Abstract
Social learning theory postulates that individuals learn to engage in aggressive behavior through observing an aggressive social model. Prior studies have shown that repeatedly observing aggression, also called “chronic passive exposure to aggression,” changes accumbal dopamine D2 receptor (D2R) and amygdaloid 5-HT1B receptor (5-HT1BR) densities in observers. But, the association between these outcomes remains unknown. Thus, our study used a rat paradigm to comprehensively examine the linkage between aggression, D2R density in the nucleus accumbens core (AcbC) and shell (AcbSh), and 5-HT1BR density in the medial (MeA), basomedial (BMA), and basolateral (BLA) amygdala following chronic passive exposure to aggression. Male Sprague-Dawley rats (N = 72) were passively exposed to either aggression or non-aggression acutely (1 day) or chronically (23 days). When observer rats were exposed to aggression chronically, they showed increased aggressive behavior and reduced D2R density in the bilateral AcbSh. On the other hand, exposure to aggression, regardless of exposure length, increased 5-HT1BR density in the bilateral BLA. Finally, low D2R in the AcbSh significantly interacted with high 5-HT1BR density in the BLA in predicting high levels of aggression in observer rats. Our results advance our understanding of the neurobiological mechanisms for observational learning of aggression, highlighting that dopamine-serotonin interaction, or AcbSh-BLA interaction, may contribute to a risk factor for aggression in observers who chronically witness aggressive interactions.
Keywords: aggression, dopamine D2 receptor, 5-HT1B receptor, nucleus accumbens, amygdala, observational learning
According to social learning theory (Albert Bandura, 1973; A. Bandura, 1977; A. Bandura, Ross, & Ross, 1961, 1963), youths are inclined to engage in aggressive behavior after they observe an aggressive adult model. Notably, this theory provides a psychosocial explanation for aggression in bystanders, who are not actually involved in violent situations. Long-lasting events of observing violence may particularly cause observer youths to adopt aggressive behavior (Huesmann & Kirwil, 2007). In fact, previous literature has shown evidence consistent with this social learning theory. For example, individuals who have witnessed community and family violence in childhood tend to show aggressive and other externalizing behaviors (Guerra, Huesmann, Tolan, Van Acker, & Eron, 1995; Holmes, 2013), child abuse (Widom, 1989), positive attitudes toward aggression (Guerra, Huesmann, & Spindler, 2003; Su, Mrug, & Windle, 2010), and aggressive fantasies (Su et al., 2010). These behavioral effects of witnessing violence, also known as “passive exposure to aggression,” have been also found in animal studies; fish and rodents show aggressive tendencies following repeatedly observing fights between conspecifics (Clotfelter & Paolino, 2003; Feldker et al., 2006; Suzuki & Lucas, 2010; Welch & Welch, 1971). Therefore, it is reasonable to suggest that chronic passive exposure to aggression is a risk factor for observers’ aggressiveness.
Yet, given that there are various forms of aggression, it is important to clarify what type of aggression particularly increases among observers who have been chronically exposed to aggressive situations. Traditionally, aggression is classified into two types: impulsive (or hostile/reactive) aggression, which is primarily driven by negative emotional states, and instrumental (or premeditated/proactive) aggression, which is a type of hurting behavior aiming to achieve some other end (Anderson & Huesmann, 2003; Nelson & Trainor, 2007; Vitiello & Stoff, 1997). Because previous research has shown that chronic passive exposure to aggression is broadly associated with impulsive, risk-taking behavior (Margolin & Gordis, 2000), including not only aggression (as discussed earlier) but also externalizing problems (Bauer et al., 2006; Emery, 2011; Fantuzzo et al., 1991) and illegal drug use (Berenson, Wiemann, & McCombs, 2001; Kilpatrick et al., 2000; Sussman, Dent, & McCullar, 2000; Sussman, Dent, & Stacy, 1999; Vermeiren, Schwab-Stone, Deboutte, Leckman, & Ruchkin, 2003), chronic exposure to aggression is conceivably associated with impulsive aggression. Furthermore, prior findings indicate that chronic passive exposure to aggression increases aggressive behavior solely, without altering defensive/submissive behavior (Suzuki & Lucas, 2010). This possibly suggests that observers increase fearless aggression (as opposed to rage or fear-induced aggression), that is, risk-seeking properties of impulsive aggression.
Indeed, the possible association between chronic passive exposure to aggression and risk-seeking/impulsive aggression has been implied by previous neurochemical studies. For instance, rats exposed to aggression for 23 consecutive days show downregulated dopamine D2 receptor (D2R) density in the shell of the nucleus accumbens (AcbSh) bilaterally, compared to those exposed to non-aggression for the same number of days (Suzuki, Han, & Lucas, 2010a). In general, the accumbal dopaminergic system has been implicated in motivation for hedonic rewards (Berridge, 2007), and dopamine release in the AcbSh is stimulated following risk-seeking/impulsive behaviors, such as alcohol consumption (Bustamante et al., 2008; van Erp & Miczek, 2007) and psychostimulant drugs (Desai, Paronis, Martin, Desai, & Bergman, 2010; Kleijn et al., 2012). Interestingly, dopamine release in the nucleus accumbens is similarly triggered following aggression (Beiderbeck et al., 2012; Ferrari, van Erp, Tornatzky, & Miczek, 2003; van Erp & Miczek, 2000), suggesting that aggression may serve as impulsively fulfilling demands for dopamine reward outputs. Moreover, a D2R antagonist (sulpiride or haloperidol) infused into the nucleus accumbens decreased aggressive behavior (Beiderbeck et al., 2012; Couppis & Kennedy, 2008), although this pharmacological manipulation broadly influenced both the core of the nucleus accumbens (AcbC) and AcbSh. Thus, the accumbal dopaminergic system may be related to the rewarding properties of aggression (Couppis & Kennedy, 2008).
Observer rats exposed to aggression for 23 days also show upregulated serotonin 5-HT1B receptor (5-HT1BR) density in the basolateral amygdala (BLA), compared to controls (Suzuki, Han, & Lucas, 2010b). The serotonergic system generally functions as regulating aggression; low 5-HT levels are often associated with aggressive traits (Caramaschi, de Boer, de Vries, & Koolhaas, 2008; Ferris et al., 1997; Ferris, Stolberg, & Delville, 1999; Pihl & Benkelfat, 2005). Among brain regions, the amygdala shows a high concentration of 5-HT, 5-HIAA (indicating 5-HT synthesis), and serotonin transporter in neurons, compared to the prefrontal cortex or hippocampus (Arrant, Jemal, & Kuhn, 2013). While aggressive motivation increased functional activation in the amygdala, including the medial (MeA), basomedial (BMA), and BLA, this amygdala activity is suppressed by a selective serotonin reuptake inhibitor (fluoxetine) (Ferris et al., 2008). This may indicate the involvement of the amygdaloid 5-HT system in aggression. Furthermore, a high number of 5-HT1BR-positive-neurons in the BLA may be associated with impulsive, “pathological” aggression (Jacobs, Van Den Broeck, & Simoens, 2007), whereas pharmacologically induced deletion of serotonergic fibers in the BLA increases fear-potentiated startle (Tran, Lasher, Young, & Keele, 2013). These findings suggest that the serotonergic system in the amygdala, especially in the BLA, may be critical in the “fight-or-flight” response to a potentially threatening situation (Cannon, 1939). That is, a “fight” response may tend to be activated more often than a “flight” response, depending on individual social experience, stress vulnerability, and 5-HT activity (D. C. Blanchard & Blanchard, 1990; D. C. Blanchard, Sakai, McEwen, Weiss, & Blanchard, 1993; D. C. Blanchard et al., 1995; R. J. Blanchard, Yudko, Dulloog, & Blanchard, 2001; Koolhaas, de Boer, Buwalda, & van Reenen, 2007; Koolhaas et al., 1999; Koolhaas, Meerlo, De Boer, Strubbe, & Bohus, 1997; Tamashiro, Nguyen, & Sakai, 2005).
Together, the accumbal dopaminergic activity and the amygdaloid serotonergic activity appear to be involved in impulsive aggressive behavior. This suggests the possibility that the alterations in D2R density in the AcbSh and 5-HT1BR density in the BLA following chronic passive exposure to aggression, as shown in prior studies (Suzuki et al., 2010a, 2010b), might contribute to impulsive aggressive behavior in observers (Clotfelter & Paolino, 2003; Feldker et al., 2006; Suzuki & Lucas, 2010; Welch & Welch, 1971). However, to our knowledge, there are no studies that have directly examined an interplay between these local receptor densities and aggressive behavior following chronic passive exposure to aggression. Furthermore, no studies have directly compared these local receptor densities in acute versus chronic passive exposure to aggression.
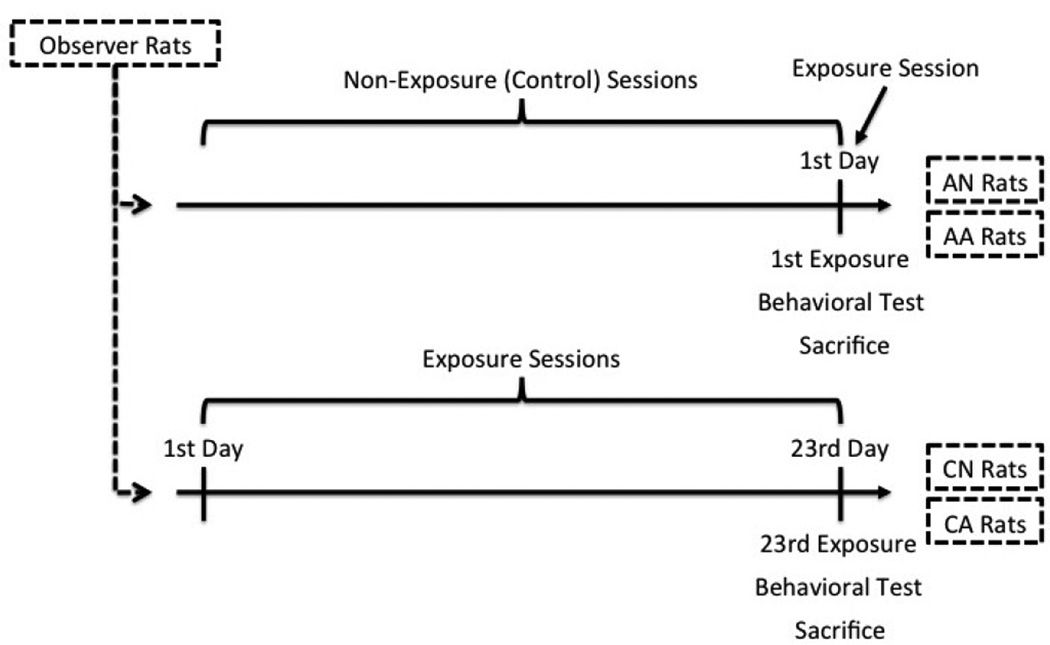
To clarify the above two questions, the present study was a follow-up on prior studies examining the effects of passive exposure to aggression. Specifically, the current study was conducted to quantify impulsive aggression, D2R density in the nucleus accumbens (AcbC and AcbSh), and 5-HT1BR density in the amygdala (MeA, BMA, and BLA) altogether within observer rats and compare them between acute and chronic passive exposure to aggression. To achieve this goal, we developed a rat paradigm specifically tailored to test our hypothesis of observer-learned aggression (Suzuki et al., 2010a, 2010b; Suzuki & Lucas, 2010). Notably, it was important to contrast acute exposure with chronic exposure in order to illustrate whether observer rats increased their aggressiveness due to “mimicry/priming” effects or “observational learning” effects (Huesmann & Kirwil, 2007; Suzuki & Lucas, 2010). That is, if chronic exposure to aggression resulted in more aggression in observer rats than acute exposure to aggression, this would be likely to indicate that observers’ aggression was induced by a long-term observational learning process, rather than just an instant imitation of aggression (which would then be seen immediately after an acute exposure). Therefore, our paradigm administered acute or chronic exposure session(s) right before a behavioral assessment of aggression for an observer rat (see Figure 1), which was suitable for our purpose.
