Article first published online 3 April 2015.
Key Words: inflammatory bowel disease, Crohn's disease, ulcerative colitis, androgens, testosterone, Nurses' Health Study
Abstract
Background:
Androgens, which are known to be altered by exogenous hormone use, have recently been linked to alterations of the gut microbiome and mucosal immune function. No study has evaluated the association between circulating levels of androgens and risk of Crohn's disease (CD) and ulcerative colitis (UC).
Methods:
We conducted a nested case–control study of women enrolled in the Nurses' Health Study and Nurses' Health Study II who provided a blood specimen. Cases of CD and UC were each matched to 2 controls. Prediagnosis plasma levels of dehydroepiandrosterone sulfate, testosterone, and sex hormone–binding globulin were measured. We examined the association of each analyte with risk of CD or UC using conditional logistic regression models.
Results:
Compared with women in the lowest quintile of testosterone, the multivariable-adjusted odds ratios for CD were 0.86 (95% confidence interval, 0.39–1.90) for women in the second quintile, 0.49 (95% confidence interval, 0.21–1.15) for the third quartile, 0.22 (0.08–0.65) for the fourth quintile, and 0.39 (95% confidence interval, 0.16–0.99) for the highest quintile (Plinear trend = 0.004). In contrast, we did not observe a consistent association between prediagnostic testosterone and risk of UC (Plinear trend = 0.84). We also did not observe any association between plasma levels of sex hormone–binding globulin or dehydroepiandrosterone sulfate and risk of UC or CD (all Plinear trends > 0.10).
Conclusions:
Among women, prediagnostic circulating testosterone is associated with a lower risk of CD but not UC. Further studies to understand the biological mechanisms by which endogenous androgens may mediate the etiopathogenesis of CD are warranted.
Crohn's disease (CD) and ulcerative colitis (UC), collectively known as inflammatory bowel disease (IBD), are chronic inflammatory disorders of the gastrointestinal tract affecting nearly 1.4 million Americans. Although the exact pathophysiology of the disease remains largely unknown, it is thought that IBD occurs as a result of inappropriate immune response to the intestinal microbiome in genetically susceptible individuals.1 In turn, the composition of human gut microbiome seems to be closely linked to environmental factors, such as diet, stress, and medications.2–4
Genetic studies have identified a number of common variants associated with risk of CD and UC.5–7 Collectively, the contribution of these variants to risk of IBD is modest. Therefore, identification of novel exogenous and endogenous factors with potential biological links to human immune function and/or the microbiome will likely better shape our overall understanding of the complex interplay of the environment and genetics on risk of CD and UC. An association between oral contraceptives (OCs) and risk of CD has been consistently demonstrated.8 Recently, we confirmed this association in 2 prospective cohorts of women.9 In addition, we have also shown an association between use of menopausal hormone therapy (MHT) and risk of UC.10 Although the exact biological mechanism underlying these associations is unknown, these medications are known to influence endogenous circulating levels of sex hormones.11–13 In turn, compelling data have linked these hormones to immune function and/or gut microbiome composition and function.14,15 To date, no studies have evaluated the association between endogenous sex hormones and risk of CD or UC.
We therefore sought to examine the association between prediagnostic levels of circulating androgens and risk of CD and UC in 2 large ongoing prospective cohort studies of U.S. women, the Nurses' Health Study (NHS) and NHSII. With more than 20 years of biennially updated and validated data on use of OC, menopausal hormones, diet, and medical diagnoses, as well as archived blood specimens, these cohorts offered us the unique opportunity to examine the association between prediagnostic measures of plasma androgens and subsequent risk of CD and UC.
METHODS
Study Population
The NHS is a prospective cohort that began in 1976 when 121,700 U.S. female registered nurses, aged 30 to 55 years, completed a mailed questionnaire. Follow-up questionnaires are mailed every 2 years to update health information. In 1989, a parallel cohort, the NHS II, enrolled 116,430 U.S. female nurses between the ages of 25 and 42 years. These women have been followed with similar biennial questionnaires. Follow-up for these participants has consistently exceeded 95%.
In 1989 to 1990, 32,826 NHS participants (aged 43–69 yr) returned a blood sample on ice packs by overnight courier and completed a short questionnaire.16 Between 1996 and 1999, 29,611 NHSII participants (aged 32–54 yr) provided blood samples and completed a short questionnaire in a similar protocol.17 Blood samples were processed on receipt and subsequently stored in the vapor phase of liquid nitrogen freezers. Our study is a nested case–control study of women with available blood samples in the 2 large prospective cohorts of NHS and NHSII. Institutional review board at the Brigham and Women's Hospital approved this study.
