Abstract
Women with BRCA1 or 2 mutations are at high risk for breast cancer. For BRCA1, a trend of increasing risk has been associated with increasing downstream (3′) location for mutations compared to the upstream (5′) mutations in the gene. For BRCA2, an increased risk of breast cancer has been associated with mutations outside of the ovarian cancer cluster region (OCCR). We sought to determine the mutation position in BRCA-associated breast cancers and whether or not there was a genotype-phenotype correlation. Breast cancer patients with BRCA1/2 mutations were identified by a search of a prospectively maintained data base. Mutation site, patient, and tumor characteristics were determined through retrospective review. One hundred and sixty-four patients with BRCA1-associated breast cancer and 109 patients with BRCA2-associated breast cancer were identified. Among patients with BRCA1-associated cancers, 86 (52%) had mutations in the 5′ half of the gene. Among patients with BRCA2-associated breast cancers, 40 (37%) had OCCR mutations. Although BRCA1-associated tumors were more likely to be ER/PR- than BRCA2-associated cancers (p < 0.0001), there was no difference in the tumor characteristics among BRCA1 or BRCA2-associated cancers based on mutation location. In this single-institution study, over half of BRCA1-associated and over a third of BRCA2-associated breast cancers were associated with putative lower risk mutations. Although we cannot exclude the possibility that mutations in these regions confer a lower relative risk for breast cancer, vigilance in cancer screening and prevention remains necessary. Further studies in genotype/phenotype correlation are needed to individualize prevention strategies.
Keywords: BRCA, Breast cancer, Genotype
Women with germline BRCA1 or 2 mutations are estimated to have a 45–70% risk of breast cancer by age 70 years (1–4). Therefore, patients with BRCA mutations are offered close surveillance with clinical breast examination, mammography, and magnetic resonance imaging, as well as breast cancer risk-reducing strategies including prophylactic mastectomy.
The identification of BRCA1 and 2 mutations was a major step in personalizing breast cancer risk assessment, screening and risk reduction strategies. Studies are ongoing to determine whether or not certain subgroups of BRCA mutation carriers may be at a higher risk for breast cancer. It has been proposed that certain BRCA mutations may confer a differential risk of future breast cancer development, suggesting an important genotype-phenotype correlation (5,6). In a recent kin-cohort study in Ontario, Risch et al. observed a trend of increasing breast cancer risk associated with increasing downstream location of BRCA1 mutation with a continuous linear trend and a 32% increase in risk associated with each additional 10%, or 559 nucleotides of downstream distance. For BRCA2, compared with no mutation, they found an increased risk associated with mutations outside of the OCCR (RR = 9.2, 95% CI = 5.4–16), but not with mutations in the OCCR (RR = 1.0, 95% CI = 0.18–5.9) (6).
We hypothesized that if BRCA1 5′ mutations and BRCA2 OCCR mutations are indeed associated with a lower risk of breast cancer, BRCA mutations in these regions would be uncommon among breast cancer patients who undergo clinical genetic testing. Thus, in this study, we sought to determine the mutation position in BRCA-associated breast cancers and whether or not there was a correlation between genotype and tumor features.
MATERIALS AND METHODS
Patient Population
We used the prospectively maintained high-risk breast cancer data base from the Clinical Cancer Genetics Program at the University of Texas M.D. Anderson Cancer Center to identify patients with BRCA mutations. We searched for patients who had undergone clinical genetic testing for BRCA1 and 2 between 1997 and 2009. Of the 3587 patients included in the data base, we identified 273 women with breast cancer and a BRCA1 or BRCA2 germ-line mutation. This study was approved by Institutional Review Board at MDACC and the need for informed consent was waived. The data collected included family history of first-degree relatives with breast and ovarian cancer, personal history of breast primaries, and tumor characteristics including age of diagnosis, tumor size, nodal status at time of diagnosis, estrogen receptor (ER), progesterone receptor (PR), HER2 receptor status (both immunohistochemistry and fluorescence in situ hybridization analysis), tumor histology, and BRCA mutation location. For this study, the OCCR region was defined as nucleotides 3035–6629 (5,6).
Statistical Analysis
Clinicopathologic data were tabulated for each mutation type. Known clinical and pathologic characteristics were compared with Chi-Square Analysis or Fisher’s Exact test as appropriate.
