Abstract
Small direct current (DC) electric fields direct some important angiogenic responses of vascular endothelial cells. Those responses indicate promising use of electric fields to modulate angiogenesis. We sought to determine the regulation of electric fields on transcription and expression of a serial of import angiogenic factors by endothelial cells themselves.
Using semi-quantitative PCR and ELISA we found that electric stimulation upregulates the levels of mRNAs and proteins of a number of angiogenic proteins, most importantly VEGF165, VEGF121 and IL-8 in human endothelial cells. The up-regulation of mRNA levels might be specific, as the mRNA encoding bFGF, TGF-beta and eNOS are not affected by DC electric stimulation at 24 h time-point. Inhibition of VEGF receptor (VEGFR1 or VEGFR2) signaling significantly decreased VEGF production and completely abolished IL-8 production.
DC electric stimulation selectively regulates production of some growth factors and cytokines important for angiogenesis through a feed-back loop mediated by VEGF receptors.
Keywords: Endothelial cells, Electrical stimulation, Angiogenesis, Gene expression
1. Introduction
Angiogenesis, the formation of new blood vessels by branching of existing capillaries, is important in many important physiological and pathological processes such as wound healing and tumor growth [1]. Endothelial cells are responsive to a large array of signals that include growth factors, cytokines, and hormones from both local and distant sources. A balance between pro-angiogenic and anti-angiogenic growth factors and cytokines tightly controls angiogenesis.
Electrical stimulation is able to enhance angiogenesis and may offer a promising new strategy to modulate angiogenesis [2–4]. Significant angiogenesis is induced by electrical stimulation in ischemic and non-ischemic rat limbs, and is mediated by increased expression of VEGF in muscle cells [2,5]. Electric stimulation therefore has an indirect effect on endothelial cells via inducing VEGF production from muscle cells. Even sub-threshold stimulation, which does not induce muscle contraction, induces VEGF production in muscle cells. More recently, DC electric stimulation was demonstrated to directly affect endothelial cells [6–8].
In addition to exogenously applied electric fields, endogenous electric fields (EFs) can be generated by active ion transport across polarized epithelia [9–15]. Endogenous EFs also are found in and around the vasculature. For example, ζ-potentials arise from blood flow in large blood vessels and are 100–400 mV at the blood endothelial cell interface [16]. Injured and ischemic tissues also are electrically polarized, and this can produce a DC EF of ~5.8 mV mm−1 across an 8-mm zone at the boundary with undamaged tissue [17].
Electric fields, as a new type of signal, have an overriding guidance effect in directing the migration of epithelial cells during wound healing [18]. In some situations where active angiogenesis happens in the presence of endogenous electric fields, as in wounds, the electric fields may contribute to angiogenesis regulation. We recently demonstrated that a small physiological EF as low as 75–100 mV mm−1 stimulated cell elongation, reoriented and directed alignment of single cells and cell monolayers, and directed cell migration. Since we use pure endothelial cultures, these effects were directly on the endothelial cells and did not need other cell types. These responses are caused by enhanced release of VEGF from endothelial cells and activation of VEGF receptors (VEGFRs), phosphatidylinositol-3-kinase (PI3K)-Akt and Rho-ROCK elements of the VEGFR signaling pathway [7,8].
We tested the hypothesis that electric fields may regulate release and transcript expression of angiogenic factors, thus inducing the pro-angiogenic response. We report here that EFs upregulate VEGF mRNA and protein expression. EFs also stimulate interleukin 8 (IL-8), another potent angiogenic factor, and IL-8 mRNA expression, in endothelial cells. HUVEC cultured in 200 mv mm−1 DC EF (relevant to the endogenous DC field of the wound) showed a fivefold elevation in interleukin-8 (IL-8) secretion at 24 h over control cells cultured in a similar condition. EFs dramatically up-regulate the mRNA expression of IL-8 at 4–24 h, and increase protein production beginning at 4 h, whereas elevated VEGF protein begins as early as 30 min after EF exposure. These results suggest a direct regulation of EFs on endothelial cells through regulation of angiogenic factors.
2. Materials and methods
2.1. Cell cultures and reagents
The HUVEC cell line from ATCC was used before passage 10. Tissue culture reagents were from Life Technologies UK. The IL-8 enzyme-linked immunosorbent assay (ELISA) kit and the VEGF (VEGF165) ELISA kit were purchased from R&D Systems (Minneapolis). Matrigel was from BD Sciences (Becton, Dickinson and Company). Acetylated low density lipoprotein (AcLDL) was from Biomedical Technologies, Inc. (Stoughton, MA). The VEGFR inhibitor (for VEGFR1 and VEGFR2, catalog number 676475, 4-[(4′-chloro-2′-fluoro)phenylamino]-6,7-dimethoxyquinazoline) and SU1498 (for VEGFR2 or KDR, catalog number 572888) were from Calbiochem. Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum (FBS) was used for culture cells and EF exposure experiments.