Ascertainment of Cases and Controls
We have previously detailed our methods for confirming self-reported cases of CD and UC.9,18,19 In brief, since 1976, participants have reported diagnoses of UC or CD through an open-ended response on biennial surveys. In addition, participants were asked about diagnoses of UC since 1982 and CD since 1992. In NHS II, we have specifically queried participants about diagnoses of both CD and UC since 1993. When a diagnosis was reported on any biennial questionnaire, a supplementary questionnaire and related medical records were requested and reviewed by 2 gastroenterologists blinded to exposure information.
We excluded participants who subsequently denied either the diagnosis on the supplementary questionnaire or permission to review their records. Data were extracted on diagnostic tests, histopathology, anatomical location of disease, and disease behavior. Using standard criteria,20–23 UC diagnosis was based on a typical clinical presentation for >4 weeks and endoscopic or surgical pathological specimen consistent with UC (e.g., evidence of colitis). CD diagnosis was based on a typical clinical history for >4 weeks and endoscopy or radiologic evaluation demonstrating small bowel findings, or surgical findings consistent with CD combined with pathology suggesting transmural inflammation or granuloma. Disagreements were resolved through consensus. Among those women from whom we received adequate medical records, the case confirmation rate for IBD was 78%.9
From among participants who provided a blood specimen, we matched 83 CD cases (NHS = 59, NHSII = 24) and 91 UC cases (NHS = 59, NHSII = 32) that were diagnosed after blood collection to 2 controls on age, menopausal status at the time of blood collection, month of blood collection, fasting status, and use of MHT at the time of blood collection. In NHSII, premenopausal blood samples that were timed to the luteal phase of the menstrual cycle were also matched on the day of the luteal phase (date of next period minus date of blood draw). Consistent with previous studies, we identified and excluded 2 outliers: 1 control with a dehydroepiandrosterone sulfate (DHEAS) <15 μg/dL and 1 control with a testosterone = 4.5 ng/dL.24 Thus, our final analysis included 83 CD cases matched to 165 controls and 91 UC cases matched to 181 controls.
Ascertainment of Exposures
Our primary exposures of interest were androgens, which included testosterone, DHEAS, and sex hormone–binding globulin (SHBG). All laboratory assays were conducted by personnel blinded to case–control status at the Mayo Clinic (Rochester, MN). Samples were assayed for testosterone in 3 batches by liquid chromatography–tandem mass spectrometry; DHEAS and SHBG were measured by a solid-phase, chemiluminescent enzyme immunoassay (Siemens Healthcare Diagnostics, Deerfield, IL). Masked replicate quality control samples (10% of the samples) were included in each batch to assess coefficients of variation. Overall coefficients of variation within batches were 6.4% to 9.6% for DHEAS, 4.0% to 5.1% for testosterone, and 4.3% to 6.3% for SHBG. Free testosterone was calculated using the formula described by Södergard et al.25
Assessment of Other Covariates
On each biennial questionnaire, women were asked about pertinent lifestyle factors, including body weight, smoking status, parity, and use of OCs and MHT. Participants' self-report of body weight, height, and use of OCs have been previously validated.26,27 At baseline, we collected data on age at menarche in both cohorts. On each questionnaire, menopausal status was determined by asking whether the participants' menstrual periods had ceased permanently and, if so, at what age and for what reason (occurring naturally or after radiation therapy or surgery). If menopause was due to surgery, the participant was asked to report the number of ovaries removed. Self-reported type of menopause and age at time of menopause was highly accurate compared with medical records.28
Information on physical activity was also collected every 2 to 4 years. Participants' self-report of physical activity and use of OC have been previously validated.26,27 Intake of dietary vitamin D was assessed using validated, self-administered, semiquantitative food frequency questionnaires administered in 1991, 1995, and 1999 in NHSII and 1984, 1986, and 1990 in NHS before blood collections. For these analyses, data on weight and menopause status were taken directly from the short questionnaires administered at the time of blood collection; data from other covariates were obtained from the general questionnaires that were completed closest to the time of blood draw.
Statistical Analysis
We examined the possibility that there is a nonlinear association between androgens and risk of CD and UC using a previously reported nonparametric cubic spline method.29 This method is unique in allowing for controlling for covariates. It also allows stepwise selection among spline variables. The output is the set of P-values from the likelihood ratio tests for nonlinearity, a linear relation, and any relation, as well as a graph of the predicted incidence rate, with or without its confidence band. Using this method, the likelihood ratio test comparing models with linear terms with those with spline terms were not statistically significant (All Pcomparisons > 0.40), indicating that the relationship between androgens and risk of CD and UC is linear. As these analyses are particularly sensitive to outliers, we performed further sensitivity analyses removing observations beyond 3 interquartile ranges. In these analyses, the likelihood ratio test comparing models with linear terms with those with spline terms continue to not be statistically significant (All Pcomparisons > 0.30). Based on these findings, we used continuous and quintile categories of androgens in our main analyses.
