1078-0432.CCR-08-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Heylbroeck C, Balachandran S, Servant MJ, Deluca C, Barber GN, Lin R, et al. The IRF3 transcription factor mediates sendai virus-induced apoptosis. J Virol. 2000;74:3781–3792. doi: 10.1128/jvi.74.8.3781-3792.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Romieu-Mourez R, Solis M, Nardin A, Goubau D, Baron-Bodo V, Lin R, et al. Distinct roles for IFN regulatory factor (IRF)-3 and IRF7 in the activation of antitumor properties of human macrophages. Cancer Res. 2006;66:10576–10585. doi: 10.1158/0008-5472.CAN-06-1279. [DOI] [PubMed] [Google Scholar]
- 193.Holm GH, Zurney J, Tumilasci V, Leveille S, Danthi P, Hiscott J, et al. Retinoic acid-inducible gene-I and interferon-beta promoter stimulator-1 augment proapoptotic responses following mammalian reovirus infection via interferon regulatory factor-3. J Biol Chem. 2007;282:21953–21961. doi: 10.1074/jbc.M702112200. [DOI] [PubMed] [Google Scholar]
- 194.Chattopadhyay S, Marques JT, Yamashita M, Peters KL, Smith K, Desai A, et al. Viral apoptosis is induced by IRF-3-mediated activation of Bax. EMBO J. 2010;29:1762–1773. doi: 10.1038/emboj.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Tokunaga T, Naruke Y, Shigematsu S, Kohno T, Yasui K, Ma Y, et al. Aberrant expression of interferon regulatory factor 3 in human lung cancer. Biochem Biophys Res Commun. 2010;397:202–207. doi: 10.1016/j.bbrc.2010.05.085. [DOI] [PubMed] [Google Scholar]
- 196.Couzinet A, Tamura K, Chen Hm, Nishimura K, Wang Z, Morishita Y, et al. A cell-type-specific requirement for IFN regulatory factor 5 (IRF5) in Fas-induced apoptosis. Proc Natl Acad Sci USA. 2008;105:2556–2561. doi: 10.1073/pnas.0712295105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yanai H, Chen Hm, Inuzuka T, Kondo S, Mak TW, Takaoka A, et al. Role of IFN regulatory factor 5 transcription factor in antiviral immunity and tumor suppression. Proc Natl Acad Sci USA. 2007;104:3402–3407. doi: 10.1073/pnas.0611559104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hu G, Barnes BJ. IRF5 is a mediator of the death receptor-induced apoptotic signaling pathway. J Biol Chem. 2009;284:2767–2777. doi: 10.1074/jbc.M804744200. [DOI] [PubMed] [Google Scholar]
- 199.Mori T, Anazawa Y, Iiizumi M, Fukuda S, Nakamura Y, Arakawa H. Identification of the interferon regulatory factor 5 gene (IRF5) as a direct target for p53. Oncogene. 2002;21:2914–2918. doi: 10.1038/sj.onc.1205459. [DOI] [PubMed] [Google Scholar]
- 200.Hrdlickova R, Nehyba J, Bose HR., Jr Regulation of telomerase activity by Interferon regulatory factors 4 and 8 in immune cells. Mol Cell Biol. 2009;29:929–941. doi: 10.1128/MCB.00961-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Burchert A, Cai D, Hofbauer L, Samuelsson MKR, Slater EP, Duyster J, et al. Interferon consensus sequence binding protein (ICSBP, IRF8) antagonizes BCR/ABL and down-regulates bcl-2. Blood. 2003;103:3480–3489. doi: 10.1182/blood-2003-08-2970. [DOI] [PubMed] [Google Scholar]
- 202.Taniguchi T, Ogasawara K, Takaoka A, Tanaka N. IRF family of transcription factors as regulators of host defense. Annu Rev Immunol. 2001;19:623–655. doi: 10.1146/annurev.immunol.19.1.623. [DOI] [PubMed] [Google Scholar]
- 203.Pagano JS. Viruses and lymphomas. N Engl J Med. 2002;347:78–79. doi: 10.1056/NEJMp020056. [DOI] [PubMed] [Google Scholar]