2.2. Electric field stimulation
The experimental setup and EF exposure protocols were reported previously [19].
2.3. Detection of IL-8 and VEGF by enzyme-linked immunosorbent assay (ELISA)
VEGF and IL-8 release was quantified with ELISA as directed by the manufacturer. Briefly, HUVECs were grown in DMEM-complete medium in a culture dish chamber. The medium was replaced by serum-free DMEM medium and the cells were starved for 4 h. Synthetic inhibitors were added to the cells for 1 h before the assay was initiated. After incubation for the indicated times with EF in medium, the culture supernatant was removed and evaluated for IL-8 and VEGF content using the corresponding ELISA kit.
2.4. Total RNA extraction and RT-PCR analysis
Total RNA was extracted using the Rneasy Mini Kit (QIAGEN, West Sussex, UK), according to the manufacturer’s instructions. Total RNA integrity and purity was verified on a 1% agarose gel, and quantities were evaluated photometrically at 260 nm. One microgram of total RNA was reverse transcribed (RT-PCR amplifier kit, BD CLONTECH, Hampshire, UK) using oligo dT (12–18 mer; Invitrogen) and 4 U reverse transcriptase (Omniscript Reverse Transcriptase; QIAGEN, West Sussex, UK), following manufacturer’s instructions. The cDNA samples were then stored at −80 °C until required for amplification with polymerase chain reaction (PCR). Sequence-specific primers for IL-8, VEGF, bFGF, TGF-beta, eNOS and β-actin were synthesized and purified commercially (Invitrogen) or purchased from BD Biosciences.
PCR assay from cDNA was performed in a final volume of 50 μl containing 2 μl of cDNA, 1 μl of each primer, 0.2 mM of each deoxyribonucleotide triphosphate, 10 mM Tris–HCl (pH 9.0), 50 mM KCl, 0.1% Triton X-100, 1.5 mM MgCl2 and 1.2 U high fidelity Pfu DNA polymerase (Promega, UK), and 3′ and 5′ primer pairs of the IL-8, VEGF, bFGF, TGF-beta and eNOS (20 μmol/l). A housekeeping gene (β-actin) was simultaneously amplified for each cDNA examined. The number of cycles was previously determined by different assays, the optimal semi-quantitative conditions falling in the linear range of the reaction, when the PCR signal intensity was not saturated. Amplifications were carried out using a thermocycler (Cyclogene; Techne, Cambridge, UK) with the following PCR profile: 1 min denaturation at 96 °C, 1 min annealing at 60 °C, followed by 1 min extension at 72 °C. Cycling was started by 5 min denaturation at 96 °C and terminated by a final extension step 7 min at 72 °C. The primers used, recognizing gene sequence of IL-8, VEGF, bFGF, TGF-beta, eNOS, and β-actin are as follows
IL-8: upstream 5′-cttcctgatttctgcagctct-3′ and downstream 5′aaacttctccacaaccctctgc-3′;
VEGF: uptream-5′-gaagtggtgaagttcatggatgtc-3′ and downstream-5′-cgatcgttctgtatcagtctttcc-3′;
bFGF: upstream-5′-gccacttcaaggaccccaag, and downstream 5′-tcagctcttagcagacattgg;
TGF-beta: upstream-5′-ccggcctttcctgcttctca, and downstream 5′-cccgggttatgctggttgta;
eNOS: upstream-5′-ccagctagccaaagtcaccat, and downstream 5′-gtctcggagccatacaggatt;
β-Actin: uptream-5′-atctggcaccacaccttctacaatgagctgcg-3′ and downstream-5′-cgtcatactcctgcttgctgatccacatctgc-3′.
For all PCR-analyses a no template negative control was included. Precautions suggested by Kwok and Higuchi [20] were strictly followed. The amplified products were resolved in a 2% agarose gel containing ethidium bromide and visualized under UV light. The specificity of the amplification was confirmed by size of the PCR products. The image of the gel was captured using Syngene (Cambridge, UK), and the intensities of the corresponding PCR products were determined using Syngene Genesnap System (Cambridge, UK). The PCR product was measured as follows: IL-8, 240 bp; VEGF165 and 121, 540 bp and 410 bp; bFGF, 398 bp; TGF-beta, 285 bp; eNOS, 354 bp; and β-actin, 838 bp.
2.5. Statistical analysis
Statistical analyses were made using unpaired, two-tailed Student’s t-test. Data are expressed as mean ± SD, unless indicated otherwise.