We calculated cutoffs for quintiles based on the distribution of each analyte within controls separately for each cohort (NHS and NHSII) to account for differences in the distribution of menopausal status between studies. We used conditional logistic regression to estimate the odds ratio (OR) and 95% confidence interval (CI). Multivariable analyses were adjusted for physical activity, body mass index (BMI), total dietary vitamin D intake, OC use, parity, menopause status (include type of menopause), cohort, and smoking because these variables have previously been associated with CD or UC or may influence the levels of androgens. We did not include use of nonsteroidal inflammatory drugs, appendectomy, age at menarche, and age at menopause because these variables did not alter our effect estimates. We also evaluated if the association between androgens and risk of CD and UC varied by menopause status (premenopausal or postmenopausal), current MHT (yes or no), BMI (≤25 or >25 kg/m2), and OC use (never or ever).
We used SAS version 9.3 (Cary, NC) for these analyses. All P-values were 2-sided and <0.05 was considered statistically significant.
RESULTS
Within both cohorts, our analysis included 83 cases of CD matched to 165 controls and 91 cases of UC matched to 181 controls with prediagnostic blood specimens (Table 1). The mean time between blood collection and diagnosis of either CD or UC or index date for controls was 5.0 years (SD = 5.1). At the time of blood collection, the age range was 36 to 68 years for the CD case–control set and 36 to 69 years for the UC case–control set. There were no significant differences in baseline characteristics of CD and UC cases with their matched controls (Table 1). The median concentration of testosterone was significantly lower in CD cases (16.0 ng/dL) compared with controls (19.0 ng/dL, Pdifference = 0.01) (Table 1). There were no significant differences in plasma concentration of SHBG and DHEAS comparing cases of CD with controls (all Pdifference > 0.30). Comparing UC cases with their matched controls, there were no significant differences in plasma concentrations of any androgens (all Pdifference > 0.40).
TABLE 1.
Baseline Characteristics of Cases and Controls at the Time of Blood Collectiona
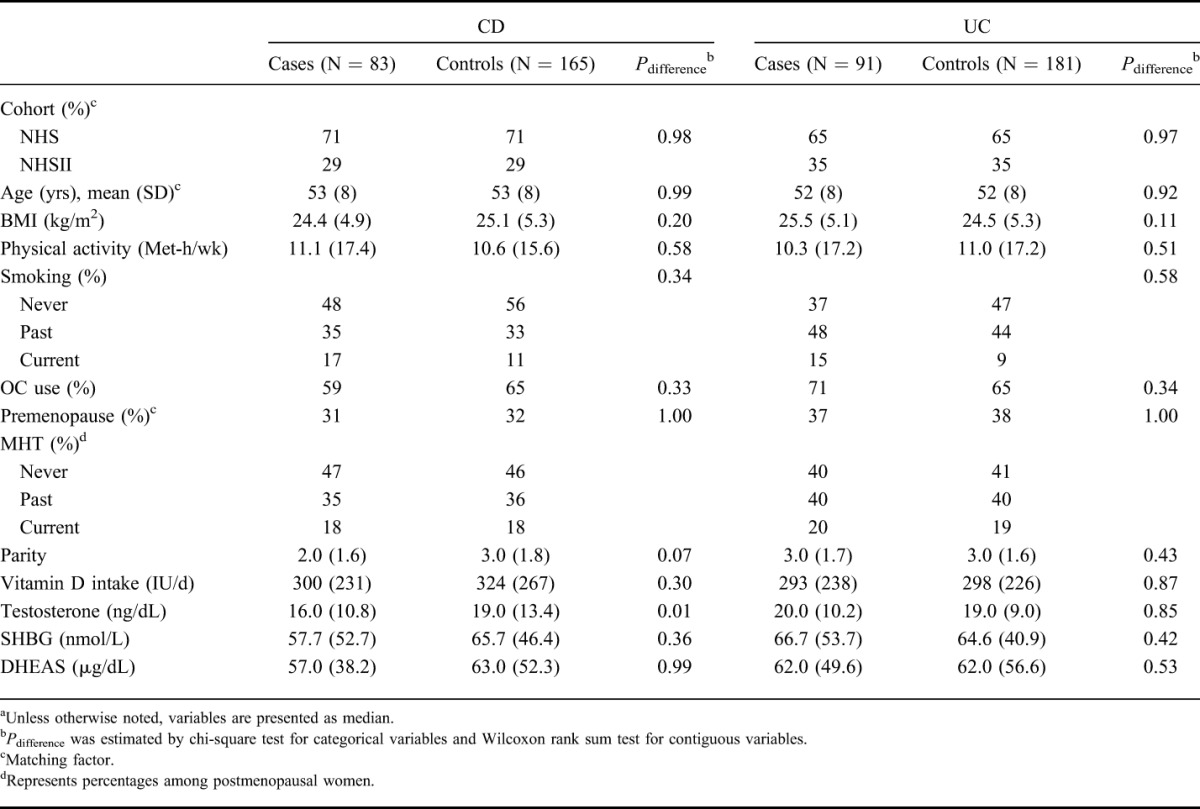
Compared with matched controls, the risk of CD decreased with higher plasma levels of testosterone (Ptrend = 0.004) (Table 2). Specifically, compared with the lowest quintile of plasma levels of testosterone, the multivariable-adjusted ORs of CD were 0.86 (95% CI, 0.39–1.90) for the second quintile, 0.49 (95% CI, 0.21–1.15) for the third quintile, 0.22 (95% CI, 0.08–0.65) for the fourth quintile, and 0.39 (95% CI, 0.16–0.99) for the highest quintile (Table 2). Although the risk of CD seemed to decrease with higher plasma levels of SHBG, the trend did not reach statistical significance (Ptrend = 0.11). We also evaluated the effect of total plasma testosterone on risk of CD using plasma testosterone as a continuous variable and found that for every 10 ng/mL increase in plasma levels of testosterone, the risk of CD decreases by 15% (OR, 0.85; 95% CI, 0.74–0.97).
