Abstract
The present study investigated the feasibility of encapsulating two drugs, fasudil and superoxide dismutase (SOD), into liposomes for targeted and inhalational delivery to the pulmonary vasculature to treat pulmonary arterial hypertension (PAH). Nanosized liposomes were prepared by a thin-film formation and extrusion method, and the drugs were encapsulated by a modified freeze-thaw technique. The peptide CARSKNKDC (CAR), a pulmonary-specific targeting sequence, was conjugated on the surface of liposomes. Formulations were optimized for various physicochemical properties, tested for their ex-vivo and in-vivo drug absorption after intratracheal administration, and evaluated for short-term safety in healthy rats. The homogenous nanosized liposomes contained both SOD (~55% entrapment) and fasudil (~40% entrapment), and were stable at 4°C and after nebulization. Liposomes released the drugs in a controlled-release fashion. Compared with plain liposomes, CAR-liposomes increased the uptake by pulmonary endothelial and smooth muscle cells by ~2-fold. CAR-liposomes extended the biological half-lives of SOD and fasudil by ~3-fold. Ex-vivo studies demonstrated that CAR-liposomes were better retained in the lungs than plain liposomes. Bronchoalveolar lavage studies indicated the safety of peptide-equipped liposomes as pulmonary delivery carriers. Overall, this study demonstrates that CAR-liposomes may be used as inhalational carriers for SOD plus fasudil-based combination therapy for PAH.
Keywords: liposomes, pulmonary delivery/absorption, toxicity, controlled release, fasudil, superoxide dismutase
INTRODUCTION
Having improved the therapeutic index of a variety of drugs by increasing the pharmacological potency and/or decreasing the toxicity of the encapsulated drug molecules, liposomes have become favored drug-delivery carriers (Mallick and Choi, 2014) over past the few years. For example, liposomal formulations of doxorubicin (Sharpe et al., 2002), paclitaxel (Main et al., 2006), cytarabine (Feldman et al., 2011), daunorubicin (Myhren et al., 2014) and vincristine (Zhigaltsev et al., 2005) have shown promise in cancer therapy; some liposomal formulations are either approved for use or in clinical trials. Liposomes can also protect the encapsulated drugs (e.g., superoxide dismutase; SOD) from proteolysis and facilitate targeted delivery of SOD to the endothelium lining the vascular lumen (Hood et al., 2011; Shuvaev et al., 2011). Despite the known advantages of combination therapies in a number of medical conditions, most liposomal formulations are still based on single-drug encapsulation. Reasons for this may be the problems in achieving stable and efficient encapsulation of two drugs in liposomes as well as challenges in controlling drug release, which is even more problematic when the two drugs are chemically and physically different. However, at least two studies have reported dual-drug encapsulation inside liposomes: paracetamol and acetylsalicylic acid (Nasedkin et al., 2013), and irinotecan and floxuridine (Tardi et al., 2007).
Whereas all of the above-mentioned formulations were designed to be delivered via the intravenous route, there have been recent major efforts to evaluate and optimize liposomes for inhalational delivery of drugs for respiratory disorders (Cipolla et al., 2013). The inhalational route significantly adds to the value of a drug whose primary site of action is the lungs. But the main challenge for pulmonary drug delivery is maintaining the stability of the formulation during the aerosolization process, which disqualifies many of the currently used drug carriers. We and others have successfully optimized liposomal formulations for pulmonary delivery of numerous drugs, including fasudil (Gupta et al., 2013), ciprofloxacin (Moazeni et al., 2010), iloprost (Jain et al., 2014), vasoactive peptides (Hajos et al., 2008), and beclomethasone (Darwis and Kellaway, 2001) among others, for the treatment of different respiratory diseases.
To our knowledge, no studies have reported encapsulation of two drugs inside inhalable liposomes for the treatment of pulmonary arterial hypertension (PAH). PAH is a rare but life-threatening disease of the small pulmonary arteries and several overlapping pathways contribute to its development and progression (Chan and Loscalzo, 2008; Klinger, 2007; McGoon and Kane, 2009). Our interest in developing liposomal formulations containing drug combinations is propelled by the fact that currently all curative PAH treatments utilize drug combinations. We hypothesize that heightened pulmonary-specific vasodilation may be achieved by simultaneously delivering and exposing anti-PAH drug combinations to PAH sites in-vivo, as compared to using single or sequentially administered agents. This approach could be of even greater importance if the formulation can be made to concentrate at the disease site. In this regard, the peptide CARSKNKDC (CAR) was recently shown to specifically bind with heparan sulfate overexpressed on PAH lesions (Jarvinen and Ruoslahti, 2007; Urakami et al., 2011). Additionally, this peptide has cell-penetrating properties and can bind to all three layers of blood vessels (Toba et al., 2014). One other approach to improving PAH therapy might be to deliver multiple liposome-based anti-PAH drugs, but the attendant higher lipid doses could have adverse effects.
In view of the above considerations, we undertook a series of studies aimed at developing liposome formulations that are able to stably co-encapsulate two drugs and coordinate the release of the two agents after either intravenous or intratracheal administration. In the present study, we describe the co-encapsulation of fasudil, a rho-kinase inhibitor, and SOD, a superoxide scavenger. This drug combination was selected because both rho-kinase activity and oxidative stress-mediated vasoconstriction and pulmonary arterial remodeling play major roles in the pathogenesis of PAH (Demarco et al., 2010; Farrow et al., 2010; Nagaoka et al., 2005; Oka et al., 2007). Thus, we prepared CAR-conjugated liposomes containing SOD and fasudil, and optimized them for favorable physicochemical properties. We then evaluated the pharmacokinetic profiles of the liposomal product, both in-vivo and ex-vivo, and investigated the safety of the particles for inhalational delivery.
MATERIALS AND METHODS
Materials
Lipids, 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), cholesterol from ovine wool (CHOL) and DSPE-PEG2000-maleimide were purchased from Avanti Polar Lipids, Inc. (Alabaster, Alabama). CAR peptide, available from and being commercially developed by Vascular Biosciences (Goleta, CA), was custom synthesized by LifeTein, LLC (South Plainfield, NJ). Fasudil hydrochloride and superoxide dismutase (SOD) were purchased from LC Laboratories, Inc. (Woburn, MA) and (EMD Millipore, Billerica, MA), respectively. Sephadex-G-25 PD-10 pre-packed columns were purchased from GE Healthcare (Piscataway, New Jersey, USA). All other chemicals, including chloroform, methanol, phosphate-buffered saline (PBS), acetonitrile and dimethyl sulfoxide (DMSO), were of analytical grade and obtained from various vendors in the United States. All chemicals were used without further purification.