3. Results
3.1. Characterization of the endothelial cells
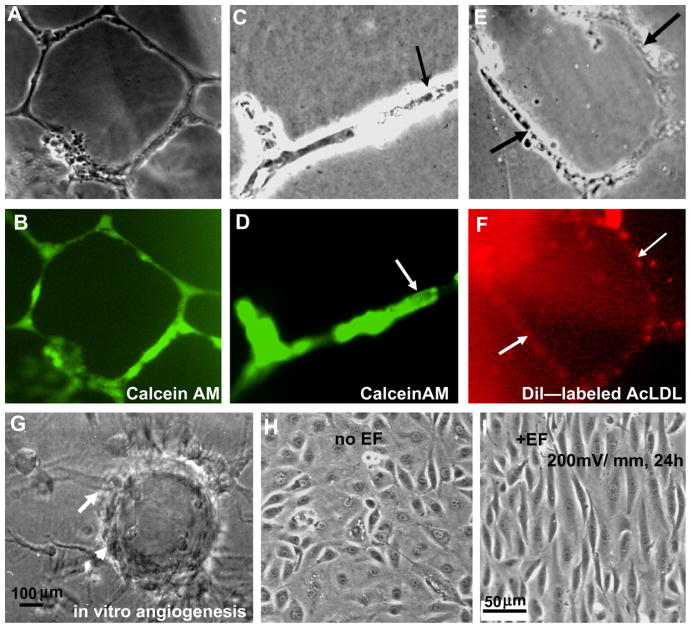
The HUVECs formed tubular-like networks on Matrigel (Fig. 1A and B), had the ability to form the lumen (Fig. 1C and D), and uptake AcLDL (Fig. 1E and F). The cells are positive for an endothelium-specific marker, von Willebrand factor (data not shown). Three dimensional cultures of the cells on microcarriers show evident vessel like structure formation (Fig. 1G). These results indicate that the cell lines we used maintain properties of endothelial cells and have the ability to form vessel-like structures in 3D culture. Cells were used between passages 4–10 for all the experiments.
Fig. 1.
Characterization of HUVEC cells and the electric field induced cellular responses. (A and B) Tubular-like networks, and (C and D) lumen formation of HUVEC cells on matrigel. (E and F) Intake of Ac-LDL. HUVECs cultured on Matrigel for 24 h. For Calcein AM staining 8 μg/ml of the stain was added to the surface. For AcLDL staining 50 μg of Dil-labled AcLDL was added. (G) An in vitro angiogenesis model shows the vessel like structure formation. (H) HUVEC cells cultured without EFs showed a typical cobblestone morphology and random orientation. (I) Cells exposed to a small applied EF (200 mV mm−1) elongate and align perpendicularly to the vector of the EF (pointing to the left).
3.2. An electric field induces pre-angiogenic responses in human vascular endothelial cells
As reported in our previous publication [7,8], HUVECs exposed to small, applied EFs (200 mV mm−1) showed pre-angiogenic responses, including directional migration, elongation, and perpendicular orientation in the EF, while control cells that were not subjected to EFs showed a typical cobblestone morphology and random orientation (Fig. 1H and I).
3.3. An electric field increases IL-8 and VEGF secretion by endothelial cells
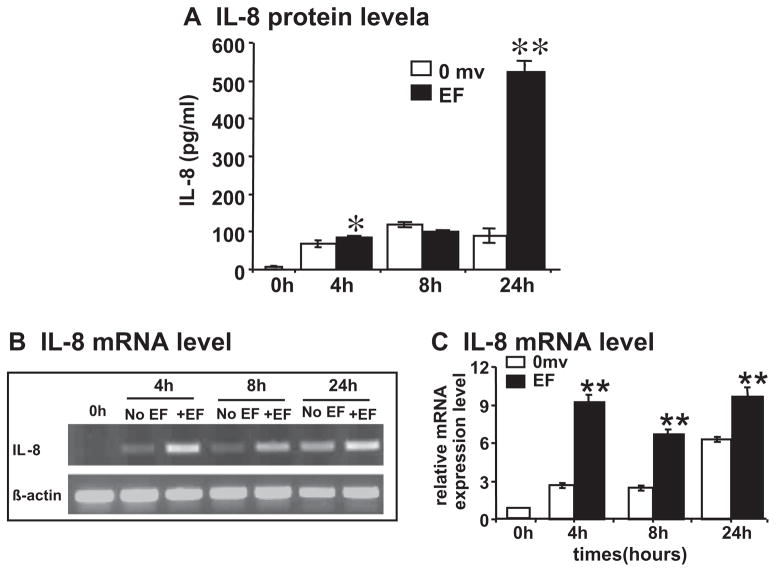
Endothelial cells may produce several types of angiogenic factors, and VEGF and IL-8 are among the most potent. We quantified the levels of IL-8 and VEGF after electric field-exposure. An EF of 200 mV mm−1 significantly enhanced levels of IL-8 release into the culture medium. Significant increases were found at 4 h in the EF. The level increased to more than fivefold after 24 h in the EFs (Fig 2A). Marked elevation of VEGF in the culture medium was observed as early as 30 min after onset of the EF. The level was reduced at 1 and 2 h in the EFs. The level of VEGF rose again at 4 h, and reached a high level by 24 h [7]. These data indicate a latent EF induced production of IL-8 (compared with that of VEGF in HUVECs).
Fig. 2.