TABLE 2.
Risk of CD According to Quintiles of Plasma Androgen Concentrations
As the level of free testosterone is strongly influenced by SHBG, the inverse association between testosterone and risk of CD was significantly attenuated in analysis of free testosterone, calculated from SHBG and total testosterone, and risk of CD (Ptrend = 0.24). In addition, we did not find an association between plasma levels of DHEAS (Ptrend = 0.46) and risk of CD.
We explored the possibility that changes in endogenous hormones due to subclinical disease may explain our observed association. Thus, we repeated our analyses after excluding cases of CD in whom blood samples were collected less than 2 years before diagnosis. Compared with women in the lowest quintile of plasma testosterone level, the multivariable-adjusted ORs of CD were 0.68 (95% CI, 0.24–1.89) among women in the second quintile, 0.24 (95% CI, 0.07–0.84) among women in the third quintile, 0.10 (95% CI, 0.02–0.49) among women in the fourth quintile, and 0.42 (95% CI, 0.14–1.27) among women in the highest quintile (Ptrend = 0.02).
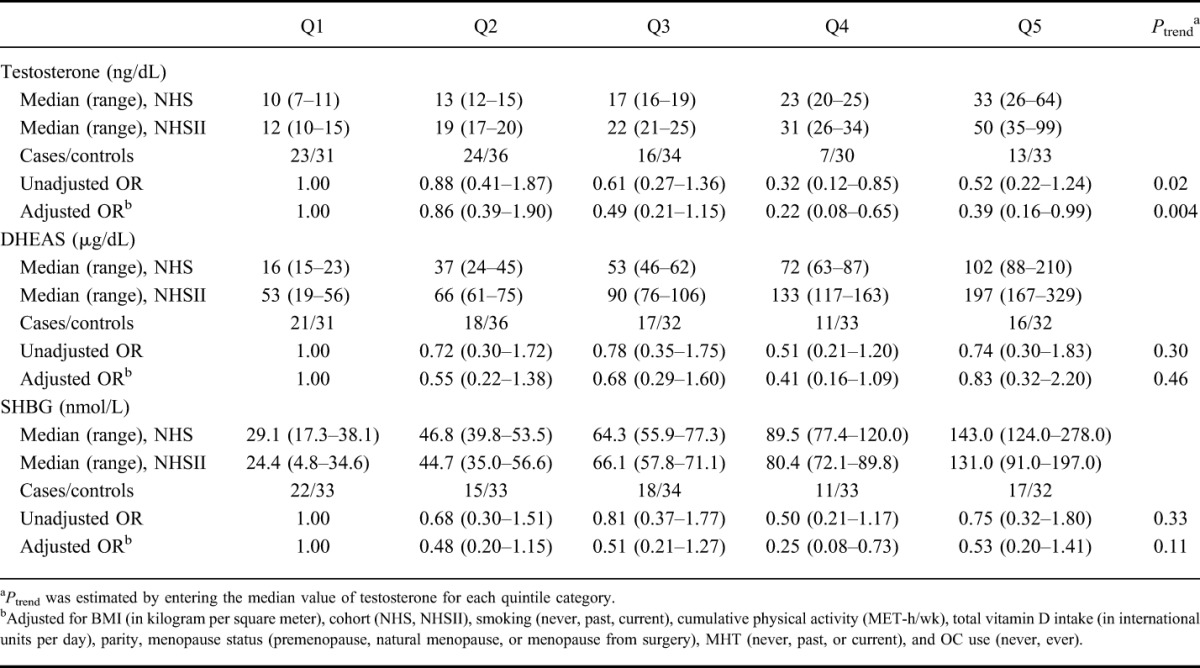
In contrast, plasma levels of testosterone or free testosterone were not associated with risk of UC (Ptrend = 0.84 and 0.25, respectively) (Table 3). Although, the risk of UC seemed to increase with increasing plasma levels of SHBG, the trend did not reach statistical significance (Ptrend = 0.09). Compared with the women in the lowest quintile of plasma levels of SHBG, the multivariable-adjusted ORs of UC were 1.00 (95% CI, 0.44–2.28) among women in the second quintile, 1.43 (95% CI, 0.55–3.69) among women in the third quintile, 1.01 (95% CI, 0.39–2.58) among women in the fourth quintile, and 2.80 (95% CI, 1.05–7.49) among women in the highest quintile. We did not find an association between plasma levels of DHEAS (Ptrend = 0.41) and risk of UC.
