Abstract
Introduction
Attention deficit hyperactivity disorder (ADHD) is associated with substantial functional impairment in children and in adults. Many individuals with ADHD have clear neurocognitive deficits, including problems with visual attention, processing speed, and set shifting. ADHD is etiologically complex, and although genetic factors play a role in its development, much of the genetic contribution to ADHD remains unidentified.
Methods
We conducted clinical and neuropsychological assessments of 294 individuals (269 with ADHD) from 163 families (48 multigenerational families created using genealogical reconstruction, 78 affected sib pair families, and 37 trios) from the Central Valley of Costa Rica (CVCR). We used principal components analysis (PCA) to group neurocognitive and behavioral variables using the subscales of the Child Behavior Checklist (CBCL) and 15 neuropsychological measures, and created quantitative traits for heritability analyses.
Results
We identified seven cognitive and two behavioral domains. Individuals with ADHD were significantly more impaired than their unaffected siblings on most behavioral and cognitive domains. The verbal IQ domain had the highest heritability (92%), followed by auditory attention (87%), visual processing speed and problem solving (85%), and externalizing symptoms (81%).
Conclusions
The quantitative traits identified here have high heritabilities, similar to the reported heritability of ADHD (70–90%), and may represent appropriate alternative phenotypes for genetic studies. The use of multigenerational families from a genetically isolated population may facilitate the identification of ADHD risk genes in the face of phenotypic and genetic heterogeneity.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a neurodevelopmental disorder that is characterized by persistent and impairing problems with inattention, hyperactivity, and impulsivity [1, 2]. Although the reported prevalence of the disorder varies greatly depending on the design of the study [3–5], worldwide prevalence rates have been estimated to be between 4 and 7%, and rates in the US between 7–12% (with higher rates reported for males and in older children and adolescents) [4–6]. ADHD is now recognized to persist into adulthood for up to 70% of youth [2, 7–11] and recent epidemiological estimates suggest that 4–5% of adults suffer from the disorder [7, 12].
ADHD is associated with multiple negative outcomes; affected individuals show higher rates of adaptive impairment and co-morbid psychiatric disorders, including disruptive behavior, substance use, anxiety, and depressive disorders, among others [7, 13, 14]. This prevalence of functional impairment, coupled with high rates of psychiatric comorbidity, persistence into adulthood in a significant proportion of cases, and in some cases, treatment resistance, results not only in high individual and family costs, but also in significant costs to health care systems and society as a whole [13].
ADHD has a complex multifactorial etiology, in which genetics play an important role. Although environmental factors are postulated to play a role in the development of ADHD, twin, adoption, and family studies have consistently demonstrated a substantial genetic predisposition for this disorder [14–16]. In fact, twin studies suggest that the heritability of ADHD is between 70–80% [14], among the highest reported for a neuropsychiatric disorder [5, 17]. There is also an increased rate of ADHD among first-degree relatives, and a 57% increased risk of ADHD among children of parents with ADHD [18]. However, despite the high heritability, the underlying genetic etiology of ADHD has yet to be fully elucidated.
The executive function abnormalities most commonly implicated in ADHD include deficits in inhibitory control, working memory, set shifting, and processing speed [14, 19–24]. From a quantitative perspective, identifying combinations of observed neurocognitive dysfunction and behavioral traits that represent potential vulnerability traits for ADHD may be of use in further elucidating the underlying etiology of this complex and heterogeneous disorder. For example, both impulsivity and attention, which are core symptoms of ADHD, have quantifiable cognitive and behavioral correlates; these may represent distinct aspects of ADHD, or they may represent alternative aspects of the same underlying vulnerability. In turn, these vulnerability traits, whether behavioral or cognitive or both, may be more closely related to underlying genetic architecture than the surface phenotype symptoms of ADHD, representing “intermediate phenotypes”. In fact, some neuropsychological traits related to the neurocognitive domains implicated in ADHD have heritabilities that are similar to, or in some cases exceed, those reported for ADHD (70–90%) [25]. In particular, visual attention and processing speed, as well as general cognitive ability, have reported heritabilities in the range of 70–80% (reviewed in Doyle et al, 2005) [26, 27]. In addition, some aspects of neurocognitive function have been associated with particular genetic variants that have previously been suggested as susceptibility genes for ADHD [28]. For example, processing speed, set-shifting, and cognitive impulsivity in children with ADHD have been found to be linked to 7 repeat allele carriers for the DRD4 gene [14, 28]. Therefore, identifying behavioral and neurocognitive intermediate phenotypes may help to distinguish genetically homogenous subgroups of individuals with ADHD, facilitating the identification of susceptibility genes [25].
Another method of identifying susceptibility genes for presumably polygenic psychiatric disorders such as ADHD relies on the use of so-called “population isolates”, i.e, relatively genetically homogenous populations that can be traced back to a small group of founders [29, 30]. One such population isolate has been identified in the Central Valley region of Costa Rica (CVCR), a mixed urban-suburban-rural area surrounding and including the country’s capital city, San José [30]. Despite its importance as a research population for a variety of complex traits, including bipolar disorder, Tourette Syndrome, schizophrenia, and obsessive-compulsive disorder [31–39], there have not yet been studies examining dimensional behavioral and neuropsychological profiles among those with ADHD in Costa Rica, or in the genetically isolated population of the CVCR.
The aim of the present study is to describe the behavioral profiles and neuropsychological characteristics of a sample of children with ADHD from the CVCR population isolate and to examine the heritability of the identified profiles. We hypothesized that such an analysis would help to identify heritable intermediate phenotypes for ADHD that may be useful for genetic studies. The identification of homogeneous subgroups based on such intermediate phenotypes in a sample recruited from this genetically homogenous population could facilitate the identification of risk genes of significant effect. Essentially, this approach seeks to combine the potential advantages of both the population isolate method and quantitative analytic approaches. The relationship between ADHD and executive function has been previously examined in population isolates (including a study of adolescents with and without ADHD from Northern Finland and a family-based study from Antioquia, Colombia) [19, 40]. There are few published studies examining the heritability of neuropsychological indices associated with ADHD in general, however, and similarly, few studies describing genetically-relevant aspects of ADHD in a population isolate, the others having been done in Colombia (a population that is very similar genetically to the CVCR) [40–42] and Finland [43].
There is some evidence to suggest that the cognitive impairments seen in ADHD may actually arise independently from the behavioral symptoms or that they may represent a separate ADHD subtype[25, 44]. However, studies have also shown that cognitive impairment is also seen in unaffected relatives of individuals with ADHD, suggesting that they may represent a core underlying endophenotype for ADHD[26, 44]. Similarly, studies examining the response of the behavioral and cognitive aspects of ADHD to treatment are mixed. Some studies suggest that the behavioral symptoms of ADHD improve with treatment and the cognitive symptoms are less responsive[45, 46], but a recent meta-analysis shows consistent improvement in cognitive symptoms across domains with stimulant treatment[47].