Figure 1.
Timeline of each condition. AN = acute exposure to non-aggression; AA = acute exposure to aggression; CN = chronic exposure to non-aggression; CA = chronic exposure to aggression.
The current study aimed to test three hypotheses. The first hypothesis was that chronic passive exposure to aggression would result in not only increased impulsive aggressive behavior, as reported previously (Suzuki & Lucas, 2010), but also downregulated D2R density in the AcbSh and upregulated 5-HT1BR density in observer rats, compared to acute exposure to aggression. The second hypothesis was changes in the identified local receptor densities, especially D2R density in the AcbSh and 5-HT1BR density in the BLA, would be associated with each other. The third hypothesis was that increased impulsive aggression would be associated with the identified local receptor densities, especially D2R density in the AcbSh and/or 5-HT1BR density in the BLA.
Method
Subjects
Seventy two young male Sprague-Dawley rats were bred in our Animal Care Facilities (ACF) and reared in a group (cage size = 47cm × 25.5cm × 21.5cm). When they weighed 150–250g, they were individually housed and equally assigned to one of four conditions (n = 18 each): (1) acute exposure to non-aggression (AN), (2) chronic exposure to non-aggression (CN), (3) acute exposure to aggression (AA), or (4) chronic exposure to aggression (CA). The purpose of having AN and AA rats was to examine the mimicry/priming effects of passive exposure to aggression, whereas the purpose of having CN and CA rats was to examine the observational learning effects of aggression (see Figure 1). This between-group design signified whether repeated exposure, with ruling out a possible priming effect immediately following exposure, was required for observer rats to behave aggressively.
The total sample size was determined by a prospective power analysis of our pilot behavioral data in the past (Suzuki & Lucas, 2010). Based on a 2 (exposure length; acute vs. chronic) × 2 (exposure condition; exposure to non-aggression vs. exposure to aggression) analysis of variance, the estimated values of Cohen’s d were following: 0.4 for the main effect of exposure length, 0.6 for the main effect of exposure condition, and 0.95 for the interaction between them. Power analysis indicated that 72 of the total sample size would attain 95% power to detect the effect of exposure length, 97% power to detect the effect of exposure condition, and 100% power to detect the interaction effect. Therefore, the present study assures adequate power.
All observer rats were given ad libitum (oval pellet-typed food for laboratory rodents, LabDiet 5001 Rodent Diet, Southern Agriculture, Tulsa, OK) and water in a climatized room (temperature = 21–22°C; humidity = 30–60%; 12 h lig ht-dark cycle; light-on at 7:00a.m., light-off at 7:00p.m.) under the approval of the Loyola University Chicago Institutional Animal Care and Use Committee (IACUC).
Additional Rats for Inducing Aggressive Contexts
Additional male Sprague-Dawley rats were inbred in our ACF and prepared to manipulate aggressive or non-aggressive control contexts that observer rats were exposed to. First, behavioral screening tests were administered to select six most non-aggressive rats and six most aggressive rats (body weight ≥ 400g). Next, starting from 2 weeks prior to an experiment, each non-aggressive rat was housed with a younger male rat (body weight = 100g less than the non-aggressive rat), whereas each aggressive rat was housed with a female rat (body weight = 250g). This 2-week cohabitation (1) allowed the non-aggressive male-male dyad to establish a social hierarchy or (2) provoked aggressive motivation among the aggressive male rats having a female partner (Suzuki et al., 2010a, 2010b; Suzuki & Lucas, 2010).
During an experiment, a non-aggressive dyad was presented to the AN and CN groups. The non-aggressive dyad was less likely to show aggression because they were motivated to maintain a social hierarchy and did not need to fight for sorting out their rank. In contrast, the aggressive male rat was separated from a female partner; paired with a younger naïve male rat (body weight = 100g less than the aggressive rats); and then presented to the AA and CA groups. Because this naïve male rat was a potential rival for mating and territory, the aggressive rat was likely to show intermale and territorial aggression (Suzuki et al., 2010a, 2010b; Suzuki & Lucas, 2010). In this way, this male-male pair served as an aggressive dyad. After the experiment, the aggressive rat was separated from the naïve rat and paired with the female partner again. All non-aggressive and aggressive dyads were repeatedly used until they no longer behaved their expected roles. The Loyola University Chicago IACUC approved the use of non-aggressive dyads, aggressive dyads, female partners, and young male rats (the approximate number of rats = 156 rats) during our experiment.
Procedure
The procedure was identical to a previously established protocol (Suzuki et al., 2010a, 2010b; Suzuki & Lucas, 2010). Under a red light illumination between 7:00p.m. and 9:00p.m., each observer rat was transferred from his home cage to a small plastic and transparent aquarium with a mesh lid (cage size = 22.9cm × 15cm × 16.5cm). Note that this aquarium had enough space where a rat freely moved around, thus a potential restraint stress was minimal. Then, the observer rat in the aquarium was placed into the cage (47cm × 25.5cm × 21.5cm) of either the non-aggressive dyad (for the AN and CN groups) or aggressive dyad (for the AA and CA groups). Importantly, the observer rats could not make any physical contact with the non-aggressive/aggressive dyad, while they could see, hear, and smell the dyad through the mesh lid or transparent barrier. This observational session took 10 min. per day and was recorded by a video camera. Immediately after the session, the observer rat was removed from the aquarium and placed back to his home cage.
The observational session was conducted only one time (for the AN and AA groups) or was repeated once daily for 23 consecutive days (for the CN and CA groups). Additionally, the CN and CA rats were cycled to pair a different dyad each day, minimizing the within-group variability of the amount of observing non-aggression or aggression.
As soon as the last observational session was done, a 10-min behavioral screening test was conducted under a red light illumination (between 7:10p.m. and 9:30p.m.) to assess aggressiveness of each observer rat. In this screening test, each observer rat was paired with another naïve male rat, called an opponent rat, in a new cage (cage size = 47cm × 25.5cm × 21.5cm), and their social interactions were recorded by a video camera. The opponent rat was weight-matched to the observer rat so that it was physically fair for both rats during a fight. Given such a non-handicapped fight, if the observer rat maintained aggressive behavior for a long time (regardless of whether the opponent rat attacked/counterattacked or even became dominant in several fights), this was operationally defined as impulsive aggression. Immediately after the final screening test, the observer rats were decapitated to collect blood and brain samples. Blood samples were centrifuged at 2500rpm at 4 °C for 15 min. to extract serum and was stored at −20°C until it was used. Brain samples were removed rapidly, frozen on powdered dry ice, and stored at −70°C until used.
Note that the behavioral screening test and the following decapitation were performed as soon as the last observational session was completed, which modified a previous protocol (Suzuki et al., 2010a, 2010b; Suzuki & Lucas, 2010). This is primarily because it was necessary to check stress hormone corticosterone levels following exposure before hormone levels returned to baseline. Given that stress potentially induced aggression (Wood, Norris, Waters, Stoldt, & McEwen, 2008; Wood, Young, Reagan, & McEwen, 2003; Yohe, Suzuki, & Lucas, 2012), the present protocol was helpful in confirming whether acute and chronic exposure to aggression did not produce unexpected stress. Furthermore, the present protocol helped us clarify whether any change in the target receptor density occurred slowly (e.g., in 24 hours following exposure session; see Suzuki et al. (2010a, 2010b)), or rapidly (e.g., even immediately following exposure session). Because of these reasons, decapitation was performed as soon as exposure session and behavioral testing were conducted.
Aggression Assessment
Trained raters counted up the amount of time (in sec.) when the observer rats, as well as the opponent rats, were engaged in aggressive behavior, using a stopwatch. Aggression of the opponent rats was used as background information.
Aggressive behavior was measured according to a previously published protocol (Miczek, 1974; Suzuki & Lucas, 2010). Specifically, the following actions were considered as aggressive behavior: attack (e.g., leaping at an opponent, pulling an opponent’s skin), threat (e.g., pushing an opponent with his back), aggressive posture (e.g., bending over an opponent with his head and forelimbs arched over an opponent), allogrooming (e.g., aggressively grooming or nibbling an opponent’s neck), mutual upright posture (e.g., standing on his hindlegs and boxing), and chasing (e.g., following an fleeing opponent). Play fighting (e.g., contacting each other’s snout, face, and nape of the neck) was excluded (Pellis & Pellis, 1987; Pellis, Pellis, & Foroud, 2005) when both the observer rats and the opponent rats were assessed. Inter-rater reliability of all behavioral scores met the acceptable level (Kline, 1999): Cronbach’s α of 0.84 for aggression of the observer rats and 0.79 for aggression of the opponent rats.
Radioimmunoassay
To check background information, levels of serum testosterone and corticosterone were assayed using the commercially available radioimmunoassay kits, Coat-A-Count Total Testosterone and Coat-A-Count Rat Corticosterone (Siemens, Los Angeles, CA). Following the protocols in the kits, concentrations of serum testosterone and corticosterone were respectively computed from a logit-log calibration curve, which was drawn from radioactive counts and concentrations of the calibrators.
Brain Sectioning and Receptor Binding Autoradiography
Coronal sections of 20µm thickness were cut on a cryostat at −15°C and tha w-mounted onto twelve glass microscope slides (Superfrost Plus, VWR West Chester PA; four sections per slide). The target sections included the accumbal areas (i.e., AcbC and AcbSh, between 2.52mm and 1.56mm prior to bregma) and the amygdaloid areas (i.e., MeA, BMA, and BLA, between 2.16mm and 3.12mm posterior to bregma), identified according to the atlas by Paxinos and Watson (2005). All brain sections were stored at −70°C until used.
At the time of chemical processing, two best, cross-matched slides were selected from each group of observer rats. Slides containing the accumbal sections were processed for D2R binding autoradiography in the following: (1) rinsing them twice with 50mM of Tris HCl (pH 7.4) for 10 min, (2) incubating them in a buffer solution (containing 50mM of Tris HCl (pH 7.4), 120mM of NaCl, 5mM of KCl, 2mM of CaCl2, 1mM of MgCl2, 100pM of [125I]2’-iodospiperone, and 50nM of ketanserin) at room temperature for 90 min, (3) rinsing them in cold 50mM of Tris HCl (pH 7.4) three times for 10 min per wash, (4) dipping them quickly in ice-cold double-distilled H2O for less than 5 sec, (5) drying them under a stream of cool air, (6) placing them in cassettes and exposing them, in addition to 125I plastic standards (ranging from 11.5µCi/g to 6000µCi/g, American Radiolabeled Chemicals, Inc., Saint Louis, MO), to BioMax MR film (Kodak), and (7) leaving them under a dark area for 8 hrs. For nonspecific binding, one additional slide from each group was processed in the same way described above, except that 100µM of SCH23390 was added to the buffer solution.
Slides containing the amygdaloid sections were processed for 5-HT1BR binding autoradiography in the following: (1) incubating them in a buffer solution (containing 170mM of Tris HCl (pH 7.4), 150mM of NaCl, 50pM of [125I]cyanopindolol, 100nM of 8-OH-DPAT, and 30µM of isoproterenol) at room temperature for 120 min, (2) rinsing them in cold binding buffer solution two times for 5 min per wash, (3) dipping them quickly in ice-cold double-distilled H2O at 4 °C for less than 5 sec, (4) drying them under a st ream of cool air, (5) placing them in cassettes and exposing them and 125I plastic standards to BioMax MR film (Kodak), and (6) leaving them under a dark area for 88 hrs. For nonspecific binding, one more slide was selected from each group and processed in the same way described above, except that 100µM of raclopride was added to the buffer solution.
The films were analyzed using computer-assisted densitometry. Intensity levels within the region of interest (ROI) and the corpus callosum (used as a local background) were measured on a 10-point optical density calibration scale (Stouffer Graphic Arts Equipment, Mishawaka, IN). Then, these relative ROI intensity levels were subtracted by the background intensity and averaged across the selected sections. Finally, 125I plastic standards were also measured on a 10-point calibration scale and used to estimate the relative ROI intensity levels in fmol/mg.