TABLE 3.
Risk of UC According to Quintiles of Plasma Androgen Concentrations
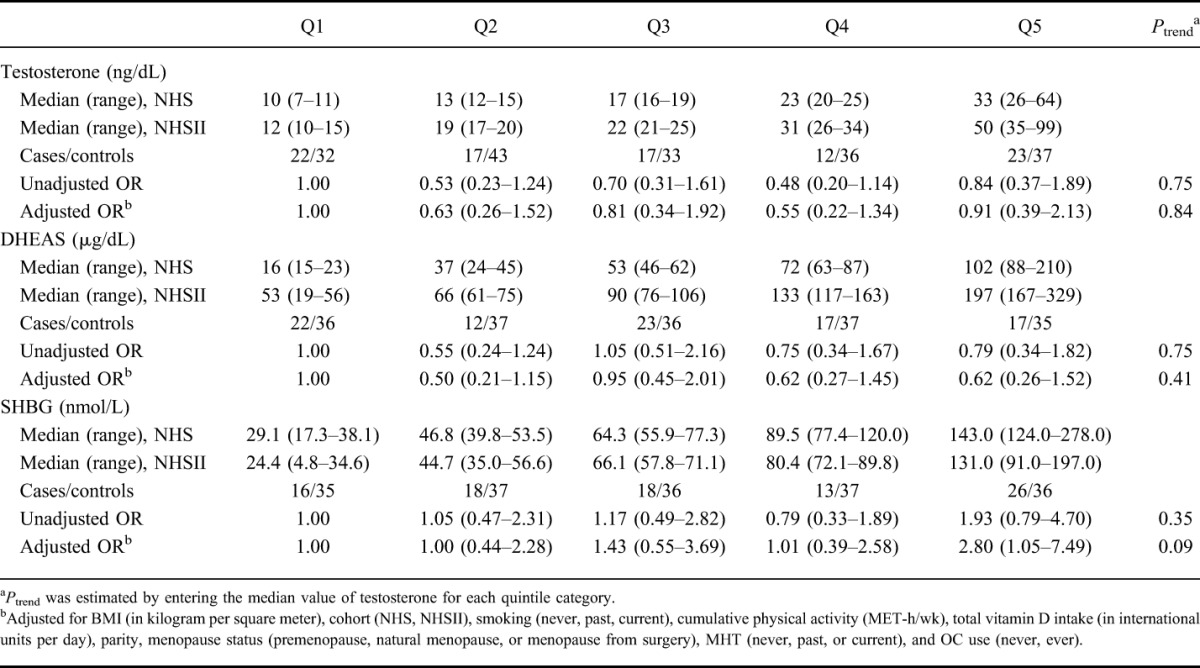
We explored the possibility that the association of testosterone with risk of CD may be modified by other putative risk factors (Table 4). The association of plasma testosterone and risk of CD did not seem to be modified by OC use, menopausal status, or BMI (all Pinteractions > 0.30). Although the effect of circulating testosterone on risk of CD seemed to be modified by use of MHT, a formal statistical test for interaction did not reach statistical significance (Pinteraction = 0.06). Specifically, among postmenopausal women who were not using MHT at the time of blood draw, the multivariable-adjusted OR of CD for every 10 ng/dL increase in testosterone was 0.66 (95% CI, 0.45–0.95). In contrast, among postmenopausal women who were using MHT at the time of blood collection, the multivariable-adjusted OR of CD for every 10 ng/dL increase in testosterone was 1.17 (95% CI, 0.88–1.56). As levels of endogenous testosterone changes significantly with age, we also assessed whether the effect of testosterone on risk of CD varies according to age as a continuous variable and observed no effect modification (Pinteraction = 0.62). We could not evaluate the possibility that smoking may modify the effect of androgens on risk of CD due to low number of CD cases among smokers (n = 13). However, among never smokers, the multivariable-adjusted OR of CD for every 10 ng/dL increase in testosterone was 0.63 (95% CI, 0.48–0.83). The association of testosterone on risk of UC did not vary by menopausal status, BMI, or use of MHT or OCs (Table 4).
TABLE 4.