We chose to include both behavioral measures and neuropsychological measures in a single analytic approach in this study because we hypothesized that these variables would not necessarily segregate independently in an ADHD sample, but rather that in some (but not all) cases, both behavioral measures and neurocognitive measures would tap into the same underlying construct (for example, impulse control). We additionally hypothesized that we would identify one or more quantitative trait(s) that were both strongly correlated with ADHD and also highly heritable in our sample, replicating at least some of the findings from a previously published study examining the heritability of neuropsychological profiles in ADHD families from Colombia[40], in addition to identifying new heritable quantitative traits. Such traits would thus represent ideal phenotypes for genetic studies aimed at identifying susceptibility loci for ADHD and ADHD-associated neurocognitive dysfunction.
Methods
Subjects
Study subjects consisted of a clinical sample of children recruited between 2002 and 2010 specifically for genetic studies of ADHD from the Central Valley of Costa Rica (CVCR). To ensure that they belonged to the relevant population isolate, all subjects were required to have at least 5 traceable grandparents from the region [48]. Participants were recruited from outpatient clinics, physicians, schools, and newspaper advertisements. Because the parent genetic study followed an affected sib pair design, the focus of recruitment was on families where multiple children were known or thought to have ADHD. During the initial screening process, or during the assessment of the identified proband, the research team took a family history and identified other potentially affected children. These children were also recruited and assessed for ADHD. In some cases, the second child was found to be unaffected, and in some cases, additional children were not available or refused to participate in the study. All available siblings were assessed using the battery of measures described below. DNA for genetic studies was obtained from all available participants, including parents. When possible, self-report data on ADHD symptoms were collected from parents; however, parents were not formally assessed. Participants were excluded if they had an IQ <70, autism, epilepsy, or known genetic disorders. Participants who were age 18 and older gave informed consent, while those 17 and under gave assent; parent permission for participation was also obtained for those under 18. This study was approved by the relevant institutional review boards in both Costa Rica and the United States.
Diagnostic Assessments
All clinical and neuropsychological assessments were conducted in Spanish, using validated Spanish translations of the instruments when available. Lifetime occurrence of ADHD and other psychiatric disorders was assessed using the Diagnostic Interview Scale for Children, Version IV (DISC IV) and supplemented with a clinical interview by a child and adolescent psychiatrist. In a small number of cases, the Schedule for Affective Disorders and Schizophrenia for School-Aged Children (K-SADS) was used in lieu of the DISC-IV. Parents and a teacher were asked to complete the Swanson, Nolan, and Pelham Version IV (SNAP-IV) rating scale for ADHD symptoms, and parents were also asked to complete the Child Behavior Checklist (CBCL) [49]. The CBCL is a standardized parent-report inventory that assesses a variety of behavioral and emotional domains in pediatric populations. Age and gender adjusted T scores are provided for eight domains of functioning: Withdrawn, Somatic Complaints, Anxious/Depressed, Social Problems, Thought Problems, Attention Problems, Delinquent Behavior and Aggressive Behavior. These domains can be divided into two categories: Internalizing Problems (Withdrawn, Somatic Complaints, Anxious/Depressed) and Externalizing Problems (Delinquent Behavior and Aggressive Behavior) [26]. There is evidence to suggest that children with ADHD, regardless of subtype, have more emotional and behavioral problems as measured by the CBCL than do children without ADHD [50].
Hospital and clinic medical records were obtained when available to supplement the questionnaire and clinical interview data. Diagnoses were made according to best estimate approaches, where consensus diagnoses were determined using DSM IV criteria by two senior investigators (DC, JJM) using all available clinical information [51]. Following our previously reported diagnostic system for genetic studies of ADHD [52], participants were given a definite ADHD diagnosis when all the DSM IV criteria were met, and a probable diagnosis when, based on the clinical information, the participant lacked one symptom item for full criteria, but functional impairment and age of onset criteria were met.
Neuropsychological assessments
Our neurocognitive battery was based on the previous literature on ADHD at the time of the initiation of the project, taking into consideration the availability of the relevant measures in Spanish and the feasibility of implementing them in our population. The following neuropsychological tests were administered to the probands and their siblings: the Wechsler Intelligence Scale for Children, third edition (WISC III) (in 5 cases the WISC-IV was used, and in the 5 adult cases, the Wechsler Adult Intelligence Scale (WAIS) was used), the Stroop Test, the Trail Making Test (TMT) parts A and B, the Rey-Osterrieth Complex Figure Test (ROCFT), and the Continuous Performance Test (CPT).
The WISC/WAIS is a widely used measure of overall intelligence, and consists of several measures of cognitive function divided into four primary domains: verbal comprehension (information, similarities, vocabulary, comprehension), perceptual organization (picture completion, picture arrangement, block design, object assembly), freedom from distractibility (digit span, arithmetic) and processing speed (symbol search, coding). Previous studies of children with ADHD have suggested a wide range of neurocognitive abnormalities as measured by the WISC, with abnormal results in at least one cognitive measure in up to 50% of ADHD participants, compared to 5–10% of controls [53–55]. Perhaps the most consistent finding is that the full scale IQ is approximately 9 points lower in individuals with ADHD than in control participants [54]. The subtest scale scores were the variables of interest for this study.
The Stroop test measures selective attention, cognitive flexibility or set shifting, and processing speed. The outcome variable used in this study was the interference T-score, which reflects cognitive flexibility and the ability to inhibit a response in order to follow the instructions of the test. Children with ADHD have been shown to have slow processing speeds and increases in the Stroop interference effect-score [56].
In the ROCFT, subjects are asked to complete a line drawing consisting of multiple complex components while viewing the figure (copy), by memory immediately after viewing (immediate recall), and then again by memory after 20 to 30 minutes (delayed recall). The ROCFT is a measure of visual spatial abilities and visual memory. The primary measures used in this study were the time spent in each of tasks (copy, immediate recall, and delayed recall) and the total scores for each task, representing the accuracy of the drawing made by the participant. Abnormalities in copy organization, recall style, and perseverative errors have all been reported on the ROCFT for children with ADHD [57, 58].
The TMT evaluates visual attention, cognitive set shifting, and information processing speed. Part A is primarily a measure of attention, while part B measures set shifting ability. The measure of interest for this study was the ratio of the Z score for part B divided by the Z score for part A, which measures set shifting ability while controlling for attention and information processing speed. Deficits in set shifting have been seen in children with ADHD, and as a result, the TMT has been proposed as part of a brief clinical screening battery for ADHD [59, 60].
The CPT measures sustained and selective attention, information processing speed, and impulsivity. CPT variables of interest for this study included the perseveration T-score (measuring the responses that take place less than 100ms after a signal happens), the hit rate T score (measuring reaction time), the omissions T score (which measures errors of omission), and the commissions T score (measuring errors of commission). A recent meta-analysis of CPT performance showed that individuals with ADHD made more errors, particularly errors of omission, but also errors of commission, and had slower and more variable reaction times than did controls [61].
Genealogical reconstructions
Using the comprehensive civil registry that documents births, deaths, and marriages throughout Costa Rica for the past several hundred years, along with supplemental church records documenting births, deaths, and marriages, our team created multigenerational families by identifying previously unknown genealogical connections between the apparently unrelated nuclear ADHD families in our study. An example is given in Figure 1. These extended pedigrees are particularly useful for assessing heritability of quantitative traits, as they allow for the comparison of traits between closely related (i.e., siblings) and more distantly related individuals (e.g., cousins, second cousins, etc).
Figure 1.
