Abstract
BACKGROUND
Inflammatory breast cancer (IBC) is a rare, aggressive form of breast cancer with poorly understood prognostic variables. The purpose of this study was to define the prognostic impact of HER-2 status on survival outcomes of patients with IBC.
METHODS
In all, 179 patients with IBC, diagnosed between 1989 and 2005, with known HER-2 status, and treated with an anthracycline-based chemotherapy regimen without trastuzumab, were included in the analysis. Patients with HER-2-positive disease who received trastuzumab at the time of disease recurrence were included. Survival outcomes were estimated by the Kaplan-Meier product limit method and compared across groups using the log-rank statistic. A Cox proportional hazards model was fitted to determine the association of survival outcomes with HER-2 status after adjusting for patient and tumor characteristics.
RESULTS
A total of 111 patients (62%) had HER-2-negative disease and 68 (38%) had HER-2-positive disease. The median follow-up among all patients was 35 months. At the time of the analysis, 62 patients (55.9%) with HER-2-negative disease and 42 patients (61.8%) with HER-2-positive disease had a recurrence. Thirty-one patients (73.8%) with HER-2-positive disease who had a disease recurrence went on to receive trastuzumab. On univariate analysis, no statistically significant difference was observed for either recurrence-free survival (P = .75) or overall survival (P = .24) between patients who had HER-2-positive disease and those who had HER-2-negative disease. In a multivariate model, HER-2 status did not appear to significantly affect recurrence-free survival (hazards ratio [HR] of 0.75; 95% confidence interval [95% CI], 0.46–1.22 [P = .241]). In the multivariate model, patients with HER-2-positive disease had a decreased hazard of death (HR of 0.56; 95% CI, 0.34–0.93 [P = .024]) compared with patients with HER-2-negative disease.
CONCLUSIONS
HER-2 status, in the absence of trastuzumab, did not appear to significantly affect recurrence-free survival. After adjusting for other characteristics, the addition of trastuzumab in the metastatic setting significantly improved survival in the HER-2-positive group above and beyond that of the HER-2-negative group. This gives us further insight into the biology of this aggressive disease and underlines the major effect of targeted intervention.
Keywords: inflammatory breast cancer, HER-2 status, survival outcomes, targeted intervention
Inflammatory breast cancer (IBC), a rare, aggressive subset of locally advanced breast cancer, accounts for approximately 1% to 6% of all breast cancer cases.1 The diagnosis of IBC relies on a set of clinical diagnostic criteria including rapid onset of breast warmth, edema (peau d’orange), and erythema, often without a palpable mass and with the early onset of axillary lymph node involvement. Despite such non-specific diagnostic criteria, which limit our ability to accurately define the true incidence of IBC, an analysis of breast cancer cases recorded between 1975 and 1992 within the Surveillance, Epidemiology, and End Results program of the National Cancer Institute has reported an approximate doubling of cases of IBC (0.3 to 0.7 per 100,000 white women and from 0.6 to 1.1 per 100,000 black women) compared with the 25% to 27% increase in the incidence of breast cancer in general.2 Although a multimodality treatment approach involving induction chemotherapy, surgery, and radiotherapy has clearly improved long-term outcomes of a once uniformly fatal disease,3–5 prognosis is still inferior to that of patients with locally advanced breast cancer,6 with 5-year survival rates ranging from 30% to 50%.1,2
The use of both prognostic factors, which can estimate an individual’s risk of harboring clinically silent micrometastatic disease, and predictive factors, which estimate the likelihood of response to therapy, has become an integral part of individualized treatment that has translated into improved survival outcomes of patients with early-stage breast cancer.7 However, unlike other forms of breast cancer, few molecular markers have been found to be characteristic of IBC8 and due to the rarity of the disease their prognostic and predictive value have not been well established. Pathologic characteristics known to be associated with IBC include high histologic grade; negative hormone receptor status; a high proliferation rate (eg, elevated MIB-1 expression); and elevated levels of expression of p53, MUC1, RhoC, E-cadherin, tumor angiogenesis-related factors (eg, vascular endothelial growth factors), chemokine receptors (eg, CXCR4 and CCR7), epidermal growth factor receptor (EGFR), and HER-2.8–13
HER-2 is a protooncogene that is located on 17q21 and is overexpressed and/or amplified in approximately 30% of human breast tumors,14 in which it is associated with increased tumor aggressiveness, increased rates of disease recurrence, and higher rates of mortality.15,16 With the introduction of trastuzumab, a monoclonal antibody that targets the HER-2 receptor, survival outcomes of patients with early and advanced stage HER-2 overexpressed/amplified tumors has significantly improved, essentially changing the natural history of this disease.17,18 Although studies have reported an increased incidence of HER-2 overexpression/amplification in patients with IBC, the prognostic significance of HER-2 overexpression/amplification in this cohort has not been clearly defined, and due to the rarity of this disease the impact of trastuzumab in this cohort is not well known.10 Using a cohort of patients treated at the University of Texas M. D. Anderson Cancer Center (MDACC), the purpose of the current study was to define the prognostic impact of HER-2 status on survival outcomes of patients with IBC.