RESULTS
One hundred and sixty-four patients with BRCA1-associated breast cancer and 109 patients with BRCA2-associated breast cancer were identified. The patient and tumor characteristics of patients with BRCA1 and BRCA2 mutations are shown in Table 1.
Table 1.
Comparison of Breast Cancer Patients with BRCA1 and BRCA2 Mutations
|
BRCA1 n = 164 |
BRCA2 n = 109 |
|||
|---|---|---|---|---|
| 40 | 20–71 | 41 | 26–67 | |
| Age years (range) | n | % | n | % |
| First-degree relatives w/breast cancer | ||||
| 0 | 77 | 47.0 | 51 | 46.8 |
| 1 | 60 | 36.6 | 43 | 39.4 |
| ≥2 | 27 | 16.5 | 15 | 13.8 |
| First-degree relatives w/ovarian cancer | ||||
| No | 126 | 76.8 | 98 | 89.9 |
| Yes | 38 | 23.2 | 11 | 10.1 |
| Invasive cancer size (cm, range) | 2.2 | (0.1–10) | 1.9 | (0.1–10) |
| ER status | ||||
| Negative | 94 | 69.1 | 19 | 22.4 |
| Positive | 42 | 30.9 | 66 | 77.6 |
| PR status | ||||
| Negative | 99 | 76.7 | 23 | 28.8 |
| Positive | 30 | 23.3 | 57 | 71.2 |
| Nodal status | ||||
| Negative | 87 | 58.8 | 54 | 57.4 |
| Positive | 61 | 41.2 | 40 | 42.6 |
Only patients with known variables are shown.
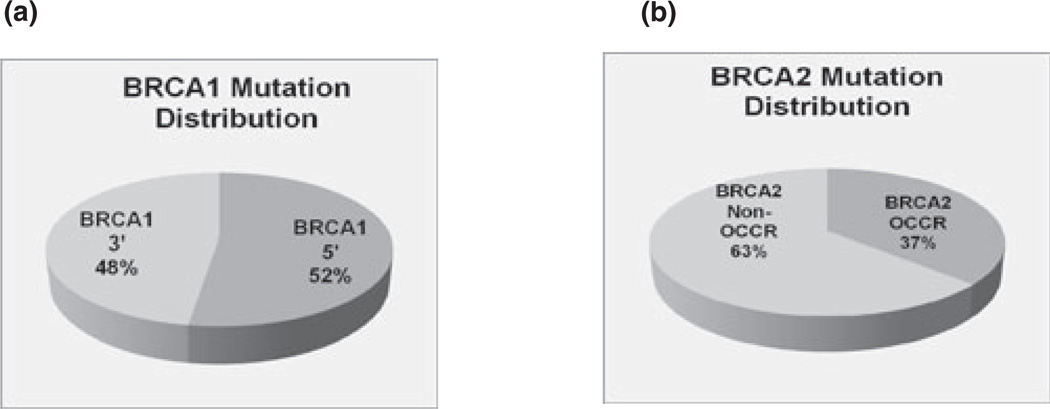
As expected, patients with BRCA1 mutations were more likely to have ER(−) tumors (69.1% for BRCA1 versus 19% for BRCA2, p < 0.0001) and more likely to have PR(−) tumors (76.7% for BRCA1 and 28.8% for BRCA2, p < 0.0001). Patients with BRCA1 mutations also were more likely to have relatives with ovarian cancer (p = 0.0094). Of 164 patients with BRCA1-associated cancers 86 (52.4%) had mutations in the 5′ half of the gene (Fig. 1a). There was no difference in average age, tumor size, ER/PR status, and nodal status between patients with 5′ versus 3′ mutations (Table 2). The results did not differ if we compared patients with mutations in the 5′ two-thirds with the 3′ third.
Figure 1.
Location of mutations in BRCA-associated breast cancers.
Table 2.