Preparation of CAR-conjugated liposomes containing fasudil and SOD
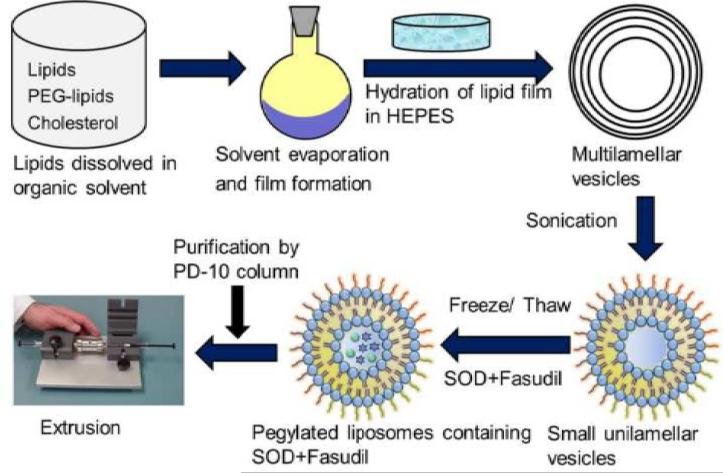
Liposomes were prepared by the thin-film formation, hydration, and extrusion method as described in our previously published studies (Gupta et al., 2013; Nahar et al., 2014) (Figure 1). Briefly, lipids were dissolved in chloroform and kept overnight on a rotary evaporator (Buchi Rotavaporator® R-114; BUCHI Labortechnik AG, Switzerland) at 40°C for solvent evaporation. The dried lipid film was hydrated in 1 ml PBS (1X, pH 7.4) for 60 minutes at high rotational speed. We prepared a total of 5 formulations with a fixed ratio of lipids [DPPC:CHOL:DSPE-PEG-MAL (6:3:1)] but with varying total lipid concentrations (10-60 mM). Later, more optimization was conducted at the encapsulation step, which was done by a freeze-thaw method. We investigated the effect of the number of freeze-thaw cycles (1-4) on encapsulation of the two drugs. Concentrations of SOD and fasudil were 20 and 30 mg/ml, respectively, for all formulations. After hydration of the lipid film, 1 ml PBS containing SOD and fasudil was added and subjected to freeze-thaw (-196°C and 70°C) cycles. Then, the formulations were extruded through polycarbonate membranes of different pore sizes (400-200 nm). Finally, unentrapped drugs were separated by passing through a PD-10 column, and again purified and concentrated using Amicon Ultra centrifugal filter units (MWCO-3000, Millipore Inc., Billerica, MA, USA) by centrifugation for 45 min at 300 × g (Centrifuge 5702-R, Eppendorf AG, Hamburg, Germany). Liposomes were then stored at 4°C for future studies. For peptide conjugation, liposomes were incubated with a solution of CAR peptide in PBS for 30 min in the dark as reported previously (Marques-Gallego and de Kroon, 2014). The free thiol group of the external cysteine linked to the CAR peptide (FAM-Cys-Linker-CAR) via a linker (6-amino hexanoic acid) was used to conjugate the peptide with the maleimide group of DSPE-PEG of the liposomal preparation. Unconjugated peptide was removed and the purified formulation was stored as described above.
Figure 1.
Schematic diagram depicting preparation of the liposomal formulation containing SOD and fasudil.
Physicochemical characterization
Particle size, polydispersity index (PDI), and zeta potential of the liposomes were measured by a Malvern® Zetasizer (Malvern® Instruments Limited, Worcestershire, UK). The amount of fasudil and SOD encapsulated in liposomes were determined by disrupting a known aliquot of formulation (20 μl) in methanol (980 μl). Fasudil was detected directly at 320 nm using a UV spectrophotometer, and SOD content was determined by a commercial assay kit (EpiQuik Superoxide Dismutase Activity/Inhibition Assay Kit, Epigentek Group, Inc, Farmingdale, NY). This colorimetric kit uses water-soluble tetrazolium salt that yields water-soluble formazan dye upon interaction with SOD. The intensity of the dye was read in a microplate reader to determine the activity of SOD, which was then converted to SOD concentration. The entrapment efficiency was calculated using the following equation: Entrapment efficiency (%) = (Amount of drug in liposomes/Amount of drug originally added) × 100.
Storage and nebulization stability
Storage stability of the liposomes was determined at 4°C for 21 days. Samples were withdrawn at predetermined intervals (day 0, 7, 14 and 21) and evaluated for changes in vesicle size and entrapment efficiency as described above. The stability of liposomes during nebulization was also assessed. Briefly, a suspension of liposomes was loaded into a Hamilton Syringe® attached to a PennCentury™ Microsprayer® (Model IA-1B, PennCentury, PA) and sprayed five times followed by collecting and analyzing the samples for particle size, PDI, and entrapment efficiency as described above.
In-vitro release studies
Drug-release studies were performed in dialysis cassettes (Slide-A-Lyzer, 3500 MWCO, 0.1-0.5 ml, Thermo-Scientific, Waltham, MA) as reported previously (Gupta et al., 2014b; Gupta et al., 2013): The cassettes were first hydrated with PBS (pH 7.4); and 500 μl of liposomes or CAR-liposomes were loaded using a syringe. Plain drugs were used as controls to evaluate whether the cassettes influence drug release. The cassettes were immersed in 100 ml PBS in a beaker and incubated at 37°C with moderate stirring. Samples were drawn at predetermined time intervals; the medium was immediately replenished with fresh PBS. The amounts of SOD and fasudil released were determined as described above.
Uptake of liposomes by rat pulmonary arterial endothelial (PAE) and smooth muscle (PASM) cells
Cellular uptake studies were performed as described by us previously (Gupta et al., 2014b; Gupta et al., 2013): Rat PAE and PASM cells were seeded on a cover slip placed in a 12-well plate at 5×103 cells/ml density. Cells were activated with tissue growth factor-beta (TGF-β, 10 ng/ml) for 12 hrs prior to uptake studies, and then treated with plain or CAR-conjugated liposomes for 60 min. Rhodamine-labeled (red) lipids were incorporated into liposomes to visualize liposomal uptake. After the treatment, cells were washed to remove the excess formulations, fixed, and stained for actin cytoskeleton by sequential incubation in monoclonal anti-β-actin primary antibody (Sigma-Aldrich, St. Louis, MO) and Alexa Fluor® 495 (green) goat anti-mouse IgG antibody (Invitrogen, Grand Island, NY). Cellular uptake was assessed by fluorescence microscopy (Olympus IX-81, Center Valley, PA).
Cellular uptake was quantitated by flow cytometry in a separate set of experiments. After the treatment, the cells were trypsinized; a cell suspension in PBS was analyzed in a flow cytometer (BD Accuri™ C6, BD Biosciences, San Jose, CA). For each sample, 10,000 cells were counted and the fluorescence intensity was recorded. Cells that received no treatment were analyzed as control samples.
Drug absorption studies of formulations
Pharmacokinetic studies of plain drugs and formulations were performed in male Sprague Dawley (SD) rats (~250-300 g) as described previously (Gupta et al., 2014b; Gupta et al., 2013). Animals were anesthetized with a cocktail of ketamine and xylazine prior to the following treatments: (i) plain SOD given intravenously (IV), (ii) plain SOD given intratracheally (IT), (iii) plain fasudil IV, (iv) plain fasudil IT, (v) plain combination SOD plus fasudil IT, (vi) plain liposomes IT, and (vii) CAR-liposomes IT. The doses of SOD and fasudil were 5 and 6 mg/kg, respectively. Intravenous injection was given via the penile vein and pulmonary administration was performed with a microsprayer for rats. At predetermined time points, blood samples were collected into citrated microcentrifuge tubes via a tail-vein milking method. Then, plasma was separated by centrifuging the blood at 1000 × g for 5 min, and stored at −20°C until further analysis. The concentration of fasudil in plasma was determined by an HPLC method (Varian ProStar 320, Walnut Creek, CA) as reported previously (Gupta et al., 2013), and the amount of SOD was determined as described above.
All animal studies were performed in accordance with NIH Guidelines for the Care and Use of Laboratory Animals under a protocol approved by TTUHSC Animal Care and Use Committee (AM-10012).