MATERIALS AND METHODS
Patient Population
Using the institutional tumor registry and Breast Medical Oncology database, we sought to identify patients with nonmetastatic IBC who had been treated at MDACC. IBC was defined according to clinical diagnostic criteria that included rapidly developing (<3 months) signs and symptoms of diffuse erythema, peau d’orange, and increasing size of the breast together with confirmation of invasive carcinoma with or without evidence of extensive dermal lymphatic invasion on core biopsy specimens. Inclusion criteria included patients with clinical stage III (T4d, any N, M0) disease (as defined by the sixth edition of the American Joint Committee on Cancer [AJCC] Staging Manual19) who had known HER-2 status, were treated with an anthracycline-based chemotherapy regimen, and had undergone definitive surgery followed by adjuvant radiotherapy. Patients who received trastuzumab as part of their primary systemic therapy were excluded. However, patients could have received trastuzumab as part of treatment for metastatic disease. Other exclusion criteria included patients who were male, had bilateral disease, and/or stage IV disease. All medical charts were reviewed to confirm accuracy of variables recorded. This retrospective review was approved by the Institutional Review Board of the MDACC.
Pathologic Review
Pathologic specimens were reviewed and histologic type and grade were defined according to the World Health Organization Classification System20 and the modified Black nuclear grading system,21 respectively. Pathologic complete response (pCR) was defined as no evidence of invasive disease in both the breast and axillary lymph nodes at the time of definitive surgery. Estrogen receptor (ER) and progesterone receptor (PR) status was determined by immunohistochemical (IHC) analysis using 4-μm sections of paraffin-embedded tissues stained with monoclonal antibodies 6f11 (Novacastra Laboratories, Burlingame, Calif) and 1A6 (Novocastra Laboratories) for ER and PR, respectively. A positive receptor status was defined when nuclear staining was >10% of invasive cancer cells. For patients diagnosed with IBC before 1993, ER and PR were determined using the dextran-coated charcoal ligand-binding method. Patients were categorized as having HER-2-positive disease if their tumor samples exhibited either gene amplification by fluorescent in situ hybridization technique (FISH)22 or overexpression (3+) by IHC (>10% of tumor cells exhibiting strong membranous staining). HER-2-negative status was assigned to those patients whose tumor samples either did not exhibit gene amplification by FISH or had no staining by IHC. Path Vysion HER-2-neu DNA probe kit (Vysis, Downers Grove, Ill) and monoclonal antibody AB8 (Neomarker/Labvision, Fremont, Calif) were used for HER-2 FISH and IHC analysis, respectively.
Statistical Methods
Patient and tumor characteristics were tabulated. The median follow-up was calculated for all patients and for those still alive at their last follow-up. Recurrence-free survival (RFS) was calculated from the date of diagnosis to the date of disease recurrence (defined as either locoregional or distant recurrence) or last follow-up. Overall survival (OS) was calculated from the date of diagnosis to the date of death from any cause or last follow-up. The Kaplan-Meier product limit method was used to estimate survival outcomes, which were compared between groups using log-rank statistics. Cox proportional hazards models were fitted to determine the association of HER-2 status with RFS and OS after adjusting for patient and tumor characteristics. A P ≤ .05 was considered statistically significant. All statistical analyses were performed using SAS 9.1 statistical software (SAS Institute Inc, Cary, NC).