A small EF induces production of angiogenic factor IL-8 from endothelial cells. (A) IL-8 protein level; (B) semi-quantitative RT-PCR with β-actin as internal control; and (C) quantification of mRNA level. HUVEC cells were cultured in serum-free DMEM and exposed to an EF of 200 mV mm−1. IL-8 protein in the medium was quantified by ELISA. Isolated total RNA from control and EF-stimulated HUVEC were analyzed by semi-quantitative RT-PCR: histogram indicating the mRNA expression of IL-8 normalized to βactin (expressed as fold change relative to the 0 h control). IL-8 protein and mRNA transcription were increased significantly at the time points 4 and 24 h, and 4–24 h, respectively (up to two- to threefold increase compared with control). The error bars represent the SE (*p < 0.05 and **p < 0.01).
3.4. An electric field specifically up-regulates expression of IL-8 and VEGF mRNA
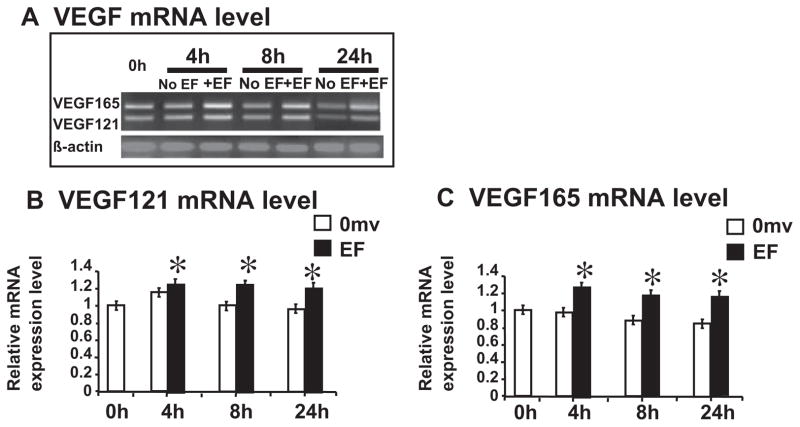
To examine whether EF stimulates IL-8 and VEGF mRNA expression in HUVECs, we used a semi-quantitative RT-PCR to detect them. We isolated total RNA from un-stimulated and EF-stimulated HUVECs (0, 4, 8 and 24 h stimulation). The results showed that an EF significantly up-regulated significantly IL-8 and VEGF mRNA expression at 4–24 h (Figs. 2B and C and 3). Both VEGF121 and VEGF165 were significantly upregulated and showed the same time course (Fig. 3).
Fig. 3.
A small EF induces significant increase of VEGF mRNA in endothelial cells. Two major VEGF isoforms, VEGF165 and 121, were amplified. (A) Semi-quantitative RT-PCR with β-actin as internal control; (B and C) quantification of mRNA levels. Isolated total RNA from control and EF-stimulated HUVEC were analyzed by semi-quantitative RTPCR: histogram indicating the mRNA expression of VEGF165 and 121 normalized to β-actin (expressed as fold change relative to the 0 h control). VEGF mRNA transcription increased significantly at all the time points 4–24 h (up to 33% increase compared with control). The error bars represent the SE (*p < 0.05).
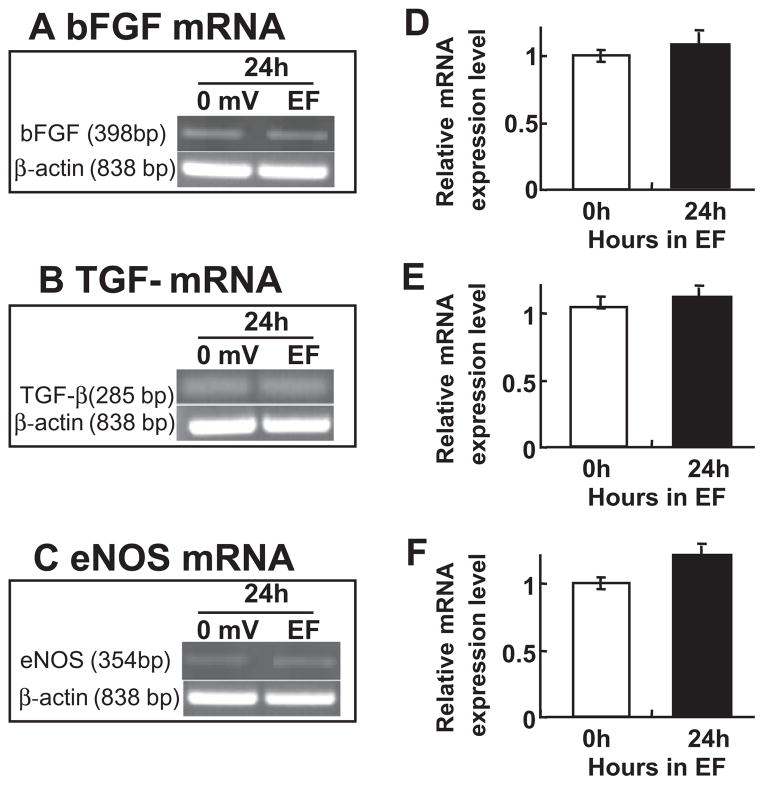
We next determined other angiogenic factors produced by the endothelial cells. Interestingly, we found that the level of mRNA coding bFGF, TGF-beta and eNOS mRNA remained unchanged at 24 h (Fig. 4) in HUVECs. It appears that the upregulation of IL-8 and VEGF mRNA levels is a direct effect of EFs. The mechanism of this selective upregulation is not clear.