Comparison of Breast Cancer Patients with 5′ versus 3′BRCA1 Mutations
|
BRCA1 5' n = 86 |
BRCA1 3' n = 78 |
|||
|---|---|---|---|---|
| 40 | 20–63 | 41 | 25–71 | |
| Age years (range) | n | % | n | % |
| First-degree relatives w/breast cancer | ||||
| 0 | 36 | 41.9 | 41 | 52.6 |
| 1 | 35 | 40.7 | 23 | 29.5 |
| ≥2 | 15 | 17.4 | 14 | 17.9 |
| First-degree relatives w/ovarian cancer | ||||
| No | 69 | 80.2 | 58 | 74.4 |
| Yes | 17 | 19.8 | 20 | 25.6 |
| Invasive cancer size (cm, range) | 2.2 | 0.6–9 | 2.0 | 0.6–7 |
| ER status | ||||
| Negative | 48 | 67.6 | 43 | 66.2 |
| Positive | 23 | 32.4 | 22 | 33.8 |
| PR status | ||||
| Negative | 57 | 81.4 | 42 | 71.2 |
| Positive | 13 | 18.6 | 17 | 28.8 |
| Nodal status | ||||
| Negative | 41 | 51.9 | 47 | 64.4 |
| Positive | 38 | 48.1 | 26 | 35.6 |
Tumor characteristics are only shown for patients with invasive cancer and known characteristics.
Of the 109 patients with BRCA2-associated breast cancers, 40 (36.7%) had OCCR mutations (Fig. 1b). There was no difference in median age, tumor size, ER/PR status, and nodal status between patients with OCCR versus non-OCCR mutations (Table 3). Although more patients with mutations in the OCCR cluster had relatives with ovarian cancer compared with patients with mutations not in the OCCR (15% versus 7.2%), this difference was not statistically significant.
Table 3.
Comparison of Breast Cancer Patients with OCCR versus Non-OCCR BRCA2 Mutations
|
BRCA2 OCCR n = 40 |
BRCA2 Non- OCCR |
|||
|---|---|---|---|---|
| 43 | 27–67 | 40 | 26–58 | |
| Age years (range) | n | % | n | % |
| First-degree relatives w/breast cancer | ||||
| 0 | 20 | 50.0 | 31 | 44.9 |
| 1 | 13 | 32.5 | 30 | 43.5 |
| ≥2 | 7 | 17.5 | 8 | 11.6 |
| First-degree relatives w/ovarian cancer | ||||
| No | 34 | 85.0 | 64 | 92.8 |
| Yes | 6 | 15.0 | 5 | 7.2 |
| Invasive cancer size (cm range) | 1.8 | 0.1–10 | 2 | 0.1–8 |
| ER status | ||||
| Negative | 5 | 17.2 | 14 | 25.9 |
| Positive | 24 | 82.8 | 40 | 74.1 |
| PR status | ||||
| Negative | 9 | 32.1 | 14 | 28.0 |
| Positive | 19 | 67.9 | 36 | 72.0 |
| Nodal status | ||||
| Negative | 19 | 61.3 | 34 | 56.7 |
| Positive | 12 | 38.7 | 26 | 43.3 |
Tumor characteristics are only shown for patients with invasive cancer and known characteristics.
The three most common mutations in our cohort were the Ashkenazi Jewish founder mutations BRCA1 187delAG, BRCA1 5385insC, and BRCA2 6174delT. These mutations are BRCA1 5′, BRCA1 3′, and BRCA2 OCCR mutations, respectively. The tumor characteristics of patients with these genotypes are shown in Table 4.
Table 4.
Comparison of Patients with the Three Most Common Founder Mutations
|
BRCA1 187delAG |
BRCA1 5385insC |
BRCA2 6174delT |
||||
|---|---|---|---|---|---|---|
| Age years (range) | 42 | 20–57 | 40 | 26–61 | 42 | 30–54 |
| n | % | n | % | n | % | |
| First-degree relatives w/breast cancer | ||||||
| No | 8 | 40 | 10 | 71.4 | 7 | 70 |
| Yes | 12 | 60 | 4 | 28.6 | 3 | 30 |
| First-degree relatives w/ovarian cancer | ||||||
| No | 16 | 80 | 11 | 73.3 | 10 | 100 |
| Yes | 4 | 20 | 4 | 26.7 | 0 | 0 |
| Invasive cancer size, cm (range) | 2.6 | 0.7–7 | 3.3 | 1.3–6.5 | 1.7 | 0.1–8.7 |
| ER status | ||||||
| Negative | 8 | 53.3 | 9 | 64.3 | 1 | 11.1 |
| Positive | 7 | 46.7 | 5 | 35.7 | 8 | 88.9 |
| PR status | ||||||
| Negative | 9 | 64.3 | 9 | 75 | 2 | 25 |
| Positive | 5 | 35.7 | 3 | 25 | 6 | 75 |
| Nodal status | ||||||
| Negative | 6 | 37.5 | 8 | 57.1 | 5 | 62.5 |
| Positive | 10 | 62.5 | 6 | 42.3 | 3 | 37.5 |
Tumor characteristics are only shown for patients with invasive cancer and known characteristics.