RESULTS
Patient and Tumor Characteristics
Between June 1989 and April 2005, 179 patients with IBC who fit eligibility criteria were identified. Table 1 summarizes patient and tumor characteristics by HER-2 status. The median age of the patients at diagnosis was 51 years (range, 29–78 years), with the dominant histology being invasive ductal carcinoma (90%). In all, 111 patients (62%) had HER-2-negative disease and 68 (38%) had HER-2-positive disease. ER and PR status were negative in 111 patients (62%) and 124 patients (69%), respectively. The majority of patients (90%) had clinical lymph node-positive disease. All patients received anthracycline-based primary systemic chemotherapy and 140 (78.2%) received taxanes. All patients underwent a modified radical mastectomy followed by comprehensive local radiotherapy to the chest wall and lymph node basins. Patients with ER-positive and/or PR-positive disease received adjuvant hormonal therapy. Twenty-three patients (13%) achieved a pCR. Detailed information regarding therapy has been previously reported.23
TABLE 1. Patient and Tumor Characteristics by HER-2 Status.
| All patients |
HER-2 negative |
HER-2 positive |
|||||
|---|---|---|---|---|---|---|---|
| No. |
No. |
No. |
|||||
| No. | 179 | Percent | 111 | Percent | 68 | Percent | P |
| Age at diagnosis, y | |||||||
| Minimum | 27 | 29 | 27 | ||||
| Median | 51 | 51 | 48.5 | ||||
| Maximum | 78 | 75 | 78 | .3796 | |||
| Histology | |||||||
| Other | 17 | 10% | 12 | 11% | 5 | 8% | |
| Ductal | 155 | 90% | 97 | 89% | 58 | 92% | .515 |
| Grade | |||||||
| 1/2 | 35 | 20% | 25 | 23% | 10 | 15% | |
| 3 | 137 | 80% | 82 | 77% | 55 | 85% | .208 |
| ER status | |||||||
| Negative | 111 | 62% | 65 | 59% | 46 | 68% | |
| Positive | 67 | 38% | 45 | 41% | 22 | 32% | .252 |
| PR status | |||||||
| Negative | 124 | 70% | 70 | 64% | 54 | 79% | |
| Positive | 53 | 30% | 39 | 36% | 14 | 21% | .032 |
| Lymphovascular invasion | |||||||
| Negative | 34 | 22% | 23 | 23% | 11 | 20% | |
| Positive | 122 | 78% | 77 | 77% | 45 | 80% | .626 |
| Lymph node classification | |||||||
| N0 | 17 | 10% | 11 | 10% | 6 | 9% | |
| N1–3 | 159 | 90% | 99 | 90% | 66 | 91% | .840 |
| Pathologic CR | |||||||
| No | 154 | 87% | 97 | 88% | 57 | 85% | |
| Yes | 23 | 13% | 13 | 12% | 10 | 15% | .551 |
ER indicates estrogen receptor, PR, progesterone receptor, CR, complete response.
Survival Outcomes
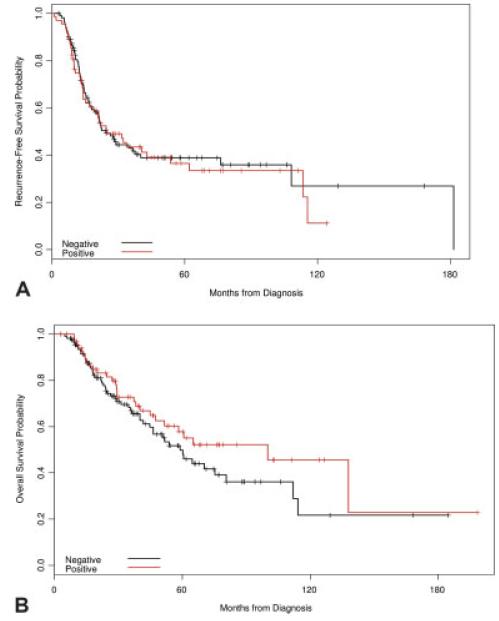
At the time of the analyses, 104 patients (58%) had experienced a disease recurrence and 78 patients (44%) had died. The median follow-up among patients who were still alive at the time of the analysis was 41 months (range, 3–198 months). For the entire cohort, the median RFS and OS were 24.5 months and 60.6 months, respectively. At the time of the analysis, 62 patients (55.9%) with HER-2-negative disease and 42 patients (61.8%) with HER-2-positive disease had a disease recurrence. Thirty-one patients (73.8%) with HER-2-positive disease who developed a disease recurrence went on to receive trastuzumab. The 5-year RFS and OS estimates are summarized in Table 2. For the entire cohort studied, the 5-year RFS and OS rates were 37.8% (95% confidence interval [95% CI], 29.9%–45.7%) and 53.1% (95% CI, 44.0%–61.3%), respectively. On univariate analysis, HER-2 status was not found to be significantly associated with RFS (P = .75) or OS (P = .24) (Fig. 1). The survival curves in Figure 1A illustrate the similarity in RFS among patients with HER-2-positive and HER-2-negative disease. Factors found to be statistically significant for improved RFS and OS included ER-positive disease, lower nuclear grade, and attaining a pCR.