Statistical Strategy
Prior to our analysis, we used Winsorising (Dixon, 1960) to reduce an effect of any outliers; we found only one outlier of aggression (CA rat, z = 4.99) and set it to the closest non-extreme value; the other cases scored within z = ±3.0. In addition, one-way ANOVAs tested any group difference in background characteristics (i.e., age, aggressive behavior of the opponent rats, testosterone, and corticosterone).
To test our first hypothesis, a two-way ANOVA was performed to test the interaction between exposure length and exposure condition on aggressive behavior of the observer rats. Moreover, three-way repeated-measures ANOVAs were used to compare D2R density in the AcbC and AcbSh (at Bonferroni-corrected significance level p = 0.05/2), as well as 5-HT1BR density in the MeA, BMA, and BLA (at Bonferroni-corrected significance level p = 0.05/3), with hemisphere as a within-subjects variable and exposure length and exposure condition as between-subjects variables. Finally, if any interaction was significant in the ANOVAs, Bonferroni-corrected post hoc tests were used to test pairwise differences. To test the second and third hypotheses, Pearson correlations and hierarchical regressions (with follow-up simple regressions, if necessary) were used to test associations among local receptor densities and aggressive behavior identified by the initial ANOVAs (at the first step) and any possible interactions (at higher-order steps).
Results
Effects of Passive Exposure to Aggression
Age, aggression of the opponent rats, testosterone, and corticosterone did not differ across the groups (see Table 1). However, although there was no age difference between the groups statistically, age was covariated in subsequent analyses in case where there might be a sensitive period in the development of aggression and/or the effect of social exposure during our experiments.
Table 1.
Background Characteristics of the Sample (N = 72).
| Variables | AN (n = 18) |
CN (n = 18) |
AA (n = 18) |
CA (n = 18) |
F (3,68) |
|---|---|---|---|---|---|
| Age (in days) | 62.50 (7.23) |
65.67 (3.53) |
62.50 (7.23) |
65.72 (3.08) |
1.94 |
| Partner’s aggressive behavior (in sec) |
9.06 (23.62) |
2.84 (5.45) |
6.16 (22.74) |
1.80 (3.89) |
0.70 |
| Testosterone (in ng/dL) | 232.40 (131.09) |
238.27 (168.54) |
185.54 (87.77) |
278.20 (209.12) |
1.07 |
| Corticosterone (in ng/mL) | 491.63 (93.91) |
539.11 (146.17) |
519.64 (61.12) |
519.62 (124.34) |
0.56 |
Note: AN = acute exposure to non-aggression; CN = chronic exposure to non-aggression; AA = acute exposure to aggression; CA = chronic exposure to aggression.
Data presented as mean (standard deviation)
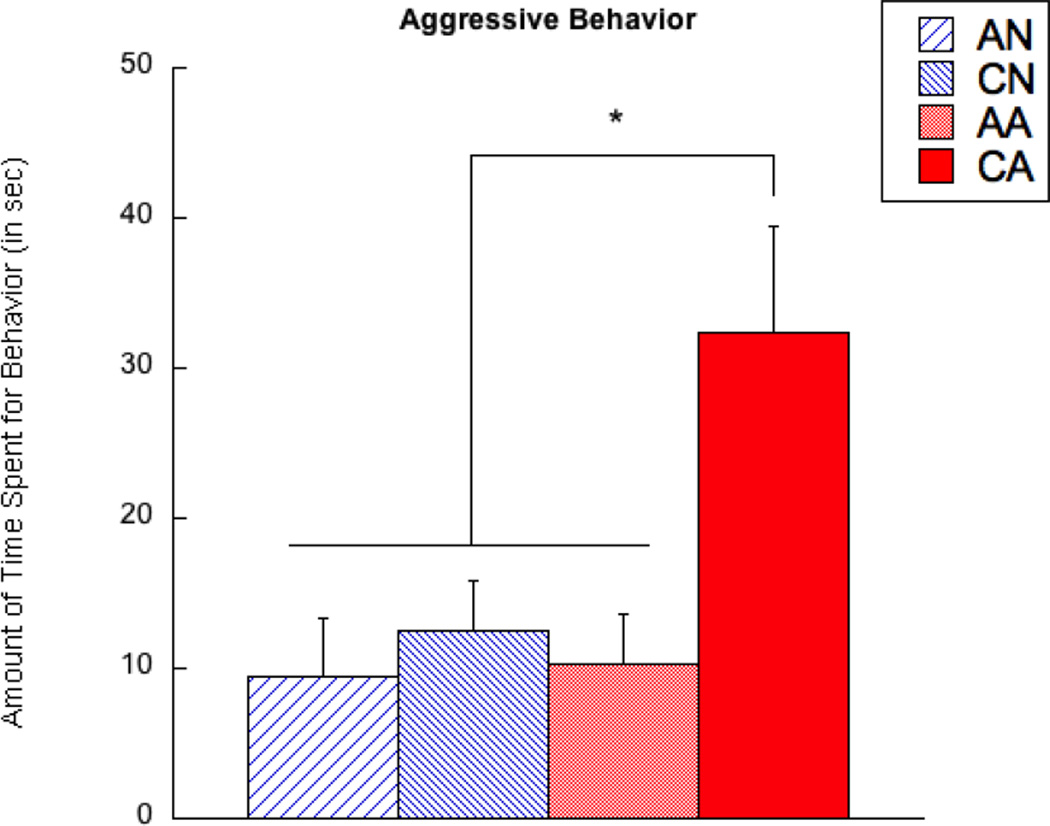
For testing aggressive behavior in the observer rats, there were significant main effects of exposure length (F(1,67) = 11.51, p < 0.01) and exposure condition (F(1,67) = 5.32, p < 0.05). Furthermore, there was a significant interaction between exposure length and exposure condition (F(1,67) = 4.44, p < 0.05). As Figure 2 illustrates, the CA group showed more aggressive than any other groups (p < 0.05), whereas there were no other pair-wise differences.
Figure 2.
Aggression of observer rats that were exposed to aggression and controls. AN = acute exposure to non-aggression; CN = chronic exposure to non-aggression; AA = acute exposure to aggression; CA = chronic exposure to aggression. A bar represents a standard error of the mean. *p < 0.05; **p < 0.01.
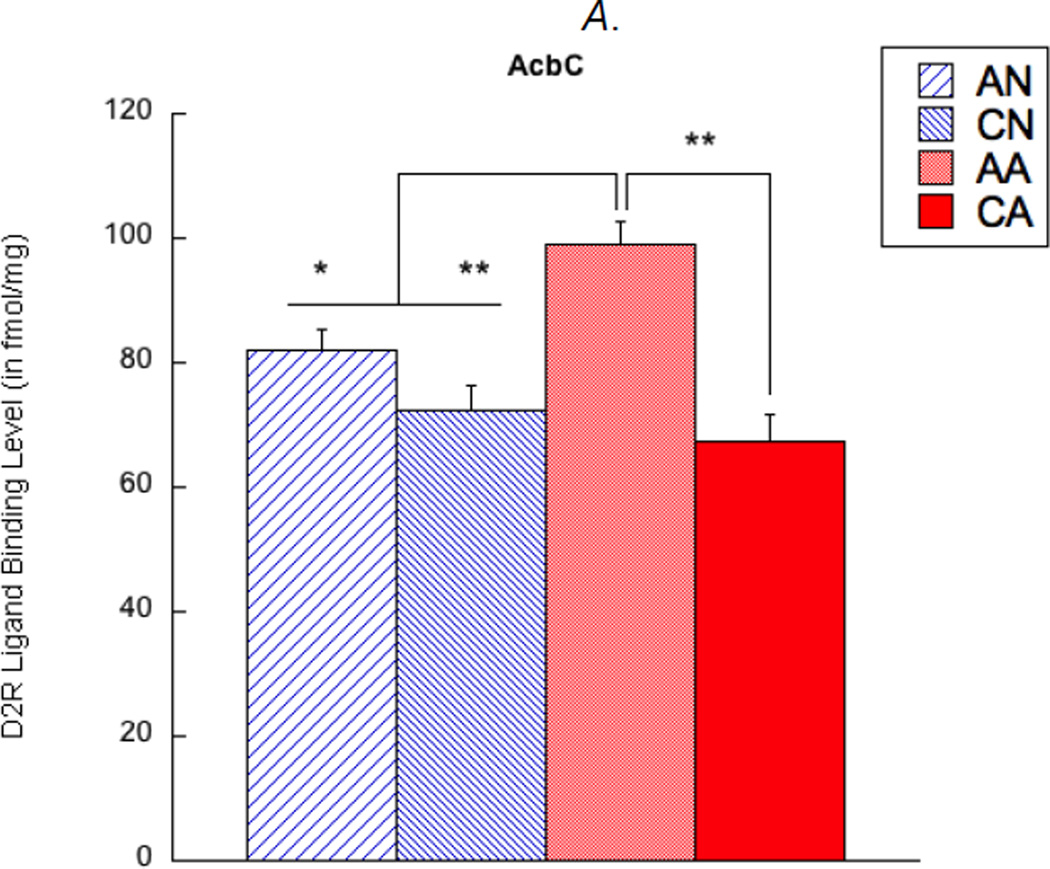
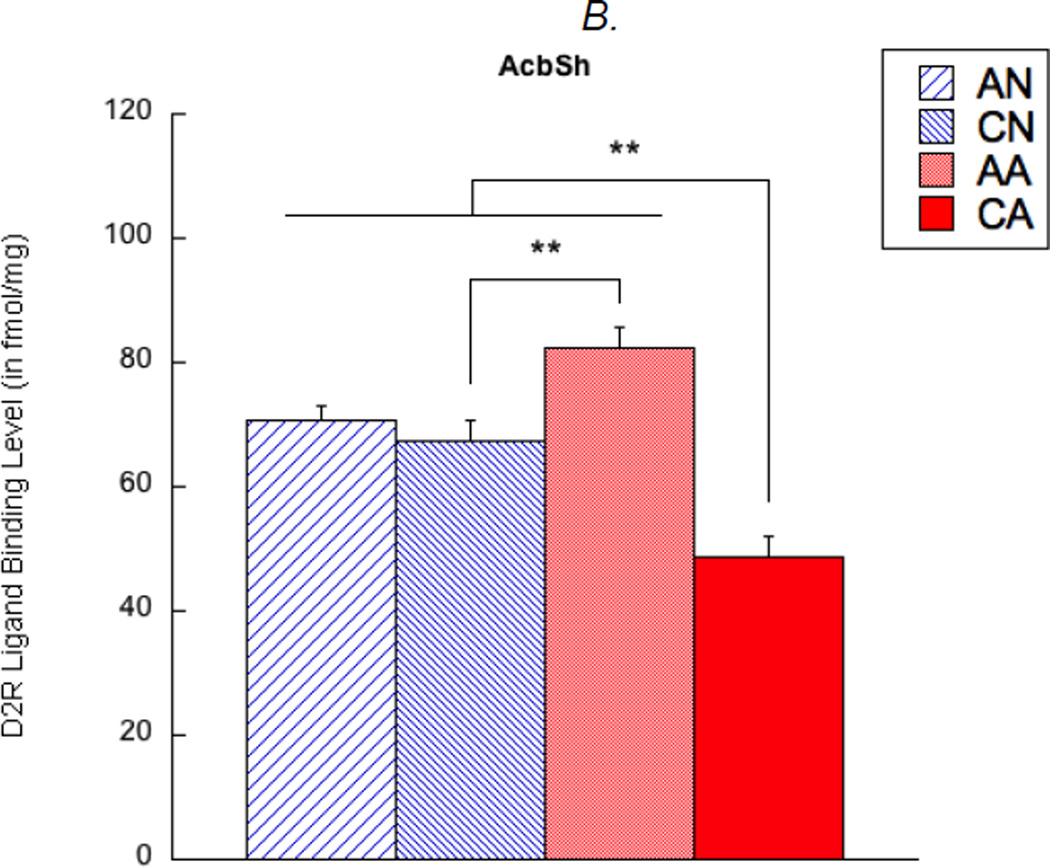
In addition, D2R density was examined in the nucleus accumbens of the observer rats. There were a main effect of exposure length (F(1,67) = 21.57, corrected p < 0.01) and an interaction effect (F(1,67) = 7.82, corrected p < 0.05) on D2R density in the AcbC. Specifically, the AA group showed higher D2R in the AcbC than any other groups (p < 0.05) (see Figure 3A). In addition, a main effect of exposure length (F(1,67) = 32.45, corrected p < 0.01) and an interaction effect (F(1,67) = 23.89, corrected p < 0.01) were found in D2R density in the AcbSh. Here, the CA group showed lower D2R in the AcbSh than any other groups (p < 0.01), and the AA group showed higher D2R than the CN group (p < 0.01; see Figure 3B). Main effects of exposure condition and hemisphere, as well as any other interactions, were not found in these analyses.
