Abstract
The immunomodulatory cytokine interleukin-10 (IL-10) plays beneficial but also potentially detrimental roles in inflammation, infection, and autoimmunity. Recent studies suggest a regulatory role for IL-10-expressing B cells in the autoimmune blistering disease pemphigus vulgaris. Here we review the studies on IL-10 in pemphigus vulgaris and discuss the potential pathophysiological significance of these findings in comparison to prior studies of IL-10 in other human conditions. A better understanding of the complex roles of IL-10 in immune regulation may improve our understanding of pemphigus pathogenesis and treatment.
1. IL-10 regulation of host immune responses
IL-10 is an immunoregulatory cytokine whose primary function is to limit inflammatory responses. Its chief role as a negative immune regulator is evidenced in IL-10−/− mice, which develop severe intestinal inflammation due to the inability to control immune responses to gut-resident bacterial flora [1, 2]. Similarly, humans with mutations in IL-10 or its receptor develop early-onset enterocolitis [3, 4]. IL-10’s myriad functions have previously been reviewed [5, 6]; its anti-inflammatory effects include inhibiting the production of pro-inflammatory cytokines such as IL-1, tumor necrosis factor alpha, and IL-12 from T cells and antigen presenting cells [7-9], inhibiting production of chemokines, downregulating expression of major histocompatibility (MHC) class II and co-stimulatory molecules such as CD86 to inhibit antigen presentation and induce tolerance [10-13], regulating immunoglobulin class switch in B cells (particularly to the IgG4 subclass) [14], and attenuating CD4+ T cell responses (Figure 1). Despite these predominantly negative immune regulatory functions, IL-10 can also stimulate immune responses by promoting the proliferation and cytotoxic activity of CD8+ T cells and natural killer cells [15-18], as well as the survival and antibody secretion of B cells [19, 20]. These effects are context-dependent, since inhibitory or pro-apoptotic effects of IL-10 on CD8+ T cells or B cells can also be observed under different experimental conditions [21, 22]. For example, IL-10 has been shown to promote apoptosis of B cells when added within 3 days of activation, whereas addition of IL-10 greater than 3 days after B cell activation supports their differentiation into antibody-secreting cells [22].
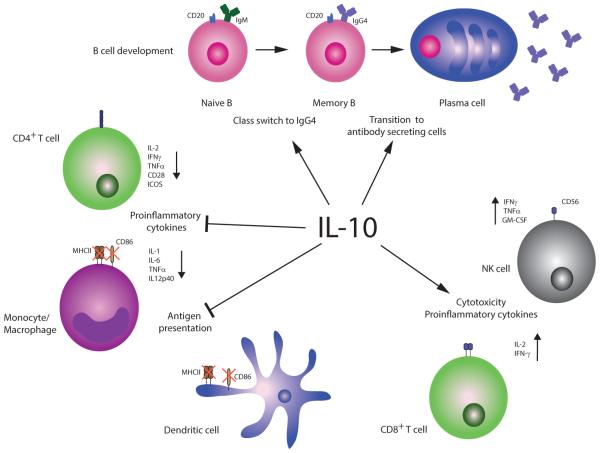
Figure 1. IL-10 in general immune homeostasis.
IL-10 inhibits proinflammatory cytokine production, including IL-2, interferon gamma (IFNγ), and tumor necrosis factor alpha (TNFα) from CD4+ T cells [7], as well as IL-1, IL-6, TNFα, and IL-12 production from monocytes and macrophages [8, 9]. IL-10 impairs CD4+ T helper cell effector and memory responses by inhibiting CD28 and inducible costimulator (ICOS) T cell signaling [10] and downregulating major histocompatibility complex class II (MHCII) and CD86 costimulatory molecules on monocytes and dendritic cells, which prevents effective antigen presentation [11-13]. IL-10 expression in B cells favors class switch to IgG4 [14] and B cell differentiation into plasma cells [31], which can have anti-inflammatory effects due to IgG4’s inability to fix complement or form immune complexes. Alongside these predominantly anti-inflammatory functions, IL-10 (in conjunction with IL-2) can increase cytotoxicity of CD8+ T cells [16] and natural killer cells [18] by upregulation of pro-inflammatory cytokines, including IFNγ, IL-2, TNFα, and/or GM-CSF. However, long-term exposure of CD4+ and CD8+ T cells to IL-10 can result in T cell exhaustion [81, 82], including lack of cytotoxicity, cytokine production, and antigen-induced proliferation.
IL-10’s beneficial and detrimental roles in host immune responses are perhaps most apparent during infection, as IL-10−/− mice demonstrate enhanced clearance of bacterial, fungal, and viral infections (reviewed in [23, 24]), but at the expense of an amplified immune response that may potentially lead to septic shock [25]. Similarly, humans with IL-10 gene polymorphisms demonstrate altered susceptibility to a variety of viral and bacterial pathogens [23, 24], consistent with their genotype-phenotype predictions. Although caution is warranted in using mouse models to understand human inflammatory conditions, IL-10 signaling pathway gene expression profiles have been reported to demonstrate the highest Pearson correlations between mouse and human models, with similar changes in expression observed for 67-79% of pathway genes [26]. Thus, there appears to be reasonable conservation of IL-10 responses among mice and humans.
IL-10 can be produced by monocytes and macrophages, T and B lymphocytes, dendritic cells, other leukocytes (including mast cells, neutrophils, and eosinophils) and some epithelial cells, including keratinocytes [27-29]. IL-10 production by CD4+ T cells is essential for immune homeostasis, since CD4+ T cell-restricted deficiency of IL-10 causes intestinal inflammation in mice similar to the colitis observed in mice globally deficient in IL-10 [30]. In T cells, IL-10 expression defines a subset of T regulatory cells (Tregs) that can develop from either CD4+CD25+FoxP3+ or CD4+CD25+FoxP3− precursors [31]. Within the B cell lineage, IL-10-producing subsets are not as restricted. Naïve CD24hiC38hi transitional cells [32], CD24hiCD27+ memory cells [33], CD73−CD25+CD71+ IgG4+ cells [34], tissue-resident marginal zone cells [35], and plasma cells [36, 37] have all been reported to express IL-10. The expression of IL-10 defines a class of B cells with regulatory functions, also known as Bregs or B10 cells [38]. However, a defining transcriptional program that is necessary and sufficient to maintain Breg functions, analogous to FoxP3 in Tregs, has not been identified. IL-10 induces the expression of plasma cell transcription factors Irf4, Xbp1, and Blimp1, with a concomitant decrease in earlier B lineage markers including Pax5 and Bcl6 [39], indicating that expression of IL-10 can directly affect B cell differentiation pathways. Thus, Bregs are not a static B cell subset defined by specific cell surface markers, but rather comprise a dynamic subset of B cells that can be induced to exhibit regulatory functions in a context-dependent manner.