TABLE 2. 5-Year Recurrence-free and Overall Survival Estimates.
| Recurrence-free survival |
Overall survival |
|||||||
|---|---|---|---|---|---|---|---|---|
| No. of events | 5–year survival | 95% CI | P | No. of events | 5-year survival | 95% CI | P | |
| All | 104 | 37.8% | (29.9–45.7%) | 78 | 53.1% | (44.0–61.3%) | ||
| Age at diagnosis, y | ||||||||
| <50 | 55 | 31.2% | (20.7–42.2%) | 38 | 52.6% | (39.4–64.2%) | ||
| ≥50 | 49 | 44.2% | (32.7–55.0%) | .035 | 40 | 53.4% | (40.7–64.6%) | .433 |
| Lymph node classification | ||||||||
| N0 | 12 | 46.3% | (22.1–67.6%) | 7 | 59.8% | (31.0–79.8%) | ||
| N1–3 | 89 | 37.6% | (29.2–46.1%) | .934 | 68 | 53.9% | (44.3–62.6%) | .337 |
| Histology | ||||||||
| Other | 9 | 47.1% | (23.0–68.0%) | 4 | 71.4% | (38.7–88.7%) | ||
| Ductal | 90 | 36.9% | (28.4–45.4%) | .488 | 70 | 52.3% | (42.6–61.1%) | .148 |
| Grade | ||||||||
| 1/2 | 18 | 51.5% | (32.2–67.7%) | 11 | 71.5% | (50.4–84.9%) | ||
| 3 | 81 | 33.9% | (25.1–42.9%) | .047 | 64 | 48.6% | (38.2–58.1%) | .030 |
| ER status | ||||||||
| Negative | 71 | 29.9% | (20.6–39.7%) | 56 | 44.1% | (33.1–54.6%) | ||
| Positive | 33 | 49.6% | (35.5–62.1%) | .004 | 22 | 66.1% | (50.1–78.1%) | .002 |
| PR status | ||||||||
| Negative | 78 | 32.5% | (23.4–41.9%) | 58 | 49.0% | (38.2–58.9%) | ||
| Positive | 25 | 49.2% | (33.6–63.1%) | .009 | 20 | 60.4% | (42.8–74.1%) | .065 |
| HER-2 status | ||||||||
| Negative | 62 | 38.8% | (28.7–48.8%) | 50 | 49.8% | (38.0–60.6%) | ||
| Positive | 42 | 36.5% | (24.0–49.0%) | .750 | 28 | 57.8% | (43.4–69.8%) | .245 |
| Lymphovascular invasion | ||||||||
| Negative | 14 | 44.5% | (21.8–5.1%) | 10 | 68.2% | (47.2–82.3%) | ||
| Positive | 74 | 36.7% | (27.4–46.1%) | .400 | 56 | 51.0% | (40.1–60.9%) | .976 |
| Pathologic CR | ||||||||
| No | 97 | 31.7% | (23.4–40.2%) | 75 | 45.1% | (35.3–54.4%) | ||
| Yes | 7 | 69.0% | (43.2–84.9%) | .003 | 3 | 95.5% | (71.9–99.3%) | .001 |
95% CI indicates 95% confidence interval; ER, estrogen receptor, PR, progesterone receptor, CR, complete response.
FIGURE 1.