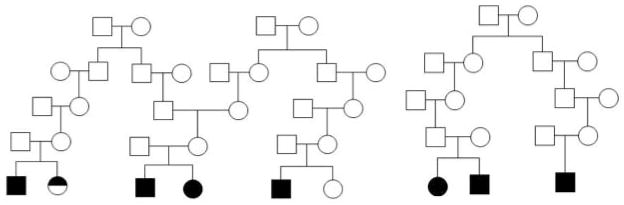
Examples of multigenerational pedigrees created through genealogical reconstruction. Circles = female; square = male. Full black symbol = definite ADHD; half black symbol = probable ADHD.
Data analysis
Demographic profiles
Clinical data were analyzed using Stata 11.2 [62]. Demographic and clinical differences between subjects with ADHD and unaffected siblings were examined using generalized estimating equations, controlling for familial relationships. Gender, and age at interview were calculated and the ADHD and unaffected siblings groups were compared. Neuropsychological and behavioral (CBCL) differences between subjects with ADHD and the unaffected sibling group were also assessed using generalized estimating equations, controlling for familial relationships.
Factor analyses
In order to minimize problems of multiple testing and to create quantitative cognitive and/or behavioral domains with relevance to ADHD, principal components analyses (PCA) were conducted in Mplus using the CBCL domain scores and the neuropsychological measures described above (Table I). A robust maximum likelihood approach was used to control for potential skewness of the variables.
Table I.
Principal components factor loadings for behavioral (CBCL) and neuropsychological measures in participants with ADHD. Bold indicates factor loadings ≥ 0.40. Gray indicates the variables included within a particular factor, determined based on the strongest factor loading for that variable.
| Verbal IQ | Internalizing symptoms | Externalizing symptoms | Information processing speed & visual problem solving | Visual sustained attention | Visuospatial ability | Impulsivity/ Perseveration | |
|---|---|---|---|---|---|---|---|
| Variable | |||||||
| WISC Information scale score | 0.77 | 0.04 | −0.23 | −0.02 | −0.13 | 0.22 | −0.05 |
| WISC Similarities scale score | 0.77 | −0.09 | 0.00 | 0.23 | 0.11 | −0.03 | −0.03 |
| WISC Arithmetic scale score | 0.56 | −0.08 | 0.10 | 0.26 | 0.01 | 0.18 | 0.03 |
| WISC Vocabulary scale score | 0.81 | −0.19 | 0.04 | 0.14 | −0.10 | 0.05 | 0.07 |
| WISC Comprehension scale score | 0.73 | 0.04 | 0.03 | −0.13 | −0.01 | 0.12 | −0.07 |
| CBCL Anxious/ Depressed T score | −0.03 | 0.80 | 0.31 | 0.10 | 0.01 | −0.12 | 0.02 |
| CBCL Withdrawn/Depressed T score | −0.04 | 0.78 | −0.06 | −0.12 | 0.09 | 0.10 | −0.12 |
| CBCL Somatic complaints T score | −0.02 | 0.64 | 0.25 | −0.11 | 0.02 | −0.23 | −0.10 |
| CBCL Social problems T score | −0.14 | 0.72 | 0.36 | 0.01 | 0.05 | −0.03 | 0.14 |
| CBCL Attention problems T score | −0.15 | 0.44 | 0.37 | 0.06 | 0.15 | −0.06 | 0.24 |
| CBCL Thought problems T score | 0.01 | 0.42 | 0.57 | −0.06 | 0.13 | −0.18 | 0.00 |
| CBCL Rule-breaking T score | 0.03 | 0.13 | 0.82 | −0.07 | 0.06 | 0.08 | 0.03 |
| CBCL Aggressive behavior T score | −0.08 | 0.32 | 0.81 | −0.02 | 0.07 | 0.01 | −0.03 |
| WISC Picture Completion scale score | 0.31 | −0.21 | 0.36 | 0.40 | 0.03 | 0.37 | 0.18 |
| WISC Coding scale score | 0.18 | −0.04 | −0.07 | 0.62 | −0.26 | 0.20 | −0.14 |
| WISC Picture Arrangement scale score | 0.40 | 0.12 | −0.15 | 0.42 | −0.01 | 0.25 | 0.11 |
| WISC Symbol Search scale score | 0.34 | 0.05 | −0.01 | 0.69 | −0.23 | 0.03 | 0.03 |
| Stroop Interference T score | −0.15 | −0.09 | −0.18 | 0.63 | 0.06 | 0.01 | −0.09 |
| CPT Errors of omission T score | −0.00 | 0.14 | 0.05 | 0.01 | 0.80 | −0.15 | 0.07 |
| CPT Hit reaction time T score | −0.08 | 0.02 | 0.10 | −0.16 | 0.86 | −0.03 | −0.19 |
| WISC Block Design scale score | 0.47 | −0.04 | 0.13 | 0.30 | −0.07 | 0.51 | −0.17 |
| WISC Object Assembly scale score | 0.23 | −0.03 | 0.18 | 0.36 | 0.01 | 0.64 | −0.07 |
| Rey-O immediate copy Z score | 0.09 | −0.09 | −0.08 | −0.04 | −0.13 | 0.86 | 0.05 |
| CPT Perseverance T score | 0.02 | −0.05 | 0.04 | −0.04 | 0.48 | 0.05 | 0.71 |
| CPT Errors of commission T score | −0.03 | 0.01 | −0.03 | −0.03 | −0.27 | −0.02 | 0.89 |
A varimax-orthogonal rotation was utilized in order to determine the loadings of the neuropsychological variables in each factor. Multiple models were examined, and the most parsimonious fit was determined based on examination of the scree plot, the eigenvalues, the variable factor loadings, and the fit statistics. Only factors with eigenvalues >1 were retained. Variables were included in the factor in which they had the highest loading. Variables were assigned to a factor if they had a loading of ≥ 0.4 on that factor. Variables that loaded on multiple factors were assigned to the factor for which they had the strongest loading. Only factors with ≥ 2 variables loading at ≥ 0.40 were retained; variables that consistently loaded as the single variable within a factor; i.e., did not load with other items, were excluded from the PCA. In subsequent analyses, these items were analyzed separately. To determine model fit, we examined the root mean square error of approximation (RMSEA), chi square, the Akaike Information Criterion (AIC) and the Bayesian Information Criterion (AIC). An RMSEA of <0.05 indicates a good fit, while smaller AIC and BIC indicate better fitting models than those with larger AIC and BIC values. We also directly compared models to assess fit (e.g., a 4 factor model compared to a 3 factor model); p-values ≤0.05 suggest that the higher order factor solution is a better fit than the lower order solution, while p-values >0.05 suggest that the higher order solution does not improve on the lower.
Secondary sensitivity analyses were also conducted to determine the stability of the final model. Although we chose to use all ADHD affected individuals in the original model to increase the size and thus the power of the sample, we also conducted PCA in a subsample of individuals that was limited to one ADHD affected individual per family. Similarly, we conducted PCA using a geomin (oblique) rotation to determine the robustness of the solutions when the variables were allowed to be correlated. Finally, because of concerns about potential bias due to variable differences in scale, we also conducted exploratory PCA using z scores instead of scale or t scores.