Risk of CD and UC According to Plasma Testosterone Concentrations in Selected Strataa
DISCUSSION
In this large, prospective, nested case–control study, we observed that higher levels of prediagnostic plasma testosterone are associated with lower risk of CD but not UC. In addition, there was a suggestion that the association was more evident among individuals who did not concurrently use MHT. There was also a trend for a direct association between plasma levels of SHBG and risk of UC as well as an inverse association between plasma levels of SHBG and risk of CD. To our knowledge, no previous study has investigated the link between endogenous levels of plasma androgens and risk of CD and UC. A previous study from our cohort has shown an association between OC use and risk of CD, which has been supported by many other studies.8,9 OC use has been shown to reduce endogenous levels of testosterone.11,13 Thus, the consistent association of OC and risk of incident CD may be mediated by the biological consequences of lower circulating testosterone. Moreover, the apparent benefit of higher circulating testosterone on risk of CD may be attenuated by the use of high doses of exogenous estrogen in the form of MHT.
Although our understanding of the pathogenesis of CD and UC remains incomplete, the discovery of distinct genetic susceptibility loci for both diseases points to potential diverging biological pathways that may be differentially influenced by endogenous levels of testosterone.5 In addition, CD and UC have immunologically distinct gastrointestinal mucosal cytokine profiles, with mucosal inflammation in CD primarily mediated by Th1-related cytokines and UC mediated by Th2-related cytokines. The potential differential effect of sex hormones including estrogen and testosterone on Th1- and Th2-mediated disease processes may partially explain the specific link between endogenous testosterone and risk of CD. In particular, testosterone has been shown to modulate immune function, including cytokine production. In animal models, endogenous levels of testosterone are linked to reduction in expression of Toll-like receptor 4 on macrophages, which play a fundamental role in pathogen recognition and innate immunity.15 In addition, recent animal data suggest that gut commensal microbes may modulate levels of endogenous testosterone, leading to development of autoimmune diseases.14 Thus, our observed association between endogenous testosterone and development of CD may be biologically mediated by a complex interaction between endogenous hormones, the gut microbiome, and immune function.
Previously, we have shown an association between MHT and increased risk of UC.10 Although the exact biological mechanism of this is unknown, estrogen compounds modify colonic barrier function,30,31 which is a biological pathway that may be uniquely related to pathogenesis of UC.32 Thus, modification of distinct biological processes by OC use compared with MHT may have potentially divergent effects on the pathogenesis of CD and UC. Of note, the apparent lack of association between free testosterone and risk of CD in our study is likely explained by the significant influence of plasma levels of SHBG on free testosterone levels, with higher SHBG levels being associated with lower free testosterone.
Our study has several notable strengths. First, the availability of detailed and validated information on BMI, use of OC or MHT, physical activity, smoking, and other reproductive factors allowed us to control for a number of potential confounders that may affect our observed associations. Second, we have confirmed all of the cases of CD and UC through medical record review, an advantage over studies that rely on self-reported or diagnoses codes that may not be accurate. Third, blood samples were collected before diagnosis of CD and UC, allowing us to assess prediagnosis levels of androgens in relation to risk of disease.
We acknowledge several limitations. First, plasma levels of androgens were based on a single measurement that may not be an accurate reflection of long-term endogenous androgen levels. However, the intraclass correlation over 3 years for these androgens in luteal phase ranged from 0.73 (for testosterone) to 0.89 (for SHBG and DHEAS) in premenopausal women33 and from 0.88 (for testosterone and DHEAS) to 0.92 (for SHBG) in postmenopausal women,34 suggesting that a single measurement reasonably reflects long-term levels. Second, unlike NHSII, blood samples collected from premenopausal women in NHS were not timed according to menstrual phase (luteal versus follicular). However, androgen levels do not vary substantially by menstrual phase.35,36 In addition, our findings were consistent across the 2 cohorts of NHS and NHSII, and premenopausal cases accounted for only 30% of all cases and controls. Third, we acknowledge that because of our small number of cases, we had limited power to detect more modest associations (e.g., SHBG). In addition, because of our sample size, the results from our stratified analyses that endogenous levels of androgens may modulate the effect of exogenous hormones on risk of CD should be interpreted with caution. Finally, only 10 participants (controls and cases) were taking OCs at the time of blood collection and therefore we were unable to fully evaluate the hypothesis that OCs through modulating the endogenous level of androgens alter risk of developing CD. We estimated that at least 40% of our participants would have had to be on OCs at the time of blood collection in order for us to have nearly 80% power to detect the potential mediation effect of testosterone on the associations between OCs and risk of CD.
In conclusion, we show that prediagnosis levels of circulating total testosterone are associated with a lower risk of CD but not UC among women. This finding may, at least in part, explain the consistent association observed between use of OC and subsequent risk of CD. Although the exact mechanism underlying the association between exogenous and endogenous sex hormones and risk of CD is largely unknown, we believe that these initial studies justify the need for further translational studies carried out at the intersection of epidemiology, sex hormones, the gut microbiome, and immune function. In particular, further investigation into evaluating the complex interaction between exogenous and endogenous sex hormones and community structure of human gut microbiome on risk and progression of CD are warranted. Finally, whether exogenous and endogenous sex hormones also play a role in IBD progression is yet to be determined and is the topic of future research.