Kaplan-Meier plots for (A) recurrence-free and (B) overall survival by HER-2 status. On univariate analysis, no significant difference was noted for recurrence-free survival (A). Univariate analysis revealed a non-significant trend in improved survival in patients with HER-2-positive disease compared with those with HER-2-negative disease.
Table 3 shows the results of the multivariate models for RFS and OS. The models were adjusted for age, grade, hormone receptor status, lymph node status, pCR, HER-2 status, and lymphovascular invasion. These terms were included in the models due to their clinical significance regardless of their statistical significance. After adjusting for these variables, HER-2 status was not found to significantly affect RFS (hazards ratio [HR] of0.75; 95% CI, 0.46–1.22 [P = .241]). In the model for OS, patients with HER-2-positive disease had a statistically significant decreased hazard of death (HR of 0.56; 95% CI, 0.34–0.93 [P = .024]) compared with patients with HER-2-negative disease. In the multivariate model, ER-positive disease and pCR were found to be independently associated with improved RFS and OS.
TABLE 3. Multivariate Models for Recurrence-free and Overall Survival.
| Hazards ratio |
Lower 95% CI |
Upper 95% CI |
P | |
|---|---|---|---|---|
| Recurrence-free survival | ||||
| Age (continuous) | 0.96 | 0.93 | 0.98 | <.0001 |
| HER-2 status (positive vs negative) | 0.75 | 0.46 | 1.22 | .241 |
| ER status (positive vs negative) | 0.51 | 0.29 | 0.90 | .020 |
| PR status (positive vs negative) | 0.70 | 0.40 | 1.25 | .227 |
| Lymphovascular invasion (yes vs no) | 1.03 | 0.56 | 1.88 | .938 |
| Grade (3 vs 1/2) | 0.92 | 0.49 | 1.71 | .787 |
| Lymph node classification (N1–3 vs N0) | 1.12 | 0.54 | 2.32 | .755 |
| Pathologic CR (yes vs no) | 0.30 | 0.11 | 0.83 | .020 |
| Overall survival | ||||
| Age (continuous) | 0.95 | 0.93 | 0.98 | .0002 |
| HER-2 status (positive vs negative) | 0.56 | 0.34 | 0.93 | .024 |
| ER status (positive vs negative) | 0.51 | 0.29 | 0.88 | .016 |
| PR status (positive vs negative) | 0.72 | 0.41 | 1.25 | .243 |
| Lymphovascular invasion (yes vs no) | 0.59 | 0.31 | 1.10 | .099 |
| Grade (3 vs 1/2) | 1.00 | 0.54 | 1.87 | .992 |
| Lymph node classification (N1–3 vs N0) | 1.49 | 0.72 | 3.08 | .282 |
| Pathologic CR (yes vs no) | 0.34 | 0.12 | 0.95 | .039 |
95% CI indicates 95% confidence interval; ER, estrogen receptor, PR, progesterone receptor, CR, complete response.
DISCUSSION
The majority of studies of IBC are hampered because of the rarity of the disease, resulting in small numbers of patients included and the reliance on non-specific clinical diagnostic criteria to identify cases retrospectively. In the current study, we report results on a large cohort of patients with IBC who were identified prospectively based on consistent diagnostic institutional criteria to define the prognostic value of HER-2 status within this cohort. Interestingly, in the absence of trastuzumab, no statistically significant difference in RFS was observed between patients with HER-2-positive disease compared with those with HER-2-negative disease. However, after adjusting for other patient and tumor characteristics, OS significantly favored patients with HER-2-positive disease, the majority of whom had received trastuzumab upon experiencing disease recurrence. In addition, variables such as ER-positive disease and attaining a pCR, factors known to have a favorable prognostic impact in other types of breast cancer, were associated with improved RFS and OS within the cohort studied.