Fig. 4.
An EF does not change mRNA level of bFGF, TGF-β and eNOS. EF stimulation did not alter mRNA levels of bFGF, TGF-beta and eNOS in HUVEC. Cells were stimulated 24 h with an EF of 200 mV mm−1. Total RNA was analyzed by RT-PCR. (A–C) Representatives of bFGF, TGF-beta and eNOS mRNA signal respectively, house-keeping gene β-actin as internal controls. (D–F) Histogram depicting bFGF, TGF-beta and eNOS mRNA levels normalized to β-actin (expressed as fold change relative to the 0 h control). The error bars represent the SE.
3.5. Inhibition of VEGF receptors abolished EF-induced VEGF and IL-8 in HUVECs
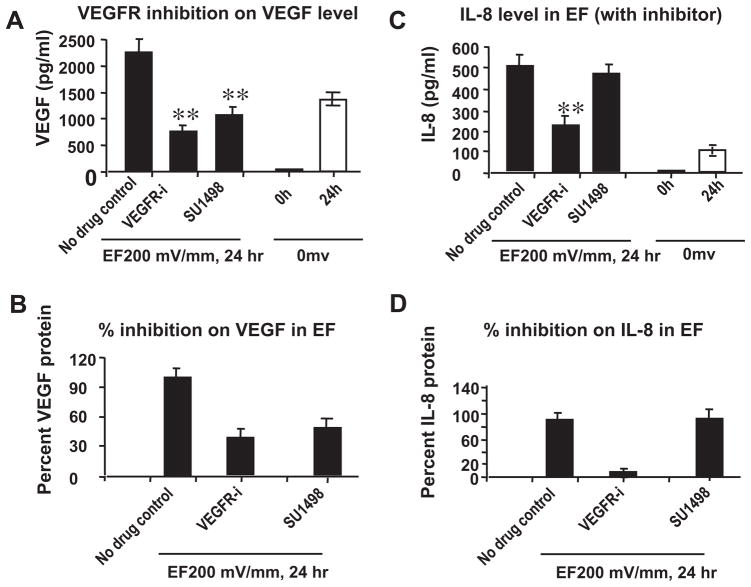
To determine the mechanism of VEGF expression upregulation, we inhibited VEGF receptors. The VEGFR inhibitors (VEGFR-i and SU1498) were used to treat HUVECs for 1 h, and EF-induced VEGF production (VEGF165) was then evaluated by ELISA. As shown in Fig. 5, 50μM VEGFR-i potently blocked EF-induced VEGF production in HUVECs. In addition, EF-induced VEGF production was only partially inhibited (significantly) by 100 μM SU-1498, an inhibitor more specific for Flk-1/KDR (Fig. 5A and B). These data demonstrate that EF stimulated VEGF molecules activate the endothelial cells through VEGF receptor signaling pathway in HUVECs.
Fig. 5.
VEGFR inhibitors decreases significantly the EF-induced increases in VEGF and IL-8 expression. HUVECs were pretreated with 50 μMVEGFR inhibitor (for both VEGFR1 and VEGFR2) or 100 μM SU1498 (for VEGFR2 only) for 1 h. Then the cells were stimulated by an EF of 200 mV mm−1 for 24 h. Cells that had not been subjected to an EF was used as the control. Culture supernatant was collected and analyzed as described under Section 2. (A and C) VEGF and IL-8 protein levels in the supernatant respectively; (B and D) percent VEGF and IL-8 proteins in culture supernatant respectively. The error bars represent the SE (**p < 0.01). VEGFR inhibitors block the EF-mediated increase in IL-8 protein levels.
Inhibition of VEGFR activation stopped or decreased IL-8 release from endothelial cells. Interestingly, inhibition of VEGFR1 and 2 completely abolished IL-8 release, whereas inhibition of VEGFR2 (SU1498) showed a partial inhibition of IL-8 protein (Fig. 5C and D). This suggests that VEGFR1 and VEGFR2, or VEGFR2 only played a differential role in IL-8 protein expression.
4. Discussion
We have shown that EF directly stimulates VEGF release in HUVECs in vitro following EF stimulation [7,8]. In the present study, we show that an EF stimulated VEGF mRNA expression, and promoted IL-8 (one of the most important angiogenic factors) transcription and expression. Together they may mediate EF-induced angiogenic responses. More importantly, the upregulation of angiogenic factors might be specific, since mRNA levels of FGFb, TGF-β and eNOS remained unaffected at 24 h time-point.