Isolated perfused rat lung (IPRL) studies
Retention of drug in the lungs after administration of plain or CAR-conjugated liposomes containing SOD and fasudil was measured in an IPRL system: The trachea of anesthetized rats (male SD, ~250-300 g) was exposed and a cannula was inserted for positive pressure ventilation. Then, the chest cavity was opened; pulmonary arterial and left ventricular cannulae were inserted for entry and exit of perfusion medium, respectively. The perfusion medium (pH 7.4) was prepared, as described earlier (Gupta et al., 2014a), and circulated through the lungs maintained at 37°C. The whole lung-heart block was removed without disturbing the cannulae or flow of perfusion medium, and placed in an artificial thoracic chamber. Negative pressure was immediately turned on to prevent lung collapse. Plain or CAR liposomes containing SOD plus fasudil, or combination of plain drugs were sprayed directly into the lungs via the tracheal cannula. SOD and fasudil were administered at a dose of 5 and 6 mg/kg, respectively. Aliquots of perfusate at various time intervals were collected over 2 hrs and analyzed as described above by HPLC and a commercial SOD detection kit. After this, the lungs were removed from the thoracic system and immediately frozen in liquid nitrogen, and kept at −80°C for further analysis. Frozen lungs were homogenized in saline, centrifuged, and the supernatant was collected; fasudil and SOD were extracted from the supernatant by precipitation with (1:1 v/v) followed by centrifugation for 10 min at 2000 × g. Both drugs were analyzed as described above.
Short-term toxicity of formulations
In-vitro toxicity of the formulations was evaluated in rat PASM cells by an MTT assay as reported previously (Gupta et al., 2014b): Cells (5×104 cells/well) were seeded in a 96-well plate and treated with (i) saline (ii) 0.1% sodium dodecyl sulfate (SDS), (iii) plain SOD, (iv) plain fasudil, (v) plain combination of SOD+fasudil, (vi) plain liposomes, and (vii) CAR-liposomes. 100 μl of either plain drugs or formulations were added to the cells for 24 hrs at the same doses listed above. Then, the cells were washed and incubated with 100 μl MTT for an additional 4 hrs. The formazan crystals thus formed were dissolved in DMSO by moderate shaking for an hour. Finally, the absorbance was recorded at 570 nm using a Synergy™MX Microplate Reader (Biotek, Winnoski, VT). Cell viability was calculated using the following equation: Percent Cell viability = (ODsample – ODblank) / (ODcontrol – ODblank) × 100; OD is optical density, sample is test compound, control is saline, and blank is no treatment.
A bronchoalveolar lavage (BAL) study was performed to evaluate the in-vivo safety of CAR-liposomes (Gupta et al., 2014b; Gupta et al., 2013): Anesthetized male SD rats were divided into three groups to receive the following treatments intratracheally: (i) saline (negative control), (ii) CAR-liposomes (equivalent to 5 and 6 mg/kg SOD and fasudil, respectively) and (iii) sodium dodecyl sulfate (SDS, 0.1%, positive control). After 12 hrs of administration, animals were weighed; lungs were surgically removed and weighed. The wet lung weights were reported as g/100 g body weight. The lungs were then lavaged by instilling 5-ml of normal saline into the trachea and collecting the fluid after 30 s. The BAL fluid was centrifuged at 500 g for 10 min and then the supernatant was stored at −20 °C. Total protein concentration (μg/ml) was determined by Bradford assay (Sigma Aldrich, St. Louis MO). The enzymatic activities of lactate dehydrogenase (LDH) and alkaline phosphatase (ALP) in BAL fluid were determined by using commercial kits (Pointe Scientific, Canton, MI).
Data analysis
The data are presented as mean ± standard deviation and were analyzed by one-way ANOVA followed by a Tukey's post-hoc test using GraphPad Prism 6.0 (p < 0.05 was considered statistically significant). Pharmacokinetic analysis was performed by standard non-compartmental analysis (WinNonlin®, Pharsight Corp., Cary, NC).
RESULTS AND DISCUSSION
Formulation and characterization of CAR-liposomes containing SOD and fasudil
Two drugs, fasudil and SOD, were encapsulated in liposomes by a freeze-thaw method. First, we prepared empty unilamellar vesicles by solvent evaporation, thin-film formation and hydration (Gupta et al., 2013). We used, initially, a 30 mM lipid concentration and optimized the number of cycles required to obtain a fair entrapment of both drugs. SOD, an enzyme, has been encapsulated efficiently by freeze-thawing (Costa et al., 2014). Importantly, this method does not disrupt the enzymatic activity and stability of SOD. We found the highest encapsulation efficiency with 4 cycles of freeze-thaw. Then, we investigated the effect of lipid concentration on entrapment and observed that drug loading increased with increasing lipid content. Entrapment efficiencies of SOD and fasudil increased from ~17% to ~41% and ~26% to ~60%, respectively, when the lipid content was increased from 10 to 60 mM. Concentrations of both drugs were kept constant during this whole procedure and the increase in encapsulation efficiency may be due to both entrapment in the core and the lipid bilayer. We used PEG-functionalized lipids to prepare liposomes because PEG-chains shield liposomes against degradation in biological fluids and clearance by macrophages; thus they increase liposomes’ residence time in the lungs (Allen et al., 1989; Wang et al., 2012). The liposomes were monodispersed (PDI = ~0.16-0.19), with an average size of ~150-160 nm (Table 1); because of the smaller size, liposomes are less likely to be phagocytosed (Gupta et al., 2013). Surface charge, an important factor for colloidal stability, keeps the particles separated and prevents them from aggregating; indeed either a large positive or negative zeta potential is warranted for superior stability of the formulations (Nahar et al., 2014). The zeta potential of the liposomes in this study was about +13 mV, which is adequate for the stability of the formulation. CAR peptide was conjugated by a simple reaction between the maleimide group of DSPE-PEG and a thiol-group present on the peptide. After purification and concentration, we again characterized the formulation for the above parameters. There were no noticeable changes in the properties of the formulation other than a slight loss of drug. The final formulation was 152.7±2.38 nm in size, with a PDI of 0.119±0.02, zeta potential of +28.17±1.77 and entrapment efficiencies for SOD and fasudil of 54.91±1.66% and 39.22±3.41%, respectively.
Table 1.
Physicochemical characteristics of liposomes containing SOD and fasudil.
| Total lipid (mM) | Size (nm) | Zeta potential (mV) | PDI | Entrapment Efficiency (%) |
|
|---|---|---|---|---|---|
| SOD | Fasudil | ||||
| 10 | 156.3±1.22 | +8.76±4.89 | 0.164±0.03 | 26.39±9.62 | 17.65±5.67 |
| 20 | 155.1±0.98 | +7.46±5.22 | 0.191±0.09 | 43.11±3.99 | 24.93±2.83 |
| 30 | 147.4±1.09 | +9.62±0.73 | 0.181±0.04 | 48.77±1.34 | 32.21±4.82 |
| 40 | 159.1±0.14 | +11.43±2.09 | 0.167±0.01 | 52.98±1.62 | 37.49±1.38 |
| 50 | 161.2±3.19 | +13.34±1.12 | 0.187±0.03 | 54.19±7.81 | 40.01±3.14 |
| 60 | 147.4±2.78 | +11.71±1.91 | 0.183±0.04 | 60.38±3.19 | 41.63±2.66 |
Data represent mean ± standard deviation (n=3).
Storage and nebulization stability
We examined the stability of the optimized formulation: Liposomes were stored at 4°C and periodically evaluated for changes in physicochemical properties. We did not observe any significant changes in particle size and drug entrapment after two weeks of storage, although there were some changes in the third week (Table 2). The minimal changes in particle size can be attributed to the steric stability provided by the PEG chains present on the surface of the liposomes and the zeta potential to some extent, which prevent the particles from coalescing and aggregating. However, when the formulations were stored at 25 and 37°C, the physicochemical properties of the liposomes changed significantly even after one week of storage (data not shown).
Table 2.
Storage stability of CAR-liposomes containing SOD and fasudil at 4°C.
| Days | Size (nm) | Entrapment Efficiency (%) |
|
|---|---|---|---|
| SOD | Fasudil | ||
| 0 | 152.7 ± 2.38 | 54.91 ± 1.66 | 39.22 ± 3.41 |
| 7 | 153.9 ± 2.89 | 53.14 ± 1.13 | 37.38 ± 2.19 |
| 14 | 159.1 ± 3.22 | 49.88 ± 1.99 | 33.91 ± 4.46 |
| 21 | 164.3 ± 5.17* | 48.31 ± 3.86 | 31.88 ± 5.67* |
Data represent mean ± standard deviation (n=3).