To our knowledge, Slamon et al.14 first reported, in a cohort of 189 women with primary breast cancers, the implications of the presence of HER-2 amplification, correlating it with shorter RFS and OS when compared with tumors that did not exhibit HER-2 amplification. The prognostic power of HER-2 amplification not only retained its significance after adjusting for other known prognostic factors, it was also found to have superior prognostic power to factors such as hormone receptor status and the presence of lymph node involvement. The introduction of a novel monoclonal antibody, trastuzumab, that targeted the HER-2 receptor, essentially changed the natural history of breast tumors that exhibited over-expression and/or amplification of HER-2, significantly improving survival outcomes in both patients with early-stage and advanced disease.17,18 Unfortunately, the majority of studies have been unable to define the prognostic significance of HER-2 status in patients with IBC, despite the finding that HER-2 amplification is common in this disease. In a small cohort of 46 women with IBC, Sawaki et al.24 concluded that HER-2 status was not a significant prognostic factor within this cohort. We believe the results of the current study give us unique insight into the biology of IBC tumors, in that the patients enrolled who had HER-2-positive disease did not receive trastuzumab as part of their primary systemic treatment; however, the majority of the same subset received trastuzumab when they developed disease recurrence, allowing us the opportunity to evaluate the impact of trastuzumab onOS as well. Although the absolute difference in RFS between patients with HER-2-positive and HER-2-negative disease was 2.3% at 5 years and tended to favor the latter group, it did not reach statistical significance (P = .750). This is further illustrated in the Kaplan-Meier curves for RFS, in which visually no difference was observed between the 2 cohorts and in the multivariate model when, after adjusting for patient and tumor characteristics, HER-2 status did not significantly affect RFS (although we acknowledge that we only had 28% power to detect a significant difference). One hypothesis to explain this may be that other molecular factors of IBC have a superior prognostic power, superseding that of HER-2 status within this cohort. Indeed, factors such as increased expression of EGFR and p53 and loss of p27 have been shown to be significantly associated with worse outcomes in patients with IBC.13,11 Another possible explanation is that HER-2-positive tumors often coexpress topoisomerase II, the target of anthracyclines.25 Because all patients received an anthracycline-containing regimen, this regimen might have been more effective in HER-2-positive tumors than in HER-2-negative tumors and in that manner might have eliminated the impact of HER-2 status in determining RFS. It is interesting to note that, in the multivariate model, after adjusting for patient and tumor characteristics, we demonstrated that patients with HER-2-positive tumors had significantly better OS compared with patients with HER-2-negative disease (HR of 0.56; 95% CI, 0.34-0.93 [P = .024]), indicating the positive effect of the trastuzumab administered in the metastatic setting. These results are important because they demonstrate that the benefit of trastuzumab also extends to patients with IBC. The results also indicate the significant affect of trastuzumab on the natural history of the disease, essentially reversing the prognostic roles of HER-2-positive and HER-2-negative disease.
Attaining a pCR has been shown in several studies to correlate with improved long-term outcome,26–28 believed to be secondary to the eradication of distant micrometastatic residual invasive disease. In a cohort of 178 patients with IBC who were diagnosed between 1973 and 1993 and treated with a doxorubicin-based primary systemic chemotherapy regimen at the MDACC, Ueno et al.5 demonstrated that disease-free survival at 15 years was 44% for those who achieved a CR, 31% for those who achieved a partial response, and 7% for those who did not respond to primary systemic chemotherapy. The results of the current study demonstrate this phenomenon to be true, with absolute increases at 5 years of 37.3% and 50.4% observed for RFS and OS, respectively, when a pCR was attained. Thus, it can be hypothesized that novel therapeutic approaches geared toward increasing pCR rates within this cohort would serve to subsequently improve survival outcomes. Buzdar et al.29 demonstrated that the addition of trastuzumab to an anthracycline/taxane-based primary systemic chemotherapy regimen increased pCR rates to approximately 60% in patients with HER-2-positive disease. Although all patients in that study had operable disease, the trastuzumab-containing regimen used may be a reasonable approach to be studied in prospective clinical trials evaluating IBC patients. Cristofanilli et al.30 recently evaluated the role of lapatinib, a tyrosine kinase inhibitor that reversibly inhibits both HER-2 and EGFR-1, in combination with paclitaxel in a cohort of patients with HER-2-overexpressing IBC and demonstrated that 95% of patients achieved a clinical response. These results will now be prospectively evaluated in a phase 3 clinical trial.