- 204.Zhang L, Zhang J, Lambert Q, Der CJ, Del Valle L, Miklossy J, et al. Interferon regulatory factor 7 is associated with Epstein-Barr virus-transformed central nervous system lymphoma and has oncogenic properties. J Virol. 2004;78:12987–12995. doi: 10.1128/JVI.78.23.12987-12995.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Alizadeh AA, Bohen SP, Lossos C, Martinez-Climent JA, Ramos JC, Cubedo-Gil E, et al. Expression profiles of adult T-cell leukemia-lymphoma and associations with clinical responses to zidovudine and interferon α. Leukemia Lymphoma. 2010;51:1200–1216. doi: 10.3109/10428191003728628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Barnes BJ, Kellum MJ, Pinder KE, Frisancho JA, Pitha PM. Interferon regulatory factor 5, a novel mediator of cell cycle arrest and cell death. Cancer Res. 2003;63:6424–6431. [PubMed] [Google Scholar]
- 207.Xiao C, Rajewsky K. MicroRNA control in the immune system: basic principles. Cell. 2009;136:26–36. doi: 10.1016/j.cell.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 208.O'Connell RM, Rao DS, Chaudhuri AA, Baltimore D. Physiological and pathological roles for microRNAs in the immune system. Nat Rev Immunol. 2010;10:111–122. doi: 10.1038/nri2708. [DOI] [PubMed] [Google Scholar]
- 209.Eis PS, Tam W, Sun L, Chadburn A, Li Z, Gomez MF, et al. Accumulation of miR-155 and BIC RNA in human B cell lymphomas. Proc Natl Acad Sci USA. 2005;102:3627–3632. doi: 10.1073/pnas.0500613102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Garzon R, Croce CM. MicroRNAs in normal and malignant hematopoiesis. Curr Opin Hematol. 2008;15:352–358. doi: 10.1097/MOH.0b013e328303e15d. [DOI] [PubMed] [Google Scholar]
- 211.Cho WC. OncomiRs: the discovery and progress of microRNAs in cancers. Mol Cancer. 2007;6:60. doi: 10.1186/1476-4598-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Gatto G, Rossi A, Rossi D, Kroening S, Bonatti S, Mallardo M. Epstein-Barr virus latent membrane protein 1 transactivates miR-155 transcription through the NF-κB pathway. Nucleic Acids Res. 2008;36:6608–6619. doi: 10.1093/nar/gkn666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Yin Q, McBride J, Fewell C, Lacey M, Wang X, Lin Z, et al. MicroRNA-155 is an Epstein-Barr virus-induced gene that modulates Epstein-Barr virus-regulated gene expression pathways. J Virol. 2008;82:5295–5306. doi: 10.1128/JVI.02380-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Rahadiani N, Takakuwa T, Tresnasari K, Morii E, Aozasa K. Latent membrane protein-1 of Epstein-Barr virus induces the expression of B-cell integration cluster, a precursor form of microRNA-155, in B lymphoma cell lines. Biochem Biophys Res Commun. 2008;377:579–583. doi: 10.1016/j.bbrc.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 215.Huang Y, Walstrom A, Zhang L, Zhao Y, Cui M, Ye L, et al. Type I interferons and interferon regulatory factors regulate TNF-related apoptosis-inducing ligand (TRAIL) in HIV-1-infected macrophages. PLoS ONE. 2009;4:e5397. doi: 10.1371/journal.pone.0005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Andrews HN, Mullan PB, McWilliams S, Sebelova S, Quinn JE, Gilmore PM, et al. BRCA1 regulates the interferon gamma-mediated apoptotic response. J Biol Chem. 2002;277:26225–26232. doi: 10.1074/jbc.M201316200. [DOI] [PubMed] [Google Scholar]
- 217.Kim TK, Kim T, Kim TY, Lee WG, Yim J. Chemotherapeutic DNA-damaging drugs activate interferon regulatory factor-7 by the mitogen-activated protein kinase kinase-4-c-Jun NH2-terminal kinase pathway. Cancer Res. 2000;60:1153–1156. [PubMed] [Google Scholar]