Values after 21 days of storage were significantly different from day 0 samples, p<0.05.
We also investigated if our formulation can resist the pressure exerted during aerosolization. Commercial nebulizers use different active and passive modes of energies to aerosolize the drugs/formulations, thus it was necessary to determine if the force applied during nebulization ruptures the liposomes and causes premature drug release. Neither the vesicle size nor drug entrapment was changed due to nebulization (Table 3), suggesting that energy produced by the microsprayer did not affect liposomal integrity.
Table 3.
Physicochemical characterization of CAR-liposomes containing SOD and fasudil before and after nebulization with a microsprayer.
| Nebulization | Size (nm) | Entrapment Efficiency (%) |
|
|---|---|---|---|
| SOD | Fasudil | ||
| Before | 152.7 ± 2.38 | 54.91 ± 1.66 | 39.22 ± 3.41 |
| After | 154.2 ± 3.61 | 52.41 ± 1.72 | 38.98 ± 2.91 |
Data represent mean ± standard deviation (n=3).
In-vitro drug release studies
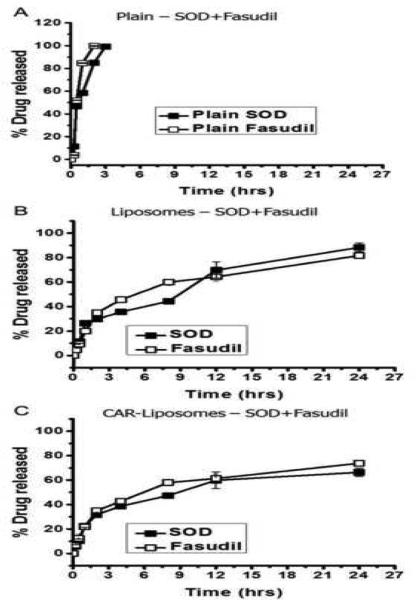
In-vitro drug release studies were performed to test the hypothesis that the formulations control the release of encapsulated SOD and fasudil in physiological fluid. The release profile of the formulations in PBS buffer at 37°C showed sustained-release behavior of both drugs for 24 hrs (Figure 2), whereas 100% of plain SOD or fasudil was released in 2-3 hrs, indicating that the dialysis cassettes were not a rate-limiting factor for drug release. Plain liposomes released ~90% of the drugs over a period of 24 hrs. We observed a similar but more controlled release pattern in the case of CAR-liposomes, which could be due to the presence of the CAR peptide on the liposomal surface. During the first 12 hrs, CAR-liposomes released 59.8±6.7% SOD and 61.3±3.72% fasudil, higher percentages than for plain liposomes. At the end of 24 hr, CAR-liposomes released 66.3±3.45% SOD and 73.7±2.48% fasudil; plain liposomes released 88.3±4.29% SOD and 81.7±2.66% fasudil. The slower release of drugs from the liposomal formulations reflects the fact that the drugs are diffusing out of the lipid bilayer instead of being released due to disruption of liposomes (Gupta et al., 2013). However, we cannot disregard the latter mechanism of drug release because a burst release was observed in the first 30 min. Overall, the CAR-liposomal formulation would be effective in delivering SOD and fasudil to the distal pulmonary arterioles for a prolonged period and would likely provide sustained therapeutic efficacy.
Figure 2.
In-vitro release profiles of (A) plain drugs, (B) liposomes containing both drugs, and (C) CAR-liposomes containing both drugs in PBS (pH 7.4, 37°C). Data represent mean ± standard deviation (n=3).
Cellular uptake of formulations
To exert therapeutic effects, formulations must be taken up by pulmonary arterial cells; therefore, we performed cellular uptake studies using rat PAE and PASM cells. The therapeutic target of fasudil is rho-kinase, which is activated by several endogenous vasoconstrictors (e.g., endothelin, serotonin, angiotensin). Fasudil inhibits rho-kinase activity, prevents smooth muscle contraction (Barman et al., 2009), and thus acts as a potent pulmonary vasodilator (Gupta et al., 2013; Nagaoka et al., 2005). SOD scavenges superoxide anions and increases the bioavailability of vasodilators such as nitric oxide and prostacyclins (Farrow et al., 2010). Further, our liposomal formulation is equipped with the homing peptide CAR. A number of studies demonstrated that the propensity of CAR to bind with and accumulate onto various layers of pulmonary arteries and arterioles (intimal, medial and adventitial layers) is much higher than its propensity to bind to negatively charged ECM (Jarvinen and Ruoslahti, 2007; Urakami et al., 2011): First, CAR binds to heparan sulfate, which is overexpressed in all layers of PAH vasculatures. Second, the negative charge density in heparan sulfate, overexpressed in PAH lesions, is much higher than in healthy ECM components. Thus, the nonspecific binding of CAR with ECM would be negligible in healthy tissues. CAR, moreover, accumulates in all layers of PAH arteries because of its cell and tissue penetrating properties.
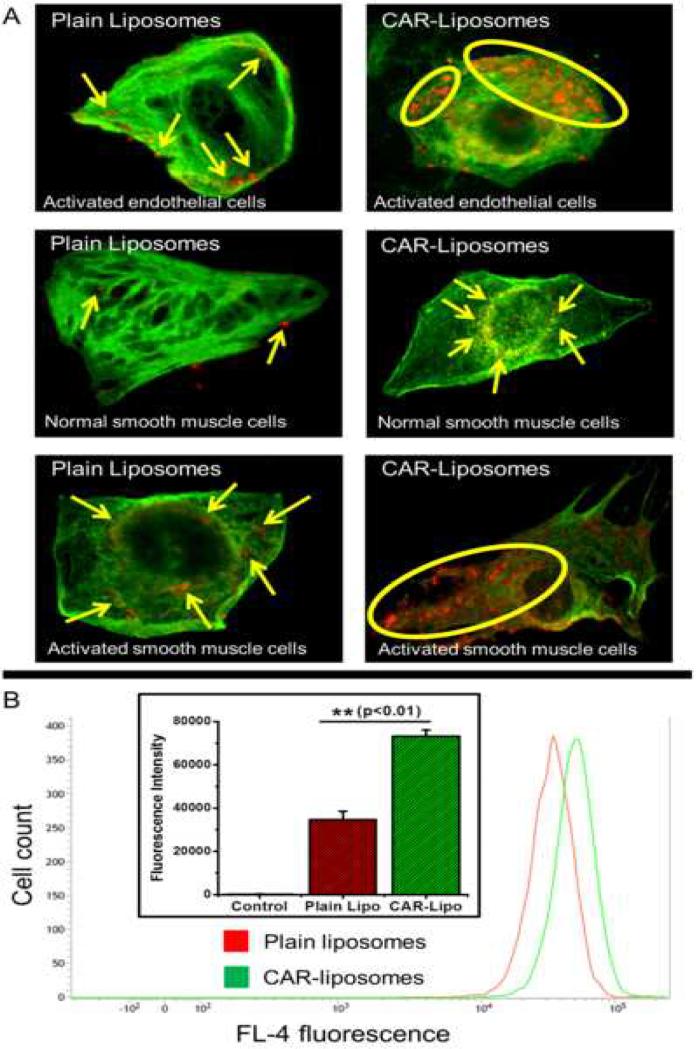
To compare binding efficiencies, we first activated PASM cells with TGF-β, which increases endogenous heparan sulfate synthesis and expression (Dodge et al., 1990). True to the previous observations, CAR-liposomes accumulated more in activated PASM cells compared to unmodified liposomes (Figure 3.A), suggesting that CAR-liposomes are preferentially taken up by cells mimicking PAH-like conditions. When normal PASM cells were used, the differences in uptake by plain versus CAR-liposomes were not remarkable. These results are encouraging, because if more liposomal particles are taken up, more fasudil and SOD should be available for interaction with their respective pathways. This supports our hypothesis that peptide-decorated particles should produce more pronounced pharmacological effects versus plain particles.