2. The role of IL-10 in PV pathogenesis
Pemphigus vulgaris (PV) is a tissue-specific autoimmune blistering disease caused by autoantibodies against the keratinocyte adhesion protein desmoglein (Dsg) 3[40]. Anti-Dsg3 IgG antibodies are both necessary and sufficient to cause characteristic suprabasal epidermal blisters in mouse passive transfer models of PV [41, 42]. Even monovalent anti-Dsg3 antibody fragments (Fab’ or single chain variable fragment monoclonal antibodies) can induce blisters in mouse and human skin [43-45], thus clearly establishing the pathogenicity of anti-Dsg3 antibodies in PV.
Because of IL-10’s diverse functions in regulating immune responses, there is great interest in understanding the role of IL-10 in the onset and treatment of autoimmunity. Recent studies have expanded our understanding of IL-10 in PV.
IL-10 in mouse models of PV
In a murine alloreactive model of PV, pathogenic and nonpathogenic Dsg3-reactive CD4+ T cell clones (isolated after immunization of Dsg3−/− mice with Dsg3) have been isolated; the former induce anti-Dsg3 IgG production and typical PV blisters after adoptive transfer with splenic Dsg3−/− B cells to Dsg3+/+Rag-2−/− mice, whereas the latter do not elicit disease [46]. IL-4 and IL-10 expression are significantly upregulated in pathogenic versus non-pathogenic anti-Dsg3 T cell clones. However, exogenous administration of IL-10 does not promote production of anti-Dsg3 IgG from primed B cells in vitro, and in vivo IL-10 blockade by expression of soluble IL-10 receptor does not affect disease incidence, whereas IL-4 promotes anti-Dsg3 IgG production and is necessary for disease onset in this model. In contrast, IL-10−/− mice demonstrate increased susceptibility to blister formation after passive transfer of PV plasma, and conversely, exogenous administration of IL-10 prevents blister formation after passive transfer of PV plasma to wild-type mice [47], indicating that IL-10 protects against PV blister formation. It is unknown whether these findings are due to loss of the immunoregulatory effects of IL-10, or whether IL-10-deficient keratinocytes may have defects in cell adhesion. In favor of the latter, IL-10 has been shown to inhibit the p38/MAPKAP-kinase 2 pathway [48], which protects against blister formation by regulating PV IgG-induced Dsg3 endocytosis in keratinocytes [49-52]. Taken together, these data are not sufficient to support a central role for IL-10 in the onset of autoimmunity in mouse PV models, although IL-10 may have other regulatory roles in preventing PV blister formation downstream of IgG binding to Dsg3.
IL-10 gene polymorphisms in PV patients
An increased frequency of the low producer IL-10 haplotype (−1082A/−819T) was identified in Argentinian PV patients, suggesting that the decreased production of IL-10 may predispose to PV, or alternatively that haplotypes conferring high and intermediate IL-10 production are protective against PV [53]. A weak association between PV and a different low producer IL-10 haplotype (−1082A/−819C/−592C) was also identified in Slovak PV patients [54]. How IL-10 polymorphisms might affect PV susceptibility in these patient populations is unknown, although the phenotype-genotype correlations are consistent with the protective role of IL-10 observed in mouse studies.
Serum levels of IL-10 in PV
Serum IL-10 levels have been reported to be elevated in PV patients with active disease compared to unaffected controls [55, 56], and in one study, levels of serum IL-10 correlate with disease activity [55]. However, two other studies were unable to detect serum IL-10 in PV patients [57, 58]. It is possible that the use of high-sensitivity assays in some studies may facilitate the detection of lower level increases in serum IL-10 in patients versus controls. IL-10 is consistently upregulated in the blister fluid of PV patients [55, 57], although elevations of IL-10 in blister fluid are also observed in bullous contact dermatitis, indicating that this finding is non-specific and likely results from local production of IL-10 by damaged keratinocytes.
IL-10 in IgG4 class switch
Although the Fc region of anti-Dsg3 IgG is not required for blister formation, active disease is associated with specific anti-Dsg3 IgG subclasses. PV patients with active disease demonstrate predominantly IgG4 followed by IgG1 autoantibodies to Dsg3, whereas patients in remission and unaffected relatives of PV patients can demonstrate anti-Dsg3 IgG1[59-63]. Furthermore, in patients with active disease, anti-Dsg3 antibodies comprise a significantly higher percentage of total serum IgG4 versus IgG1[64], indicating selective enrichment of PV autoantibodies in the IgG4 subclass.
IL-10 can induce naïve CD40-primed B cells to class switch and secrete antibody [65], preferentially of the IgG4 subclass [14, 66, 67]. IgG4 possesses unique structural features within the hinge region that prevent it from crosslinking antigen, forming immune complexes, or activating complement [68-70]. These anti-inflammatory functions of IgG4 in part underlie the effectiveness of allergen desensitization therapy. Repetitive antigen stimulation induces an influx of IL-10, made initially by T regulatory cells, then B cells and monocytes. IL-10 promotes the production of antigen-specific IgG4, which blocks the pro-inflammatory effects of IgE, and IL-10 also causes antigen-specific T cells to become anergic or exhausted (discussed further below) [14, 71, 72]. In beekeepers tolerant to bee venom antigens and patients after allergen-specific immunotherapy, IgG4 production has been shown to be restricted to IL-10+ B cells [34].
Interestingly, novice beekeepers initially demonstrate IgG1 serum responses to phospholipase A antigens in bee venom, but within 3 years virtually all beekeepers have an IgG4-predominant response [73], similar to the evolution of autoantibody profiles in PV patients. Thus, repetitive antigen exposure triggers a stereotyped immune response, characterized by an increase in IL-10+ T and B cells and a shift toward antigen-specific IgG4, which in the setting of allergy is protective. However, in the setting of PV, the switch to antigen-specific IgG4 does not alleviate disease, but instead becomes the defining serologic feature of active disease.