- 218.Nonkwelo C, Ruf IK, Sample J. Interferon-independent and -induced regulation of Epstein-Barr virus EBNA-1 gene transcription in Burkitt lymphoma. J Virol. 1997;71:6887–6897. doi: 10.1128/jvi.71.9.6887-6897.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Schaefer BC, Paulson E, Strominger JL, Speck SH. Constitutive activation of Epstein-Barr virus (EBV) nuclear antigen 1 gene transcription by IRF1 and IRF2 during restricted EBV latency. Mol Cell Biol. 1997;17:873–886. doi: 10.1128/mcb.17.2.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Negishi H, Ohba Y, Yanai H, Takaoka A, Honma K, Yui K, et al. Negative regulation of Toll-like-receptor signaling by IRF4. Proc Natl Acad Sci USA. 2005;102:15989–15994. doi: 10.1073/pnas.0508327102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Chen H, Huang J, Wu FY, Liao G, Hutt-Fletcher L, Hayward SD. Regulation of expression of the Epstein-Barr virus BamHI-A rightward transcripts. J Virol. 2005;79:1724–1733. doi: 10.1128/JVI.79.3.1724-1733.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Lo AKF, To KF, Lo KW, Lung RWM, Hui JWY, Liao G, et al. Modulation of LMP1 protein expression by EBV-encoded microRNAs. Proc Natl Acad Sci USA. 2007;104:16164–16169. doi: 10.1073/pnas.0702896104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Xu D, Brumm K, Zhang L. The latent membrane protein 1 of Epstein-Barr virus (EBV) primes EBV latency cells for type I interferon production. J Biol Chem. 2006;281:9163–9169. doi: 10.1074/jbc.M511884200. [DOI] [PubMed] [Google Scholar]
- 224.Zhang J, Das SC, Kotalik C, Pattnaik AK, Zhang L. The latent membrane protein 1 of Epstein-Barr virus establishes an antiviral state via induction of interferon-stimulated genes. J Biol Chem. 2004;279:46335–46342. doi: 10.1074/jbc.M403966200. [DOI] [PubMed] [Google Scholar]
- 225.Kim WU, Sreih A, Bucala R. Toll-like receptors in systemic lupus erythematosus: prospects for therapeutic intervention. Autoimmun Rev. 2009;8:204–208. doi: 10.1016/j.autrev.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Baccala R, Hoebe K, Kono DH, Beutler B, Theofilopoulos AN. TLR-dependent and TLR-independent pathways of type I interferon induction in systemic autoimmunity. Nat Med. 2007;13:543–551. doi: 10.1038/nm1590. [DOI] [PubMed] [Google Scholar]
- 227.Graham RR, Kozyrev SV, Baechler EC, Reddy MVP, Plenge RM, Bauer JW, et al. Collaborative Groupsthe Argentine and Spanish. A common haplotype of interferon regulatory factor 5 (IRF5) regulates splicing and expression and is associated with increased risk of systemic lupus erythematosus. Nat Genet. 2006;38:550–555. doi: 10.1038/ng1782. [DOI] [PubMed] [Google Scholar]
- 228.Pinheiro RF, Metze K, Silva MRR, de Lourdes Lopes Ferrari Chauffaille M. The ambiguous role of interferon regulatory factor-1 (IRF-1) immunoexpression in myelodysplastic syndrome. Leukemia Res. 2009;33:1308–1312. doi: 10.1016/j.leukres.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 229.Honda K, Yanai H, Takaoka A, Taniguchi T. Regulation of the type I IFN induction: a current view. Int Immunol. 2005;17:1367–1378. doi: 10.1093/intimm/dxh318. [DOI] [PubMed] [Google Scholar]
- 230.Okabe Y, Kawane K, Nagata S. IFN regulatory factor (IRF) 3/7-dependent and -independent gene induction by mammalian DNA that escapes degradation. Eur J Immunol. 2008;38:3150–3158. doi: 10.1002/eji.200838559. [DOI] [PubMed] [Google Scholar]