Figure 3.
(A) Uptake of fluorescent liposomes by rat pulmonary arterial endothelial (normal) and smooth muscle cells (normal and activated). (B) Flow cytometric analysis of uptake of plain and CAR-liposomes by activated smooth muscle cells. Data represent mean ± standard deviation (n=3).
Further, we confirmed our microscopy results by flow cytometric analysis. The cellular uptake of CAR-liposomes was ~2.1-fold higher than that of plain liposomes (Figure 3.B). This was observed as a shift in the peak fluorescence intensity, which is consistent with more formulation being either bound or taken up by cells. Based on the above results, we believe our formulation should provide superior pharmacological efficacy in-vivo.
Pharmacokinetics of CAR-liposomes containing SOD and fasudil
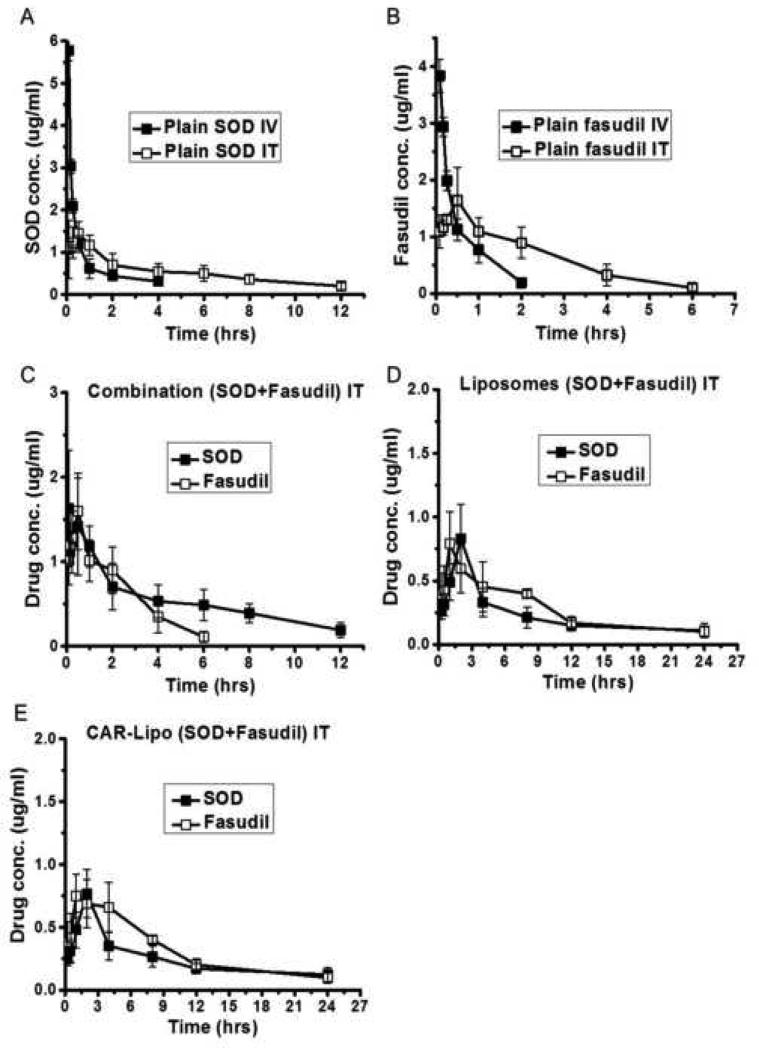
In a pharmacokinetic study, the pulmonary absorption profile of CAR-liposomes was compared with those of plain liposomes or combined plain drugs. IV administration of plain drugs alone showed a quick rise in drug plasma concentrations (Cmax = 3.83±0.29 and 5.78±0.24 μg/ml for fasudil and SOD, respectively) followed by a rapid decline (Figures 4.A and 4.B), whereas IT administration reduced the drug concentrations in the plasma (Cmax = 1.64±0.31 and 1.45±0.28 μg/ml for fasudil and SOD, respectively), suggesting the drugs tended to remain in the lungs. Further, the elimination half-lives of the drugs after IT administration were significantly higher (~2.5-fold) than after IV administration. The absorption profiles of the plain combination of drugs given IT were not different from the profiles of the individual plain drugs (Figure 4.C). However, the profiles of both the plain and CAR-conjugated liposomal formulations were very different from those of the plain drugs (combined or alone) (Figures 4.D and 4.E). CAR-liposomes showed Cmax values of 0.74±0.37 and 0.77±0.19 μg/ml for fasudil and SOD, respectively, at 1- to 2-hr time points as compared to either the plain combination or drugs alone, which showed Cmax values at earlier time points. This difference could be due to the controlled release of drugs from the liposomal bilayer. Pharmacokinetic analysis of the in-vivo data revealed that CAR-liposomes increased the elimination half-lives of both drugs (t1/2 of 4.53±0.26 and 6.75±0.18 hrs for fasudil and SOD, respectively) (Table 4). In fact, the t1/2s of fasudil and SOD encapsulated in CAR-liposomes were ~3 and ~7.9 times higher, respectively, than for plain drugs after either IT or IV administration. Based on the plasma concentration-time data of the CAR-liposomes containing SOD and fasudil, we expect that the formulation will maintain therapeutic concentrations of both drugs for an extended period, and hence reduce the dosing frequency.
Figure 4.
In-vivo absorption profiles of (A) plain SOD administered either intravenously (IV) or intratracheally (IT), (B) plain fasudil via IT or IV, (C) plain combination of SOD+fasudil IT, (D) unmodified liposomes containing both drugs IT, and (E) CAR-liposomes containing both drugs IT. Doses of SOD and fasudil were 5 and 6 mg/kg, respectively. Data represent mean ± standard deviation (n=3).
Table 4.
Pharmacokinetic parameters of plain drugs, SOD and fasudil, and formulations containing both drugs.
| Drug | Treatment / Route | Cmax (μg/ml) | t½ (hrs) | AUC0→inf (μg/ml*hr) |
|---|---|---|---|---|
| Fasudil | Plain IV | 3.83±0.29 | 0.57±0.03 | 7.17±3.42 |
| Plain IT | 1.64±0.31 | 1.49±0.09 | 15.62±4.21 | |
| Plain combination IT | 1.59±0.45 | 1.52±0.05 | 16.83±3.99 | |
| Liposomes IT | 0.79±0.25 | 4.21±0.17 | 14.01±3.27 | |
| CAR-Liposomes IT | 0.74±0.37 | 4.53±0.26 | 13.85±5.82 | |
| SOD | Plain IV | 5.78±0.24 | 0.89±0.04 | 9.31±2.75 |
| Plain IT | 1.45±0.28 | 2.24±0.03 | 27.78±6.91 | |
| Plain combination IT | 1.41±0.57 | 2.35±0.08 | 27.69±8.19 | |
| Liposomes IT | 0.83±0.27 | 6.32±0.29 | 18.41±9.81 | |
| CAR-Liposomes IT | 0.77±0.19 | 6.75±0.18 | 19.75±5.28 | |
Data represent mean ± standard deviation (n=3).