Figure 3.
Dopamine D2 receptor (D2R) ligand binding levels in the nucleus accumbens core (AcbC, shown in A) and shell (AcbSh, shown in B). AN = acute exposure to non-aggression; CN = chronic exposure to non-aggression; AA = acute exposure to aggression; CA = chronic exposure to aggression. A bar represents a standard error of the mean. *p < 0.05; **p < 0.01.
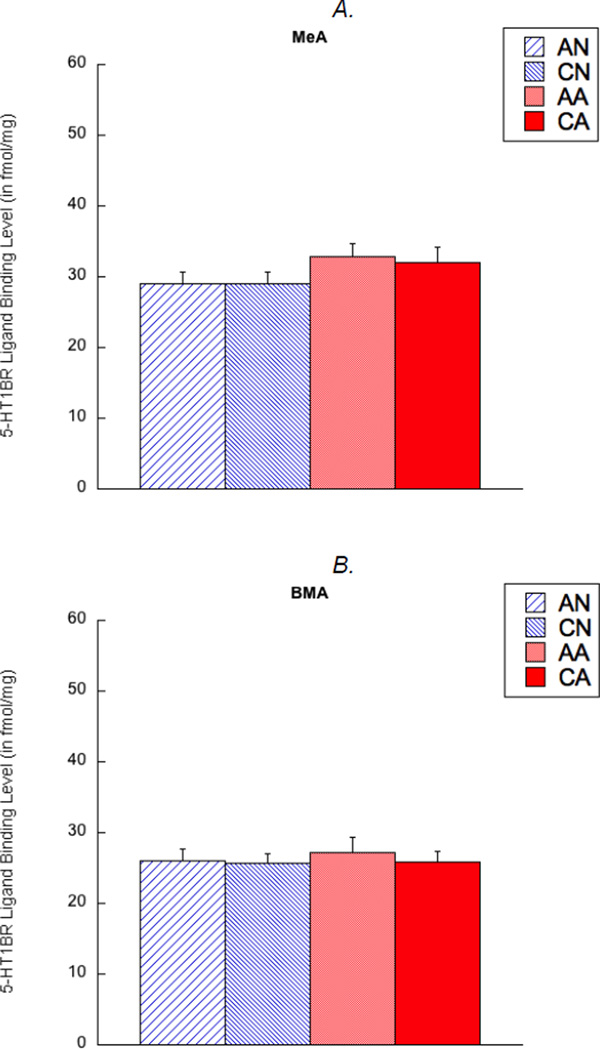
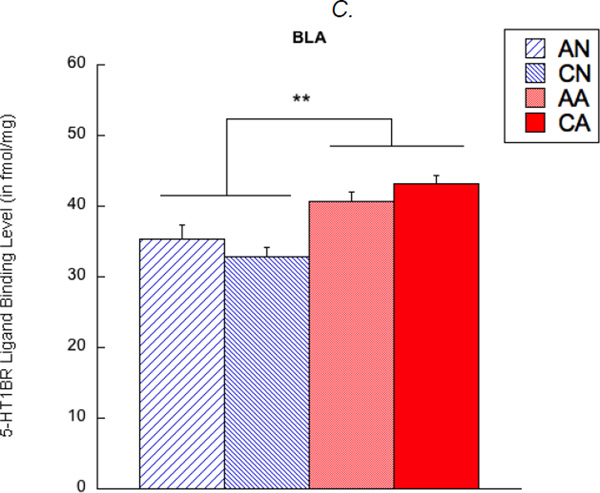
5-HT1BR density was also examined in the amygdala of the observer rats. A main effect of exposure condition was found in 5-HT1BR density in the BLA (F(1,67) = 28.80, corrected p < 0.01); the rats exposed to an aggression (AA and CA rats) showed higher 5-HT1BR density than the rats exposed to a non-aggression (AN and CN rats) (see Figure 5C). In contrast, no main effects of exposure length and hemisphere, as well as any interaction, were found.
Figure 5.
5-HT1B receptor (5-HT1BR) ligand binding levels in the medial amygdala (MeA, shown in A), basomedial amygdala (BMA, shown in B), and basolateral amygdala (BLA, shown in C). AN = acute exposure to non-aggression; CN = chronic exposure to non-aggression; AA = acute exposure to aggression; CA = chronic exposure to aggression. A bar represents a standard error of the mean. *p < 0.05; **p < 0.01.
Associations among D2R and 5-HT1BR
The above ANOVA results identified three biomarkers for passive exposure to aggression: D2R densities in the AcbC and AcbSh and 5-HT1BR density in the BLA. Thus, we further examined whether these three local receptor densities were correlated with each other. Because there was no effect of hemisphere in the above results, each local receptor density value was averaged over the left and right hemispheres to simplify our subsequent analyses and to control for Type II error rate.
Table 2 illustrates results of partial correlations (with age as a covariate) with Bonferroni correction in pooled subjects, as well as within each group. In general, rats showed a significant positive correlation between D2R density in the AcbC and D2R density in the AcbSh. Nonetheless, any other partial correlations were not found, although there were marginal correlations between (1) D2R density in the AcbC and 5-HT1BR density in the BLA among the acute exposure groups (p = 0.057) and (2) D2R density in the AcbSh and 5-HT1BR density in the BLA among the chronic exposure groups (p = 0.051).
Table 2.
Partial Correlations Among Exposure Length, Exposure Condition, and Identified Receptor Densities (N = 72)
| D2R in AcbSh | 5-HT1BR in BLA | |||||||
|---|---|---|---|---|---|---|---|---|
| All groups | ||||||||
| D2R in AcbC | 0.84** | 0.16 | ||||||
| D2R in AcbSh | --- | −0.05 | ||||||
| Exposure length | Acute | Chronic | Acute | Chronic | ||||
| D2R in AcbC | 0.79** | 0.79** | 0.40 | −0.08 | ||||
| D2R in AcbSh | --- | --- | 0.28 | −0.40 | ||||
| Exposure condition | Non-aggression | Aggression | Non-aggression | Aggression | ||||
| D2R in AcbC | 0.87** | 0.87** | 0.26 | −0.07 | ||||
| D2R in AcbSh | --- | --- | 0.19 | −0.14 | ||||
| Exposure length × exposure condition | AN | CN | AA | CA | AN | CN | AA | CA |
| D2R in AcbC | 0.75** | 0.94** | 0.72** | 0.81** | 0.50 | −0.06 | −0.06 | 0.10 |
| D2R in AcbSh | --- | --- | --- | --- | 0.31 | 0.04 | −0.03 | −0.07 |
Note: D2R = dopamine D2 receptor density; 5-HT1BR = 5-HT1B receptor density; AcbC = core of the nucleus accumbens; AcbSh = shell of the nucleus accumbens; BLA = basolateral amygdala; AN = acute exposure to non-aggression; CN = chronic exposure to non-aggression; AA = acute exposure to aggression; CA = chronic exposure to aggression.
Age was covariated in all analyses.
p < 0.05;
p < 0.01.
D2R and 5-HT1BR Densities in Relation to Aggressive Behavior
We further used a hierarchical regression to predict aggressive behavior in the observer rats; the first step included all main effects of the identified receptor densities, the second step added all possible two-way interactions, and the third step added the three-way interaction (see Table 3). In pooled subjects, the first step (F(4,67) = 4.84, p < .01) revealed that, with other variables constant, D2R densities in the AcbC and AcbSh respectively contributed to predicting aggressive behavior. Specifically, aggression increased as D2R density in the AcbC increased or as D2R density in the AcbSh decreased. In contrast, 5-HT1BR density in the BLA was not associated with aggression directly. The second step (F(7,64) = 3.53, p < .01) showed that the two-way interaction between D2R density in the AcbSh and 5-HT1BR density in the BLA, but not the other two-way interactions, significantly contributed to predicting aggressive behavior. Finally, the third step (F(8,63) = 3.04, p < .01) indicated that the three-way interaction was not a significant predictor of aggression. We also performed simple regression analysis within each group using Bonferroni corrections. Nevertheless, none of the above identified effects remained significant, although this might be due to reduced statistical power. Therefore, aggressive behavior was associated with (1) D2R density in the AcbC and (2) a combination of D2R density in the AcbSh and 5-HT1BR density in the BLA respectively, regardless of exposure to aggression.
Table 3.
Summary of Hierarchical Regression Analysis Predicting Aggressive Behavior in Pooled Subjects (N = 72) and Post Hoc Simple Regression Analysis in Each Group (n = 18)
| All | AN | CN | AA | CA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables |
B (SE B) |
β |
B (SE B) |
Β |
B (SE B) |
β |
B (SE B) |
β |
B (SE B) |
β |
| Step 1 | ||||||||||
| AcbC | 0.71 (0.23) |
0.67** | 0.23 (0.28) |
0.19 | 0.37 (0.19) |
0.46 | 0.13 (0.21) |
0.15 | 0.42 (0.35) |
0.26 |
| AcbSh | −0.99 (0.26) |
−0.81** | 0.12 (0.39) |
0.07 | 0.50 (0.23) |
0.50 | −0.01 (0.24) |
−0.01 | −0.12 (0.51) |
−0.05 |
| BLA | 0.02 (0.34) |
0.01 | 0.48 (0.45) |
0.24 | −0.01 (0.62) |
−0.00 | −0.79 (0.65) |
−0.31 | −0.15 (1.43) |
−0.02 |
| Step 2 | ||||||||||
| AcbC | 0.59 (0.24) |
0.56* | --- | --- | --- | --- | --- | --- | --- | --- |
| AcbSh | −0.78 (0.27) |
−0.64** | --- | --- | --- | --- | --- | --- | --- | --- |
| BLA | 0.05 (0.34) |
0.02 | --- | --- | --- | --- | --- | --- | --- | --- |
| AcbC × AcbSh | 0.00 (0.01) |
0.06 | −0.01 (0.02) |
−0.17 | 0.01 (0.01) |
0.17 | −0.00 (0.01) |
−0.12 | 0.01 (0.02) |
0.05 |
| AcbC × BLA | 0.05 (0.03) |
0.29 | 0.00 (0.04) |
0.01 | −0.04 (0.02) |
−0.37 | −0.02 (0.02) |
−0.22 | 0.05 (0.05) |
0.25 |
| AcbSh × BLA | −0.08 (0.04) |
−0.42* | 0.02 (0.05) |
0.08 | −0.08 (0.04) |
−0.49 | −0.02 (0.04) |
−0.12 | −0.02 (0.05) |
−0.08 |
| Step 3 | ||||||||||
| AcbC | 0.60 (0.24) |
0.56* | --- | --- | --- | --- | --- | --- | --- | --- |
| AcbSh | −0.78 (0.28) |
−0.64** | --- | --- | --- | --- | --- | --- | --- | --- |
| BLA | 0.00 (0.45) |
0.00 | --- | --- | --- | --- | --- | --- | --- | --- |
| AcbC × AcbSh | 0.00 (0.01) |
0.06 | --- | --- | --- | --- | --- | --- | --- | --- |
| AcbC × BLA | 0.04 (0.03) |
0.28 | --- | --- | --- | --- | --- | --- | --- | --- |
| AcbSh × BLA | −0.08 (0.04) |
−0.41 | --- | --- | --- | --- | --- | --- | --- | --- |
| AcbC × AcbSh × BLA | 0.00 (0.00) |
0.02 | 0.00 (0.00) |
0.23 | 0.00 (0.00) |
−0.20 | 0.00 (0.00) |
−0.13 | 0.00 (0.00) |
0.03 |
Note: R2 = 0.22 for Step 1 in all rats; ΔR2 = 0.05 for Step 2 in all rats; ΔR2 = 0.00 for Step 3 in all rats. AcbC = dopamine D2 receptor density in the core of the nucleus accumbens; AcbSh = dopamine D2 receptor density in the shell of the nucleus accumbens; BLA = 5-HT1B receptor density in the basolateral amygdala. AN = acute exposure to non-aggression; CN = chronic exposure to non-aggression; AA = acute exposure to aggression; CA = chronic exposure to aggression.