IL-10+ T cells in PV
The critical role of Tregs in maintaining immune homeostasis is evidenced by individuals with IPEX (immunodysregulation polyendocrinopathy enteropathy X-linked) syndrome, caused by mutations in the Treg transcription factor FOXP3 [74]. Patients develop a broad range of autoimmune and inflammatory conditions, including enterocolitis, dermatitis, type 1 diabetes, and autoimmune thyroiditis. Subsequently, there have been numerous studies on the role of Tregs in autoimmunity. Tregs are reduced in patients with systemic lupus erythematosus, correlating with disease severity (reviewed in [75]). However, studies in type 1 diabetes, multiple sclerosis, and rheumatoid arthritis have not shown consistent changes in Treg frequencies in the peripheral blood, suggesting that qualitative rather than quantitative defects in Tregs, or Treg deficiencies in affected tissues, may be more important in these diseases. A complicating factor in determining the role of IL-10 in Treg function and autoimmunity is that IL-10 expression can occur in FoxP3− T cells and not all FoxP3+ Tregs express IL-10 [31], indicating that IL-10 expression does not define the Treg population and conversely that Tregs do not always depend on IL-10 for their regulatory functions.
Studies in PV have shown that similar low frequencies of Dsg3-autoreactive T cells are found in PV patients and asymptomatic carriers of PV-associated HLA class II alleles, but not healthy HLA-unmatched controls [76]. There are no marked differences in Dsg3 peptides recognized by T cells from PV patients and HLA-matched unaffected individuals, indicating that T cell autoreactivity to Dsg3 is primarily dictated by HLA class II expression and is not sufficient to produce disease [77]. In contrast, IL-10+ Dsg3-reactive Tregs, capable of suppressing T cell proliferation in response to Dsg3 in an IL-10-dependent manner, are found in 17% of PV patients, compared to 80% of HLA-matched unaffected controls [78]. Although FoxP3 expression was not directly assayed in these studies, collectively the data suggest that IL-10+ Dsg3-reactive Tregs and T helper cells are critical for maintaining tolerance to Dsg3 and controlling disease onset in PV.
As discussed above, IL-10 production by T cells is critical for limiting host immune responses, but these protective functions can become detrimental in different contexts. During chronic viral infection, both CD4 and CD8 T cells can become functionally exhausted [79, 80]. These cells lose the capacity to eliminate target cells, secrete pro-inflammatory cytokines, and proliferate in response to antigen which results in the inability to control viral replication. IL-10 plays a critical role in mediating T cell exhaustion. In a mouse model of chronic lymphocytic choriomeningitis virus infection, treatment of mice with an IL-10 receptor blocking antibody reestablishes cytotoxic T cell functions and promotes viral clearance [81, 82]. Similarly, in vitro blockade of IL-10 signaling in exhausted CD4 T cells isolated from chronically infected HIV or hepatitis C individuals restores T cell proliferation and secretion of antiviral cytokines in response to antigen [83, 84]. Autoimmunity, like chronic infection, is characterized by the inability to clear antigen, leading to repetitive antigen stimulation and IL-10 induction. Although T cell exhaustion is detrimental in the setting of chronic infection, autoantigen-specific T cell exhaustion in PV would be desirable, as the blunting of immune responses by IL-10 may reestablish tolerance to self-antigen and potentially induce disease remission.
IL-10+ B regulatory cells in PV
The functional significance of Bregs was first described in a mouse model of experimental autoimmune encephalomyelitis, in which recovery from disease was dependent on IL-10+ autoantigen-reactive B cells [85]. B cells exhibiting regulatory functions have subsequently been identified in humans, where they are thought to be most highly enriched in the CD19+CD24hiCD38hi B cell subset [32]. The physiologic and pathophysiologic roles of Bregs have previously been reviewed [38, 86]. Pemphigus patients with active disease have an increased percentage of CD19+CD24hiCD38hi IL-10-expressing Bregs in the peripheral blood compared to unaffected controls or pemphigus patients in remission [87]. However, Bregs in pemphigus patients showed reduced ability to secrete IL-10 and suppress interferon-gamma expression by CD4+ T cells. These findings are generally consistent with prior studies, which have identified an expansion of Bregs in other autoimmune disease patients [33], but with qualitative defects in Breg IL-10 production and T cell suppressive capacity [32].
A recent study has shown that pemphigus patients achieving long-term complete remission of disease after B-cell depletion therapy with the anti-CD20 monoclonal antibody rituximab demonstrate significantly higher numbers of IL-10-expressing CD19+CD24hiCD38hi Bregs compared to unaffected controls or pemphigus patients in incomplete remission [88]. The elevation of CD19+CD24hiCD38hi transitional B cells in patients in long-term remission could simply reflect a delay in B cell repopulation due to more complete B cell depletion in responders. Alternatively, the expansion of IL-10+ Bregs in long-term responders may be an independent consequence of rituximab therapy, since B cell depletion causes a temporary rise in the levels of serum B cell activating factor [89], which can induce the transcription of IL-10 in B cells [90]. Thus, it is unclear whether prolonged remission after rituximab in PV is due to the absence of anti-Dsg3 antibody-secreting cells or requires the additional protective effect of IL-10+ Bregs. Supporting a direct role for IL-10+ Bregs in disease remission, a study of myasthenia gravis patients treated with rituximab showed that disease remission is associated with early B10 cell repopulation, whereas delayed B10 cell repopulation is associated with lack of response to therapy [91].
3. Therapeutic potential of IL-10 in PV
Due to IL-10’s complex roles in maintaining immune homeostasis, the challenge for therapy is to understand the contexts in which IL-10 can be effectively and safely modulated to treat disease. Systemic lupus erythematosus (SLE) patients demonstrate elevated levels of IL-10 production by peripheral blood mononuclear cells [92, 93], elevations in serum IL-10 correlate with SLE disease activity [94, 95], and IL-10 gene polymorphisms conferring high IL-10 production are significantly associated with risk of developing SLE [96, 97]. An IL-10 blocking monoclonal antibody prevents onset of lupus-like disease in NZB/W F1 mice [98] and also reduces polyclonal IgM and IgG production by B cells isolated from SLE patients [99, 100]. These and other studies have led to the theory that SLE is a state of polyclonal B cell hyperactivity and that IL-10 may help drive disease by promoting the continuous differentiation of B cells into antibody-secreting cells, with their autoantibodies being one of the hallmark diagnostic features of SLE. Subsequently, a clinical trial of an IL-10 blocking monoclonal antibody in 6 SLE patients demonstrated clinical efficacy and safety, with decreased disease activity, decreased markers of immune cell hyperreactivity, and no serious adverse events at six months follow up [101].