ACKNOWLEDGMENTS
The authors would like to thank the Mayo Clinic Laboratory for preforming measurements of androgens in our cohorts.
Authors Ccontributions: Study concept and design, acquisition of data, statistical analysis, interpretation of data, and drafting of the manuscript, H. Khalili; acquisition of data and critical revision of the manuscript, A. N. Ananthakrishnan; acquisition of data and critical revision of the manuscript, G. G. Konijeti, S. S. Tworoger, S. E. Hankinson; acquisition of data and critical revision of the manuscript for important intellectual content, L. M. Higuchi; study concept and design, acquisition of data, and critical revision of the manuscript, J. M. Richter; acquisition of data; and critical revision of the manuscript, S. S. Tworoger; acquisition of data and critical revision of the manuscript, S. E. Hankinson; study concept and design, analysis and interpretation of data, drafting of the manuscript, and critical revision of the manuscript, A. T. Chan.
Footnotes
Supported by R01 CA137178, R01 CA050385, P01 CA87969, CA49449, CA67262, P30 DK043351, K23 DK099681, K08 DK064256, K24 098311, and K23 DK091742. A. T. Chan is supported by a senior investigator grant from the Crohn's and Colitis Foundation of America. H. Khalili is supported by a career development award from the American Gastroenterological Association and by the National Institute of Diabetes and Digestive and Kidney Diseases (K23 DK099681). L. M. Higuchi is supported by National Institute of Diabetes and Digestive and Kidney Diseases (K08 DK064256).
A. N. Ananthakrishnan is a member of the scientific advisory board for Prometheus, Inc., and Janssen, Inc., Abbvie, and Cubist Pharmaceuticals. J. M. Richter is a consultant for policy analysis. A. T. Chan has served as a consultant for Bayer Healthcare, Pfizer, Inc., and Pozen, Inc. The remaining authors have no conflicts of interest to disclose.
The institutional review board at the Partners Healthcare and Brigham and Women's Hospital approved this study.
Requests for access to data, statistical code, questionnaires, and technical processes may be made by contacting the corresponding author at hkhalili@mgh.harvard.edu.
REFERENCES
- 1.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Filippo C, Cavalieri D, Di Paola M, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107:14691–14696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luna RA, Foster JA. Gut brain axis: diet microbiota interactions and implications for modulation of anxiety and depression. Curr Opin Biotechnol. 2014;32C:35–41. [DOI] [PubMed] [Google Scholar]
- 4.Fricke WF, Maddox C, Song Y, et al. Human microbiota characterization in the course of renal transplantation. Am J Transplant. 2014;14:416–427. [DOI] [PubMed] [Google Scholar]
- 5.Jostins L, Ripke S, Weersma RK, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson CA, Boucher G, Lees CW, et al. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet. 2011;43:246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franke A, McGovern DP, Barrett JC, et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nat Genet. 2010;42:1118–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornish JA, Tan E, Simillis C, et al. The risk of oral contraceptives in the etiology of inflammatory bowel disease: a meta-analysis. Am J Gastroenterol. 2008;103:2394–2400. [DOI] [PubMed] [Google Scholar]
- 9.Khalili H, Higuchi LM, Ananthakrishnan AN, et al. Oral contraceptives, reproductive factors and risk of inflammatory bowel disease. Gut. 2013;62:1153–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khalili H, Higuchi LM, Ananthakrishnan AN, et al. Hormone therapy increases risk of ulcerative colitis but not Crohn's disease. Gastroenterology. 2012;143:1199–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan MF, Dowsett M, Folkerd E, et al. Past oral contraceptive and hormone therapy use and endogenous hormone concentrations in postmenopausal women. Menopause. 2008;15:332–339. [DOI] [PubMed] [Google Scholar]
- 12.Tworoger SS, Missmer SA, Barbieri RL, et al. Plasma sex hormone concentrations and subsequent risk of breast cancer among women using postmenopausal hormones. J Natl Cancer Inst. 2005;97:595–602. [DOI] [PubMed] [Google Scholar]
- 13.Zimmerman Y, Eijkemans MJ, Coelingh Bennink HJ, et al. The effect of combined oral contraception on testosterone levels in healthy women: a systematic review and meta-analysis. Hum Reprod Update. 2014;20:76–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Markle JG, Frank DN, Mortin-Toth S, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339:1084–1088. [DOI] [PubMed] [Google Scholar]