Creation of factor sum scores
Following determination of the final model from the PCA, sum scores were created for each identified factor. These variables were created by dividing the number of items endorsed by the total number of items for which there were available data within a particular factor, thus accounting for any missing data on individual measures. The advantage of a sum score over a traditional factor score is that the sum score does not rely on factor loadings, but rather on number of symptoms endorsed within a particular factor, and thus is generalizable to multiple datasets[63]. In contrast, factor scores are specific to the model and sample in which they were generated, and are thus not generalizable to other samples. Sum scores, which are quantitative variables representing the constructs identified in each factor, and are generalizable to other datasets, were then used in generalized estimating equations to explore the association between ADHD (outcome variable) and the behavioral or cognitive domains represented by the factors (predictor variables), correcting for age, gender, and familial relationships. Sum scores were also used as quantitative traits in the heritability analyses described below.
Heritability analyses
Heritability estimates were obtained for each factor using the Sequential Oligogenic Linkage Analysis Routine (SOLAR) statistical package, version 7.2.5 [64]. SOLAR uses a variance components approach to give an estimate of additive genetic variance (VA) and the environmental variance (VE) based on all information available from pedigree family members. The heritability that results from this calculation (h2, defined by VA/(VA+VE)) is based on a maximum-likelihood-based variance decomposition approach, utilizes all available family relationship information (e.g, sibships, cousinships, etc) using kinship coefficients, and does not assume a particular inheritance model. Proband status, age, and gender were controlled for in all analyses. The shared genetic and environmental variance of ADHD (as a categorical variable and using quantitative SWAN scores) with each factor was assessed with bivariate analyses, and the proportion of shared variance due to environment (RhoE) and additive genetic factors (RhoG) was calculated [65, 66].
Results
Sample characteristics
Of the 365 participants in the parent genetic study, 294 individuals from 213 nuclear families had sufficient neuropsychological data for inclusion in this study. Using genealogical reconstruction, we were able to link 98 of these nuclear families to construct 48 multigenerational families incorporating ≥ 2 nuclear families. Of the remainder, 78 families had at least two siblings, while 37 families had only one child. The total number of independent families was 163.
The ages of the participants ranged from 6 to 28 years old (mean: 10 and SD: 3.5). 90.6% were 14 years old or younger. 91.5% of the sample (N = 269) met diagnostic criteria for ADHD, while 8.5% were unaffected (N = 25). About 70% of those with ADHD diagnoses met criteria for the combined type (N = 189), 3% met criteria for hyperactive impulsive type (N = 8) and 27% met criteria for inattentive subtype (N = 72). Individuals with ADHD were approximately 2 years younger than the unaffected siblings, on average, and were more likely to be male.
Neuropsychological characteristics
Subjects with ADHD had a mean full-scale IQ of 91.1 (SD = 15), while the unaffected siblings had a mean full-scale IQ of 97.1 (SD = 10.8), (z = −1.98, p = 0.05). Subjects with ADHD had mean verbal IQ scores of 91.8 (SD = 13.6) compared with a mean score of 94.5 (SD = 10.9) for unaffected siblings (z = −1.12, p = 0.26); mean performance IQ scores were 92.7 (SD = 14.7) for those with ADHD and 99.5 (SD=12.4) for unaffected siblings (z = 2.16, p = 0.03). Participants with ADHD scored significantly lower on the Picture Arrangement subscale of the WISC than did unaffected siblings (scale scores of 7.8 and 9.3 respectively, z= −2.04, p=0.04), as well as on the ROCFT Immediate Recall (scale scores of 11.7 and 15.4 respectively, z= 2.25, p=0.03), and on the CPT errors of commission measure (scores of 50.8 and 41.8 respectively, z= 2.20, p=0.03). They showed better performance relative to their unaffected siblings on the CPT errors of omission measure (scores of 52.8 and 67.2 respectively, z= −2.65, p=0.008). See Supplemental Table I for details.
As expected, total CBCL T scores were also significantly different between ADHD-affected individuals and their unaffected siblings (Supplemental Table I). The CBCL total T score for ADHD-affected participants was 66.6 (SD = 8.3) compared to a total T score of 57.1 (SD = 8.9) in the unaffected individuals (t = 125.1; p < 0.0001). All CBCL domains with the exception of the anxious/depressed and withdrawn/depressed domains showed statistically significant differences between ADHD and unaffected participants. The most prominent were increased scores among individuals with ADHD in the attention problems, aggression, and rule breaking domains of the CBCL (See Supplement).
Factor analysis
We explored models with between three and nine factors using PCA and examined a variety of metrics to determine the best fit. The eight and nine factor models did not converge, and therefore were not further examined. The initial exploratory models included all variables described above; however, the TMT (which is a measure of cognitive set shifting) and the WAIS Digit Span subtest (which is a measure of auditory attention) were subsequently dropped from the factor analysis, as they consistently loaded uniquely into separate factors of their own. These measures were subsequently examined as separate variables in the heritability analyses. The AIC and BIC values did not distinguish between the models, and are therefore not reported. The RMSEA, chi-square and model comparisons, as well as examination of the eigenvalues and factor loadings, suggested that the best-fit model for the PCA was a seven-factor model (Tables I and II). This model accounted for 64% of the variance. The final model included the following factors: 1) verbal IQ, 2) internalizing symptoms, 3) externalizing symptoms, 4) information processing speed and visual problem solving, 5) visual sustained attention, 6) visuospatial ability, and 7) impulsivity and perseveration. The sensitivity analyses using 1) one ADHD affected individual per family, 2) geomin rather than varimax rotation, or 3) z scores rather than t or scale scores all resulted in similar solutions; although the individual factor loadings changed and the order of the variables within each factor changed, the overall factor structure did not change from analysis to analysis (data not shown).
Table II.
Fit statistics for principal components factor analysis, 3 to 7 factor models. The 8 and 9 factor models did not converge. RMSEA = root mean square error of approximation. RMSEA values <0.05 indicate a good fit for the data. The chi square test indicates that the 6 or 7 factor model is a good fit, while the RMSEA favors the 7 factor model. The model comparison suggests that the 7 factor model is better than the 6 factor model, which is better than the 5 factor model, etc.
| Number of factors | 3 | 4 | 5 | 6 | 7 |
| Chi square (df) | 590.9 (297) | 506.3 (272) | 389.1 (248) | 286.8 (225) | 216.4 (203) |
| P value | <0.00001 | <0.00001 | <0.00001 | 0.003 | 0.25 |
| RMSEA estimate (90% CI) | 0.061 (0.054—0.068) | 0.057 (0.049—0.065) | 0.046 (0.037—0.055) | 0.032 (0.019—0.043) | 0.016 (0.00—0.03) |
| Model comparison chi square, df (all p values <0.00001) | N/A | 84.68, 25 | 117.17, 24 | 102.24, 23 | 70.48, 22 |
Six of the associated domains were significantly different between individuals with ADHD and those without ADHD (verbal IQ, internalizing symptoms, externalizing symptoms, information processing speed/visual problem solving, and visual spatial ability, and impulsivity/perseveration) (Table III). Overall, individuals with ADHD scored lower (worse performance) on domains measuring executive function and higher (more pathology) on behavioral domains.
Table III.