Rheumatoid arthritis (RA) patients demonstrate many of the same features of disease as patients with SLE in regard to IL-10, including elevated IL-10 production by peripheral blood mononuclear cells [92], increased serum (as well as synovial) IL-10 levels [102], and decreased antibody secretion by B cells after IL-10 blockade [100], suggesting that IL-10 blockade could be similarly beneficial for the treatment of RA. However, in mouse models of collagen induced arthritis, transfer of CD40-primed, IL-10+ B cells prevents disease onset and also ameliorates established disease [103]. These effects are dependent on IL-10, since an IL-10 blocking monoclonal antibody inhibits the protective effects of IL-10+ B cells, and transfer of CD40-primed IL-10−/− B cells do not prevent disease onset. Additionally, in vitro culture of human RA synovial cells with IL-10 decreases their ability to act as antigen presenting cells [104], suggesting a suppressive role of IL-10 in this context. Based on the hypothesis that IL-10 would act as an anti-inflammatory and tolerogenic agent in RA, a trial of subcutaneous IL-10 in six RA patients was performed. However, RA patients demonstrate no clinical benefit after IL-10 treatment [105]. IL-10 therapy is associated with increased Fc gamma receptor expression on monocytes and correspondingly increased tumor necrosis factor alpha production by monocytes after immune complex stimulation, as well as a non-significant trend toward elevated C-reactive protein levels, suggesting that IL-10 is not effective as a systemic anti-inflammatory agent in RA. As an alternative approach, targeted delivery of IL-10 to the sites of inflammation has proven effective in preclinical studies of RA [106], and a Phase I clinical trial to deliver IL-10 specifically to the synovium is currently underway [107]. These studies underscore IL-10’s potentially beneficial and detrimental roles in regulating inflammation and the challenges involved in harnessing IL-10’s biological functions to effectively treat disease.
IL-10 may also be a double-edged sword for PV. Ideally, IL-10 could be delivered to induce Dsg3-specific T cell exhaustion in order to attenuate the autoimmune response. However, IL-10 could also further stimulate anti-Dsg3 IgG4 production, and this in fact may be the predominant response early on in therapy, with tolerogenic effects occurring only after chronic administration. Alternatively, administration of IL-10 after B cell depletion may promote tolerance and avoid further stimulation of IgG4 class switch and antibody secretion due to prior depletion of the memory B cell pool. Such a strategy may help re-establish the immune regulatory network and prevent disease relapse after B cell depletion, which affects approximately 80% of PV patients treated with rituximab [88]. An additional strategy for therapy could be to restore IL-10’s regulatory functions in Bregs and Tregs, which are thought to be dysfunctional in PV patients. Targeted re-expression of IL-10 in these cell subsets may be more effective than systemic IL-10, given its myriad and potentially conflicting roles in different cell types.
The dual nature of IL-10 in PV (detrimental during active disease but beneficial for disease remission) is depicted in Figure 2. Future studies are necessary to clarify the role of IL-10 in PV patients throughout the course of disease and therapy. A better understanding of the context-dependent roles of IL-10 in immune regulation may not only help improve our understanding of PV pathogenesis, but also lead to the design of novel strategies for disease treatment.
Figure 2. A model for the dual roles of IL-10 in PV during active disease and remission after B cell depletion therapy.
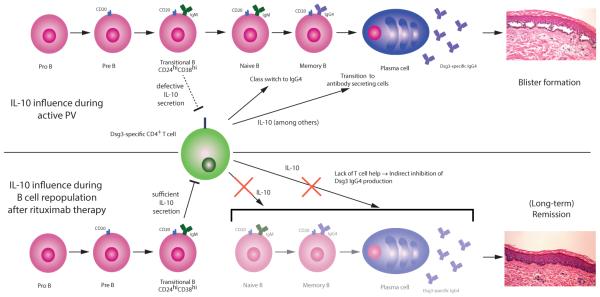
In active disease, IL-10 favors class switch to IgG4, the characteristic PV autoantibody subclass. Additionally, B cell differentiation into antibody secreting cells is promoted by IL-10, which results in higher anti-Dsg3 antibody concentrations, leading to disease symptoms. Bregs in active PV are defective, with reduced IL-10 secretion and inability to suppress CD4+ T cell responses, which perpetuates the autoimmune reaction. After B cell depletion with rituximab, repopulating CD24hiCD38hi transitional B cells produce IL-10 that can inhibit CD4+ T cells, thereby preventing the stimulation of pathogenic anti-Dsg3 B cell populations, leading to disease remission.
Highlights.
The cytokine IL-10 has myriad functions in maintaining immune homeostasis
IL-10 may have both harmful and beneficial regulatory roles in pemphigus vulgaris
IL-10 based therapies in pemphigus vulgaris may be a novel strategy for treatment
Acknowledgements
This work was supported in part by grants AR064220 (ASP) and T32-AR007465 (MJC) from NIAMS/NIH, as well as EL711/1-1 (CTE) from Deutsche Forschungsgemeinschaft. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIAMS or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- [1].Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–74. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- [2].Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224–31. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Glocker EO, Kotlarz D, Boztug K, Gertz EM, Schaffer AA, Noyan F, et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med. 2009;361:2033–45. doi: 10.1056/NEJMoa0907206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Glocker EO, Frede N, Perro M, Sebire N, Elawad M, Shah N, et al. Infant colitis--it's in the genes. Lancet. 2010;376:1272. doi: 10.1016/S0140-6736(10)61008-2. [DOI] [PubMed] [Google Scholar]
- [5].Moore KW, de Waal MR, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- [6].Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- [7].Brooks DG, Walsh KB, Elsaesser H, Oldstone MB. IL-10 directly suppresses CD4 but not CD8 T cell effector and memory responses following acute viral infection. Proc Natl Acad Sci U S A. 2010;107:3018–23. doi: 10.1073/pnas.0914500107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–22. [PubMed] [Google Scholar]