- 15.Rettew JA, Huet-Hudson YM, Marriott I. Testosterone reduces macrophage expression in the mouse of toll-like receptor 4, a trigger for inflammation and innate immunity. Biol Reprod. 2008;78:432–437. [DOI] [PubMed] [Google Scholar]
- 16.Hankinson SE. Circulating levels of sex steroids and prolactin in premenopausal women and risk of breast cancer. Adv Exp Med Biol. 2008;617:161–169. [DOI] [PubMed] [Google Scholar]
- 17.Tworoger SS, Sluss P, Hankinson SE. Association between plasma prolactin concentrations and risk of breast cancer among predominately premenopausal women. Cancer Res. 2006;66:2476–2482. [DOI] [PubMed] [Google Scholar]
- 18.Khalili H, Huang ES, Ananthakrishnan AN, et al. Geographical variation and incidence of inflammatory bowel disease among US women. Gut. 2012;61:1686–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khalili H, Ananthakrishnan AN, Konijeti GG, et al. Physical activity and risk of inflammatory bowel disease: prospective study from the Nurses' Health Study cohorts. BMJ. 2013;347:f6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loftus EV, Jr, Silverstein MD, Sandborn WJ, et al. Crohn's disease in Olmsted County, Minnesota, 1940-1993: incidence, prevalence, and survival. Gastroenterology. 1998;114:1161–1168. [DOI] [PubMed] [Google Scholar]
- 21.Loftus EV, Jr, Silverstein MD, Sandborn WJ, et al. Ulcerative colitis in Olmsted County, Minnesota, 1940-1993: incidence, prevalence, and survival. Gut. 2000;46:336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fonager K, Sorensen HT, Rasmussen SN, et al. Assessment of the diagnoses of Crohn's disease and ulcerative colitis in a Danish hospital information system. Scand J Gastroenterol. 1996;31:154–159. [DOI] [PubMed] [Google Scholar]
- 23.Moum B, Vatn MH, Ekbom A, et al. Incidence of inflammatory bowel disease in southeastern Norway: evaluation of methods after 1 year of registration. Southeastern Norway IBD Study Group of Gastroenterologists. Digestion. 1995;56:377–381. [DOI] [PubMed] [Google Scholar]
- 24.Tworoger SS, Lee IM, Buring JE, et al. Plasma androgen concentrations and risk of incident ovarian cancer. Am J Epidemiol. 2008;167:211–218. [DOI] [PubMed] [Google Scholar]
- 25.Södergard R, Backstrom T, Shanbhag V, et al. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16:801–810. [DOI] [PubMed] [Google Scholar]
- 26.Hunter DJ, Manson JE, Colditz GA, et al. Reproducibility of oral contraceptive histories and validity of hormone composition reported in a cohort of US women. Contraception. 1997;56:373–378. [DOI] [PubMed] [Google Scholar]
- 27.Troy LM, Hunter DJ, Manson JE, et al. The validity of recalled weight among younger women. Int J Obes Relat Metab Disord. 1995;19:570–572. [PubMed] [Google Scholar]
- 28.Colditz GA, Stampfer MJ, Willett WC, et al. Reproducibility and validity of self-reported menopausal status in a prospective cohort study. Am J Epidemiol. 1987;126:319–325. [DOI] [PubMed] [Google Scholar]
- 29.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–561. [DOI] [PubMed] [Google Scholar]
- 30.Braniste V, Jouault A, Gaultier E, et al. Impact of oral bisphenol A at reference doses on intestinal barrier function and sex differences after perinatal exposure in rats. Proc Natl Acad Sci U S A. 2010;107:448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Looijer-van Langen M, Hotte N, Dieleman LA, et al. Estrogen receptor-beta signaling modulates epithelial barrier function. Am J Physiol Gastrointest Liver Physiol. 2011;300:G621–G626. [DOI] [PubMed] [Google Scholar]
- 32.Lees CW, Barrett JC, Parkes M, et al. New IBD genetics: common pathways with other diseases. Gut. 2011;60:1739–1753. [DOI] [PubMed] [Google Scholar]
- 33.Missmer SA, Spiegelman D, Bertone-Johnson ER, et al. Reproducibility of plasma steroid hormones, prolactin, and insulin-like growth factor levels among premenopausal women over a 2- to 3-year period. Cancer Epidemiol Biomarkers Prev. 2006;15:972–978. [DOI] [PubMed] [Google Scholar]
- 34.Hankinson SE, Manson JE, Spiegelman D, et al. Reproducibility of plasma hormone levels in postmenopausal women over a 2-3-year period. Cancer Epidemiol Biomarkers Prev. 1995;4:649–654. [PubMed] [Google Scholar]
- 35.Longcope C, Franz C, Morello C, et al. Steroid and gonadotropin levels in women during the peri-menopausal years. Maturitas. 1986;8:189–196. [DOI] [PubMed] [Google Scholar]
- 36.Rannevik G, Jeppsson S, Johnell O, et al. A longitudinal study of the perimenopausal transition: altered profiles of steroid and pituitary hormones, SHBG and bone mineral density. Maturitas. 1995;21:103–113. [DOI] [PubMed] [Google Scholar]