- 231.Harley JB, Alarcon-Riquelme ME, Criswell LA, Jacob CO, Kimberly RP, Moser KL, et al. Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nat Genet. 2008;40:204–210. doi: 10.1038/ng.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Salloum R, Franek BS, Kariuki SN, Rhee L, Mikolaitis RA, Jolly M, et al. Genetic variation at the IRF7/PHRF1 locus is associated with autoantibody profile and serum interferon- alpha activity in lupus patients. Arthritis Rheum. 2010;62:553–561. doi: 10.1002/art.27182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Fu Q, Zhao J, Qian X, Wong JL, Kaufman KM, Yu CY, et al. Hwee Siew Howe the TTSH Lupus Study Group. Association of a functional IRF7 variant with systemic lupus erythematosus. Arthritis Rheumatism. 2011;63:749–754. doi: 10.1002/art.30193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Heinig M, Petretto E, Wallace C, Bottolo L, Rotival M, Lu H, et al. A trans-acting locus regulates an anti-viral expression network and type 1 diabetes risk. Nature. 2010;467:460–464. doi: 10.1038/nature09386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Battistini A. Interferon regulatory factors in hematopoietic cell differentiation and immune regulation. J Interferon Cytokine Res. 2009;29:765–780. doi: 10.1089/jir.2009.0030. [DOI] [PubMed] [Google Scholar]
- 236.Lien C, Fang CM, Huso D, Livak F, Lu R, Pitha PM. Critical role of IRF-5 in regulation of B-cell differentiation. Proc Natl Acad Sci USA. 2010;107:4664–4668. doi: 10.1073/pnas.0911193107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Lu R, Pitha PM. Monocyte differentiation to macrophage requires interferon regulatory factor 7. J Biol Chem. 2001;276:45491–45496. doi: 10.1074/jbc.C100421200. [DOI] [PubMed] [Google Scholar]
- 238.Pietila TE, Veckman V, Lehtonen A, Lin R, Hiscott J, Julkunen I. Multiple NF-kappaB and IFN regulatory factor family transcription factors regulate CCL19 gene expression in human monocyte-derived dendritic cells. J Immunol. 2007;178:253–261. doi: 10.4049/jimmunol.178.1.253. [DOI] [PubMed] [Google Scholar]
- 239.Lin R, Genin P, Mamane Y, Hiscott J. Selective DNA binding and association with the CREB binding protein coactivator contribute to differential activation of α/β interferon genes by interferon regulatory factors 3 and 7. Mol Cell Biol. 2000;20:6342–6353. doi: 10.1128/mcb.20.17.6342-6353.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Sgarbanti M, Marsili G, Remoli AL, Orsatti R, Battistini A. IRF7: new role in the regulation of genes involved in adaptive immunity. Ann N Y Acad Sci. 2007;1095:325–333. doi: 10.1196/annals.1397.036. [DOI] [PubMed] [Google Scholar]
- 241.Barnes BJ, Richards J, Mancl ME, Hanash S, Beretta L, Pitha PM. Global and specific targets of IRF5 and IRF7 during innate response to viral infection. J Biol Chem. 2004;279:45194–45207. doi: 10.1074/jbc.M400726200. [DOI] [PubMed] [Google Scholar]
- 242.Zhao Y, Xu D, Jiang Y, Zhang L. Dual functions of interferon regulatory factors 7C in Epstein-Barr virus-mediated transformation of human B lymphocytes. PLoS ONE. 2010;5:e9459. doi: 10.1371/journal.pone.0009459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Green DR, Kroemer G. Cytoplasmic functions of the tumour suppressor p53. Nature. 2009;458:1127–1130. doi: 10.1038/nature07986. [DOI] [PMC free article] [PubMed] [Google Scholar]