- [9].Murphy EE, Terres G, Macatonia SE, Hsieh CS, Mattson J, Lanier L, et al. B7 and interleukin 12 cooperate for proliferation and interferon gamma production by mouse T helper clones that are unresponsive to B7 costimulation. J Exp Med. 1994;180:223–31. doi: 10.1084/jem.180.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Taylor A, Akdis M, Joss A, Akkoc T, Wenig R, Colonna M, et al. IL-10 inhibits CD28 and ICOS costimulations of T cells via src homology 2 domain-containing protein tyrosine phosphatase 1. J Allergy Clin Immunol. 2007;120:76–83. doi: 10.1016/j.jaci.2007.04.004. [DOI] [PubMed] [Google Scholar]
- [11].Koppelman B, Neefjes JJ, de Vries JE, de Waal MR. Interleukin-10 down-regulates MHC class II alphabeta peptide complexes at the plasma membrane of monocytes by affecting arrival and recycling. Immunity. 1997;7:861–71. doi: 10.1016/s1074-7613(00)80404-5. [DOI] [PubMed] [Google Scholar]
- [12].Buelens C, Willems F, Delvaux A, Pierard G, Delville JP, Velu T, et al. Interleukin-10 differentially regulates B7-1 (CD80) and B7-2 (CD86) expression on human peripheral blood dendritic cells. Eur J Immunol. 1995;25:2668–72. doi: 10.1002/eji.1830250940. [DOI] [PubMed] [Google Scholar]
- [13].Mitra RS, Judge TA, Nestle FO, Turka LA, Nickoloff BJ. Psoriatic skin-derived dendritic cell function is inhibited by exogenous IL-10. Differential modulation of B7-1 (CD80) and B7-2 (CD86) expression. J Immunol. 1995;154:2668–77. [PubMed] [Google Scholar]
- [14].Akdis CA, Blesken T, Akdis M, Wuthrich B, Blaser K. Role of interleukin 10 in specific immunotherapy. J Clin Invest. 1998;102:98–106. doi: 10.1172/JCI2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rowbottom AW, Lepper MA, Garland RJ, Cox CV, Corley EG. Interleukin-10-induced CD8 cell proliferation. Immunology. 1999;98:80–9. doi: 10.1046/j.1365-2567.1999.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Santin AD, Hermonat PL, Ravaggi A, Bellone S, Pecorelli S, Roman JJ, et al. Interleukin-10 increases Th1 cytokine production and cytotoxic potential in human papillomavirus-specific CD8(+) cytotoxic T lymphocytes. J Virol. 2000;74:4729–37. doi: 10.1128/jvi.74.10.4729-4737.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Schwarz MA, Hamilton LD, Tardelli L, Narula SK, Sullivan LM. Stimulation of cytolytic activity by interleukin-10. J Immunother Emphasis Tumor Immunol. 1994;16:95–104. doi: 10.1097/00002371-199408000-00003. [DOI] [PubMed] [Google Scholar]
- [18].Carson WE, Lindemann MJ, Baiocchi R, Linett M, Tan JC, Chou CC, et al. The functional characterization of interleukin-10 receptor expression on human natural killer cells. Blood. 1995;85:3577–85. [PubMed] [Google Scholar]
- [19].Rousset F, Garcia E, Defrance T, Peronne C, Vezzio N, Hsu DH, et al. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci U S A. 1992;89:1890–3. doi: 10.1073/pnas.89.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Levy Y, Brouet JC. Interleukin-10 prevents spontaneous death of germinal center B cells by induction of the bcl-2 protein. J Clin Invest. 1994;93:424–8. doi: 10.1172/JCI116977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Groux H, Bigler M, de Vries JE, Roncarolo MG. Inhibitory and stimulatory effects of IL-10 on human CD8+ T cells. J Immunol. 1998;160:3188–93. [PubMed] [Google Scholar]
- [22].Itoh K, Hirohata S. The role of IL-10 in human B cell activation, proliferation, and differentiation. J Immunol. 1995;154:4341–50. [PubMed] [Google Scholar]
- [23].Mege JL, Meghari S, Honstettre A, Capo C, Raoult D. The two faces of interleukin 10 in human infectious diseases. Lancet Infect Dis. 2006;6:557–69. doi: 10.1016/S1473-3099(06)70577-1. [DOI] [PubMed] [Google Scholar]
- [24].Iyer SS, Cheng G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit Rev Immunol. 2012;32:23–63. doi: 10.1615/critrevimmunol.v32.i1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gazzinelli RT, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, Kuhn R, et al. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J Immunol. 1996;157:798–805. [PubMed] [Google Scholar]
- [26].Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013;110:3507–12. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Saraiva M, O'Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170–81. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- [28].Enk AH, Katz SI. Identification and induction of keratinocyte-derived IL-10. J Immunol. 1992;149:92–5. [PubMed] [Google Scholar]
- [29].Rivas JM, Ullrich SE. Systemic suppression of delayed-type hypersensitivity by supernatants from UV-irradiated keratinocytes. An essential role for keratinocyte-derived IL-10. J Immunol. 1992;149:3865–71. [PubMed] [Google Scholar]
- [30].Roers A, Siewe L, Strittmatter E, Deckert M, Schluter D, Stenzel W, et al. T cell-specific inactivation of the interleukin 10 gene in mice results in enhanced T cell responses but normal innate responses to lipopolysaccharide or skin irritation. J Exp Med. 2004;200:1289–97. doi: 10.1084/jem.20041789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Maynard CL, Harrington LE, Janowski KM, Oliver JR, Zindl CL, Rudensky AY, et al. Regulatory T cells expressing interleukin 10 develop from Foxp3+ and Foxp3− precursor cells in the absence of interleukin 10. Nat Immunol. 2007;8:931–41. doi: 10.1038/ni1504. [DOI] [PubMed] [Google Scholar]
- [32].Blair PA, Norena LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. 2010;32:129–40. doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- [33].Iwata Y, Matsushita T, Horikawa M, Dilillo DJ, Yanaba K, Venturi GM, et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117:530–41. doi: 10.1182/blood-2010-07-294249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].van de Veen W, Stanic B, Yaman G, Wawrzyniak M, Sollner S, Akdis DG, et al. IgG4 production is confined to human IL-10-producing regulatory B cells that suppress antigen-specific immune responses. J Allergy Clin Immunol. 2013;131:1204–12. doi: 10.1016/j.jaci.2013.01.014. [DOI] [PubMed] [Google Scholar]