Age was covaried in all steps.
p < 0.05;
p < 0.01.
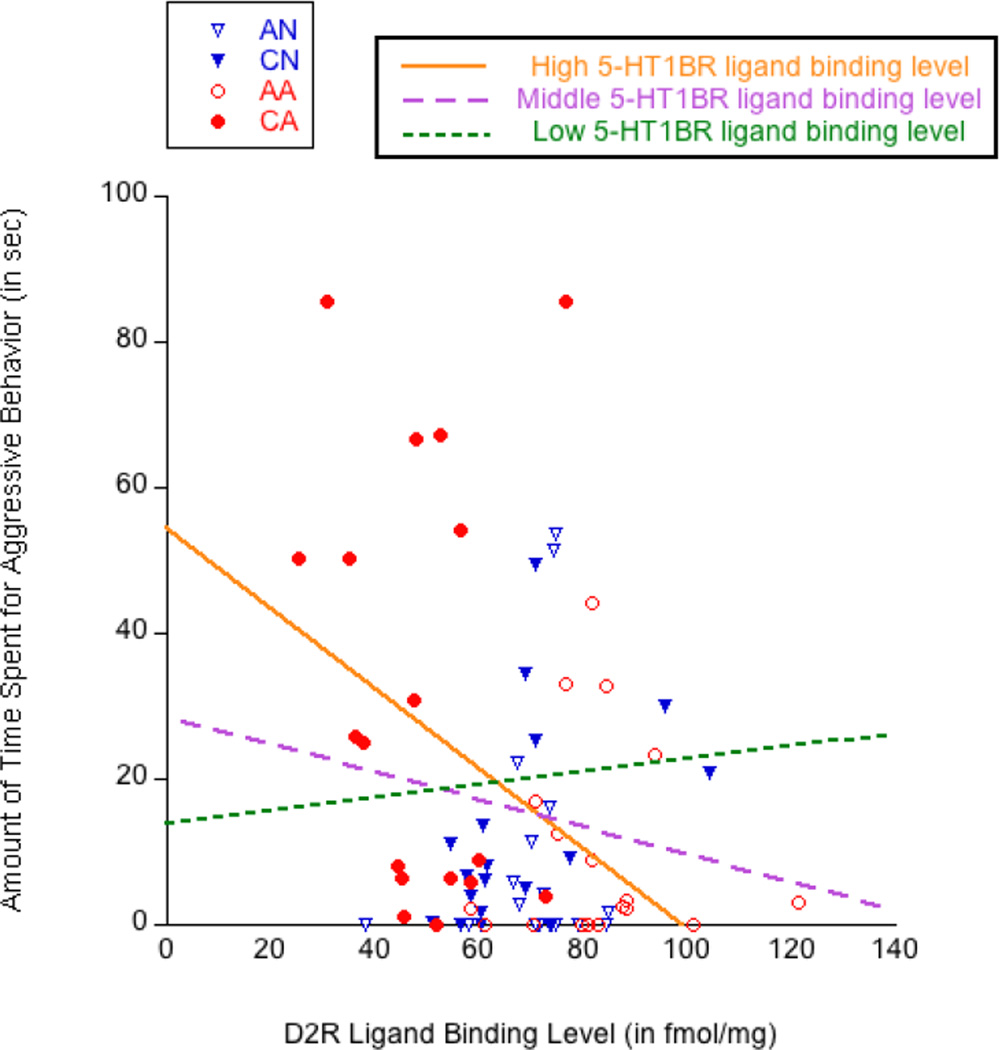
To visualize the interaction between D2R density in the AcbSh and 5-HT1BR density in the BLA, Figure 7 shows a scatter plot describing the association between D2R density in the AcbSh and aggression, moderated by three regression lines with different levels of 5-HT1BR density in the BLA. Each line represents the slope for aggression on D2R density in the AcbSh, while 5-HT1BR density in the BLA was held at either a high value (centered around its mean plus one standard deviation), a middle value (centered around its mean), or a low value (centered around its mean minus one standard deviation). A orange straight line, representing the condition of high 5-HT1BR density in the BLA, indicates the stronger negative association between D2R density in the AcbSh and aggression (B = −0.55, constant = 54.35) than a magenta dashed line, representing the condition of average 5-HT1BR density in the BLA (B = −0.19, constant = 28.40), and a green dotted line, representing the condition of low 5-HT1BR density in the BLA (B = 0.17, constant = 2.45). Therefore, aggression increased as D2R density in the AcbSh was low, especially when 5-HT1BR density in the BLA was high.
Figure 7.
Scatter plot representing the relationship between aggressive behavior and dopamine D2 receptor (D2R) density in the shell of the nucleus accumbens (AcbSh), moderated by 5-HT1B receptor (5-HT1BR) density in the basolateral amygdala (BLA) at a high value (orange line), middle value (purple line), and low value (green line).
The purpose of this study was to test our hypotheses that, in contrast to acute exposure, chronic exposure to aggression would lead observer rats to show (1) higher levels of impulsive aggression, (2) lower D2R density in the AcbSh, and (3) higher 5-HT1BR density in the BLA. We also hypothesized any association between D2R density and 5-HT1BR density, which altogether predicted impulsive aggressive behavior. Four major findings were obtained.
First, our results revealed that observer rats showed increased impulsive aggressive behavior only when they were passively exposed to aggressive situations chronically. In contrast, acute exposure to aggression did not increase impulsivity/aggressiveness in observer rats, compared to chronic exposure to aggression. These findings exactly replicated previous study (Suzuki & Lucas, 2010). Therefore, while a single-time observation of aggression does not necessarily lead to social learning of aggression in observers, repeated observation of aggression is a risk factor socializing observers to learn aggressive manners (Huesmann & Kirwil, 2007).
Second, all accumbal regions, regardless of hemisphere, generally showed lower D2R density in chronic exposure conditions than acute exposure conditions, and this effect further depended on whether observer rats were exposed to aggression or not. In particular, compared with non-aggression exposure control conditions, acute passive exposure to aggression increased D2R density in the AcbC, whereas chronic passive exposure to aggression downregulated D2R density in the AcbSh. These contrasting patterns may reflect that the AcbC and AcbSh actually have differential functions. For example, Bassareo et al. (2002) found that, although dopaminergic activity in both AcbC and AcbSh is activated by novel appetitive stimuli, only dopamine response in the AcbSh is then habituated and reduced following repeated appetitive stimuli. Thus, assuming that aggression has some rewarding properties (May & Kennedy, 2009), acute passive exposure to aggression may rapidly enhance dopaminergic activity by upregulating D2R density in the AcbC and AcbSh, as seen in the AA group (see Figure 3A). However, once dopaminergic activity became habituated by repeated exposure to aggression, this might abruptly reduce D2R density in the AcbSh (but not AcbC), as seen in the CA group (see Figure 3B). Future research needs to test this hypothesis.
Interestingly, a similar downregulated D2R density in the AcbSh has been found following chronic administration of cocaine (Moore, Vinsant, Nader, Porrino, & Friedman, 1998; Nader et al., 2002), morphine (Hemby, 2004), and anabolic-androgenic steroids (Kindlundh, Lindblom, Bergstrom, & Nyberg, 2003). On the other hand, other studies have addressed the issue that chronic use of psychostimulants induces extra release of dopamine in the nucleus accumbens (Hernandez & Hoebel, 1988; Weiss, Paulus, Lorang, & Koob, 1992). Taken together, high dopamine release may be correlated with low D2R density in the AcbSh, suggesting that downregulated D2R may result from a compensatory function to maintain dopamine activity. In the present study, chronic passive exposure to aggression may produce effects on D2R similar to long-term dose of psychostimulants, as indicated by low D2R density in the CA group. Alternatively, the downregulation of D2R following chronic passive exposure to aggression may be subject to increased dopamine release in the AcbSh, which would be an intrinsically rewarding/salient signal for observer rats. In contrast, note that chronic stress is not associated with a compensatory downregulation of D2R density in the AcbSh immediately after stress (Lucas, Wang, McCall, & McEwen, 2007) or even after a recovery period (Lucas et al., 2004; Yohe et al., 2012). Accordingly, our findings were less likely to be confounded with any social stress effect (Tzanoulinou, Riccio, de Boer, & Sandi, 2014; Wommack & Delville, 2007), as no group difference was observed in the levels of serum corticosterone immediately following passive exposure (see Table 1).
The third major finding was that 5-HT1BR density in the BLA, but not the other amygdaloid nuclei, was bilaterally upregulated in the observer rats exposed to aggression, and this finding was present regardless of exposure length. A previous study has reported that increased 5-HT1BR density in the BLA was identified following chronic passive exposure to aggression (Suzuki et al., 2010b), but our current results extended these findings. That is, 5-HT1BR density in the BLA can be rapidly upregulated following even a single-time exposure to aggression. The subregional difference in 5-HT1BR density might explain some features of aggressive behavior in observer rats. For example, the MeA plays a role of emotion generation, such as fear-induced aggression (Siegel, Bhatt, Bhatt, & Zalcman, 2007), and has neural projections to the hypothalamus (Sah, Faber, Lopez De Armentia, & Power, 2003), which is essentially related to fearful and subordinate behavior in a social context (Motta et al., 2009). In contrast, passive exposure to aggression did not affect 5-HT1BR density in the MeA and, thus, was not presumably related to self-defensive aggression or any fear-related aggression. Rather, passive exposure to aggression changed structure of the BLA, which is involved in associative learning of emotions (e.g., emotional acquisition and conditioning) and shows neural projections to the striatum, nucleus accumbens, and prefrontal cortex (Sah et al., 2003). This suggests that exposure to aggression might initiate an emotional learning process to make aggression accessible as the socio-behavioral repertoire. Further studies should clarify this hypothesis.
Finally, Table 3 shows that increased impulsive aggression was associated with (1) high D2R density in the AcbC, (2) low D2R density in the AcbSh, and (3) a combination of low D2R density in the AcbSh and high 5-HT1BR density in the BLA. However, our follow-up, subgroup analysis of simple regressions showed that these identified associations did not remain significant in the condition of passive exposure to aggression. Although a lack of findings in our subgroup analysis might be due to the small group size (n = 18 each), our results indicated that the accumbal D2R and/or amygdaloid 5HT1BR was/were generally linked with aggression, regardless of passive exposure to aggression. The positive association between impulsive aggression and D2R density in the AcbC was somewhat unexpected because D2R density in the AcbC was actually lower in the CA group (which exclusively showed increased aggression) than in the AN and CN control groups. But, regardless of D2R levels in the AcbC, D2R density in the AcbSh showed a negative association with impulsive aggression, and this association was moderated by high 5-HT1BR density in the BLA (see Figure 7). Because these behavioral and neurochemical outcomes resulted from chronic passive exposure to aggression, we propose that the interaction between D2R in the AcbSh and 5-HT1BR in the BLA on impulsive aggression provides a neurobiological perspective of why observers exposed to aggression chronically are at high risk for being aggressive. That is, chronic passive exposure to aggression downregulates D2R density in the AcbSh and upregulates 5-HT1BR density in the BLA in observers, and these neurochemical profiles are significantly associated with increased impulsive aggression.