Relationship between behavioral and cognitive domains and ADHD diagnosis. Gender, age, and familial relationships are controlled for in all analyses.
| Domain | Coefficient (95% CI) | Z score | p value |
|---|---|---|---|
| Verbal IQ | −1.47 (−2.71— −0.24) | −2.35 | 0.019 |
| Internalizing symptoms | 5.01 (0.35—0.67) | 2.11 | 0.035 |
| Externalizing symptoms | 6.44 (1.75—11.13) | 2.69 | 0.007 |
| Information processing speed and visual problem solving | −1.40 (−2.61— −0.19) | −2.26 | 0.024 |
| Visual spatial ability | −16.0 (−23.78— −8.20) | −4.02 | <0.0001 |
| Visual sustained attention | −1.14 (−2.45—0.18) | −1.70 | 0.09 |
| Impulsivity/perseveration | 10.00 (0.99—19.02) | 2.17 | 0.03 |
| Auditory attention | −1.58 (−3.68—0.52) | −1.48 | 0.14 |
| Cognitive set shifting | 0.03 (−2.54—2.60) | 0.03 | 0.98 |
Heritability analyses
Heritability estimates and the corresponding standard errors and p values for each factor sum score, as well as for the cognitive set shifting and auditory attention variables are reported in Table IV. Because the quantitative traits were derived from an ADHD-affected sample rather than from a non-clinical sample, some of the measures were not normally distributed (Supplemental Figure I). Deviation from normality can, in some instances, result in a kurtosis that potentially affects interpretability of the heritability estimates. Therefore, all variables were transformed using an inverse normal transformation, and heritability for each domain was subsequently assessed using the transformed variables. The inverse normalization process ranks observations and replaces them by the expected value for that rank using a standard normal distribution. A value of p < .05 (2-sided) was considered statistically significant and we calculated 95% confidence intervals for all point estimates. The highest heritability estimates were seen for the verbal IQ factor, with 92% heritability, followed by auditory attention (90% heritability), information processing speed and visual problem solving (85% heritability), and the externalizing symptoms factor (83% heritability). Internalizing symptoms, visual spatial ability, and visual sustained attention were also heritable, although at lower rates (Table IV). Heritability estimates for the impulsivity/perseveration factor and cognitive set shifting were not statistically significant. Age was a significant covariate for most of the domains. Gender was a significant covariate for externalizing symptoms and auditory attention only.
Table IV.
Heritability estimates (H2) and corresponding p values for behavioral and cognitive quantitative traits. Factors = factor sum scores (transformed using an inverse normal transformation).
| Phenotype | H2 (SE) | p-value | |
|---|---|---|---|
| Factor 1 | Verbal IQ2 | 0.92 (0.18) | 0.00002 |
| Factor 2 | Internalizing symptoms1 | 0.63 (0.17) | 0.0004 |
| Factor 3 | Externalizing symptoms1,2 | 0.83 (0.16) | 0.000002 |
| Factor 4 | IPS and visual problem solving1 | 0.85 (0.18) | 0.00003 |
| Factor 5 | Visual spatial ability1 | 0.66 (0.24) | 0.006 |
| Factor 6 | Visual sustained attention1 | 0.45 (0.22) | 0.02 |
| Factor 7 | Impulsivity/ perseveration1 | 0.17 (0.26) | NS |
| DS | Auditory attention1,2 | 0.90 (0.18) | 0.000002 |
| TMT | Cognitive set shifting | 0.07 (0.25) | NS |
| SWAN | SWAN total score2 | 0.66 (0.19) | 0.0005 |
IPS = information processing speed. NS = not significant.
age is a significant covariate.
gender is a significant covariate.
We also performed bivariate heritability analyses using ADHD diagnosis or a quantitative measure of ADHD symptomatology (total SWAN score) and the transformed factor sum scores. Because of the small number of individuals without ADHD who had neurocognitive data available, the point estimates for shared genetic and environmental influences between ADHD and the factors had large standard errors, and none reached the threshold for statistical significance. Nevertheless, the results of these analyses generally supported a role for both shared environment and shared genetic factors between ADHD and the behavioral and cognitive domains, with over 60% of the genetic variance shared (data not shown). When the total SWAN score was used, the results were similar, although the point estimates were approximately 30% lower. However, only the genetic correlation with factor 3 (externalizing symptoms) reached the threshold for statistical significance (RhoG = −0.43, SE = 0.17, p = 0.04), primarily due to large standard errors.
Discussion
The primary goal of this study was to describe the behavioral profiles and neuropsychological characteristics of a sample of children with ADHD from the CVCR population isolate and to examine the heritability of the identified profiles to determine their utility as quantitative phenotypes for future genetic studies. As noted in a review by Doyle et al., ADHD is very heterogeneous, both clinically and etiologically, and the identification of quantitative phenotypes associated with ADHD can be useful for molecular genetic studies[26]. This is true even if the identified quantitative phenotypes have lower heritability than the categorical diagnosis of ADHD, as the magnitude of the effect of a particular gene or gene pathway may be higher for the quantitative phenotype than for the global syndrome [26].
ADHD is a multidimensional disorder consisting of varying levels of inattention, hyperactivity, and impulsivity, and also is characterized by neurocognitive deficits, including deficits in executive function, motivation, and perhaps also reinforcement and reward processing[67]. Several models have been developed to explain the underlying deficits in ADHD, and to link them to the pathognomonic symptoms. One of the most influential has been the dual pathway model, which posits abnormalities both in executive function (the cognitive pathway, leading to problems with behavioral dysregulation and poor planning), and in reward learning (the motivational pathway, leading to performance deficits in the context of delayed reward in particular)[44, 68]. In this study, we used multiple measures of executive function and behavioral/emotional symptomatology, and identified several quantitative constructs, both neurocognitive and behavioral, that were associated with ADHD in our sample. In contrast to our original hypothesis, we did not identify symptom domains that included both cognitive measures and behavioral variables using factor analytic approaches; rather, all of the identified domains contained either purely cognitive measures or purely behavioral measures. This finding supports the idea that, as has been previously suggested, different mechanisms may underlie the executive dysfunction and the behavioral dysregulation seen in ADHD[44, 55, 68]. It is also possible that such “mixed” domains do exist in ADHD, but that the current study as designed is not able to identify them, and that more sensitive measures than were employed here are needed.
Although impairments or abnormalities in most of the domains identified in this study have been previously associated with ADHD (e.g., verbal IQ, attention, impulsivity, perseveration, etc.), three stood out as being of potential interest for genetic studies: verbal IQ, information processing speed and visual problem solving, and externalizing symptoms. It should be noted however that, with the exception of impulsivity/perseveration and cognitive set shifting, the heritability point estimates for all of the other domains were fairly high, suggesting that these domains may also be of interest for genetic studies if they can be replicated.