- [35].Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–50. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- [36].Madan R, Demircik F, Surianarayanan S, Allen JL, Divanovic S, Trompette A, et al. Nonredundant roles for B cell-derived IL-10 in immune counter-regulation. J Immunol. 2009;183:2312–20. doi: 10.4049/jimmunol.0900185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Dang VD, Hilgenberg E, Ries S, Shen P, Fillatreau S. From the regulatory functions of B cells to the identification of cytokine-producing plasma cell subsets. Curr Opin Immunol. 2014;28:77–83. doi: 10.1016/j.coi.2014.02.009. [DOI] [PubMed] [Google Scholar]
- [38].Candando KM, Lykken JM, Tedder TF. B10 cell regulation of health and disease. Immunol Rev. 2014;259:259–72. doi: 10.1111/imr.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Maseda D, Smith SH, Dilillo DJ, Bryant JM, Candando KM, Weaver CT, et al. Regulatory B10 cells differentiate into antibody-secreting cells after transient IL-10 production in vivo. J Immunol. 2012;188:1036–48. doi: 10.4049/jimmunol.1102500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Payne AS, Hanakawa Y, Amagai M, Stanley JR. Desmosomes and disease: pemphigus and bullous impetigo. Curr Opin Cell Biol. 2004;16:536–43. doi: 10.1016/j.ceb.2004.07.006. [DOI] [PubMed] [Google Scholar]
- [41].Amagai M, Karpati S, Prussick R, Klaus-Kovtun V, Stanley JR. Autoantibodies against the amino-terminal cadherin-like binding domain of pemphigus vulgaris antigen are pathogenic. J Clin Invest. 1992;90:919–26. doi: 10.1172/JCI115968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Amagai M, Hashimoto T, Shimizu N, Nishikawa T. Absorption of pathogenic autoantibodies by the extracellular domain of pemphigus vulgaris antigen (Dsg3) produced by baculovirus. J Clin Invest. 1994;94:59–67. doi: 10.1172/JCI117349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Mascaro JM, Jr, Espana A, Liu Z, Ding X, Swartz SJ, Fairley JA, et al. Mechanisms of acantholysis in pemphigus vulgaris: role of IgG valence. Clin Immunol Immunopathol. 1997;85:90–6. doi: 10.1006/clin.1997.4408. [DOI] [PubMed] [Google Scholar]
- [44].Payne AS, Ishii K, Kacir S, Lin C, Li H, Hanakawa Y, et al. Genetic and functional characterization of human pemphigus vulgaris monoclonal autoantibodies isolated by phage display. J Clin Invest. 2005;115:888–99. doi: 10.1172/JCI24185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Cho MJ, Lo ASY, Mao X, Nagler AR, Ellebrecht CT, Mukherjee EM, et al. Shared VH1-46 gene usage by pemphigus vulgaris autoantibodies indicates common humoral immune responses among patients. Nature Commun. 2014 doi: 10.1038/ncomms5167. doi: 10.1038/ncomms5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Takahashi H, Amagai M, Nishikawa T, Fujii Y, Kawakami Y, Kuwana M. Novel system evaluating in vivo pathogenicity of desmoglein 3-reactive T cell clones using murine pemphigus vulgaris. J Immunol. 2008;181:1526–35. doi: 10.4049/jimmunol.181.2.1526. [DOI] [PubMed] [Google Scholar]
- [47].Toto P, Feliciani C, Amerio P, Suzuki H, Wang B, Shivji GM, et al. Immune modulation in pemphigus vulgaris: role of CD28 and IL-10. J Immunol. 2000;164:522–9. doi: 10.4049/jimmunol.164.1.522. [DOI] [PubMed] [Google Scholar]
- [48].Kontoyiannis D, Kotlyarov A, Carballo E, Alexopoulou L, Blackshear PJ, Gaestel M, et al. Interleukin-10 targets p38 MAPK to modulate ARE-dependent TNF mRNA translation and limit intestinal pathology. EMBO J. 2001;20:3760–70. doi: 10.1093/emboj/20.14.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Berkowitz P, Hu P, Warren S, Liu Z, Diaz LA, Rubenstein DS. p38MAPK inhibition prevents disease in pemphigus vulgaris mice. Proc Natl Acad Sci USA. 2006;103:12855–60. doi: 10.1073/pnas.0602973103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Jolly PS, Berkowitz P, Bektas M, Lee HE, Chua M, Diaz LA, et al. p38MAPK signaling and desmoglein-3 internalization are linked events in pemphigus acantholysis. J Biol Chem. 2010;285:8936–41. doi: 10.1074/jbc.M109.087999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Mao X, Sano Y, Park JM, Payne AS. p38 MAPK activation is downstream of the loss of intercellular adhesion in pemphigus vulgaris. J Biol Chem. 2011;286:1283–91. doi: 10.1074/jbc.M110.172874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Mao X, Li H, Sano Y, Gaestel M, Park JM, Payne AS. MAPKAP kinase 2 (MK2)-dependent and -independent models of blister formation in pemphigus vulgaris. J Invest Dermatol. 2014;134:68–76. doi: 10.1038/jid.2013.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Eberhard Y, Burgos E, Gagliardi J, Vullo CM, Borosky A, Pesoa S, et al. Cytokine polymorphisms in patients with pemphigus. Arch Dermatol Res. 2005;296:309–13. doi: 10.1007/s00403-004-0528-6. [DOI] [PubMed] [Google Scholar]
- [54].Javor J, Chmurova N, Parnicka Z, Ferencik S, Grosse-Wilde H, Buc M, et al. TNF-alpha and IL-10 gene polymorphisms show a weak association with pemphigus vulgaris in the Slovak population. J Eur Acad Dermatol Venereol. 2010;24:65–8. doi: 10.1111/j.1468-3083.2009.03260.x. [DOI] [PubMed] [Google Scholar]
- [55].Bhol KC, Rojas AI, Khan IU, Ahmed AR. Presence of interleukin 10 in the serum and blister fluid of patients with pemphigus vulgaris and pemphigoid. Cytokine. 2000;12:1076–83. doi: 10.1006/cyto.1999.0642. [DOI] [PubMed] [Google Scholar]
- [56].Satyam A, Khandpur S, Sharma VK, Sharma A. Involvement of T(H)1/T(H)2 cytokines in the pathogenesis of autoimmune skin disease-Pemphigus vulgaris. Immunol Invest. 2009;38:498–509. doi: 10.1080/08820130902943097. [DOI] [PubMed] [Google Scholar]
- [57].Baroni A, Perfetto B, Ruocco E, Greco R, Criscuolo D, Ruocco V. Cytokine pattern in blister fluid and sera of patients with pemphigus. Dermatology. 2002;205:116–21. doi: 10.1159/000063895. [DOI] [PubMed] [Google Scholar]
- [58].Keskin DB, Stern JN, Fridkis-Hareli M, Ahmed AR. Cytokine profiles in pemphigus vulgaris patients treated with intravenous immunoglobulins as compared to conventional immunosuppressive therapy. Cytokine. 2008;41:315–21. doi: 10.1016/j.cyto.2007.12.007. [DOI] [PubMed] [Google Scholar]