Isolated perfused rat lung studies
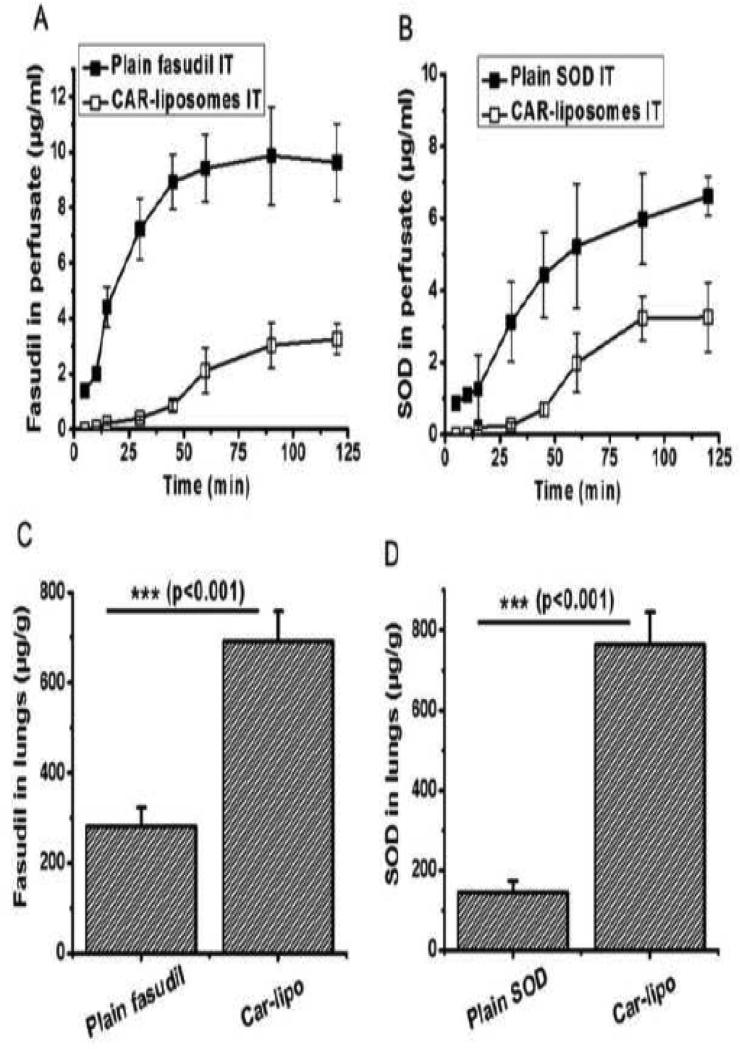
We performed IPRL studies to assess the fraction-drug-retained in the lungs after IT administration of either the plain combination of SOD+fasudil or CAR-liposomes containing both drugs. IT administration is expected to limit the drugs’ systemic exposure and reduce side-effects. Compared to the plain combination, for which both drugs were detected within 5 min, the release of drugs from CAR-liposomes was slower, and drug concentrations were detectable only after 15 min (Figures 5.A and 5.B). Plain drugs absorbed very fast via the lung epithelium, whereas the formulation retained the drugs in the lungs for longer times. Although the concentration of each plain drug increased in the perfusate over time, the concentrations of drugs released from liposomes plateaued, suggesting an enhanced retention in the lungs. We also quantified the amount of each drug remained in the lungs by homogenizing the tissue. The amounts of fasudil and SOD remaining in the lungs treated with CAR-liposomes were ~2.5 and ~5.3 times higher, respectively, compared with lungs that received the plain combination drugs (Figures 5.C and 5.D). Later, we calculated the mass balance using the following equation: Amount of drug administered = Amount of drug retained in the lungs + Amount of drug released in the perfusate. The weight of the lungs was ~1.8 g and the total volume of perfusate was 50 ml. We administered a combination of fasudil (~1.5 mg) and SOD (~1.3 mg) either as a plain form or as CAR-liposomes. In case of SOD, the recovery was ~53% for plain SOD (~353 μg in perfusate and ~330 μg in lungs) and ~86% for CAR-liposomes (~165 μg in perfusate and ~950 μg in lungs). Similarly, ~71% plain fasudil was recovered (~490 μg in perfusate and ~580 μg in lungs) but ~91% of the drug was in the lungs when administered in CAR-liposomes (~160 μg in perfusate and ~1200 μg in lungs).
Figure 5.
Isolated perfused rat lung studies showing the concentration of plain and CAR-liposomal (A) fasudil and (B) SOD. Amount of plain and CAR-liposomal (C) fasudil and (D) SOD remaining in the lungs (tissue homgenate) after perfusion for 2 hrs. Data represent mean ± standard deviation (n=3).
In fact, drug solubility can influence the absorption of a drug/formulation from the lung epithelium. Upon deposition of drug particles in the airway, drugs undergo wetting and dissolution in the airway lining fluid (Sahib et al., 2011). Water-soluble drugs can escape the clearance mechanisms (e.g. phagocytosis and mucociliary clearance) and quickly cross the alveolar epithelial barrier. But poorly soluble drugs take longer to partition between the phases of the lining fluid, which facilitates its clearance. Because our formulations contain highly soluble PEG chains, the formulations will readily dissolve in the respiratory fluid and release the drug, which may then reach the lumen directly or after crossing the adventitia, media and intima of the blood capillaries (Gomberg-Maitland and Olschewski, 2008). Overall, the data point toward increased residence time of the drugs in the lungs when administered as a liposomal formulation, which is likely to translate into pulmonary-preferential and prolonged vasodilation in-vivo.
However, a recent study has demonstrated that unconjugated intravenous CAR increases the accumulation of drugs in PAH lungs by phenomenon called the bystander effect (Toba et al., 2014). In contrast to this observation, we believe that CAR-induced bystander effect is biologically unfeasible because CAR alone has no anti-PAH effect neither can it pull or sequester circulating the drug molecules to its sites of accumulation, PAH lesions. We hypothesize that in the aqueous solution, CAR forms either electrostatic or non-covalent inclusion complex with the drugs. Upon intravenous administration, the non-covalent CAR-drug complex accumulates in PAH afflicted arterioles. In aqueous solutions, CAR may also form micelles and encapsulate the drugs in the micellar cavity. To accept or reject the hypotheses of a bystander effect and CAR-drug conjugation, one must administer CAR and drug at two different time points with a lag time between administrations of CAR and the drug (administer CAR first, allow 30 minutes for the peptide to distribute in the body and then administer the drug). If CAR produces any bystander effect, the drug should produce pulmonary selective vasodilation even 30 minutes after administration of the CAR.
Even if CAR has any bystander effect, intravenous administration of unconjugated CAR plus drug would not reduce the dosing frequency; neither can this approach eliminate the complications of needle-based drug administration nor circumvent the requirement of multiple dosing. CAR-conjugated liposomal formulations will be long acting, needle free, and patient compliant. This study established the proof-of-concept for combination therapy of PAH using inhalable CAR-conjugated liposomes. In the future, we will test the hypothesis of complexation versus bystander effect.