In conclusion, the results of the current study provide insight into the prognostic significance of HER-2 status and evaluate its predictive power with regard to trastuzumab treatment in a cohort of patients with IBC, providing valuable information into the biology of this very aggressive disease. However, we do acknowledge that although it may be one of the largest studies conducted in IBC patients, the current study is still small and largely underpowered to detect moderate differences in outcome with statistical significance. Although a multimodality approach to the treatment of IBC has certainly improved outcomes, the 5-year RFS and OS rates in our cohort were only 37.8% and 53.1%, respectively, well below those of other forms of breast cancer. The finding that our results demonstrated no difference in RFS between HER-2-positive and HER-2-negative groups in the absence of trastuzumab, but indicated a significant difference when trastuzumab was added after disease recurrence, underlines the major effect of this targeted intervention. It is thus clear that more specific prognostic and predictive markers, with the subsequent development of novel therapeutic regimens, are needed to individualize treatment and thus improve survival outcomes.
Acknowledgments
Supported in part by the Susan G. Komen Foundation, the Nellie B. Connally Fund for Breast Cancer Research, and the Inflammatory Breast Cancer Research Group.
Dr. Gabriel N. Hortobagyi is supported by DAMD17-02-1-0694 01, 2P30 CA016672 28(PP-4), and P50 CA116199-01.
Dr. Ana M. Gonzalez-Angulo is supported by K23CA121994-01 and an American Society of Clinical Oncology Career Development Award.
FUNDING STATEMENT:
This article was supported by NIH. The grant number is P30 CA016672.
REFERENCES
- 1.Jaiyesimi IA, Buzdar AU, Hortobagyi G. Inflammatory breast cancer: a review. J Clin Oncol. 1992;10:1014–1024. doi: 10.1200/JCO.1992.10.6.1014. [DOI] [PubMed] [Google Scholar]
- 2.Chang S, Parker S, Pham T, Buzdar AU, Hursting SD. Inflammatory breast carcinoma incidence and survival: the SEER program of the National Cancer Institute. Cancer. 1998;82:2366–2372. [PubMed] [Google Scholar]
- 3.Cristofanilli M, Buzdar AU, Hortobagyi GN. Update on the management of inflammatory breast cancer. Oncologist. 2003;8:141–148. doi: 10.1634/theoncologist.8-2-141. [DOI] [PubMed] [Google Scholar]
- 4.Buzdar AU, Singeltary SE, Booser DJ, Frye DK, Wasaff B, Hortobagyi GN. Combined modality treatment of stage III and inflammatory breast cancer. M.D Anderson Cancer Center Experience. Surg Oncol Clin N Am. 1995;4:715–734. [PubMed] [Google Scholar]
- 5.Ueno NT, Buzdar AU, Singeltary SE, et al. Combined-modality treatment of inflammatory breast carcinoma: twenty years of experience at M.D Anderson Cancer Center. Cancer Chemother Pharmacol. 1997;40:321–329. doi: 10.1007/s002800050664. [DOI] [PubMed] [Google Scholar]
- 6.Low JA, Berman AW, Steinberg SM, Lippman ME, Swain SM. Long-term follow-up for inflammatory (IBC) and non-inflammatory (NIBC) stage III breast cancer patients treated with combination chemotherapy. Proc Am Soc Clin Oncol. 2002;21:63a. [Google Scholar]
- 7.Early Breast Cancer Trialists’ Collaborative Group Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomized trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 8.Aziz SA, Pervez S, Khan S, Kayani N, Azam SI, Rahbar MH. Case control study of prognostic markers and disease outcome in inflammatory carcinoma of the breast: a unique clinical experience. Breast J. 2001;7:398–404. doi: 10.1046/j.1524-4741.2001.07604.x. [DOI] [PubMed] [Google Scholar]
- 9.McCarthy NJ, Yang X, Linnoila, et al. Microvessel density, expression of estrogen receptor, MIB-1, p53, and c-erbB2 in inflammatory breast cancer. Clin Cancer Res. 2002;8:3857–3862. [PubMed] [Google Scholar]
- 10.Turpin E, Bieche I, Berthaeau P, et al. The increased incidence of ERBB2 over expression and TP53 mutation in inflammatory breast cancer. Oncogene. 2002;21:7593–7597. doi: 10.1038/sj.onc.1205932. [DOI] [PubMed] [Google Scholar]