Several previous studies have examined the utility of quantitative phenotypes in genetic linkage studies of ADHD, and some have examined a wide range of neurocognitive phenotypes [40, 69–75]. One study of particular relevance to our work examined the heritability of a variety of cognitive measures in a population isolate that is genetically closely related to our CVCR population(the region of Antioquia, Colombia)[40]. This study identified several highly heritable quantitative phenotypes of potential use in genetic studies of ADHD, including visuospatial ability, full scale IQ, performance IQ, and continuous vigilance (or sustained attention) [40]. The findings in the Colombian sample are remarkably similar to those in our Costa Rican sample, where IQ (in this case, verbal IQ), visual sustained attention, and information processing speed/visuospatial problem solving were all highly heritable. These studies have all suggested that quantitative neurocognitive phenotypes can be useful in elucidating the complex genetic underpinnings of ADHD, and a few genome-wide linkage studies using such phenotypes have identified candidate genomic regions of interest [72, 73]. However, the data have yet to converge, and although a few candidate genes/genomic regions for ADHD susceptibility have emerged, particularly genes in the dopamine system (the dopamine transporter, dopamine receptors, etc.), much of the genetic risk for ADHD has yet to be identified. Therefore, additional studies, both of ADHD as a categorical phenotype, and of quantitative phenotypes that are associated with ADHD, are needed. The quantitative phenotypes, both behavioral and neurocognitive, that were associated with ADHD in our population and had high heritabilities represent potential phenotypes for quantitative trait locus genetic linkage studies in our ADHD families; such studies are currently underway. The examination of such phenotypes in multigenerational ADHD families from a genetically isolated population such as the CVCR decreases the likelihood of substantial genetic heterogeneity, and thus increases the likelihood of finding additional genes that contribute to ADHD susceptibility.
Limitations
The results of this study must be interpreted in the context of several limitations. First, this is a relatively small sample, with very few unaffected siblings. As a result, we were unable to interpret the genetic relationships between ADHD and cognitive and behavioral domains with any degree of certainty, due to reduced variability within the sample and large standard errors. Second, the fact that phenotypic and neurocognitive data were not available for parents reduced the amount of information available for the heritability studies. Fortunately, this problem is somewhat counterbalanced by our ability to link nuclear families into larger multigenerational pedigrees, allowing us to leverage information from more distant relationships (e.g., cousinships), therefore mitigating the influence of shared environment in the heritability estimates and increasing their precision. Third, although most of the behavioral and cognitive measures loaded uniquely and strongly onto a single factor, a few loaded on two factors. For the sake of clarity, we chose to include these measures in the factors for which they loaded most strongly, but recognize that the reported factor structure may in fact be an over-simplification of the actual underlying relationships between the phenotypes. Fourth, the measures we used, although well validated in both nonclinical and ADHD populations, were limited in scope and sophistication by the logistical constraints of the study, and we did not have the ability to examine reward processing, motivation, or delay aversion, all areas of potential future research. Fifth, the mix of assessments, which included questionnaires, non-timed neurocognitive measures, and timed neurocognitive measures, may have led to artifactual clustering of the variables based on similarities and differences in scale of measurement, error measures, etc. Although we have partially addressed the scale of measurement concern by conducting a sensitivity analysis with z scored data, we cannot completely address the possibility of artifactual clustering of the data. Finally, although we identified a number of heritable quantitative traits that were associated with ADHD in our sample, it is always possible that these traits may not actually be etiologically related to ADHD and thus, may not be useful for genetic studies of ADHD. However, we note that even if this is the case, identifying quantitative trait loci that are associated with these neurocognitive domains may be of interest in its own right.
Summary
In spite of the limitations discussed above, in this study we identified both behavioral and neurocognitive quantitative phenotypes that were associated with ADHD and showed strong evidence of heritability in our CVCR families. Our results extend the previous work on neurocognitive profiles for ADHD, confirming the findings that deficits in multiple cognitive domains are seen in children with ADHD, and perhaps more importantly, providing further evidence that such quantitative phenotypes are heritable, and therefore potentially useful for genetic studies. Future studies will include genome-wide linkage studies in this sample, both of the categorical ADHD phenotype, and of the most heritable of the quantitative phenotypes identified here. We anticipate that such studies will identify new genomic regions of interest for ADHD, and perhaps also for cognitive function more generally.
Supplementary Material
Acknowledgments
This research was supported by grant R01 NS048376 to CAM and grant K08 MH081065 to RSM. We would like to thank the individuals and families who participated in this work.
Footnotes
Conflicts of interest: none.
References
- 1.APA. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 2.Sadock BJSVARP. Kaplan and Sadock’s Comprehensive Textbook of Psychiatry. II. Lippincott Williams and Wilkins; 2009. [Google Scholar]
- 3.Faraone SV, et al. The worldwide prevalence of ADHD: is it an American condition? World Psychiatry. 2003;2(2):104–13. [PMC free article] [PubMed] [Google Scholar]
- 4.Willcutt EG. The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurotherapeutics. 2012;9(3):490–9. doi: 10.1007/s13311-012-0135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spencer TJ, Biederman J, Mick E. Attention-deficit/hyperactivity disorder: diagnosis, lifespan, comorbidities, and neurobiology. J Pediatr Psychol. 2007;32(6):631–42. doi: 10.1093/jpepsy/jsm005. [DOI] [PubMed] [Google Scholar]
- 6.Perou R, et al. Mental health surveillance among children--United States, 2005–2011. MMWR Surveill Summ. 2013;62(Suppl 2):1–35. [PubMed] [Google Scholar]
- 7.Kessler RC, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163(4):716–23. doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barkley RA, et al. The persistence of attention-deficit/hyperactivity disorder into young adulthood as a function of reporting source and definition of disorder. J Abnorm Psychol. 2002;111(2):279–89. [PubMed] [Google Scholar]
- 9.Hurtig T, et al. ADHD symptoms and subtypes: relationship between childhood and adolescent symptoms. J Am Acad Child Adolesc Psychiatry. 2007;46(12):1605–13. doi: 10.1097/chi.0b013e318157517a. [DOI] [PubMed] [Google Scholar]
- 10.Wilens TE, Biederman J, Spencer TJ. Attention deficit/hyperactivity disorder across the lifespan. Annu Rev Med. 2002;53:113–31. doi: 10.1146/annurev.med.53.082901.103945. [DOI] [PubMed] [Google Scholar]