DISCUSSION
To personalize risk reduction strategies, it is critical to be able to accurately assess an individual’s breast cancer risk. Currently, women who are carriers of deleterious BRCA mutations are considered to be at high risk of breast cancer development and are closely screened and offered surgical risk reduction. It would be important to determine whether or not there is a genotype-phenotype correlation that can assist in identifying BRCA carriers that are at low risk of breast cancer development. It has been reported that 5′ mutations in BRCA1, and OCCR mutations of BRCA2 are associated with a lower risk of breast cancer development than mutations in other regions (6). We thus sought to determine the mutation position in BRCA-associated breast cancers at our institution. We found that over half of BRCA1-associated breast cancers and over a third of BRCA2-associated breast cancers were associated with putative lower risk mutation positions.
The effect of genotype on relative breast and ovarian cancer risk has been assessed in several studies to date. Gayther et al. have studied the risk of breast and ovarian cancer related to mutation location, and reported that truncating mutations in the first two-thirds of the coding region of BRCA1 are associated with a higher ovarian cancer risk than breast cancer risk (7). In another study, Gayther et al. reported that mutations in OCCR are associated with a higher ovarian cancer risk, compared to breast cancer risk (5). Lubinski et al. confirmed that families with ovarian cancer were more likely to harbor mutations in the OCCR than elsewhere in the BRCA2 gene (OR = 2.21; p = 0.0002) (8). Risch et al. reported that for BRCA1, there is a trend of increasing risk associated with increasing downstream (i.e., 3′) location of mutations compared to the upstream (i.e., 5′) mutations (6). For BRCA2, an increased risk of breast cancer was associated with mutations outside of the “ovarian cancer cluster region” (OCCR). These studies suggest that patients with 5′ BRCA1 mutations and BRCA OCCR mutations may not be at increased risk for breast cancer as currently thought. However, it would be critical to validate these results, and determine if BRCA mutation location can indeed be used for further risk stratification.
We found that over half of BRCA1-associated breast cancers and over a third of BRCA2-associated breast cancers were associated with putative lower risk mutation positions. These results suggest that even these lower risk regions are associated with a significant number of BRCA-associated breast cancers, and argue against using genotype for risk counseling in the absence of better validated risk assessment tools. However, we already have some additional clinical-pathologic information that can be used for risk counseling in BRCA mutation carriers. A rapid decrease in the relative risk of BRCA-associated breast cancer is noted with increasing age (9). Family history is important even among BRCA mutation carriers; breast cancer risk is higher among first-degree relatives of probands with breast cancer rather than ovarian cancer (10). Furthermore, an oophorectomy not only decreases ovarian cancer risk but also significantly decreases breast cancer risk. However, further risk stratification among BRCA mutation carriers is still necessary. Along these lines, major effort has been made into identifying other genetic modifiers of breast cancer risk among BRCA carriers (11,12). It is likely in the near future we will be able to more accurately predict an individual’s breast cancer risk by combining genotype and clinical characteristics.
Our study has several limitations. First, it is of a limited sample size. Due to our small sample size, we focused on site of mutation, but did not further classify by type of mutation (e.g., missense versus truncating mutations). Second, it is of a retrospective nature, with patients identified through a prospectively maintained high-risk breast cancer data base from Clinical Cancer Genetics. Patients referred to Clinical Cancer Genetics may have a stronger family history, or earlier age of onset cancer, and thus, more penetrant genotypes may be identified. Third, we studied BRCA mutations identified in patients with breast cancer and a deleterious BRCA mutation. By study design, we do not know the prevalence of selected BRCA mutations in populations that form our referral basin. The relative risk of specific BRCA mutations would be best assessed in studies of BRCA mutation carriers with long follow-up. Our study design does not allow us to determine the relative risk of breast cancer conferred by BRCA mutations at different locations, and we cannot exclude the possibility that the relative risk of breast cancer conferred by BRCA1 5′, and BRCA2 OCCR mutations is less. We also do not have any information on genotype of other genes that may be modifiers of risk.