- 11.Resetkova E, Gonzalez-Angulo AM, Sneige N, et al. Prognostic value of p53, MDM-2, and MUC-1 for patients with inflammatory breast carcinoma. Cancer. 2004;101:913–917. doi: 10.1002/cncr.20465. [DOI] [PubMed] [Google Scholar]
- 12.van Golen KL, Wu ZF, Qiao XT, et al. Rho C GTPase, a novel transforming oncogene for human mammary epithelial cells that partially recapitulates the inflammatory breast cancer phenotype. Cancer Res. 2000;60:5832–6838. [PubMed] [Google Scholar]
- 13.Cabioglu N, Gong Y, Islam R, et al. Expression of growth factor and chemokine receptors: new insights in the biology of inflammatory breast cancer. Ann Oncol. 2007;18:1021–1029. doi: 10.1093/annonc/mdm060. [DOI] [PubMed] [Google Scholar]
- 14.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 15.Borg A, Tandon AK, Sigurdsson H, et al. HER-2/neu amplification predicts poor survival in node-positive breast cancer. Cancer Res. 1990;50:4322–4327. [PubMed] [Google Scholar]
- 16.Winstanley J, Cooke T, Murray GD, et al. The long term prognostic significance of c-erb-2 in primary breast cancer. Br J Cancer. 1991;63:447–450. doi: 10.1038/bjc.1991.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 18.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 19.Green FL, Page DL, Flemming ID, Fritz AG, Balch CM, Haller DG, editors. AJCC cancer staging manual. 6th ed. Springer; New York: 2002. Breast; pp. 225–281. [Google Scholar]
- 20.Anonymous, The World Health Organization The World Health Organization Histological Typing of Breast Tumors. Am J Clin Pathol. (2nd ed.) 1982;78:806–816. doi: 10.1093/ajcp/78.6.806. [DOI] [PubMed] [Google Scholar]
- 21.Black MM, Speer FD. Nuclear structure in cancer tissues. Surg Gynecol Obstet. 1957;105:97–102. [PubMed] [Google Scholar]
- 22.Gong Y, Booser DJ, Sneige N. Comparison of HER2-neu status determined by fluorescence in situ hybridization in primary and metastatic breast carcinoma. Cancer. 2005;103:1763–1769. doi: 10.1002/cncr.20987. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez-Angulo AM, Hennessy BT, Cristofanilli M. Trends for inflammatory breast cancer: is survival improving? Breast Cancer Res Treat. 2006;100(suppl 1):S266. [Google Scholar]
- 24.Sawaki M, Ito Y, Akiyama F, et al. High prevelance of HER-2/neu and p53 overexpression in inflammatory breast cancer. Breast Cancer. 2006;13:172–178. doi: 10.2325/jbcs.13.172. [DOI] [PubMed] [Google Scholar]
- 25.Tanner M, Isola J, Wiklund T, et al. Topoisomerase IIa gene amplification predicts favorable treatment response to tailored and dose-escalated anthracycline-based adjuvant chemotherapy in HER-2/neu-amplified breast cancer: Scandinavian Breast Group Trial 9401. J Clin Oncol. 2006;24:2428–2436. doi: 10.1200/JCO.2005.02.9264. [DOI] [PubMed] [Google Scholar]
- 26.Kuerer HM, Newman LA, Smith TL, et al. Clinical course of breast cancer patients with complete pathological primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999;17:460–469. doi: 10.1200/JCO.1999.17.2.460. [DOI] [PubMed] [Google Scholar]
- 27.Bear HD, Anderson S, Smith RE, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer. National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2006;24:2019–2027. doi: 10.1200/JCO.2005.04.1665. [DOI] [PubMed] [Google Scholar]
- 28.Wolmark N, Wang J, Mamounas E, et al. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from the National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001;30:96–102. doi: 10.1093/oxfordjournals.jncimonographs.a003469. [DOI] [PubMed] [Google Scholar]
- 29.Buzdar AU, Ibrahim NK, Francis D, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23:3676–3685. doi: 10.1200/JCO.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 30.Cristofanilli M, Boussen H, Baselga J, et al. A phase II combination study of lapatinib and paclitaxel as a neoadjuvant therapy in patients with newly diagnosed inflammatory breast cancer (IBC) Breast Cancer Res Treat. 2006;100(suppl 1):S5. [Google Scholar]