- 11.Biederman J, et al. Predictors of persistence and remission of ADHD into adolescence: results from a four-year prospective follow-up study. J Am Acad Child Adolesc Psychiatry. 1996;35(3):343–51. doi: 10.1097/00004583-199603000-00016. [DOI] [PubMed] [Google Scholar]
- 12.Fayyad J, et al. Cross-national prevalence and correlates of adult attention-deficit hyperactivity disorder. Br J Psychiatry. 2007;190:402–9. doi: 10.1192/bjp.bp.106.034389. [DOI] [PubMed] [Google Scholar]
- 13.Klassen AF, Miller A, Fine S. Health-related quality of life in children and adolescents who have a diagnosis of attention-deficit/hyperactivity disorder. Pediatrics. 2004;114(5):e541–7. doi: 10.1542/peds.2004-0844. [DOI] [PubMed] [Google Scholar]
- 14.Biederman JSV Faraone. Attention-deficit hyperactivity disorder. Lancet. 2005;366(9481):237–48. doi: 10.1016/S0140-6736(05)66915-2. [DOI] [PubMed] [Google Scholar]
- 15.Mick ESV Faraone. Genetics of attention deficit hyperactivity disorder. Child Adolesc Psychiatr Clin N Am. 2008;17(2):261–84. vii–viii. doi: 10.1016/j.chc.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Sherman DK, McGue MK, Iacono WG. Twin concordance for attention deficit hyperactivity disorder: a comparison of teachers’ and mothers’ reports. Am J Psychiatry. 1997;154(4):532–5. doi: 10.1176/ajp.154.4.532. [DOI] [PubMed] [Google Scholar]
- 17.Franke B, et al. The genetics of attention deficit/hyperactivity disorder in adults, a review. Mol Psychiatry. 2012;17(10):960–87. doi: 10.1038/mp.2011.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biederman J, et al. High risk for attention deficit hyperactivity disorder among children of parents with childhood onset of the disorder: a pilot study. Am J Psychiatry. 1995;152(3):431–5. doi: 10.1176/ajp.152.3.431. [DOI] [PubMed] [Google Scholar]
- 19.Loo SK, et al. Executive functioning among Finnish adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(12):1594–604. doi: 10.1097/chi.0b013e3181575014. [DOI] [PubMed] [Google Scholar]
- 20.Barkley RA. Genetics of childhood disorders: XVII. ADHD, Part 1: The executive functions and ADHD. J Am Acad Child Adolesc Psychiatry. 2000;39(8):1064–8. doi: 10.1097/00004583-200008000-00025. [DOI] [PubMed] [Google Scholar]
- 21.Schachar R, et al. Restraint and cancellation: multiple inhibition deficits in attention deficit hyperactivity disorder. J Abnorm Child Psychol. 2007;35(2):229–38. doi: 10.1007/s10802-006-9075-2. [DOI] [PubMed] [Google Scholar]
- 22.de Zeeuw P, et al. Inhibitory performance, response speed, intraindividual variability, and response accuracy in ADHD. J Am Acad Child Adolesc Psychiatry. 2008;47(7):808–16. doi: 10.1097/CHI.0b013e318172eee9. [DOI] [PubMed] [Google Scholar]
- 23.Doyle AE. Executive functions in attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2006;67(Suppl 8):21–6. [PubMed] [Google Scholar]
- 24.Martel M, Nikolas M, Nigg JT. Executive function in adolescents with ADHD. J Am Acad Child Adolesc Psychiatry. 2007;46(11):1437–44. doi: 10.1097/chi.0b013e31814cf953. [DOI] [PubMed] [Google Scholar]
- 25.Rommelse N, et al. Relationship between endophenotype and phenotype in ADHD. Behav Brain Funct. 2008;4:4. doi: 10.1186/1744-9081-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doyle AE, et al. Are endophenotypes based on measures of executive functions useful for molecular genetic studies of ADHD? J Child Psychol Psychiatry. 2005;46(7):774–803. doi: 10.1111/j.1469-7610.2005.01476.x. [DOI] [PubMed] [Google Scholar]
- 27.Lee T, et al. Genetic influences on four measures of executive functions and their covariation with general cognitive ability: the Older Australian Twins Study. Behav Genet. 2012;42(4):528–38. doi: 10.1007/s10519-012-9526-1. [DOI] [PubMed] [Google Scholar]
- 28.Kebir O, et al. Candidate genes and neuropsychological phenotypes in children with ADHD: review of association studies. J Psychiatry Neurosci. 2009;34(2):88–101. [PMC free article] [PubMed] [Google Scholar]
- 29.Arcos-Burgos MM Muenke. Genetics of population isolates. Clin Genet. 2002;61(4):233–47. doi: 10.1034/j.1399-0004.2002.610401.x. [DOI] [PubMed] [Google Scholar]
- 30.Mathews CA, et al. Genetic studies of neuropsychiatric disorders in Costa Rica: a model for the use of isolated populations. Psychiatr Genet. 2004;14(1):13–23. doi: 10.1097/00041444-200403000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Ophoff RA, et al. Genomewide linkage disequilibrium mapping of severe bipolar disorder in a population isolate. Am J Hum Genet. 2002;71(3):565–74. doi: 10.1086/342291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeLisi LE, et al. Genome-wide scan for linkage to schizophrenia in a Spanish-origin cohort from Costa Rica. Am J Med Genet. 2002;114(5):497–508. doi: 10.1002/ajmg.10538. [DOI] [PubMed] [Google Scholar]
- 33.McInnes LA, et al. Fine-scale mapping of a locus for severe bipolar mood disorder on chromosome 18p11.3 in the Costa Rican population. Proc Natl Acad Sci U S A. 2001;98(20):11485–90. doi: 10.1073/pnas.191519098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Escamilla MA, et al. Genome screening for linkage disequilibrium in a Costa Rican sample of patients with bipolar-I disorder: a follow-up study on chromosome 18. Am J Med Genet. 2001;105(2):207–13. doi: 10.1002/ajmg.1205. [DOI] [PubMed] [Google Scholar]
- 35.McInnes LA, et al. A complete genome screen for genes predisposing to severe bipolar disorder in two Costa Rican pedigrees. Proc Natl Acad Sci U S A. 1996;93(23):13060–5. doi: 10.1073/pnas.93.23.13060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Escamilla MA, et al. Use of linkage disequilibrium approaches to map genes for bipolar disorder in the Costa Rican population. Am J Med Genet. 1996;67(3):244–53. doi: 10.1002/(SICI)1096-8628(19960531)67:3<244::AID-AJMG2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 37.Walss-Bass C, et al. Linkage disequilibrium analyses in the Costa Rican population suggests discrete gene loci for schizophrenia at 8p23.1 and 8q13.3. Psychiatr Genet. 2006;16(4):159–68. doi: 10.1097/01.ypg.0000218616.27515.67. [DOI] [PubMed] [Google Scholar]
- 38.Moon E, et al. Lack of association to a NRG1 missense polymorphism in schizophrenia or bipolar disorder in a Costa Rican population. Schizophr Res. 2011;131(1–3):52–7. doi: 10.1016/j.schres.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ross J, et al. Genomewide linkage analysis in Costa Rican families implicates chromosome 15q14 as a candidate region for OCD. Hum Genet. 2011;130(6):795–805. doi: 10.1007/s00439-011-1033-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pineda DA, et al. Potential cognitive endophenotypes in multigenerational families: segregating ADHD from a genetic isolate. Atten Defic Hyperact Disord. 2011;3(3):291–9. doi: 10.1007/s12402-011-0061-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arcos-Burgos M, et al. Attention-deficit/hyperactivity disorder in a population isolate: linkage to loci at 4q13.2, 5q33.3, 11q22, and 17p11. Am J Hum Genet. 2004;75(6):998–1014. doi: 10.1086/426154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palacio JD, et al. Attention-deficit/hyperactivity disorder and comorbidities in 18 Paisa Colombian multigenerational families. J Am Acad Child Adolesc Psychiatry. 2004;43(12):1506–15. doi: 10.1097/01.chi.0000142279.79805.dc. [DOI] [PubMed] [Google Scholar]
- 43.Nyman ES, et al. ADHD candidate gene study in a population-based birth cohort: association with DBH and DRD2. J Am Acad Child Adolesc Psychiatry. 2007;46(12):1614–21. doi: 10.1097/chi.0b013e3181579682. [DOI] [PubMed] [Google Scholar]