Small DC EFs direct the migration of many cell types including endothelial cells and other vascular cells [6,21–29]. More recently, we demonstrated that small DC EFs are an overriding guidance cue that directs corneal epithelial cell migration in wound healing. In endothelial cells, small EFs induce directional cell migration, cell alignment, cell elongation and production of VEGF [6–8]. The EFs therefore may provide a new approach to modulate angiogenesis. Indeed, electric stimulation in vivo has shown promising results that angiogenesis can be significantly enhanced even with subcontraction stimulation [2–3]. EF-induced cellular responses may be mediated through Ca2+ and Na+ channels [23,30], cell surface charge [14,31–33], growth factors, and protein kinases [34,35].
VEGF production by muscle cells underlies EF-stimulated angiogenesis both in vitro and in vivo. The VEGF from muscle cells act on endothelial cells through paracrine pathway [2,5]. Another possibility is the autocrine pathway, in which the EFs induce production of angiogenic factors directly by stimulation of the endothelial cells without the presence of other cell types [6–8]. Application of DC EFs directly upregulates mRNA and protein levels of IL-8 and VEGF in endothelial cells (Figs. 2 and 3). Two major splicing isoforms of VEGF – VEGF121 and VEGF165 are upregulated (Fig. 3). Interestingly, EF stimulation did not upregulate mRNA expression of bFGF, TGF-beta, and eNOS (Fig. 4), which are also angiogenic factors. These results suggest that EFs selectively regulate angiogenic factors.
How EFs induce transcriptional changes and expression are not clear. In brain blood endothelial cells, VEGF regulates the functional expression of IL-8 [36]. One recent study demonstrated that the effect of VEGF on expression of IL-8 and its receptor in bovine theca cells [37]. Our results suggest that inhibition of the VEGF receptor not only inhibits the VEGF production, but also IL-8 release. It appears that VEGF receptor 1 mediates the EF-induced upregulation of VEGF and IL-8. The VEGF receptor 2 (KDR) specific inhibitor only partially inhibits IL-8 production (10% inhibition), whereas inhibition of both VEGFR1 and VEGFR2 showed a nearly complete inhibition (92% inhibition) of IL-8 release.
EF induced increases in VEGF and IL-8 may stimulate angiogenic responses through binding to VEGF and chemokine receptors. VEGF receptor activation will instigate several signaling pathways, including MAP kinase (ERK1/2), PI3-kinase, protein kinase C, and calcium in endothelial cells [38,39]. PI3-kinase inhibitor LY294002 significantly inhibited the pre-angiogenic response of endothelial cells in an EF [7].
IL-8 binds chemokine receptors CXCR 1 to CXCR 3 to induce the angiogenic response [40]. Those receptors are found in several type of endothelial cells, including HUVECs [40]. Endothelial cell production of IL-8 in an EF suggested that autocrine is a pathway of EF induced angiogenic responses. EFs can upregulate expression of some growth factor receptors, such as EGF receptors on cell membranes in corneal epithelial cells [15]. Whether EFs also upregulate IL-8 receptor (CXCR 1–3) expression on endothelial cells remains to be determined.
In conclusion, EF stimulation upregulates the mRNA and protein levels of VEGF and IL-8 in HUVECs. Such an upregulation is likely a specific effect, at least for the time points tested. Activation of VEGF receptor 1 is required for the upregulation of both VEGF and IL-8. Small EF-induced angiogenic factors may orchestrate in directing endothelial behaviors and angiogenic responses.
Acknowledgments
We thank the Wellcome Trust for continuous support. This work is also supported in part by the British Heart Foundation (FS/2000056 and PG/99191), Department start fund from UC Davis Medical School (to MZ), and from the National Natural Science Foundation of China (30872774 to HB), NSFC Grant (30628026), and Sichuan Provincial Department of Science and Technology (2008JY0049 to HB). MZ is also supported by grants from California Institute of Regenerative Medicine RB1-01417, NIH 1R01EY019101, NSF MCB-0951199. We are grateful to Dr. Erica M. Chedin (School of Medicine, University of California, Davis) for editorial assistance.