Our findings on the interaction between the AcbSh and BLA may have some implications in social learning of aggression. Generally, the BLA receives sensory inputs from the thalamus, hippocampus, and cortex (Davis & Whalen, 2001) and is involved in associative learning of emotional behavior (Sah et al., 2003), such as contextual fear conditioning (Fenton, Spicer, Halliday, Mason, & Stevenson, 2013; Herry et al., 2008; Maren, Poremba, & Gabriel, 1991) and social defeat conditioning (Morrison & Cooper, 2012). 5-HT1BR in the BLA is specifically associated with impulsive/aggressive trends, as evident by a higher amount of binding of 5-HT1BR in pathologically aggressive animals than normally behaving animals (Jacobs et al., 2007). In our paradigm, circumstances that provided an aggressive situation upregulated 5-HT1BR density in the BLA in passive observers. This may reflect associative learning of aggression, such that observer rats learned to associate an aggressive social interaction and its consequence (e.g., defeat) in a social encounter. Our behavioral results indeed demonstrated that repeatedly observing aggressive circumstances was necessary to reinforce observers’ aggressive responses in later social encounters. We expect that such reinforcing effects were probably related to D2R in the AcbSh because dopaminergic activity in the AcbSh, which is actually modulated by the BLA (Jackson & Moghaddam, 2001), plays important roles in motivational valence (i.e., aversive vs. rewarding) (Bassareo et al., 2002; Jentsch & Taylor, 1999; Shirayama & Chaki, 2006). Interestingly, the intra-AcbSh infusion of a D2R antagonist, which acts to simulate low D2R availability, (1) switches an animal’s response from aversion to reward (Bernal et al., 2008; Laviolette, Lauzon, Bishop, Sun, & Tan, 2008), (2) disrupts the inhibitory control of hedonic behavior (Halpern et al., 2013), (3) increases appetitive social interaction (Thompson, Leonard, & Brudzynski, 2006), (4) facilitates self-administration of cocaine (Bachtell, Whisler, Karanian, & Self, 2005), and (5) increases impulsive behavior (Besson et al., 2010). Thus, low D2R in the AcbSh is related to high reward-seeking behavior. Furthermore, reduced D2R density could reflect a compensatory function for excessive dopamine release, which induces intrinsic rewards; although, to our knowledge, no studies have directly examined the relation between D2R and extracellular concentration of dopamine, a number of separate studies on drug use has consistently shown that chronic use of psychostimulants results in low D2R density in the AcbSh (Hemby, 2004; Kindlundh et al., 2003; Moore et al., 1998; Nader et al., 2002) and excessive dopamine release in the nucleus accumbens (Hernandez & Hoebel, 1988; Weiss et al., 1992). Based on these findings, in our paradigm, repeatedly observing aggressive circumstances might accumulatively activate dopamine release in the nucleus accumbens. Consequently, D2R binding in the AcbSh was reduced as a compensatory function. Nevertheless, the drawback of the compensatory reduction of D2R density is that postsynaptic sensitivity to dopamine neurotransmission could be blunted if presynaptic dopamine release recovered to the baseline. Accordingly, after being removed from chronic passive exposure to aggression, observer rats may experience blunted sensitivity to dopamine release (i.e., deficiency in dopamine-related rewards) and be motivated to fulfill their demands for dopamine. Their deficiency in dopamine may be treated by reward-seeking behavior, such as performing aggressive behavior (May & Kennedy, 2009). Taken all of the above environmental, psychological, and neurochemical factors together, our results indicated that the combined effects of high 5-HT1BR density in the BLA (which may represent associative learning of aggression processed by exposure to aggression) and low D2R density in the AcbSh (which may represent reinforcing and rewarding qualities of aggression increased by repeated exposure to aggression) motivated observer rats to interact with a naïve rat aggressively.
Nevertheless, the following study limitations need to be noted: although our findings indicated the linkage among aggressive behavior, D2R density, and 5-HT1BR density, there is still uncertainty with respect to a causal relationship among them. Moreover, it is still unclear whether there is an age difference in vulnerability to chronic exposure to aggression. On average, the postnatal day (P) in our sample of observer rats was specifically 44 days at the beginning of our exposure paradigm and 64 days at the time of assessing aggressive behavior. In a rat’s lifespan, P44 is around the late stage of periadolescence, and P64 is at the stage of young adulthood (Sengupta, 2013). A replication of our results may depend on the timing of being exposed to aggression (Mrug et al., 2014; Veenit, Cordero, Tzanoulinou, & Sandi, 2013) and/or the timing of onset aggression (Cleverley, Szatmari, Vaillancourt, Boyle, & Lipman, 2012; Hartup, 2005). More research is needed to clarify the developmental vulnerability to chronic exposure to aggression.
In summary, this current study used a novel rat paradigm to examine the behavioral and neurochemical effects of passive exposure to aggression. Within this paradigm, it was demonstrated that chronic passive exposure to aggression increased impulsive aggressive behavior and reduced D2R density in the AcbSh among observer rats; in contrast, these effects were not found in acute exposure to aggression. In addition, as soon as observer rats were exposed to aggression, 5-HT1BR density in the BLA also increased. Furthermore, we also found that a combination of low D2R density in the AcbSh and high 5-HT1BR density in the BLA was associated with a high risk for impulsivity/aggressiveness. Taken together, we conclude that repeated observations of aggression promote a number of neurobiological effects by downregulating D2R density in the AcbSh and upregulating 5-HT1B in the BLA, whereby observers are inclined to show increased impulsive aggression.
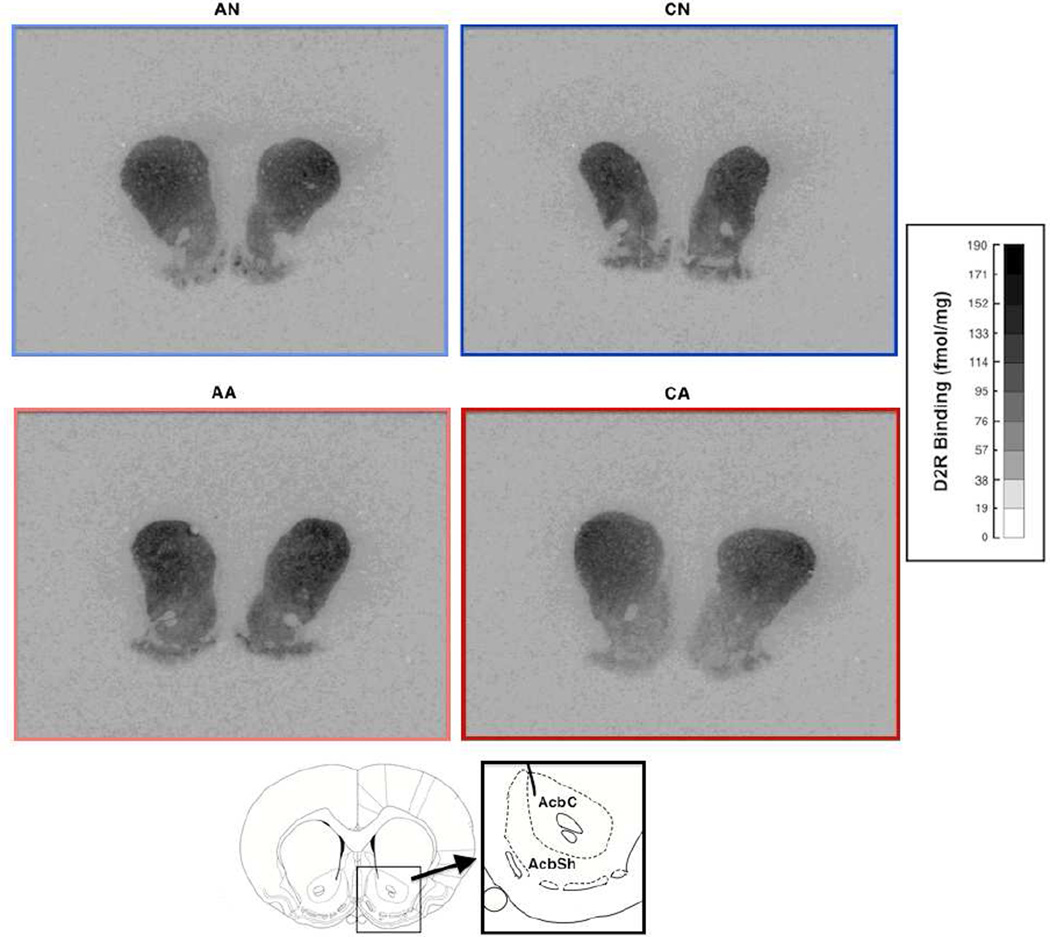
Figure 4.
Representative images of dopamine D2 receptor density in the nucleus accumbens. Darker gray indicates higher density. AN = acute exposure to non-aggression; CN = chronic exposure to non-aggression; AA = acute exposure to aggression; CA = chronic exposure to aggression. The atlas was cited from Paxinos and Watson (2005).
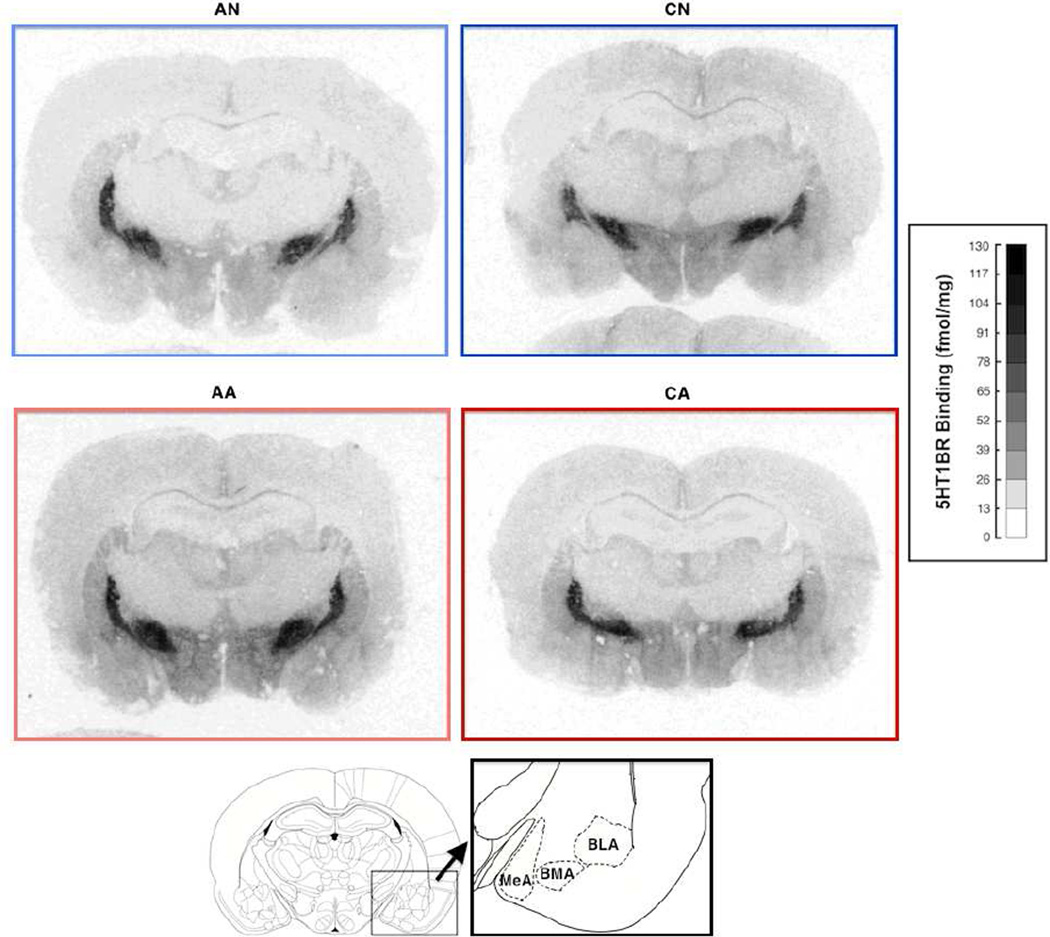
Figure 6.
Representative images of 5-HT1B receptor density in the amygdala. Darker gray indicates higher density. AN = acute exposure to non-aggression; CN = chronic exposure to non-aggression; AA = acute exposure to aggression; CA = chronic exposure to aggression. *p < 0.05; **p < 0.01. The atlas was cited from Paxinos and Watson (2005).