- [59].Jones CC, Hamilton RG, Jordon RE. Subclass distribution of human IgG autoantibodies in pemphigus. J Clin Immunol. 1988;8:43–9. doi: 10.1007/BF00915155. [DOI] [PubMed] [Google Scholar]
- [60].Bhol K, Mohimen A, Ahmed AR. Correlation of subclasses of IgG with disease activity in pemphigus vulgaris. Dermatology. 1994;189(Suppl 1):85–9. doi: 10.1159/000246938. [DOI] [PubMed] [Google Scholar]
- [61].Bhol K, Natarajan K, Nagarwalla N, Mohimen A, Aoki V, Ahmed AR. Correlation of peptide specificity and IgG subclass with pathogenic and nonpathogenic autoantibodies in pemphigus vulgaris: a model for autoimmunity. Proc Natl Acad Sci USA. 1995;92:5239–43. doi: 10.1073/pnas.92.11.5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Kricheli D, David M, Frusic-Zlotkin M, Goldsmith D, Rabinov M, Sulkes J, et al. The distribution of pemphigus vulgaris-IgG subclasses and their reactivity with desmoglein 3 and 1 in pemphigus patients and their first-degree relatives. Br J Dermatol. 2000;143:337–42. doi: 10.1046/j.1365-2133.2000.03659.x. [DOI] [PubMed] [Google Scholar]
- [63].Futei Y, Amagai M, Ishii K, Kuroda-Kinoshita K, Ohya K, Nishikawa T. Predominant IgG4 subclass in autoantibodies of pemphigus vulgaris and foliaceus. Journal of Dermatological Science. 2001;26:55–61. doi: 10.1016/s0923-1811(00)00158-4. [DOI] [PubMed] [Google Scholar]
- [64].Funakoshi T, Lunardon L, Ellebrecht CT, Nagler AR, O'Leary CE, Payne AS. Enrichment of total serum IgG4 in patients with pemphigus. Br J Dermatol. 2012;167:1245–53. doi: 10.1111/j.1365-2133.2012.11144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Malisan F, Briere F, Bridon JM, Harindranath N, Mills FC, Max EE, et al. Interleukin-10 induces immunoglobulin G isotype switch recombination in human CD40-activated naive B lymphocytes. J Exp Med. 1996;183:937–47. doi: 10.1084/jem.183.3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Satoguina JS, Weyand E, Larbi J, Hoerauf A. T regulatory-1 cells induce IgG4 production by B cells: role of IL-10. J Immunol. 2005;174:4718–26. doi: 10.4049/jimmunol.174.8.4718. [DOI] [PubMed] [Google Scholar]
- [67].Jeannin P, Lecoanet S, Delneste Y, Gauchat JF, Bonnefoy JY. IgE versus IgG4 production can be differentially regulated by IL-10. J Immunol. 1998;160:3555–61. [PubMed] [Google Scholar]
- [68].van der Neut KM, Schuurman J, Losen M, Bleeker WK, Martinez-Martinez P, Vermeulen E, et al. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science. 2007;317:1554–7. doi: 10.1126/science.1144603. [DOI] [PubMed] [Google Scholar]
- [69].van der Zee JS, van SP, Aalberse RC. Inhibition of complement activation by IgG4 antibodies. Clin Exp Immunol. 1986;64:415–22. [PMC free article] [PubMed] [Google Scholar]
- [70].Bindon CI, Hale G, Bruggemann M, Waldmann H. Human monoclonal IgG isotypes differ in complement activating function at the level of C4 as well as C1q. J Exp Med. 1988;168:127–42. doi: 10.1084/jem.168.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Jutel M, Jaeger L, Suck R, Meyer H, Fiebig H, Cromwell O. Allergen-specific immunotherapy with recombinant grass pollen allergens. J Allergy Clin Immunol. 2005;116:608–13. doi: 10.1016/j.jaci.2005.06.004. [DOI] [PubMed] [Google Scholar]
- [72].Senti G, Crameri R, Kuster D, Johansen P, Martinez-Gomez JM, Graf N, et al. Intralymphatic immunotherapy for cat allergy induces tolerance after only 3 injections. J Allergy Clin Immunol. 2012;129:1290–6. doi: 10.1016/j.jaci.2012.02.026. [DOI] [PubMed] [Google Scholar]
- [73].Aalberse RC, van der Gaag R, van Leeuwen J. Serologic aspects of IgG4 antibodies .1. Prolonged immunization results in an IgG4-restricted response. J Immunol. 1983;130:722–6. [PubMed] [Google Scholar]
- [74].Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–1. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- [75].Long SA, Buckner JH. CD4+FOXP3+ T regulatory cells in human autoimmunity: more than a numbers game. J Immunol. 2011;187:2061–6. doi: 10.4049/jimmunol.1003224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Veldman C, Stauber A, Wassmuth R, Uter W, Schuler G, Hertl M. Dichotomy of autoreactive Th1 and Th2 cell responses to desmoglein 3 in patients with pemphigus vulgaris (PV) and healthy carriers of PV-associated HLA class II alleles. J Immunol. 2003;170:635–42. doi: 10.4049/jimmunol.170.1.635. [DOI] [PubMed] [Google Scholar]
- [77].Veldman CM, Gebhard KL, Uter W, Wassmuth R, Grotzinger J, Schultz E, et al. T cell recognition of desmoglein 3 peptides in patients with pemphigus vulgaris and healthy individuals. J Immunol. 2004;172:3883–92. doi: 10.4049/jimmunol.172.6.3883. [DOI] [PubMed] [Google Scholar]
- [78].Veldman C, Höhne A, Dieckmann D, Schuler G, Hertl M. Type I regulatory T cells specific for desmoglein 3 are more frequently detected in healthy individuals than in patients with pemphigus vulgaris. J Immunol. 2004;172:6468–75. doi: 10.4049/jimmunol.172.10.6468. [DOI] [PubMed] [Google Scholar]
- [79].Shoukry NH, Cawthon AG, Walker CM. Cell-mediated immunity and the outcome of hepatitis C virus infection. Annu Rev Microbiol. 2004;58:391–424. doi: 10.1146/annurev.micro.58.030603.123836. [DOI] [PubMed] [Google Scholar]
- [80].Blackburn SD, Wherry EJ. IL-10, T cell exhaustion and viral persistence. Trends Microbiol. 2007;15:143–6. doi: 10.1016/j.tim.2007.02.006. [DOI] [PubMed] [Google Scholar]
- [81].Ejrnaes M, Filippi CM, Martinic MM, Ling EM, Togher LM, Crotty S, et al. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J Exp Med. 2006;203:2461–72. doi: 10.1084/jem.20061462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MB. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med. 2006;12:1301–9. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Clerici M, Wynn TA, Berzofsky JA, Blatt SP, Hendrix CW, Sher A, et al. Role of interleukin-10 in T helper cell dysfunction in asymptomatic individuals infected with the human immunodeficiency virus. J Clin Invest. 1994;93:768–75. doi: 10.1172/JCI117031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Rigopoulou EI, Abbott WG, Haigh P, Naoumov NV. Blocking of interleukin-10 receptor--a novel approach to stimulate T-helper cell type 1 responses to hepatitis C virus. Clin Immunol. 2005;117:57–64. doi: 10.1016/j.clim.2005.06.003. [DOI] [PubMed] [Google Scholar]