References
- 1.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 2.Kanno S, Oda N, Abe M, Saito S, Hori K, Handa Y, et al. Establishment of a simple and practical procedure applicable to therapeutic angiogenesis. Circulation. 1999;99:2682–7. doi: 10.1161/01.cir.99.20.2682. [DOI] [PubMed] [Google Scholar]
- 3.Patterson C, Runge MS. Therapeutic angiogenesis: the new electrophysiology? Circulation. 1999;99:2614–6. doi: 10.1161/01.cir.99.20.2614. [DOI] [PubMed] [Google Scholar]
- 4.Linderman JR, Kloehn MR, Greene AS. Development of an implantable muscle stimulator: measurement of stimulated angiogenesis and poststimulus vessel regression. Microcirculation. 2000;7:119–28. [PubMed] [Google Scholar]
- 5.Hang J, Kong L, Gu JW, Adair TH. VEGF gene expression is upregulated in electrically stimulated rat skeletal muscle. Am J Physiol. 1995;269:H1827–31. doi: 10.1152/ajpheart.1995.269.5.H1827. [DOI] [PubMed] [Google Scholar]
- 6.Li X, Kolega J. Effects of direct current electric fields on cell migration and actin filament distribution in bovine vascular endothelial cells. J Vasc Res. 2002;39:391–404. doi: 10.1159/000064517. [DOI] [PubMed] [Google Scholar]
- 7.Zhao M, Bai H, Wang E, Forrester JV, McCaig CD. Electrical stimulation directly induces pre-angiogenic responses in vascular endothelial cells by signaling through VEGF receptors. J Cell Sci. 2004;117(Pt 3):397–405. doi: 10.1242/jcs.00868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bai H, McCaig CD, Forrester JV, Zhao M. DC electric fields induce distinct preangiogenic responses in microvascular and macrovascular cells. Arterioscler Thromb Vasc Biol. 2004;24:1234–9. doi: 10.1161/01.ATV.0000131265.76828.8a. [DOI] [PubMed] [Google Scholar]
- 9.Barker AT, Jaffe LF, Vanable JW., Jr The glabrous epidermis of cavies contains a powerful battery. Am J Physiol. 1982;242:R358–66. doi: 10.1152/ajpregu.1982.242.3.R358. [DOI] [PubMed] [Google Scholar]
- 10.Jaffe LF, Stern CD. Strong electrical currents leave the primitive streak of chick embryos. Science. 1979;206:569–71. doi: 10.1126/science.573921. [DOI] [PubMed] [Google Scholar]
- 11.Jaffe LF, Vanable JW., Jr Electric fields and wound healing. Clin Dermatol. 1984;2:34–44. doi: 10.1016/0738-081x(84)90025-7. [DOI] [PubMed] [Google Scholar]
- 12.Nuccitelli R. Endogenous ionic currents and DC electric fields in multicellular animal tissues. Bioelectromagnetics. 1992;(Suppl 1):147–57. doi: 10.1002/bem.2250130714. [DOI] [PubMed] [Google Scholar]
- 13.Hotary KB, Robinson KR. Evidence of a role for endogenous electrical fields in chick embryo development. Development. 1992;114:985–96. doi: 10.1242/dev.114.4.985. [DOI] [PubMed] [Google Scholar]
- 14.McCaig CD, Zhao M. Physiological electrical fields modify cell behaviour. Bioessays. 1997;19:819–26. doi: 10.1002/bies.950190912. [DOI] [PubMed] [Google Scholar]
- 15.Zhao M, Dick A, Forrester JV, McCaig CD. Electric field-directed cell motility involves up-regulated expression and asymmetric redistribution of the epidermal growth factor receptors and is enhanced by fibronectin and laminin. Mol Biol Cell. 1999;10:1259–76. doi: 10.1091/mbc.10.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sawyer PN, Himmelfarb E, Lustrin I, Ziskind H. Measurement of streaming potentials of mammalian blood vessels, aorta and vena cava, in vivo. Biophys J. 1966;6:641–51. doi: 10.1016/S0006-3495(66)86683-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coronel R, Wilms-Schopman FJG, Opthof T, van Capelle FJL, Janse MJ. Injury current and gradient of diastolic stimulation threshold, TQ potential and extracellular potassium concentration during acute regional ischemia in the isolated perfused pig heart. Circ Res. 1991;68:1241–9. doi: 10.1161/01.res.68.5.1241. [DOI] [PubMed] [Google Scholar]
- 18.Zhao M, Song B, Pu J, Wada T, Reid B, Tai G, et al. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature. 2006;442:457–60. doi: 10.1038/nature04925. [DOI] [PubMed] [Google Scholar]
- 19.Zhao M, Agius-Fernandez A, Forrester JV, McCaig CD. Orientation and directed migration of cultured corneal epithelial cells in small electric fields are serum dependent. J Cell Sci. 1996;109:1405–14. doi: 10.1242/jcs.109.6.1405. [DOI] [PubMed] [Google Scholar]
- 20.Kwok S, Higuchi R. Avoiding false positives with PCR. Nature. 1989;18(339):237–8. doi: 10.1038/339237a0. [DOI] [PubMed] [Google Scholar]