Acknowledgments
This work was supported by Loyola University Chicago Research Support grant (L.R.L). H.S.’s time was supported by NIH Grants MH090786 and MH098099.
Footnotes
There is no conflict of interests.
References
- Anderson CA, Huesmann LR. Human aggression: A social-cognitive view. In: Hogg MA, Cooper JC, editors. The Sage handbook of social psychology. Thousand Oaks: Sage Publications; 2003. pp. 296–323. [Google Scholar]
- Arrant AE, Jemal H, Kuhn CM. Adolescent male rats are less sensitive than adults to the anxiogenic and serotonin-releasing effects of fenfluramine. Neuropharmacology. 2013;65:213–222. doi: 10.1016/j.neuropharm.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachtell RK, Whisler K, Karanian D, Self DW. Effects of intra-nucleus accumbens shell administration of dopamine agonists and antagonists on cocaine-taking and cocaine-seeking behaviors in the rat. Psychopharmacology. 2005;183(1):41–53. doi: 10.1007/s00213-005-0133-1. [DOI] [PubMed] [Google Scholar]
- Bandura A. Aggression: a social learning analysis. Englewood Cliffs, NJ: Prentice-Hall; 1973. [Google Scholar]
- Bandura A. Social learning theory. Englewood Cliffs, NJ: Prentice-Hall; 1977. [Google Scholar]
- Bandura A, Ross D, Ross SA. Transmission of aggression through imitation of aggressive models. Journal of abnormal and social psychology. 1961;63:575–582. doi: 10.1037/h0045925. [DOI] [PubMed] [Google Scholar]
- Bandura A, Ross D, Ross SA. Imitation of film-mediated agressive models. Journal of abnormal and social psychology. 1963;66:3–11. doi: 10.1037/h0048687. [DOI] [PubMed] [Google Scholar]
- Bassareo V, De Luca MA, Di Chiara G. Differential Expression of Motivational Stimulus Properties by Dopamine in Nucleus Accumbens Shell versus Core and Prefrontal Cortex. J Neurosci. 2002;22(11):4709–4719. doi: 10.1523/JNEUROSCI.22-11-04709.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer NS, Herrenkohl TI, Lozano , Rivara FP, Hill KG, Hawkins JD. Childhood bullying involvement and exposure to intimate partner violence. Pediatrics. 2006;118(2):e235–e242. doi: 10.1542/peds.2005-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beiderbeck DI, Reber SO, Havasi A, Bredewold R, Veenema AH, Neumann ID. High and abnormal forms of aggression in rats with extremes in trait anxiety--involvement of the dopamine system in the nucleus accumbens. Psychoneuroendocrinology. 2012;37(12):1969–1980. doi: 10.1016/j.psyneuen.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Berenson AB, Wiemann CM, McCombs S. Exposure to violence and associated health-risk behaviors among adolescent girls. Archives of Pediatrics & Adolescent Medicine. 2001;155(11):1238–1242. doi: 10.1001/archpedi.155.11.1238. [DOI] [PubMed] [Google Scholar]
- Bernal SY, Dostova I, Kest A, Abayev Y, Kandova E, Touzani K, Bodnar RJ. Role of dopamine D1 and D2 receptors in the nucleus accumbens shell on the acquisition and expression of fructose-conditioned flavor-flavor preferences in rats. Behav Brain Res. 2008;190(1):59–66. doi: 10.1016/j.bbr.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology. 2007;191(3):391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Besson M, Belin D, McNamara R, Theobald DE, Castel A, Beckett VL, Dalley JW. Dissociable control of impulsivity in rats by dopamine d2/3 receptors in the core and shell subregions of the nucleus accumbens. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35(2):560–569. doi: 10.1038/npp.2009.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard DC, Blanchard RJ. Behavioral correlates of chronic dominance-subordination relationships of male rats in a seminatural situation. Neuroscience & Biobehavioral Reviews. 1990;14(4):455–462. doi: 10.1016/s0149-7634(05)80068-5. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Sakai RR, McEwen B, Weiss SM, Blanchard RJ. Subordination stress: behavioral, brain, and neuroendocrine correlates. Behav Brain Res. 1993;58(1–2):113–121. doi: 10.1016/0166-4328(93)90096-9. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Spencer RL, Weiss SM, Blanchard RJ, Mcewen B, Sakai RR. Visible burrow system as a model of chronic social stress: Behavioral and neuroendocrine correlates. Psychoneuroendocrinology. 1995;20(2):117–134. doi: 10.1016/0306-4530(94)e0045-b. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Yudko E, Dulloog L, Blanchard DC. Defense changes in stress nonresponsive subordinate males in a visible burrow system. Physiol Behav. 2001;72(5):635–642. doi: 10.1016/s0031-9384(00)00449-2. [DOI] [PubMed] [Google Scholar]
- Bustamante D, Quintanilla ME, Tampier L, Gonzalez-Lira V, Israel Y, Herrera-Marschitz M. Ethanol induces stronger dopamine release in nucleus accumbens (shell) of alcohol-preferring (bibulous) than in alcohol-avoiding (abstainer) rats. European journal of pharmacology. 2008;591(1–3):153–158. doi: 10.1016/j.ejphar.2008.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon WB. The wisdom of the body. New York: W. W. Norton; 1939. [Google Scholar]
- Caramaschi D, de Boer SF, de Vries H, Koolhaas JM. Development of violence in mice through repeated victory along with changes in prefrontal cortex neurochemistry. Behav Brain Res. 2008;189(2):263–272. doi: 10.1016/j.bbr.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Cleverley K, Szatmari , Vaillancourt T, Boyle M, Lipman E. Developmental trajectories of physical and indirect aggression from late childhood to adolescence: sex differences and outcomes in emerging adulthood. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51(10):1037–1051. doi: 10.1016/j.jaac.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Clotfelter ED, Paolino AD. Bystanders to contests between conspecifics are primed for increased aggression in male fighting fish. Animal Behaviour. 2003;66:343–347. [Google Scholar]
- Couppis MH, Kennedy CH. The rewarding effect of aggression is reduced by nucleus accumbens dopamine receptor antagonism in mice. Psychopharmacology. 2008;197(3):449–456. doi: 10.1007/s00213-007-1054-y. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular psychiatry. 2001;6(1):13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Desai RI, Paronis CA, Martin J, Desai R, Bergman J. Monoaminergic psychomotor stimulants: discriminative stimulus effects and dopamine efflux. The Journal of pharmacology and experimental therapeutics. 2010;333(3):834–843. doi: 10.1124/jpet.110.165746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon WJ. Simplified estimation from censored normal samples. The Annals of Mathematical Statistics. 1960;31(2):269–556. [Google Scholar]
- Emery CR. Controlling for selection effects in the relationship between child behavior problems and exposure to intimate partner violence. Journal of interpersonal violence. 2011;26(8):1541–1558. doi: 10.1177/0886260510370597. [DOI] [PubMed] [Google Scholar]
- Fantuzzo JW, DePaola LM, Lambert L, Martino T, Anderson G, Sutton S. Effects of interparental violence on the psychological adjustment and competencies of young children. Journal of consulting and clinical psychology. 1991;59(2):258–265. doi: 10.1037//0022-006x.59.2.258. [DOI] [PubMed] [Google Scholar]
- Feldker DE, Morsink MC, Veenema AH, Datson NA, Proutski V, Lathouwers D, Vreugdenhil E. The effect of chronic exposure to highly aggressive mice on hippocampal gene expression of non-aggressive subordinates. Brain Res. 2006;1089(1):10–20. doi: 10.1016/j.brainres.2006.02.110. [DOI] [PubMed] [Google Scholar]
- Fenton GE, Spicer CH, Halliday DM, Mason R, Stevenson CW. Basolateral amygdala activity during the retrieval of associative learning under anesthesia. Neuroscience. 2013;233:146–156. doi: 10.1016/j.neuroscience.2012.12.039. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, van Erp AM, Tornatzky W, Miczek KA. Accumbal dopamine and serotonin in anticipation of the next aggressive episode in rats. Eur J Neurosci. 2003;17(2):371–378. doi: 10.1046/j.1460-9568.2003.02447.x. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Melloni RH, Jr, Koppel G, Perry KW, Fuller RW, Delville Y. Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. The Journal of Neuroscience. 1997;17(11):4331–4340. doi: 10.1523/JNEUROSCI.17-11-04331.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CF, Stolberg T, Delville Y. Serotonin regulation of aggressive behavior in male golden hamsters (Mesocricetus auratus) Behav Neurosci. 1999;113(4):804–815. doi: 10.1037//0735-7044.113.4.804. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Stolberg T, Kulkarni , Murugavel M, Blanchard R, Blanchard DC, Simon NG. Imaging the neural circuitry and chemical control of aggressive motivation. BMC Neurosci. 2008;9:111. doi: 10.1186/1471-2202-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra NG, Huesmann LR, Spindler A. Community violence exposure, social cognition, and aggression among urban elementary school children. Child development. 2003;74(5):1561–1576. doi: 10.1111/1467-8624.00623. [DOI] [PubMed] [Google Scholar]
- Guerra NG, Huesmann LR, Tolan PH, Van Acker R, Eron LD. Stressful events and individual beliefs as correlates of economic disadvantage and aggression among urban children. Journal of consulting and clinical psychology. 1995;63(4):518–528. doi: 10.1037//0022-006x.63.4.518. [DOI] [PubMed] [Google Scholar]
- Halpern CH, Tekriwal A, Santollo J, Keating JG, Wolf JA, Daniels D, Bale TL. Amelioration of binge eating by nucleus accumbens shell deep brain stimulation in mice involves D2 receptor modulation. J Neurosci. 2013;33(17):7122–7129. doi: 10.1523/JNEUROSCI.3237-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartup WW, editor. The development of aggression. New York: The Guilford Press; 2005. [Google Scholar]
- Hemby SE. Morphine-induced alterations in gene expression of calbindin immunopositive neurons in nucleus accumbens shell and core. Neuroscience. 2004;126(3):689–703. doi: 10.1016/j.neuroscience.2004.01.056. [DOI] [PubMed] [Google Scholar]
- Hernandez L, Hoebel BG. Food reward and cocaine increase extracellular dopamine in the nucleus accumbens as measured by microdialysis. Life sciences. 1988;42(18):1705–1712. doi: 10.1016/0024-3205(88)90036-7. [DOI] [PubMed] [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Muller C, Luthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454(7204):600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- Holmes MR. The sleeper effect of intimate partner violence exposure: long-term consequences on young children’s aggressive behavior. Journal of child psychology and psychiatry, and allied disciplines. 2013;54(9):986–995. doi: 10.1111/jcpp.12071. [DOI] [PubMed] [Google Scholar]
- Huesmann LR, Kirwil L. Why observing violence increases the risk of violent behavior by the observer. In: Flannery DJ, Vazsonyi AT, Waldman ID, editors. The Cambridge handbook of violent behavior and aggression. New York: Cambridge University Press; 2007. pp. 545–570. [Google Scholar]
- Jackson ME, Moghaddam B. Amygdala regulation of nucleus accumbens dopamine output is governed by the prefrontal cortex. J Neurosci. 2001;21(2):676–681. doi: 10.1523/JNEUROSCI.21-02-00676.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs C, Van Den Broeck W, Simoens Neurons expressing serotonin-1B receptor in the basolateral nuclear group of the amygdala in normally behaving and aggressive dogs. Brain Res. 2007;1136(1):102–109. doi: 10.1016/j.brainres.2006.11.096. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146(4):373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Kilpatrick DG, Acierno R, Saunders B, Resnick HS, Best CL, Schnurr PP. Risk factors for adolescent substance abuse and dependence: data from a national sample. Journal of consulting and clinical psychology. 2000;68(1):19–30. doi: 10.1037//0022-006x.68.1.19. [DOI] [PubMed] [Google Scholar]
- Kindlundh AM, Lindblom J, Bergstrom L, Nyberg F. The anabolic-androgenic steroid nandrolone induces alterations in the density of serotonergic 5HT1B and 5HT2 receptors in the male rat brain. Neuroscience. 2003;119(1):113–120. doi: 10.1016/s0306-4522(03)00120-9. [DOI] [PubMed] [Google Scholar]