- 44.Nigg JT, et al. Causal heterogeneity in attention-deficit/hyperactivity disorder: do we need neuropsychologically impaired subtypes? Biol Psychiatry. 2005;57(11):1224–30. doi: 10.1016/j.biopsych.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 45.Rhodes SM, Coghill DR, Matthews K. Acute neuropsychological effects of methylphenidate in stimulant drug-naive boys with ADHD II--broader executive and non-executive domains. J Child Psychol Psychiatry. 2006;47(11):1184–94. doi: 10.1111/j.1469-7610.2006.01633.x. [DOI] [PubMed] [Google Scholar]
- 46.Yang P, et al. Rapid improvement in academic grades following methylphenidate treatment in attention-deficit hyperactivity disorder. Psychiatry Clin Neurosci. 2004;58(1):37–41. doi: 10.1111/j.1440-1819.2004.01190.x. [DOI] [PubMed] [Google Scholar]
- 47.Coghill DR, et al. Effects of methylphenidate on cognitive functions in children and adolescents with attention-deficit/hyperactivity disorder: evidence from a systematic review and a meta-analysis. Biol Psychiatry. 2014;76(8):603–15. doi: 10.1016/j.biopsych.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 48.Schuler J, et al. Characteristics and comorbidity of ADHD sib pairs in the Central Valley of Costa Rica. Compr Psychiatry. 2012;53(4):379–86. doi: 10.1016/j.comppsych.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rubio-Stipec M, et al. The internal consistency and concurrent validity of a Spanish translation of the Child Behavior Checklist. J Abnorm Child Psychol. 1990;18(4):393–406. doi: 10.1007/BF00917642. [DOI] [PubMed] [Google Scholar]
- 50.Graetz BW, et al. Validity of DSM-IVADHD subtypes in a nationally representative sample of Australian children and adolescents. J Am Acad Child Adolesc Psychiatry. 2001;40(12):1410–7. doi: 10.1097/00004583-200112000-00011. [DOI] [PubMed] [Google Scholar]
- 51.Leckman JF, et al. Best estimate of lifetime psychiatric diagnosis: a methodological study. Arch Gen Psychiatry. 1982;39(8):879–83. doi: 10.1001/archpsyc.1982.04290080001001. [DOI] [PubMed] [Google Scholar]
- 52.Smalley SL, et al. Familial clustering of symptoms and disruptive behaviors in multiplex families with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2000;39(9):1135–43. doi: 10.1097/00004583-200009000-00013. [DOI] [PubMed] [Google Scholar]
- 53.Seidman LJ. Neuropsychological functioning in people with ADHD across the lifespan. Clin Psychol Rev. 2006;26(4):466–85. doi: 10.1016/j.cpr.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 54.Frazier TW, Demaree HA, Youngstrom EA. Meta-analysis of intellectual and neuropsychological test performance in attention-deficit/hyperactivity disorder. Neuropsychology. 2004;18(3):543–55. doi: 10.1037/0894-4105.18.3.543. [DOI] [PubMed] [Google Scholar]
- 55.Willcutt EG, et al. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry. 2005;57(11):1336–46. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 56.Jacobson LA, et al. Working memory influences processing speed and reading fluency in ADHD. Child Neuropsychol. 2011;17(3):209–24. doi: 10.1080/09297049.2010.532204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sami N, et al. Performance of girls with ADHD and comparison girls on the Rey-Osterrieth Complex Figure: evidence for executive processing deficits. Child Neuropsychol. 2003;9(4):237–54. doi: 10.1076/chin.9.4.237.23514. [DOI] [PubMed] [Google Scholar]
- 58.Seidman LJ, et al. Performance of children with ADHD on the Rey-Osterrieth complex figure: a pilot neuropsychological study. J Child Psychol Psychiatry. 1995;36(8):1459–73. doi: 10.1111/j.1469-7610.1995.tb01675.x. [DOI] [PubMed] [Google Scholar]
- 59.Rohlf H, et al. Set shifting and working memory in adults with attention-deficit/hyperactivity disorder. J Neural Transm. 2012;119(1):95–106. doi: 10.1007/s00702-011-0660-3. [DOI] [PubMed] [Google Scholar]
- 60.Hale JB, et al. Development and validation of an attention-deficit/hyperactivity disorder (ADHD) executive function and behavior rating screening battery. J Clin Exp Neuropsychol. 2009;31(8):897–912. doi: 10.1080/13803390802687423. [DOI] [PubMed] [Google Scholar]
- 61.Huang-Pollock CL, et al. Evaluating vigilance deficits in ADHD: a meta-analysis of CPT performance. J Abnorm Psychol. 2012;121(2):360–71. doi: 10.1037/a0027205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.StataCorp. Stata 11.2. StataCorp LP; College Station, TX: 2009. [Google Scholar]
- 63.Katerberg H, et al. Symptom dimensions in OCD: item-level factor analysis and heritability estimates. Behav Genet. 2010;40(4):505–17. doi: 10.1007/s10519-010-9339-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blangero J, Lange K, Almasy L, Dyer T, Göring H, Williams J, Peterson J. T.B.R. Institute, editor. Sequential Oligogenic Linkage Analysis Routine (SOLAR) 2013. [Google Scholar]
- 65.Almasy LJ Blangero. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62(5):1198–211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Greenwood TA, et al. Initial heritability analyses of endophenotypic measures for schizophrenia: the consortium on the genetics of schizophrenia. Arch Gen Psychiatry. 2007;64(11):1242–50. doi: 10.1001/archpsyc.64.11.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marx I, et al. The impact of financial reward contingencies on cognitive function profiles in adult ADHD. PLoS One. 2013;8(6):e67002. doi: 10.1371/journal.pone.0067002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sonuga-Barke EJ. The dual pathway model of AD/HD: an elaboration of neuro-developmental characteristics. Neurosci Biobehav Rev. 2003;27(7):593–604. doi: 10.1016/j.neubiorev.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 69.Arias-Vasquez A, et al. CDH13 is associated with working memory performance in attention deficit/hyperactivity disorder. Genes Brain Behav. 2011;10(8):844–51. doi: 10.1111/j.1601-183X.2011.00724.x. [DOI] [PubMed] [Google Scholar]
- 70.Konrad K, et al. Familiality and molecular genetics of attention networks in ADHD. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(1):148–58. doi: 10.1002/ajmg.b.30967. [DOI] [PubMed] [Google Scholar]
- 71.Goos LM, et al. Validation and extension of the endophenotype model in ADHD patterns of inheritance in a family study of inhibitory control. Am J Psychiatry. 2009;166(6):711–7. doi: 10.1176/appi.ajp.2009.08040621. [DOI] [PubMed] [Google Scholar]
- 72.Doyle AE, et al. Multivariate genomewide linkage scan of neurocognitive traits and ADHD symptoms: suggestive linkage to 3q13. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(8):1399–411. doi: 10.1002/ajmg.b.30868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rommelse NN, et al. Neuropsychological endophenotype approach to genome-wide linkage analysis identifies susceptibility loci for ADHD on 2q21.1 and 13q12.11. Am J Hum Genet. 2008;83(1):99–105. doi: 10.1016/j.ajhg.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Johnson KA, et al. Absence of the 7-repeat variant of the DRD4 VNTR is associated with drifting sustained attention in children with ADHD but not in controls. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(6):927–37. doi: 10.1002/ajmg.b.30718. [DOI] [PubMed] [Google Scholar]
- 75.Cho SC, et al. Possible association of the alpha-2A-adrenergic receptor gene with response time variability in attention deficit hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(6):957–63. doi: 10.1002/ajmg.b.30725. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