Safety studies of the formulation
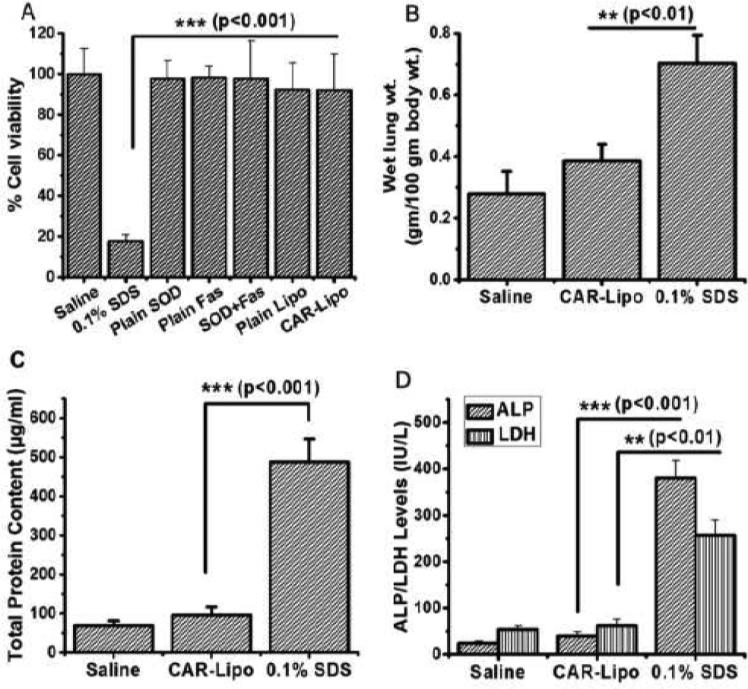
An in-vitro cytotoxicity study was performed to determine if the formulation injures or kills healthy cells. Cells treated with saline (negative control) or 0.1% SDS (positive control) showed ~99.8% and ~17.5% viabilities, respectively. Cells treated with either plain drugs or formulations showed at least 92% viabilities, suggesting the formulations were not toxic to primary rat PASM cells (Figure 6.A). We expected these results because the lipids used to prepare the formulations have little or no cytotoxicity.
Figure 6.
Safety of the formulation. (A) Cytotoxicity studies of CAR-liposomes containing SOD+fasudil in rat pulmonary arterial smooth muscle cells (n=12). Effect of the formulation on (B) wet lung weight, (C) total protein content and (D) levels of lung injury markers (ALP and LDH) in bronchoalveolar lavage fluid. Data represent mean ± standard deviation (n=4).
To further evaluate the safety, we performed BAL studies. First, wet lung weights were recorded after treatment with saline or 0.1% SDS: the lung weight of the saline-treated rats was ~0.28 g/100 g of body weight, whereas that of the SDS-treated rats was ~0.7 g/100 g of body weight, suggesting accumulation of extracellular fluid in epithelial cells of the respiratory wall and subsequent development of edema in the SDS-treated lungs. Lungs treated with CAR-liposomes showed a slight (but not significant) increase in wet lung weight (~4 g/100 g body weight) compared with saline-treated lungs, pointing to no major injury or edema formation (Figure 6.B). Similarly, the total protein content (Figure 6.C) and levels of two lung injury markers, ALP and LDH, in the BAL fluid obtained from CAR-liposome-treated rats were not significantly different from the levels in saline-treated lungs (Figure 6.D). In contrast, the BAL fluid of SDS-treated animals produced higher levels of injury markers. However, these data are valid for the acute safety of CAR-liposomes after IT administration; chronic safety studies are required to establish the product's suitability for long-term use.
CONCLUSIONS
This is the first study to investigate the feasibility of encapsulating two anti-PAH drugs in peptide-conjugated liposomal formulations. CAR-liposomes were stable at 4°C, and continuously released fasudil and SOD. The formulation was efficiently taken up by both endothelial and smooth muscle cells. Compared with the plain counterparts, CAR-liposomes significantly extended the biological half-lives of fasudil and SOD. IPRL studies demonstrated that CAR-liposomes were better retained in the lungs. BAL studies indicated the safety of peptide-equipped liposomes as pulmonary delivery carriers. However, in-vivo efficacy studies in a PAH animal model are needed to support the clinical feasibility of this liposome-based combination therapy.
ACKNOWLEDGEMENTS
We thank Drs. Eva Nozik-Grayck and Kurt Stenmark at the University of Colorado, Denver, for providing us with PAE and PASM cell lines. This work was supported in part by an American Recovery and Reinvestment Act Fund, NIH 1R15HL103431, to Dr. Fakhrul Ahsan.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Allen TM, Hansen C, Rutledge J. Liposomes with prolonged circulation times: factors affecting uptake by reticuloendothelial and other tissues. Biochim Biophys Acta. 1989;981:27–35. doi: 10.1016/0005-2736(89)90078-3. ;1; [DOI] [PubMed] [Google Scholar]
- Barman SA, Zhu S, White RE. RhoA/Rho-kinase signaling: a therapeutic target in pulmonary hypertension. Vasc Health Risk Manag. 2009;5:663–671. doi: 10.2147/vhrm.s4711. ;1; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SY, Loscalzo J. Pathogenic mechanisms of pulmonary arterial hypertension. J Mol Cell Cardiol. 2008;44:14–30. doi: 10.1016/j.yjmcc.2007.09.006. ;1; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolla D, Gonda I, Chan HK. Liposomal formulations for inhalation. Ther Deliv. 2013;4:1047–1072. doi: 10.4155/tde.13.71. ;1; [DOI] [PubMed] [Google Scholar]
- Costa AP, Xu X, Burgess DJ. Freeze-anneal-thaw cycling of unilamellar liposomes: effect on encapsulation efficiency. Pharm Res. 2014;31:97–103. doi: 10.1007/s11095-013-1135-z. ;1; [DOI] [PubMed] [Google Scholar]
- Darwis Y, Kellaway IW. Nebulisation of rehydrated freeze-dried beclomethasone dipropionate liposomes. Int J Pharm. 2001;215:113–121. doi: 10.1016/s0378-5173(00)00670-0. ;1; [DOI] [PubMed] [Google Scholar]
- Demarco VG, Whaley-Connell AT, Sowers JR, Habibi J, Dellsperger KC. Contribution of oxidative stress to pulmonary arterial hypertension. World J Cardiol. 2010;2:316–324. doi: 10.4330/wjc.v2.i10.316. ;1; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge GR, Kovalszky I, Hassell JR, Iozzo RV. Transforming growth factor beta alters the expression of heparan sulfate proteoglycan in human colon carcinoma cells. J Biol Chem. 1990;265:18023–18029. ;1; [PubMed] [Google Scholar]
- Farrow KN, Lakshminrusimha S, Czech L, Groh BS, Gugino SF, Davis JM, Russell JA, Steinhorn RH. SOD and inhaled nitric oxide normalize phosphodiesterase 5 expression and activity in neonatal lambs with persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2010;299:L109–116. doi: 10.1152/ajplung.00309.2009. ;1; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman EJ, Lancet JE, Kolitz JE, Ritchie EK, Roboz GJ, List AF, Allen SL, Asatiani E, Mayer LD, Swenson C, Louie AC. First-in-man study of CPX-351: a liposomal carrier containing cytarabine and daunorubicin in a fixed 5:1 molar ratio for the treatment of relapsed and refractory acute myeloid leukemia. J Clin Oncol. 2011;29:979–985. doi: 10.1200/JCO.2010.30.5961. ;1; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomberg-Maitland M, Olschewski H. Prostacyclin therapies for the treatment of pulmonary arterial hypertension. Eur Respir J. 2008;31:891–901. doi: 10.1183/09031936.00097107. ;1; [DOI] [PubMed] [Google Scholar]
- Gupta N, Ibrahim HM, Ahsan F. Peptide-micelle hybrids containing fasudil for targeted delivery to the pulmonary arteries and arterioles to treat pulmonary arterial hypertension. J Pharm Sci. 2014a;103:3743–3753. doi: 10.1002/jps.24193. ;1; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Patel B, Ahsan F. Nano-engineered erythrocyte ghosts as inhalational carriers for delivery of fasudil: preparation and characterization. Pharm Res. 2014b;31:1553–1565. doi: 10.1007/s11095-013-1261-7. ;1; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta V, Gupta N, Shaik IH, Mehvar R, McMurtry IF, Oka M, Nozik-Grayck E, Komatsu M, Ahsan F. Liposomal fasudil, a rho-kinase inhibitor, for prolonged pulmonary preferential vasodilation in pulmonary arterial hypertension. J Control Release. 2013;167:189–199. doi: 10.1016/j.jconrel.2013.01.011. ;1; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajos F, Stark B, Hensler S, Prassl R, Mosgoeller W. Inhalable liposomal formulation for vasoactive intestinal peptide. Int J Pharm. 2008;357:286–294. doi: 10.1016/j.ijpharm.2008.01.046. ;1; [DOI] [PubMed] [Google Scholar]