- Kleijn J, Wiskerke J, Cremers TI, Schoffelmeer AN, Westerink BH, Pattij T. Effects of amphetamine on dopamine release in the rat nucleus accumbens shell region depend on cannabinoid CB1 receptor activation. Neurochemistry international. 2012;60(8):791–798. doi: 10.1016/j.neuint.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Kline . The handbook of psychological testing. 2nd ed. London: Routledge; 1999. [Google Scholar]
- Koolhaas JM, de Boer SF, Buwalda B, van Reenen K. Individual variation in coping with stress: a multidimensional approach of ultimate and proximate mechanisms. Brain Behav Evol. 2007;70(4):218–226. doi: 10.1159/000105485. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, Van Reenen CG, Hopster H, Blokhuis HJ. Coping styles in animals: current status in behavior and stress-physiology. Neuroscience & Biobehavioral Reviews. 1999;23(7):925–935. doi: 10.1016/s0149-7634(99)00026-3. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Meerlo , De Boer SF, Strubbe JH, Bohus B. The temporal dynamics of the stress response. Neuroscience & Biobehavioral Reviews. 1997;21(6):775–782. doi: 10.1016/s0149-7634(96)00057-7. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, Lauzon NM, Bishop SF, Sun N, Tan H. Dopamine signaling through D1-like versus D2-like receptors in the nucleus accumbens core versus shell differentially modulates nicotine reward sensitivity. J Neurosci. 2008;28(32):8025–8033. doi: 10.1523/JNEUROSCI.1371-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas LR, Celen Z, Tamashiro KL, Blanchard RJ, Blanchard DC, Markham C, McEwen BS. Repeated exposure to social stress has long-term effects on indirect markers of dopaminergic activity in brain regions associated with motivated behavior. Neuroscience. 2004;124(2):449–457. doi: 10.1016/j.neuroscience.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Lucas LR, Wang CJ, McCall TJ, McEwen BS. Effects of immobilization stress on neurochemical markers in the motivational system of the male rat. Brain Res. 2007;1155:108–115. doi: 10.1016/j.brainres.2007.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Poremba A, Gabriel M. Basolateral amygdaloid multi-unit neuronal correlates of discriminative avoidance learning in rabbits. Brain research. 1991;549(2):311–316. doi: 10.1016/0006-8993(91)90473-9. [DOI] [PubMed] [Google Scholar]
- Margolin G, Gordis EB. The effects of family and community violence on children. Annual review of psychology. 2000;51:445–479. doi: 10.1146/annurev.psych.51.1.445. [DOI] [PubMed] [Google Scholar]
- May ME, Kennedy CH. Aggression as Positive Reinforcement in Mice under Various Ratio- and Time-Based Reinforcement Schedules. Journal of the Experimental Analysis of behavior. 2009;91(2):185–196. doi: 10.1901/jeab.2009.91-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA. Intraspecies aggression in rats: Effects of d-amphetamine and chlordiazepoxide. Psychopharmacologia. 1974;39(4):275–301. doi: 10.1007/BF00422968. [DOI] [PubMed] [Google Scholar]
- Moore RJ, Vinsant SL, Nader MA, Porrino LJ, Friedman DP. Effect of cocaine self-administration on dopamine D2 receptors in rhesus monkeys. Synapse. 1998;30(1):88–96. doi: 10.1002/(SICI)1098-2396(199809)30:1<88::AID-SYN11>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Morrison KE, Cooper MA. A role for 5-HT1A receptors in the basolateral amygdala in the development of conditioned defeat in Syrian hamsters. Pharmacology, biochemistry, and behavior. 2012;100(3):592–600. doi: 10.1016/j.pbb.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta SC, Goto M, Gouveia FV, Baldo MV, Canteras NS, Swanson LW. Dissecting the brain’s fear system reveals the hypothalamus is critical for responding in subordinate conspecific intruders. Proc Natl Acad Sci U S A. 2009;106(12):4870–4875. doi: 10.1073/pnas.0900939106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrug S, Elliott MN, Davies S, Tortolero SR, Cuccaro , Schuster MA. Early puberty, negative peer influence, and problem behaviors in adolescent girls. Pediatrics. 2014;133(1):7–14. doi: 10.1542/peds.2013-0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader MA, Daunais JB, Moore T, Nader SH, Moore RJ, Smith HR, Porrino LJ. Effects of cocaine self-administration on striatal dopamine systems in rhesus monkeys: initial and chronic exposure. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2002;27(1):35–46. doi: 10.1016/S0893-133X(01)00427-4. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Trainor BC. Neural mechanisms of aggression. Nat Rev Neurosci. 2007;8(7):536–546. doi: 10.1038/nrn2174. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5th ed ed. San Diego, CA: Elsevier Academic Press; 2005. [Google Scholar]
- Pellis SM, Pellis VC. Play-fighting differs from serious fighting in both target of attack and tactics of fighting in the laboratory rat rattus norvegicus. Aggressive behavior. 1987;13:227–242. [Google Scholar]
- Pellis SM, Pellis VC, Foroud A. Play fighting: Aggression, affiliation, and the development of nuanced social skills. In: Tremblay RE, Hartup WW, Archer J, editors. Developmental origins of aggression. New York: Guilford; 2005. pp. 47–62. [Google Scholar]
- Pihl RO, Benkelfat C, editors. Neuromodulators in the development and expression of inhibition and aggression. New York: The Guilford Press; 2005. [Google Scholar]
- Sah , Faber ESL, Lopez De Armentia M, Power J. The amygdala complex: Anatomy and Physiology. Physiological Reviews. 2003;83(3):803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Sengupta The Laboratory Rat: Relating Its Age With Human’s. International journal of preventive medicine. 2013;4(6):624–630. [PMC free article] [PubMed] [Google Scholar]
- Shirayama Y, Chaki S. Neurochemistry of the nucleus accumbens and its relevance to depression and antidepressant action in rodents. Current neuropharmacology. 2006;4(4):277–291. doi: 10.2174/157015906778520773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel A, Bhatt S, Bhatt R, Zalcman SS. The neurobiological bases for development of pharmacological treatments of aggressive disorders. Curr Neuropharmacol. 2007;5(2):135–147. doi: 10.2174/157015907780866929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su W, Mrug S, Windle M. Social cognitive and emotional mediators link violence exposure and parental nurturance to adolescent aggression. Journal of clinical child and adolescent psychology : the official journal for the Society of Clinical Child and Adolescent Psychology, American Psychological Association, Division 53. 2010;39(6):814–824. doi: 10.1080/15374416.2010.517163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman S, Dent CW, McCullar WJ. Group self-identification as a prospective predictor of drug use and violence in high-risk youth. Psychology of addictive behaviors : journal of the Society of Psychologists in Addictive Behaviors. 2000;14(2):192–196. [PubMed] [Google Scholar]
- Sussman S, Dent CW, Stacy AW. The association of current stimulant use with demographic, substance use, violence-related, social and intrapersonal variables among high risk youth. Addictive behaviors. 1999;24(6):741–748. doi: 10.1016/s0306-4603(98)00134-8. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Han SD, Lucas LR. Chronic passive exposure to aggression decreases D(2) and 5-HT(1B) receptor densities. Physiol Behav. 2010a doi: 10.1016/j.physbeh.2010.01.018. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Han SD, Lucas LR. Increased 5-HT1B receptor density in the basolateral amygdala of passive observer rats exposed to aggression. Brain Res Bull. 2010b;83(1–2):38–43. doi: 10.1016/j.brainresbull.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Lucas LR. Chronic passive exposure to aggression escalates aggressiveness of rat observers. Aggressive behavior. 2010;36(1):54–66. doi: 10.1002/ab.20333. [DOI] [PubMed] [Google Scholar]
- Tamashiro KL, Nguyen MM, Sakai RR. Social stress: from rodents to primates. Front Neuroendocrinol. 2005;26(1):27–40. doi: 10.1016/j.yfrne.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Thompson B, Leonard KC, Brudzynski SM. Amphetamine-induced 50 kHz calls from rat nucleus accumbens: a quantitative mapping study and acoustic analysis. Behav Brain Res. 2006;168(1):64–73. doi: 10.1016/j.bbr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Tran L, Lasher BK, Young KA, Keele NB. Depletion of serotonin in the basolateral amygdala elevates glutamate receptors and facilitates fear-potentiated startle. Translational psychiatry. 2013;3:e298. doi: 10.1038/tp.2013.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzanoulinou S, Riccio O, de Boer MW, Sandi C. Peripubertal stress-induced behavioral changes are associated with altered expression of genes involved in excitation and inhibition in the amygdala. Translational psychiatry. 2014;4:e410. doi: 10.1038/tp.2014.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Erp AM, Miczek KA. Aggressive behavior, increased accumbal dopamine, and decreased cortical serotonin in rats. J Neurosci. 2000;20(24):9320–9325. doi: 10.1523/JNEUROSCI.20-24-09320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Erp AM, Miczek KA. Increased accumbal dopamine during daily alcohol consumption and subsequent aggressive behavior in rats. Psychopharmacology (Berl) 2007;191(3):679–688. doi: 10.1007/s00213-006-0637-3. [DOI] [PubMed] [Google Scholar]
- Veenit V, Cordero MI, Tzanoulinou S, Sandi C. Increased corticosterone in peripubertal rats leads to long-lasting alterations in social exploration and aggression. Frontiers in behavioral neuroscience. 2013;7:26. doi: 10.3389/fnbeh.2013.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeiren R, Schwab-Stone M, Deboutte D, Leckman PE, Ruchkin V. Violence exposure and substance use in adolescents: Findings from three countries. Pediatrics. 2003;111(3):535–540. doi: 10.1542/peds.111.3.535. [DOI] [PubMed] [Google Scholar]
- Vitiello B, Stoff DM. Subtypes of aggression and their relevance to child psychiatry. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(3):307–315. doi: 10.1097/00004583-199703000-00008. [DOI] [PubMed] [Google Scholar]
- Weiss F, Paulus MP, Lorang MT, Koob GF. Increases in extracellular dopamine in the nucleus accumbens by cocaine are inversely related to basal levels: effects of acute and repeated administration. J Neurosci. 1992;12(11):4372–4380. doi: 10.1523/JNEUROSCI.12-11-04372.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch AS, Welch BL. Isolation, reactivity and aggression: Evidence for an involvement of brain catecholamines and serotonin. In: Eleftheriou BE, Scott JP, editors. The physiology of aggression and defeat: Proceedings of a symposium held during the meeting of the American Association for the Advancement of Science in Dallas, Texas, in December 1968. New York: Plenum Press; 1971. pp. 91–142. [Google Scholar]
- Widom CS. Does violence beget violence? A critical examination of the literature. Psychological bulletin. 1989;106(1):3–28. doi: 10.1037/0033-2909.106.1.3. [DOI] [PubMed] [Google Scholar]
- Wommack JC, Delville Y. Stress, aggression, and puberty: neuroendocrine correlates of the development of agonistic behavior in golden hamsters. Brain, behavior and evolution. 2007;70(4):267–273. doi: 10.1159/000105490. [DOI] [PubMed] [Google Scholar]
- Wood GE, Norris EH, Waters E, Stoldt JT, McEwen BS. Chronic immobilization stress alters aspects of emotionality and associative learning in the rat. Behav Neurosci. 2008;122(2):282–292. doi: 10.1037/0735-7044.122.2.282. [DOI] [PubMed] [Google Scholar]
- Wood GE, Young LT, Reagan LP, McEwen BS. Acute and chronic restraint stress alter the incidence of social conflict in male rats. Horm Behav. 2003;43(1):205–213. doi: 10.1016/s0018-506x(02)00026-0. [DOI] [PubMed] [Google Scholar]
- Yohe LR, Suzuki H, Lucas LR. Aggression is suppressed by acute stress but induced by chronic stress: immobilization effects on aggression, hormones, and cortical 5-HT(1B)/ striatal dopamine D(2) receptor density. Cogn Affect Behav Neurosci. 2012;12(3):446–459. doi: 10.3758/s13415-012-0095-9. [DOI] [PubMed] [Google Scholar]