- [85].Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–50. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- [86].Yang M, Rui K, Wang S, Lu L. Regulatory B cells in autoimmune diseases. Cell Mol Immunol. 2013;10:122–32. doi: 10.1038/cmi.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Zhu HQ, Xu RC, Chen YY, Yuan HJ, Cao H, Zhao XQ, et al. Impaired Function of CD19 CD24 CD38 Regulatory B Cells in Pemphigus Patients. Br J Dermatol. 2014 doi: 10.1111/bjd.13192. in press. [DOI] [PubMed] [Google Scholar]
- [88].Colliou N, Picard D, Caillot F, Calbo S, Le CS, Lim A, et al. Long-term remissions of severe pemphigus after rituximab therapy are associated with prolonged failure of desmoglein B cell response. Sci Transl Med. 2013;5:175ra30. doi: 10.1126/scitranslmed.3005166. [DOI] [PubMed] [Google Scholar]
- [89].Vallerskog T, Heimburger M, Gunnarsson I, Zhou W, Wahren-Herlenius M, Trollmo C, et al. Differential effects on BAFF and APRIL levels in rituximab-treated patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Res Ther. 2006;8:R167. doi: 10.1186/ar2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Yang M, Sun L, Wang S, Ko KH, Xu H, Zheng BJ, et al. Novel function of B cell-activating factor in the induction of IL-10-producing regulatory B cells. J Immunol. 2010;184:3321–5. doi: 10.4049/jimmunol.0902551. [DOI] [PubMed] [Google Scholar]
- [91].Sun F, Ladha SS, Yang L, Liu Q, Shi SX, Su N, et al. Interleukin-10 producing-B cells and their association with responsiveness to rituximab in myasthenia gravis. Muscle Nerve. 2014;49:487–94. doi: 10.1002/mus.23951. [DOI] [PubMed] [Google Scholar]
- [92].Llorente L, Richaud-Patin Y, Fior R, Alcocer-Varela J, Wijdenes J, Fourrier BM, et al. In vivo production of interleukin-10 by non-T cells in rheumatoid arthritis, Sjogren's syndrome, and systemic lupus erythematosus. A potential mechanism of B lymphocyte hyperactivity and autoimmunity. Arthritis Rheum. 1994;37:1647–55. doi: 10.1002/art.1780371114. [DOI] [PubMed] [Google Scholar]
- [93].Grondal G, Gunnarsson I, Ronnelid J, Rogberg S, Klareskog L, Lundberg I. Cytokine production, serum levels and disease activity in systemic lupus erythematosus. Clin Exp Rheumatol. 2000;18:565–70. [PubMed] [Google Scholar]
- [94].Houssiau FA, Lefebvre C, Vanden Berghe M, Lambert M, Devogelaer JP, Renauld JC. Serum interleukin 10 titers in systemic lupus erythematosus reflect disease activity. Lupus. 1995;4:393–5. doi: 10.1177/096120339500400510. [DOI] [PubMed] [Google Scholar]
- [95].Park YB, Lee SK, Kim DS, Lee J, Lee CH, Song CH. Elevated interleukin-10 levels correlated with disease activity in systemic lupus erythematosus. Clin Exp Rheumatol. 1998;16:283–8. [PubMed] [Google Scholar]
- [96].Lazarus M, Hajeer AH, Turner D, Sinnott P, Worthington J, Ollier WE, et al. Genetic variation in the interleukin 10 gene promoter and systemic lupus erythematosus. J Rheumatol. 1997;24:2314–7. [PubMed] [Google Scholar]
- [97].Gibson AW, Edberg JC, Wu J, Westendorp RG, Huizinga TW, Kimberly RP. Novel single nucleotide polymorphisms in the distal IL-10 promoter affect IL-10 production and enhance the risk of systemic lupus erythematosus. J Immunol. 2001;166:3915–22. doi: 10.4049/jimmunol.166.6.3915. [DOI] [PubMed] [Google Scholar]
- [98].Ishida H, Muchamuel T, Sakaguchi S, Andrade S, Menon S, Howard M. Continuous administration of anti-interleukin 10 antibodies delays onset of autoimmunity in NZB/W F1 mice. J Exp Med. 1994;179:305–10. doi: 10.1084/jem.179.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Llorente L, Zou W, Levy Y, Richaud-Patin Y, Wijdenes J, Alcocer-Varela J, et al. Role of interleukin 10 in the B lymphocyte hyperactivity and autoantibody production of human systemic lupus erythematosus. J Exp Med. 1995;181:839–44. doi: 10.1084/jem.181.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].al-Janadi M, al-Dalaan A, al-Balla S, al-Humaidi M, Raziuddin S. Interleukin-10 (IL-10) secretion in systemic lupus erythematosus and rheumatoid arthritis: IL-10-dependent CD4+CD45RO+ T cell-B cell antibody synthesis. J Clin Immunol. 1996;16:198–207. doi: 10.1007/BF01541225. [DOI] [PubMed] [Google Scholar]
- [101].Llorente L, Richaud-Patin Y, Garcia-Padilla C, Claret E, Jakez-Ocampo J, Cardiel MH, et al. Clinical and biologic effects of anti-interleukin-10 monoclonal antibody administration in systemic lupus erythematosus. Arthritis Rheum. 2000;43:1790–800. doi: 10.1002/1529-0131(200008)43:8<1790::AID-ANR15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- [102].Cush JJ, Splawski JB, Thomas R, McFarlin JE, Schulze-Koops H, Davis LS, et al. Elevated interleukin-10 levels in patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:96–104. doi: 10.1002/art.1780380115. [DOI] [PubMed] [Google Scholar]
- [103].Mauri C, Gray D, Mushtaq N, Londei M. Prevention of arthritis by interleukin 10-producing B cells. J Exp Med. 2003;197:489–501. doi: 10.1084/jem.20021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Kawakami A, Eguchi K, Matsuoka N, Tsuboi M, Urayama S, Kawabe Y, et al. Inhibitory effects of interleukin-10 on synovial cells of rheumatoid arthritis. Immunology. 1997;91:252–9. doi: 10.1046/j.1365-2567.1997.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].van Roon J, Wijngaarden S, Lafeber FP, Damen C, van de Winkel J, Bijlsma JW. Interleukin 10 treatment of patients with rheumatoid arthritis enhances Fc gamma receptor expression on monocytes and responsiveness to immune complex stimulation. J Rheumatol. 2003;30:648–51. [PubMed] [Google Scholar]
- [106].Schwager K, Kaspar M, Bootz F, Marcolongo R, Paresce E, Neri D, et al. Preclinical characterization of DEKAVIL (F8-IL10), a novel clinical-stage immunocytokine which inhibits the progression of collagen-induced arthritis. Arthritis Res Ther. 2009;11:R142. doi: 10.1186/ar2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Combination Therapy of F8-IL10 and Methotrexate in Rheumatoid Arthritis Patients. 2014. www clinicaltrials gov. NCT02076659.