- 21.Robinson KR. The responses of cells to electrical fields: a review. J Cell Biol. 1985;101:2023–7. doi: 10.1083/jcb.101.6.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erickson CA, Nuccitelli R. Embryonic fibroblast motility and orientation can be influenced by physiological electric fields. J Cell Biol. 1984;98:296–307. doi: 10.1083/jcb.98.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soon HK, Parkinson WC, Bafna S, Sulik GL, Huang S. Movements of cornel epithelial cells and stromal fibroblasts in electric fields. Invest Ophthalmol Visual Sci. 1990;31:2278–82. [PubMed] [Google Scholar]
- 24.Cooper MS, Schliwa M. Motility of cultured fish epidermal cells in the presence and absence of direct current electric fields. J Cell Biol. 1986;102:1384–99. doi: 10.1083/jcb.102.4.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luther PW, Peng HB, Lin JJ. Changes in cell shape and actin distribution induced by constant electric fields. Nature. 1983;303:61–4. doi: 10.1038/303061a0. [DOI] [PubMed] [Google Scholar]
- 26.Sulik GL, Soong HK, Chang PC, Parkinson WC, Elner SG, Elner VM. Effects of steady electric fields on human retinal pigment epithelial cell orientation and migration in culture. Acta Ophthamol (Copenh) 1992;70:115–22. doi: 10.1111/j.1755-3768.1992.tb02102.x. [DOI] [PubMed] [Google Scholar]
- 27.Yang WP, Onuma EK, Hui SW. Response of C3H/10T1/2 fibroblasts to an external steady electric field stimulation. Reorientation, shape change, con A receptor and intramembranous particle distribution and cytoskeletal reorganization. Exp Cell Res. 1984;155:92–104. doi: 10.1016/0014-4827(84)90770-5. [DOI] [PubMed] [Google Scholar]
- 28.Chang PC, Soolik GL, Parkinson WC, Qu X, Meyer RF. Galvanotropic and glavanotaxic responses of corneal endothelial cells are inhibited by cytochalasin D and W-7. Invest Ophthalmol Visual Sci. 1991;32(Suppl):1219. [Google Scholar]
- 29.Orita N, Fekdman JD. Directional protrusive pseudopodial activity and motility in macrophages induced by extracellular electric fields. Cell Motil Cytoskeleton. 1982;2:243–55. doi: 10.1002/cm.970020305. [DOI] [PubMed] [Google Scholar]
- 30.Djamgoz MBA, Mycielska M, Madeja Z, Fraser SP, Korohoda W. Directional movement of rat prostate cancer cells in direct-current electric field: involvement of voltage-gated Na+ channel activity. J Cell Sci. 2001;114:2697–705. doi: 10.1242/jcs.114.14.2697. [DOI] [PubMed] [Google Scholar]
- 31.Poo M, Lam JW, Orida N, Chao AW. Electrophoresis and diffusion in the plane of the cell membrane. Biophys J. 1979;26:1–21. doi: 10.1016/S0006-3495(79)85231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Onuma EK, Hui S-W. Electric field-directed cell shape changes, displacement, and cytoskeletal reorganization are calcium dependent. J Cell Biol. 1988;106:2067–75. doi: 10.1083/jcb.106.6.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown MJ, Loew LM. Electric field-directed fibroblast locomotion involves cell surface molecular reorganization and is calcium independent. J Cell Biol. 1994;127:117–28. doi: 10.1083/jcb.127.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keenan C, Kelleher D. Protein kinase C and the cytoskeleton. Cell Signal. 1998;10:225–32. doi: 10.1016/s0898-6568(97)00121-6. [DOI] [PubMed] [Google Scholar]
- 35.Nuccitelli R, Smart T, Ferguson J. Protein kinases are required for embryonic neural crest cell galvanotaxis. Cell Motil Cytoskel. 1993;24:54–6. doi: 10.1002/cm.970240107. [DOI] [PubMed] [Google Scholar]
- 36.Lee TH, Avraham H, Lee SH, Avraham S. Vascular endothelial growth factor modulates neutrophil transendothelial migration via up-regulation of interleukin-8 in human brain microvascular endothelial cells. J Biol Chem. 2002;22(277):10445–51. doi: 10.1074/jbc.M107348200. [DOI] [PubMed] [Google Scholar]
- 37.Murayama C, Kaji A, Miyauchi K, Matsui M, Miyamoto A, Shimizu T. Effect of VEGF (vascular endothelial growth factor) on expression of IL-8 (interleukin-8), IL-1beta and their receptors in bovine theca cells. Cell Biol Int. 2010;34:531–6. doi: 10.1042/CBI20090498. [DOI] [PubMed] [Google Scholar]
- 38.Cross MJ, Dixelius J, Matsumoto T, Claesson-Welsh L. VEGF-receptor signal transduction. Trends Biochem Sci. 2003;28:488–94. doi: 10.1016/S0968-0004(03)00193-2. [DOI] [PubMed] [Google Scholar]
- 39.Thakker GD, Hajjar DP, Muller WA, Rosengart TK. The role of phosphatidylinositol 3-kinase in vascular endothelial growth factor signaling. J Biol Chem. 1999;274:10002–7. doi: 10.1074/jbc.274.15.10002. [DOI] [PubMed] [Google Scholar]
- 40.Salcedo R, Resau JH, Halverson D, Hudson EA, Dambach M, Powell D, et al. Differential expression and responsiveness of chemokine receptors (CXCR 1–3) by human microvascular endothelial cells and umbilical vein endothelial cells. FASEB J. 2000;14:2055–64. doi: 10.1096/fj.99-0963com. [DOI] [PubMed] [Google Scholar]