- Hood E, Simone E, Wattamwar P, Dziubla T, Muzykantov V. Nanocarriers for vascular delivery of antioxidants. Nanomedicine (Lond) 2011;6:1257–1272. doi: 10.2217/nnm.11.92. ;1; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain PP, Leber R, Nagaraj C, Leitinger G, Lehofer B, Olschewski H, Olschewski A, Prassl R, Marsh LM. Liposomal nanoparticles encapsulating iloprost exhibit enhanced vasodilation in pulmonary arteries. Int J Nanomedicine. 2014;9:3249–3261. doi: 10.2147/IJN.S63190. ;1; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvinen TA, Ruoslahti E. Molecular changes in the vasculature of injured tissues. Am J Pathol. 2007;171:702–711. doi: 10.2353/ajpath.2007.061251. ;1; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger JR. Pulmonary arterial hypertension: an overview. Semin Cardiothorac Vasc Anesth. 2007;11:96–103. doi: 10.1177/1089253207301447. ;1; [DOI] [PubMed] [Google Scholar]
- Main C, Bojke L, Griffin S, Norman G, Barbieri M, Mather L, Stark D, Palmer S, Riemsma R. Topotecan, pegylated liposomal doxorubicin hydrochloride and paclitaxel for second-line or subsequent treatment of advanced ovarian cancer: a systematic review and economic evaluation. Health Technol Assess. 2006;10:1–132. iii–iv. doi: 10.3310/hta10090. ;1; [DOI] [PubMed] [Google Scholar]
- Mallick S, Choi JS. Liposomes: versatile and biocompatible nanovesicles for efficient biomolecules delivery. J Nanosci Nanotechnol. 2014;14:755–765. doi: 10.1166/jnn.2014.9080. ;1; [DOI] [PubMed] [Google Scholar]
- Marques-Gallego P, de Kroon AI. Ligation strategies for targeting liposomal nanocarriers. Biomed Res Int. 2014;2014:129458. doi: 10.1155/2014/129458. ;1; [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGoon MD, Kane GC. Pulmonary hypertension: diagnosis and management. Mayo Clin Proc. 2009;84:191–207. doi: 10.4065/84.2.191. ;1; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moazeni E, Gilani K, Sotoudegan F, Pardakhty A, Najafabadi AR, Ghalandari R, Fazeli MR, Jamalifar H. Formulation and in vitro evaluation of ciprofloxacin containing niosomes for pulmonary delivery. J Microencapsul. 2010;27:618–627. doi: 10.3109/02652048.2010.506579. ;1; [DOI] [PubMed] [Google Scholar]
- Myhren L, Nilssen IM, Nicolas V, Doskeland SO, Barratt G, Herfindal L. Efficacy of multi-functional liposomes containing daunorubicin and emetine for treatment of acute myeloid leukaemia. Eur J Pharm Biopharm. 2014;88:186–193. doi: 10.1016/j.ejpb.2014.04.002. ;1; [DOI] [PubMed] [Google Scholar]
- Nagaoka T, Fagan KA, Gebb SA, Morris KG, Suzuki T, Shimokawa H, McMurtry IF, Oka M. Inhaled Rho kinase inhibitors are potent and selective vasodilators in rat pulmonary hypertension. Am J Respir Crit Care Med. 2005;171:494–499. doi: 10.1164/rccm.200405-637OC. ;1; [DOI] [PubMed] [Google Scholar]
- Nahar K, Absar S, Patel B, Ahsan F. Starch-coated magnetic liposomes as an inhalable carrier for accumulation of fasudil in the pulmonary vasculature. Int J Pharm. 2014;464:185–195. doi: 10.1016/j.ijpharm.2014.01.007. ;1; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasedkin A, Davidsson J, Kumpugdee-Vollrath M. Determination of nanostructure of liposomes containing two model drugs by X-ray scattering from a synchrotron source. J Synchrotron Radiat. 2013;20:721–728. doi: 10.1107/S0909049513020074. ;1; [DOI] [PubMed] [Google Scholar]
- Oka M, Homma N, Taraseviciene-Stewart L, Morris KG, Kraskauskas D, Burns N, Voelkel NF, McMurtry IF. Rho kinase-mediated vasoconstriction is important in severe occlusive pulmonary arterial hypertension in rats. Circ Res. 2007;100:923–929. doi: 10.1161/01.RES.0000261658.12024.18. ;1; [DOI] [PubMed] [Google Scholar]
- Sahib MN, Darwis Y, Peh KK, Abdulameer SA, Tan YT. Rehydrated sterically stabilized phospholipid nanomicelles of budesonide for nebulization: physicochemical characterization and in vitro, in vivo evaluations. Int J Nanomedicine. 2011;6:2351–2366. doi: 10.2147/IJN.S25363. ;1; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe M, Easthope SE, Keating GM, Lamb HM. Polyethylene glycolliposomal doxorubicin: a review of its use in the management of solid and haematological malignancies and AIDS-related Kaposi's sarcoma. Drugs. 2002;62:2089–2126. doi: 10.2165/00003495-200262140-00012. ;1; [DOI] [PubMed] [Google Scholar]
- Shuvaev VV, Han J, Yu KJ, Huang S, Hawkins BJ, Madesh M, Nakada M, Muzykantov VR. PECAM-targeted delivery of SOD inhibits endothelial inflammatory response. FASEB J. 2011;25:348–357. doi: 10.1096/fj.10-169789. ;1; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardi PG, Gallagher RC, Johnstone S, Harasym N, Webb M, Bally MB, Mayer LD. Coencapsulation of irinotecan and floxuridine into low cholesterol-containing liposomes that coordinate drug release in vivo. Biochim Biophys Acta. 2007;1768:678–687. doi: 10.1016/j.bbamem.2006.11.014. ;1; [DOI] [PubMed] [Google Scholar]
- Toba M, Alzoubi A, O'Neill K, Abe K, Urakami T, Komatsu M, Alvarez D, Jarvinen TA, Mann D, Ruoslahti E, McMurtry IF, Oka M. A novel vascular homing peptide strategy to selectively enhance pulmonary drug efficacy in pulmonary arterial hypertension. Am J Pathol. 2014;184:369–375. doi: 10.1016/j.ajpath.2013.10.008. ;1; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urakami T, Jarvinen TA, Toba M, Sawada J, Ambalavanan N, Mann D, McMurtry I, Oka M, Ruoslahti E, Komatsu M. Peptide-Directed Highly Selective Targeting of Pulmonary Arterial Hypertension. Am J Pathol. 2011;178:2489–2495. doi: 10.1016/j.ajpath.2011.02.032. ;1; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Xiao R, Zeng Z, Xu L, Wang J. Application of poly(ethylene glycol)-distearoylphosphatidylethanolamine (PEG-DSPE) block copolymers and their derivatives as nanomaterials in drug delivery. Int J Nanomedicine. 2012;7:4185–4198. doi: 10.2147/IJN.S34489. ;1; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhigaltsev IV, Maurer N, Akhong QF, Leone R, Leng E, Wang J, Semple SC, Cullis PR. Liposome-encapsulated vincristine, vinblastine and vinorelbine: a comparative study of drug loading and retention. J Control Release. 2005;104:103–111. doi: 10.1016/j.jconrel.2005.01.010. ;1; [DOI] [PubMed] [Google Scholar]