In conclusion, in this study, we sought to determine the mutation position in BRCA-associated breast cancers and whether or not there was a genotype-phenotype correlation. We found that a substantial portion of BRCA-associated breast cancers had mutations in the putative lower risk mutation positions; over half of BRCA1-associated breast cancers were associated with mutations in the 5′ portion of BRCA1 and over a third of BRCA2-associated breast cancers were associated with the OCCR region. Although we cannot exclude the possibility that patients with mutations in these regions have a lower relative risk for breast cancer, vigilance in cancer screening and prevention remains necessary. Further studies in genotype/phenotype correlation are needed to individualize cancer prevention strategies.
Acknowledgments
The authors thank DaRonia Taylor for her assistance in manuscript preparation and Julie M. Eggington, Ph.D. from Myriad Genetics (Salt lake City, UT), for her assistance in genotype classification.
Footnotes
DISCLOSURE
None.
REFERENCES
- 1.Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brose MS, Rebbeck TR, Calzone KA, Stopfer JE, Nathanson KL, Weber BL. Cancer risk estimates for BRCA1 mutation carriers identified in a risk evaluation program. J Natl Cancer Inst. 2002;18:1365–1372. doi: 10.1093/jnci/94.18.1365. [DOI] [PubMed] [Google Scholar]
- 3.Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1998;62:676–689. doi: 10.1086/301749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Struewing JP, Hartge P, Wacholder S, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med. 1997;336:1401–1408. doi: 10.1056/NEJM199705153362001. [DOI] [PubMed] [Google Scholar]
- 5.Gayther SA, Mangion J, Russell P, et al. Variation of risks of breast and ovarian cancer associated with different germline mutations of the BRCA2 gene. Nat Genet. 1997;15:103–105. doi: 10.1038/ng0197-103. [DOI] [PubMed] [Google Scholar]
- 6.Risch HA, McLaughlin JR, Cole DE, et al. Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: a kin-cohort study in Ontario, Canada. J Natl Cancer Inst. 2006;98:1694–1706. doi: 10.1093/jnci/djj465. [DOI] [PubMed] [Google Scholar]
- 7.Gayther SA, Warren W, Mazoyer S, et al. Germline mutations of the BRCA1 gene in breast and ovarian cancer families provide evidence for a genotype-phenotype correlation. Nat Genet. 1995;11:428–433. doi: 10.1038/ng1295-428. [DOI] [PubMed] [Google Scholar]
- 8.Lubinski J, Phelan CM, Ghadirian P, et al. Cancer variation associated with the position of the mutation in the BRCA2 gene. Fam Cancer. 2004;3:1–10. doi: 10.1023/B:FAME.0000026816.32400.45. [DOI] [PubMed] [Google Scholar]
- 9.Chen HX, Mooney M, Boron M, et al. Phase II multicenter trial of bevacizumab plus fluorouracil and leucovorin in patients with advanced refractory colorectal cancer: an NCI Treatment Referral Center Trial TRC-0301. J Clin Oncol. 2006;24:3354–3360. doi: 10.1200/JCO.2005.05.1573. [DOI] [PubMed] [Google Scholar]
- 10.Gronwald J, Huzarski T, Byrski B, et al. Cancer risks in first degree relatives of BRCA1 mutation carriers: effects of mutation and proband disease status. J Med Genet. 2006;43:424–428. doi: 10.1136/jmg.2005.036921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antoniou AC, Beesley J, McGuffog L, et al. Common breast cancer susceptibility alleles and the risk of breast cancer for BRCA1 and BRCA2 mutation carriers: implications for risk prediction. Cancer Res. 2010;70:9742–9754. doi: 10.1158/0008-5472.CAN-10-1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antoniou AC, Wang X, Fredericksen ZS, et al. A locus on 19p13 modifies risk of breast cancer in BRCA1 mutation carriers and is associated with hormone receptor-negative breast cancer in the general population. Nat Genet. 2010;42:885–892. doi: 10.1038/ng.669. [DOI] [PMC free article] [PubMed] [Google Scholar]